Fly Ash as a Potential Adsorbent for Removing Radionuclides from Aqueous Solutions in an Adsorption-Membrane Assisted Process Compared to Batch Adsorption
Abstract
1. Introduction
- ✓
- Coal mining and coal combustion residuals,
- ✓
- Hard rock metal, rare earth uranium, or copper mining and production wastes,
- ✓
- Oil and gas mining,
- ✓
- Production of fertilizers.
- ✓
- Military explosions and nuclear accidents,
- ✓
- Nuclear fuel reprocessing.
- ✓
- High selectivity is achieved by proper selection of the sorbent,
- ✓
- Ability to separate metal ions even from very diluted solutions,
- ✓
- Relatively high speed of the purification process,
- ✓
- Low energy demand,
- ✓
- The relatively small size of the installation,
- ✓
- The simplicity of the operation,
- ✓
- Small operation costs as compared with other purification methods.
2. Experimental
2.1. Materials
2.2. Adsorption of the Radionuclides
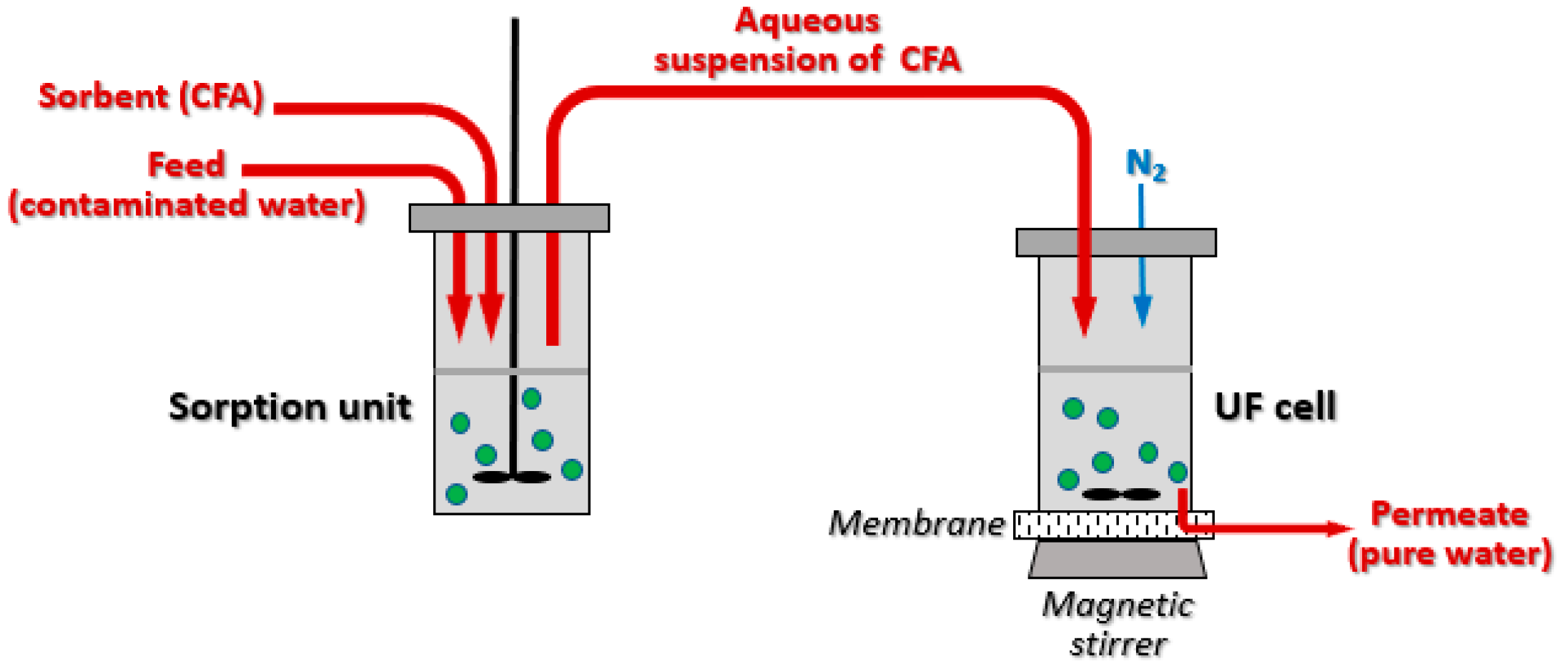
2.3. Laboratory Ultrafiltration Kit: Adsorption-Membrane Filtration (AMF) Studies
2.4. Methods Used in the Work and the Relevant Instrumentation
2.4.1. Characterisation of the FA
Surface Characterization of the FA
Electrical Potential of the Interface between Bulk Water and a Solvent Attached to the Surface of a Sorbent (Zeta-Potential)
Thermal Analysis
2.4.2. Radioanalytical Methods
Radiometric Analyses of the Initial- and Equilibrium Aqueous Solutions/Fractions Collected in the AMF
Checking the Purity of the Radionuclides
Production of the Saline Aqueous Solutions Containing Tc-99m
2.4.3. Supporting Analytical Methods
3. Results and Discussion
3.1. General Characterization of the Fly Ash (FA)
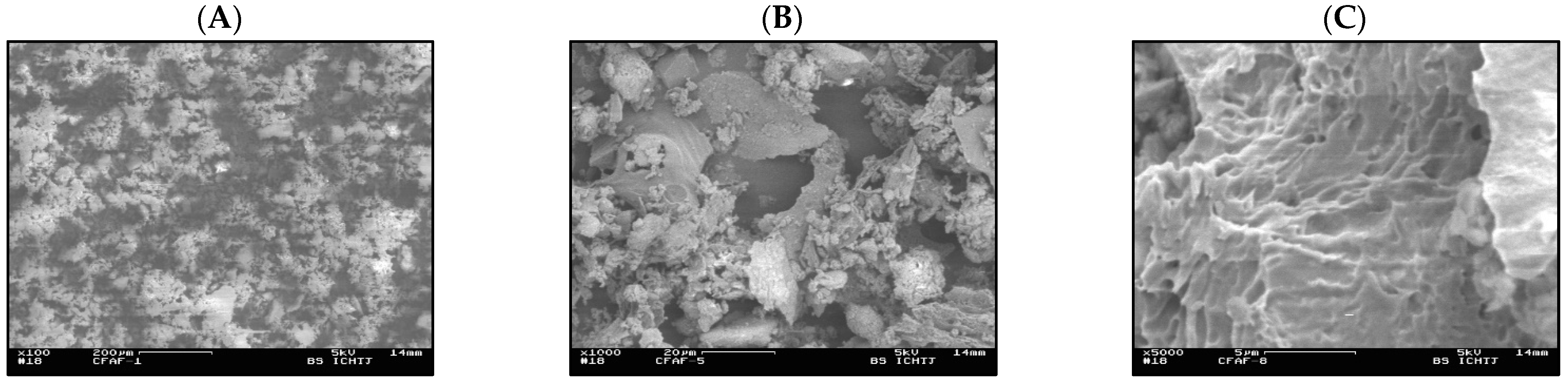
3.2. Characterization of the FA Surface by Scanning Electron Microscopy (SEM)
3.3. Thermogravimetric Analysis of the Coal Fly Ash (FA)
3.4. Zeta Potential of the FA Surface
3.5. Batch Sorption of the Cationic Radionuclides
