Advanced Oxidation Processes Coupled to Nanofiltration Membranes with Catalytic Fe0 Nanoparticles in Symmetric and Asymmetric Polyelectrolyte Multilayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication and Surface Characterizations of Catalytic Membranes with Fe0 on or in the Selective Layer
2.3. Measurements of Water Permeability, Naproxen Rejection, and Degradation Efficiency
3. Results and Discussion
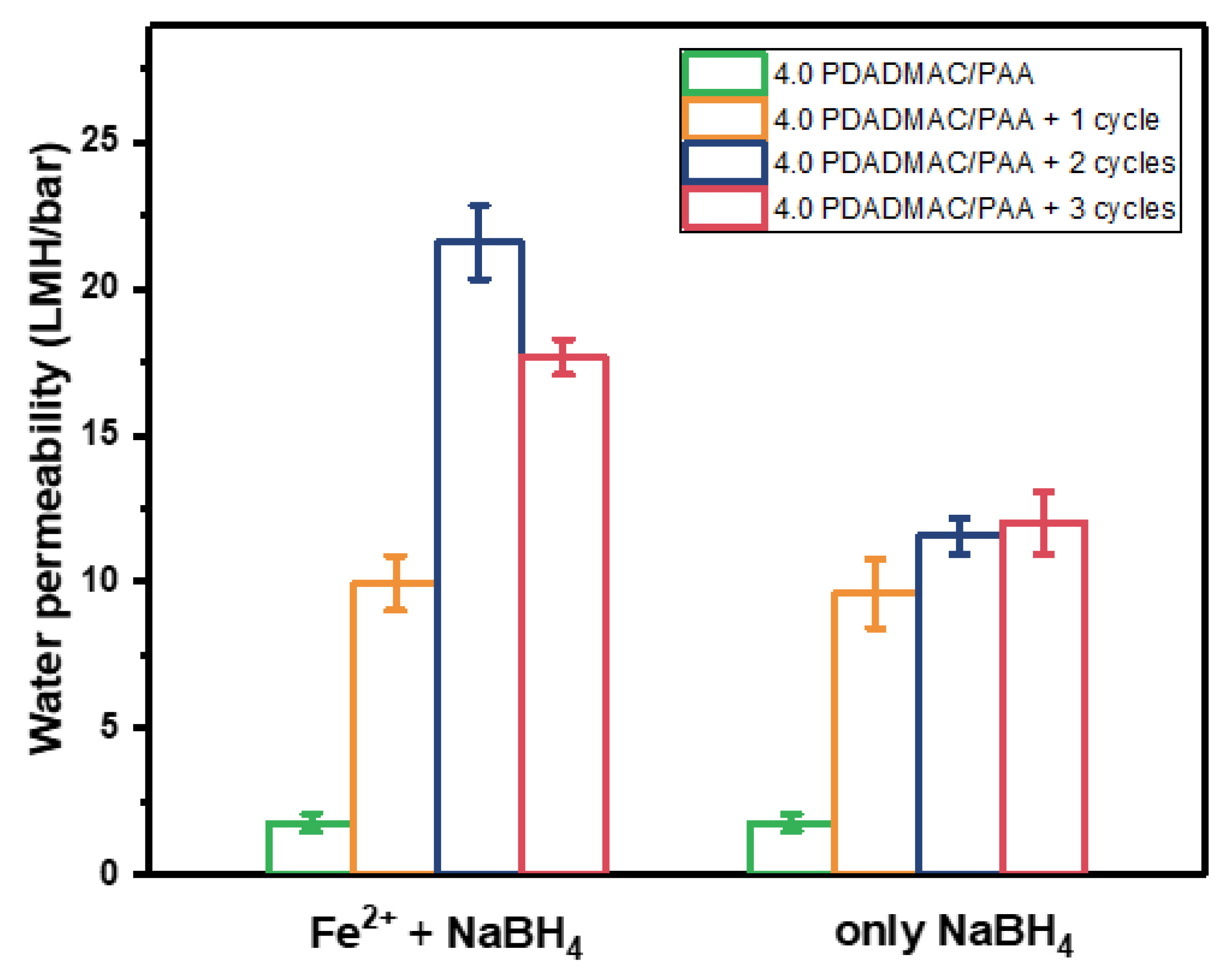
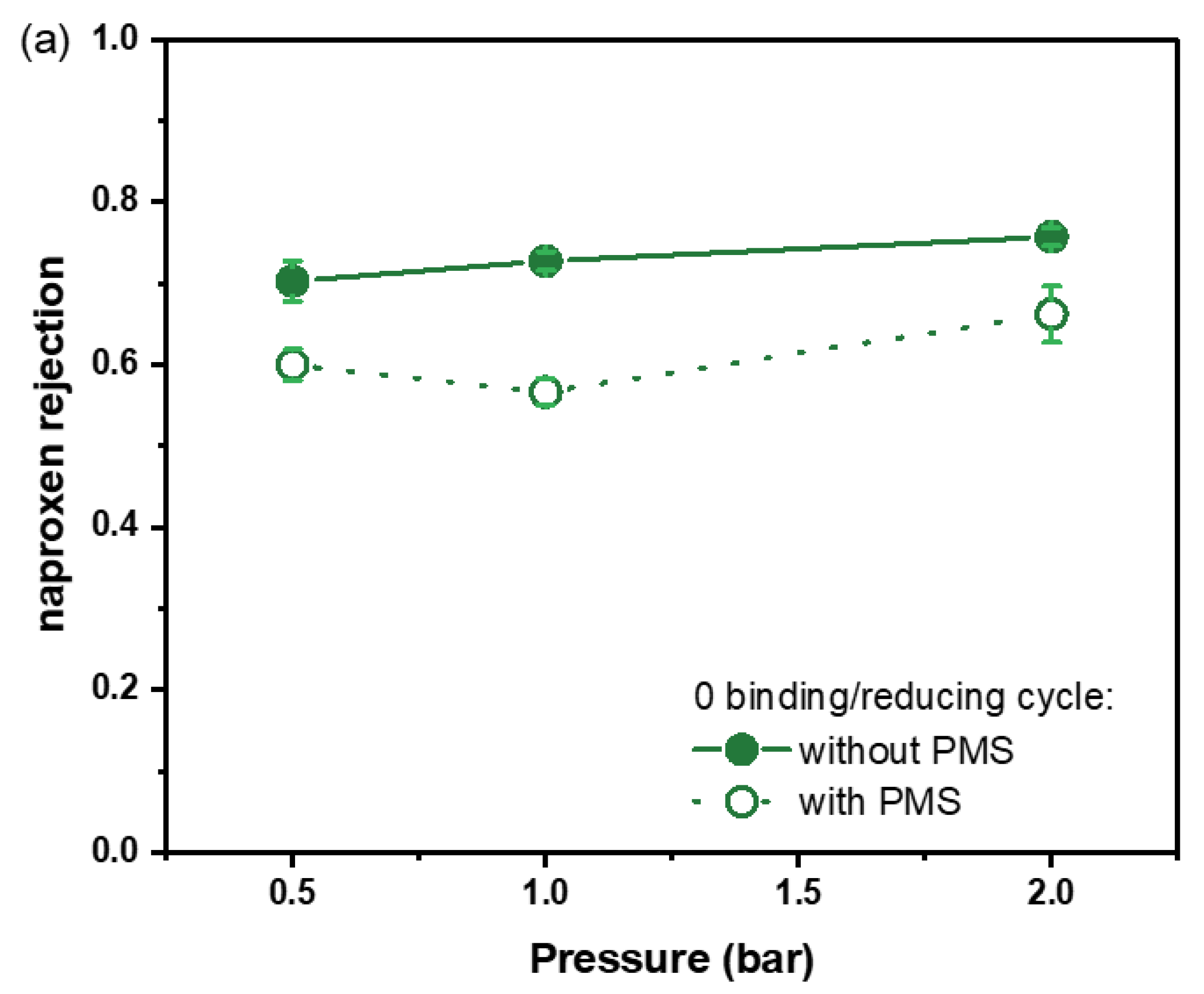
3.1. The Effects of Fe0 Synthesized on Symmetric Multilayers
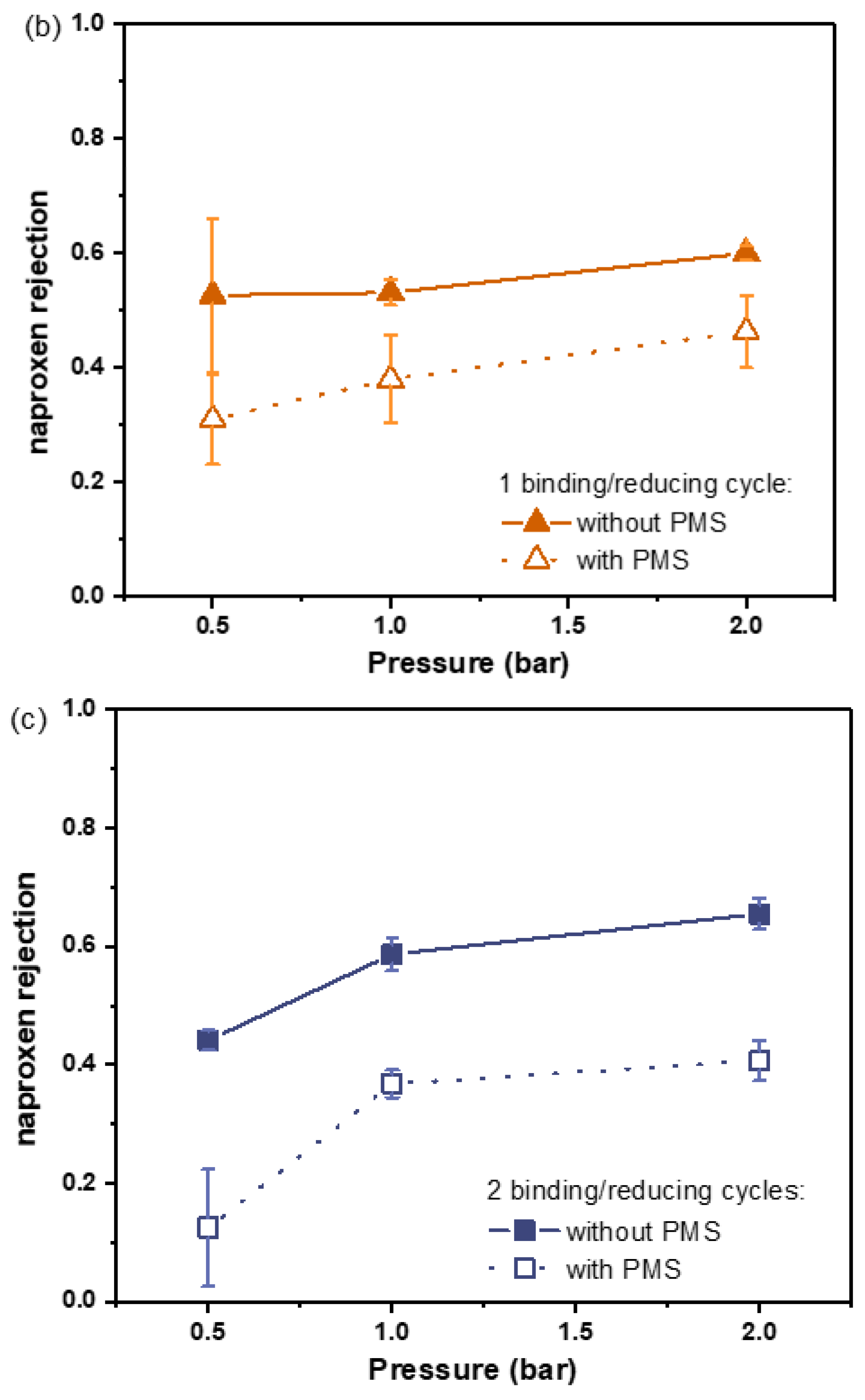
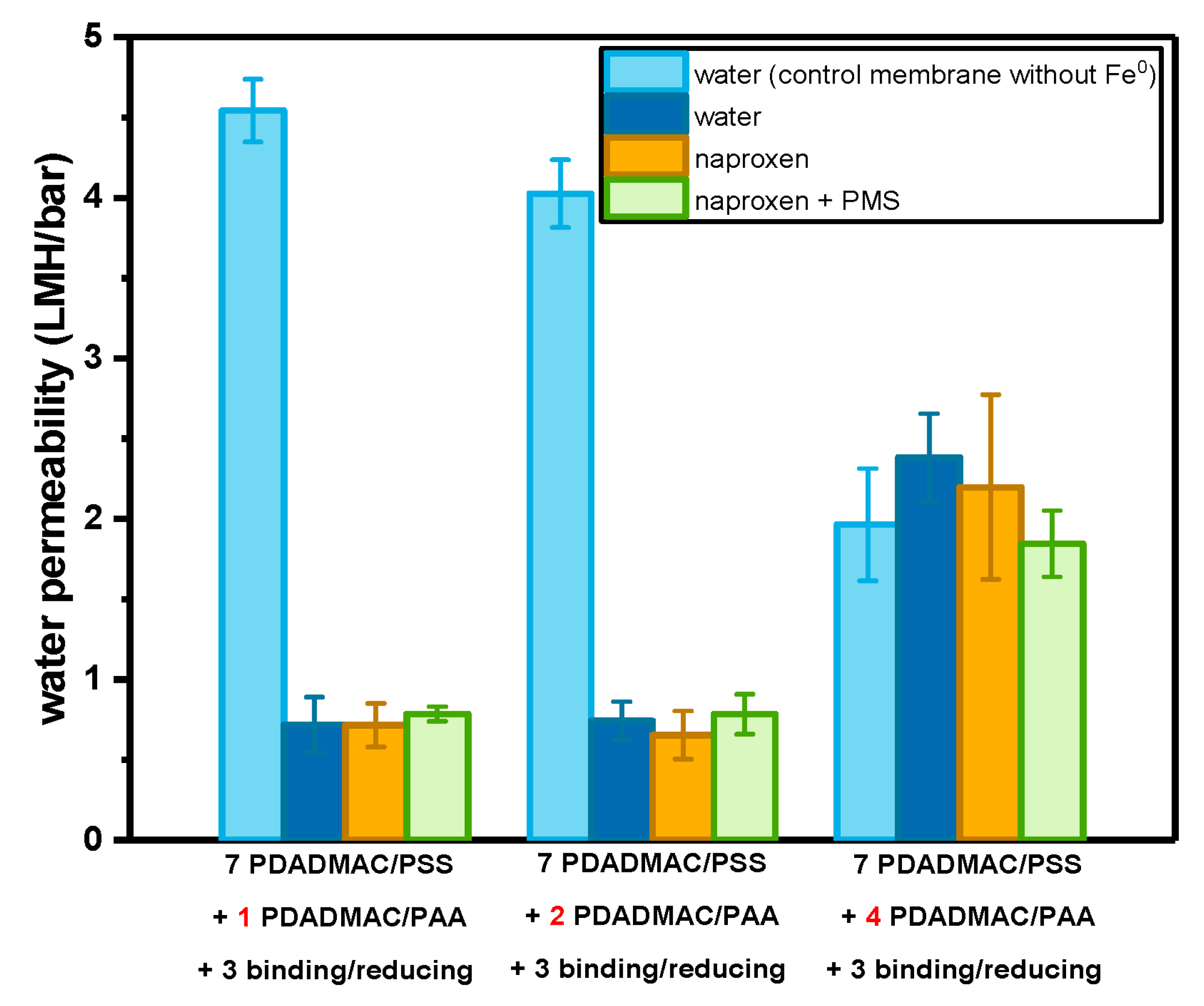
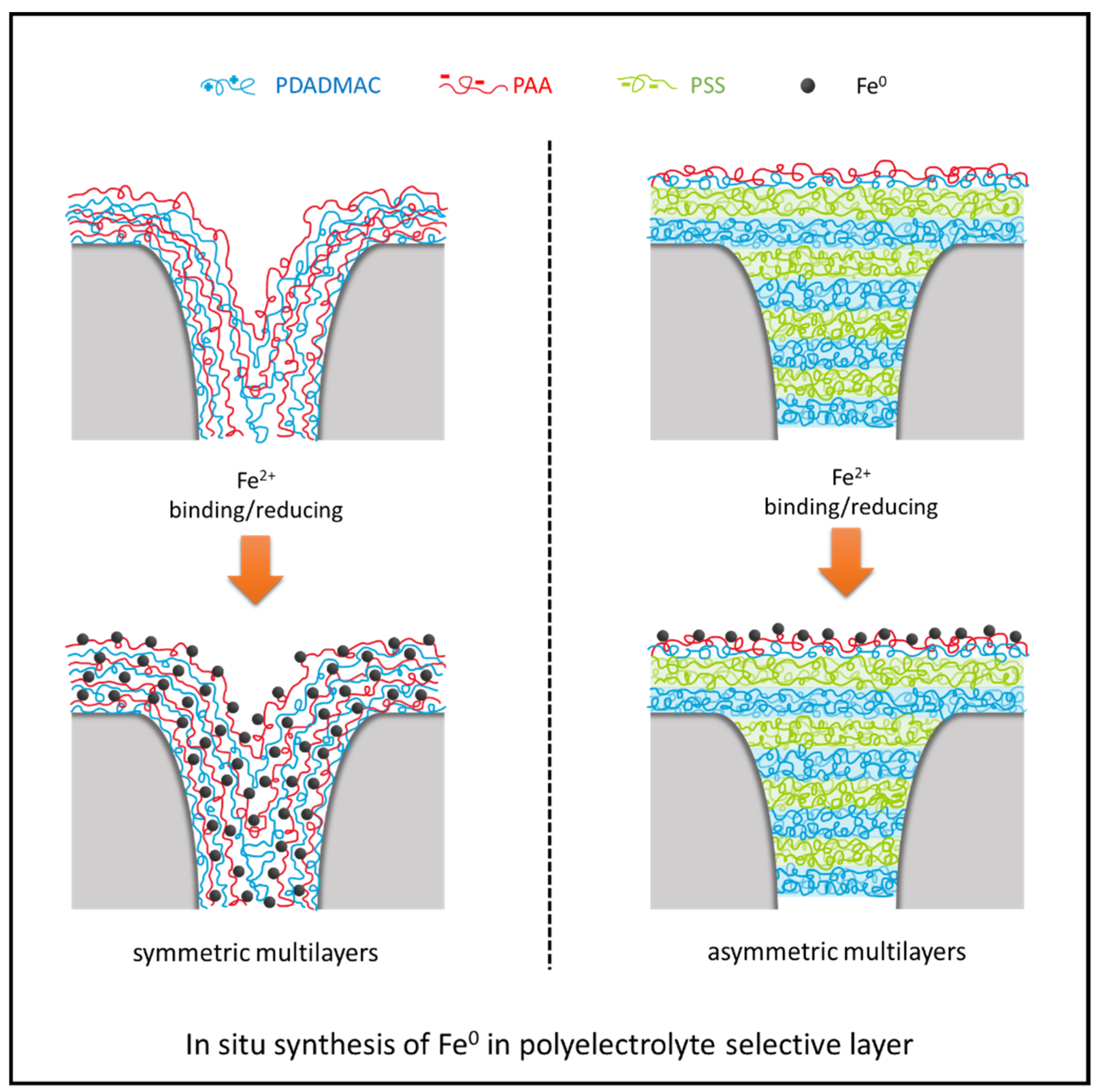
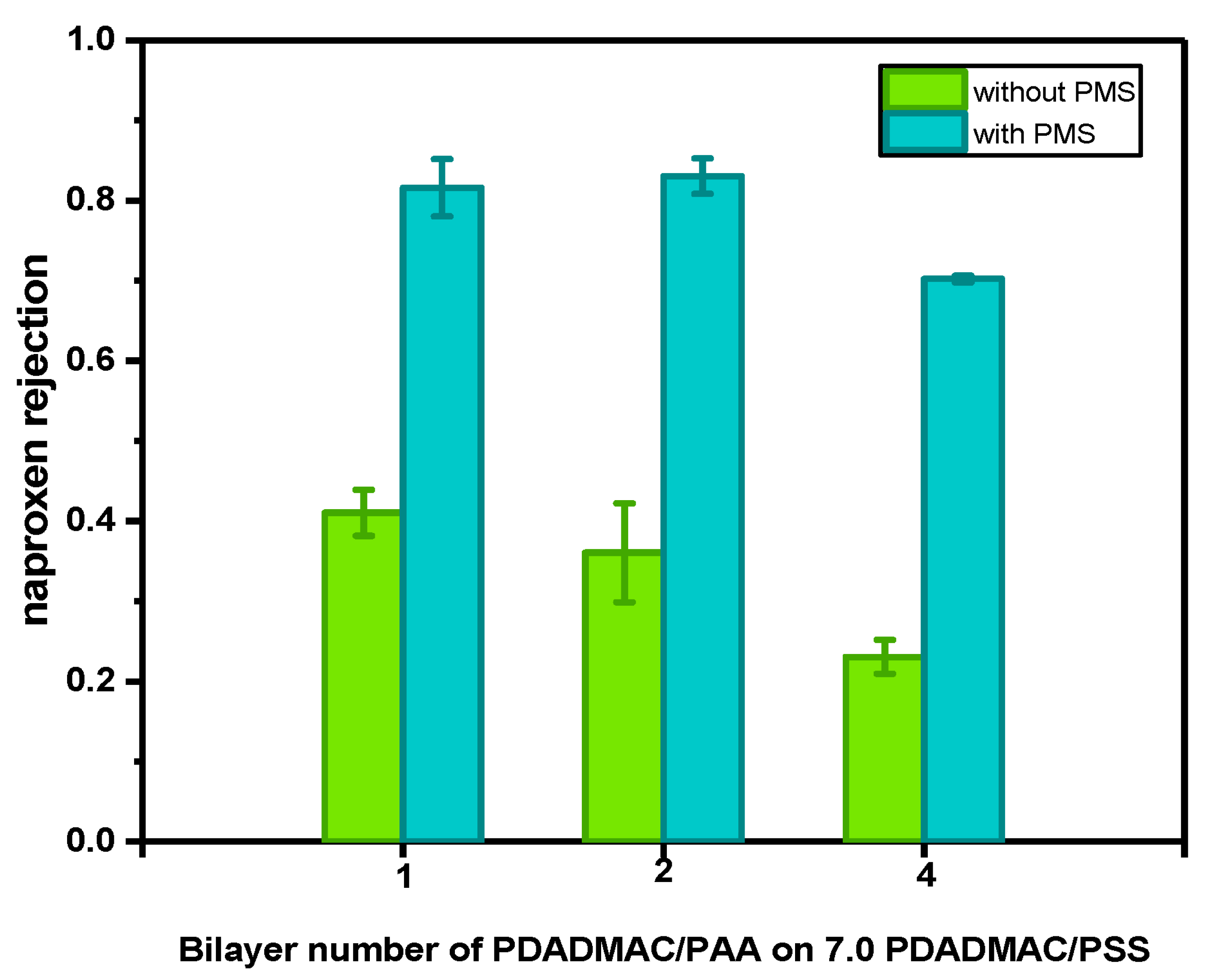
3.2. Asymmetric Selective Layer with Fe0 Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.K.; Zoh, K.D. Occurrence and Removals of Micropollutants in Water Environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef]
- Margot, J.; Rossi, L.; Barry, D.A.; Holliger, C. A Review of the Fate of Micropollutants in Wastewater Treatment Plants. WIREs Water 2015, 2, 457–487. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R.S.; Jegatheesan, V. Treatment of Micropollutants in Water and Wastewater; IWA Publishing: London, UK, 2010; Volume 9. [Google Scholar] [CrossRef]
