Recent Advanced Synthesis Strategies for the Nanomaterial-Modified Proton Exchange Membrane in Fuel Cells
Abstract
1. Introduction
2. Nanomaterials Doped PEM
- Cost-effective: Nanomaterials can make PEMs more affordable by lowering the number of expensive components used to create high-performance PEMs [55].
- Durability: PEMs treated with nanomaterials have demonstrated enhanced toughness and resilience to deterioration, making them more appropriate for long-term usage in fuel cells [56].
- High Thermal Stability: PEMs enhanced with nanomaterials have better thermal stability, which makes them more resistant to heat damage, which is crucial for high-temperature PEM fuel cells [57].
- Lower Membrane Crossover: It has been demonstrated that PEMs modified with nanomaterials have lower membrane crossover, which refers to the unintended passage of reactants over the membrane in fuel cells. This increases the effectiveness of the fuel cell and reduces reactant loss [58].
- Compatibility with Different Polymers: Composite PEMs that are compatible with a variety of fuel cell technologies may be created by combining nanomaterials with several kinds of polymers. As a result, the design of fuel cells has more freedom [59].
2.1. Metalloids or Metal Oxide-Based Nanomaterials
2.1.1. Silica Nanoparticles
2.1.2. Titanium Dioxide
2.1.3. Zirconium Dioxide
2.2. Carbon-Based Nanomaterials
2.2.1. CNTs
2.2.2. Graphene
2.2.3. Fullerenes
2.2.4. Nanodiamonds
2.3. Polymeric Nanomaterials
3. Synthesis Methods of Nanomaterials Modified PEM
- In situ polymerization: To obtain a copolymer, the polymerization process is carried out either with or without additives, leading to the formation of a composite. When monomers and nanoadditives are present during synthesis, the resulting copolymer incorporates the nanoadditives. Following the polymerization process, the product undergoes purification, and subsequent drying yields a polymer powder. To prepare a membrane, a solution is typically prepared from the polymer powder, and then casting techniques are employed.
- Solution casting: In this method, the nanomaterial is first dispersed in a solvent, and then the polymer solution is added to it. The resulting mixture is then cast into a thin film and dried to form the nanocomposite membrane.
- Electrospinning: In this method, a polymer solution containing the nanomaterial is electrospun into a nanofiber membrane. The nanofiber membrane has a high surface area and excellent mechanical strength.
- Inclusion of nanomaterials in the pre-formed membrane: This method involves the incorporation of nanomaterials into pre-formed proton exchange membranes. The nanomaterials can be added by impregnation, in situ growth, or coating.
- Layer-by-layer assembly: This method involves the layer-by-layer deposition of nanomaterials and polyelectrolytes to form a nanocomposite membrane. The resulting membrane has a well-defined structure and high proton conductivity.
3.1. In Situ Polymerization
3.2. Solution Casting
3.3. Electrospinning
3.4. Layer-by-Layer Assembly
4. Properties of Nanomaterial Modified PEMs
4.1. Electric Properties
4.2. Thermal Properties
4.3. Structural Properties
5. Future Scope of Nanomaterials Modified PEM
- Performance improvement: Improving PEM performance is one of the main goals. To increase efficiency and durability, scientists are creating novel materials, enhancing the electrolyte’s conductivity, and enhancing membrane structure.
- Cost reduction: The high price of fuel cells is one of the main obstacles to their broad implementation. Researchers are aiming to lower the cost of fuel cells by using inexpensive materials and new, less energy-intensive production techniques.
- Durability: The short lifespan of PEMs is another significant obstacle. New materials that are more resilient and can resist challenging operating circumstances are now being developed by researchers.
- Miniaturization: Another interesting development is the miniaturization of fuel cells. In order to power portable electronics, such as laptops, cell phones, and other electronic devices, researchers are striving to create miniature fuel cells.
- Integration: Another area of study is the integration of fuel cells with other energy storage technologies. For the purpose of developing hybrid energy storage systems that can serve as a dependable and sustainable source of power, researchers are looking into the feasibility of combining fuel cells with batteries and supercapacitors.
