Application of Hybrid Electrobaromembrane Process for Selective Recovery of Lithium from Cobalt- and Nickel-Containing Leaching Solutions
Abstract
1. Introduction
2. Materials and Methods
3. Results
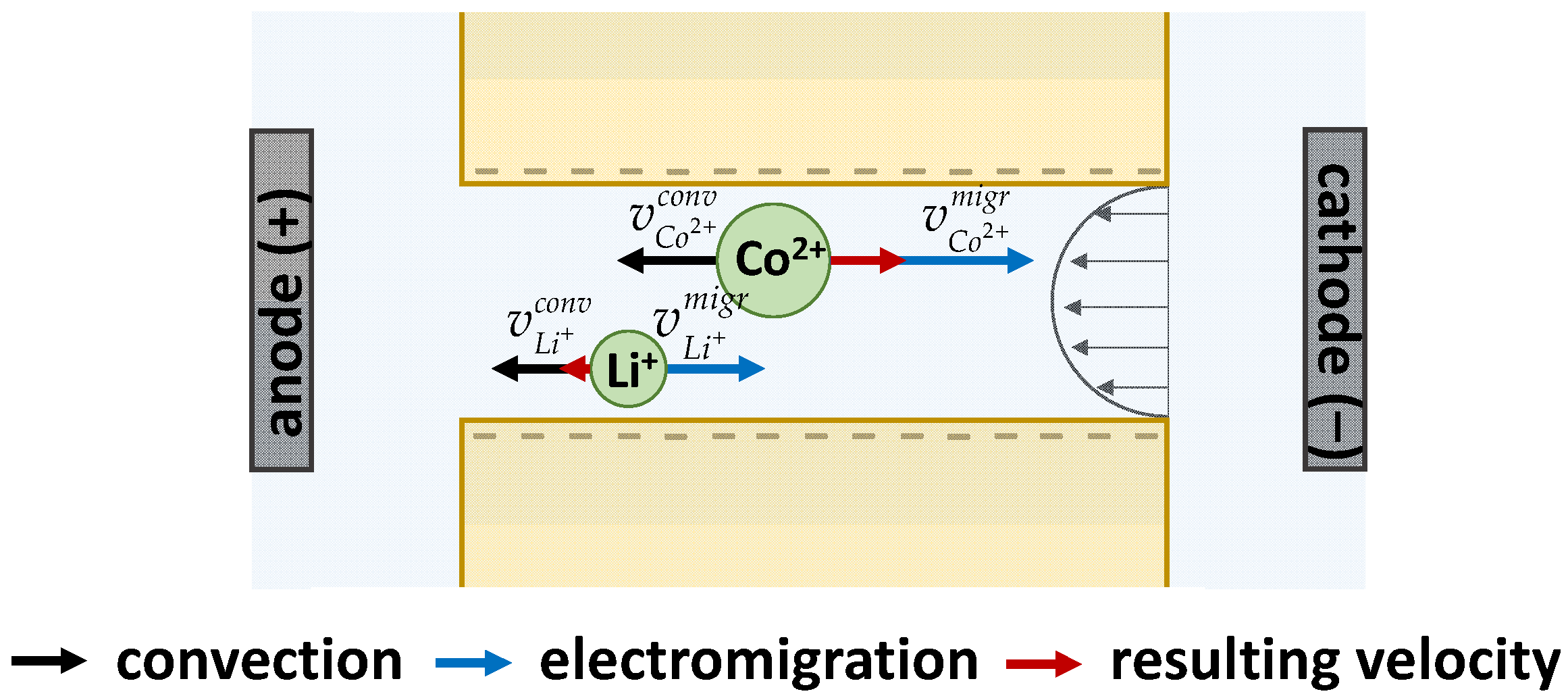
3.1. Theory of EBM Separation
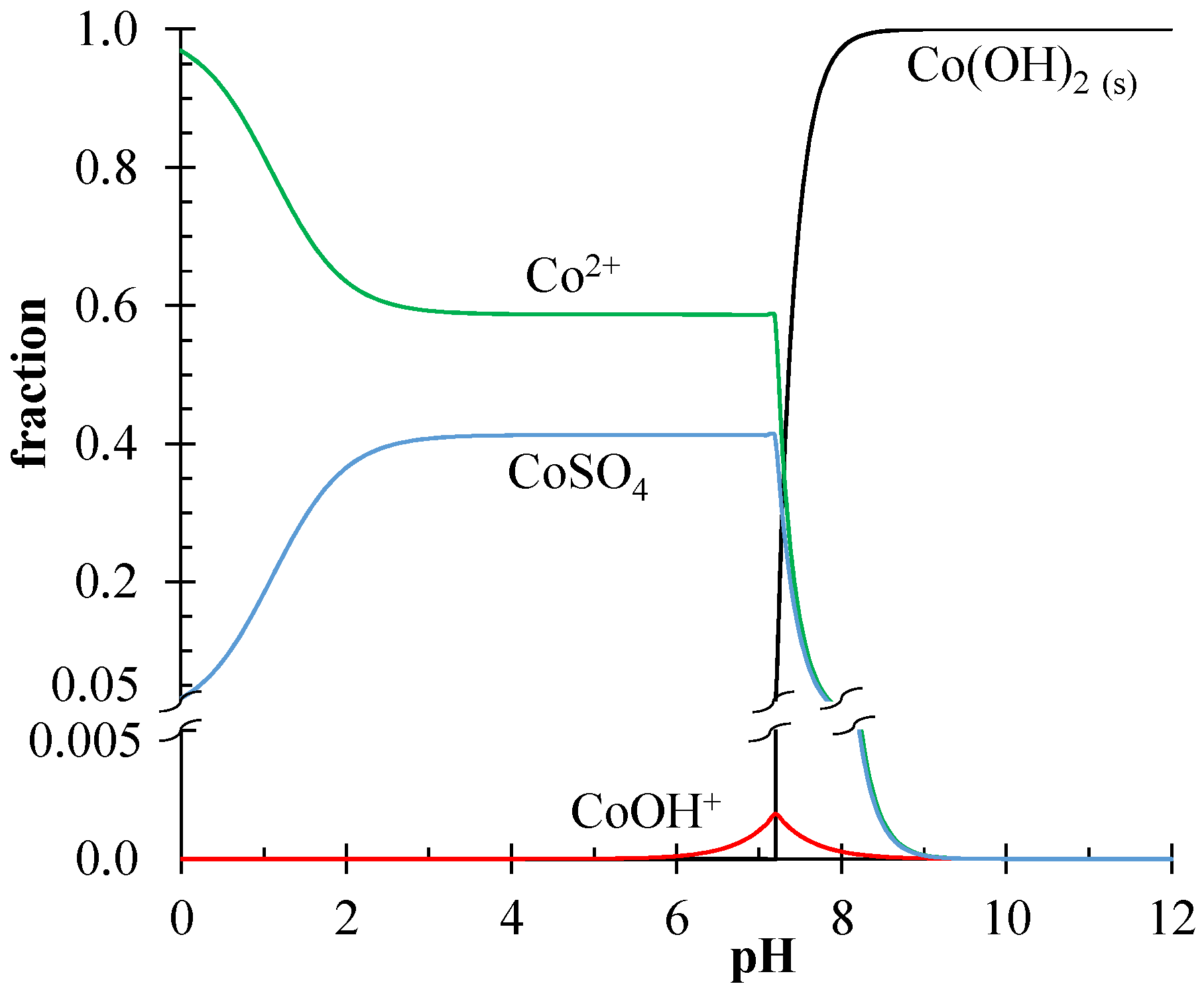
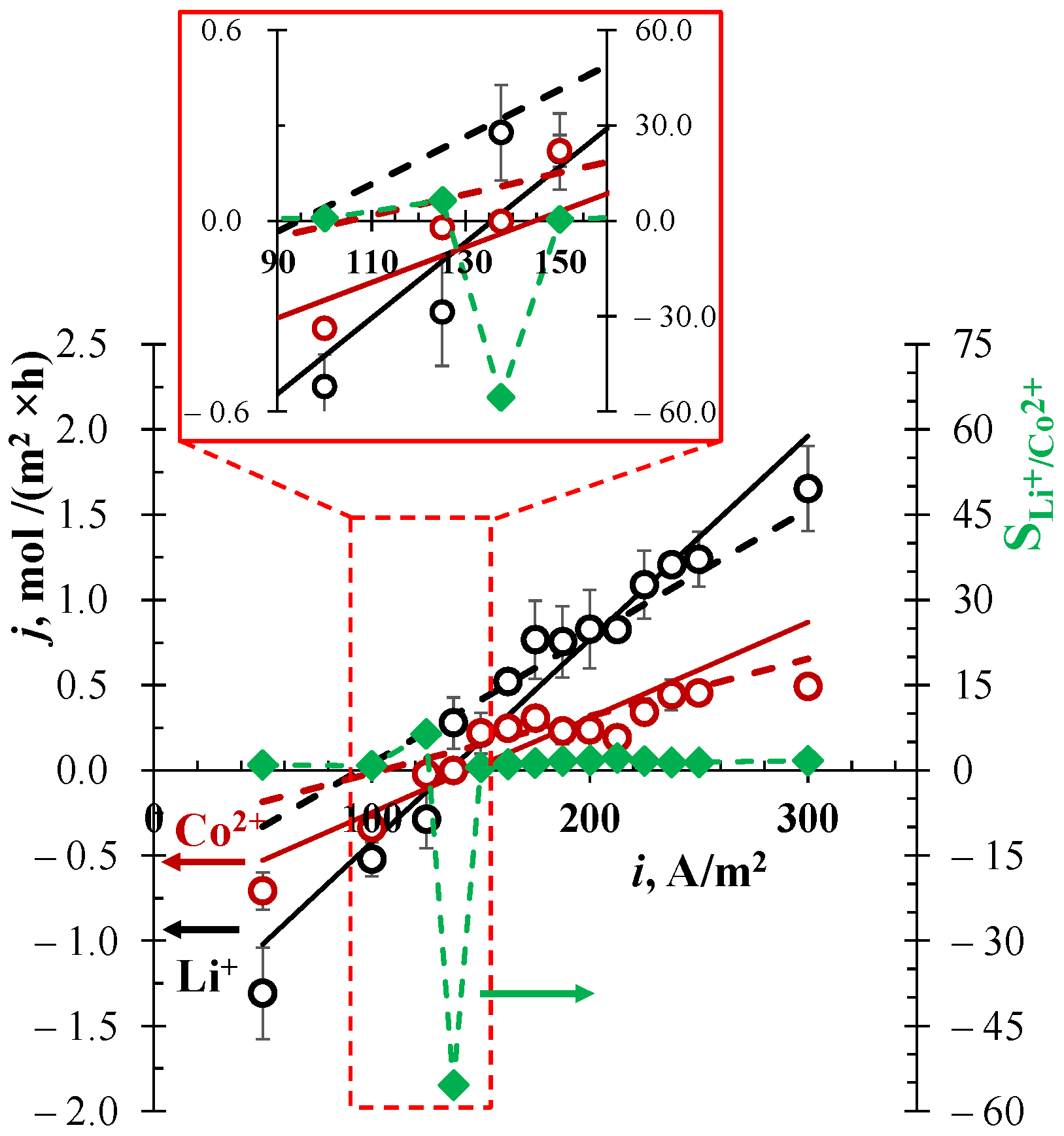
3.2. Separation of Li+/Co2+-Ions
3.3. Separation of Li+/Co2+/Ni2+-Ions
3.4. Analysis of Obtained Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butylskii, D.Y.; Dammak, L.; Larchet, C.; Pismenskaya, N.D.; Nikonenko, V.V. Selective recovery and re-utilization of lithium: Prospects for the use of membrane methods. Russ. Chem. Rev. 2023, 92, RCR5074. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Q.; Cao, J.; Li, H.; Bian, Z. Precious metal recovery. Joule 2021, 5, 3097–3115. [Google Scholar] [CrossRef]
- Coman, V.; Robotin, B.; Ilea, P. Nickel recovery/removal from industrial wastes: A review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M. Reproduction of Li battery LiNixMnyCo1−x−yO2 positive electrode material from the recycling of waste battery. Int. J. Hydrogen Energy 2017, 42, 18189–18195. [Google Scholar] [CrossRef]
- Chan, K.H.; Anawati, J.; Malik, M.; Azimi, G. Closed-Loop Recycling of Lithium, Cobalt, Nickel, and Manganese from Waste Lithium-Ion Batteries of Electric Vehicles. ACS Sustain. Chem. Eng. 2021, 9, 4398–4410. [Google Scholar] [CrossRef]