- Schwarzenbach, R.; Anderson, M.G.; Mcdonnell, J.; Ximing, C.; Cline, S.A.; Balance, W.W.; Rockstrom, J.; Daily, G.C.; Ehrlich, P.R.; Reidy, C.a.; et al. The Challenge of Micropollutants. Sci. Technol. 2006, 313, 1072–1077. [Google Scholar]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic Membrane-Based Oxidation-Filtration Systems for Organic Wastewater Purification: A Review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef]
- Mansas, C.; Mendret, J.; Brosillon, S.; Ayral, A. Coupling Catalytic Ozonation and Membrane Separation: A Review. Sep. Purif. Technol. 2020, 236, 116221. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Li, Z.; Yang, D.; Li, C.; Yan, Y.; Dai, J. 2D Confinement Freestanding Graphene Oxide Composite Membranes with Enriched Oxygen Vacancies for Enhanced Organic Contaminants Removal via Peroxymonosulfate Activation. J. Hazard. Mater. 2021, 417, 126028. [Google Scholar] [CrossRef]
- Wu, H.; Xu, X.; Shi, L.; Yin, Y.; Zhang, L.C.; Wu, Z.; Duan, X.; Wang, S.; Sun, H. Manganese Oxide Integrated Catalytic Ceramic Membrane for Degradation of Organic Pollutants Using Sulfate Radicals. Water Res. 2019, 167, 115110. [Google Scholar] [CrossRef]
