ZIF-67 Incorporated Sulfonated Poly (Aryl Ether Sulfone) Mixed Matrix Membranes for Pervaporation Separation of Methanol/Methyl Tert-Butyl Ether Mixture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ZIF-67 Preparation
2.3. Membrane Preparation
2.4. Membrane Characterization
2.5. Swelling and Sorption Experiments
2.6. Pervaporation Performance Testing
3. Results and Discussion
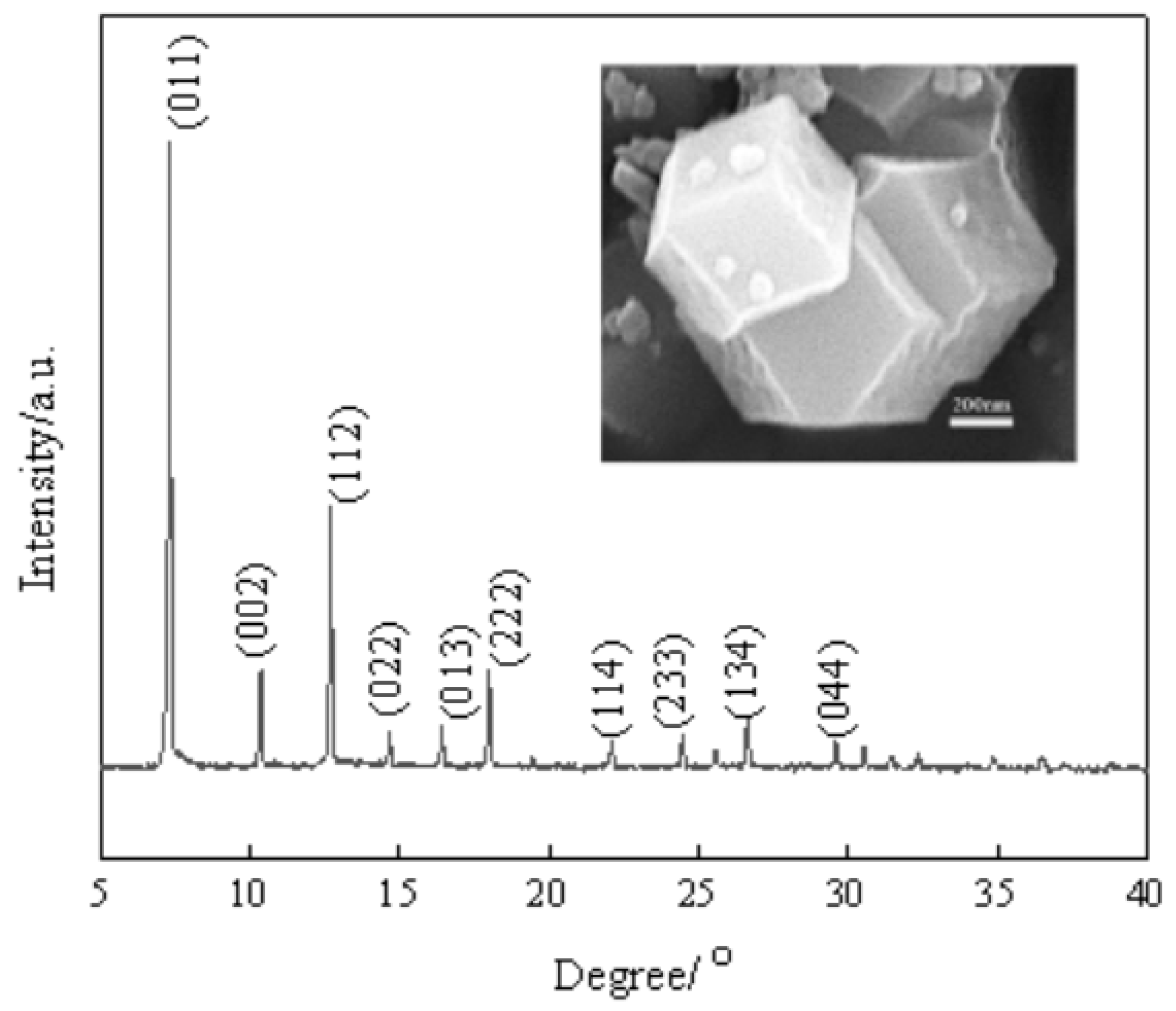
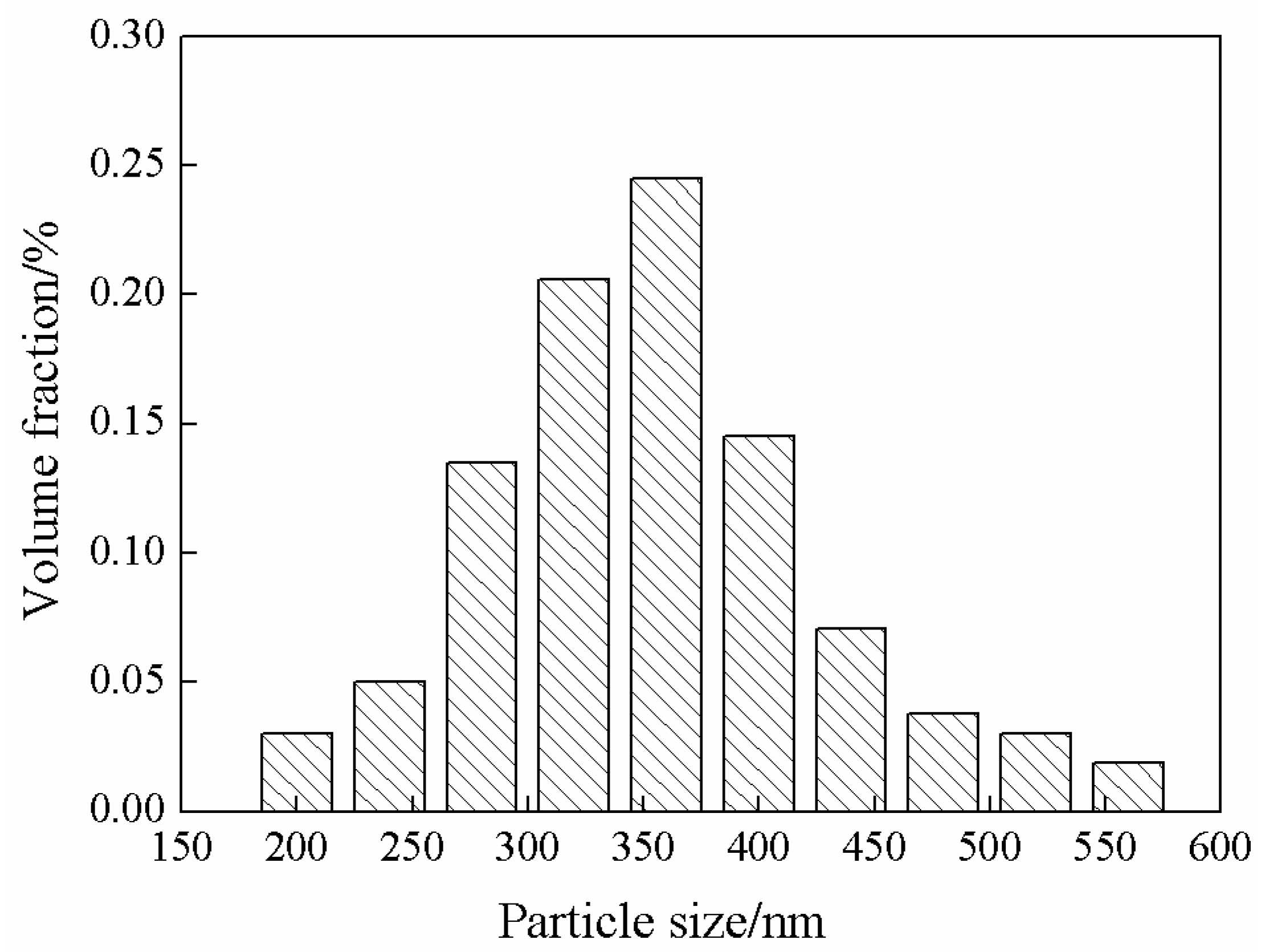
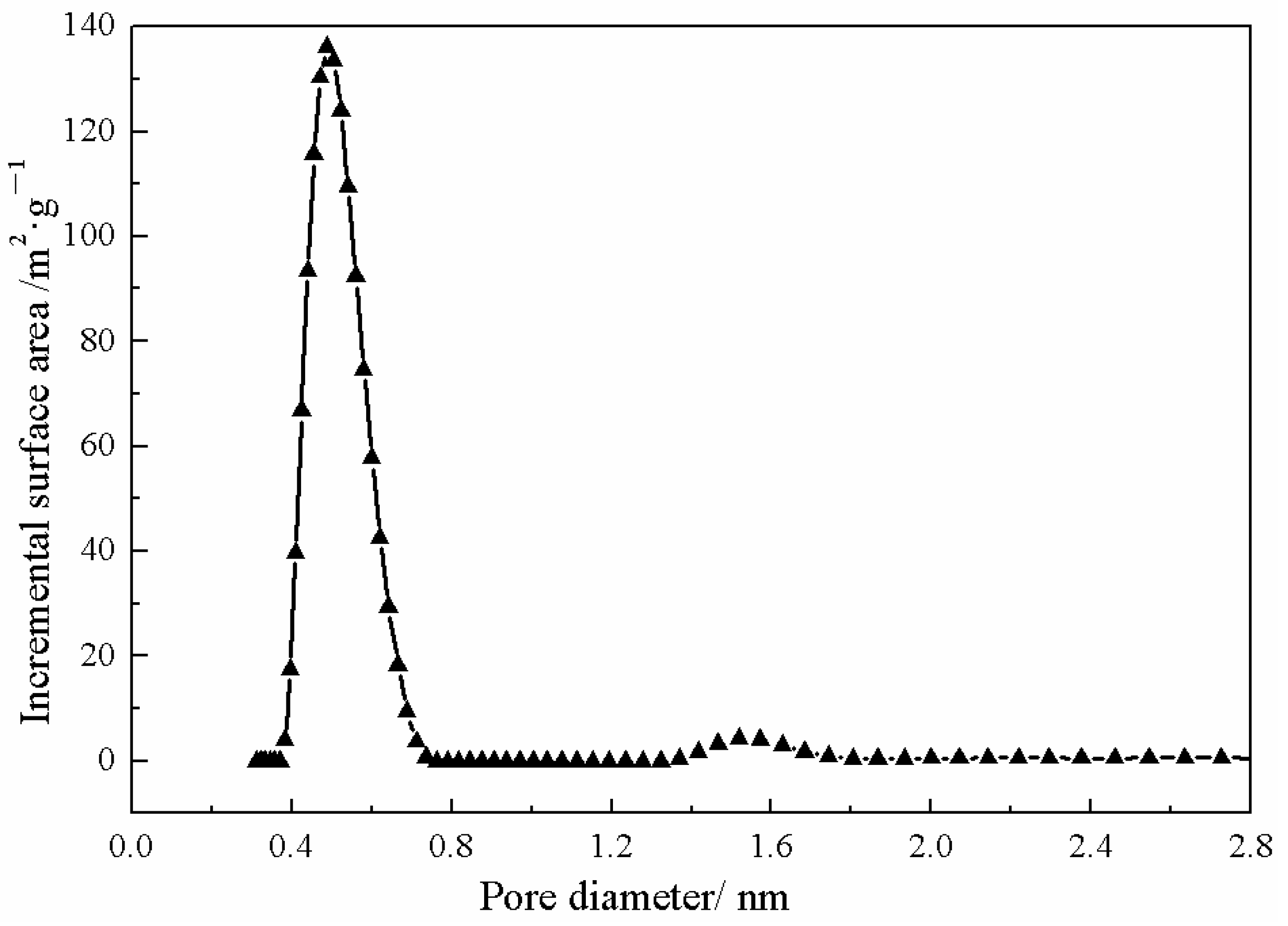
3.1. ZIF-67 Characterization
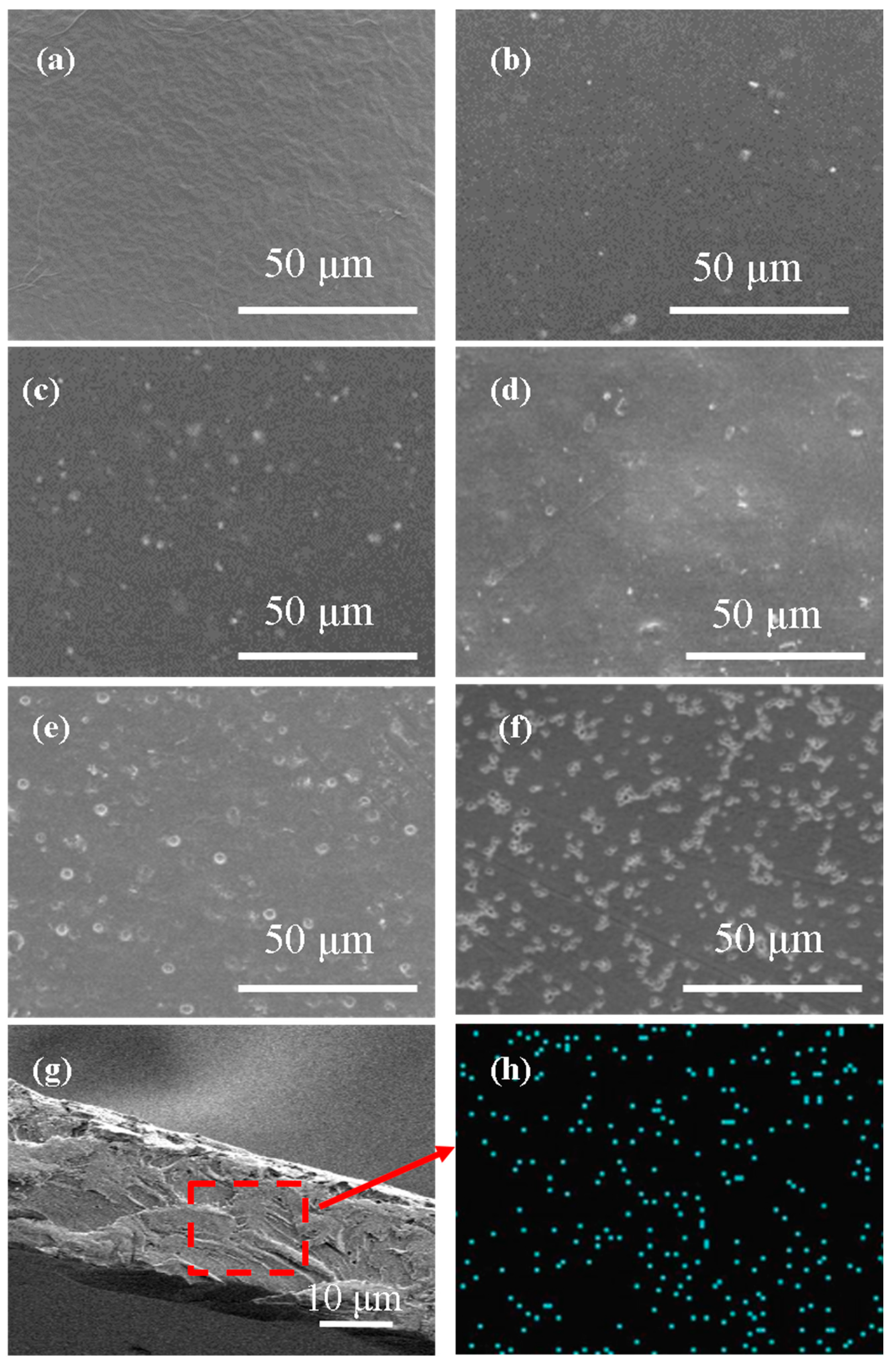
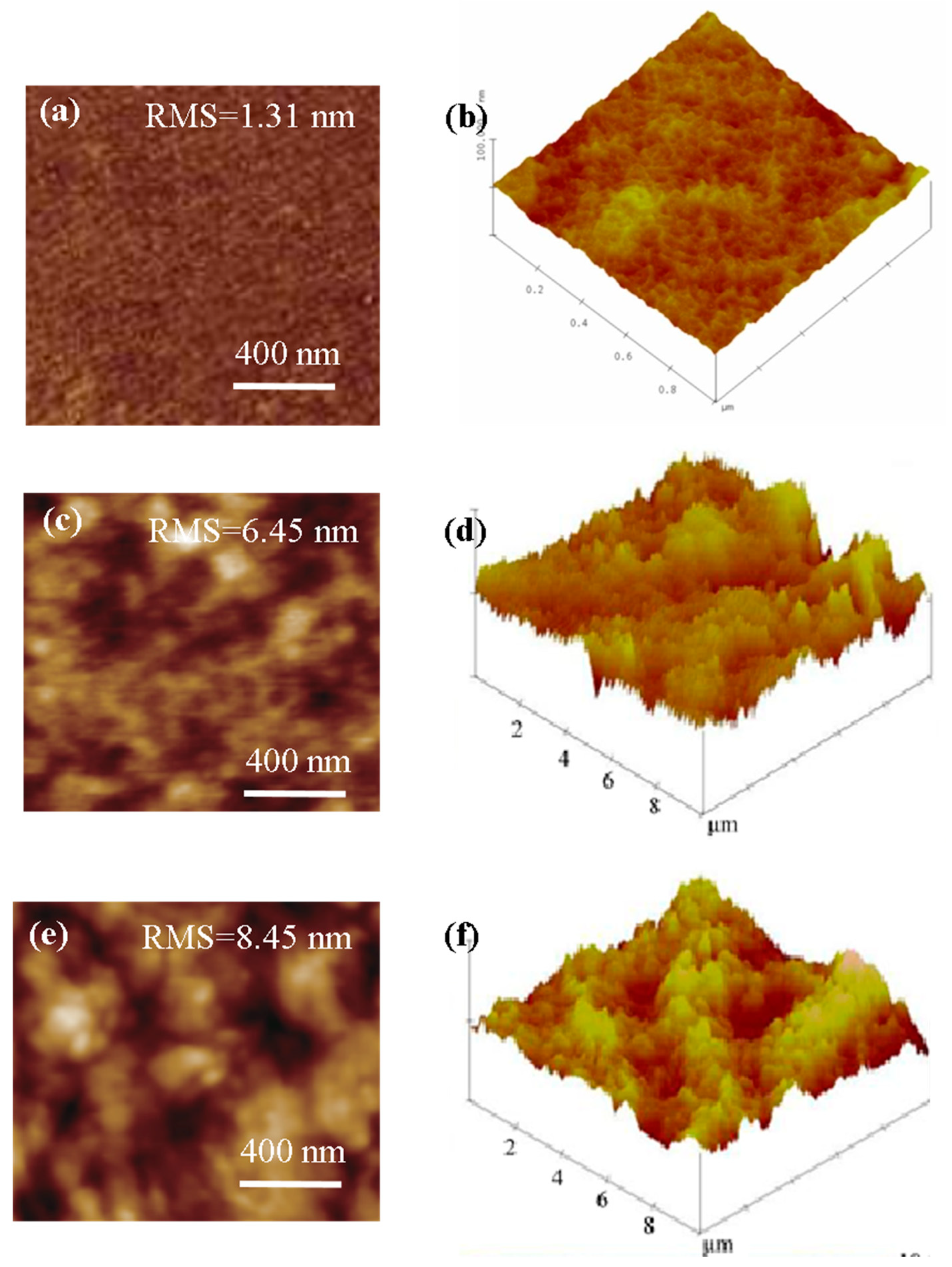
3.2. Micromorphology of Mixed Matrix Membranes
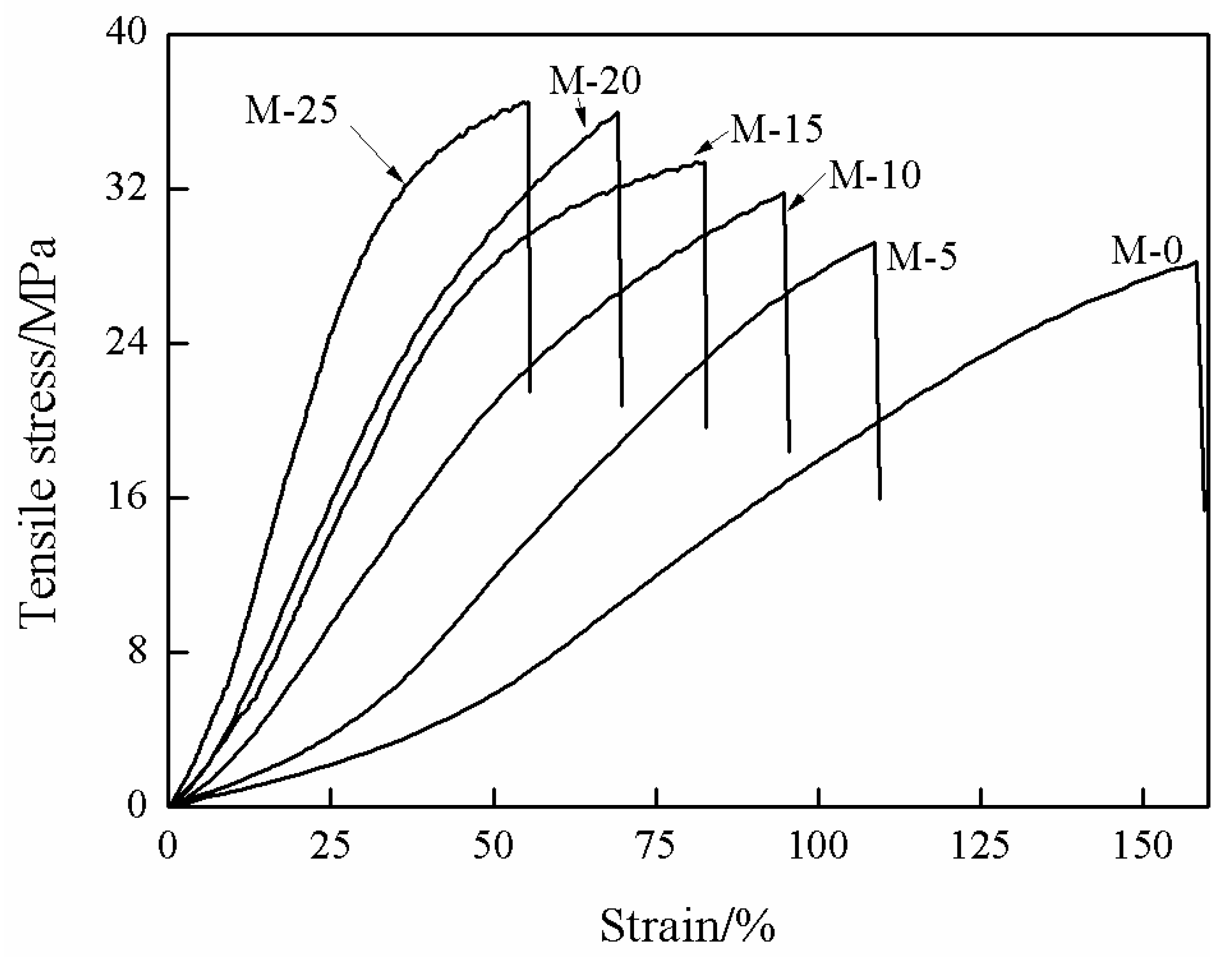
3.3. Mechanical Properties of MMMs
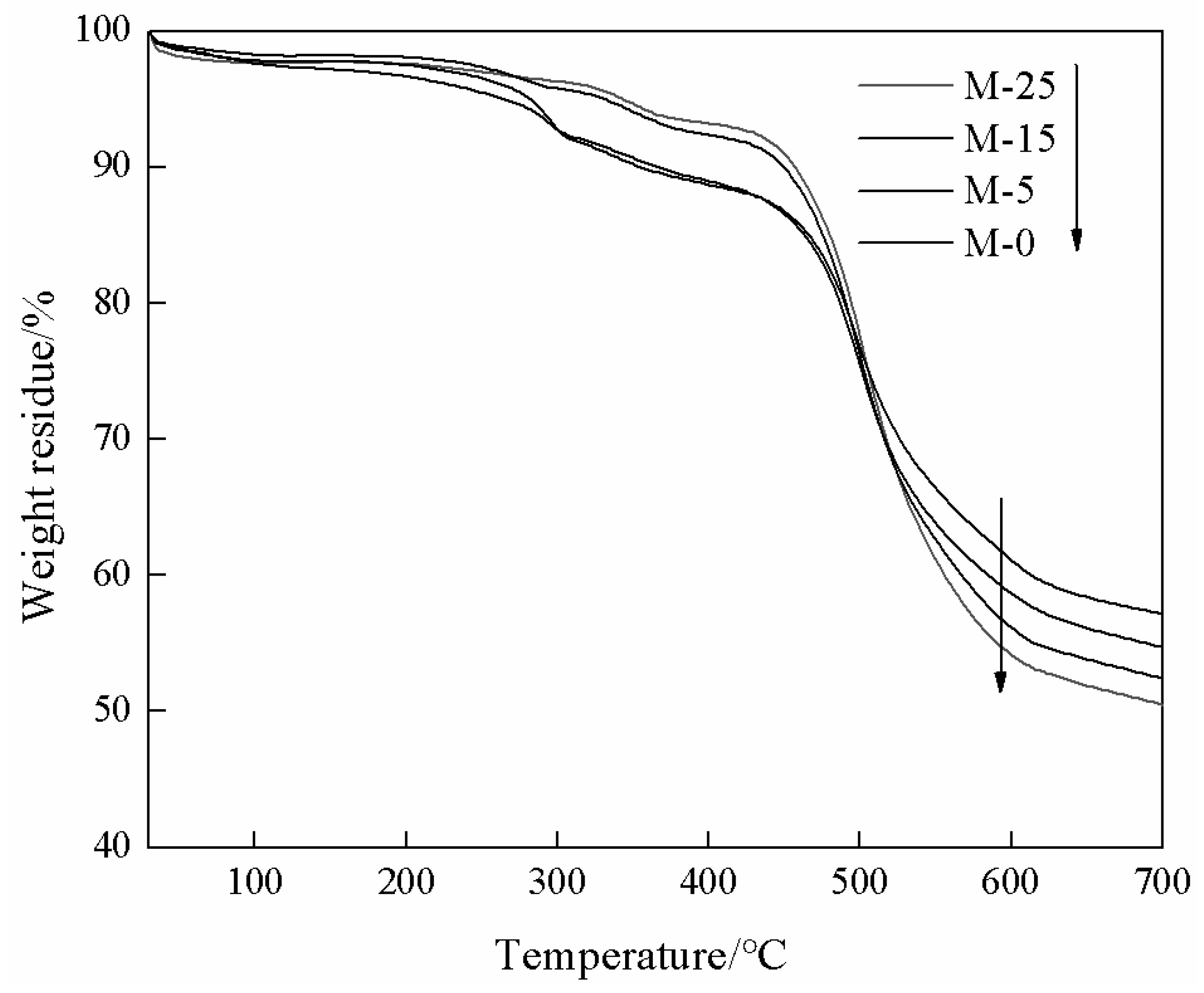
3.4. TGA Analysis
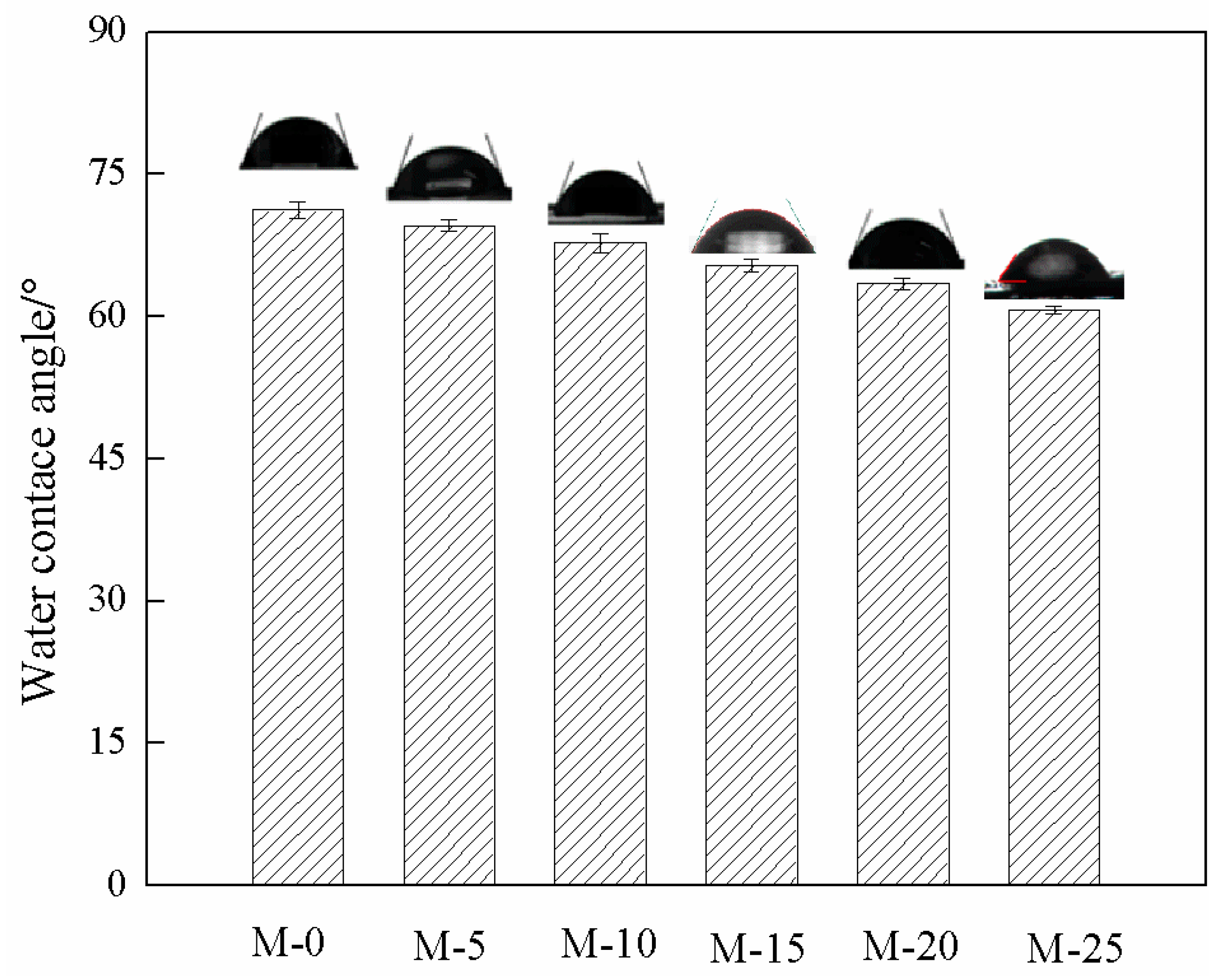
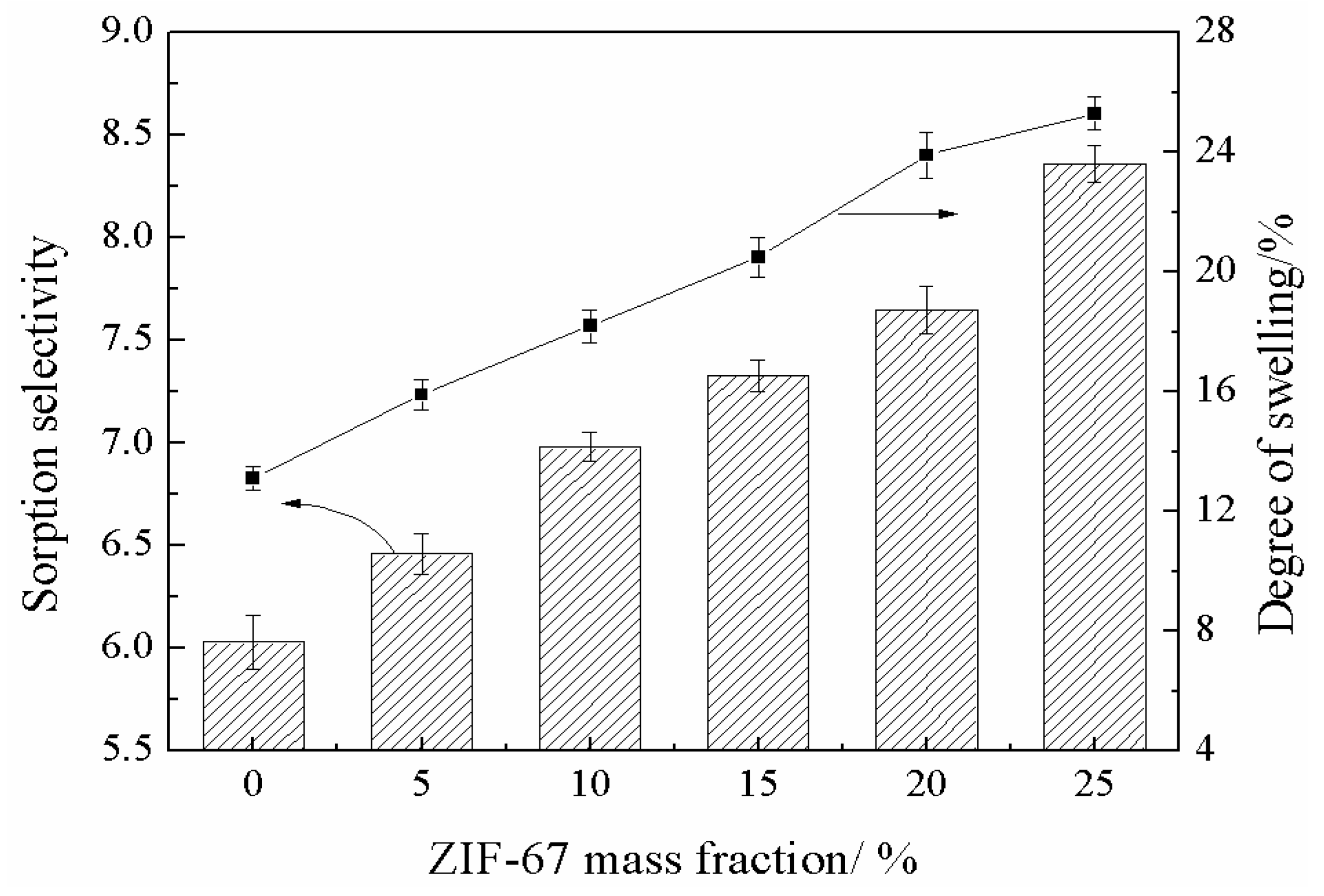
3.5. Membrane Hydrophilicity and Degree of Swelling
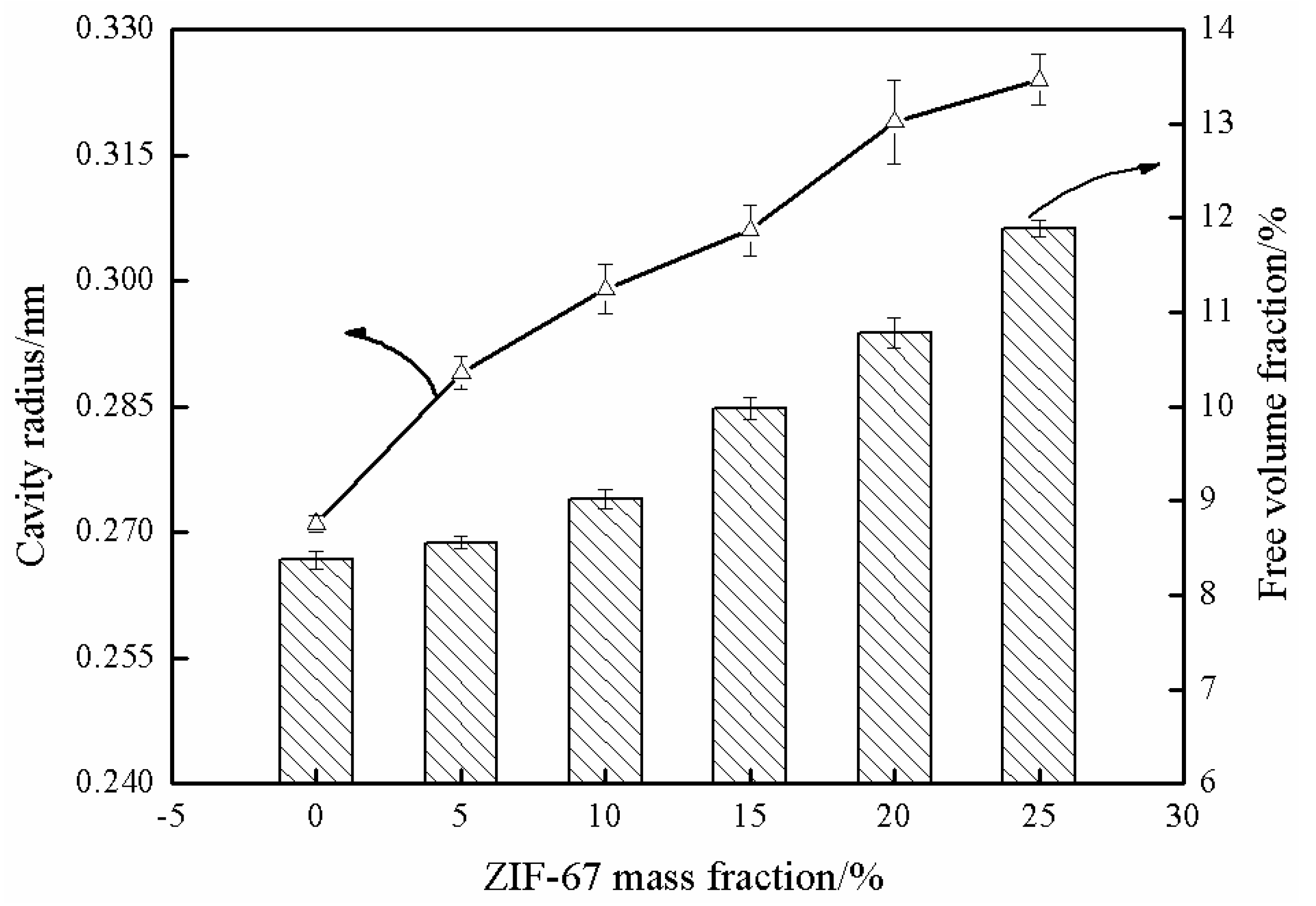
3.6. Free Vloume Parameters of MMMs
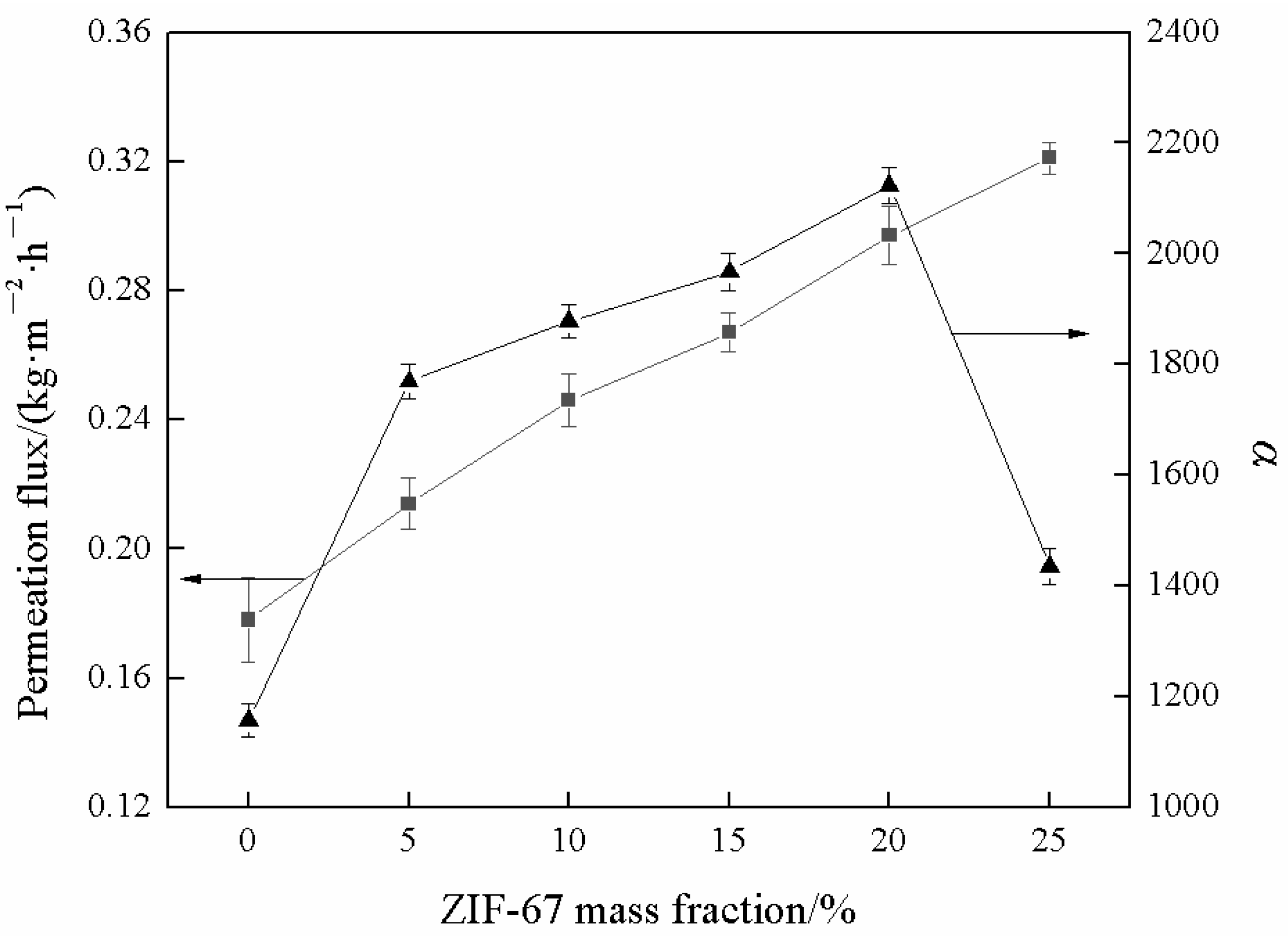
3.7. Pervaporation Performance of MMMs
3.8. Comparison of the Present Work and the Literature Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adoor, S.G.; Bhat, S.D.; Dionysiou, D.D.; Nadagouda, M.N.; Aminabhavi, T.M. Pervaporation separation of water–isopropanol mixtures using silicotungstic acid loaded sulfonated poly(ether ether ketone) composite membranes. RSC Adv. 2014, 4, 52571–52582. [Google Scholar] [CrossRef]
- Ji, C.-H.; Xue, S.-M.; Xu, Z.-L. Novel Swelling-Resistant Sodium Alginate Membrane Branching Modified by Glycogen for Highly Aqueous Ethanol Solution Pervaporation. ACS Appl. Mater. Interfaces 2016, 8, 27243–27253. [Google Scholar] [CrossRef] [PubMed]
- Dudek, G.; Turczyn, R. New type of alginate/chitosan microparticle membranes for highly efficient pervaporative dehydration of ethanol. RSC Adv. 2018, 8, 39567–39578. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xing, R.; Wu, H.; Pan, F.; Zhang, J.; Ding, H.; Jiang, Z. Nanocomposite membranes based on alginate matrix and high loading of pegylated POSS for pervaporation dehydration. J. Membr. Sci. 2017, 538, 86–95. [Google Scholar] [CrossRef]
