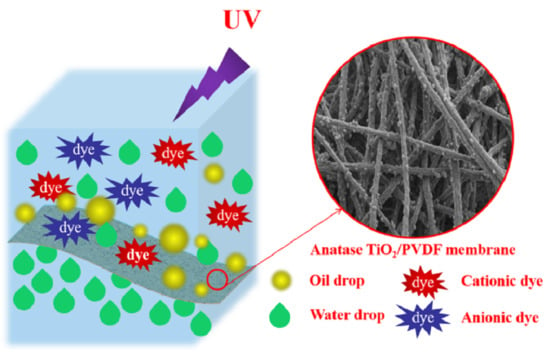
Fabrication of Anatase TiO2/PVDF Composite Membrane for Oil-in-Water Emulsion Separation and Dye Photocatalytic Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Carboxy-Rich PVDF Composite Membrane
2.3. Preparation of Bifunctional Composite Membrane Loading Anatase TiO2 with Ti3+ and Ov
2.4. Emulsion Separation
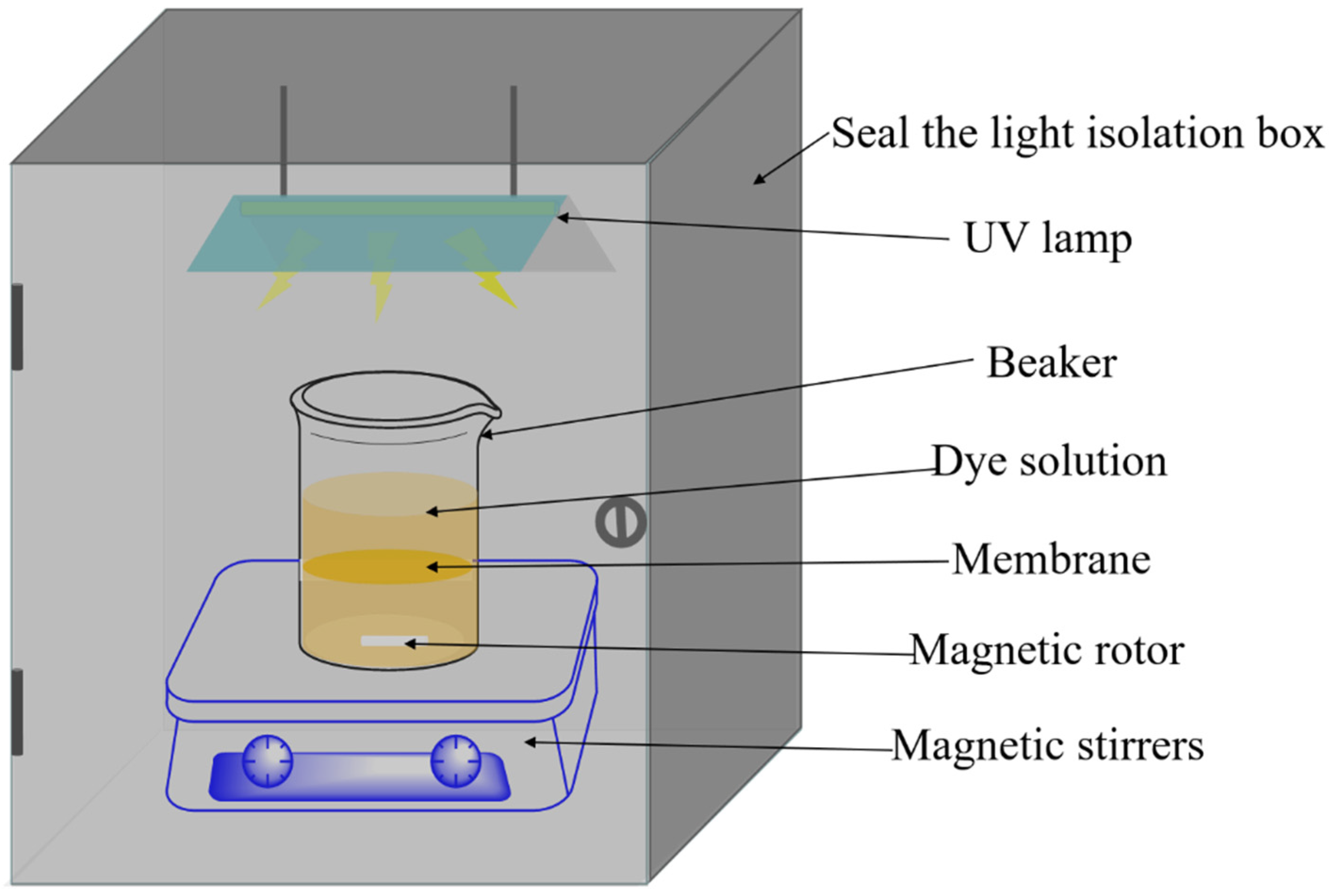
2.5. Photocatalytic Degradation Performance of Membranes
2.6. Characterization