- Yao, Y.; Lian, C.; Hu, Y.; Zhang, J.; Gao, M.; Zhang, Y.; Wang, S. Heteroatoms Doped Metal Iron–Polyvinylidene Fluoride (PVDF) Membrane for Enhancing Oxidation of Organic Contaminants. J. Hazard. Mater. 2017, 338, 265–275. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Ojajuni, O.; Saroj, D.; Cavalli, G. Removal of Organic Micropollutants Using Membrane-Assisted Processes: A Review of Recent Progress. Environ. Technol. Rev. 2015, 4, 17–37. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of Endocrine Disrupting Compounds and Pharmaceuticals by Nanofiltration and Ultrafiltration Membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of Sulfate Radical through Heterogeneous Catalysis for Organic Contaminants Removal: Current Development, Challenges and Prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Ozdemir, S.S.; Buonomenna, M.G.; Drioli, E. Catalytic Polymeric Membranes: Preparation and Application. Appl. Catal. A Gen. 2006, 307, 167–183. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic Degradation of Bisphenol-A with Immobilized Laccase on TiO2 Sol-Gel Coated PVDF Membrane. J. Memb. Sci. 2014, 469, 19–30. [Google Scholar] [CrossRef]
- Choo, K.H. Modeling Photocatalytic Membrane Reactors; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128135495. [Google Scholar]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of Organic Micropollutants Using Advanced Membrane-Based Water and Wastewater Treatment: A Review. J. Memb. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Wang, T.; de Vos, W.M.; de Grooth, J. CoFe2O4-Peroxymonosulfate Based Catalytic UF and NF Polymeric Membranes for Naproxen Removal: The Role of Residence Time. J. Memb. Sci. 2021, 646, 120209. [Google Scholar] [CrossRef]
- Kong, W.; Yue, Q.; Gao, Y.; Li, Q.; Xu, X.; Kong, Y.; Gao, B. Enhanced Photodegradation of Sulfadimidine via PAA/g-C3N4-Fe0 Polymeric Catalysts under Visible Light. Chem. Eng. J. 2021, 413, 127456. [Google Scholar] [CrossRef]
- Huang, Q.; Shi, X.; Pinto, R.A.; Petersen, E.J.; Weber, W.J. Tunable Synthesis and Immobilization of Zero-Valent Iron Nanoparticles for Environmental Applications. Environ. Sci. Technol. 2008, 42, 8884–8889. [Google Scholar] [CrossRef]
- Barbosa Ferreira, M.; Souza, F.L.; Muñoz-Morales, M.; Sáez, C.; Cañizares, P.; Martínez-Huitle, C.A.; Rodrigo, M.A. Clopyralid Degradation by AOPs Enhanced with Zero Valent Iron. J. Hazard. Mater. 2020, 392, 122282. [Google Scholar] [CrossRef]
- Cuervo Lumbaque, E.; Lopes Tiburtius, E.R.; Barreto-Rodrigues, M.; Sirtori, C. Current Trends in the Use of Zero-Valent Iron (Fe0) for Degradation of Pharmaceuticals Present in Different Water Matrices. Trends Environ. Anal. Chem. 2019, 24, e00069. [Google Scholar] [CrossRef]