- Cheng, X.; Cai, W.; Chen, X.; Shi, Z.; Li, J. Preparation of graphene oxide/poly(vinyl alcohol) composite membrane and pervaporation performance for ethanol dehydration. RSC Adv. 2019, 9, 15457–15465. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, L.; Wang, N.; Li, J.; Ji, S.; Guo, H.; Zhang, G.; Zhang, Z. In situ ultraviolet-light-induced TiO2 nanohybrid super hydrophilic membrane for pervaporation dehydration. Sep. Purif. Technol. 2014, 122, 32–40. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Li, L.; Xiao, Z.; Zhang, Z.; Tan, S. Pervaporation of acetic acid/water mixtures through carbon molecular sieve-filled PDMSmembranes. Chem. Eng. J. 2004, 97, 83–86. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.-H.; Zhang, A.-S.; Xu, L.-H.; Lu, J.-J.; Zhao, Z.-P. Novel MOF-capped halloysite nanotubes/PDMS mixed matrix membranes for enhanced n-butanol permselective pervaporation. J. Membr. Sci. 2020, 595, 117543. [Google Scholar] [CrossRef]
- Dave, H.K.; Nath, K. Graphene oxide incorporated novel polyvinyl alcohol composite membrane for pervaporative recovery of acetic acid from vinegar wastewater. J. Water Process Eng. 2016, 14, 124–134. [Google Scholar] [CrossRef]
- Izák, P.; Köckerling, M.; Kragl, U. Solute transport from aqueous mixture throught supported ionic liquid membrane by pervaporation. Desalination 2006, 199, 96–98. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lin, Y.; Chen, C. Separation of dimethyl carbonate/methanol mixtures by pervaporation with poly (acrylic acid)/poly (vinyl alcohol) blend membranes. J. Membr. Sci. 2007, 305, 238–246. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S. Separation of organic mixtures by pervaporation using crosslinked and filled rubber membranes. J. Membr. Sci. 2006, 285, 108–119. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Z.; Luo, Y.; Yu, P. Pervaporation separation of dimethyl carbonate/methanol azeotrope through cross-linked PVA–poly(vinyl pyrrolidone)/PAN composite membranes. Desalin. Water Treat. 2013, 51, 5485–5493. [Google Scholar] [CrossRef]
