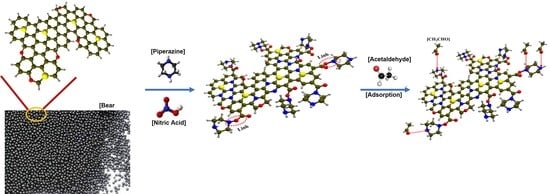
Effective Removal of Acetaldehyde Using Piperazine/Nitric Acid Co-Impregnated Bead-Type Activated Carbon
Abstract
1. Introduction
| References | Activated Carbon Type | Impregnated Material | Mechanism |
|---|---|---|---|
| [29] | AC (Calgon, Norit, and Westvaco) | nitric acid | (1) When very small pores as close as the size of the acetaldehyde molecule and oxygen-containing groups are present (to a certain extent) within AC, the heat of adsorption reaches its maximum value. (2) A low density of surface groups can enhance the heat of adsorption, whereas extensive oxidation leads to a decrease in the strength of adsorption forces. This happens due to the blockage of the pore entrances containing functional groups and the decrease in the accessibility of hydrophobic surface where the dispersive interactions of hydrocarbon moiety can be enhanced. |
| [30] | AC (Calgon and Westvaco) | urea (450/950 °C) | (1) The adsorption forces are strong in small pores, and their volume governs the adsorbed amount. (2) The absorbed amount can be enhanced when functional groups bearing nitrogen are present. (3) These groups can provide additional adsorption centers when the small pores are filled with acetaldehyde molecules. |
| [26] | AC (coconut-shell and coal-base) | amine | 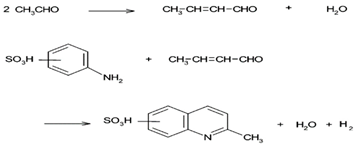 |
| [31] | AC (corn grain) | KOH | (1) The effects of acetaldehyde adsorption on ACs were investigated in terms of textural properties, energetic heterogeneity, and surface chemistries. (2) The adsorption properties of water vapor were explained by the effect of the oxygen-containing groups on the surface of ACs over acetaldehyde adsorption. (3) The influences of pore size distribution (below 8 A˚) and energetic heterogeneity of ACs on acetaldehyde adsorption were highly predominant compared to that of specific surface area and surface chemistry. |
| [32] | AC (coconut base) | - | The study established a semi-quantitative relationship between pore size distribution and energy in relation to adsorption kinetics; the wider and more heterogeneous porosities resulted in higher rate constants for the resin-based carbon when compared to the ultramicroporous nutshell material. |