- Smuleac, V.; Bachas, L.; Bhattacharyya, D. Aqueous-Phase Synthesis of PAA in PVDF Membrane Pores for Nanoparticle Synthesis and Dichlorobiphenyl Degradation. J. Memb. Sci. 2010, 346, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.L.S.; Abdelraheem, W.; Nadagouda, M.N.; Rocco, A.M.; Dionysiou, D.D.; Fonseca, F.V.; Borges, C.P. Novel Microwave-Driven Synthesis of Hydrophilic Polyvinylidene Fluoride/Polyacrylic Acid (PVDF/PAA) Membranes and Decoration with Nano Zero-Valent-Iron (NZVI) for Water Treatment Applications. J. Memb. Sci. 2021, 620, 118817. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Z.; Yan, B.; Li, Y.; Li, H.; Liu, D.; Wang, P.; Cui, F.; Shi, W. Interfacial Catalytic Oxidation for Membrane Fouling Mitigation during Algae-Laden Water Filtration: Higher Efficiency without Algae Integrity Loss. Sep. Purif. Technol. 2020, 251, 117366. [Google Scholar] [CrossRef]
- Dai, J.; Bruening, M.L. Catalytic Nanoparticles Formed by Reduction of Metal Ions in Multilayered Polyelectrolyte Films. Nano Lett. 2002, 2, 497–501. [Google Scholar] [CrossRef]
- Te Brinke, E.; Reurink, D.M.; Achterhuis, I.; de Grooth, J.; de Vos, W.M. Asymmetric Polyelectrolyte Multilayer Membranes with Ultrathin Separation Layers for Highly Efficient Micropollutant Removal. Appl. Mater. Today 2020, 18, 100471. [Google Scholar] [CrossRef]