3. Results and Discussion
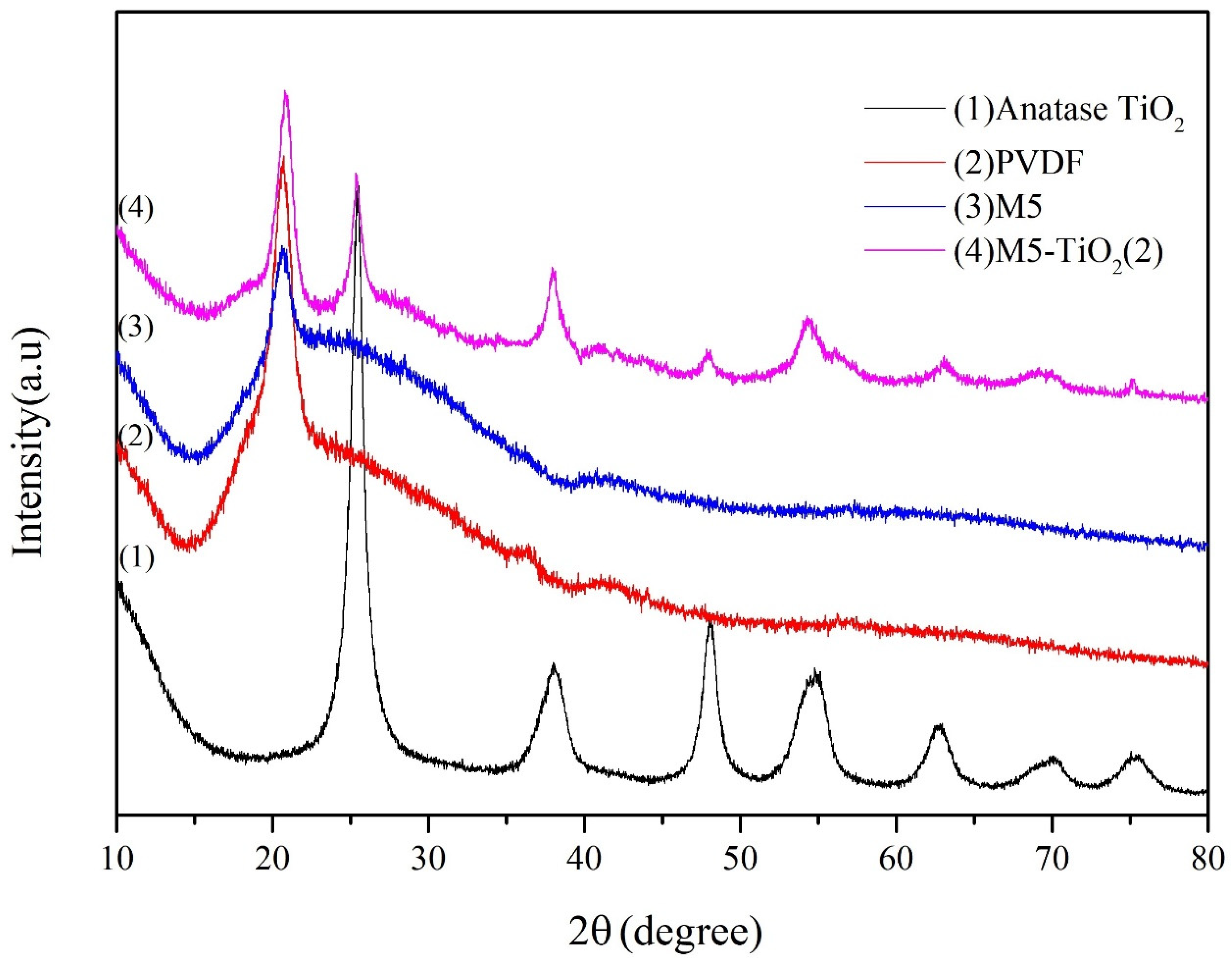
3.1. XRD Analysis
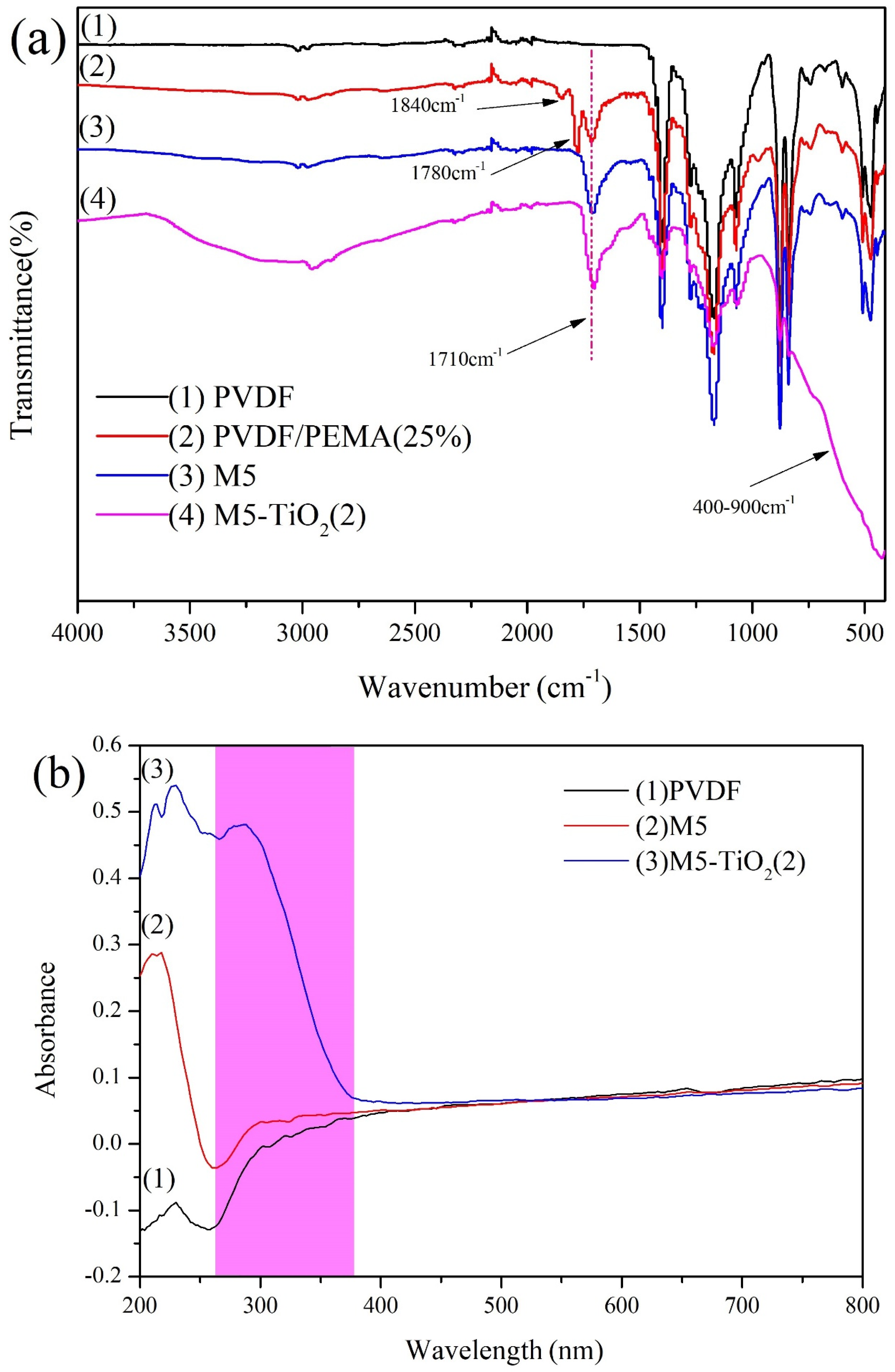
3.2. The Compositions of Membranes