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lucia, U. Overview on fuel cells. Renew. Sustain. Energy Rev. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Li, D.; Guo, H.; Jiang, S.; Zeng, G.; Zhou, W.; Li, Z. Microstructures and electrochemical performances of TiO2-coated Mg–Zr co-doped NCM as a cathode material for lithium-ion batteries with high power and long circular life. New J. Chem. 2021, 45, 19446–19455. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@ Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. [Google Scholar] [CrossRef] [PubMed]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Muduli, R.C.; Kale, P. Silicon nanostructures for solid-state hydrogen storage: A review. Int. J. Hydrogen Energy 2023, 48, 1401–1439. [Google Scholar] [CrossRef]
- Zhao, W.; Zeng, Y.; Zhao, Y.; Wu, X. Recent advances in metal-organic framework-based electrode materials for supercapacitors: A review. J. Energy Storage 2023, 62, 106934. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel cells: Principles, types, fuels, and applications. ChemPhysChem 2000, 1, 162–193. [Google Scholar] [CrossRef]
- Setzler, B.P.; Zhuang, Z.; Wittkopf, J.A.; Yan, Y. Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 2016, 11, 1020–1025. [Google Scholar] [CrossRef]
- Madheswaran, D.K.; Jayakumar, A. Recent advancements on non-platinum based catalyst electrode material for polymer electrolyte membrane fuel cells: A mini techno-economic review. Bull. Mater. Sci. 2021, 44, 287. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental aspects of fuel cells: A review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef]
- Pan, Z.; An, L.; Wen, C. Recent advances in fuel cells based propulsion systems for unmanned aerial vehicles. Appl. Energy 2019, 240, 473–485. [Google Scholar] [CrossRef]
- Kishore, S.C.; Perumal, S.; Atchudan, R.; Alagan, M.; Sundramoorthy, A.K.; Lee, Y.R. A Critical Review on Artificial Intelligence for Fuel Cell Diagnosis. Catalysts 2022, 12, 743. [Google Scholar] [CrossRef]
- Ye, Y.; Gong, L.; Xiang, S.; Zhang, Z.; Chen, B. Metal–organic frameworks as a versatile platform for proton conductors. Adv. Mater. 2020, 32, 1907090. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Fuel cells, an alternative to standard sources of energy. Renew. Sustain. Energy Rev. 2002, 6, 295–304. [Google Scholar] [CrossRef]
- Ehteshami, S.M.M.; Chan, S. The role of hydrogen and fuel cells to store renewable energy in the future energy network–potentials and challenges. Energy Policy 2014, 73, 103–109. [Google Scholar] [CrossRef]
- Nimir, W.; Al-Othman, A.; Tawalbeh, M.; Al Makky, A.; Ali, A.; Karimi-Maleh, H.; Karimi, F.; Karaman, C. Approaches towards the development of heteropolyacid-based high temperature membranes for PEM fuel cells. Int. J. Hydrogen Energy 2023, 48, 6638–6656. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Dutta, K.; Khoo, K.S.; Soto-Moscoso, M. An overview on light assisted techniques for waste-derived hydrogen fuel towards aviation industry. Fuel 2023, 334, 126645. [Google Scholar] [CrossRef]
- Pramuanjaroenkij, A.; Kakaç, S. The fuel cell electric vehicles: The highlight review. Int. J. Hydrogen Energy 2023, 48, 9401–9425. [Google Scholar] [CrossRef]
- Chehrmonavari, H.; Kakaee, A.; Hosseini, S.E.; Desideri, U.; Tsatsaronis, G.; Floerchinger, G.; Braun, R.; Paykani, A. Hybridizing solid oxide fuel cells with internal combustion engines for power and propulsion systems: A review. Renew. Sustain. Energy Rev. 2023, 171, 112982. [Google Scholar] [CrossRef]
- Scrosati, B. Power sources for portable electronics and hybrid cars: Lithium batteries and fuel cells. Chem. Rec. 2005, 5, 286–297. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Potential carbon nanomaterials as additives for state-of-the-art Nafion electrolyte in proton-exchange membrane fuel cells: A concise review. RSC Adv. 2021, 11, 18351–18370. [Google Scholar] [CrossRef]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Jurado, R.J.; Colomer, M.T. Protonic conductors for proton exchange membrane fuel cells: An overview. Hem. Ind. 2002, 56, 264–272. [Google Scholar] [CrossRef]
- Athanasaki, G.; Jayakumar, A.; Kannan, A. Gas diffusion layers for PEM fuel cells: Materials, properties and manufacturing–A review. Int. J. Hydrogen Energy 2023, 48, 2294–2313. [Google Scholar] [CrossRef]
- Bóna, Á.; Galambos, I.; Nemestóthy, N. Progress towards Stable and High-Performance Polyelectrolyte Multilayer Nanofiltration Membranes for Future Wastewater Treatment Applications. Membranes 2023, 13, 368. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells–a review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Schmittinger, W.; Vahidi, A. A review of the main parameters influencing long-term performance and durability of PEM fuel cells. J. Power Sources 2008, 180, 1–14. [Google Scholar] [CrossRef]
- Litster, S.; McLean, G. PEM fuel cell electrodes. J. Power Sources 2004, 130, 61–76. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Zhao, X.; Chen, L.; Peng, S.; Ma, C.; Duan, G.; Liu, Z.; Wang, H.; Yuan, Y. A poly (amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater. e-Polymers 2022, 22, 399–410. [Google Scholar] [CrossRef]
