Evaluation of Efficiently Removing Secondary Effluent Organic Matters (EfOM) by Al-Based Coagulant for Wastewater Recycling: A Case Study with an Industrial-Scale Food-Processing Wastewater Treatment Plant
Abstract
1. Introduction
2. Material and Methods
2.1. Experiment Setup and Operation
2.2. Jar Test Procedure
2.3. FT-IR
2.4. Dissolved Organic Matter (DOM) Fluorescence Characteristics Analysis
2.4.1. Three-Dimensional Excitation-Emission-Matrix (3D-EEM) and Parallel Factor Analysis (PARAFAC)
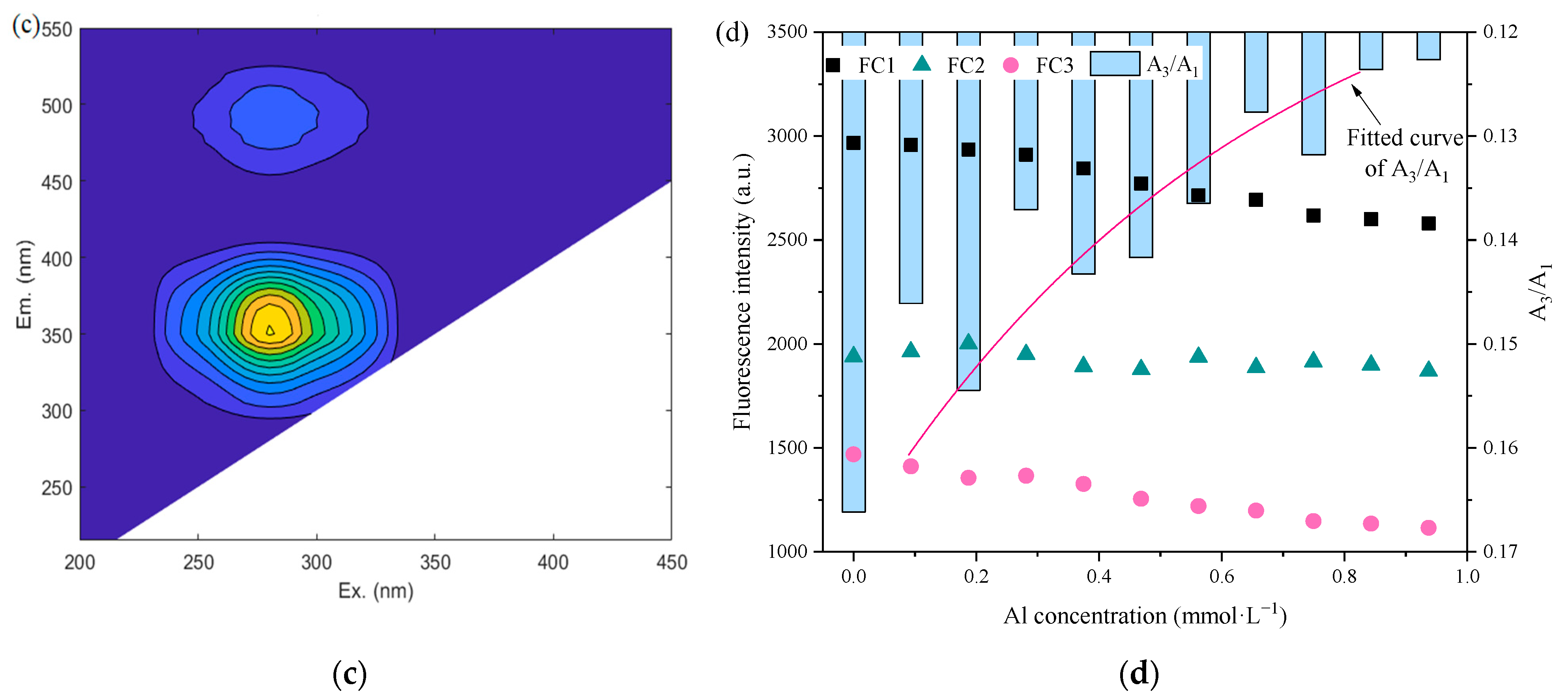
2.4.2. Fluorescence Quenching Titration
2.4.3. Two-Dimensional Synchronous Fluorescence Correlation Spectroscopy (2D-SF-COS)
2.5. Analytical Methods
3. Results and Discussion
3.1. Performance of the Coagulation Process
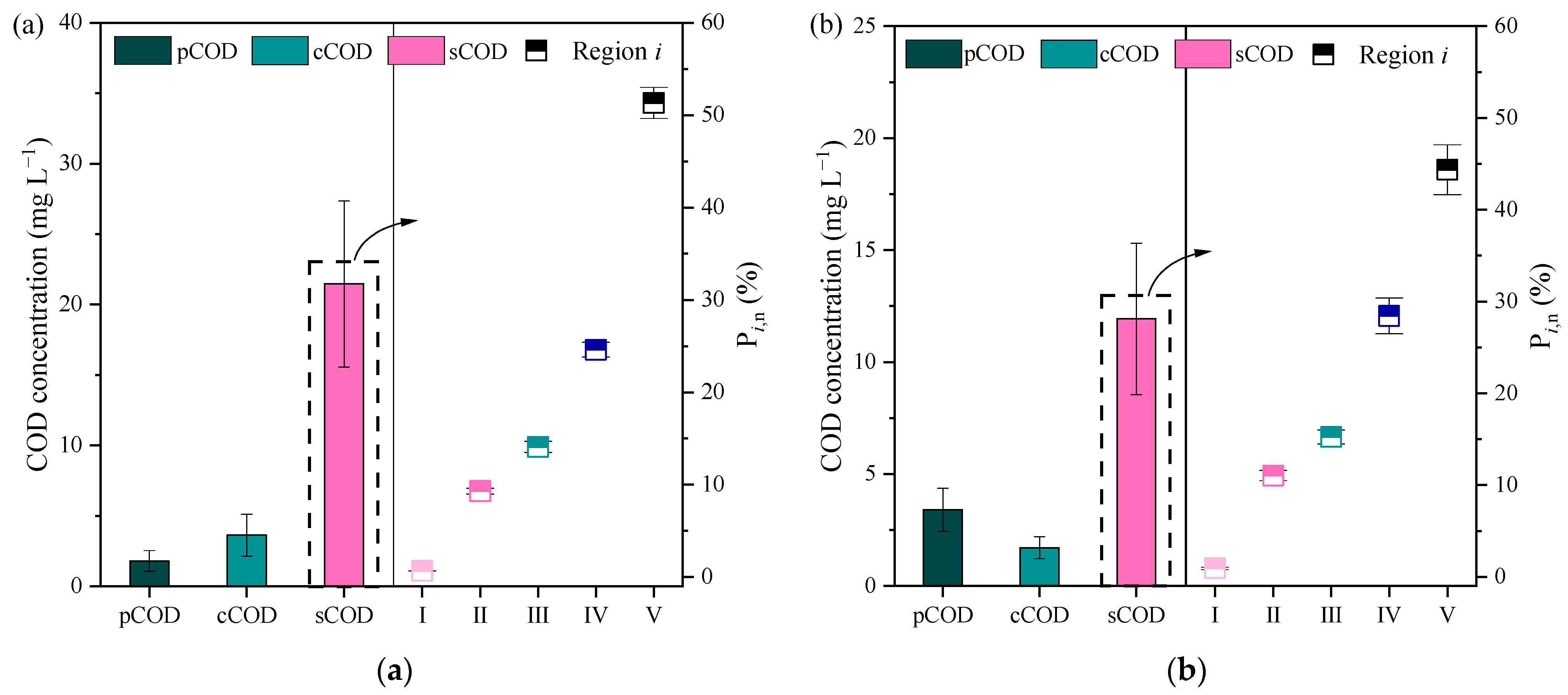
3.2. Change in COD Fractions
3.3. Influence on DOM Components
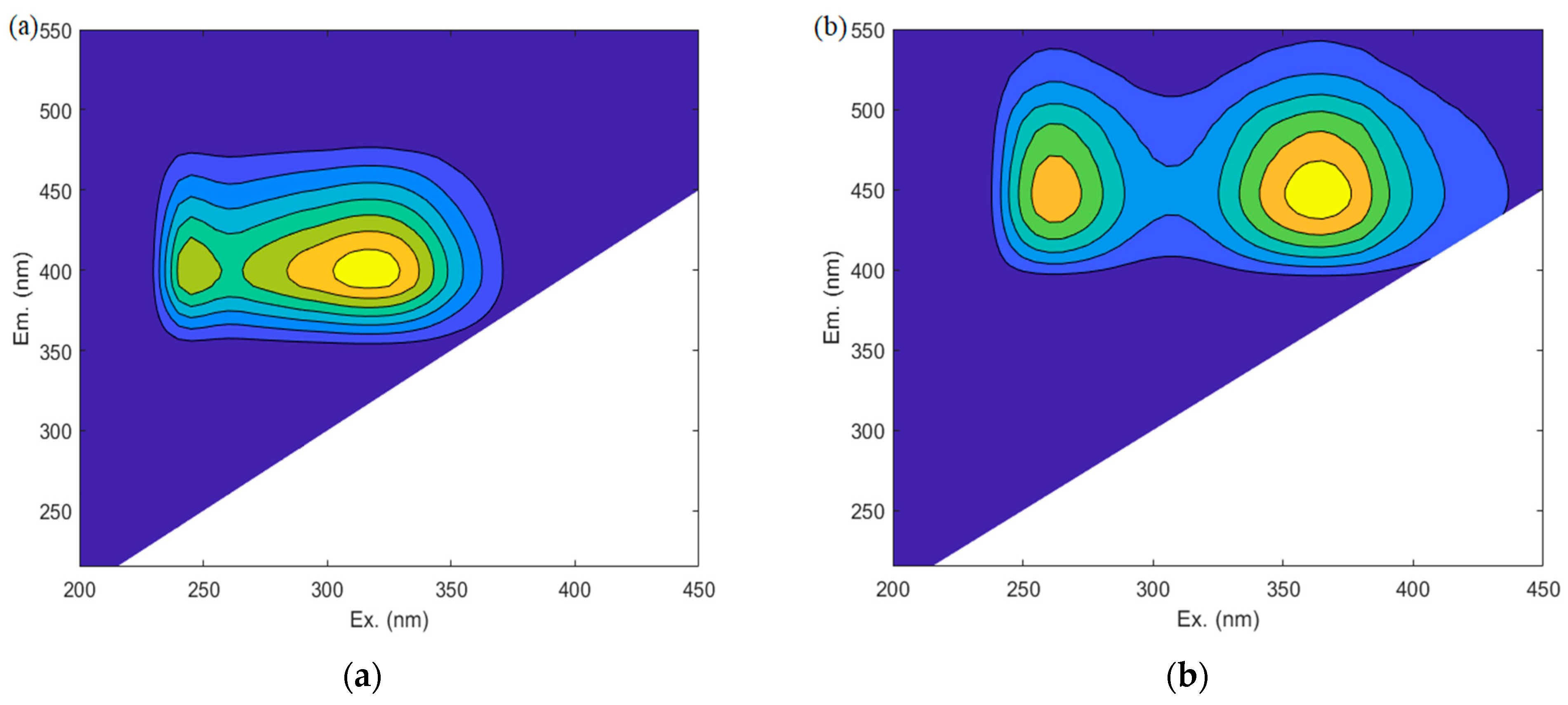
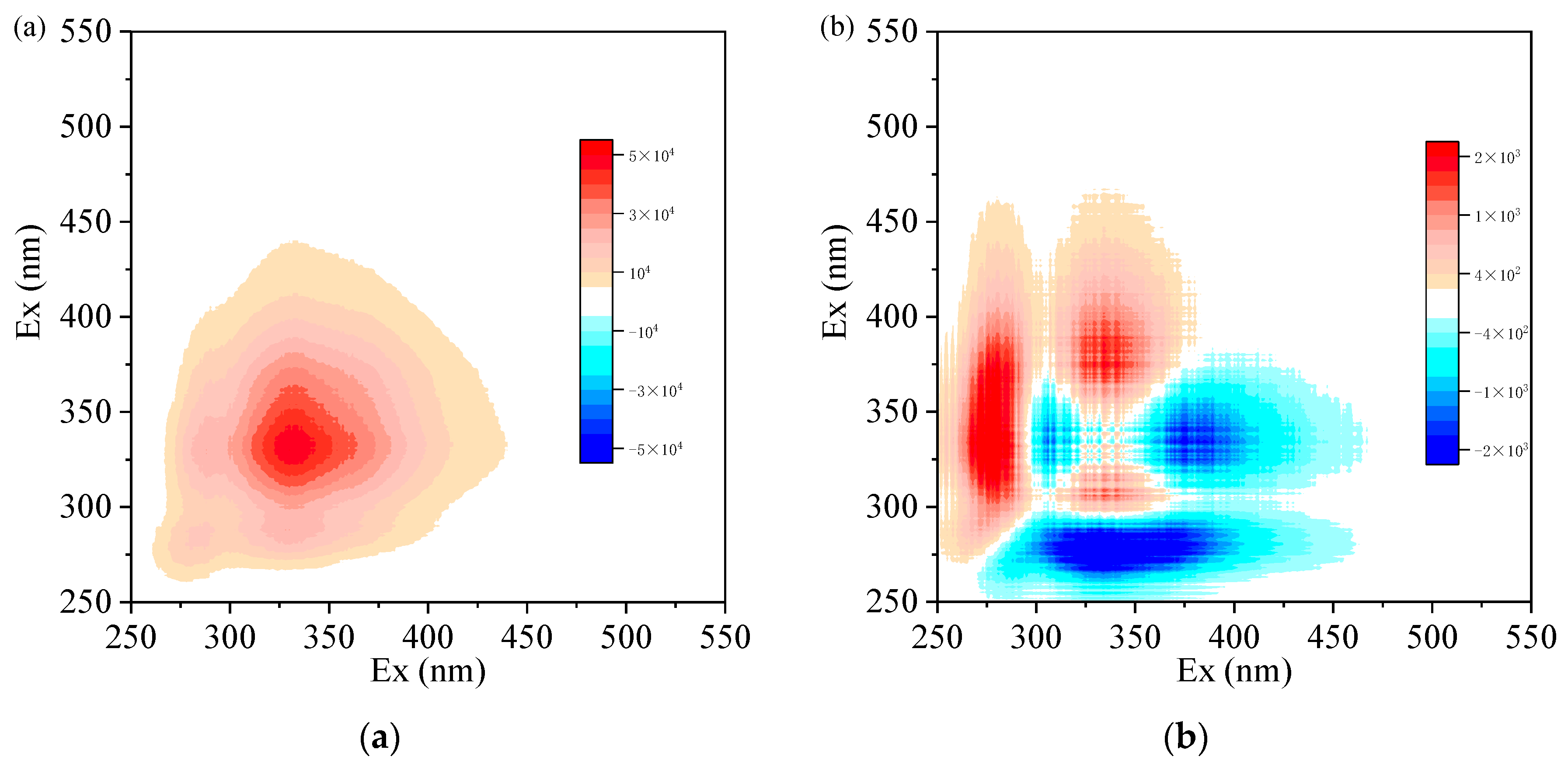