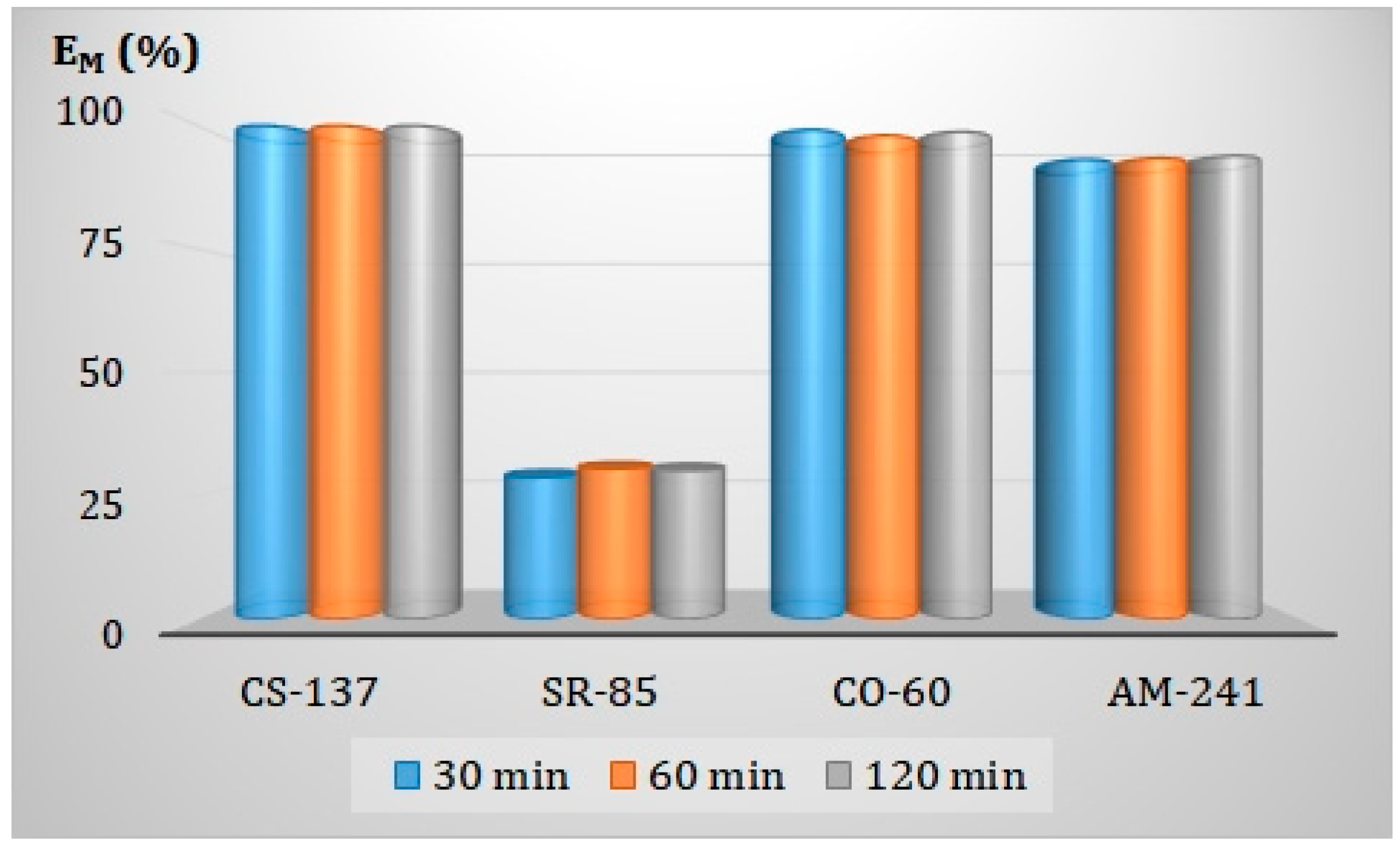
3.5.1. Effect of Contact Time of the Phases on Adsorption
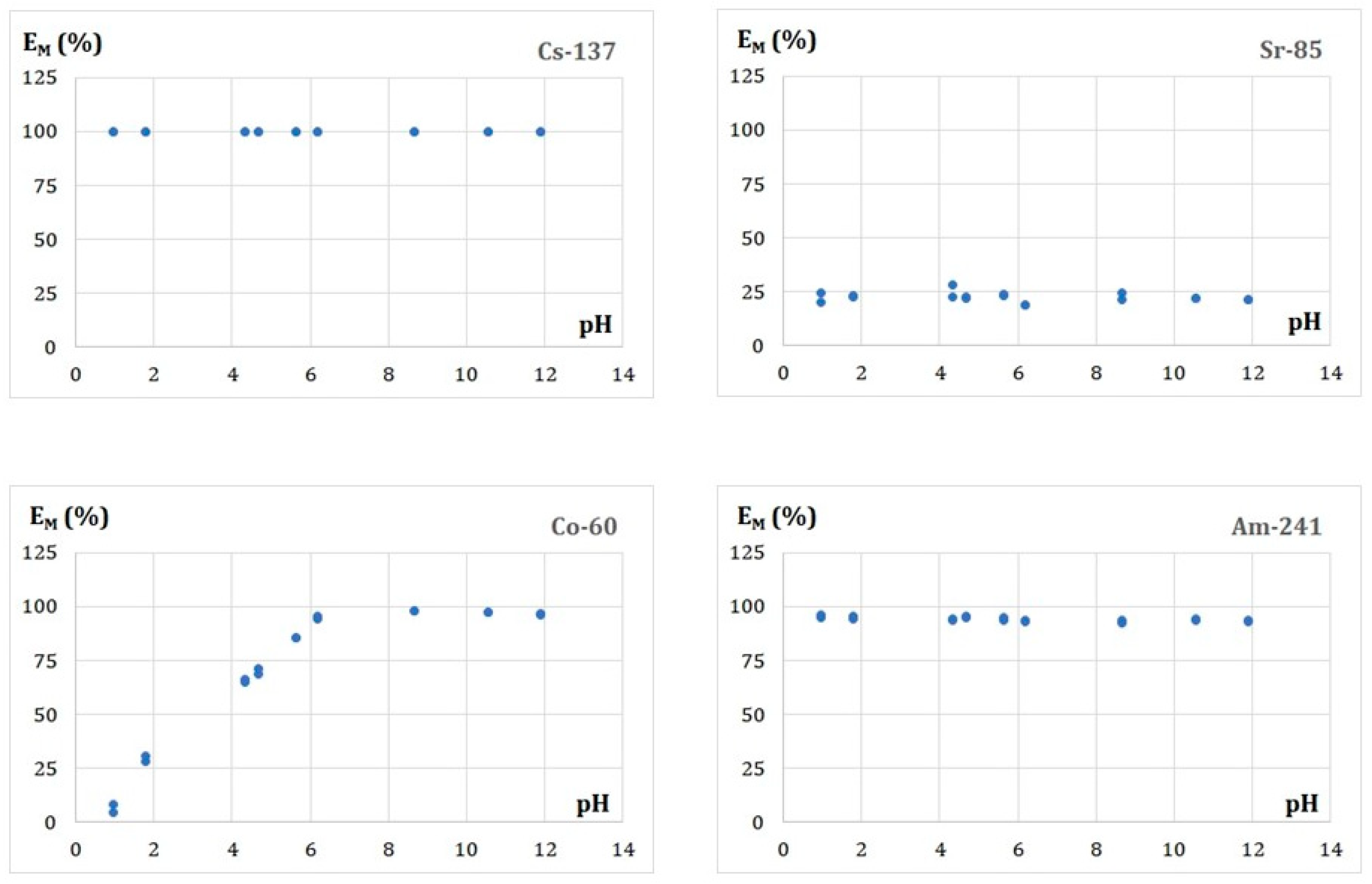
3.5.2. Effect of the Solution pH on Adsorption of the Radionuclides
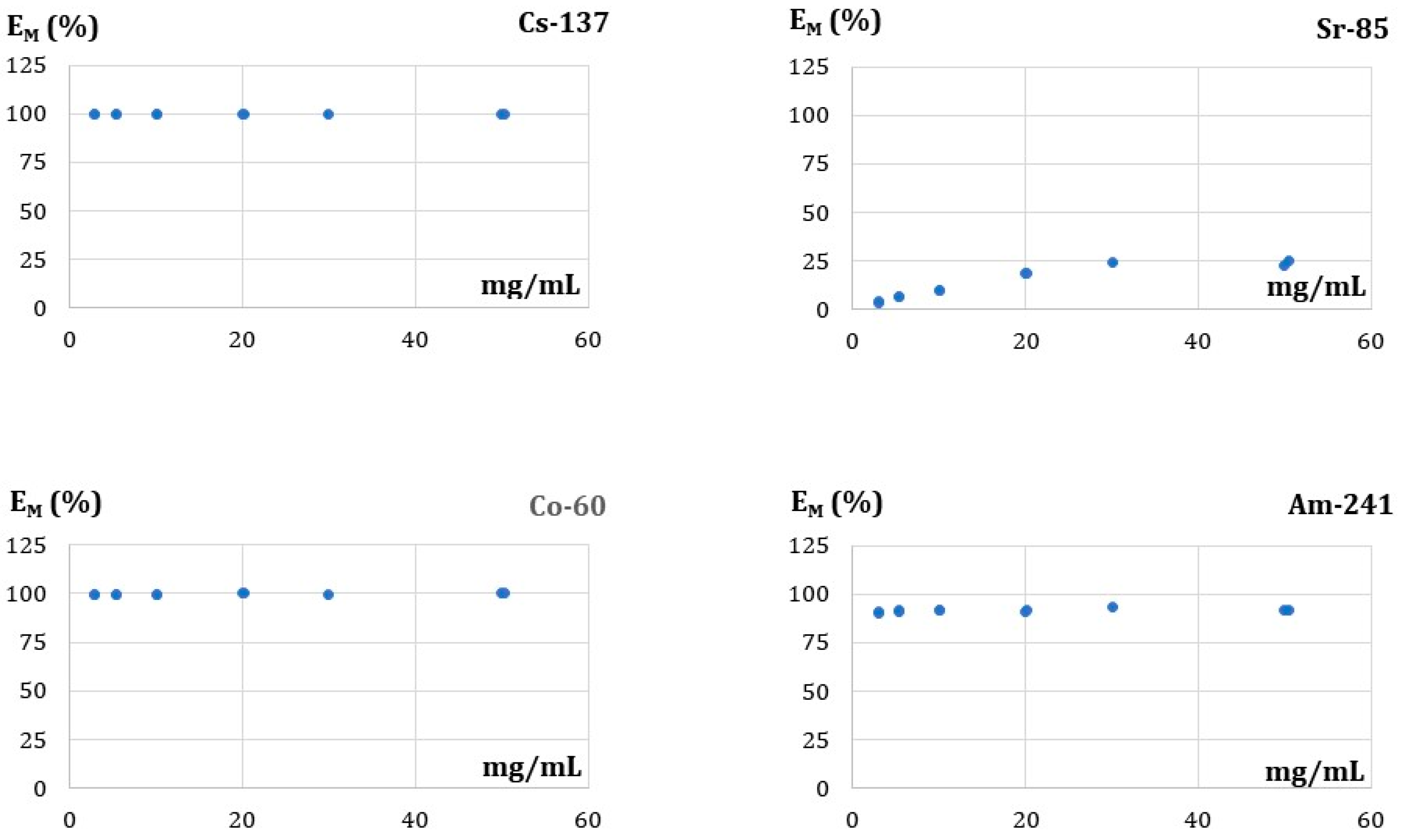
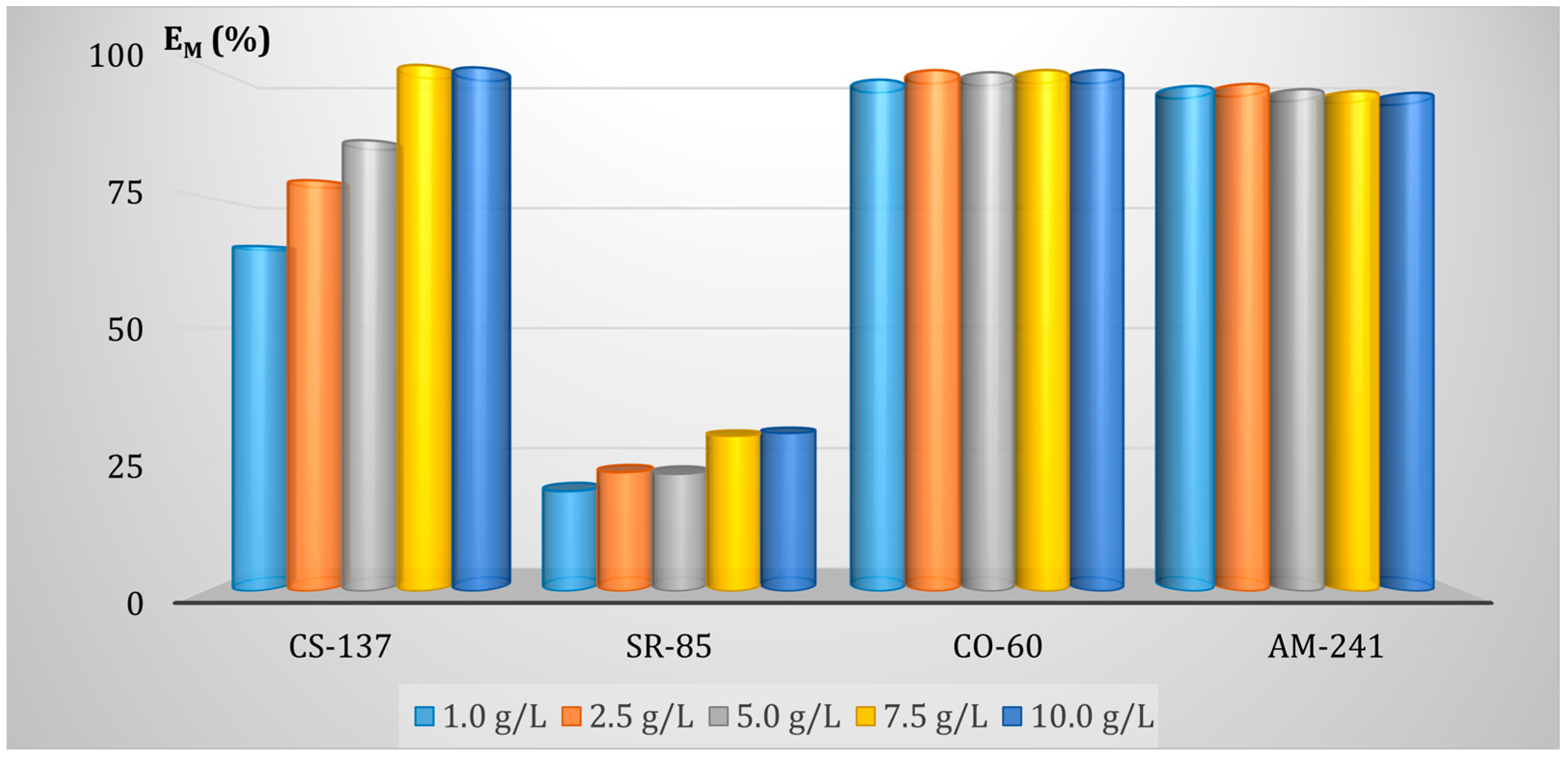
3.5.3. Effect of the FA Dose on Adsorption
3.5.4. Simulated Liquid Radioactive Waste
3.6. The Adsorption-Membrane Filtration (AMF) Hybrid Process of Removal of the Cationic Radionuclides
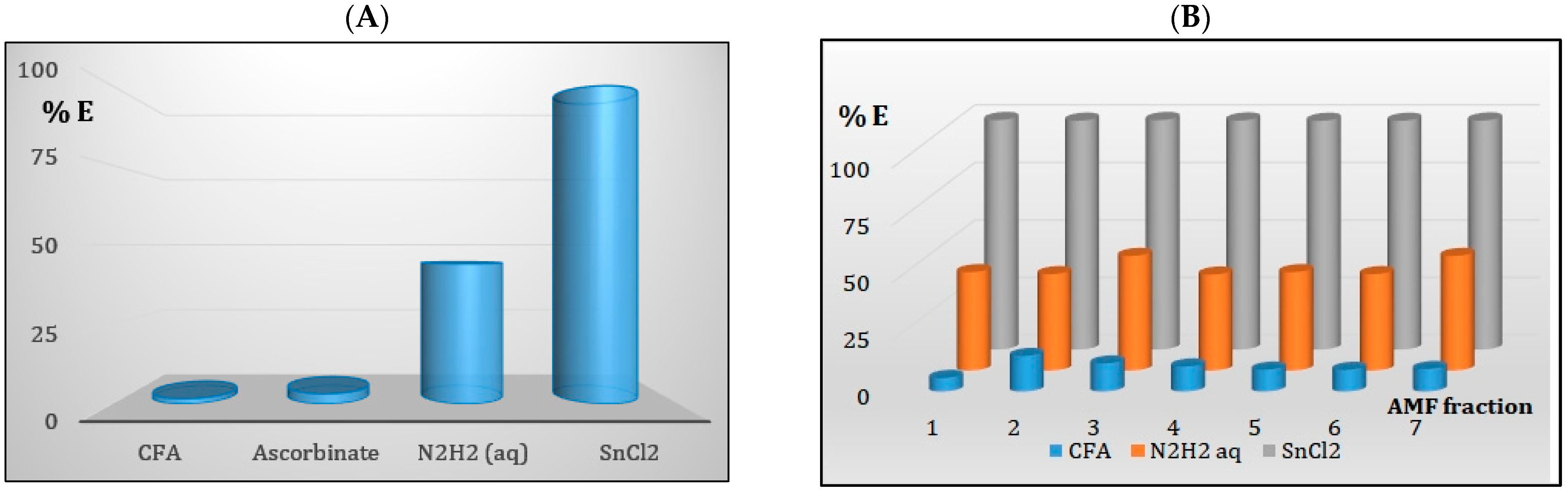
3.6.1. Choice of the Membrane
3.6.2. Effect of Mass of the Fly Ash Used as Sorbent in the Adsorption-Membrane Filtration (AMF) Hybrid Process
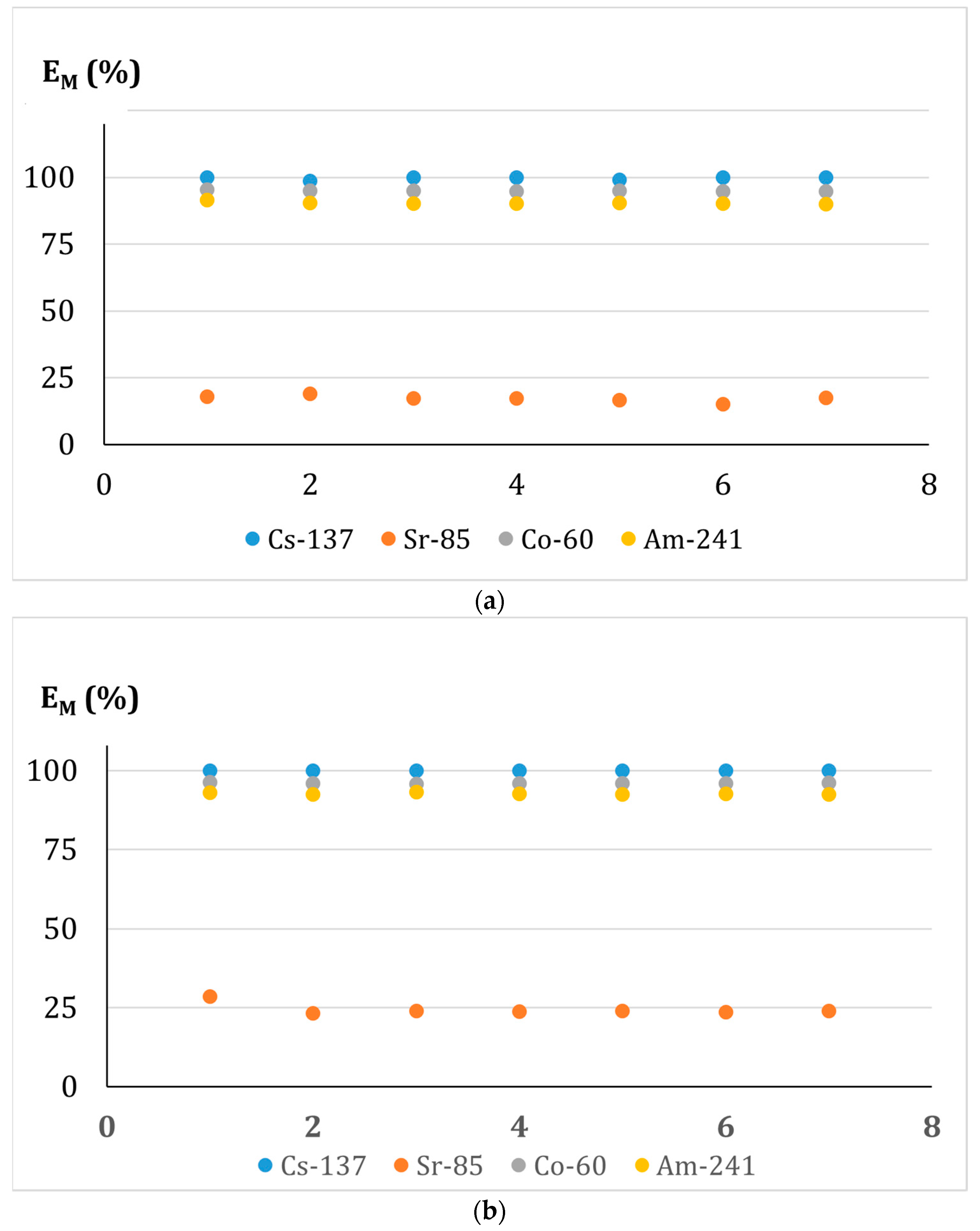
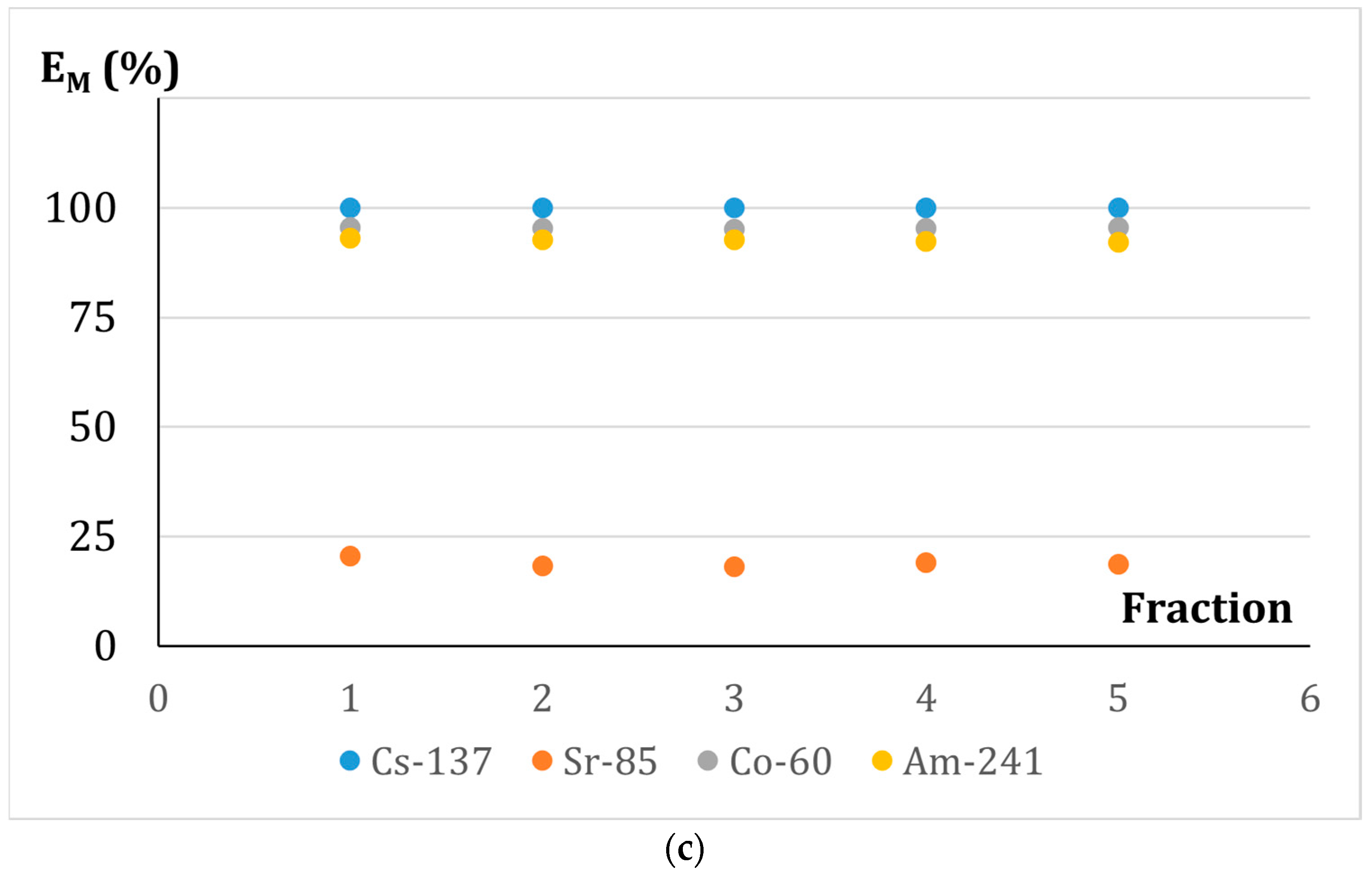
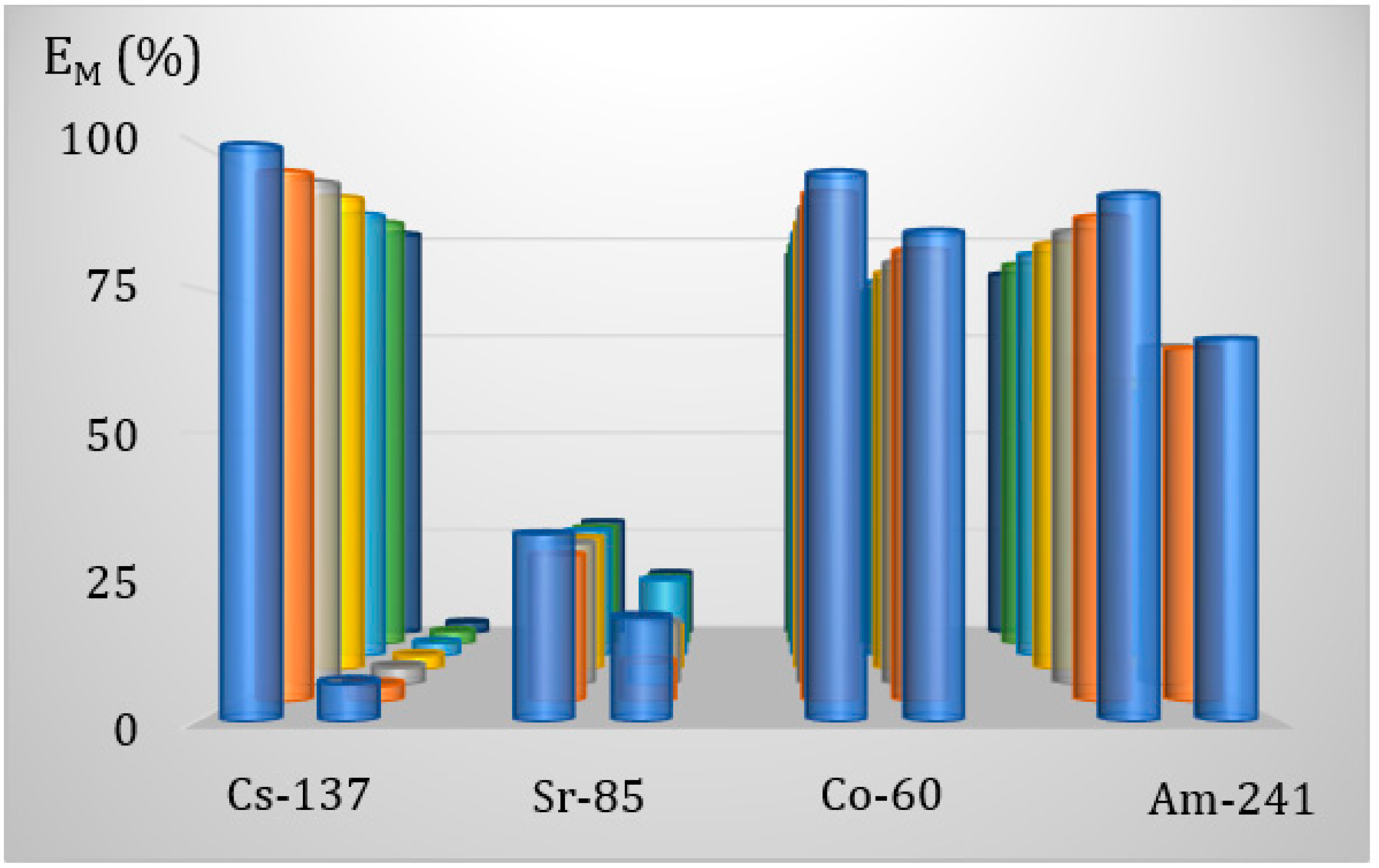
3.6.3. Effect of the Contact Time of the Phases in the Adsorption-Membrane Filtration (AMF) Hybrid Process
3.6.4. Membrane Fouling
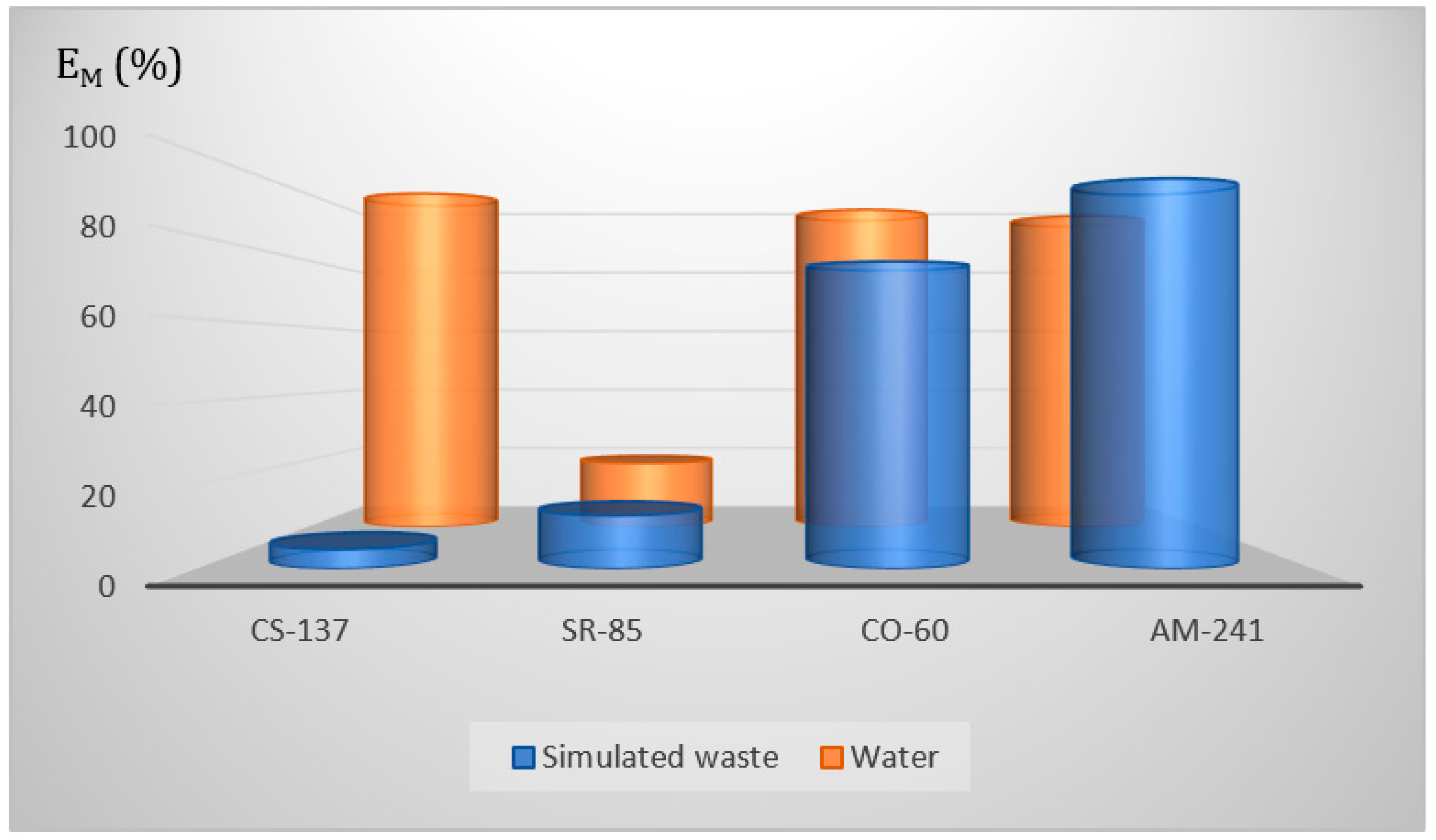
3.6.5. Simulated Liquid Radioactive Waste
3.7. Removal of the Metal Oxo-Anionic Radioactive Contaminants from Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davoodbeygi, Y.; Askari, M.; Salehi, E.; Kheirieh, S. A review on hybrid membrane-adsorption systems for intensified water and wastewater treatment: Process configurations, separation targets, and materials applied. J. Environ. Manag. 2023, 335, 117577. [Google Scholar] [CrossRef]
- Kim, S.; Nam, S.-N.; Jang, A.; Jang, M.; Park, C.M.; Son, A.; Her, N.; Heo, J.; Yoon, Y. Review of adsorption–membrane hybrid systems for water and wastewater treatment. Chemosphere 2022, 286, 131916. [Google Scholar] [CrossRef]
- Koltuniewicz, A.B.; Witek, A.; Bezak, K. Efficiency of membrane-sorption integrated processes. J. Membr. Sci. 2004, 239, 129–141. [Google Scholar] [CrossRef]