- Xu, P.; Dai, Q.; Gao, H.; Liu, H.; Zhang, M.; Li, M.; Chen, Y.; An, K.; Meng, Y.S.; Liu, P.; et al. Efficient Direct Recycling of Lithium-Ion Battery Cathodes by Targeted Healing. Joule 2020, 4, 2609–2626. [Google Scholar] [CrossRef]
- Yu, M.; Bai, B.; Xiong, S.; Liao, X. Evaluating environmental impacts and economic performance of remanufacturing electric vehicle lithium-ion batteries. J. Clean. Prod. 2021, 321, 128935. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Zhao, Y.; Rüther, T.; Staines, J. Australian Landscape for Lithium-Ion Battery Recycling and Reuse in 2020; Future Battery Industries CRC: Bentley, WA, Australia, 2021. [Google Scholar]
- Bhandari, N.; Cai, A.; Yuzawa, K.; Zhang, J.; Joshi, V.; Fang, F.; Lee, G.; Harada, R.; Shin, S. Global Batteries the Greenflation Challenge; Goldman Sachs &, Co.: New York, NY, USA, 2022. [Google Scholar]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Siekierka, A.; Yalcinkaya, F. Selective cobalt-exchange membranes for electrodialysis dedicated for cobalt recovery from lithium, cobalt and nickel solutions. Sep. Purif. Technol. 2022, 299, 121695. [Google Scholar] [CrossRef]
- Chan, K.H.; Malik, M.; Azimi, G. Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis. Resour. Conserv. Recycl. 2022, 178, 106076. [Google Scholar] [CrossRef]
- Afifah, D.N.; Ariyanto, T.; Supranto, S.; Prasetyo, I. Separation of Lithium Ion from Lithium-Cobalt Mixture using Electrodialysis Monovalent Membrane. Eng. J. 2018, 22, 165–179. [Google Scholar] [CrossRef]