3.3.1. Component Separation and Dehumification Analysis
3.3.2. Variation in SMP
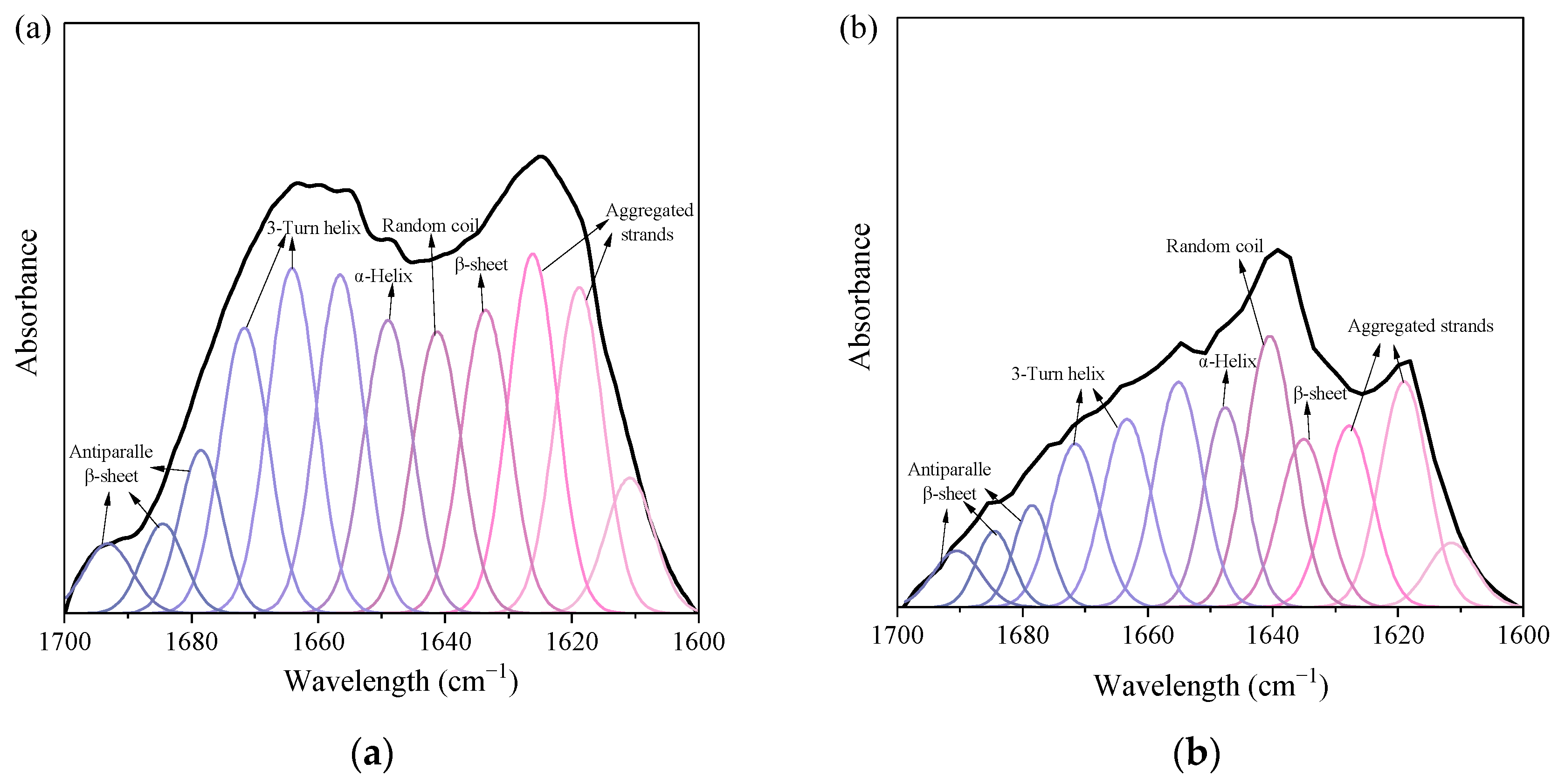
3.3.3. Characteristics of DOM Removal
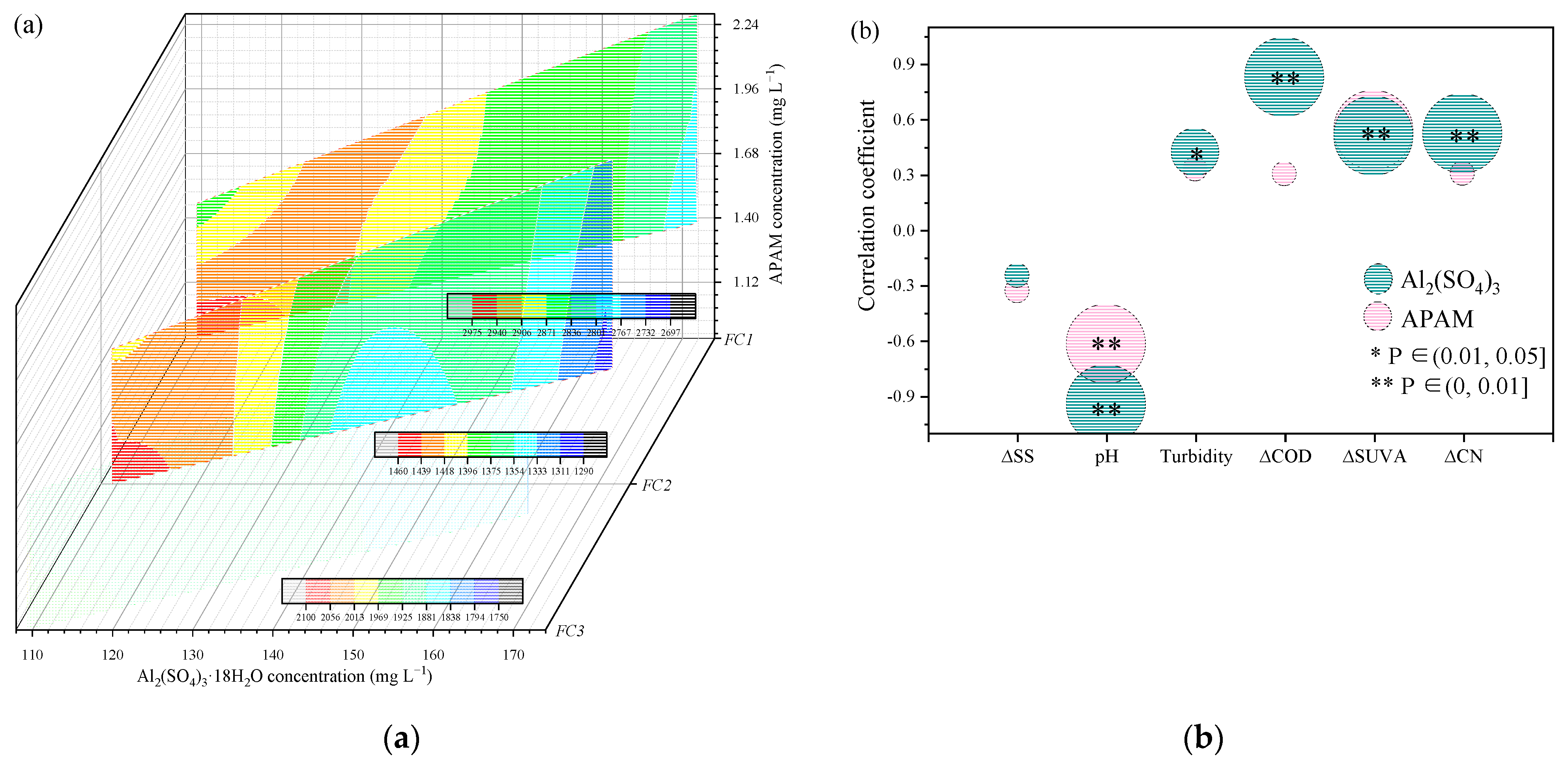
3.4. Optimization of Coagulation/Flocculation Efficiency
3.5. Integrated System Economic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Jin, X.; Zhuo, N.; Zhu, G.; Cai, Z. Interaction-sedimentation strategy for highly efficient removal of refractory humic substances in biologically treated wastewater effluent: From mechanistic investigation to full-scale application. J. Hazard. Mater. 2021, 418, 126145. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Wang, Z.; Zhou, K.; Chen, X.; Cheng, Y.; Zhang, G.; Wu, D.W.; Sun, S.P. Advanced treatment of secondary effluent organic matters (EfOM) from an industrial park wastewater treatment plant by Fenton oxidation combining with biological aerated filter. Sci. Total Environ. 2021, 784, 147204. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Zhao, M.F.; Duan, C.; Yue, P.; Li, T.G. Removal characteristics of dissolved organic matter and membrane fouling in ultrafiltration and reverse osmosis membrane combined processes treating the secondary effluent of wastewater treatment plant. Water Sci. Technol. 2021, 83, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Liu, J.D.; Li, L.; Zhen, G.Y.; Lu, X.Q.; Zhang, J.; Liu, H.B.; Zhou, Z.; Wu, Z.C.; Zhang, X.D. Prospects for humic acids treatment and recovery in wastewater: A review. Chemosphere 2023, 312, 137193. [Google Scholar] [CrossRef]
- Qu, J.H.; Dai, X.H.; Hu, H.Y.; Huang, X.; Chen, Z.; Li, T.; Cao, Y.S.; Daigger, G.T. Emerging Trends and Prospects for Municipal Wastewater Management in China. ACS EST Eng. 2022, 2, 323–336. [Google Scholar] [CrossRef]
- De Oliveira Anício, S.; dos Santos Lopes, V.; de Oliveira, A.L. PSD and Fractal Dimension for flocculation with different parameters and ferric chloride, aluminium polychloride and aluminium sulfate as coagulants. J. Water Process Eng. 2021, 43, 102180. [Google Scholar] [CrossRef]