- Rasouli, Y.; Abbasi, M.; Hashemifard, S.A. Oily wastewater treatment by adsorption–membrane filtration hybrid process using powdered activated carbon, natural zeolite powder and low cost ceramic membranes. Water Sci. Technol. 2017, 76, 895–908. [Google Scholar] [CrossRef]
- Chmielewska-Śmietanko, D.; Miśkiewicz, A. Batch, fixed-bed column and hybrid microfiltration process studies of radiocesium removal from contaminated water by nanocomposite SiEA KNiFe sorbent. In Waste PET-MOF-Cleanwater: Waste PET-Derived Metal-Organic Framework (MOFs) as Cost-Effective Adsorbents for Removal of Hazardous Elements from Polluted Water; Ren, J., Nomngongo, P.N., Jen, T.-C., Eds.; University of Johannesburg Press: Auckland Park, South Africa, 2022; pp. 33–44. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Osibote, O.A.; Darmokoesoemo, H.; Kusuma, H.S. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: A review. J. Mat. Res. Technol. 2021, 14, 2751–2774. [Google Scholar] [CrossRef]
- Tofan, L.; Paduraru, C.; Volf, I.; Wenkert, R. Comparative study concerning the kinetic and thermodynamic description of some heavy metal ions sorption on fly ash. J. Optoelectron. Adv. Mat. 2011, 13, 892–896. [Google Scholar]
- Fomenko, A.I.; Sokolov, L.I. Sorption properties of fly ash microspheres of thermal power plants. Ecol. Ind. Russ. 2019, 23, 50–54. [Google Scholar]
- Apak, R.; Tütem, E.; Hügül, M.; Hizal, J. Heavy metal cation retention by unconventional sorbents (red muds and fly ashes). Water Res. 1998, 32, 430–440. [Google Scholar]
- Hu, X.; Huang, X.; Zhao, H.; Liu, F.; Wang, L.; Zhao, X.; Gao, P.; Li, X.; Ji, P. Possibility of using modified fly ash and organic fertilizers for remediation of heavy-metal-contaminated soils. J. Clean. Prod. 2021, 284, 124713. [Google Scholar]
- Mohan, S.; Gandhimath, R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J. Hazard. Mater. 2009, 169, 351–359. [Google Scholar]