| [33] | ACF | metal oxide | ACF-K-20/5%MgO revealed three types of surface adsorption sites: one was assigned to physisorption on the surface O-containing carbon groups and two other sites are placed on a MgO surface and provide acetaldehyde chemisorption in two different modes. |
| [34] | ACF (cellulose base) | aniline-ethanol | (1) CH3CHO(g) → CH3CHO(AD) [Adsorption] (2) CH3CHO(AD) + O2 → CH3COOH [Oxidation] (3) 2CH3COOH → (CH3CO)2O + H2O [Dehydration] (4) (CH3CO)2O + C6H5NH2 → C6H5NCH3CO − CH3COOH |
| [35] | ACF (HDPE fiber) | Ag | (1) Ag particles were precipitated on the surface of ACF through interactive affinity, and the carbonyl group of AA is in creased to show that AA is adsorbed on the AC surface. (2) The AA adsorption of ACF and Ag/ACF composites performed in this study was suitable for the dose–response model, and the experimental data showing the asymmetric shape of the AA adsorption breakthrough curve for ACF and Ag/ACF composites were satisfactorily fitted. |
| [36] | AC and ACF | amine | (1) The high BET surface area provides more sites for acetaldehyde adsorption. (2) ACF has a systematic open macrostructure, which drives a low-pressure drop and allows fast adsorption without diffu sion hindrance. |
2. Experimental Section
2.1. Materials and Sample Preparation
2.2. Methods
2.2.1. Preliminary Characterization of BACs
2.2.2. N2 Sorption
2.2.3. CHNS Elemental Analysis
2.2.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.2.5. X-ray Photoelectron Spectroscopy (XPS)
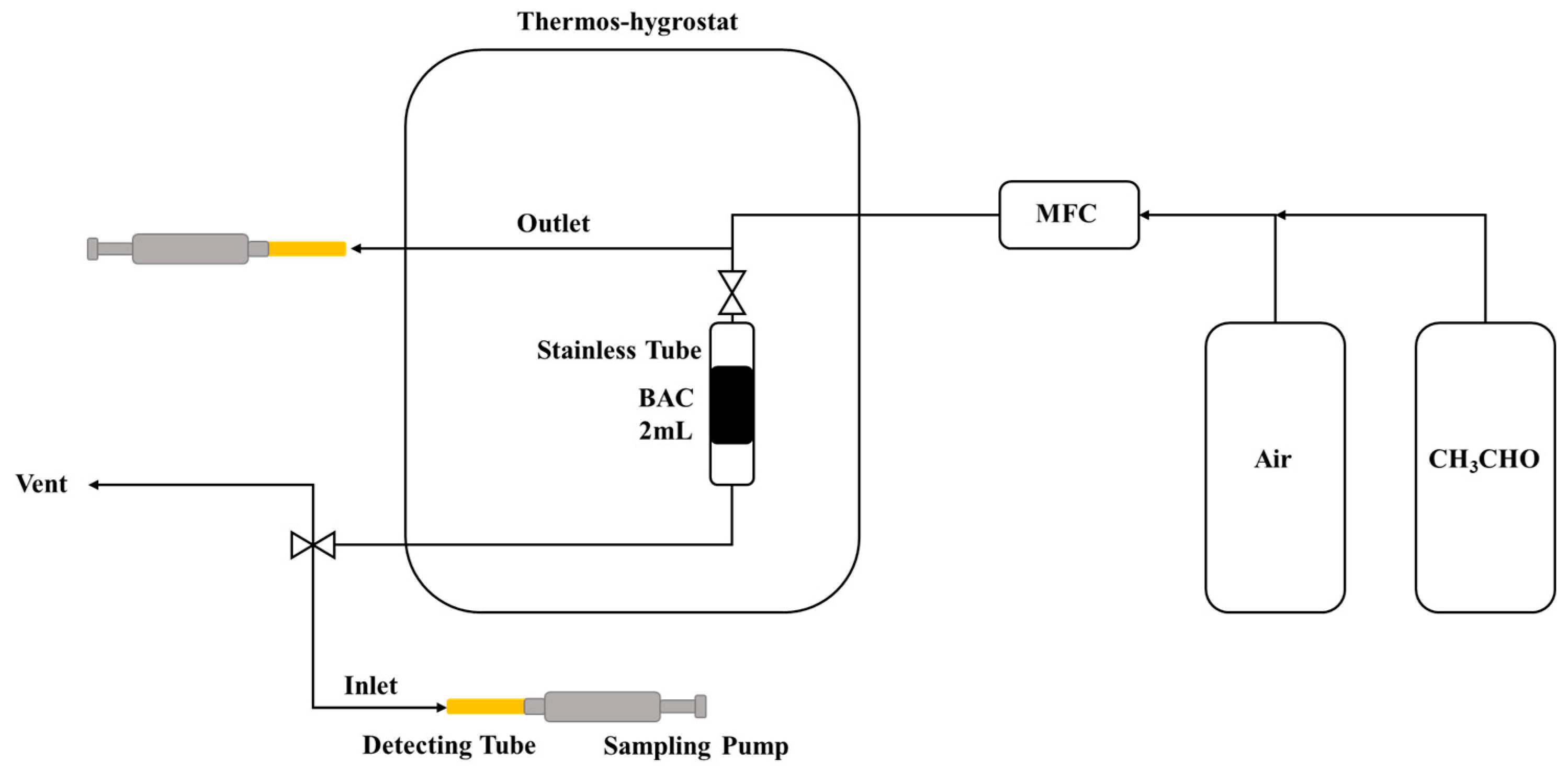
2.2.6. CH3CHO Adsorption
2.2.7. Thermal Regeneration
3. Results
3.1. Characterizations of BAC
3.1.1. Textural Structure
3.1.2. CHNS Elemental Analysis
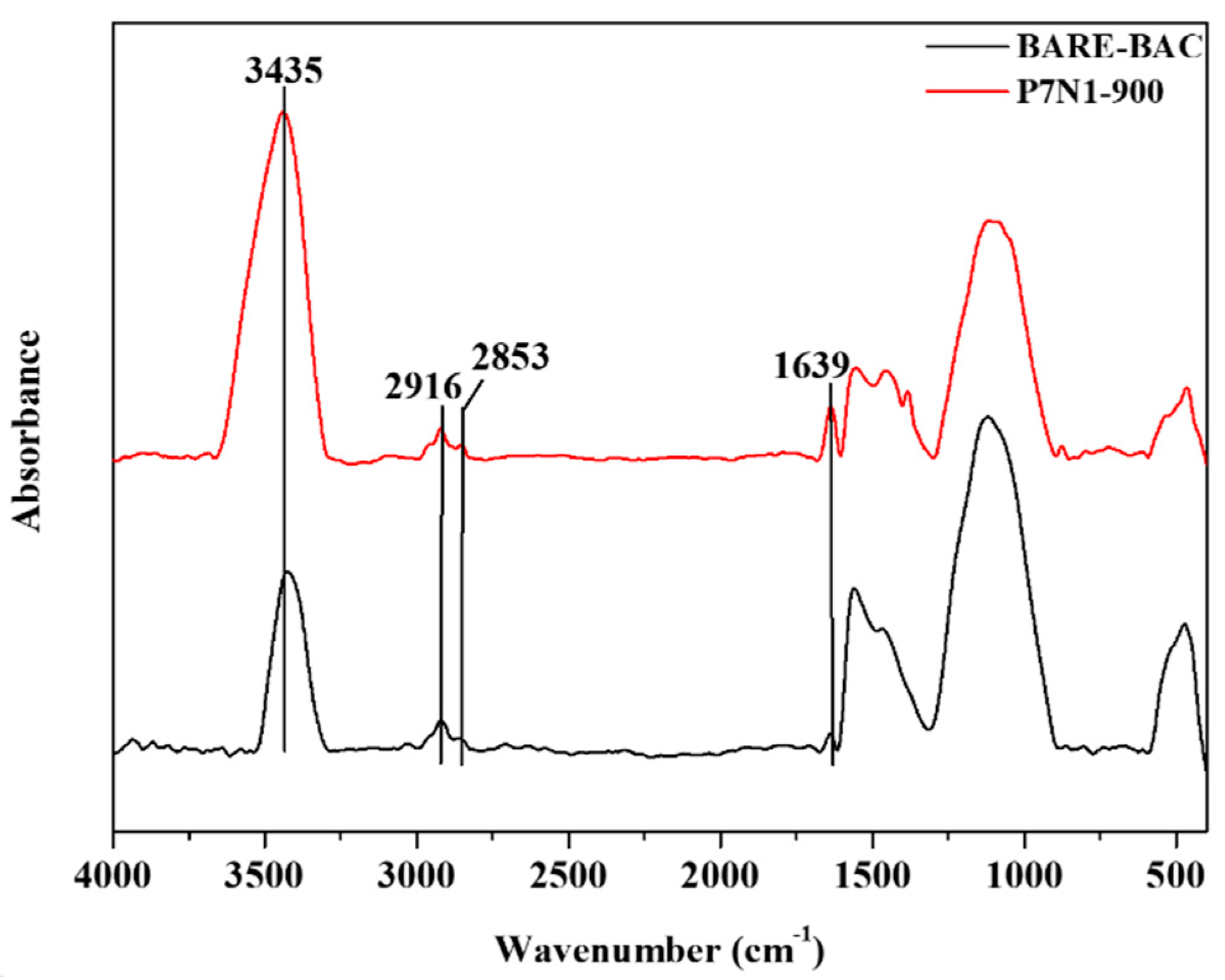
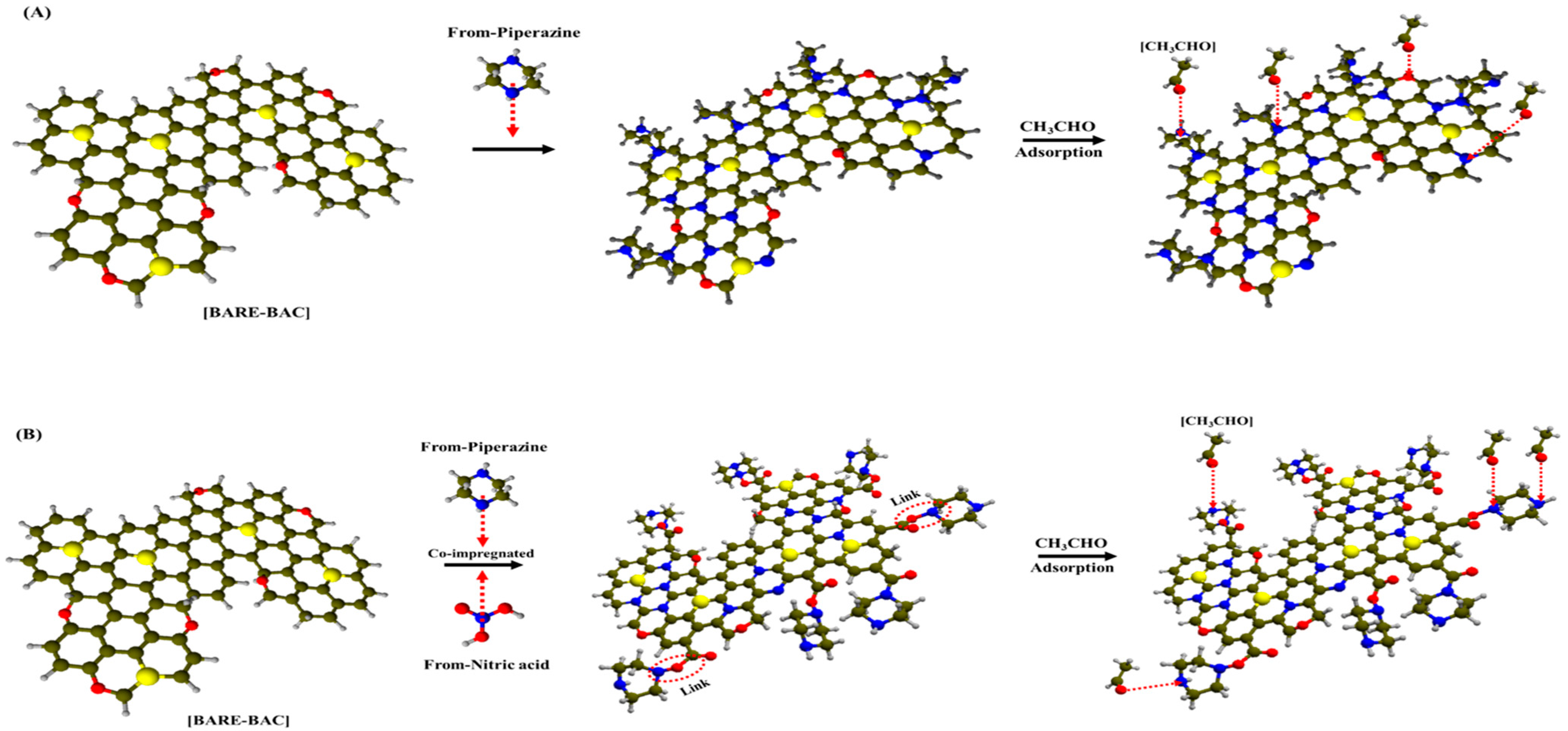
3.1.3. Chemical Characterization
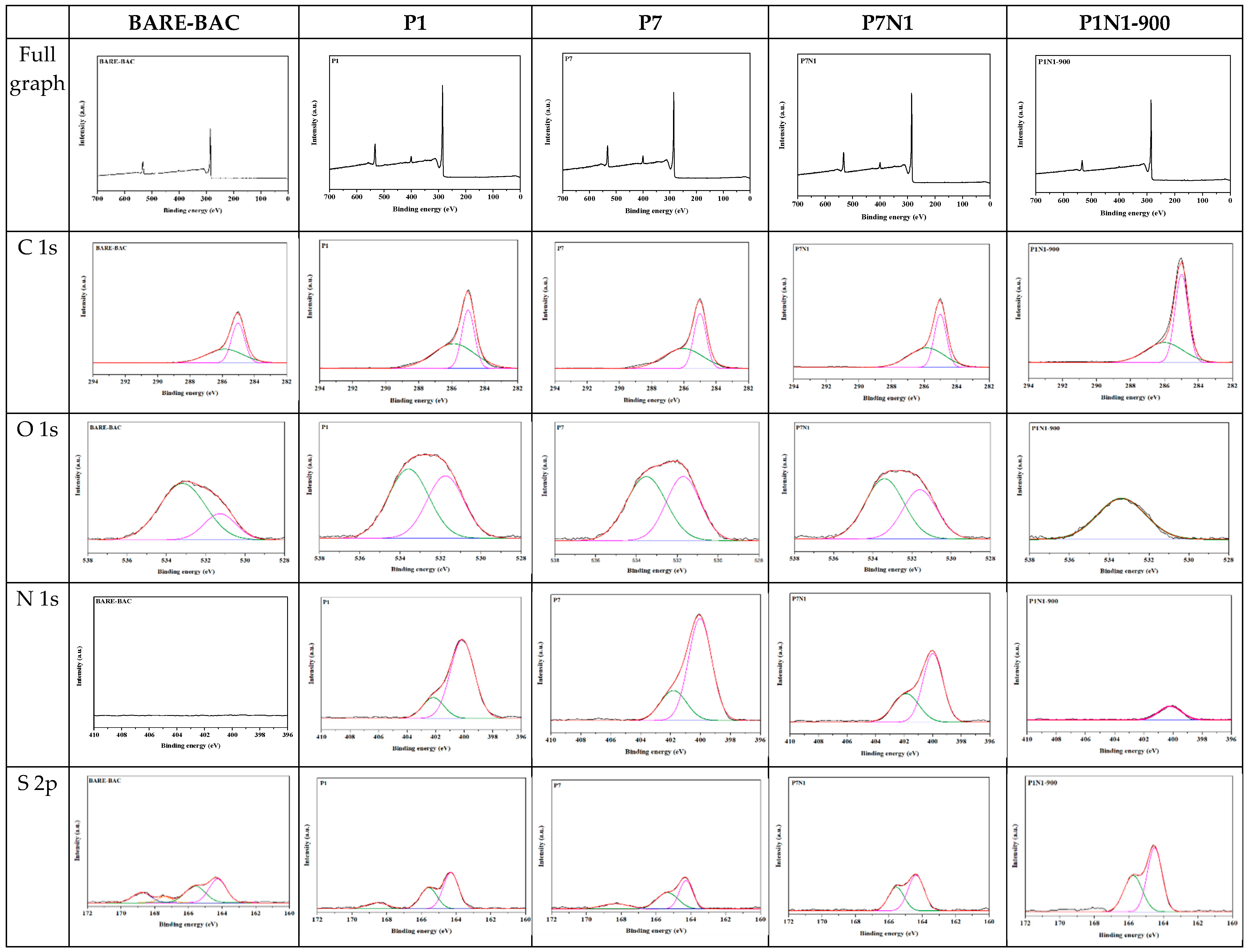
3.1.4. XPS
| Bond Assignment | Energy [eV] | BARE-BAC [%] | P1 [%] | P7 [%] | P7N1 [%] | P1N1-900 [%] | P3N1-900 [%] | P5N1-900 [%] | P7N1-900 [%] | P10N1-900 [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| C 1s | ||||||||||