- te Brinke, E.; Achterhuis, I.; Reurink, D.M.; de Grooth, J.; de Vos, W.M. Multiple Approaches to the Buildup of Asymmetric Polyelectrolyte Multilayer Membranes for Efficient Water Purification. ACS Appl. Polym. Mater. 2020, 2, 715–724. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate Activation on Crystallographic Manganese Oxides: Mechanism of Singlet Oxygen Evolution for Nonradical Selective Degradation of Aqueous Contaminants. Environ. Sci. Technol. 2019, 53, 307–315. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.Y.; Li, J.; Lu, X.T.; Yuan, L.P. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015, 49, 12941–12950. [Google Scholar] [CrossRef]
- Kimura, K.; Amy, G.; Drewes, J.E.; Heberer, T.; Kim, T.U.; Watanabe, Y. Rejection of Organic Micropollutants (Disinfection by-Products, Endocrine Disrupting Compounds, and Pharmaceutically Active Compounds) by NF/RO Membranes. J. Memb. Sci. 2003, 227, 113–121. [Google Scholar] [CrossRef]
- Chandia, N.P.; Canales, J.C.; Azocar, I.; Pawar, V.G.; De Borggraeve, W.M.; Dehaen, W. Reductions of Organic Functional Groups Using NaBH4 OR NaBH4/LiCl in Diglyme at 125 to 162 °C. Synth. Commun. An Int. J. Rapid Commun. Synth. Org. Chem. 2009, 139, 927–939. [Google Scholar]