- Cretescu, I.; Harja, M.; Teodosiu, C.; Isopescu, D.N.; Chok, M.F.; Sluser, B.M.; Salleh, M.A.M. Synthesis and characterisation of a binder cement replacement based on alkali activation of fly ash waste. Process Saf. Environ. Prot. 2018, 119, 23–35. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and characterization of zeolites prepared from industrial fly ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible applications of coal fly ash in wastewater treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Singh, N.B.; Agarwal, A.; De, A.; Singh, P. Coal fly ash: An emerging material for water remediation. Int. J. Coal Sci. Technol. 2022, 9, 44. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A. Adsorption of 2,4-Dichlorophenoxyacetic Acid from an Aqueous Solution on Fly Ash. Water Environ. Res. 2016, 88, 231–238. [Google Scholar] [CrossRef]
- Gadore, V.; Ahmaruzzaman, M. Tailored fly ash materials: A recent progress of their properties and applications for remediation of organic and inorganic contaminants from water. J. Water Process Eng. 2021, 41, 101910. [Google Scholar] [CrossRef]
- Hosseini Asl, S.M.; Javadian, H.; Khavarpour, M.; Belviso, C.; Taghavi, M.; Maghsudi, M. Porous adsorbents de-rived from coal fly ash as cost-effective and environmentally-friendly sources of aluminosilicate for se-questration of aqueous and gaseous pollutants: A review. J. Clean. Prod. 2019, 208, 1131–1147. [Google Scholar] [CrossRef]
- Fuks, L.; Miśkiewicz, A.; Zakrzewska-Kołtuniewicz, G. Sorption-assisted ultrafiltration hybrid method for treatment of the radioactive aqueous solutions. Chemistry 2022, 4, 1076–1091. [Google Scholar] [CrossRef]
- Cho, H.; Oh, D.; Kim, K. A study on removal characteristics of heavy metals from aqueous solution by fly ash. J. Hazard. Mater. 2005, 127, 187–195. [Google Scholar] [CrossRef]