| C-C sp2 | 284.8 | 51.15 | 41.23 | 46.46 | 47.57 | 62.49 | 55.21 | 60.32 | 69.02 | 66.84 |
| C-O (phenol, alcohol, ether), C=N (amine, amide) | 286.0–286.3 | 48.85 | 58.77 | 53.54 | 52.43 | 37.51 | 44.79 | 39.68 | 30.98 | 33.16 |
| O 1s | ||||||||||
| O-C/O-S (in phenol/ epoxy or thioethers/sulfonic) | 533.3–533.6 | 77.78 | 55.29 | 54.16 | 59.19 | 100 | 100 | 100 | 100 | 100 |
| O=C/O=S (in carboxy/carbonyl or sulfoxides/sulfones) | 532.0–532.5 | 22.22 | 44.71 | 45.84 | 40.81 | - | - | - | - | - |
| N 1s | ||||||||||
| N-(C)3 (tertiary nitrogen, secondary amine) | 399.1–400.0 | - | 83.51 | 76.46 | 68.18 | 100 | 100 | 63.78 | 67.99 | 70.61 |
| C-N+O-C (oxidized nitrogen functionalities) | 402.3 | - | 16.49 | 23.54 | 31.82 | - | - | 36.22 | 32.01 | 29.39 |
| S 2p | ||||||||||
| C-S-C (in sulfides); R-S-S-OR (in thioethers) | 164.5–166.0 | 81.74 | 96.35 | 93.56 | 93.56 | 100 | 100 | 100 | 100 | 100 |
| R2-S=O (in sulfoxides) | 167.0–167.3 | 2.28 | - | - | - | - | - | - | - | - |
| R-SO2-R (in sulfones) | 168.4–168.6 | 15.98 | 3.65 | 6.44 | 6.44 | - | - | - | - | - |
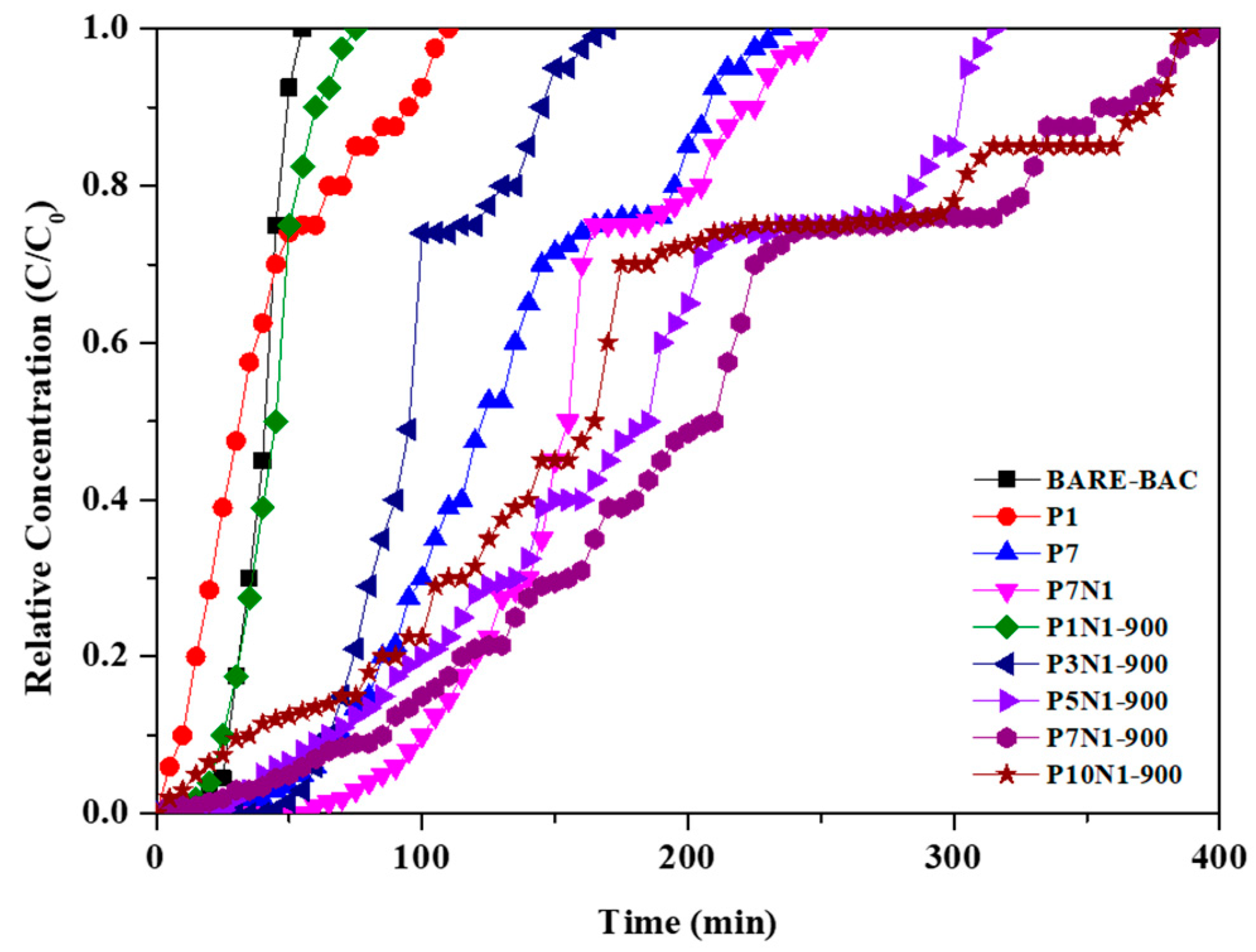
3.2. CH3CHO Adsorption
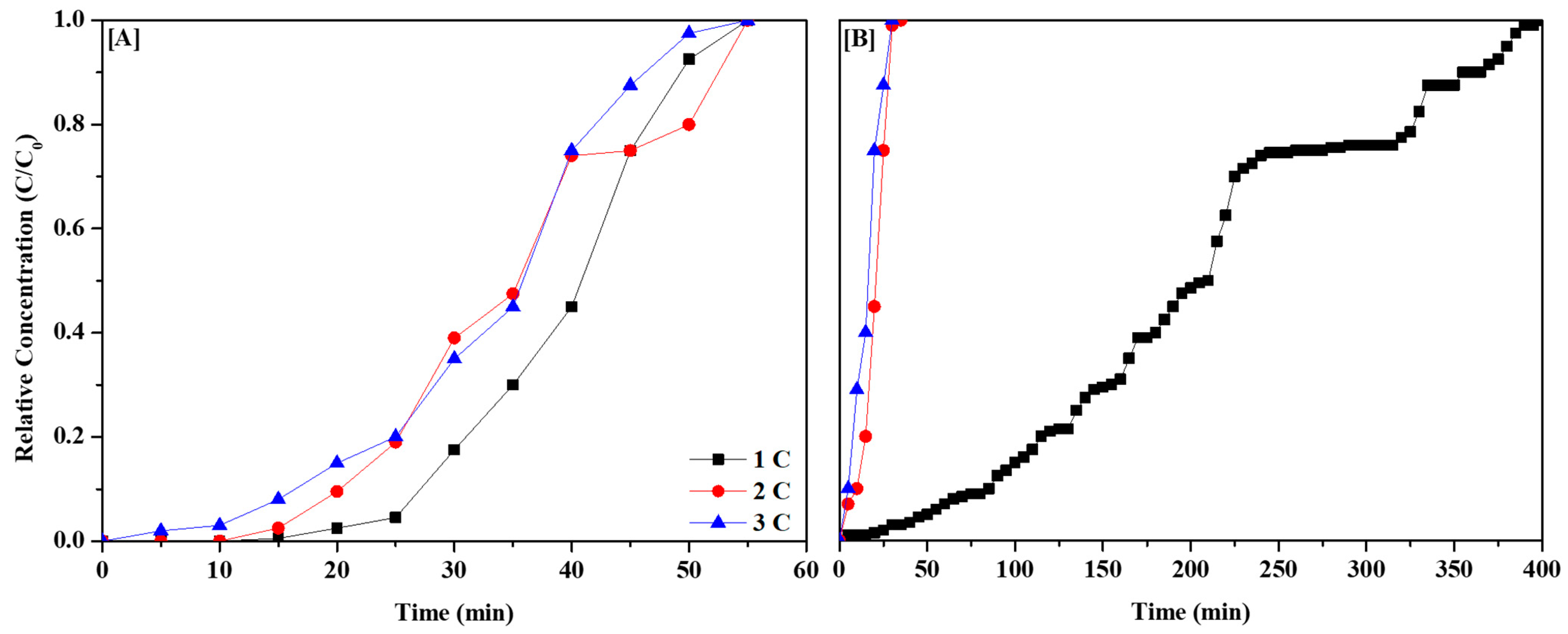
3.3. Effect of Thermal Regeneration on CH3CHO Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.W.; Kim, S.; Ryu, C.; Park, S.H.; Park, Y.K. Review of the use of activated biochar for energy and environmental applications. Carbon Lett. 2018, 26, 1–10. [Google Scholar]
- Isinkaralar, K.; Turkyilmaz, A. Simultaneous adsorption of selected VOCs in the gas environment by low-cost adsorbent from Ricinus communis. Carbon Lett. 2022, 32, 1781–1789. [Google Scholar] [CrossRef]
- Ryu, D.Y.; Kim, D.W.; Kang, Y.J.; Lee, Y.J.; Nakabayashi, K.; Miyawaki, J.; Park, J.I.; Yoon, S.H. Preparation of environmental-friendly N-rich chitin-derived activated carbon for the removal of formaldehyde. Carbon Lett. 2022, 32, 1473–1479. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, H.M.; Kim, B.J. Facile microwave treatment of activated carbons and its effects on hydrocarbon adsorption/desorption behaviors. Carbon Lett. 2023, 33, 1–10. [Google Scholar] [CrossRef]