- Asensio, J.A.; Sánchez, E.M.; Gomez-Romero, P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem. Soc. Rev. 2010, 39, 3210–3239. [Google Scholar] [CrossRef]
- Kunitskaya, L.; Zheltonozhskaya, T.; Nesin, S.; Klepko, V.; Minenko, N. Dielectric Behavior of Solid Polymer Electrolyte Films Formed by Double Hydrophilic Block Copolymers. In Proceedings of the Nanomaterials and Nanocomposites, Nanostructure Surfaces, and Their Applications: Selected Proceedings of the IX International Conference Nanotechnology and Nanomaterials (NANO2021), Lviv, Ukraine, 25–28 August 2021; pp. 493–504. [Google Scholar]
- Awang, N.; Ismail, A.; Jaafar, J.; Matsuura, T.; Junoh, H.; Othman, M.; Rahman, M. Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. React. Funct. Polym. 2015, 86, 248–258. [Google Scholar] [CrossRef]
- Yang, X.; Qi, H.; Jiang, S.; Zhang, C.; Dong, X. Electrospun dual-aeolotropic conductive exceptive Janus membrane and Janus tubule functionalized by up-/down-converting fluorescence and magnetism. Mater. Chem. Front. 2022, 6, 3431–3441. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Organic–inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Bouziane, K.; Zamel, N.; Francois, X.; Meyer, Y.; Candusso, D. Effects of mechanical compression on the performance of Polymer Electrolyte Fuel Cells and analysis through in-situ characterisation techniques—A review. J. Power Sources 2019, 424, 8–26. [Google Scholar]
- Shen, X.; Liang, X.; Xu, Y.; Yu, W.; Li, Q.; Ge, X.; Wu, L.; Xu, T. In-situ growth of PPy/MnOx radical quenching layer for durability enhancement of proton exchange membrane in PEMFCs. J. Membr. Sci. 2023, 675, 121556. [Google Scholar] [CrossRef]
- Saqib, J.; Aljundi, I.H. Membrane fouling and modification using surface treatment and layer-by-layer assembly of polyelectrolytes: State-of-the-art review. J. Water Process Eng. 2016, 11, 68–87. [Google Scholar] [CrossRef]
- Nechitailov, A.A.; Volovitch, P.; Glebova, N.V.; Krasnova, A. Features of the Degradation of the Proton-Conducting Polymer Nafion in Highly Porous Electrodes of PEM Fuel Cells. Membranes 2023, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zou, Y.; Zhang, X.; Jin, Z.; Yang, Y.; Yang, L.; Duan, G.; Xu, Y.; Li, Y. Degradable and recyclable solar desalination membranes based on naturally occurring building blocks. Chem. Mater. 2022, 34, 10399–10408. [Google Scholar] [CrossRef]
- Raduwan, N.F.; Shaari, N.; Kamarudin, S.K.; Masdar, M.S.; Yunus, R.M. An overview of nanomaterials in fuel cells: Synthesis method and application. Int. J. Hydrogen Energy 2022, 47, 18468–18495. [Google Scholar] [CrossRef]
- Jayakumar, A.; Madheswaran, D.K.; Velu, R. Metal Additive Manufacturing of PEM Fuel Cell Flow Field Plates and the Scope of Nanomaterials for Its Fabrication. Nanotechnol.-Based Addit. Manuf. Prod. Des. Prop. Appl. 2023, 1, 103–129. [Google Scholar]
- Sharma, R.; Cherusseri, J.; Kar, K.K. Polymer electrolyte membrane fuel cells: Role of carbon nanotubes/graphene in cathode catalysis. In Handbook of Polymer Nanocomposites. Processing, Performance and Application: Volume B: Carbon Nanotube Based Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2015; pp. 361–390. [Google Scholar]
- Bet-Moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, S.P. Nanostructured Organic–Inorganic Hybrid Membranes for High-Temperature Proton Exchange Membrane Fuel Cells. In Functional Organic and Hybrid Nanostructured Materials: Fabrication, Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 383–418. [Google Scholar]
- Gao, J.; Dong, X.; Tian, Q.; He, Y. Carbon nanotubes reinforced proton exchange membranes in fuel cells: An overview. Int. J. Hydrogen Energy 2023, 48, 3216–3231. [Google Scholar] [CrossRef]
- Liu, G. Progress of high temperature polybenzimidazole proton exchange membrane: A systematic review. J. Phys. Conf. Ser. 2021, 2076, 012032. [Google Scholar] [CrossRef]
- Alabi, A.S.; Popoola, A.P.; Popoola, O.; Mathe, N.; Abdulwahab, M. Materials for electrocatalysts in proton exchange membrane fuel cell: A brief review. Front. Energy Res. 2023, 11, 112. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Lim, K.L. Protic ionic liquids as next-generation proton exchange membrane materials: Current status & future perspectives. React. Funct. Polym. 2022, 171, 105160. [Google Scholar]
- Saha, M.S.; Neburchilov, V.; Ghosh, D.; Zhang, J. Nanomaterials-supported Pt catalysts for proton exchange membrane fuel cells. Wiley Interdiscip. Rev. Energy Environ. 2013, 2, 31–51. [Google Scholar] [CrossRef]
- Scofield, M.E.; Liu, H.; Wong, S.S. A concise guide to sustainable PEMFCs: Recent advances in improving both oxygen reduction catalysts and proton exchange membranes. Chem. Soc. Rev. 2015, 44, 5836–5860. [Google Scholar] [CrossRef]
