Self-Phosphorylated Polybenzimidazole: An Environmentally Friendly and Economical Approach for Hydrogen/Air High-Temperature Polymer-Electrolyte Membrane Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gel Permeation Chromatography
2.3. Thermogravimetric Analysis
2.4. Differential Scanning Calorimetry
2.5. Nuclear Magnetic Resonance
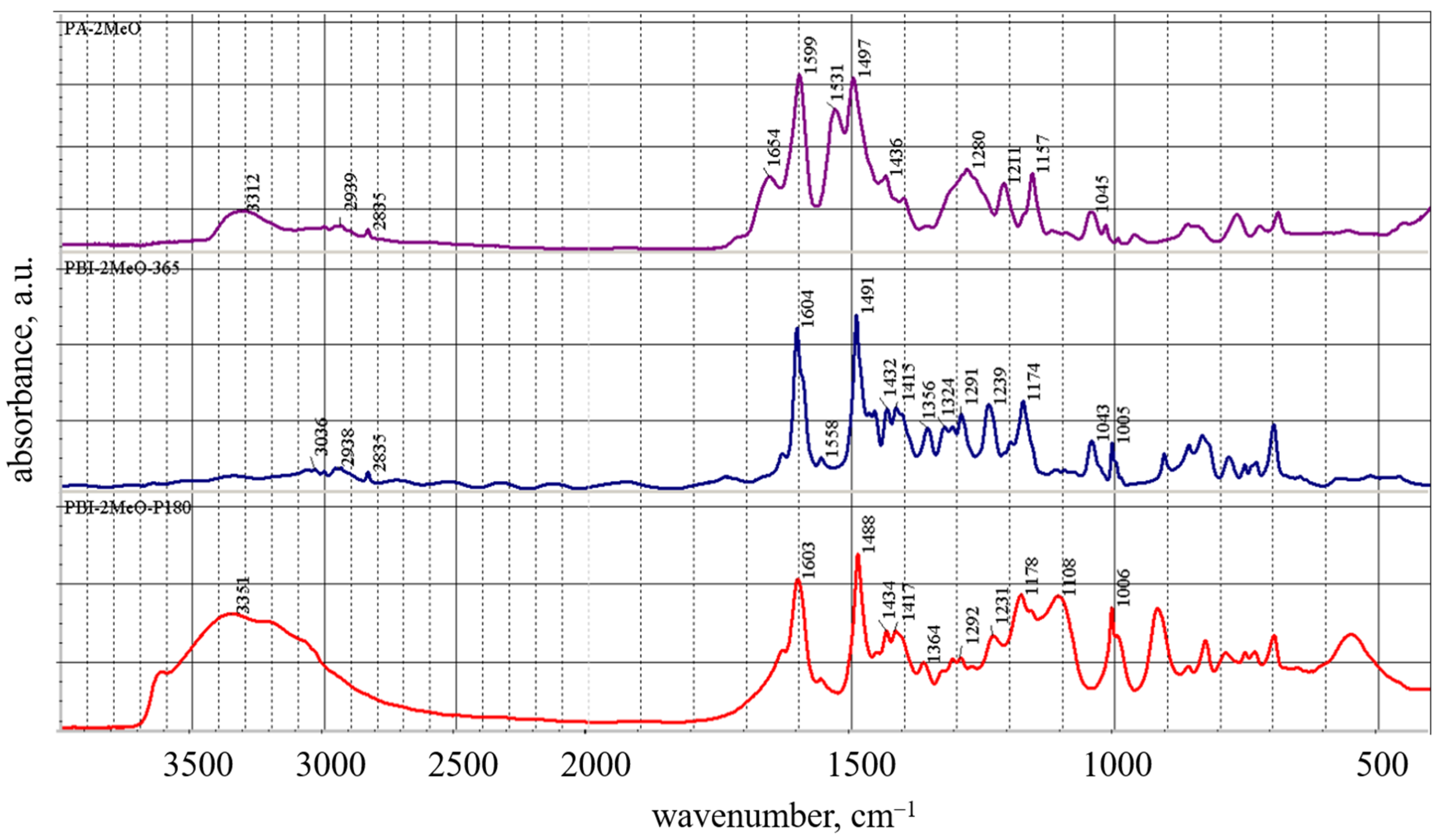
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. Elemental Analysis
2.8. X-ray Analysis
2.9. Synthesis
2.9.1. Synthesis of N,N′-Bis(3-methoxyphenyl)-4,6-dinitro-1,3-benzenediamine
2.9.2. Synthesis of N1,N5-Bis(3-methoxyphenyl)-1,2,4,5-benzenetetramine (1)
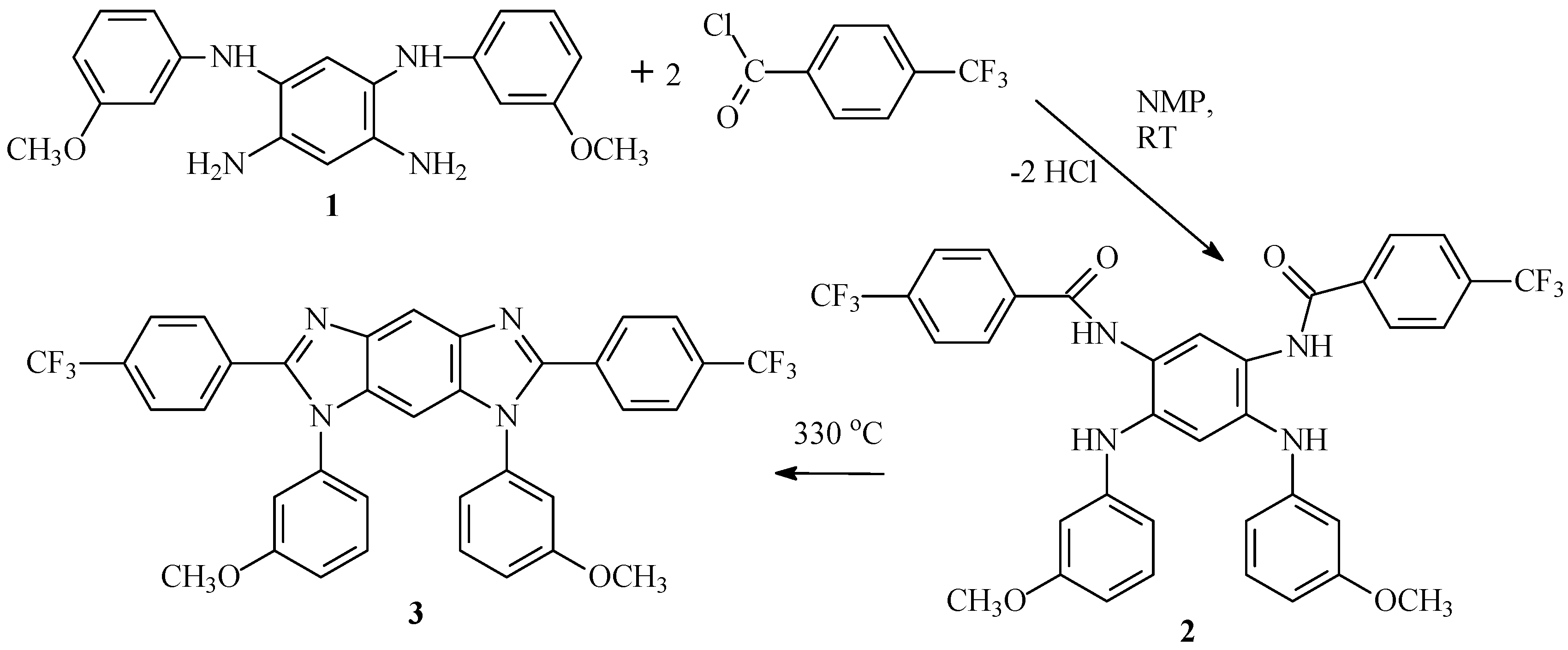
