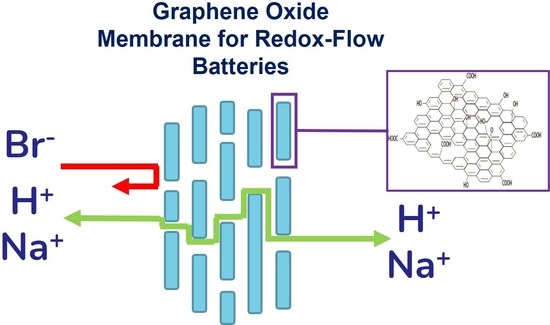
Permselectivity and Ionic Conductivity Study of Na+ and Br− Ions in Graphene Oxide-Based Membranes for Redox Flow Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Nafion 117 Membrane Activation Treatment
2.2. GO Membrane Fabrication
2.3. Characterization
2.4. Permselectivity and Ionic Conductivity Measurement
3. Results
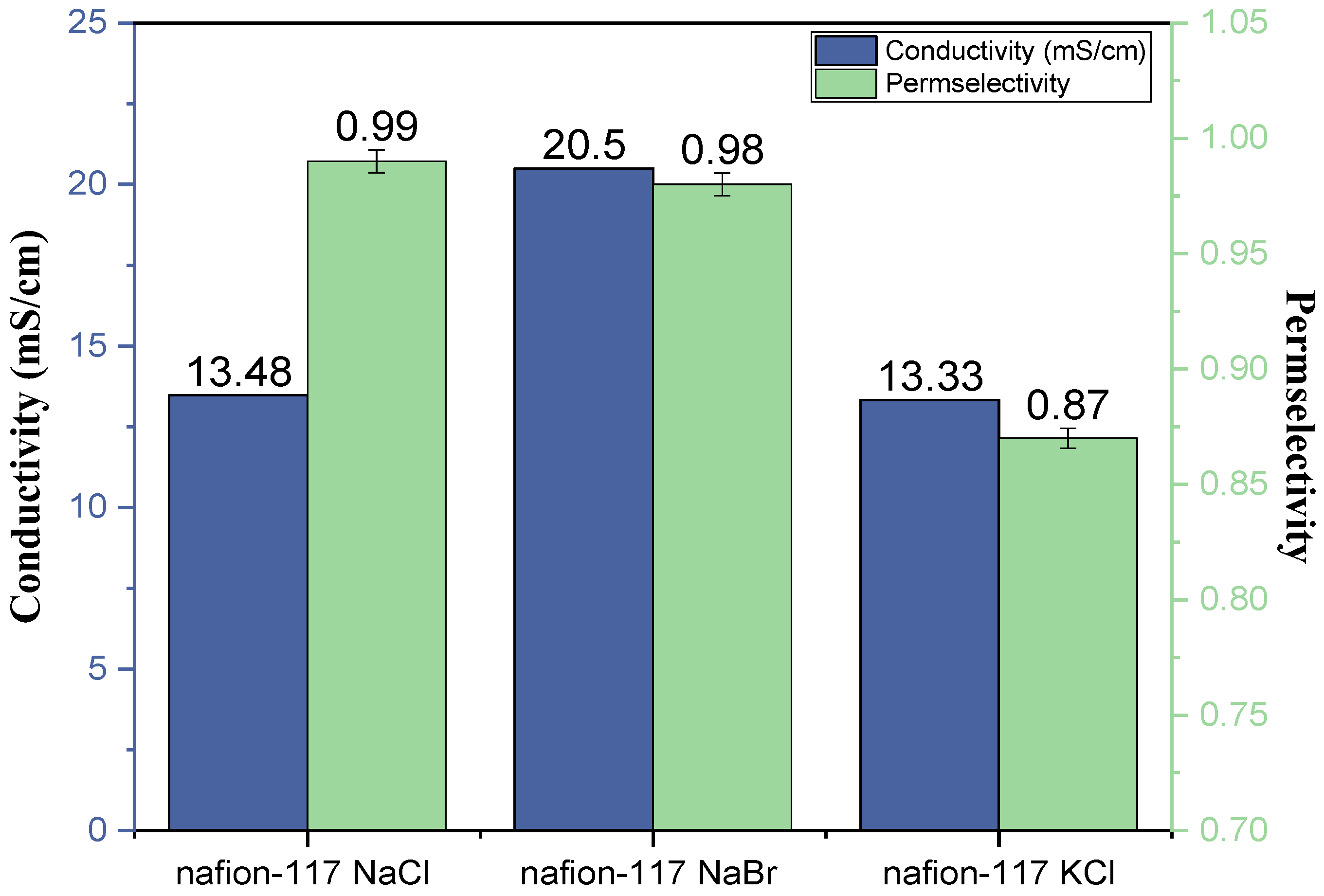
3.1. Electrochemical Characterization (Ionic Conductivity–Permselectivity)
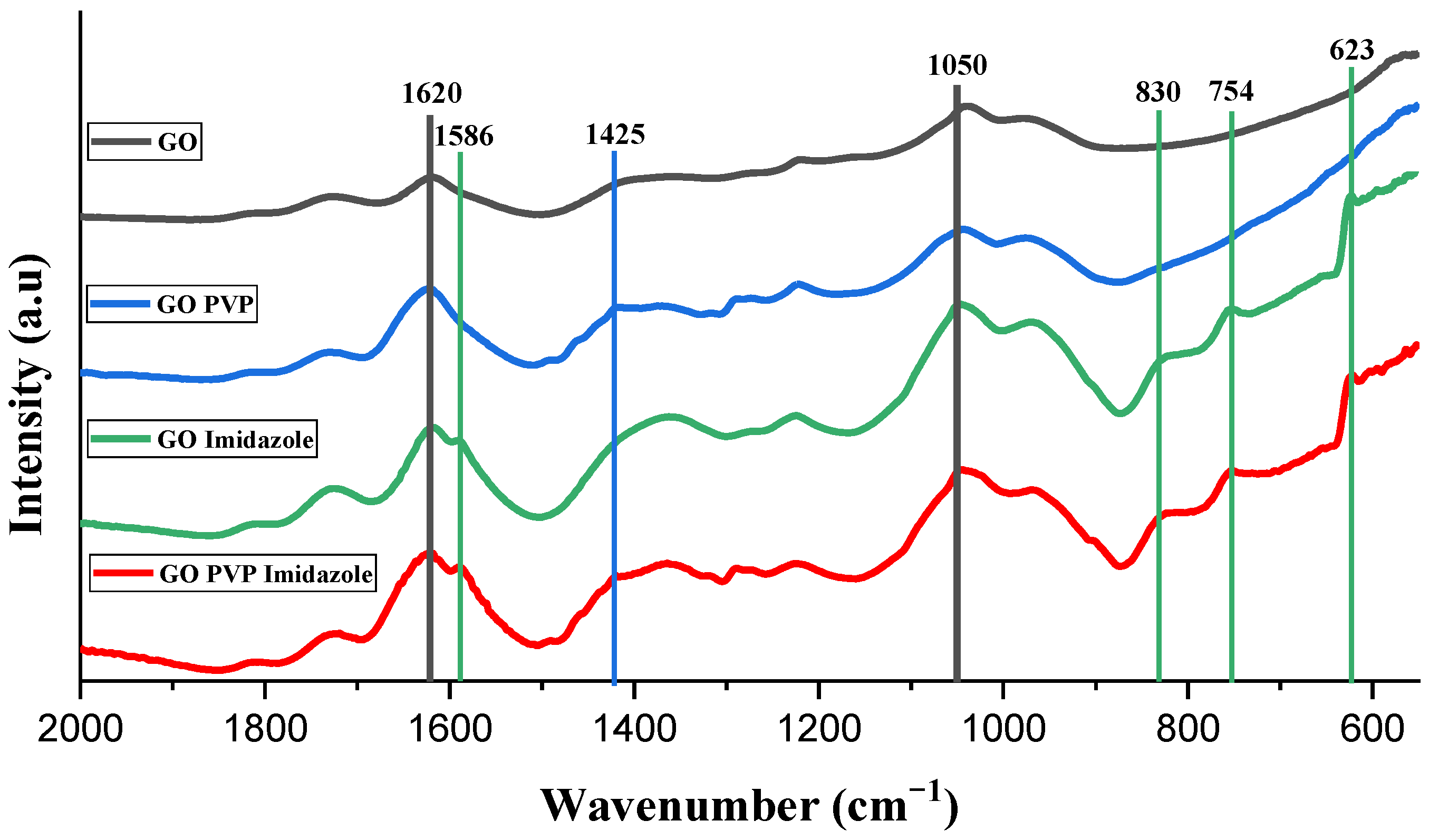
3.2. IR Spectroscopy
3.3. Raman
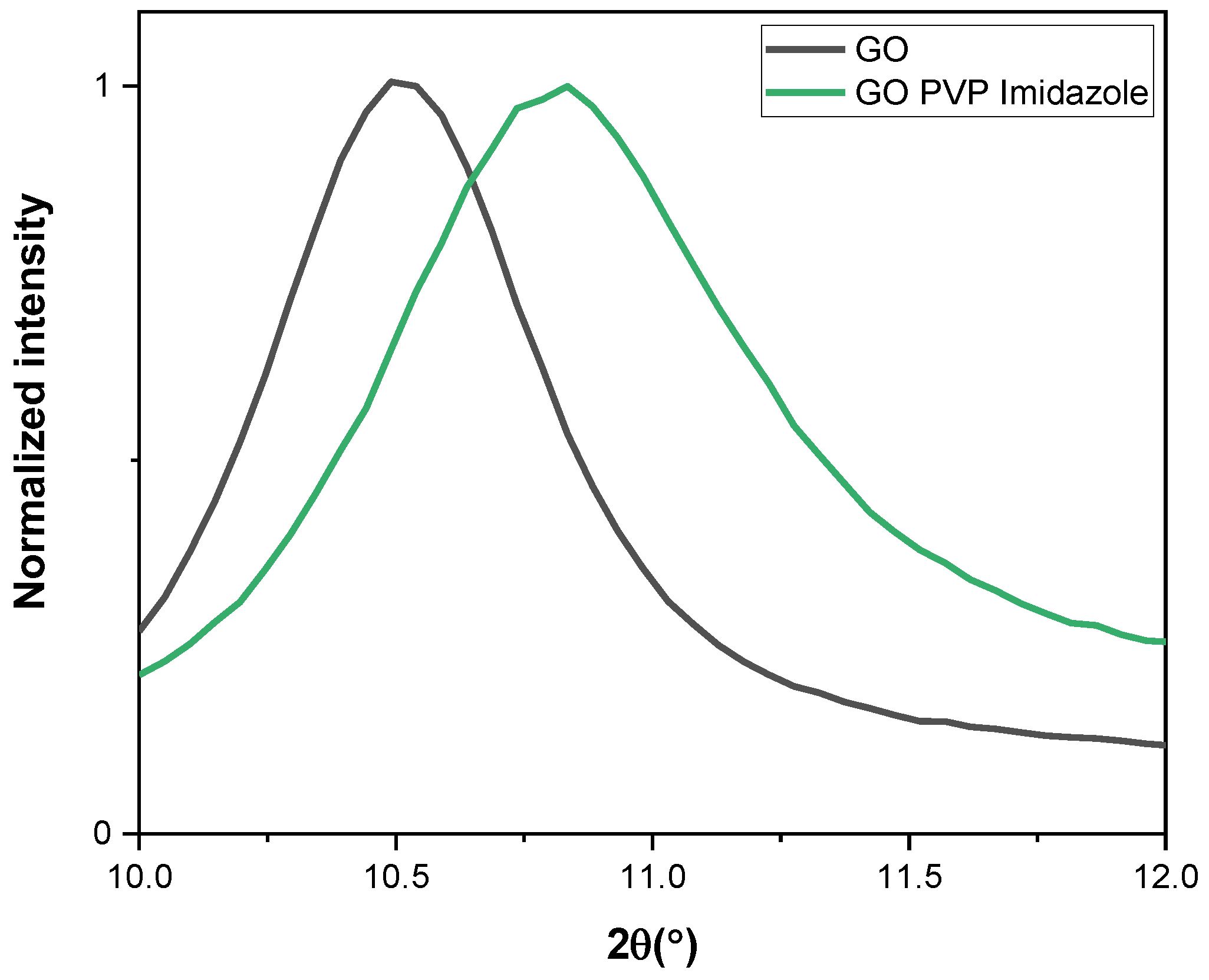
3.4. XRD
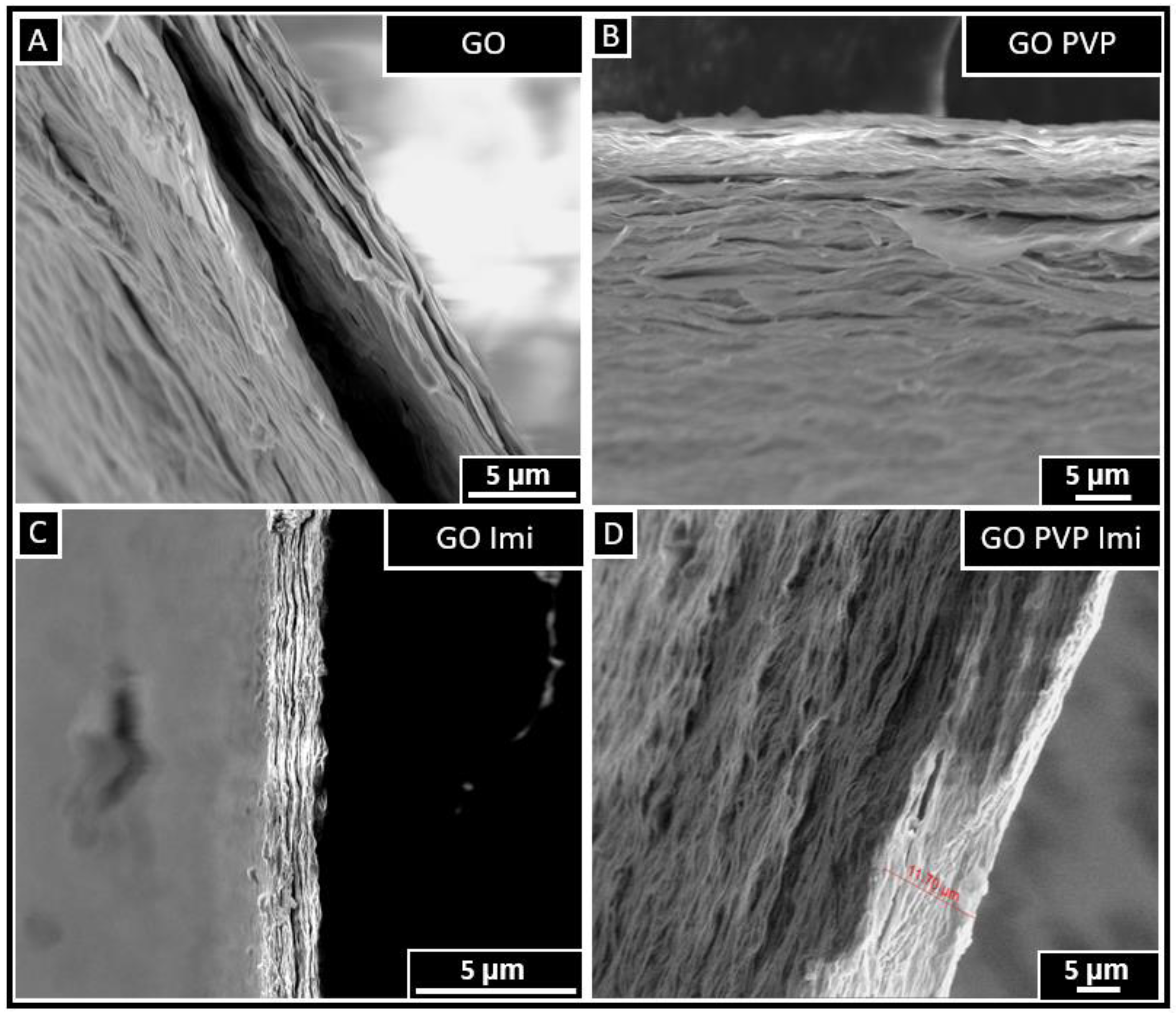
3.5. SEM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, A.Z.; Mench, M.; Meyers, J.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Küttinger, M.; Wlodarczyk, J.K.; Daubner, D.; Fischer, P.; Tübke, J. High Energy Density Electrolytes for H2/Br2 Redox Flow Batteries, Their Polybromide Composition and Influence on Battery Cycling Limits. RSC Adv. 2021, 11, 5218–5229. [Google Scholar] [CrossRef]
- Saadi, K.; Fan, X.; Hardisty, S.S.; Pintauro, P.; Zitoun, D. Ultralow Platinum Loading for Redox-Flow Battery by Electrospinning the Electrocatalyst and the Ionomer in Core-Shell Fibers. J. Energy Storage 2023, 59, 106430. [Google Scholar] [CrossRef]
- Saadi, K.; Hardisty, S.S.; Tatus-Portnoy, Z.; Zitoun, D. Influence of Loading, Metallic Surface State and Surface Protection in Precious Group Metal Hydrogen Electrocatalyst for H2/Br2 Redox-Flow Batteries. J. Power Sources 2022, 536, 231494. [Google Scholar] [CrossRef]
- Saadi, K.; Kuettinger, M.; Fischer, P.; Zitoun, D. Hydrogen-Bromine Redox-Flow Battery Cycling with Bromine Complexing Agent: On the Benefits of Nanoporous Separator Versus Proton Exchange Membrane. Energy Technol. 2021, 9, 2000978. [Google Scholar] [CrossRef]