- Azimi, H.; Tezel, F.; Thibault, J. Effect of embedded activated carbon nanoparticles on the performance of polydimethylsiloxane (PDMS) membrane for pervaporation separation of butanol. J. Chem. Technol. Biotechnol. 2017, 92, 2901–2910. [Google Scholar] [CrossRef]
- Bhat, S.D.; Aminabhavi, T.M. Zeolite K-LTL-loaded sodium alginate mixed matrix membranes for pervaporation dehydration of aqueous–organic mixtures. J. Membr. Sci. 2007, 306, 173–185. [Google Scholar] [CrossRef]
- Flynn, E.; Keane, D.; Tabari, P.; Morris, M. Pervaporation performance enhancement through the incorporation of mesoporous silica spheres into PVA membranes. Sep. Purif. Technol. 2013, 118, 73–80. [Google Scholar] [CrossRef]
- Ismail, A.; Goh, P.; Sanip, S.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Sardarabadi, H.; Kiani, S.; Karkhanechi, H.; Mousavi, S.M.; Saljoughi, E.; Matsuyama, H. Effect of Nanofillers on Properties and Pervaporation Performance of Nanocomposite Membranes: A Review. Membranes 2022, 12, 1232. [Google Scholar] [CrossRef]
- Penkova, A.V.; Polotskaya, G.A.; Gavrilova, V.A.; Toikka, A.M.; Liu, J.-C.; Trchová, M.; Šlouf, M.; Pientka, Z. Polyamide membranes modified by carbon nanotubes: Application for pervaporation. Sep. Sci. Technol. 2009, 45, 35–41. [Google Scholar] [CrossRef]
- Peng, F.; Pan, F.; Sun, H.; Lu, L.; Jiang, Z. Novel nanocomposite pervaporation membranes composed of poly (vinyl alcohol) and chitosan-wrapped carbon nanotube. J. Membr. Sci. 2007, 300, 13–19. [Google Scholar] [CrossRef]
- Shirazi, Y.; Tofighy, M.A.; Mohammadi, T. Synthesis and characterization of carbon nanotubes/poly vinyl alcohol nanocomposite membranes for dehydration of isopropanol. J. Membr. Sci. 2011, 378, 551–561. [Google Scholar] [CrossRef]