- Xing, Y.; Li, H.; Avgouropoulos, G. Research progress of proton exchange membrane failure and mitigation strategies. Materials 2021, 14, 2591. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton exchange membrane fuel cells (PEMFCs): Advances and challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Zhang, L.; Chae, S.-R.; Hendren, Z.; Park, J.-S.; Wiesner, M.R. Recent advances in proton exchange membranes for fuel cell applications. Chem. Eng. J. 2012, 204, 87–97. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Lai, J.-Y.; Liu, Y.-L. Polybenzimidazole (PBI)-functionalized silica nanoparticles modified PBI nanocomposite membranes for proton exchange membranes fuel cells. J. Membr. Sci. 2012, 403, 1–7. [Google Scholar]
- Du, L.; Yan, X.; He, G.; Wu, X.; Hu, Z.; Wang, Y. SPEEK proton exchange membranes modified with silica sulfuric acid nanoparticles. Int. J. Hydrogen Energy 2012, 37, 11853–11861. [Google Scholar] [CrossRef]
- Su, Y.-H.; Liu, Y.-L.; Sun, Y.-M.; Lai, J.-Y.; Wang, D.-M.; Gao, Y.; Liu, B.; Guiver, M.D. Proton exchange membranes modified with sulfonated silica nanoparticles for direct methanol fuel cells. J. Membr. Sci. 2007, 296, 21–28. [Google Scholar] [CrossRef]
- Nayak, J.K.; Shankar, U.; Samal, K. Fabrication and development of SPEEK/PVdF-HFP/SiO2 proton exchange membrane for microbial fuel cell application. Chem. Eng. J. Adv. 2023, 14, 100459. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Park, B.H.; Han, M.K.; Yoo, D.J. Enhanced performance and durability of composite membranes containing anatase titanium oxide for fuel cells operating under low relative humidity. Int. J. Energy Res. 2022, 46, 4835–4851. [Google Scholar] [CrossRef]
- Sidharthan, K.A.; Joseph, S. Performance evaluation of passive alkaline direct methanol fuel cell-the effect of inorganic salt and TiO2 nano fillers. Sustain. Energy Technol. Assess. 2022, 53, 102673. [Google Scholar] [CrossRef]
- Elumalai, V.; Deenadhayalan, T.; Kathleen Asitha, A.; Joel Kirubhakaran, D.; Sangeetha, D. Preparation of tungstic acid functionalized titanium oxide nanotubes and its effect on proton exchange membrane fuel cell. SN Appl. Sci. 2019, 1, 348. [Google Scholar] [CrossRef]
- Lee, S.; Seo, K.; Ghorpade, R.V.; Nam, K.-H.; Han, H. High temperature anhydrous proton exchange membranes based on chemically-functionalized titanium/polybenzimidazole composites for fuel cells. Mater. Lett. 2020, 263, 127167. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Vatanparast, M. Ultrasonic-assisted synthesis of ZrO2 nanoparticles and their application to improve the chemical stability of Nafion membrane in proton exchange membrane (PEM) fuel cells. J. Colloid Interface Sci. 2016, 483, 1–10. [Google Scholar] [CrossRef]
- Lee, C.; Park, J.; Jeon, Y.; Park, J.-I.; Einaga, H.; Truong, Y.B.; Kyratzis, I.L.; Mochida, I.; Choi, J.; Shul, Y.-G. Phosphate-modified TiO2/ZrO2 nanofibrous web composite membrane for enhanced performance and durability of high-temperature proton exchange membrane fuel cells. Energy Fuels 2017, 31, 7645–7652. [Google Scholar] [CrossRef]
- Mossayebi, Z.; Saririchi, T.; Rowshanzamir, S.; Parnian, M.J. Investigation and optimization of physicochemical properties of sulfated zirconia/sulfonated poly (ether ether ketone) nanocomposite membranes for medium temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 12293–12306. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Moghaddam, J.A. Investigation of physicochemical and electrochemical properties of recast Nafion nanocomposite membranes using different loading of zirconia nanoparticles for proton exchange membrane fuel cell applications. Mater. Sci. Energy Technol. 2018, 1, 146–154. [Google Scholar] [CrossRef]
- Ketpang, K.; Son, B.; Lee, D.; Shanmugam, S. Porous zirconium oxide nanotube modified Nafion composite membrane for polymer electrolyte membrane fuel cells operated under dry conditions. J. Membr. Sci. 2015, 488, 154–165. [Google Scholar] [CrossRef]
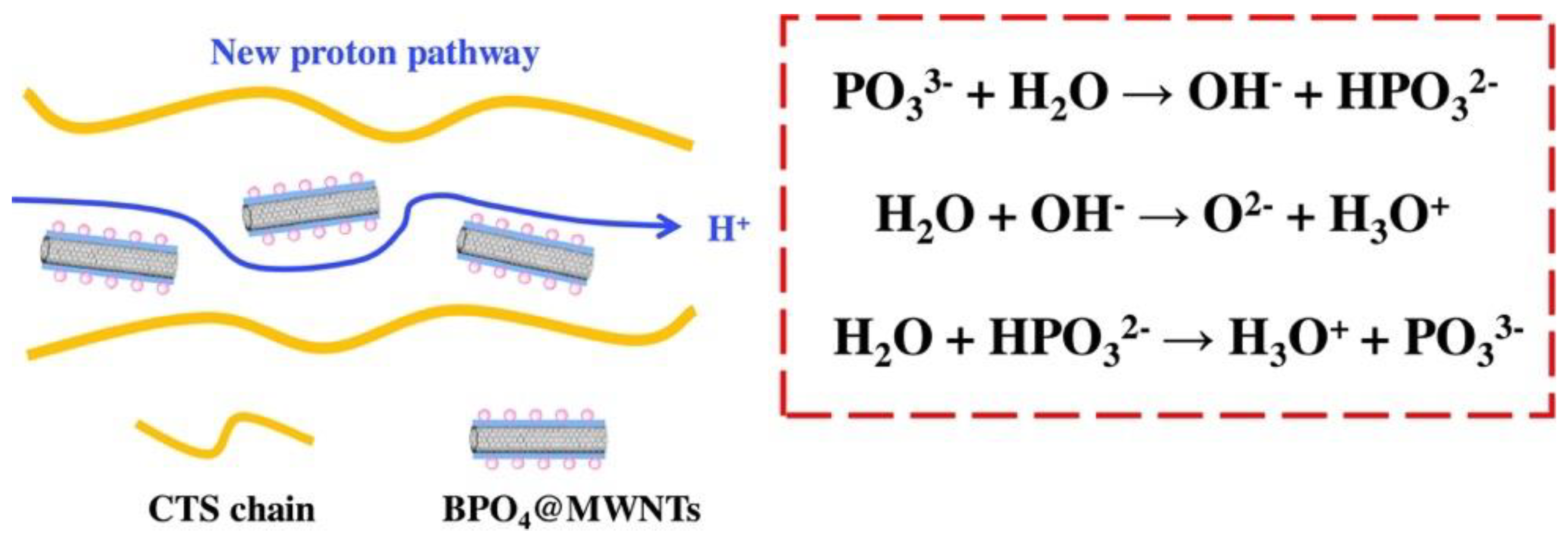
- Wang, J.; Qu, T.; Ni, J.; Cheng, F.; Hu, F.; Ou, Y.; Gong, C.; Wen, S.; Chen, X.; Liu, H. Composite proton exchange membranes based on inorganic proton conductor boron phosphate functionalized multi-walled carbon nanotubes and chitosan. Surf. Interfaces 2023, 36, 102557. [Google Scholar] [CrossRef]
- Fu, J.; Ni, J.; Wang, J.; Qu, T.; Hu, F.; Liu, H.; Zhang, Q.; Xu, Z.; Gong, C.; Wen, S. Highly proton conductive and mechanically robust SPEEK composite membranes incorporated with hierarchical metal–organic framework/carbon nanotubes compound. J. Mater. Res. Technol. 2023, 22, 2660–2672. [Google Scholar] [CrossRef]
- Wu, S.; Feng, X.; Zhong, F.; Zhang, B.; Wang, J.; Qu, T.; Ni, J.; Liu, H.; Gong, C.; Hu, F. The Construction and Application of Dual-Modified Carbon Nanotubes in Proton Exchange Membranes with Enhanced Performances. Macromol. Mater. Eng. 2021, 306, 2100519. [Google Scholar] [CrossRef]
