Analysis of the Polyphenolic Composition of Vaccinium L. Extracts and Their Protective Effect on Red Blood Cell Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Standards
2.2. Plant Material
2.3. Red Blood Cells
2.4. Erythrocyte Membranes
2.5. Lipid Membranes
2.6. Extracts and HPLC–DAD and UPLC–ESI–MS Analysis
2.7. Antioxidant Activity of Extracts
2.8. Hemolytic Activity of Extracts and Osmotic Resistance of Erythrocytes
2.9. Erythrocyte Shapes
2.10. Fluidity and Packing Arrangement of the Membranes
2.11. Statistical Analysis
3. Results
3.1. HPLC–DAD and UPLC–ESI–MS Analysis
3.2. Antioxidant Activity of Extracts
3.3. Hemolytic Activity of Extracts and Osmotic Resistance of Erythrocytes
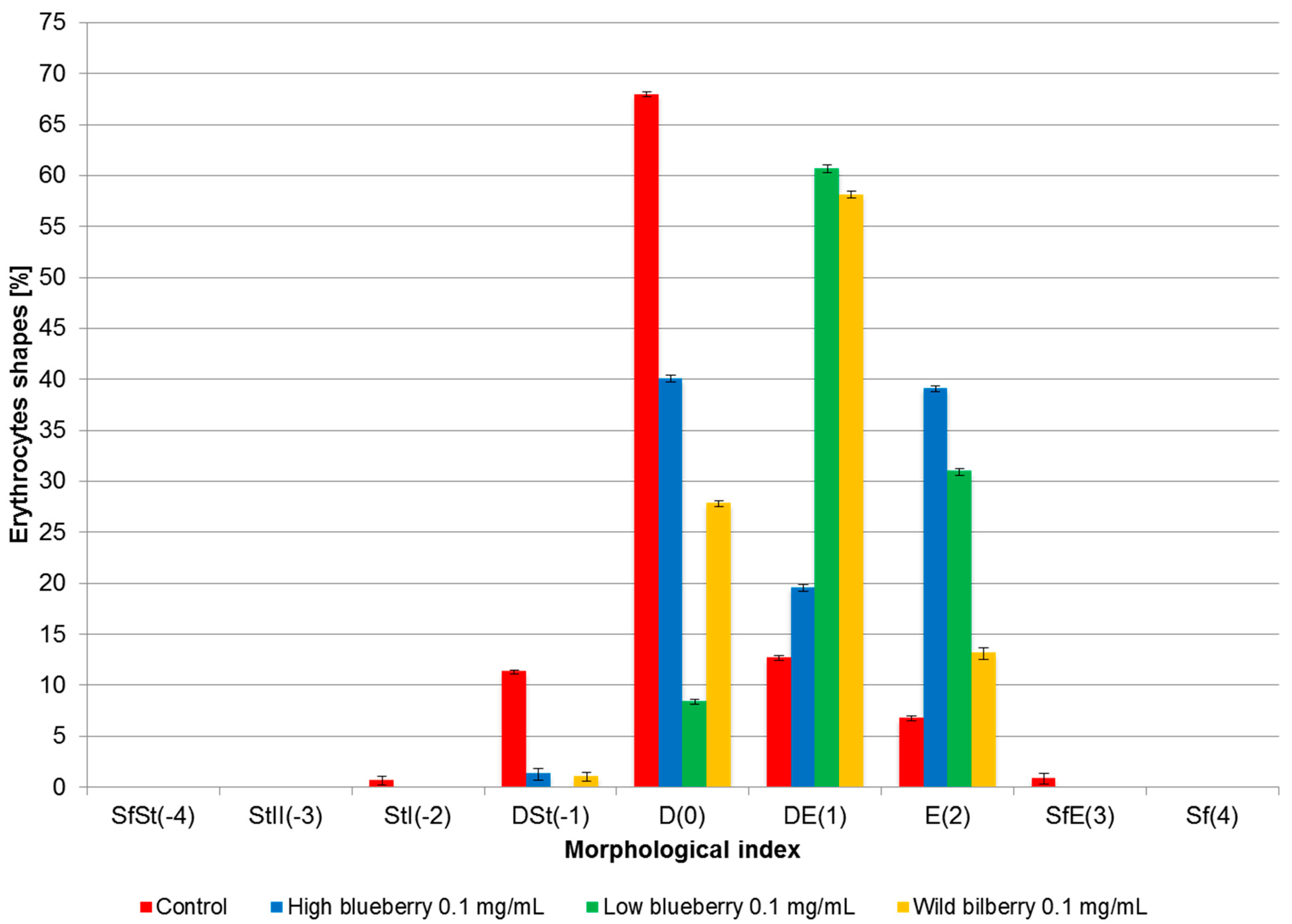
3.4. Erythrocyte Shapes
3.5. Fluidity and Packing Arrangement of the Membrane
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Šaponjac, V.T.; Čanadanović-Brunet, J.; Ćetković, G.; Djilas, S.; Četojević-Simin, D. Dried bilberry (Vaccinium myrtillus L.) extract fractions as antioxidants and cancer cell growth inhibitors. LWT 2015, 61, 615–621. [Google Scholar] [CrossRef]
- Felgus-Lavefve, L.; Howard, L.; Adams, S.H.; Baum, J.I. The Effects of Blueberry Phytochemicals on Cell Models of Inflammation and Oxidative Stress. Adv. Nutr. Int. Rev. J. 2022, 13, 1279–1309. [Google Scholar] [CrossRef]
- Zou, H.; Ye, H.; Zhang, J.; Ren, L. Recent advances in nuclear receptors-mediated health benefits of blueberry. Phytomedicine 2022, 100, 154063. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Ren, L.; Xie, Y. Advances in structures required of polyphenols for xanthine oxidase inhibition. Food Front. 2020, 1, 152–167. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health-promoting properties of blueberries. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef]
- Tran, P.H.; Tran, T.T. Blueberry Supplementation in Neuronal Health, and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins. Biomolecules 2021, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gallegos, J.L.; Haskell-Ramsay, C.; Lodge, J.K. Effects of Blueberry Consumption on Cardiovascular Health in Healthy Adults: A Cross-Over Randomised Controlled Trial. Nutrients 2022, 14, 2562. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. Int. Rev. J. 2019, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Mauramo, M.; Onali, T.; Wahbi, W.; Vasara, J.; Lampinen, A.; Mauramo, E.; Kivimäki, A.; Martens, S.; Häggman, H.; Sutinen, M.; et al. Bilberry (Vaccinium myrtillus L.) Powder Has Anticarcinogenic Effects on Oral Carcinoma In Vitro and In Vivo. Antioxidants 2021, 10, 1319. [Google Scholar] [CrossRef]
- Najjar, R.S.; Schwartz, A.M.; Wong, B.J.; Mehta, P.K.; Feresin, R.G. Berries and Their Polyphenols as a Potential Therapy for Coronary Microvascular Dysfunction: A Mini-Review. Int. J. Mol. Sci. 2021, 22, 3373. [Google Scholar] [CrossRef]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of phenolic compounds from bilberry (Vaccinium myrtillus L.) press residue: Effects on phenolic composition and cell proliferation. LWT 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Bomser, J.; Madhavi, D.; Singletary, K.; Smith, M. In Vitro Anticancer Activity of Fruit Extracts from Vaccinium Species. Planta Med. 1996, 62, 212–216. [Google Scholar] [CrossRef]