- Ratajczak, T.; Gaweł, A.; Górniak, K.; Muszyński, M.; Szydłak, T.; Wyszomirski, P. Charakterystyka popiołów lotnych ze spalania niektórych węgli kamiennych i brunatnych (Characteristics of volatile ash from the combustion of certain hard and lignite coals). Pol. Tow. Mineral.—Pr. Spec. 1999, 13, 9–34. (In Polish) [Google Scholar]
- Naqvi, S.Z.; Ramkumar, J.; Kar, K.K. Coal-based fly ash. In Handbook of Fly Ash; Kar, K.K., Ed.; Butterworth-Heinemann: Oxford, UK, 2021; pp. 3–33. ISBN 9780128176863. [Google Scholar]
- Suraneni, P.; Burris, L.; Shearer, C.R.; Hooton, R.D. ASTM C618 fly ash specification: Comparison with other specifications, shortcomings, and solutions. ACI Mater. J. 2021, 118, 157–167. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Fly ash from coal combustion: Dependence of the concentration of various elements on the particle size. Fuel 2018, 228, 263–271. [Google Scholar] [CrossRef]
- Marruzzo, G.; Medici, F.; Panei, L.; Piga, L.; Rinaldi, G. Characteristics and Properties of a Mixture Containing Fly Ash, Hydrated Lime, and an Organic Additive. Environ. Eng. Sci. 2001, 18, 159–165. [Google Scholar] [CrossRef]
- Fuks, L.; Herdzik-Koniecko, I.; Rogowski, M. Carbon obtained from waste polyethylene terephthalate (PET) containers as potential sorbent of radionuclides from the contaminated aqueous solutions. Int. J. Environ. Sci. Technol. 2021, 18, 3527–3538. [Google Scholar] [CrossRef]
- Siva, T.; Muralidharan, S.; Sadagopan, S.; Manikandan, E. Enhanced Polymer Induced Precipitation of Polymorphous in Calcium Carbonate: Calcite Aragonite Vaterite Phases. J. Inorg. Organomet. Polym. 2017, 27, 770–778. [Google Scholar] [CrossRef]
- Delgado, A.V.; Gonzalez-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1753–1805. [Google Scholar] [CrossRef]
- Nägele, E.; Schneider, U. The zeta-potential of blast furnace slag and fly ash. Cem. Concr. Res. 1989, 19, 811–820. [Google Scholar] [CrossRef]