- Liu, H.; Gong, C.; Wang, J.; Liu, X.; Liu, H.; Cheng, F.; Wang, G.; Zheng, G.; Qin, C.; Wen, S. Chitosan/silica coated carbon nanotubes composite proton exchange membranes for fuel cell applications. Carbohydr. Polym. 2016, 136, 1379–1385. [Google Scholar] [CrossRef]
- Ahmed, S.; Ali, M.; Cai, Y.; Lu, Y.; Ahmad, Z.; Khannal, S.; Xu, S. Novel sulfonated multi-walled carbon nanotubes filled chitosan composite membrane for fuel-cell applications. J. Appl. Polym. Sci. 2019, 136, 47603. [Google Scholar] [CrossRef]
- Muhmed, S.; Jaafar, J.; Ahmad, S.; Mohamed, M.; Ismail, A.; Ilbeygi, H.; Othman, M.; Rahman, M.A. Incorporating functionalized graphene oxide in green material-based membrane for proton exchange membrane fuel cell application. J. Environ. Chem. Eng. 2023, 11, 109547. [Google Scholar] [CrossRef]
- Rath, R.; Mohanty, S.; Kumar, P.; Nayak, S.K.; Unnikrishnan, L. Synergistic effect of silica-covered graphene oxide (in-situ) hybrid nanocomposites for use as a polymer electrolyte membrane for fuel cell applications. Surf. Interfaces 2023, 38, 102761. [Google Scholar] [CrossRef]
- Huang, D.; Hwang, J.-Y. Dual functionalized graphene oxide incorporated with sulfonated polyolefin proton exchange membrane for fuel cell application. Solid State Ion. 2023, 392, 116149. [Google Scholar] [CrossRef]
- Theerthagiri, S.; Krishnan, S.; Deivanayagam, P.; Muthiah, C.; Kannaiyan, D. TiO2-graphene dispersed sulfonated polyphenylenesulfide sulfone nanocomposites for medium temperature PEMFCs. Polym. Adv. Technol. 2023. [Google Scholar] [CrossRef]
- Devrim, Y.; Durmuş, G.N.B. Composite membrane by incorporating sulfonated graphene oxide in polybenzimidazole for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2022, 47, 9004–9017. [Google Scholar] [CrossRef]
- Rambabu, G.; Nagaraju, N.; Bhat, S.D. Functionalized fullerene embedded in Nafion matrix: A modified composite membrane electrolyte for direct methanol fuel cells. Chem. Eng. J. 2016, 306, 43–52. [Google Scholar] [CrossRef]
- Tasaki, K.; Gasa, J.; Wang, H.; DeSousa, R. Fabrication and characterization of fullerene–Nafion composite membranes. Polymer 2007, 48, 4438–4448. [Google Scholar] [CrossRef]
- Hou, H.; Polini, R.; Di Vona, M.L.; Liu, X.; Sgreccia, E.; Chailan, J.-F.; Knauth, P. Thermal crosslinked and nanodiamond reinforced SPEEK composite membrane for PEMFC. Int. J. Hydrogen Energy 2013, 38, 3346–3351. [Google Scholar] [CrossRef]
- Postnov, V.; Mel’nikova, N.; Shul’meister, G.; Novikov, A.; Murin, I.; Zhukov, A. Nafion-and aquivion-based nanocomposites containing detonation nanodiamonds. Russ. J. Gen. Chem. 2017, 87, 2754–2755. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Primachenko, O.; Odinokov, A.; Shvidchenko, A.; Bairamukov, V.Y.; Gofman, I.; Lebedev, V.; Ivanchev, S.; Vul, A.Y.; Kuklin, A. Composite proton-conducting membranes with nanodiamonds. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 140–146. [Google Scholar] [CrossRef]
- Primachenko, O.N.; Kulvelis, Y.V.; Odinokov, A.S.; Glebova, N.V.; Krasnova, A.O.; Antokolskiy, L.A.; Nechitailov, A.A.; Shvidchenko, A.V.; Gofman, I.V.; Marinenko, E.A. New Generation of Compositional Aquivion®-Type Membranes with Nanodiamonds for Hydrogen Fuel Cells: Design and Performance. Membranes 2022, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, F.; Ma, X.; Guan, X.; Chen, D.; Hickner, M.A. Sulfonated polymers containing polyhedral oligomeric silsesquioxane (POSS) core for high performance proton exchange membranes. Int. J. Hydrogen Energy 2015, 40, 7135–7143. [Google Scholar] [CrossRef]
- Chen, F.; Dong, W.; Lin, F.; Ren, W.; Ma, X. Composite proton exchange membrane with balanced proton conductivity and swelling ratio improved by gradient-distributed POSS nanospheres. Compos. Commun. 2021, 24, 100676. [Google Scholar] [CrossRef]
- Guan, Z.; Jin, Y.; Shi, S.; Jin, B.; Zhang, M.; Zhao, L. Self-assembled proton conduction networks consisting of SPEEK, NH2-POSS, and IL with enhanced proton conduction and decreased IL loss. Polymer 2022, 254, 125011. [Google Scholar] [CrossRef]
- Sang, J.; Wang, Z.; Zhu, H. Functionalized POSS-Modified SEBS-Based Composite Anion-Exchange Membranes for AEMFCs. Energy Fuels 2022, 36, 12780–12790. [Google Scholar] [CrossRef]
- Wang, G.; Yang, S.; Kang, N.Y.; Lu, M.; Hua, B.; Wei, H.; Kang, J.; Tang, W.; Lee, Y.M. Sulfonated graphene oxide doped sulfonated polybenzothiazoles for proton exchange membrane fuel cells. J. Membr. Sci. 2023, 668, 121239. [Google Scholar] [CrossRef]
- Lin, B.; Yuan, W.; Xu, F.; Chen, Q.; Zhu, H.; Li, X.; Yuan, N.; Chu, F.; Ding, J. Protic ionic liquid/functionalized graphene oxide hybrid membranes for high temperature proton exchange membrane fuel cell applications. Appl. Surf. Sci. 2018, 455, 295–301. [Google Scholar] [CrossRef]