- Saadi, K.; Nanikashvili, P.; Tatus-Portnoy, Z.; Hardisty, S.; Shokhen, V.; Zysler, M.; Zitoun, D. Crossover-Tolerant Coated Platinum Catalysts in Hydrogen/Bromine Redox Flow Battery. J. Power Sources 2019, 422, 84–91. [Google Scholar] [CrossRef]
- Suresh, S.; Ulaganathan, M.; Venkatesan, N.; Periasamy, P.; Ragupathy, P. High Performance Zinc-Bromine Redox Flow Batteries: Role of Various Carbon Felts and Cell Configurations. J. Energy Storage 2018, 20, 134–139. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for Redox Flow Battery Applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef]
- Gubler, L. Membranes and Separators for Redox Flow Batteries. Curr. Opin. Electrochem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, L.; Chen, Y.; Peng, S.; Yu, G. A Chemistry and Microstructure Perspective on Ion-Conducting Membranes for Redox Flow Batteries. Angew. Chem. Int. Ed. 2021, 60, 24770–24798. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, R.; Hegde, M.; Wang, J.; Kusoglu, A.; You, W.; Coronell, O. Tunable Anion Exchange Membrane Conductivity and Permselectivity via Non-Covalent, Hydrogen Bond Cross-Linking. ACS Appl. Mater. Interfaces 2021, 13, 52647–52658. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, H.; Xiao, C.; Tong, X.; Chen, Y. The Trade-off between Membrane Permselectivity and Conductivity: A Percolation Simulation of Mass Transport. J. Membr. Sci. 2020, 597, 117751. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Pourcelly, G.; Yaroslavtsev, A.B. Permselectivity and Ion-Conductivity of Grafted Cation-Exchange Membranes Based on UV-Oxidized Polymethylpenten and Sulfonated Polystyrene. Sep. Purif. Technol. 2018, 207, 329–335. [Google Scholar] [CrossRef]