- Diana, P.; Alexander, O.; Tikhon, K.; Eugenii, G.; Elena, I.; Alexander, Z. Carbonization of oriented poly(vinyl alcohol) fibers impregnated with potassium bisulfate. Carbon Lett. 2020, 30, 637–650. [Google Scholar]
- Azuma, K.; Uchiyama, I.; Uchiyama, S.; Kunugita, N. Assessment of inhalation exposure to indoor air pollutants: Screening for health risks of multiple pollutants in Japanese dwellings. Environ. Res. 2016, 145, 39–49. [Google Scholar] [CrossRef]
- Ghaffarianhoseini, A.; AlWaer, H.; Omrany, H.; Ghaffarianhoseini, A.; Alalouch, C.; Clements-Croome, D.; Tookey, J. Sick building syndrome: Are we doing enough? Archit. Sci. Rev. 2018, 61, 99–121. [Google Scholar] [CrossRef]
- Jansz, J. Theories and knowledge about sick building syndrome. SBS 2011, 591, 25–58. [Google Scholar]
- Naddafi, K.; Nabizadeh, R.; Rostami, R.; Ghaffari, H.R.; Fazlzadeh, M. Formaldehyde and acetaldehyde in the indoor air of waterpipe cafés: Measuring exposures and assessing health effects. Build. Environ. 2019, 165, 106392. [Google Scholar] [CrossRef]
- World Health Organization. Acetaldehyde: Health and safety guide. Health Saf. Guide 1994, 90, 32. [Google Scholar]
- Nikawa, T.; Naya, S.I.; Tada, H. Rapid removal and decomposition of gaseous acetaldehyde by the thermo-and photo-catalysis of gold nanoparticle-loaded anatase titanium (IV) oxide. J. Colloid Interface Sci. 2015, 456, 161–165. [Google Scholar] [CrossRef]
- Gabelman, A.; Hwang, S.T. Hollow fiber membrane contactors. J. Membr. Sci. 1999, 159, 61–106. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Kennes, C.; Thalasso, F. Waste gas biotreatment technology. J. Chem. Technol. Biotechnol. 1998, 72, 303–319. [Google Scholar] [CrossRef]
- Lahousse, C.; Bernier, A.; Grange, P.; Delmon, B.; Papaefthimiou, P.; Ioannides, T.; Verykios, X. Evaluation of γ-MnO2 as a VOC removal catalyst: Comparison with a noble metal catalyst. J. Catal. 1998, 178, 214–225. [Google Scholar] [CrossRef]