- Sun, F.; Qin, L.-L.; Zhou, J.; Wang, Y.-K.; Rong, J.-Q.; Chen, Y.-J.; Ayaz, S.; Hai-Yin, Y.; Liu, L. Friedel-Crafts self-crosslinking of sulfonated poly (etheretherketone) composite proton exchange membrane doped with phosphotungstic acid and carbon-based nanomaterials for fuel cell applications. J. Membr. Sci. 2020, 611, 118381. [Google Scholar] [CrossRef]
- Yu, D.M.; Yoon, Y.J.; Kim, T.-H.; Lee, J.Y.; Hong, Y.T. Sulfonated poly (arylene ether sulfone)/sulfonated zeolite composite membrane for high temperature proton exchange membrane fuel cells. Solid State Ion. 2013, 233, 55–61. [Google Scholar] [CrossRef]
- Yang, T.; Li, Z.; Lyu, H.; Zheng, J.; Liu, J.; Liu, F.; Zhang, Z.; Rao, H. A graphene oxide polymer brush based cross-linked nanocomposite proton exchange membrane for direct methanol fuel cells. RSC Adv. 2018, 8, 15740–15753. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-J.; Lai, J.-Y.; Liu, Y.-L. Preparation of poly (styrenesulfonic acid) grafted Nafion with a Nafion-initiated atom transfer radical polymerization for proton exchange membranes. RSC Adv. 2017, 7, 37255–37260. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Prasad, A.K.; Advani, S.G. Effect of ceria loading on performance and durability of sulfonated poly (ether ether ketone) nanocomposite membranes for proton exchange membrane fuel cell applications. J. Membr. Sci. 2018, 565, 342–357. [Google Scholar] [CrossRef]
- Zarrin, H.; Higgins, D.; Jun, Y.; Chen, Z.; Fowler, M. Functionalized graphene oxide nanocomposite membrane for low humidity and high temperature proton exchange membrane fuel cells. J. Phys. Chem. C 2011, 115, 20774–20781. [Google Scholar] [CrossRef]
- Chuang, S.-W.; Hsu, S.L.-C.; Liu, Y.-H. Synthesis and properties of fluorine-containing polybenzimidazole/silica nanocomposite membranes for proton exchange membrane fuel cells. J. Membr. Sci. 2007, 305, 353–363. [Google Scholar] [CrossRef]
- Vani, R.; Ramaprabhu, S.; Haridoss, P. Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells. Sustain. Energy Fuels 2020, 4, 1372–1382. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Kowsari, E. Synthesis and characterization of poly (vinyl alcohol)/sulfonated graphene oxide nanocomposite membranes for use in proton exchange membrane fuel cells (PEMFCs). Ind. Eng. Chem. Res. 2014, 53, 16621–16632. [Google Scholar] [CrossRef]
- Hwang, C.-K.; Lee, K.A.; Lee, J.; Kim, Y.; Ahn, H.; Hwang, W.; Ju, B.-K.; Kim, J.Y.; Yeo, S.Y.; Choi, J. Perpendicularly stacked array of PTFE nanofibers as a reinforcement for highly durable composite membrane in proton exchange membrane fuel cells. Nano Energy 2022, 101, 107581. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Zhang, Y.; Li, C.; Dong, J.; Liu, Y.; Cheng, H. Electrospun multifunctional sulfonated carbon nanofibers for design and fabrication of SPEEK composite proton exchange membranes for direct methanol fuel cell application. Int. J. Hydrogen Energy 2017, 42, 10275–10284. [Google Scholar] [CrossRef]
- Awang, N.; Yajid, M.; Jaafar, J. Impact of exfoliated structure on the performance of electrospun SPEEK/cloisite nanocomposite membranes as proton exchange membranes for direct methanol fuel cell application. J. Environ. Chem. Eng. 2021, 9, 105319. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mohammadi, N.; Mehdipour-Ataei, S. On the preparation of thin nanofibers of polysulfone polyelectrolyte for improving conductivity of proton-exchange membranes by electrospinning: Taguchi design, response surface methodology, and genetic algorithm. Int. J. Hydrogen Energy 2020, 45, 34110–34124. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhuang, X.; Cheng, B.; Wang, W.; Kang, W.; Shi, L.; Li, H. Modification of Nafion membrane with biofunctional SiO2 nanofiber for proton exchange membrane fuel cells. J. Power Sources 2017, 340, 201–209. [Google Scholar] [CrossRef]
- Wang, H.; Sun, N.; Zhang, L.; Zhou, R.; Ning, X.; Zhuang, X.; Long, Y.; Cheng, B. Ordered proton channels constructed from deoxyribonucleic acid-functionalized graphene oxide for proton exchange membranes via electrostatic layer-by-layer deposition. Int. J. Hydrogen Energy 2020, 45, 27772–27778. [Google Scholar] [CrossRef]
- Jia, T.; Shen, S.; Zhao, J.; Jin, J.; Pan, B.; Duan, X.; Meng, C.; Che, Q. Ultrathin membranes formation via the layer by layer self-assembly of carbon nanotubes-based inorganics as high temperature proton exchange membranes. Int. J. Hydrogen Energy 2020, 45, 14517–14527. [Google Scholar] [CrossRef]
- Jia, T.; Shen, S.; Xiao, L.; Jin, J.; Zhao, J.; Che, Q. Constructing multilayered membranes with layer-by-layer self-assembly technique based on graphene oxide for anhydrous proton exchange membranes. Eur. Polym. J. 2020, 122, 109362. [Google Scholar] [CrossRef]