- Xiong, X.; Wu, X.; Zhang, B.; Xu, H.; Wang, D. The interaction between effluent organic matter fractions and Al2(SO4)3 identified by fluorescence parallel factor analysis and FT-IR spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 418–428. [Google Scholar] [CrossRef]
- Yu, H.; Qu, F.; Sun, L.; Liang, H.; Han, Z.; Chang, H.; Shao, S.; Li, G. Relationship between soluble microbial products (SMP) and effluent organic matter (EfOM): Characterized by fluorescence excitation emission matrix coupled with parallel factor analysis. Chemosphere 2015, 121, 101–109. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, W.J.; Yang, X.Y.; Xiao, P.; Wang, D.S.; Song, Y. Advanced treatment of effluent from municipal WWTP with different metal salt coagulants: Contaminants treatability and floc properties. Sep. Purif. Technol. 2013, 120, 123–128. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, T.; Cui, F.; Shi, W. Effect of reused alum-humic-flocs on coagulation performance and floc characteristics formed by aluminum salt coagulants in humic-acid water. Chem. Eng. J. 2016, 287, 225–232. [Google Scholar] [CrossRef]
- Jin, P.; Song, J.; Wang, X.C.; Jin, X. Two-dimensional correlation spectroscopic analysis on the interaction between humic acids and aluminum coagulant. J. Environ. Sci. 2018, 64, 181–189. [Google Scholar] [CrossRef]
- Song, F.; Wu, F.; Feng, W.; Tang, Z.; Giesy, J.P.; Guo, F.; Shi, D.; Liu, X.; Qin, N.; Xing, B.; et al. Fluorescence regional integration and differential fluorescence spectroscopy for analysis of structural characteristics and proton binding properties of fulvic acid sub-fractions. J. Environ. Sci. 2018, 74, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Cheng, Y.; Tian, K.; Xie, P.; Ren, X.H.; Li, Y.; Kou, Y.; Chon, K.; Hwang, M.H.; Ko, M.H. Insights into the minimization of excess sludge production in micro-aerobic reactors coupled with a membrane bioreactor: Characteristics of extracellular polymeric substances. Chemosphere 2022, 292, 133434. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Wang, H.; An, Y.; Guo, X.; He, L. Insight into the binding properties of carbamazepine onto dissolved organic matter using spectroscopic techniques during grassy swale treatment. Ecotoxicol. Environ. Saf. 2019, 173, 444–451. [Google Scholar] [CrossRef]
- Rahman, A.; De Clippeleir, H.; Thomas, W.; Jimenez, J.A.; Wett, B.; Al-Omari, A.; Murthy, S.; Riffat, R.; Bott, C. A-stage and high-rate contact-stabilization performance comparison for carbon and nutrient redirection from high-strength municipal wastewater. Chem. Eng. J. 2019, 357, 737–749. [Google Scholar] [CrossRef]
- HJ828; Water Quality-Determination of the Chemical Oxygen Demand-Dichromate Method. Ministry of Ecology and Environmental of the People’s Republic of China: Beijing, China, 2017.