2.9.3. Synthesis of N2,N4-Bis(p-trifluorobenzoyl)-N1,N5-bis(3-methoxyphenyl)-1,2,4,5-benzenetetramine (2)
2.9.4. Synthesis of 2,6-Bis(p-trifluorophenyl)-1,7-dihydro-1,7-Bis(m-methoxyphenyl)-benzo [1,2-d:4,5-d′]diimidazole (3)
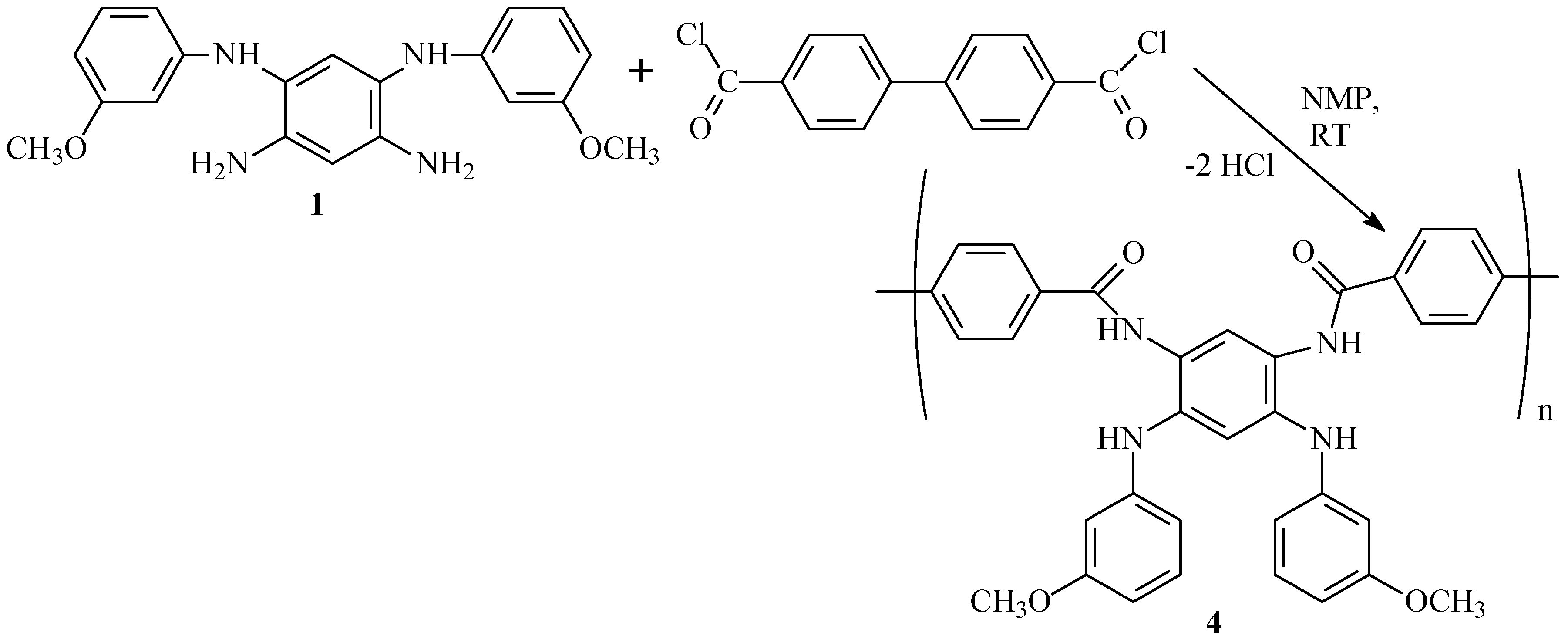
2.9.5. Synthesis of Polyamide Pre-Polymer (4)
2.9.6. Thermal Heterocyclizaton
2.10. HT-PEM Fuel Cell Operation
2.11. Electrochemical Impedance Spectroscopy
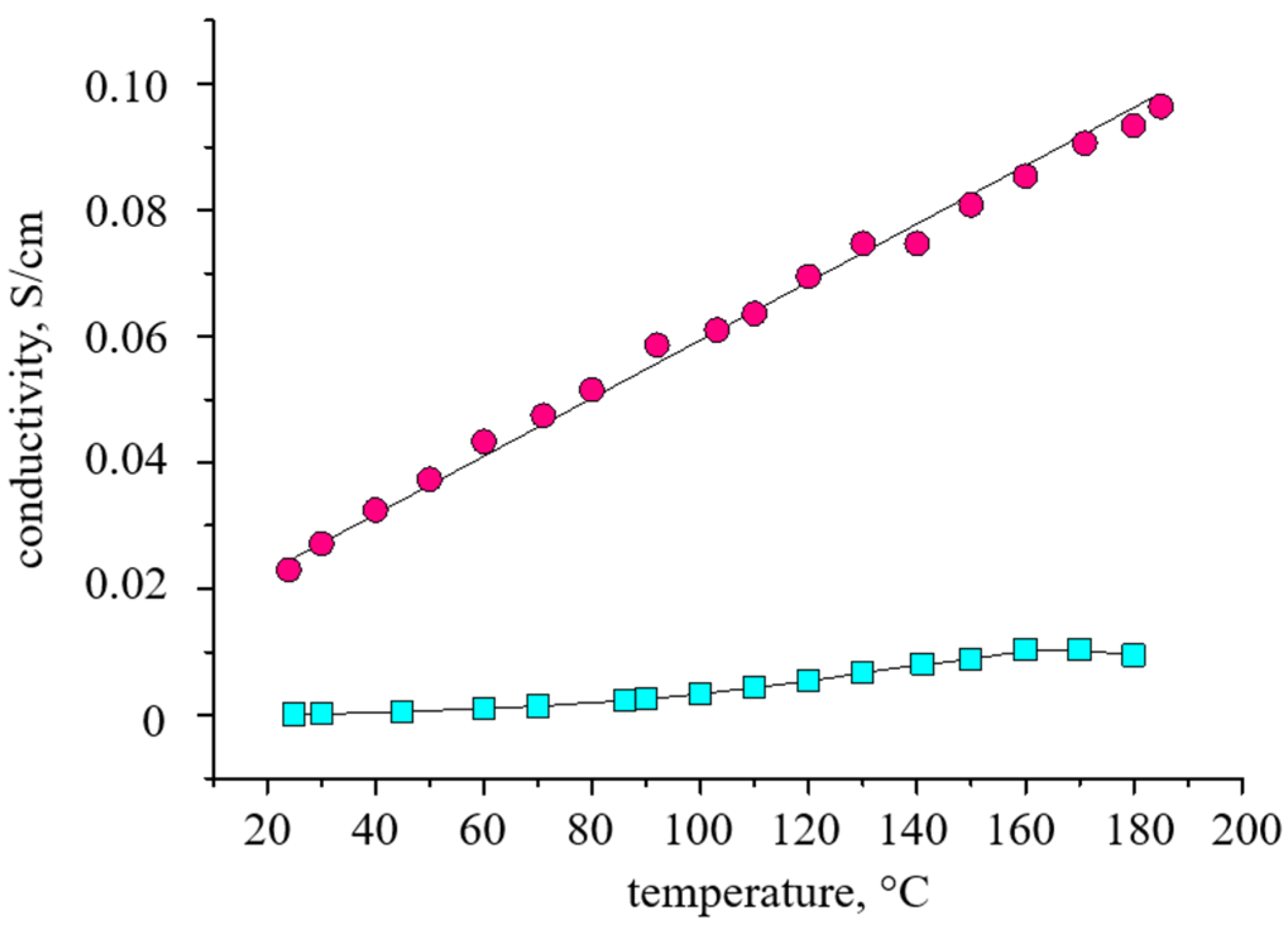
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powers, E.J.; Serad, G.A. History and Development of Polybenzimidazoles. In High Performance Polymers: Their Origin and Development; Seimour, R.B., Kirschenbaum, G.S., Eds.; Springer: Dordrecht, Germany, 1986; pp. 355–373. [Google Scholar] [CrossRef]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Song, K.; Lan, Y.; Zhang, X.; Jiang, J.; Sun, C.; Yang, G.; Yang, F.; Lan, H. A Review on Interoperability of Wireless Charging Systems for Electric Vehicles. Energies 2023, 16, 1653. [Google Scholar] [CrossRef]
- Wainright, J.S.; Wang, J.-T.; Weng, D.; Savinell, R.F.; Litt, M. Acid-Doped Polybenzimidazoles: A New Polymer Electrolyte. J. Electrochem. Soc. 1995, 142, L121. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Zhigalina, O.M.; Skupov, K.M.; Modestov, A.D.; Basu, V.G.; Sufiyanova, A.E.; Ponomarev, I.I.; Razorenov, D.Y. Preparation and thermal treatment influence on Pt decorated electrospun carbon nanofiber electrocatalysts. RSC Adv. 2019, 9, 27406–27418. [Google Scholar] [CrossRef] [PubMed]
- Skupov, K.M.; Ponomarev, I.I.; Razorenov, D.Y.; Zhigalina, V.G.; Zhigalina, O.M.; Ponomarev, I.I.; Volkova, Y.A.; Volfkovich, Y.M.; Sosenkin, V.E. Carbon nanofiber paper cathode modification for higher performance of phosphoric acid fuel cells on polybenzimidazole membrane. Russ. J. Electrochem. 2017, 53, 728–733. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Zhigalina, O.M.; Naumkin, A.V.; Modestov, A.D.; Basu, V.G.; Sufiyanova, A.E.; Razorenov, D.Y.; Ponomarev, I.I. New Carbon Nanofiber Composite Materials Containing Lanthanides and Transition Metals Based on Electrospun Polyacrylonitrile for High Temperature Polymer Electrolyte Membrane Fuel Cell Cathodes. Polymers 2020, 12, 1340. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers, Fundamentals and Applications; Springer: London, UK, 2008. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di Noto, V. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ion. 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Vogel, H.; Marvel, S. Polybenzimidazoles, new thermally stable polymers. J. Polym. Sci. 1961, 50, 511–539. [Google Scholar] [CrossRef]
- About Celazole® PBI. Available online: https://pbipolymer.com/about/about-celazole-pbi/ (accessed on 4 May 2023).