- Liu, K.; Zuo, T.; Wei, X.; Hu, S.; Che, Q. Constructing High Temperature Proton Exchange Membranes with Sandwich Structure based on Graphene Oxide Nanosheets and Electrospinning Polyvinyl Chloride Nanofibers. J. Mol. Liq. 2023, 381, 121808. [Google Scholar] [CrossRef]
- Huang, L.; He, Y.; Jin, L.; Hou, X.; Miao, L.; Lü, C. Fabrication and properties of graphene oxide/sulfonated polyethersulfone layer-by-layer assembled polyester fiber composite proton exchange membranes. Chem. Res. Chin. Univ. 2018, 34, 318–325. [Google Scholar] [CrossRef]
- Peng, K.-J.; Lai, J.-Y.; Liu, Y.-L. Nanohybrids of graphene oxide chemically-bonded with Nafion: Preparation and application for proton exchange membrane fuel cells. J. Membr. Sci. 2016, 514, 86–94. [Google Scholar] [CrossRef]
- Dong, F.; Li, Z.; Wang, S.; Wang, Z. Synthesis and characteristics of proton-conducting membranes based on cerium sulfophenyl phosphate and poly (2, 5-benzimidazole) by hot-pressing method. Int. J. Hydrogen Energy 2011, 36, 11068–11074. [Google Scholar] [CrossRef]
- Tinh, V.D.C.; Thuc, V.D.; Jeon, Y.; Gu, G.-Y.; Kim, D. Highly durable poly (arylene piperidinium) composite membranes modified with polyhedral oligomeric silsesquioxane for fuel cell and water electrolysis application. J. Membr. Sci. 2022, 660, 120903. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Chen, D.; Jin, Q.; Chen, J.; Cao, Y. Preparation of phosphotungstic acid hybrid proton exchange membranes by constructing proton transport channels for direct methanol fuel cells. Polymer 2023, 265, 125589. [Google Scholar] [CrossRef]
- Jun, Y.; Zarrin, H.; Fowler, M.; Chen, Z. Functionalized titania nanotube composite membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 6073–6081. [Google Scholar] [CrossRef]
- Feng, K.; Tang, B.; Wu, P. “Evaporating” graphene oxide sheets (GOSs) for rolled up GOSs and its applications in proton exchange membrane fuel cell. ACS Appl. Mater. Interfaces 2013, 5, 1481–1488. [Google Scholar] [CrossRef]
- Devrim, Y.; Devrim, H.; Eroglu, I. Polybenzimidazole/SiO2 hybrid membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 10044–10052. [Google Scholar] [CrossRef]
- Devrim, Y.; Erkan, S.; Bac, N.; Eroğlu, I. Preparation and characterization of sulfonated polysulfone/titanium dioxide composite membranes for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 3467–3475. [Google Scholar] [CrossRef]
- Nawn, G.; Pace, G.; Lavina, S.; Vezzù, K.; Negro, E.; Bertasi, F.; Polizzi, S.; Di Noto, V. Nanocomposite membranes based on polybenzimidazole and ZrO2 for high-temperature proton exchange membrane fuel cells. ChemSusChem 2015, 8, 1381–1393. [Google Scholar] [CrossRef]
- Shao, Z.-G.; Joghee, P.; Hsing, I.-M. Preparation and characterization of hybrid Nafion–silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells. J. Membr. Sci. 2004, 229, 43–51. [Google Scholar] [CrossRef]
- Maiti, T.K.; Dixit, P.; Singh, J.; Talapatra, N.; Ray, M.; Chattopadhyay, S. A novel strategy toward the advancement of proton exchange membranes through the incorporation of propylsulfonic acid-functionalized graphene oxide in crosslinked acid-base polymer blends. Int. J. Hydrogen Energy 2023, 48, 1482–1500. [Google Scholar] [CrossRef]
- Eskitoros-Togay, Ş.M.; Bulbul, Y.E.; Cınar, Z.K.; Sahin, A.; Dilsiz, N. Fabrication of PVP/sulfonated PES electrospun membranes decorated by sulfonated halloysite nanotubes via electrospinning method and enhanced performance of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2023, 48, 280–290. [Google Scholar] [CrossRef]
- Nawn, G.; Pace, G.; Lavina, S.; Vezzu, K.; Negro, E.; Bertasi, F.; Polizzi, S.; Di Noto, V. Interplay between composition, structure, and properties of new H3PO4-doped PBI4N–HfO2 nanocomposite membranes for high-temperature proton exchange membrane fuel cells. Macromolecules 2015, 48, 15–27. [Google Scholar] [CrossRef]

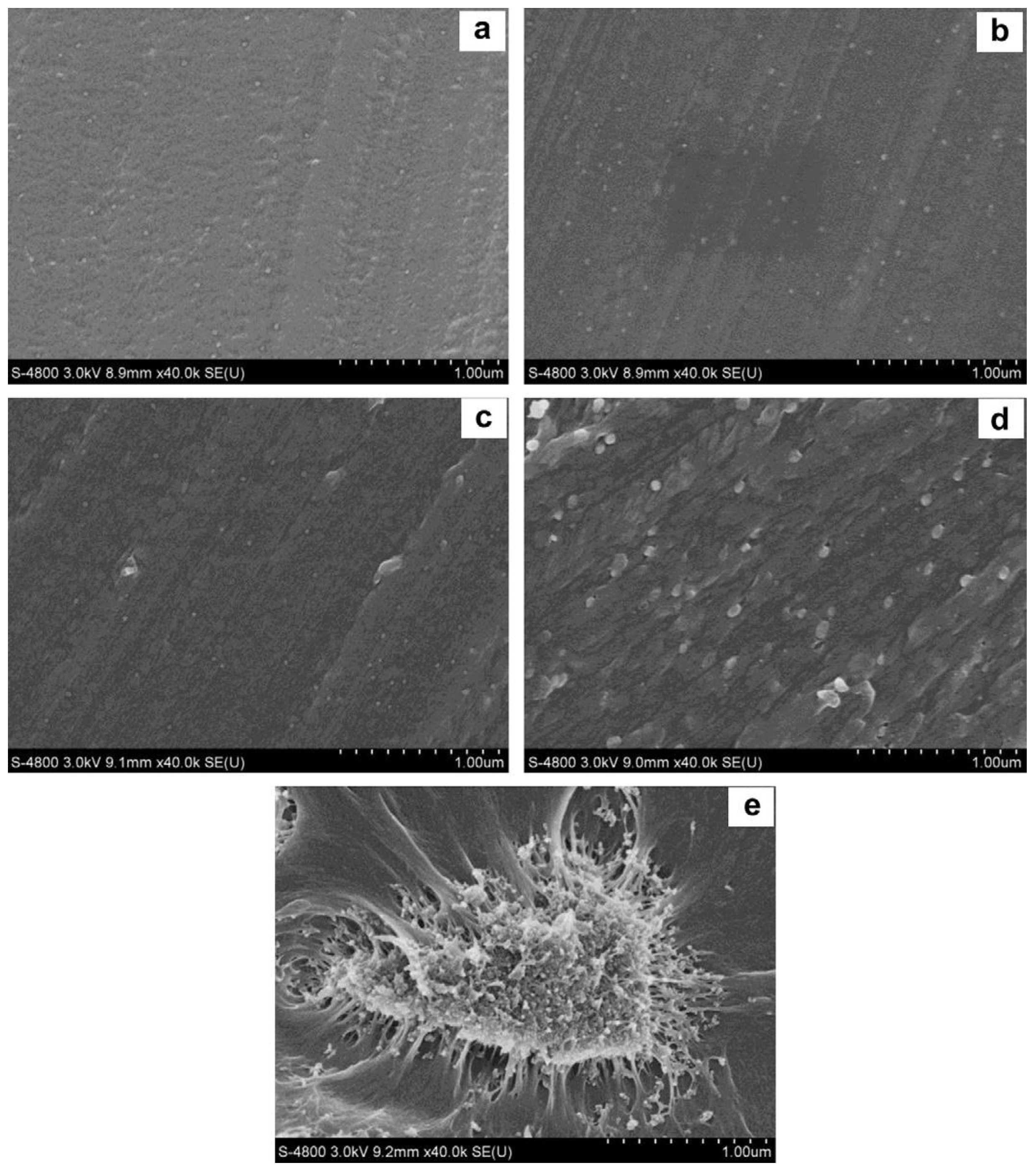
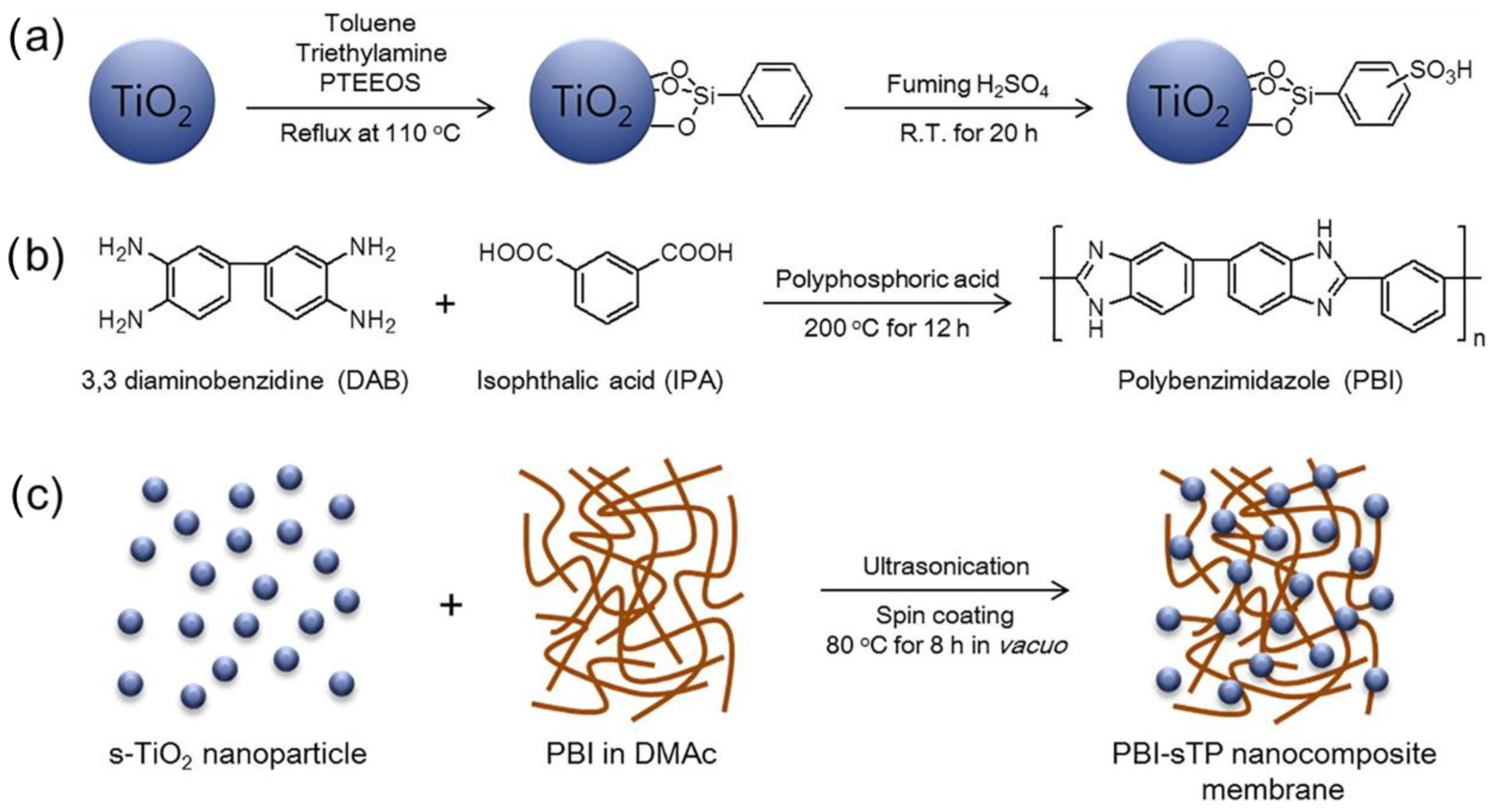



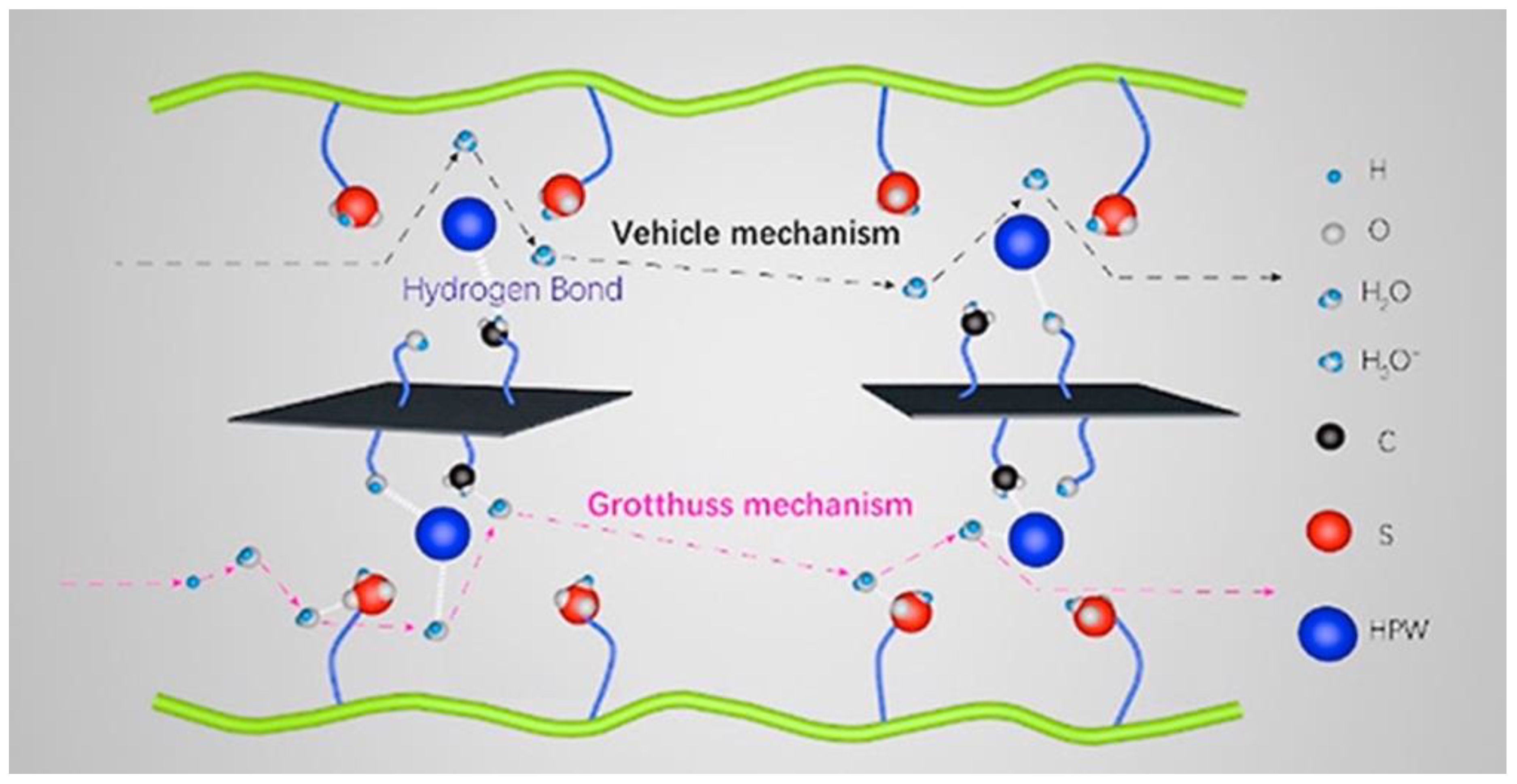

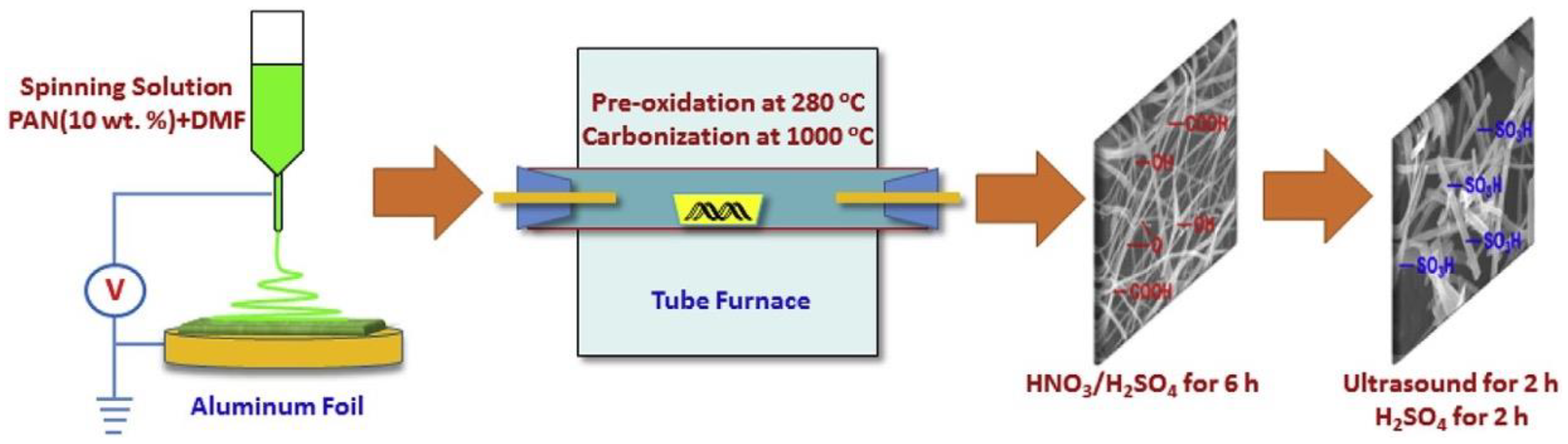
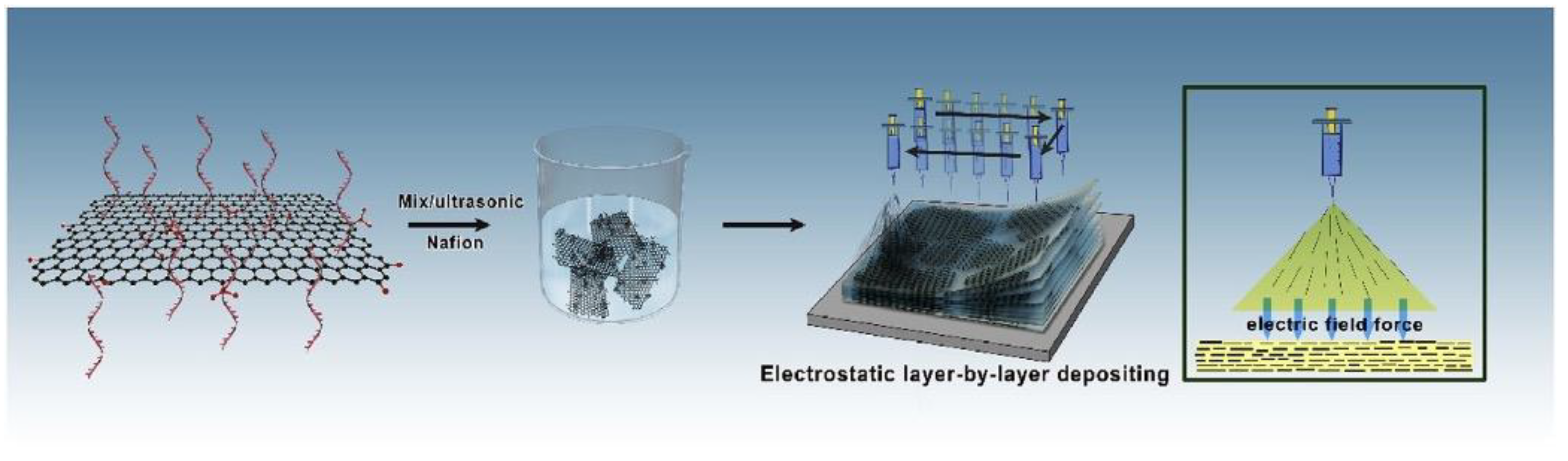
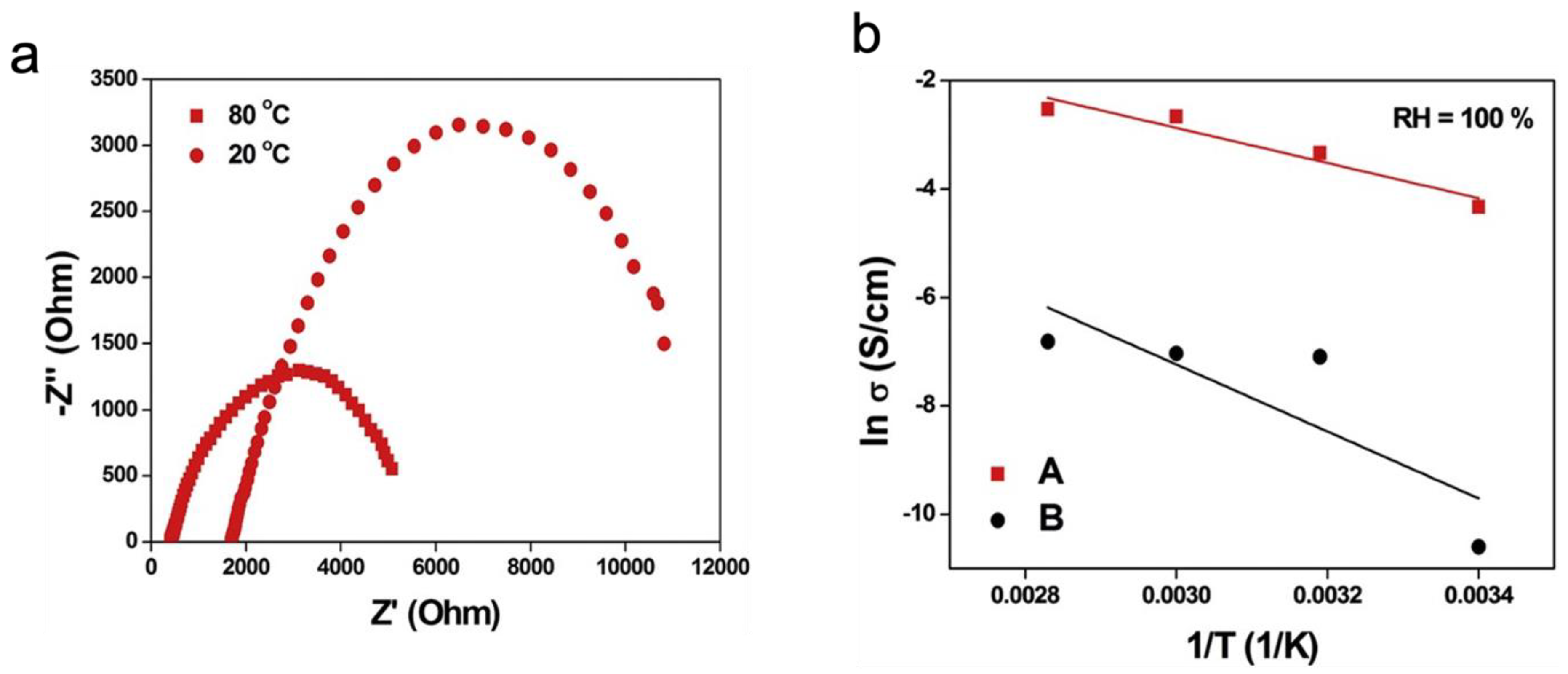

| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Sol-Gel Method | Versatile and enables fine composition and structure modification | Plenty of stages with complicated procedure |
| Can include a variety of nanomaterials | Gel formation involves high temperatures | |
| Improves thermal and mechanical stability | Risk for nanoparticle agglomeration | |
| Electrospinning | Produce fibers with a high surface area to volume ratio that are nanoscale | Minimal capacity to regulate fiber orientation and alignment |
| Enables the inclusion of various nanoparticles | Mass production is only partially scalable. | |