- Parmar, G.R.; Rao, N.N. Emerging control technologies for volatile organic compounds. Crit. Rev. Environ. Sci. Technol. 2008, 39, 41–78. [Google Scholar] [CrossRef]
- Gil, E.R.; Ruiz, B.; Lozano, M.S.; Martín, M.J.; Fuente, E. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem. Eng. J. 2014, 245, 80–88. [Google Scholar] [CrossRef]
- Kang, Y.J.; Jo, H.K.; Jang, M.H.; Ma, X.; Jeon, Y.; Oh, K.; Park, J.I. A Brief Review of Formaldehyde Removal through Activated Carbon Adsorption. Appl. Sci. 2022, 12, 5025. [Google Scholar] [CrossRef]
- Zaitan, H.; Manero, M.H.; Valdés, H. Application of high silica zeolite ZSM-5 in a hybrid treatment process based on sequential adsorption and ozonation for VOCs elimination. J. Environ. Sci. 2016, 41, 59–68. [Google Scholar] [CrossRef]
- Kim, K.J.; Ahn, H.G. The effect of pore structure of zeolite on the adsorption of VOCs and their desorption properties by microwave heating. Microporous Mesoporous Mater. 2012, 152, 78–83. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Y.; Shao, Q.; Long, C. Porous polymeric resin for adsorbing low concentration of VOCs: Unveiling adsorption mechanism and effect of VOCs’ molecular properties. Sep. Purif. Technol. 2019, 228, 115755. [Google Scholar] [CrossRef]
- Bradley, R.H. Recent developments in the physical adsorption of toxic organic vapours by activated carbons. Adsorpt. Sci. Technol. 2011, 29, 1–28. [Google Scholar] [CrossRef]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Singh, K.P.; Mohan, D.; Tandon, G.S.; Gupta, G.S.D. Vapor-phase adsorption of hexane and benzene on activated carbon fabric cloth: Equilibria and rate studies. Ind. Eng. Chem. 2002, 41, 2480–2486. [Google Scholar] [CrossRef]
- Hayashi, T.; Kumita, M.; Otani, Y. Removal of acetaldehyde vapor with impregnated activated Carbons: Effects of steric structure on impregnant and acidity. Environ. Sci. Technol. 2005, 39, 5436–5441. [Google Scholar] [CrossRef]
- Vikrant, K.; Qu, Y.; Kim, K.H.; Boukhvalov, D.W.; Ahn, W.S. Amine-functionalized microporous covalent organic polymers for adsorptive removal of a gaseous aliphatic aldehyde mixture. Environ. Sci. Nano 2020, 7, 3447–3468. [Google Scholar] [CrossRef]