- Smith, M.A.L.; Marley, K.A.; Seigler, D.; Singletary, K.W.; Meline, B. Bioactive Properties of Wild Blueberry Fruits. J. Food Sci. 2000, 65, 352–356. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Howell, A.B.; McEniry, B.; Knight, C.T.; Seigler, D.; Erdman, J.W.; Lila, M.A. Effective Separation of Potent Antiproliferation and Antiadhesion Components from Wild Blueberry (Vaccinium angustifolium Ait.) Fruits. J. Agric. Food Chem. 2004, 52, 6433–6442. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Erdman, J.W.; Lila, M.A. Differential effects of blueberry proanthocyanidins on androgen sensitive and insensitive human prostate cancer cell lines. Cancer Lett. 2006, 231, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrich, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef]
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry anthocyanins in health promotion: A metabolic overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Adams, L.S.; Kanaya, N.; Phung, S.; Liu, Z.; Chen, S. Whole Blueberry Powder Modulates the Growth and Metastasis of MDA-MB-231 Triple Negative Breast Tumors in Nude Mice. J. Nutr. 2011, 141, 1805–1812. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries Decrease Cardiovascular Risk Factors in Obese Men and Women with Metabolic Syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Vieira, A. Protective activities of Vaccinium antioxidants with potential relevance to mitochondrial dysfunction and neurotoxicity. Neurotoxicology 2007, 28, 93–100. [Google Scholar] [CrossRef]
- Grace, M.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Chen, F.-X.; Wang, L.; Wang, J.; Jin, S.; Ma, Y.-M. Protective effects of blueberries (Vaccinium corymbosum L.) extract against cadmium-induced hepatotoxicity in mice. Environ. Toxicol. Pharmacol. 2014, 37, 1015–1027. [Google Scholar] [CrossRef]
- Sancho, R.A.S.; Pastore, G.M. Evaluation of the effects of anthocyanins in type 2 diabetes. Food Res. Int. 2012, 46, 378–386. [Google Scholar] [CrossRef]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-Week Consumption of a Wild Blueberry Powder Drink Increases Bifidobacteria in the Human Gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds1. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Michałowicz, J.; Krokosz, A.; Sicińska, P. Comparison of the effect of phenol and its derivatives on protein and free radical formation in human erythrocytes (in vitro). Blood Cells Mol. Dis. 2007, 39, 238–244. [Google Scholar] [CrossRef]
- Suwalsky, M.; Vargas, P.; Avello, M.; Villena, F.; Sotomayor, C.P. Human erythrocytes are affected in vitro by flavonoids of Aristotelia chilensis (Maqui) leaves. Int. J. Pharm. 2008, 363, 85–90. [Google Scholar] [CrossRef]
- Chen, J.-R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. The Tea Catechin Epigallocatechin Gallate Suppresses Cariogenic Virulence Factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity, and impact on the health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Açaì (Euterpe oleracea) Extract Protects Human Erythrocytes from Age-Related Oxidative Stress. Cells 2022, 11, 2391. [Google Scholar] [CrossRef] [PubMed]