| Has excellent mechanical characteristics | Vulnerable to fiber damage when handled | |
| In situ Polymerization | Nanomaterials are dispersed uniformly | The ability to modify the size and shape of nanoparticles is inadequate |
| Strong interfacial bonding between polymer matrix and nanomaterials | May require optimization of reaction conditions | |
| Enhances mechanical and thermal properties | Difficulty in achieving high nanoparticle loading | |
| Scalable and suitable for mass production | ||
| Solution Casting | Quick and easy process | Inadequate control over the dispersion of nanoparticles |
| For production on a large scale, scaling is simple | Possibility of nanoparticle loss during washing processes | |
| Economically feasible compared to other approaches | It could be necessary to perform post-treatment to incorporate all nanoparticles | |
| Layer-by-Layer Assembly | Allows for precise oversight of the deposition of nanoparticles | A labor-intensive and tedious process |
| Provides flexibility in layer composition and thickness | Large-scale production is only partially scalable | |
| Enables regulated and sequential deposition | Interfacial defect possibilities between layers | |
| Permits the development of many layers for superior properties |
| Membrane | Preparation Method | Conductivity/Current Density | Temperature | Peak Power Density | Fuel | Ref. |
|---|---|---|---|---|---|---|
| PBI/SNP-PBI nanocomposites | Solution-casting | 50 mS cm−1 | 160 °C | 650 mW cm−2 | Hydrogen | [60] |
| SPEEK/PVdF-HFP/SiO2 | Solution-casting | 8 × 10−2 S cm−1 | 90 °C | 1.5 mW m−2 | Microbial | [63] |
| SPEEK 6% W-TNT | Solution-casting | 690 mA cm−2 | 80 °C | 352 mW cm−2 | Hydrogen | [66] |
| PA-doped PBI-sTP2 | Spin coating | 0.096 S cm−1 | 150 °C | 621 mW cm−2 | Hydrogen | [67] |
| Aquivion/TiO2/ZrO2 | Impregnation | 0.027 S cm−1 | 75 °C | 1120 mW cm−2 | Hydrogen | [69] |
| The Nafion-ZrNT | Solution-casting | 140 mS cm−1 | 80 °C | 982 mW cm−2 | Hydrogen | [72] |
| CTS/BPO4@MWNT | Solution-casting | 0.040 S cm−1 | 80 °C | 49.0 mW cm−2 | Methanol | [73] |
| SPEEK/ZSC | Solution-casting | 38.10 mS cm−1 | 80 °C | 38.9 mW cm−2 | Methanol | [74] |
| CS/SSiO2@CNTs | Solution casting | 35.8 mS cm−1 | 70 °C | 60.7 mW cm−2 | Methanol | [75] |
| NCC/PVA-SHGO-1.0 | Solution casting | 1.1 × 10−2 S cm−1 | 80 °C | 31.4 mW cm−2 | Hydrogen | [78] |
| PEM doped with 8% PS-MGO | Grafting | 0.084 S cm−1 | 25 °C | 78 mW cm−2 | Methanol | [80] |
| polybenzimidazole (PBI)/sulfonated graphene oxide (sGO) | Solution casting | 0.118 S cm−1 | 160 °C | 364 mW cm−2 | Hydrogen | [82] |
| PAP100-POSSI5.0 | Solution casting | 0.105 S cm−1 | 80 °C | 152.37 mW cm−2 | Hydrogen | [116] |
| Pi-POSS15%/Pi-SEBS | Solution casting | 69.11 mS cm−1 | 80 °C | 219 mW cm−2 | Hydrogen | [92] |
| sPBT-E62.5/SGO3 | In situ polymerization | 0.139 S cm−1 | 80 °C | 519.9 mW cm−2 | Hydrogen | [93] |
| C-SPEEK/HPW/GO | In situ polymerization | 119.04 mS cm−1 | 80 °C | 876.80 mW cm−2 | Hydrogen | [95] |
| CA-PTFE RCMs | Solution casting | 0.210 S cm−1 | 80 °C | 0.85 W cm−2 | Hydrogen | [104] |
| SPEEK/cloisite fibre mats | Solution casting | 7.73 mA cm−2 | 60 °C | 1.18 mW cm−2 | Methanol | [106] |
| ss-DNA@GO | Electrostatic layer-by-layer assembly | 351.8 mS cm−1 | 80 °C | 255.33 mW cm−2 | Methanol | [109] |
| PU/CNT-CdTe/PU/CS)150/60%PA | Layer-by-layer assembly | 6.82 × 10−2 S cm−1 | 150 °C | - | Methanol | [110] |
| PU/GO/PDDA/GO)200/60%PA | Layer-by-layer assembly | 1.83 × 10−1 S cm−1 | 150 °C | - | Methanol | [111] |
| PNs/GO/PNs)es/PA | Layer-by-layer assembly | 9.26 × 10−2 S cm−1 | 150 °C | - | Methanol | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra Kishore, S.; Perumal, S.; Atchudan, R.; Alagan, M.; Wadaan, M.A.; Baabbad, A.; Manoj, D. Recent Advanced Synthesis Strategies for the Nanomaterial-Modified Proton Exchange Membrane in Fuel Cells. Membranes 2023, 13, 590. https://doi.org/10.3390/membranes13060590
Chandra Kishore S, Perumal S, Atchudan R, Alagan M, Wadaan MA, Baabbad A, Manoj D. Recent Advanced Synthesis Strategies for the Nanomaterial-Modified Proton Exchange Membrane in Fuel Cells. Membranes. 2023; 13(6):590. https://doi.org/10.3390/membranes13060590
Chicago/Turabian StyleChandra Kishore, Somasundaram, Suguna Perumal, Raji Atchudan, Muthulakshmi Alagan, Mohammad Ahmad Wadaan, Almohannad Baabbad, and Devaraj Manoj. 2023. "Recent Advanced Synthesis Strategies for the Nanomaterial-Modified Proton Exchange Membrane in Fuel Cells" Membranes 13, no. 6: 590. https://doi.org/10.3390/membranes13060590
APA StyleChandra Kishore, S., Perumal, S., Atchudan, R., Alagan, M., Wadaan, M. A., Baabbad, A., & Manoj, D. (2023). Recent Advanced Synthesis Strategies for the Nanomaterial-Modified Proton Exchange Membrane in Fuel Cells. Membranes, 13(6), 590. https://doi.org/10.3390/membranes13060590