- Gmar, S.; Chagnes, A.; Lutin, F.; Muhr, L. Application of Electrodialysis for the Selective Lithium Extraction Towards Cobalt, Nickel and Manganese from Leach Solutions Containing High Divalent Cations/Li Ratio. Recycling 2022, 7, 14. [Google Scholar] [CrossRef]
- Brewer, A.K.; Madorsky, S.L.; Westhaver, J.W. The Concentration of 39K and 41K by Balanced. Ion Migration in a Counterflowing Electrolyte. Science 1946, 104, 156–157. [Google Scholar] [CrossRef]
- Forssell, P.; Kontturi, K. Experimental Verification of Separation of Ions Using Countercurrent Electrolysis in a Thin, Porous Membrane. Sep. Sci. Technol. 1983, 18, 205–214. [Google Scholar] [CrossRef]
- Kontturi, K.; Forssell, P.; Ekman, A. Separation of Ions Using Countercurrent Electrolysis in a Thin, Porous Membrane. Sep. Sci. Technol. 1982, 17, 1195–1204. [Google Scholar] [CrossRef]
- Butylskii, D.; Troitskiy, V.; Chuprynina, D.; Kharchenko, I.; Ryzhkov, I.; Apel, P.; Pismenskaya, N.; Nikonenko, V. Selective Separation of Singly Charged Chloride and Dihydrogen Phosphate Anions by Electrobaromembrane Method with Nanoporous Membranes. Membranes 2023, 13, 455. [Google Scholar] [CrossRef]
- Butylskii, D.Y.; Pismenskaya, N.D.; Apel, P.Y.; Sabbatovskiy, K.G.; Nikonenko, V.V. Highly selective separation of singly charged cations by countercurrent electromigration with a track-etched membrane. J. Memb. Sci. 2021, 635, 119449. [Google Scholar] [CrossRef]