- Lee, C.; Wang, X.; Peng, J.-K.; Katzenberg, A.; Ahluwalia, R.K.; Kusoglu, A.; Komini Babu, S.; Spendelow, J.S.; Mukundan, R.; Borup, R.L. Toward a Comprehensive Understanding of Cation Effects in Proton Exchange Membrane Fuel Cells. ACS Appl. Mater. Interfaces 2022, 14, 35555–35568. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next Generation of Proton-Exchange Membrane Fuel Cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Hugo, Y.A.; Kout, W.; Sikkema, F.; Borneman, Z.; Nijmeijer, K. Performance mapping of cation exchange membranes for hydrogen-bromine flow batteries for energy storage. J. Memb. Sci. 2018, 566, 406–414. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, K.-S. Fuel Cell Membrane Characterizations. Polym. Rev. 2015, 55, 330–370. [Google Scholar] [CrossRef]
- Woo Park, J.; Wycisk, R.; Lin, G.; Chong, P.Y.; Powers, D.; Van Nguyen, T.; Dowd, R.P., Jr.; Pintauro, P.N. Electrospun Nafion/PVDF single-fiber blended membranes for regenerative H2/Br2 fuel cells. J. Memb. Sci. 2017, 541, 85–92. [Google Scholar] [CrossRef]
- Kontturi, K.; Mafé, S.; Manzanares, J.A.; Murtomäki, L.; Viinikka, P. Limiting Currents and Sodium Transport Numbers in Nafion Membranes. Electrochim. Acta 1994, 39, 883–888. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, H.; Xu, W.; Yuan, Z.; Li, X. Mechanism and Transfer Behavior of Ions in Nafion Membranes under Alkaline Media. J. Membr. Sci. 2018, 566, 8–14. [Google Scholar] [CrossRef]