- Guo, R.; Ma, X.; Hu, C.; Jiang, Z. Novel PVA–silica nanocomposite membrane for pervaporative dehydration of ethylene glycol aqueous solution. Polymer 2007, 48, 2939–2945. [Google Scholar] [CrossRef]
- Hu, S.Y.; Zhang, Y.; Lawless, D.; Feng, X. Composite membranes comprising of polyvinylamine-poly (vinyl alcohol) incorporated with carbon nanotubes for dehydration of ethylene glycol by pervaporation. J. Membr. Sci. 2012, 417, 34–44. [Google Scholar] [CrossRef]
- Liu, G.; Wang, W.; Jin, W.; Xu, N. Polymer/ceramic composite membranes and their application in pervaporation process. Chin. J. Chem. Eng. 2012, 20, 62–70. [Google Scholar] [CrossRef]
- Bakhtiari, O.; Mosleh, S.; Khosravi, T.; Mohammadi, T. Mixed matrix membranes for pervaporative separation of isopropanol/water mixtures. Desalination Water Treat. 2012, 41, 45–52. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A.; Fotouhi, M. Preparation and characterization of thin film nanocomposite membrane for pervaporative dehydration of aqueous alcohol solutions. Desalination 2013, 314, 20–27. [Google Scholar] [CrossRef]
- Sunitha, K.; Rani, K.Y.; Moulik, S.; Satyanarayana, S.; Sridhar, S. Separation of NMP/water mixtures by nanocomposite PEBA membrane: Part, I. Membrane synthesis, characterization and pervaporation performance. Desalination 2013, 330, 16. [Google Scholar] [CrossRef]
- Yadav, A.; Lind, M.L.; Ma, X.; Lin, Y. Nanocomposite silicalite-1/polydimethylsiloxane membranes for pervaporation of ethanol from dilute aqueous solutions. Ind. Eng. Chem. Res. 2013, 52, 5207–5212. [Google Scholar] [CrossRef]
- Jullok, N.; Van Hooghten, R.; Luis, P.; Volodin, A.; Van Haesendonck, C.; Vermant, J.; Van der Bruggen, B. Effect of silica nanoparticles in mixed matrix membranes for pervaporation dehydration of acetic acid aqueous solution: Plant-inspired dewatering systems. J. Clean. Prod. 2016, 112, 4879–4889. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Ji, S.; Wang, N.; An, W. Layer-by-layer assembled nanohybrid multilayer membranes for pervaporation dehydration of acetone–water mixtures. J. Membr. Sci. 2012, 415, 745–757. [Google Scholar] [CrossRef]