- Tang, C.; Bondarenko, M.P.; Yaroshchuk, A.; Bruening, M.L. Highly selective ion separations based on counter-flow electromigration in nanoporous membranes. J. Memb. Sci. 2021, 638, 119684. [Google Scholar] [CrossRef]
- Tang, C.; Yaroshchuk, A.; Bruening, M.L. Ion Separations Based on Spontaneously Arising Streaming Potentials in Rotating Isoporous Membranes. Membranes 2022, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Kislyi, A.G.; Butylskii, D.Y.; Mareev, S.A.; Nikonenko, V. V Model of Competitive Ion Transfer in an Electro-Baromembrane System with Track-Etched Membrane. Membr. Membr. Technol. 2021, 3, 131–137. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005; ISBN 0-8493-0485-7. [Google Scholar]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Apel, P.Y. Track-Etching. In Encyclopedia of Membrane Science and Technology; Hoek, E.M.V., Tarabara, V.V., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 332–355. [Google Scholar]
- Sarapulova, V.V.; Pasechnaya, E.L.; Titorova, V.D.; Pismenskaya, N.D.; Apel, P.Y.; Nikonenko, V.V. Electrochemical Properties of Ultrafiltration and Nanofiltration Membranes in Solutions of Sodium and Calcium Chloride. Membr. Membr. Technol. 2020, 2, 332–350. [Google Scholar] [CrossRef]
- Puigdomenech, I. HYDRA (Hydrochemical Equilibrium-Constant Database) and Medusa (Make Equilibrium Diagrams Using Sophisticated Algorithms). Available online: https://www.kth.se/che/medusa/ (accessed on 11 April 2023).