- Ling, X.; Bonn, M.; Domke, K.F.; Parekh, S.H. Correlated Interfacial Water Transport and Proton Conductivity in Perfluorosulfonic Acid Membranes. Proc. Natl. Acad. Sci. USA 2019, 116, 8715–8720. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ikhsan, M.M.; Jin, K.S.; Henkensmeier, D. Nanostructure-property relationship of two perfluorinated sulfonic acid (PFSA) membranes. Int. J. Energy Res. 2022, 46, 11265–11277. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Kim, S.; Wang, H.; Lee, Y.M. 2D Nanosheets and Their Composite Membranes for Water, Gas, and Ion Separation. Angew. Chem. Int. Ed. 2019, 58, 17512–17527. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, G.; Zhao, J.; Jin, W. Two-Dimensional-Material Membranes: Manipulating the Transport Pathway for Molecular Separation. Acc. Mater. Res. 2021, 2, 114–128. [Google Scholar] [CrossRef]
- Wang, W.; Wei, Y.; Fan, J.; Cai, J.; Lu, Z.; Ding, L.; Wang, H. Recent Progress of Two-Dimensional Nanosheet Membranes and Composite Membranes for Separation Applications. Front. Chem. Sci. Eng. 2021, 15, 793–819. [Google Scholar] [CrossRef]
- Taufiq Musa, M.; Shaari, N.; Kamarudin, S.K. Carbon Nanotube, Graphene Oxide and Montmorillonite as Conductive Fillers in Polymer Electrolyte Membrane for Fuel Cell: An Overview. Int. J. Energy Res. 2021, 45, 1309–1346. [Google Scholar] [CrossRef]
- Baudino, L.; Pedico, A.; Bianco, S.; Periolatto, M.; Pirri, C.F.; Lamberti, A. Crown-Ether Functionalized Graphene Oxide Membrane for Lithium Recovery from Water. Membranes 2022, 12, 233. [Google Scholar] [CrossRef]
- Epsztein, R.; DuChanois, R.M.; Ritt, C.L.; Noy, A.; Elimelech, M. Towards Single-Species Selectivity of Membranes with Subnanometre Pores. Nat. Nanotechnol. 2020, 15, 426–436. [Google Scholar] [CrossRef]