- Lin, W.; Zhu, T.; Li, Q.; Yi, S.; Li, Y. Study of pervaporation for dehydration of caprolactam through PVA/nano silica composite membranes. Desalination 2012, 285, 39–45. [Google Scholar] [CrossRef]
- Olukman, M.; Sanlı, O. A novel in situ synthesized magnetite containing acrylonitrile and 2-hydroxyethyl methacrylate grafted poly (vinyl alcohol) nanocomposite membranes for pervaporation separation of acetone/water mixtures. Chem. Eng. Process. Process Intensif. 2015, 98, 60–70. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shahi, V.K. Functionalized silica–chitosan hybrid membrane for dehydration of ethanol/water azeotrope: Effect of cross-linking on structure and performance. J. Membr. Sci. 2013, 444, 116–126. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Mohammadi, T. Effect of annealing temperature and time on structure and performance of poly (vinyl) alcohol nanocomposite membranes. Polym. Eng. Sci. 2010, 50, 2392–2399. [Google Scholar] [CrossRef]
- Sairam, M.; Naidu, B.V.K.; Nataraj, S.K.; Sreedhar, B.; Aminabhavi, T.M. Poly (vinylalcohol)-ironoxidenanocompositemembranes for pervaporation dehydration of isopropanol, 1, 4-dioxane and tetrahydrofuran. J. Membr. Sci. 2006, 283, 65–73. [Google Scholar] [CrossRef]
- Das, P.; Ray, S.K. Synthesis and characterization of biopolymer based mixed matrix membranes for pervaporative dehydration. Carbohydr. Polym. 2014, 103, 274–284. [Google Scholar] [CrossRef]
- Jose, T.; George, S.C. Induced Hydrophilicity of the Nanoclay on the Pervaporation Performance of Cross-Linked Poly (vinyl alcohol) Nanocomposite Membranes. Polym. Plast. Technol. Eng. 2016, 55, 1266–1281. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Separation of ethanol from water by pervaporation using mixed matrix copolymer membranes. Sep. Purif. Technol. 2015, 146, 176–186. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadatnia, B. Poly (vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 469, 34. [Google Scholar] [CrossRef]