- Sata, T.; Sata, T.; Yang, W. Studies on cation-exchange membranes having permselectivity between cations in electrodialysis. J. Memb. Sci. 2002, 206, 31–60. [Google Scholar] [CrossRef]
- Wang, W.; Liu, R.; Tan, M.; Sun, H.; Niu, Q.J.; Xu, T.; Nikonenko, V.; Zhang, Y. Evaluation of the ideal selectivity and the performance of selectrodialysis by using TFC ion exchange membranes. J. Memb. Sci. 2019, 582, 236–245. [Google Scholar] [CrossRef]
- Karpenko, T.V.; Kovalev, N.V.; Kirillova, K.R.; Achoh, A.R.; Melnikov, S.S.; Sheldeshov, N.V.; Zabolotsky, V.I. Competing Transport of Malonic and Acetic acids across Commercial and Modified RALEX AMH Anion-Exchange Membranes. Membr. Membr. Technol. 2022, 4, 118–126. [Google Scholar] [CrossRef]
- Kontturi, K.; Ojala, T.; Forssell, P. Transport of acetate and chloroacetate weak electrolytes through a thin porous membrane in counter-current electrolysis. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1984, 80, 3379. [Google Scholar] [CrossRef]
- Nichka, V.S.; Mareev, S.A.; Apel, P.Y.; Sabbatovskiy, K.G.; Sobolev, V.D.; Nikonenko, V.V. Modeling the Conductivity and Diffusion Permeability of a Track-Etched Membrane Taking into Account a Loose Layer. Membranes 2022, 12, 1283. [Google Scholar] [CrossRef]
- Apel, P.; Koter, S.; Yaroshchuk, A. Time-resolved pressure-induced electric potential in nanoporous membranes: Measurement and mechanistic interpretation. J. Membr. Sci. 2022, 653, 120556. [Google Scholar] [CrossRef]
- Apel, P. Track etching technique in membrane technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Tang, C.; Yaroshchuk, A.; Bruening, M.L. Flow through negatively charged, nanoporous membranes separates Li+ and K+ due to induced electromigration. Chem. Commun. 2020, 56, 10954–10957. [Google Scholar] [CrossRef]
- Ji, P.-Y.; Ji, Z.-Y.; Chen, Q.-B.; Liu, J.; Zhao, Y.-Y.; Wang, S.-Z.; Li, F.; Yuan, J.-S. Effect of coexisting ions on recovering lithium from high Mg2+/Li+ ratio brines by selective-electrodialysis. Sep. Purif. Technol. 2018, 207, 1–11. [Google Scholar] [CrossRef]
- Iizuka, A.; Yamashita, Y.; Nagasawa, H.; Yamasaki, A.; Yanagisawa, Y. Separation of lithium and cobalt from waste lithium-ion batteries via bipolar membrane electrodialysis coupled with chelation. Sep. Purif. Technol. 2013, 113, 33–41. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, C.; Ha, G.-S.; Park, Y.-K.; Ali Khan, M.; Jang, M.; Kim, S.-H.; Amin, M.A.; Gacem, A.; Jeon, B.-H. Downstream recovery of Li and value-added metals (Ni, Co, and Mn) from leach liquor of spent lithium-ion batteries using a membrane-integrated hybrid system. Chem. Eng. J. 2022, 447, 137507. [Google Scholar] [CrossRef]

| Ion | Symbol | Diffusion Coefficient, 10−9 m2/s [27] | Stokes Radius, Å [28] |