- Hussain, S.; van Leeuwen, J.; Chow, C.; Beecham, S.; Kamruzzaman, M.; Wang, D.; Drikas, M.; Aryal, R. Removal of organic contaminants from river and reservoir waters by three different aluminum-based metal salts: Coagulation adsorption and kinetics studies. Chem. Eng. J. 2013, 225, 394–405. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, Z.; He, J.; Li, Q. Novel strategy for controlling colloidal instability during the flocculation pretreatment of landfill leachate. Chemosphere 2022, 287, 132051. [Google Scholar] [CrossRef]
- Van Gijn, K.; Chen, Y.L.; van Oudheusden, B.; Gong, S.; de Wilt, H.A.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Optimizing biological effluent organic matter removal for subsequent micropollutant removal. J. Environ. Chem. Eng. 2021, 9, 106247. [Google Scholar] [CrossRef]
- Lal, K.; Garg, A. Effectiveness of synthesized aluminum and iron based inorganic polymer coagulants for pulping wastewater treatment. J. Environ. Chem. Eng. 2019, 7, 103204. [Google Scholar] [CrossRef]
- Prokopova, M.; Novotna, K.; Pivokonska, L.; Cermakova, L.; Cajthaml, T.; Pivokonsky, M. Coagulation of polyvinyl chloride microplastics by ferric and aluminium sulphate: Optimisation of reaction conditions and removal mechanisms. J. Environ. Chem. Eng. 2021, 9, 106465. [Google Scholar] [CrossRef]
- Lapointe, M.; Papineau, I.; Peldszus, S.; Peleato, N.; Barbeau, B. Identifying the best coagulant for simultaneous water treatment objectives: Interactions of mononuclear and polynuclear aluminum species with different natural organic matter fractions. J. Water Process Eng. 2021, 40, 101829. [Google Scholar] [CrossRef]
- Wang, P.; Ding, S.; An, G.; Qu, R.; Liu, X.; Fang, C.; Chu, W. Removal of disinfection by-product precursors by Al-based coagulants: A comparative study on coagulation performance. J. Hazard. Mater. 2021, 420, 126558. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zheng, X.; Li, X.; Liu, T.; Wang, Y.; Ma, Y.; Ji, Y.; Qiu, X.; Wan, Y.; Pan, B. Variation of effluent organic matter (EfOM) during anaerobic/anoxic/oxic (A2O) wastewater treatment processes. Water Res. 2020, 178, 115830. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Song, Y.; Xi, B.; Xia, X.; He, X.; Tu, X. Application of chemometrics to spectroscopic data for indicating humification degree and assessing salinization processes of soils. J. Soils Sediments 2012, 12, 341–353. [Google Scholar] [CrossRef]
- Ding, A.; Lin, W.; Chen, R.; Ngo, H.H.; Zhang, R.; He, X.; Nan, J.; Li, G.; Ma, J. Improvement of sludge dewaterability by energy uncoupling combined with chemical re-flocculation: Reconstruction of floc, distribution of extracellular polymeric substances, and structure change of proteins. Sci. Total Environ. 2022, 816, 151646. [Google Scholar] [CrossRef]
- Guo, X.-J.; Li, Y.-Z.; Feng, Y.-H.; Yuan, D.-H. Using fluorescence quenching combined with two-dimensional correlation fluorescence spectroscopy to characterise the binding-site heterogeneity of dissolved organic matter with copper and mercury in lake sediments. Environ. Chem. 2017, 14, 91–98. [Google Scholar] [CrossRef]
- Noda, I.; Ozaki, Y. Two-Dimensional Correlation Spectroscopy: Applications in Vibrational and Optical Spectroscopy, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Luo, Y.L.; Yang, Z.H.; Xu, Z.Y.; Zhou, L.J.; Zeng, G.M.; Huang, J.; Xiao, Y.; Wang, L.K. Effect of trace amounts of polyacrylamide (PAM) on long-term performance of activated sludge. J. Hazard. Mater. 2011, 189, 69–75. [Google Scholar] [CrossRef]