- Cyboran-Mikołajczyk, S.; Męczarska, K.; Solarska-Ściuk, K.; Ratajczak-Wielgomas, K.; Oszmiański, J.; Jencova, V.; Bonarska-Kujawa, D. Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria Vesca L. And Rubus Idaeus L. Leaves Extracts—The Mechanism of Action. Molecules 2022, 27, 5865. [Google Scholar] [CrossRef]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Maddy, A.; Dunn, M.; Kelly, P. The characterisation of membrane proteins by centrifugation and gel electrophoresis a comparison of proteins prepared by different methods. Biochim. Biophys. Acta Biomembr. 1972, 288, 263–276. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Cyboran-Mikołajczyk, S.; Kleszczyńska, H. Molecular mechanism of action of chlorogenic acid on erythrocyte and lipid membranes. Mol. Membr. Biol. 2015, 32, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Gasiorowski, K.; Szyba, K.; Brokos, B.; Kołaczyńska, B.; Jankowiak-Włodarczyk, M.; Oszmiański, J. Antimutagenic activity of anthocyanins isolated from Aronia melanocarpa fruits. Cancer Lett. 1997, 119, 37–46. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdylło, A.; Gorzelany, J.; Kapusta, I. Identification and Characterization of Low Molecular Weight Polyphenols in Berry Leaf Extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A.; Kolniak, J. Effect of Enzymatic Mash Treatment and Storage on Phenolic Composition, Antioxidant Activity, and Turbidity of Cloudy Apple Juice. J. Agric. Food Chem. 2009, 57, 7078–7085. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Pruchnik, H.; Kleszczyńska, H. Interaction of selected anthocyanins with erythrocytes and liposome membranes. Cell. Mol. Biol. Lett. 2012, 17, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Cyboran, S.; Bonarska-Kujawa, D.; Kapusta, I.; Oszmiański, J.; Kleszczyńska, H. Antioxidant potentials of polyphenolic extracts from leaves of trees and fruit bushes. Curr. Top. Biophys. 2011, 34, 15–21. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Cyboran, S.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Biological Activity of Blackcurrant Extracts (Ribes Nigrum L.) in Relation to Erythrocyte Membranes. BioMed Res. Int. 2014, 2014, 783059. [Google Scholar] [CrossRef]
- Brecher, G.; Bessis, M. Present status of spiculed red cells and their relationship to the discocyte-echinocyte transformation: A critical review. Blood 1972, 40, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Bonarska-Kujawa, D.; Pruchnik, H.; Oszmiański, J.; Sarapuk, J.; Kleszczyńska, H. Changes Caused by Fruit Extracts in the Lipid Phase of Biological and Model Membranes. Food Biophys. 2010, 6, 58–67. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Fluorescence polarization. In Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA; London, UK, 2006; pp. 353–382. [Google Scholar]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Goiffon, J.-P.; Mouly, P.P.; Gaydou, E.M. Anthocyanic pigment determination in red fruit juices, concentrated juices and syrups using liquid chromatography. Anal. Chim. Acta 1999, 382, 39–50. [Google Scholar] [CrossRef]