- Goutham, S.; Keerthi, A.; Ismail, A.; Bhardwaj, A.; Jalali, H.; You, Y.; Li, Y.; Hassani, N.; Peng, H.; Martins, M.V.S.; et al. Beyond Steric Selectivity of Ions Using Ångström-Scale Capillaries. Nat. Nanotechnol. 2023, 18, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Pedico, A.; Fontana, M.; Bianco, S.; Kara, S.; Periolatto, M.; Carminati, S.; Pirri, C.F.; Tresso, E.; Lamberti, A. Graphene Oxide Membranes for Trace Hydrocarbon Contaminant Removal from Aqueous Solution. Nanomaterials 2020, 10, 2242. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Liu, J.; Chen, L.; Liang, S.; Fang, H. Unexpected Ion Sieving in Graphene Oxide Membranes. J. Phys. Chem. C 2022, 126, 9572–9579. [Google Scholar] [CrossRef]
- Liu, M.; Weston, P.J.; Hurt, R.H. Controlling Nanochannel Orientation and Dimensions in Graphene-Based Nanofluidic Membranes. Nat. Commun. 2021, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, M.; Wang, B.; Yang, F.; Quan, X.; Tang, C.Y.; Dong, Y. Cross-Linked Graphene Oxide Framework Membranes with Robust Nano-Channels for Enhanced Sieving Ability. Environ. Sci. Technol. 2020, 54, 15442–15453. [Google Scholar] [CrossRef]
- Pedico, A.; Baudino, L.; Aixalà-Perelló, A.; Lamberti, A. Green Methods for the Fabrication of Graphene Oxide Membranes: From Graphite to Membranes. Membranes 2023, 13, 429. [Google Scholar] [CrossRef]
- Ying, L.; Guo, X.; Fang, J. Synthesis, Freestanding Membrane Formation, and Properties of Novel Sulfonated Hyperbranched Polyimides. High Perform. Polym. 2018, 30, 3–15. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Alomari, N.; Aparicio, S.; Fleming, P.D.; Pekarovicova, A.; Wu, Q.; Atilhan, M. Understanding of Three Different Polyvinylpyrrolidone (PVP) Based Battery Binders Blends on Graphene Surfaces from First Principles via DFT Simulations. Mater. Chem. Phys. 2023, 301, 127548. [Google Scholar] [CrossRef]