- Cheng, W.; Liu, C.; Tong, T.; Epsztein, R.; Sun, M.; Verduzco, R.; Ma, J.; Elimelech, M. Selective Removal of Divalent Cations by Polyelectrolyte Multilayer Nanofiltration Membrane: Role of Polyelectrolyte Charge, Ion Size, and Ionic Strength. J. Memb. Sci. 2018, 559, 98–106. [Google Scholar] [CrossRef]
- Alonso, T.; Irigoyen, J.; Iturri, J.J.; Larena, I.L.; Moya, S.E. Study of the Multilayer Assembly and Complex Formation of Poly(Diallyldimethylammonium Chloride) (PDADMAC) and Poly(Acrylic Acid) (PAA) as a Function of PH. Soft Matter 2013, 9, 1920–1928. [Google Scholar] [CrossRef]
- Elshof, M.G.; de Vos, W.M.; de Grooth, J.; Benes, N.E. On the Long-Term PH Stability of Polyelectrolyte Multilayer Nanofiltration Membranes. J. Memb. Sci. 2020, 615, 118532. [Google Scholar] [CrossRef]
- Cuhorka, J.; Wallace, E.; Mikulášek, P. Removal of Micropollutants from Water by Commercially Available Nanofiltration Membranes. Sci. Total Environ. 2020, 720, 137474. [Google Scholar] [CrossRef]
- De Grooth, J. A Tale of Two Charges: Zwitterionic Polyelectrolyte Multilayer Membranes. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2015. [Google Scholar]
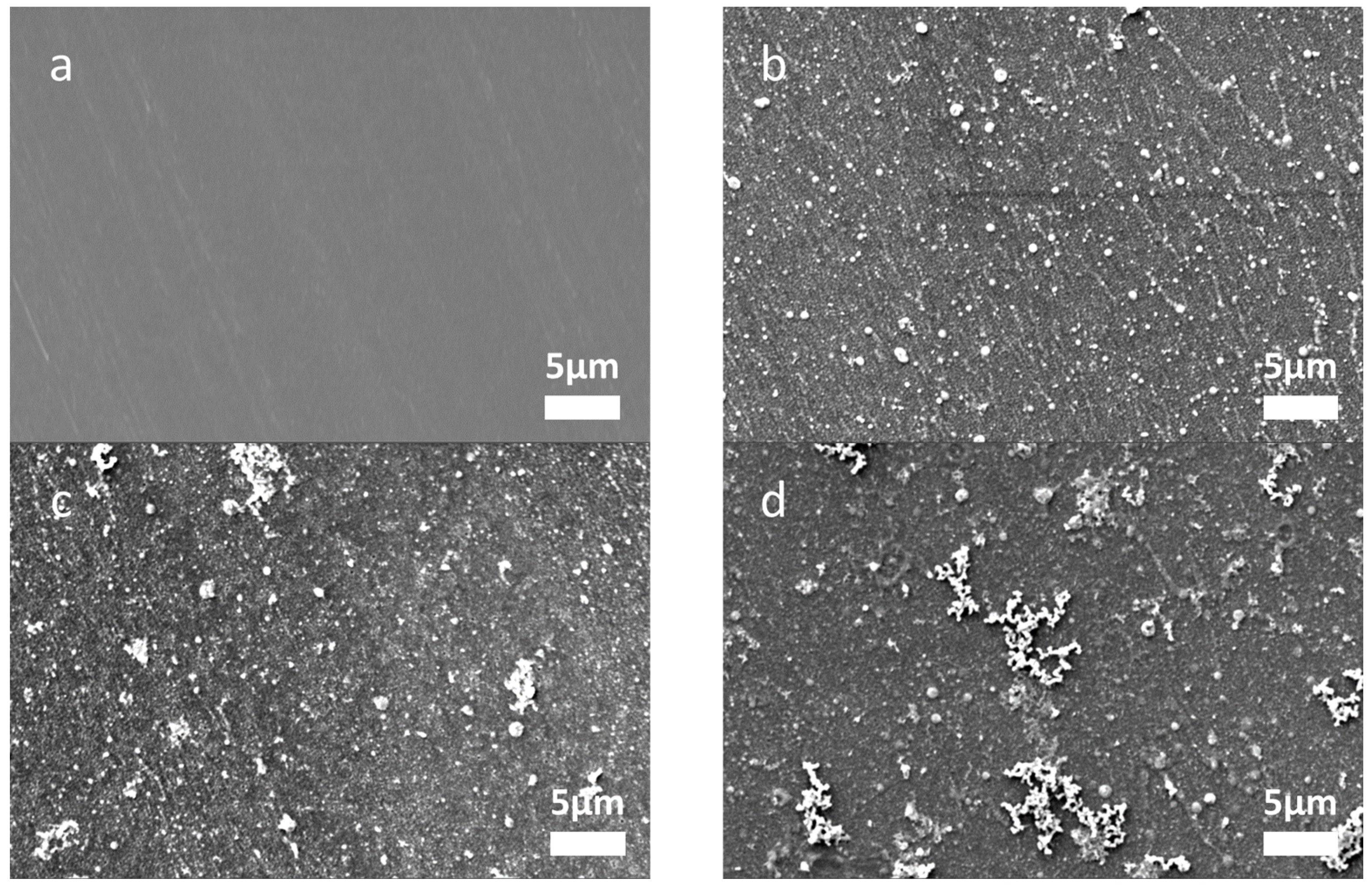

| Atom % | Mass % | |||||
|---|---|---|---|---|---|---|
| Element | C | O | Fe | C | O | Fe |
| 0 cycle | 72.0 | 27.7 | 0.3 | 65.2 | 33.3 | 1.5 |
| 1 cycle | 62.9 | 15.0 | 22.1 | 33.9 | 10.8 | 55.3 |