- Shi, G.M.; Chen, H.; Jean, Y.; Chung, T.S. Sorption, swelling, and free volume of polybenzimidazole (PBI) and PBI/zeolitic imidazolate framework (ZIF-8) nano-composite membranes for pervaporation. Polymer 2013, 54, 774–783. [Google Scholar] [CrossRef]
- Khoonsap, S.; Supanchaiyamat, N.; Hunt, A.J.; Klinsrisuk, S.; Amnuaypanich, S. Improving water selectivity of poly (vinyl alcohol) (PVA)–Fumed silica (FS) nanocomposite membranes by grafting of poly (2-hydroxyethyl methacrylate) (PHEMA) on fumed silica particles. Chem. Eng. Sci. 2015, 122, 373–383. [Google Scholar] [CrossRef]
- Wang, N.; Shi, G.; Gao, J.; Li, J.; Wang, L.; Guo, H.; Zhang, G.; Ji, S. MCM-41@ ZIF-8/PDMS hybrid membranes with micro-and nanoscaled hierarchical structure for alcohol permselective pervaporation. Sep. Purif. Technol. 2015, 153, 146–155. [Google Scholar] [CrossRef]
- Yan, H.; Li, J.; Fan, H.; Ji, S.; Zhang, G.; Zhang, Z. Sonication-enhanced in situ assembly of organic/inorganic hybrid membranes: Evolution of nanoparticle distribution and pervaporation performance. J. Membr. Sci. 2015, 481, 94–105. [Google Scholar] [CrossRef]
- Shi, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415, 577–586. [Google Scholar] [CrossRef]
- Han, G.L.; Zhang, Q.G.; Liu, Q.L. Separation of methanol/methyl tert-butyl ether using sulfonated polyarylethersulfone with cardo (SPES-C) membranes. J. Membr. Sci. 2013, 430, 180–187. [Google Scholar] [CrossRef]
- Pei, N.; Ma, C.C.; Liu, H.O.; Qiu, J.S.; Zhang, X.F. High-performance Co-based ZIF-67 tubular membrane achieved by ZnO-induced synthesis for highly efficient Pervaporation separation of methanol/methyl tert-butyl ether mixture. Ind. Eng. Chem. Res. 2019, 58, 15297–15306. [Google Scholar]
- Ma, X.; Hu, C.; Guo, R.; Fang, X.; Wu, H.; Jiang, Z. HZSM5-filled cellulose acetate membranes for pervaporation separation of methanol/MTBE mixtures. Sep. Purif. Technol. 2008, 59, 34–42. [Google Scholar] [CrossRef]