- Yamashita, K.; Noguchi, M.; Mizukoshi, A.; Yanagisawa, Y. Acetaldehyde removal from indoor air through chemical absorption using L-cysteine. Int. J. Environ. Res. Public Health 2010, 7, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Son, B.C.; Park, C.H.; Kim, C.S. Fabrication of antimicrobial nanofiber air filter using activated carbon and cinnamon essential oil. J. Nanosci. Nanotechnol. 2020, 20, 4376–4380. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Y.; Bandosz, T.J. A study of acetaldehyde adsorption on activated carbons. J. Colloid Interface Sci. 2001, 242, 44–51. [Google Scholar] [CrossRef]
- El-Sayed, Y.; Bandosz, T.J. Acetaldehyde adsorption on nitrogen-containing activated carbons. Langmuir 2002, 18, 3213–3218. [Google Scholar] [CrossRef]
- Park, K.H.; Shim, W.G.; Shon, H.K.; Lee, S.G.; Ngo, H.H.; Vigneswaran, S.; Moon, H. Adsorption characteristics of acetaldehyde on activated carbons prepared from corn-based biomass precursor. Sep. Sci. Technol. 2010, 45, 1084–1091. [Google Scholar] [CrossRef]
- Branton, P.; Bradley, R.H. Effects of active carbon pore size distributions on adsorption of toxic organic compounds. Adsorption 2011, 17, 293–301. [Google Scholar] [CrossRef]
- Baur, G.B.; Yuranov, I.; Kiwi-Minsker, L. Activated carbon fibers modified by metal oxide as effective structured adsorbents for acetaldehyde. Catal. Today 2015, 249, 252–258. [Google Scholar] [CrossRef]
- Ryu, D.Y.; Nakabayashi, K.; Shimohara, T.; Morio, U.; Mochida, I.; Miyawaki, J.; Jeon, Y.; Park, J.I.; Yoon, S.H. Behaviors of cellulose-based activated carbon fiber for acetaldehyde adsorption at low concentration. Appl. Sci. 2019, 10, 25. [Google Scholar] [CrossRef]
- Kim, B.J.; An, K.H.; Shim, W.G.; Park, Y.K.; Park, J.; Lee, H.; Jung, S.C. Acetaldehyde Adsorption Characteristics of Ag/ACF Composite Prepared by Liquid Phase Plasma Method. Nano Mater. 2021, 11, 2344. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yaqub, M.; Lee, S.; Lee, W. Adsorption of acetaldehyde from air by activated carbon and carbon fibers. Environ. Eng. Res. 2022, 27, 2. [Google Scholar] [CrossRef]