- Vogel, H.; Marvel, S. Polybenzimidazoles. J., II. Polym. Sci. Part A 1963, 1, 1131–1541. [Google Scholar] [CrossRef]
- Iwakura, Y.; Uno, K.; Imai, Y. Polyphenylenebenzimidazoles. J. Polym. Sci. Part A 1964, 2, 2605–2615. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, S.Y.; An, S.J.; Eun, Y.C.; Kim, J.-Y.; Yoon, H.-K.; Kweon, H.-J.; Yew, K.H. Synthesis of Poly(2,5-benzimidazole) for Use as a Fuel-Cell Membrane. Macromol. Rapid Commun. 2004, 25, 894–897. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Razorenov, D.Y.; Ponomarev, I.I.; Volkova, Y.A.; Skupov, K.M.; Lysova, A.A.; Yaroslavtsev, A.B.; Modestov, A.D.; Buzin, M.I.; Klemenkova, Z.S. Polybenzimidazoles via polyamidation: A more environmentally safe process to proton conducting membrane for hydrogen HT-PEM fuel cell. Eur. Polym. J. 2021, 156, 110613. [Google Scholar] [CrossRef]
- Melchior, J.-P.; Majerb, G.; Kreuer, K.-D. Why do proton conducting polybenzimidazole phosphoric acid membranes perform well in high-temperature PEM fuel cells? Phys. Chem. Chem. Phys. 2017, 19, 601–612. [Google Scholar] [CrossRef]
- Eberhardt, S.H.; Marone, F.; Stampanoni, M.; Buchi, F.N.; Schmidt, T.J. Operando X-ray Tomographic Microscopy Imaging of HT-PEFC: A Comparative Study of Phosphoric Acid Electrolyte Migration. J. Electrochem. Soc. 2016, 163, F842–F847. [Google Scholar] [CrossRef]
- Rikukawa, M.; Sanui, K. Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog. Polym. Sci. 2000, 25, 1463–1502. [Google Scholar] [CrossRef]
- Rusanov, A.L.; Kostoglodov, P.V.; Abadie, M.J.M.; Voytekunas, V.Y.; Likhachev, D.Y. Proton-conducting polymers and membranes carrying phosphonic acid groups. In Fuel Cells II. Advances in Polymer Science; Scherer, G.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 216, pp. 125–155. [Google Scholar] [CrossRef]
- Lafitte, B.; Jannasch, P. Chapter Three—On the Prospects for Phosphonated Polymers as Proton-Exchange Fuel Cell Membranes. In Advances in Fuel Cells; Zhao, T.S., Kreuer, K.-D., Nguyen, Y.V., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2007; Volume 1, pp. 119–185. [Google Scholar] [CrossRef]
- Ngamsantivongsa, P.; Lin, H.-L.; Yu, T.L. Properties and fuel cell applications of polybenzimidazole and ethyl phosphoric acid grafted polybenzimidazole blend membranes. J. Membr. Sci. 2015, 491, 10–21. [Google Scholar] [CrossRef]
- Marestin, C.; Chatti, S.; Mercier, R. Synthesis of poly(aryl ether)s bearing phosphonated side-chains from phosphonate ester-containing bisphenols. Polymer 2021, 222, 123647. [Google Scholar] [CrossRef]
- Allcock, H.R.; Hofmann, M.A.; Ambler, C.M.; Morford, R.V. Phenylphosphonic Acid Functionalized Poly[aryloxyphosphazenes]. Macromolecules 2002, 35, 3484–3489. [Google Scholar] [CrossRef]