- Tian, Q.; Giusti, M.M.; Stoner, G.D.; Schwartz, S.J. Screening for anthocyanins using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry with precursor-ion analysis, product-ion analysis, common-neutral-loss analysis, and selected reaction monitoring. J. Chromatogr. A 2005, 1091, 72–82. [Google Scholar] [CrossRef]
- Harris, C.S.; Burt, A.J.; Saleem, A.; Le, P.M.; Martineau, L.C.; Haddad, P.S.; Bennett, S.A.L.; Arnason, J.T. A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root, and fruit extracts ofVaccinium angustifolium. Phytochem. Anal. 2007, 18, 161–169. [Google Scholar] [CrossRef]
- Nakajima, J.; Tanaka, I.; Seo, S.; Yamazaki, M.; Saito, K. LC/PDA/ESI-MS Profiling and Radical Scavenging Activity of Anthocyanins in Various Berries. J. Biomed. Biotechnol. 2004, 2004, 241–247. [Google Scholar] [CrossRef]
- Werber, J.; Wang, Y.J.; Milligan, M.; Li, X.; Ji, J.A. Analysis of 2,2′-Azobis (2-Amidinopropane) Dihydrochloride Degradation and Hydrolysis in Aqueous Solutions. J. Pharm. Sci. 2011, 100, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Bonarska-Kujawa, D.; Cyboran, S.; Oszmiański, J.; Kleszczyńska, H. Extracts from Apple Leaves and Fruits as Effective Antioxidants. J. Med. Plants Res. 2011, 5, 2339–2347. [Google Scholar]
- Deuticke, B. Transformation and restoration of biconcave shape of human erythrocytes induced by amphiphilic agents and changes of ionic environment. Biochim. Biophys. Acta Biomembr. 1968, 163, 494–500. [Google Scholar] [CrossRef]
- Iglič, A.; Kralj-Iglič, V.; Hägerstrand, H. Amphiphile induced echinocyte-spheroechinocyte transformation of red blood cell shape. Eur. Biophys. J. 1998, 27, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2017, 58, 1310–1329. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Lipid Oxidation; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 51–64. [Google Scholar]
- Kochevar, I.E. UV-induced protein alterations and lipid oxidation in erythrocyte membranes. Photochem. Photobiol. 1990, 52, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Neff, W.E.; Selke, E.; Weisleder, D. Photosensitized oxidation of methyl linoleate: Secondary and volatile thermal decomposition products. Lipids 1982, 17, 11–18. [Google Scholar] [CrossRef]
- Oszmiański, J.; Nowicka, P.; Teleszko, M.; Wojdyło, A.; Cebulak, T.; Oklejewicz, K. Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruits. Int. J. Mol. Sci. 2015, 16, 14540–14553. [Google Scholar] [CrossRef]
- Valentová, K.; Ulrichová, J.; Cvak, L.; Šimánek, V. Cytoprotective effect of a bilberry extract against oxidative damage of rat hepatocytes. Food Chem. 2007, 101, 912–917. [Google Scholar] [CrossRef]
- Calò, R.; Marabini, L. Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B Biol. 2014, 132, 27–35. [Google Scholar] [CrossRef]
- Cyboran, S.; Bonarska-Kujawa, D.; Pruchnik, H.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Phenolic content and biological activity of extracts of blackcurrant fruit and leaves. Food Res. Int. 2014, 65, 47–58. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Pruchnik, H.; Cyboran-Mikołajczyk, S.