- Ramezanipour Penchah, H.; Ghaemi, A.; Jafari, F. Piperazine-modified activated carbon as a novel adsorbent for CO2 capture: Modeling and characterization. Environ. Sci. Pollut. Res. 2022, 29, 5134–5143. [Google Scholar] [CrossRef]
- Karnati, S.R.; Høgsaa, B.; Zhang, L.; Fini, E.H. Developing carbon nanoparticles with tunable morphology and surface chemistry for use in construction. Constr. Build. Mater. 2020, 262, 120780. [Google Scholar] [CrossRef]
- Kang, Y.J.; Jo, H.K.; Jang, M.J.; Han, G.J.; Yoon, S.J.; Oh, K.; Park, J.I. Acid treatment enhances performance of beads activated carbon for formaldehyde removal. Carbon Lett. 2023, 33, 397–408. [Google Scholar] [CrossRef]
- Seredych, M.; Łoś, S.; Giannakoudakis, D.A.; Rodríguez-Castellón, E.; Bandosz, T.J. Photoactivity of g-C3N4 /S-Doped Porous Carbon Composite: Synergistic Effect of Composite Formation. ChemSusChem 2016, 9, 795–799. [Google Scholar] [CrossRef]


| Sample | Piperazine % [w/v%] | Nitric Acid % [w/v%] | Heat Treatment Temp. [°C] |
|---|---|---|---|
| P1 | 1 | - | - |
| P7 | 7 | - | - |
| P7N1 | 7 | 1 | - |
| P1N1-900 | 1 | 1 | 900 |
| P3N1-900 | 3 | 1 | 900 |
| P5N1-900 | 5 | 1 | 900 |
| P7N1-900 | 7 | 1 | 900 |
| P10N1-900 | 10 | 1 | 900 |
| Sample | SBET [m2/g] | SMicro [m2/g] | VTotal [cm3/g] | VMicro [cm3/g] | Average Pore Diameter [nm] |
|---|---|---|---|---|---|
| BARE-BAC | 1442.1 | 1437.3 | 0.6284 | 0.6189 | 1.7429 |
| P1 | 921.5 | 916.9 | 0.4123 | 0.4033 | 1.7898 |
| P7 | 794.5 | 791.0 | 0.3533 | 0.3462 | 1.7788 |
| P7N1 | 1141.3 | 1137.1 | 0.5001 | 0.4916 | 1.7528 |
| P1N1-900 | 1347.2 | 1341.2 | 0.5905 | 0.5788 | 1.7533 |
| P3N1-900 | 1275.3 | 1270.2 | 0.5612 | 0.5508 | 1.7602 |
| P5N1-900 | 1191.6 | 1185.8 | 0.5259 | 0.5142 | 1.7652 |
| P7N1-900 | 1115.3 | 1110.0 | 0.4818 | 0.4711 | 1.7281 |
| P10N1-900 | 983.8 | 979.3 | 0.4390 | 0.4298 | 1.7850 |
| Sample | C [%] | H [%] | N [%] | S [%] |
|---|---|---|---|---|
| BARE-BAC | 93.84 | 0.45 | * ND | 1.31 |
| P1 | 80.18 | 2.16 | 0.63 | 1.01 |
| P7 | 87.00 | 1.64 | 3.52 | 1.13 |
| P7N1 | 85.90 | 1.74 | 1.89 | 1.21 |
| P1N1-900 | 83.02 | 2.30 | 0.39 | 0.97 |