|---|---|---|---|
| Lithium | Li+ | 1.04 | 2.38 |
| Cobalt | Co2+ | 0.73 | 3.35 |
| Nickel | Ni2+ | 0.66 | 2.92 |
| Sulfate | SO42− | 1.06 | 2.30 |
| Parameter | Value |
|---|---|
| Thickness | 10 μm |
| Density (dry) * | 1.10 ± 0.05 g/cm3 |
| Pore density ** | 5.0 × 109 pores/cm2 |
| Pore diameter | 35 ± 3.0 nm ** |
| 28 ± 2.0 nm *** | |
| Surface porosity | 5.3% ± 1.0% |
| Water uptake | 5% |
| Hydraulic permeability | 0.10 ± 0.02 cm3/(cm2 × min × bar) |
| Functional groups | hydroxyl and carboxyl groups [29] |
| Exchange capacity * | 0.064 ± 0.003 mmol/gwet |
| Electrical conductivity (0.1 M NaCl) * | 0.81 mS/cm |
| Integral diffusion permeability coefficient (0.1 M NaCl) * | 2.8 × 10−7 cm2/s |
| Current Density, i, A/m2 | Ions in the Feed Solution | |||
|---|---|---|---|---|
| Li+/Co2+-containing feed solution | ||||
| 137.5 | Li+ | 0.69 | 0.28 | – |
| Co2+ | 2.95 | –0.0025 | n/a | |
| 125 | Li+ | 0.69 | –0.29 | – |
| Co2+ | 2.95 | –0.022 | 8 | |
| Li+/Co2+/Ni2+-containing feed solution | ||||
| 137.5 | Li+ | 0.69 | 0.33 | – |
| Co2+ | 1.47 | 0.02 | 4 | |
| Ni2+ | 1.47 | 0.04 | 2 | |
| 125 | Li+ | 0.69 | 0.30 | – |
| Co2+ | 1.47 | –0.02 | n/a | |
| Ni2+ | 1.47 | –0.005 | n/a | |
| Method | Membrane | Feed Solution | Experiment Details | Competing Cation, C+ | |||
|---|---|---|---|---|---|---|---|
| Selective electrodialysis Ref. [18] | Cell with monovalent selective Selemion CSO (Asahi Glass, Tokyo, Japan) or Neosepta CIMS membranes, as well as Neosepta AMX (Astom, Shunan, Japan) | Leach solution of NMC111 cathodic materials: 2.60 g/L Li+, 7.88 g/L Co2+, 8.01 g/L Ni2+, 4.40 g/L Mn2+, 51.45 g/L SO42– (pH = 2.8) | 125 A/m2 | 1.92 over the Selemion CSO | Co2+ | 0.56 | 1.25 |
| Ni2+ | 0.54 | 1.3 | |||||
| Mn2+ | 0.29 | 1.4 | |||||
| 3.05 over the Neosepta CIMS | Co2+ | 0.20 | 5.6 | ||||
| Ni2+ | 0.18 | 6.1 | |||||
| Mn2+ | 0.12 | 5.4 | |||||
| Selective electrodialysis Ref. [17] | 5 cell pair with monovalent selective PC-MVK & PC-MVA membranes (PCA GmbH, Heusweiler, Germany) | 0.1 g/L Li+& 0.3 g/L Co2+ | 5 V (1 V/cell) ~15 A/m2 | 0.1 | Co2+ | 8.8 × 10−3 | 4 |
| Selective electrodialysis Ref. [15] | Cell with laboratory-made selective PAN-5C8Q membrane as well as Neosepta ASE (Astom, Shunan, Japan) | 0.027 g/L Li+, 0.108 g/L Co2+, 0.049 g/L Ni2+ | 5 V | 0.047 | Co2+ | 0.044 | 0.5 |
| Ni2+ | 1.5 × 10−3 | 7 | |||||
| Conventional electrodialysis + complexation & selective electrodialysis Ref. [16] | Cell with monovalent selective Neosepta CMS membrane, as well as Neosepta AMX, Neosepta CMX (Astom, Japan) and PCA PC 400D (PCA GmbH, Heusweiler, Germany) | Leach solution of NMC111 cathodic materials: 0.07 g/L Li+, 0.2 g/L Co2+, 0.2 g/L Ni2+, 0.18 g/L Mn2+ & SO42– (pH ~ 1.5) | 18 V (Stage 1) | 0.165 over the Neosepta CMS (Stage 3) | Ni-EDTAn– over the PCA PC 400D (Stage 1) | 0.057 | n/a |