- Allcock, H.R.; Hofmann, M.A.; Ambler, C.M.; Lvov, S.N.; Zhou, X.Y.; Chalkova, E.; Weston, J. Phenyl phosphonic acid functionalized poly[aryloxyphosphazenes] as proton-conducting membranes for direct methanol fuel cells. J. Membr. Sci. 2002, 201, 47–54. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Rybkin, Y.Y.; Goryunov, E.I.; Petrovskii, P.V.; Lyssenko, K.A. Synthesis and properties of 10-hydroxy-10-oxo-10H-10λ5-phenoxaphosphine-2,8-dicarboxylic acid and related polybenzoimidazoles. Russ. Chem. Bull. 2004, 53, 2020–2024. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Petrovskii, P.V.; Volkova, Y.A.; Razorenov, D.Y.; Goryunova, I.B.; Starikova, Z.A.; Fomenkov, A.I.; Khokhlov, A.R. Synthesis of N-phosphonoethylated cardo poly(benzimidazole) and testing of proton-conducting membranes made of it. Dokl. Chem. 2010, 432, 168–174. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Goryunov, E.I.; Petrovskii, P.V.; Ponomarev, I.I.; Volkova, Y.A.; Razorenov, D.Y.; Khokhlov, A.R. Synthesis of new monomer 3,3′-diamino-4,4′-bis{p-[(diethoxyphosphoryl)methyl]phenylamino}diphenyl sulfone and polybenzimidazoles on its basis. Dokl. Chem. 2009, 429, 315–320. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Ponomarev, I.I.; Goryunov, E.I.; Volkova, Y.A.; Razorenov, D.Y.; Starikova, Z.A.; Blagodatskikh, I.V.; Buzin, M.I.; Khokhlov, A.R. Chemical modification of cardo poly(benzimidazole) using “click” reaction for membranes of high-temperature hydrogen fuel cells. Dokl. Chem. 2012, 447, 227–232. [Google Scholar] [CrossRef]
- Kondratenko, M.S.; Ponomarev, I.I.; Gallyamov, M.O.; Razorenov, D.Y.; Volkova, Y.A.; Kharitonova, E.P.; Khokhlov, A.R. Novel composite Zr/PBI-O-PhT membranes for HT-PEFC applications. Beilstein J. Nanotechnol. 2013, 4, 481–492. [Google Scholar] [CrossRef]
- Lysova, A.A.; Yurova, P.A.; Stenina, I.A.; Ponomarev, I.I.; Pourcelly, G.; Yaroslavtsev, A.B. Hybrid membranes based on polybenzimidazoles and silica with imidazoline-functionalized surface, candidates for fuel cells applications. Ionics 2020, 26, 1853–1860. [Google Scholar] [CrossRef]
- Kaboudin, B.; Abedi, Y. A Novel Synthesis of Aryl Mesylates via One-Pot Demethylation-Mesylation of Aryl Methyl Ethers Using a Mixture of Phosphorus Pentoxide in Methanesulfonic Acid. Synthesis 2009, 2025–2028. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Baurmeister, J. Properties of high temperature PEFC Celtec®-P 1000 MEAs in start/stop operation mode. J. Power Sources 2008, 176, 428–434. [Google Scholar] [CrossRef]
- Ahmed, S.; Arshad, T.; Zada, A.; Afzal, A.; Khan, M.; Hussain, A.; Hassan, M.; Ali, M.; Xu, S. Preparation and Characterization of a Novel Sulfonated Titanium Oxide Incorporated Chitosan Nanocomposite Membranes for Fuel Cell Application. Membranes 2021, 11, 450. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. Phys. Chem. 1889, 4U, 226–248. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sones: Chichester, UK, 2004. [Google Scholar]