- Babu, S. Advances in Chemical Mechanical Planarization (CMP), 2nd ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2016; pp. 309–312. [Google Scholar]
- Fuks, L.; Oszczak, A.; Dudek, J.; Majdan, M.; Trytek, M. Removal of the radionuclides from aqueous solutions by biosorption on the roots of the dandelion (taraxacum officinale). Int. J. Environ. Sci. Technol. 2016, 13, 2339–2352. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Kadu, M.S.; Pandhurnekar, C.P.; Muthreja, I.L. Kinetics study on the adsorption of Ni2+ ions onto fly ash. J. Chem. Technol. Metall. 2015, 50, 601–605. [Google Scholar]
- Abou-Lilah, R.A.; Rizk, H.E.; Elshorbagy, M.A.; Gamal, A.M.; Ali, A.M.; Badawy, N.A. Efficiency of bentonite in removing cesium, strontium, cobalt and uranium ions from aqueous solution: Encapsulation with alginate for column application. Int. J. Environ. Anal. Chem. 2022, 102, 2913–2936. [Google Scholar] [CrossRef]
- Hamed, M.M.; Aly, M.I.; Nayl, A.A. Kinetics and thermodynamics studies of cobalt, strontium, and caesium sorption on marble from aqueous solution. Chem. Ecol. 2016, 32, 68–87. [Google Scholar] [CrossRef]
- Atwood, D.A. Radionuclides in the Environment; John Wiley and Sons: Chichester, UK, 2010; pp. 85–86. [Google Scholar]
- McCarthy, G.; Johansen, D.; Steinwand, S.; Thedchanamoorthy, A. X-Ray Diffraction Analysis of Fly Ash. Adv. X-ray Anal. 1987, 31, 331–342. [Google Scholar] [CrossRef]
- Schneider, H.; Schreuer, J.; Hildmann, B. Structure and properties of mullite—A review. J. Eur. Ceram. Soc. 2008, 28, 329–344. [Google Scholar] [CrossRef]
- Todorović, M.; Milonjić, S.K.; Čomor, J.J.; Gal, I.J. Adsorption of Radioactive Ions 137Cs, 85Sr, and 60Co on natural magnetite and hematite. Sep. Sci. Technol. 1992, 27, 671–679. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.S.; Al Zboom, K.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly ash based geo-polymer for heavy metal removal: A case study on copper removal. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Yildiz, E. Phosphate removal from water by fly ash using crossflow microfiltration. Sep. Purif. Technol. 2004, 35, 241–252. [Google Scholar] [CrossRef]