- Meese, A.F.; Kim, D.J.; Wu, X.H.; Le, L.; Napier, C.; Hernandez, M.T. Opportunities and Challenges for Industrial Water Treatment and Reuse. ACS EST Eng. 2022, 8, 465–488. [Google Scholar] [CrossRef]
- Hao, X.-D.; Gan, W.; Li, J.; Cao, D.-Q. Effectiveness of ozonation on oxidizing secondary effluent organic matter (EfOM) from WWTP. China Water Wastewater 2021, 37, 1–7. (In Chinese) [Google Scholar] [CrossRef]
- Lu, H.Y.; Qian, C.Y.; Luo, S.G.; Zhu, Y.G.; Liu, R.Q.; Wu, M.R. Study on the influence and mechanism of polyferric sulfate on COD removal and reuse of scheelite flotation wastewater. Min. Eng. 2023, 191, 107940. [Google Scholar] [CrossRef]
| Parameter | Influent | Effluent | Removal Efficiency (%) |
|---|---|---|---|
| TCOD (mg L−1) | 26.36 ± 7.20 | 14.41 ± 3.57 | 44.61 ± 6.53 |
| UVA254 (cm−1) | 0.263 ± 0.002 | 0.197 ± 0.005 | 25.13 ± 1.64 |
| SUVA (L mg−1 m−1) | 2.25 ± 0.03 | 2.07 ± 0.03 | 9.13 ± 1.35 |
| CN (cm−1) | 0.020 ± 0.001 | 0.012 ± 0.002 | 36.65 ± 9.49 |
| SS (mg L−1) | 4.56 ± 1.50 | 0.66 ± 0.58 | 85.01 ± 12.00 |
| Turbidity (NTU) | 1.19 ± 0.28 | 0.63 ± 0.45 | 50.35 ± 34.43 |
| pH | 7.92 ± 0.28 | 7.00 ± 0.05 | 11.52 ± 1.25 |
| Component | Log Km | f | R2 |
|---|---|---|---|
| FC1 | 4.12 | 0.12 | 0.9197 |
| FC2 | 4.10 | 0.22 | 0.7289 |
| Wavelength cm−1 | Secondary Structures | Influent | Effluent |
|---|---|---|---|
| Proportion of Area (%) | Proportion of Area (%) | ||
| 1616–1626 | Aggregated strands | 20.86 | 18.04 |
| 1629–1640 | β-sheet | 10.43 | 8.95 |
| 1640–1645 | Random coil | 10.43 | 15.77 |
| 1649–1650 | α-Helix | 10.39 | 12.24 |
| 1655–1669 | 3-Turn helix | 20.78 | 17.20 |
| 1676–1694 | Antiparallel β-sheet/aggregated strands α-Helix/(β-sheet +random coil) | 14.21 | 12.80 |
| Parameter | Value |
|---|---|
| Q | 150 m3 d−1 |
| No. of working days per year | 365 d |
| tw | 7 h d−1 |
| Construction cost (designing & treatment units manufacturing & mechanical cost) | CNY 4,000,000 |
| Electrical costs | CNY 0.98 t−1 |
| Chemical costs Al2(SO4)3·18 H2O | CNY 0.45 t−1 |
| APAM | CNY 0.08 t−1 |
| Maintenance cost | CNY 5000 a−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Cheng, Q.; Zhao, C.; Ren, X.; Wang, Y.; Kou, Y.; Chon, K.; Ko, M.-H.; Hwang, M.-H. Evaluation of Efficiently Removing Secondary Effluent Organic Matters (EfOM) by Al-Based Coagulant for Wastewater Recycling: A Case Study with an Industrial-Scale Food-Processing Wastewater Treatment Plant. Membranes 2023, 13, 510. https://doi.org/10.3390/membranes13050510
Cheng Y, Cheng Q, Zhao C, Ren X, Wang Y, Kou Y, Chon K, Ko M-H, Hwang M-H. Evaluation of Efficiently Removing Secondary Effluent Organic Matters (EfOM) by Al-Based Coagulant for Wastewater Recycling: A Case Study with an Industrial-Scale Food-Processing Wastewater Treatment Plant. Membranes. 2023; 13(5):510. https://doi.org/10.3390/membranes13050510
Chicago/Turabian StyleCheng, Yu, Qiangqiang Cheng, Chengjin Zhao, Xianghao Ren, Yu Wang, Yingying Kou, Kangmin Chon, Myung-Han Ko, and Moon-Hyun Hwang. 2023. "Evaluation of Efficiently Removing Secondary Effluent Organic Matters (EfOM) by Al-Based Coagulant for Wastewater Recycling: A Case Study with an Industrial-Scale Food-Processing Wastewater Treatment Plant" Membranes 13, no. 5: 510. https://doi.org/10.3390/membranes13050510
APA StyleCheng, Y., Cheng, Q., Zhao, C., Ren, X., Wang, Y., Kou, Y., Chon, K., Ko, M.-H., & Hwang, M.-H. (2023). Evaluation of Efficiently Removing Secondary Effluent Organic Matters (EfOM) by Al-Based Coagulant for Wastewater Recycling: A Case Study with an Industrial-Scale Food-Processing Wastewater Treatment Plant. Membranes, 13(5), 510. https://doi.org/10.3390/membranes13050510