- Wu, H.; Fang, X.; Zhang, X.; Jiang, Z.; Li, B.; Ma, X. Cellulose acetate–poly(N-vinyl-2-pyrrolidone) blend membrane for pervaporation separation of methanol/MTBE mixtures. Sep. Purif. Technol. 2008, 64, 183–191. [Google Scholar] [CrossRef]
- Villaluenga, J.P.G.; Godino, P.; Khayet, M.; Seoane, B.; Mengual, J.I. Pervaporation of alcohols and methyl tert-butyl ether through a dense poly(2,6-dimethyl-1,4-phenylene oxide) membrane. Ind. Eng. Chem. Res. 2004, 43, 2548–2555. [Google Scholar] [CrossRef]
- Villegas, M.; Vidaurre, E.F.C.; Habert, A.C.; Gottifredi, J.C. Sorption and pervaporation with poly(3-hydroxybutyrate) membranes: Methanol/methyl tert-butyl ether mixtures. J. Membr. Sci. 2011, 367, 103–109. [Google Scholar] [CrossRef]
- Zereshki, S.; Figoli, A.; Madaeni, S.S.; Simone, S.; Drioli, E. Pervaporation separation of methanol/methyl tert-butyl ether with poly(lactic acid) membranes. J. Appl. Polym. Sci. 2010, 118, 1364–1371. [Google Scholar] [CrossRef]
- Han, G.L.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Pervaporation separation of methanol/ methyl tert-butyl ether mixtures using polyarylethersulfone with cardo membranes. Sep. Purif. Technol. 2013, 107, 211–218. [Google Scholar] [CrossRef]
- Rhim, J.W.; Kim, Y.K. Pervaporation separation of MTBE–methanol mixtures using cross-linked PVA membranes. J. Appl. Polym. Sci. 2000, 75, 1699–1707. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Moon, G.Y.; Pal, R. Chitosan/anionic surfactant complex membranes for the pervaporation separation of methanol/MTBE and characterization of the polymer/surfactant system. J. Membr. Sci. 2001, 184, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, L.R.; Luo, G.S.; Dai, Y.Y. Preparation of cellulose acetate membrane filled with metal oxide particles for the pervaporation separation of methanol/methyl tert-butyl ether mixtures. Chem. Eng. J. 2009, 146, 6–10. [Google Scholar] [CrossRef]
- Han, G.L.; Gong, Y.; Zhang, Q.G.; Zhu, A.M.; Ye, M.L.; Liu, Q.L. Facile preparation of homogeneous polyelectrolyte complex membranes for separation of methanol/ methyl tert-butyl ether mixtures. J. Membr. Sci. 2013, 447, 246–252. [Google Scholar] [CrossRef]