- Saıdi, S.; Khmakem, S.; Larbot, A.; Elloumi-Ammar, N.; Fourati, A.; Charfi, A.; Ben Amar, R. New ceramic microfiltration membranes from mineral coal fly ash. Arab. J. Chem. 2009, 2, 31–39. [Google Scholar]
- Fuks, L.; Herdzik-Koniecko, I. Metal-selective sorbents. In Handbooks in Separation Science—Solid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 185–213. [Google Scholar] [CrossRef]
- Schwochau, K. Technetium: Chemistry and Radiopharmaceutical Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; p. 43. [Google Scholar]
- Lee, M.S.; Um, W.; Wang, G.; Kruger, A.A.; Lukens, W.W.; Rousseau, R.; Glezakou, V.A. Impeding 99Tc(IV) mobility in novel waste forms. Nat. Commun. 2016, 7, 12067–12072. [Google Scholar] [CrossRef]
- Fuks, L.; Herdzik-Koniecko, I. Vermiculite as a potential component of the engineered barriers in low- and medium-level radioactive waste repositories. Appl. Clay Sci. 2018, 161, 139–150. [Google Scholar] [CrossRef]
- Littlewood, J.; Shaw, S.; Bots, P.; Peacock, C.; Trivedi, D.; Burke, I. Effect of solution composition on the recrystallization of kaolinite to feldspathoids in hyperalkaline conditions: Limitations of pertechnetate in-corporation by ion competition effects. Mineral. Mag. 2015, 79, 1379–1388. [Google Scholar] [CrossRef]
- Saha, G.B. Fundamentals of Nuclear Pharmacy; Springer International Publishing: Cham, Switzerland, 2018; p. 111. ISBN 978-3-319-57579-7. [Google Scholar]


| Parameter | Value | ||
|---|---|---|---|
| Moisture | 1.1% | ||
| Grain size (diameter) | 0.20–0.10 mm (41.2%) 0.10–0.05 mm (48.2%) 0.05–0.03 mm (8.6%) | ||
| Bulk density: | 1.79 g/mL | ||
| Mass loss below 650 °C | 15% | ||
| Mass loss above 650 °C | 2% | ||