| 18 V (Stage 2) | Co-EDTAn– over the PCA PC 400D (Stage 2) | 0.042 | n/a | ||||
| 3 V (Stage 3) | Mn2+ over the Neosepta CMS (Stage 3) | 5.7 × 10−4 | 92 | ||||
| Bipolar membrane electrodialysis + complexation Ref. [41] | Cell with Neosepta BP-1E bipolar membrane (Astom, Japan), as well as Selemion CMV and Selemion AMV (Asahi Glass, Tokyo, Japan) | 0.14 g/L Li+, 1.18 g/L Co2+, 3.72 g/L NO3–, 1.84 g/L Na+ (pH = 7.0) | 20 V | 0.77 over the Selemion CMV | Co-EDTAn– over the Selemion AMV | 0.33 | n/a |
| Nanofiltration Ref. [42] | Ccell with VNF2 nanofiltration membrane (Vontron Membrane Technology Ltd., Beijing, China) | Leach liquor of lithium-iron-phosphate spent LiBs: 23.9 g/L Li+, 0.78 g/L Ni2+, 0.58 g/L Co2+, 0.67 g/L Mn2+, 27.3 g/L Fe3+, 0.18 g/L Al3+, 0.28 g/L Cr3+, 0.059 g/L Cu2+, 11.0 g/L PO43– (pH = 2.2) | 10 bar | 0.67 | Co2+ | 3.0 × 10−4 | 6.2 |
| Ni2+ | 5.7 × 10−4 | 4.7 | |||||
| Mn2+ | 3.0 × 10−4 | 8.2 | |||||
| Hybrid electro-baromembrane (EBM) method [this study] | Cell with TEM #811 track-etched membrane, as well as two MA-41 (JCC Shchekinoazot, Pervomayskiy, Russia) | 0.69 g/L Li+, 2.95 g/L Co2+, 9.6 g/L SO42– (pH = 4.2–4.4) or 0.69 g/L Li+, 1.47 g/L Co2+, 1.47 g/L Ni2+, 9.6 g/L SO42– (pH = 5.2) | 137.5 A/m2 0.3 bar | 0.36 * | Co2+ | 0.01 * | 18 * |
| 0.33 | Co2+ | 0.02 | 4 | ||||
| Ni2+ | 0.04 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butylskii, D.; Troitskiy, V.; Chuprynina, D.; Dammak, L.; Larchet, C.; Nikonenko, V. Application of Hybrid Electrobaromembrane Process for Selective Recovery of Lithium from Cobalt- and Nickel-Containing Leaching Solutions. Membranes 2023, 13, 509. https://doi.org/10.3390/membranes13050509
Butylskii D, Troitskiy V, Chuprynina D, Dammak L, Larchet C, Nikonenko V. Application of Hybrid Electrobaromembrane Process for Selective Recovery of Lithium from Cobalt- and Nickel-Containing Leaching Solutions. Membranes. 2023; 13(5):509. https://doi.org/10.3390/membranes13050509
Chicago/Turabian StyleButylskii, Dmitrii, Vasiliy Troitskiy, Daria Chuprynina, Lasâad Dammak, Christian Larchet, and Victor Nikonenko. 2023. "Application of Hybrid Electrobaromembrane Process for Selective Recovery of Lithium from Cobalt- and Nickel-Containing Leaching Solutions" Membranes 13, no. 5: 509. https://doi.org/10.3390/membranes13050509
APA StyleButylskii, D., Troitskiy, V., Chuprynina, D., Dammak, L., Larchet, C., & Nikonenko, V. (2023). Application of Hybrid Electrobaromembrane Process for Selective Recovery of Lithium from Cobalt- and Nickel-Containing Leaching Solutions. Membranes, 13(5), 509. https://doi.org/10.3390/membranes13050509