- Han, G.L.; Gong, Y.; Zhang, Q.G.; Liu, Q.L. Polyarylethersulfone with cardo/poly (vinyl pyrrolidone) blend membrane for pervaporation of methanol/methyl tertbutyl ether mixtures. J. Membr. Sci. 2013, 448, 55–61. [Google Scholar] [CrossRef]
| Membranes | MeOH in Feed | Temperature (°C) | J (kg·m−2·h−1) | α | Reference |
|---|---|---|---|---|---|
| HZSM5/CA | 20 wt% | 30 | 0.226 | 346 | [48] |
| CA/PVP | 20 wt% | 40 | 0.430 | 411 | [49] |
| PEEK-C | 20 wt% | 20 | 0.050 | 15 | [50] |
| PHB | 40 mol% | 40 | 0.387 | 9 | [51] |
| PLA | 20 wt% | 30 | 0.552 | 6 | [52] |
| PES-C | 40 wt% | 40 | 0.108 | 99 | [53] |
| PVA/PAA | 15 wt% | 50 | 0.010 | 4000 | [54] |
| CS/surfactants | 20 wt% | 25 | 0.653 | 231 | [55] |
| CA/ZnO | 31 wt% | 40 | 0.115 | 760 | [56] |
| SPES-C/PEI | 15 wt% | 40 | 0.194 | 1860 | [57] |
| PES-C/PVP | 15 wt% | 40 | 0.172 | 889 | [58] |
| M-20 | 15 wt% | 40 | 0.297 | 2123 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Lv, J.; Chen, M. ZIF-67 Incorporated Sulfonated Poly (Aryl Ether Sulfone) Mixed Matrix Membranes for Pervaporation Separation of Methanol/Methyl Tert-Butyl Ether Mixture. Membranes 2023, 13, 389. https://doi.org/10.3390/membranes13040389
Han G, Lv J, Chen M. ZIF-67 Incorporated Sulfonated Poly (Aryl Ether Sulfone) Mixed Matrix Membranes for Pervaporation Separation of Methanol/Methyl Tert-Butyl Ether Mixture. Membranes. 2023; 13(4):389. https://doi.org/10.3390/membranes13040389
Chicago/Turabian StyleHan, Guanglu, Jie Lv, and Mohan Chen. 2023. "ZIF-67 Incorporated Sulfonated Poly (Aryl Ether Sulfone) Mixed Matrix Membranes for Pervaporation Separation of Methanol/Methyl Tert-Butyl Ether Mixture" Membranes 13, no. 4: 389. https://doi.org/10.3390/membranes13040389
APA StyleHan, G., Lv, J., & Chen, M. (2023). ZIF-67 Incorporated Sulfonated Poly (Aryl Ether Sulfone) Mixed Matrix Membranes for Pervaporation Separation of Methanol/Methyl Tert-Butyl Ether Mixture. Membranes, 13(4), 389. https://doi.org/10.3390/membranes13040389