- Li, Q.; Aili, D.; Hjuler, H.A.; Jensen, J.O. High Temperature Polymer Electrolyte Membrane Fuel Cells, Approaches, Status and Perspectives; Springer: London, UK, 2016. [Google Scholar] [CrossRef]
- Lysova, A.A.; Ponomarev, I.I.; Skupov, K.M.; Vtyurina, E.S.; Lysov, K.A.; Yaroslavtsev, A.B. Effect of Organo-Silanes Structure on the Properties of Silane-Crosslinked Membranes Based on Cardo Polybenzimidazole PBI-O-PhT. Membranes 2023, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.N.; Konovalova, A.; Aili, D.; Li, Q.; Park, H.S.; Jang, J.H.; Kim, H.-J.; Henkensmeier, D. Thermally crosslinked sulfonated polybenzimidazole membranes and their performance in high temperature polymer electrolyte fuel cells. J. Membr. Sci. 2019, 588, 117218. [Google Scholar] [CrossRef]
- Neyerlin, K.C.; Singh, A.; Chu, D. Kinetic characterization of a Pt–Ni/C catalyst with a phosphoric acid doped PBI membrane in a proton exchange membrane fuel cell. J. Power Sources 2008, 176, 112–117. [Google Scholar] [CrossRef]

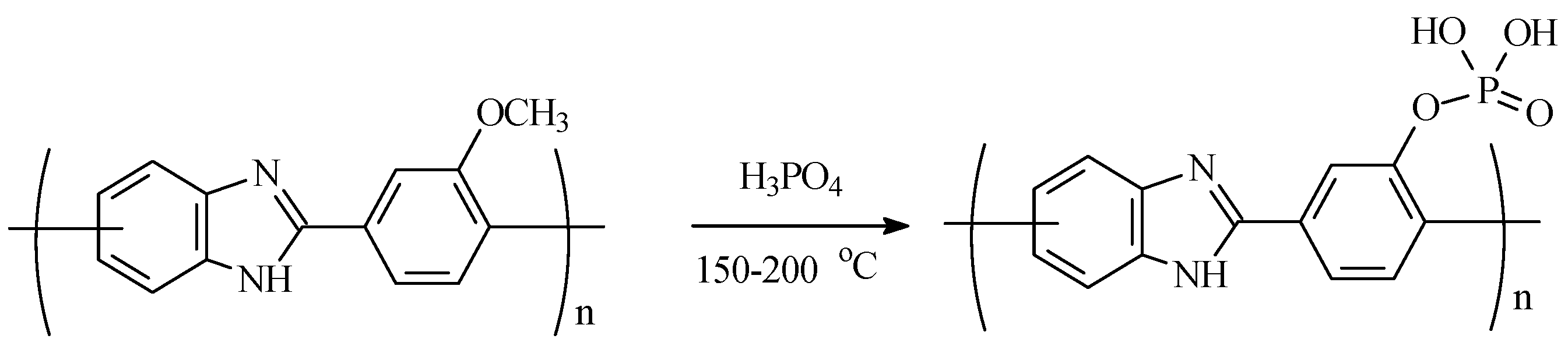








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponomarev, I.I.; Razorenov, D.Y.; Skupov, K.M.; Ponomarev, I.I.; Volkova, Y.A.; Lyssenko, K.A.; Lysova, A.A.; Vtyurina, E.S.; Buzin, M.I.; Klemenkova, Z.S. Self-Phosphorylated Polybenzimidazole: An Environmentally Friendly and Economical Approach for Hydrogen/Air High-Temperature Polymer-Electrolyte Membrane Fuel Cells. Membranes 2023, 13, 552. https://doi.org/10.3390/membranes13060552
Ponomarev II, Razorenov DY, Skupov KM, Ponomarev II, Volkova YA, Lyssenko KA, Lysova AA, Vtyurina ES, Buzin MI, Klemenkova ZS. Self-Phosphorylated Polybenzimidazole: An Environmentally Friendly and Economical Approach for Hydrogen/Air High-Temperature Polymer-Electrolyte Membrane Fuel Cells. Membranes. 2023; 13(6):552. https://doi.org/10.3390/membranes13060552
Chicago/Turabian StylePonomarev, Igor I., Dmitry Y. Razorenov, Kirill M. Skupov, Ivan I. Ponomarev, Yulia A. Volkova, Konstantin A. Lyssenko, Anna A. Lysova, Elizaveta S. Vtyurina, Mikhail I. Buzin, and Zinaida S. Klemenkova. 2023. "Self-Phosphorylated Polybenzimidazole: An Environmentally Friendly and Economical Approach for Hydrogen/Air High-Temperature Polymer-Electrolyte Membrane Fuel Cells" Membranes 13, no. 6: 552. https://doi.org/10.3390/membranes13060552
APA StylePonomarev, I. I., Razorenov, D. Y., Skupov, K. M., Ponomarev, I. I., Volkova, Y. A., Lyssenko, K. A., Lysova, A. A., Vtyurina, E. S., Buzin, M. I., & Klemenkova, Z. S. (2023). Self-Phosphorylated Polybenzimidazole: An Environmentally Friendly and Economical Approach for Hydrogen/Air High-Temperature Polymer-Electrolyte Membrane Fuel Cells. Membranes, 13(6), 552. https://doi.org/10.3390/membranes13060552