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Biophysical Mechanism of the Protective Effect of Blue Honeysuckle (Lonicera caerulea L. var. kamtschatica Sevast.) Polyphenols Extracts Against Lipid Peroxidation of Erythrocyte and Lipid Membranes. J. Membr. Biol. 2014, 247, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Cyboran-Mikołajczyk, S.; Solarska-Ściuk, K.; Mieszała, K.; Glatzel-Plucińska, N.; Matczak, K.; Kleszczyńska, H. The Impact of O-Glycosylation on Cyanidin Interaction with RBCs and HMEC-1 Cells—Structure–Activity Relationships. Int. J. Mol. Sci. 2019, 20, 1928. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Masters, B.R. Principles of Fluorescence Spectroscopy, Third Edition. J. Biomed. Opt. 2008, 13, 029901. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S.; Muzafarov, E.N.; Kim, Y. Rafts making and rafts braking: How plant flavonoids may control membrane heterogeneity. Mol. Cell. Biochem. 2008, 314, 65–71. [Google Scholar] [CrossRef]
- Koren, E.; Kohen, R.; Ginsburg, I. Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp. Biol. Med. 2010, 235, 689–699. [Google Scholar] [CrossRef]
- Colina, J.R.; Suwalsky, M.; Manrique-Moreno, M.; Petit, K.; Aguilar, L.; Jemiola-Rzeminska, M.; Strzalka, K. An in vitro study of the protective effect of caffeic acid on human erythrocytes. Arch. Biochem. Biophys. 2019, 662, 75–82. [Google Scholar] [CrossRef]
- Suwalsky, M.; Duguet, J.; Speisky, H. An In Vitro Study of the Antioxidant and Antihemolytic Properties of Buddleja globosa (Matico). J. Membr. Biol. 2017, 250, 239–248. [Google Scholar] [CrossRef]
- Roy, A.; Dutta, R.; Kundu, N.; Banik, D.; Sarkar, N. A Comparative Study of the Influence of Sugars Sucrose, Trehalose, and Maltose on the Hydration and Diffusion of DMPC Lipid Bilayer at Complete Hydration: Investigation of Structural and Spectroscopic Aspect of Lipid–Sugar Interaction. Langmuir 2016, 32, 5124–5134. [Google Scholar] [CrossRef]
- Pruchnik, H.; Bonarska-Kujawa, D.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Application of the DSC and spectroscopy methods in the analysis of the protective effect of extracts from the blueberry fruit of the genus Vaccinium in relation to the lipid membrane. J. Therm. Anal. Calorim. 2018, 134, 679–689. [Google Scholar] [CrossRef]

| Compounds | Content [%] HB | Content [%] LB | Content [%] WB |
|---|---|---|---|
| Chlorogenic acid | 11.26 | 11.82 | 1.19 |
| Delphinidin-3-O-galactoside | 2.65 | 7.97 | 5.72 |
| Delphinidin-3-O-glucoside | 2.75 | 4.16 | 6.12 |
| Cyanidin-3-O-galactoside | 6.30 | 2.61 | 2.60 |
| Delphinidin-3-O-arabinoside | 1.58 | 3.53 | 3.87 |
| Cyanidin-3-O-glucoside | 0.89 | 1.35 | 1.82 |
| Petunidin-3-O-galactoside | 0.64 | 1.73 | 0.62 |
| Cyanidin-3-O-arabinoside | 2.64 | 1.12 | 1.84 |
| Petunidin-3-O-glucoside | 0.87 | 2.02 | 1.35 |
| Peonidin-3-O-galactoside | 1.46 | 1.81 | 0.43 |
| Petunidin-3-O-arabinoside | 1.79 | 0.69 | 0.41 |
| Peonidin-3-O-glucoside | 0.92 | 0.12 | 1.87 |
| Malvidin-3-O-galactoside | 0.81 | 0.04 | 2.60 |
| Peonidin-3-O-arabinoside | 0.87 | 0.14 | 0.98 |
| Malvidin-3-O-glucoside | 0.21 | 0.04 | 0.85 |
| Malvidin-3-O-arabinoside | 0.73 | 0.21 | 0.44 |
| Quercetin-3-O-arabinoside | 0.15 | 0.05 | - |
| Quercetin-3-O-rhamnoside | 0.86 | 0.33 | - |
| Quercetin-3-O-galactoside | 0.29 | 3.50 | 0.72 |
| Quercetin-3-O-glucoside | 0.16 | 0.66 | 0.55 |
| Total | 37.83 | 43.90 | 33.98 |