- Aslan, M.; Weingarth, D.; Jäckel, N.; Atchison, J.S.; Grobelsek, I.; Presser, V. Polyvinylpyrrolidone as Binder for Castable Supercapacitor Electrodes with High Electrochemical Performance in Organic Electrolytes. J. Power Sources 2014, 266, 374–383. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, M.; Cheng, L.; Yuan, J.; Liu, G.; Huang, L.; Barboiu, M.; Jin, W. Bola-Amphiphile-Imidazole Embedded GO Membrane with Enhanced Solvent Dehydration Properties. J. Membr. Sci. 2020, 595, 117545. [Google Scholar] [CrossRef]
- Aixalà-Perelló, A.; Pedico, A.; Laurenti, M.; Fontananova, E.; Bocchini, S.; Ferrari, I.V.; Lamberti, A. Scalable and Highly Selective Graphene-Based Ion-Exchange Membranes with Tunable Permselectivity. NPJ 2D Mater. Appl. 2023, 7, 46. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Renishaw Apply Innovation. Raman Measurements on Graphene; AS039(EN)-01-A; Spectroscopy Product Division: New Mills, UK, 2014. [Google Scholar]
- Thomson, S. Tuning the Photoluminescence of Graphene Oxide. Edinburgh 2018. Available online: https://www.edinst.com/fr/library/tuning-the-photoluminescence-of-graphene-oxide/ (accessed on 29 May 2023).
- Thi, T.M.; Tinh, L.V.; Van, B.H.; Ben, P.V.; Trung, V.Q. The Effect of Polyvinylpyrrolidone on the Optical Properties of the Ni-Doped ZnS Nanocrystalline Thin Films Synthesized by Chemical Method. J. Nanomater. 2012, 2012, 528047. [Google Scholar] [CrossRef]
- Razzano, V.; Paolino, M.; Reale, A.; Giuliani, G.; Artusi, R.; Caselli, G.; Visintin, M.; Makovec, F.; Donati, A.; Villafiorita-Monteleone, F.; et al. Development of Imidazole-Reactive Molecules Leading to a New Aggregation-Induced Emission Fluorophore Based on the Cinnamic Scaffold. ACS Omega 2017, 2, 5453–5459. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sadanandhan, A.M.; Jain, S.L. Silver doped reduced graphene oxide as promising plasmonic photocatalyst for oxidative coupling of benzylamines under visible light irradiation. New J. Chem. 2019, 43, 9116–9122. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Flotron, S.; Zhu, S.; Call, D.; Coronell, O. Junction Potentials Bias Measurements of Ion Exchange Membrane Permselectivity. Environ. Sci. Technol. 2018, 52, 4929–4936. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flack, R.; Aixalà-Perelló, A.; Pedico, A.; Saadi, K.; Lamberti, A.; Zitoun, D. Permselectivity and Ionic Conductivity Study of Na+ and Br− Ions in Graphene Oxide-Based Membranes for Redox Flow Batteries. Membranes 2023, 13, 695. https://doi.org/10.3390/membranes13080695
Flack R, Aixalà-Perelló A, Pedico A, Saadi K, Lamberti A, Zitoun D. Permselectivity and Ionic Conductivity Study of Na+ and Br− Ions in Graphene Oxide-Based Membranes for Redox Flow Batteries. Membranes. 2023; 13(8):695. https://doi.org/10.3390/membranes13080695
Chicago/Turabian StyleFlack, Raphael, Anna Aixalà-Perelló, Alessandro Pedico, Kobby Saadi, Andrea Lamberti, and David Zitoun. 2023. "Permselectivity and Ionic Conductivity Study of Na+ and Br− Ions in Graphene Oxide-Based Membranes for Redox Flow Batteries" Membranes 13, no. 8: 695. https://doi.org/10.3390/membranes13080695
APA StyleFlack, R., Aixalà-Perelló, A., Pedico, A., Saadi, K., Lamberti, A., & Zitoun, D. (2023). Permselectivity and Ionic Conductivity Study of Na+ and Br− Ions in Graphene Oxide-Based Membranes for Redox Flow Batteries. Membranes, 13(8), 695. https://doi.org/10.3390/membranes13080695