| ζ-potential (pH 5) | −21.6 mV | ||
| ζ-potential (pH 8) | −24.8 mV | ||
| Element | Element concentration in %, based on actual atomic mass (EDX) * | Constituent | wt. (%) |
| Oxygen | 42.90 ± 0.80 | ||
| Carbon | 26.49 ± 1.65 | 26.5 | |
| Silicon | 12.08 ± 1.76 | SiO2 | 25.9 |
| Aluminium | 9.12 ± 1.21 | Al2O3 | 17.2 |
| Calcium | 3.42 ± 0.41 | CaO | 4.8 |
| Iron | 3.05 ± 0.18 | Fe2O3 | 8.7 |
| Potassium | 1.27 ± 0.20 | K2O | 3.1 |
| Sulfur | 0.76 ± 0.17 | SO2 | 1.5 |
| Titanium | 0.64 ± 0.04 | TiO2 | 1.1 |
| Magnesium | 0.26 ± 0.10 | MgO | 0.4 |
| Other ** | 10.8 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuks, L.; Miśkiewicz, A.; Herdzik-Koniecko, I.; Zakrzewska-Kołtuniewicz, G. Fly Ash as a Potential Adsorbent for Removing Radionuclides from Aqueous Solutions in an Adsorption-Membrane Assisted Process Compared to Batch Adsorption. Membranes 2023, 13, 572. https://doi.org/10.3390/membranes13060572
Fuks L, Miśkiewicz A, Herdzik-Koniecko I, Zakrzewska-Kołtuniewicz G. Fly Ash as a Potential Adsorbent for Removing Radionuclides from Aqueous Solutions in an Adsorption-Membrane Assisted Process Compared to Batch Adsorption. Membranes. 2023; 13(6):572. https://doi.org/10.3390/membranes13060572
Chicago/Turabian StyleFuks, Leon, Agnieszka Miśkiewicz, Irena Herdzik-Koniecko, and Grażyna Zakrzewska-Kołtuniewicz. 2023. "Fly Ash as a Potential Adsorbent for Removing Radionuclides from Aqueous Solutions in an Adsorption-Membrane Assisted Process Compared to Batch Adsorption" Membranes 13, no. 6: 572. https://doi.org/10.3390/membranes13060572
APA StyleFuks, L., Miśkiewicz, A., Herdzik-Koniecko, I., & Zakrzewska-Kołtuniewicz, G. (2023). Fly Ash as a Potential Adsorbent for Removing Radionuclides from Aqueous Solutions in an Adsorption-Membrane Assisted Process Compared to Batch Adsorption. Membranes, 13(6), 572. https://doi.org/10.3390/membranes13060572