| Extract/Inductor | Concentration IC50 [µg/mL] ± SD | |
|---|---|---|
| AAPH | UVC | |
| Wild bilberry (WB) | 7.42 ± 0.37 | 21.19 ± 1.06 |
| High blueberry (HB) | 4.63 ± 0.23 | 26.34 ± 1.32 |
| Low blueberry (LB) | 4.96 ± 0.25 | 47.77 ± 2.38 |
| Trolox® | 3.90 ± 0.30 | 14.60 ± 1.30 |
| chlorogenic acid * | 0.90 ± 0.08 | 7.40 ± 0.50 |
| Membrane | Erythrocyte Ghost | Liposomes from Erythrocyte’s Lipids |
|---|---|---|
| Concentrations [µg/mL] | Anisotropy(A) ± SD | |
| wild bilberry (WB) | ||
| Control | 0.24 ± 0.01 | 0.21 ± 0.01 |
| 5.0 | 0.24 ± 0.01 | 0.20 ± 0.01 |
| 7.5 | 0.25 ± 0.01 | 0.21 ± 0.01 |
| 10.0 | 0.23 ± 0.01 | 0.21 ± 0.01 |
| 25.0 | 0.23 ± 0.01 | 0.21 ± 0.01 |
| high blueberry (HB) | ||
| Control | 0.24 ± 0.01 | 0.21 ± 0.01 |
| 5.0 | 0.24 ± 0.02 | 0.20 ± 0.01 |
| 7.5 | 0.22 ± 0.02 | 0.20 ± 0.01 |
| 10.0 | 0.23 ± 0.01 | 0.20 ± 0.01 |
| 25.0 | 0.22 ± 0.01 * | 0.20 ± 0.01 |
| low blueberry (LB) | ||
| Control | 0.24 ± 0.01 | 0.21 ± 0.01 |
| 5.0 | 0.24 ± 0.01 | 0.20 ± 0.01 |
| 7.5 | 0.24 ± 0.02 | 0.20 ± 0.01 |
| 10.0 | 0.22 ± 0.01 | 0.21 ± 0.01 |
| 25.0 | 0.22 ± 0.01 * | 0.21 ± 0.01 |
| Membrane | Erythrocyte Ghost | Liposomes from Erythrocyte’s Lipids |
|---|---|---|
| Concentrations [µg/mL] | Generalized Polarization (GP) ± SD | |
| wild bilberry (WB) | ||
| Control | 0.33 ± 0.01 | 0.27 ± 0.01 |
| 5.0 | 0.31 ± 0.02 | 0.27 ± 0.01 |
| 7.5 | 0.29 ± 0.01 * | 0.27 ± 0.01 |
| 10.0 | 0.28 ± 0.02 * | 0.26 ± 0.02 |
| 25.0 | 0.22 ± 0.03 * | 0.27 ± 0.01 |
| high blueberry (HB) | ||
| Control | 0.33 ± 0.01 | 0.27 ± 0.01 |
| 5.0 | 0.29 ± 0.03 | 0.27 ± 0.01 |
| 7.5 | 0.30 ± 0.01 * | 0.27 ± 0.01 |
| 10.0 | 0.26 ± 0.02 * | 0.27 ± 0.02 |
| 25.0 | 0.22 ± 0.02 * | 0.26 ± 0.02 |
| low blueberry (LB) | ||
| Control | 0.33 ± 0.01 | 0.27 ± 0.01 |
| 5.0 | 0.33 ± 0.01 | 0.27 ± 0.01 |
| 7.5 | 0.26 ± 0.01 * | 0.28 ± 0.01 |
| 10.0 | 0.24 ± 0.03 * | 0.27 ± 0.01 |
| 25.0 | 0.20 ± 0.03 * | 0.25 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak, T.; Bonarska-Kujawa, D.; Męczarska, K.; Cyboran-Mikołajczyk, S.; Oszmiański, J.; Kapusta, I. Analysis of the Polyphenolic Composition of Vaccinium L. Extracts and Their Protective Effect on Red Blood Cell Membranes. Membranes 2023, 13, 589. https://doi.org/10.3390/membranes13060589
Kaźmierczak T, Bonarska-Kujawa D, Męczarska K, Cyboran-Mikołajczyk S, Oszmiański J, Kapusta I. Analysis of the Polyphenolic Composition of Vaccinium L. Extracts and Their Protective Effect on Red Blood Cell Membranes. Membranes. 2023; 13(6):589. https://doi.org/10.3390/membranes13060589
Chicago/Turabian StyleKaźmierczak, Teresa, Dorota Bonarska-Kujawa, Katarzyna Męczarska, Sylwia Cyboran-Mikołajczyk, Jan Oszmiański, and Ireneusz Kapusta. 2023. "Analysis of the Polyphenolic Composition of Vaccinium L. Extracts and Their Protective Effect on Red Blood Cell Membranes" Membranes 13, no. 6: 589. https://doi.org/10.3390/membranes13060589
APA StyleKaźmierczak, T., Bonarska-Kujawa, D., Męczarska, K., Cyboran-Mikołajczyk, S., Oszmiański, J., & Kapusta, I. (2023). Analysis of the Polyphenolic Composition of Vaccinium L. Extracts and Their Protective Effect on Red Blood Cell Membranes. Membranes, 13(6), 589. https://doi.org/10.3390/membranes13060589