| P3N1-900 | 93.39 | 0.79 | 1.23 | 1.25 |
| P5N1-900 | 81.99 | 2.67 | 1.52 | 0.94 |
| P7N1-900 | 83.21 | 2.21 | 2.13 | 0.98 |
| P10N1-900 | 80.89 | 2.77 | 2.67 | 0.89 |
| Band Position [cm−1] | Component | Intensity | |
|---|---|---|---|
| BARE-BAC | P7N1-900 | ||
| 3435 | O-H | 3.40 | 6.95 |
| 2916 | Saturated aliphatic CH2 | 0.42 | 0.46 |
| 2853 | Saturated aliphatic CH2 | 0.16 | 0.21 |
| 1639 | Amie, primary/secondary NH | 0.29 | 1.23 |
| Sample | Cin [ppm] | Wad [mg/g] |
|---|---|---|
| BARE-BAC | 200 | 17.17 |
| P1 | 200 | 18.73 |
| P7 | 200 | 50.05 |
| P7N1 | 200 | 57.44 |
| P1N1-900 | 200 | 18.84 |
| P3N1-900 | 200 | 54.48 |
| P5N1-900 | 200 | 62.95 |
| P7N1-900 | 200 | 72.34 |
| P10N1-900 | 200 | 61.38 |
| Sample | Number of Cycle | Adsorption Amount [mg/g] | Regeneration Efficiency of 3 Cycles [%] |
|---|---|---|---|
| BARE-BAC | 1 | 17.17 | 86.78 |
| 2 | 15.89 | ||
| 3 | 14.90 | ||
| P7N1-900 | 1 | 72.34 | 8.77 |
| 2 | 6.13 | ||
| 3 | 5.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.-J.; Kim, Y.-J.; Yoon, S.-J.; Seo, D.-J.; Cho, H.-R.; Oh, K.; Yoon, S.-H.; Park, J.-I. Effective Removal of Acetaldehyde Using Piperazine/Nitric Acid Co-Impregnated Bead-Type Activated Carbon. Membranes 2023, 13, 595. https://doi.org/10.3390/membranes13060595
Kang Y-J, Kim Y-J, Yoon S-J, Seo D-J, Cho H-R, Oh K, Yoon S-H, Park J-I. Effective Removal of Acetaldehyde Using Piperazine/Nitric Acid Co-Impregnated Bead-Type Activated Carbon. Membranes. 2023; 13(6):595. https://doi.org/10.3390/membranes13060595
Chicago/Turabian StyleKang, Yu-Jin, Yu-Jin Kim, Seong-Jin Yoon, Dong-Jin Seo, Hye-Ryeong Cho, Kyeongseok Oh, Seong-Ho Yoon, and Joo-Il Park. 2023. "Effective Removal of Acetaldehyde Using Piperazine/Nitric Acid Co-Impregnated Bead-Type Activated Carbon" Membranes 13, no. 6: 595. https://doi.org/10.3390/membranes13060595
APA StyleKang, Y.-J., Kim, Y.-J., Yoon, S.-J., Seo, D.-J., Cho, H.-R., Oh, K., Yoon, S.-H., & Park, J.-I. (2023). Effective Removal of Acetaldehyde Using Piperazine/Nitric Acid Co-Impregnated Bead-Type Activated Carbon. Membranes, 13(6), 595. https://doi.org/10.3390/membranes13060595