| 2 cycles | 54.4 | 12.4 | 33.2 | 24.1 | 7.3 | 68.6 |
| 3 cycles | 47.3 | 10.1 | 42.6 | 18.3 | 5.2 | 76.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Bachs, E.S.; de Grooth, J.; de Vos, W.M. Advanced Oxidation Processes Coupled to Nanofiltration Membranes with Catalytic Fe0 Nanoparticles in Symmetric and Asymmetric Polyelectrolyte Multilayers. Membranes 2023, 13, 388. https://doi.org/10.3390/membranes13040388
Wang T, Bachs ES, de Grooth J, de Vos WM. Advanced Oxidation Processes Coupled to Nanofiltration Membranes with Catalytic Fe0 Nanoparticles in Symmetric and Asymmetric Polyelectrolyte Multilayers. Membranes. 2023; 13(4):388. https://doi.org/10.3390/membranes13040388
Chicago/Turabian StyleWang, Tao, Enrique Serra Bachs, Joris de Grooth, and Wiebe M. de Vos. 2023. "Advanced Oxidation Processes Coupled to Nanofiltration Membranes with Catalytic Fe0 Nanoparticles in Symmetric and Asymmetric Polyelectrolyte Multilayers" Membranes 13, no. 4: 388. https://doi.org/10.3390/membranes13040388
APA StyleWang, T., Bachs, E. S., de Grooth, J., & de Vos, W. M. (2023). Advanced Oxidation Processes Coupled to Nanofiltration Membranes with Catalytic Fe0 Nanoparticles in Symmetric and Asymmetric Polyelectrolyte Multilayers. Membranes, 13(4), 388. https://doi.org/10.3390/membranes13040388