3.3. Membrane Morphologies
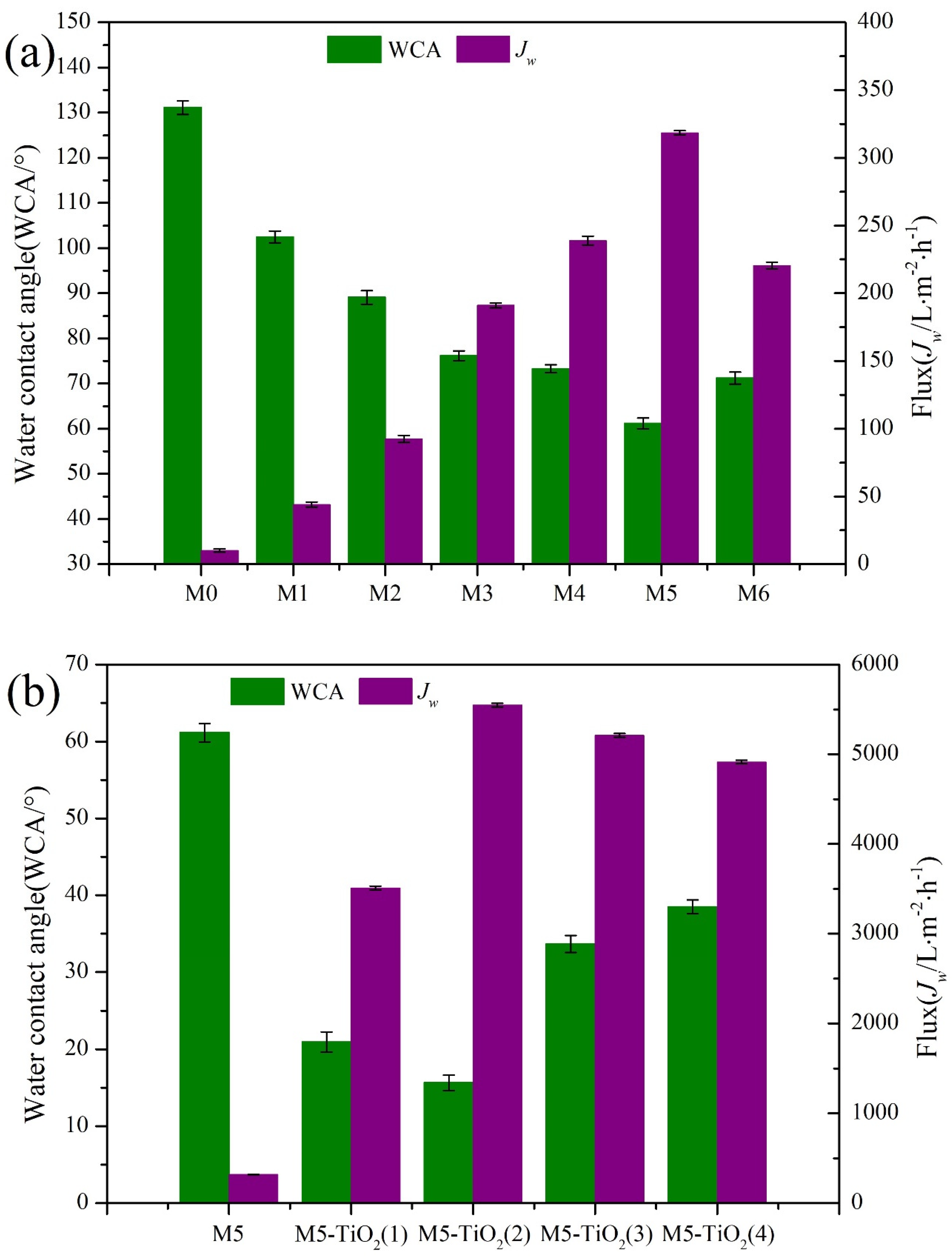
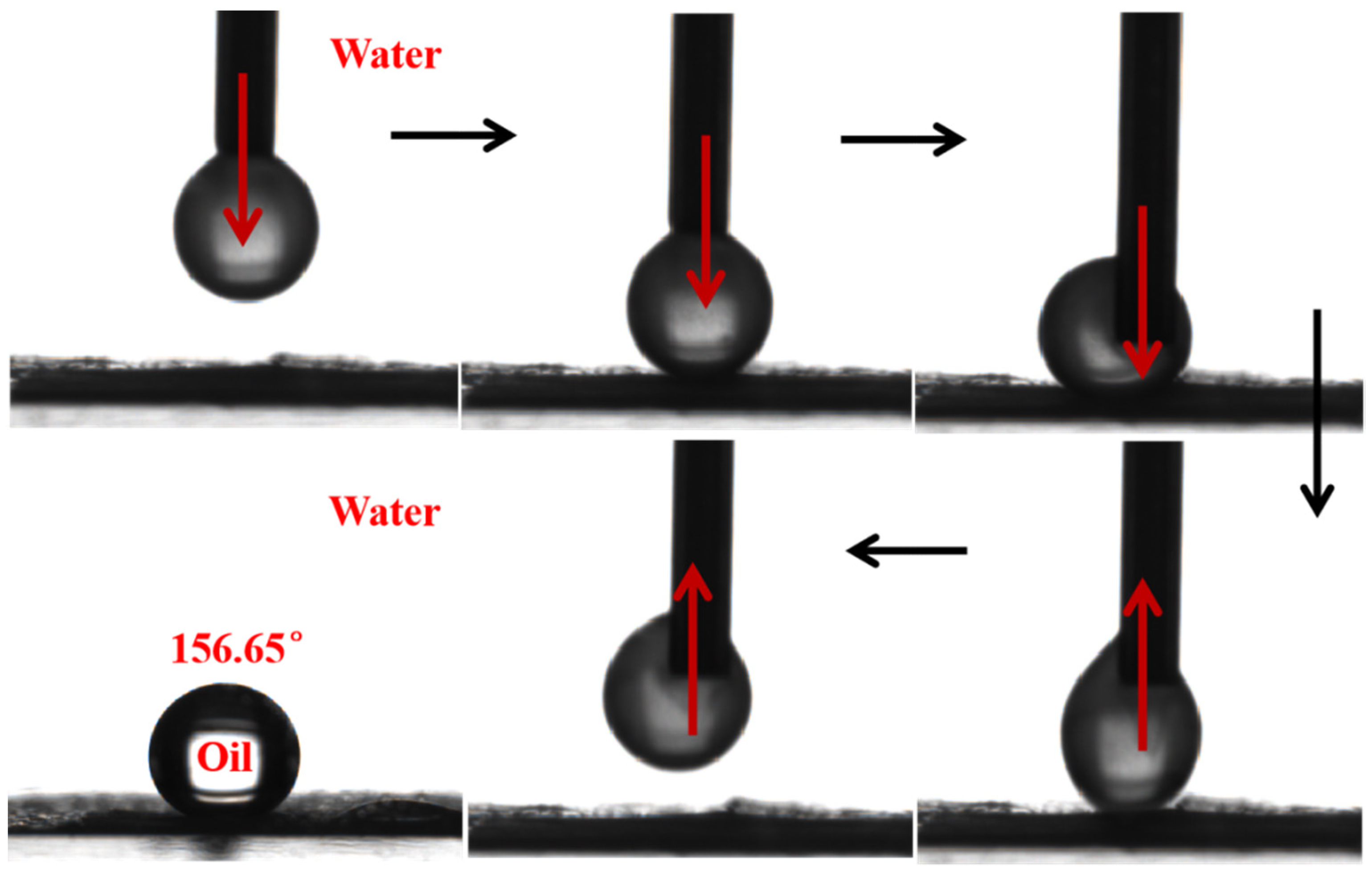
3.4. Hydrophilicity of Membranes
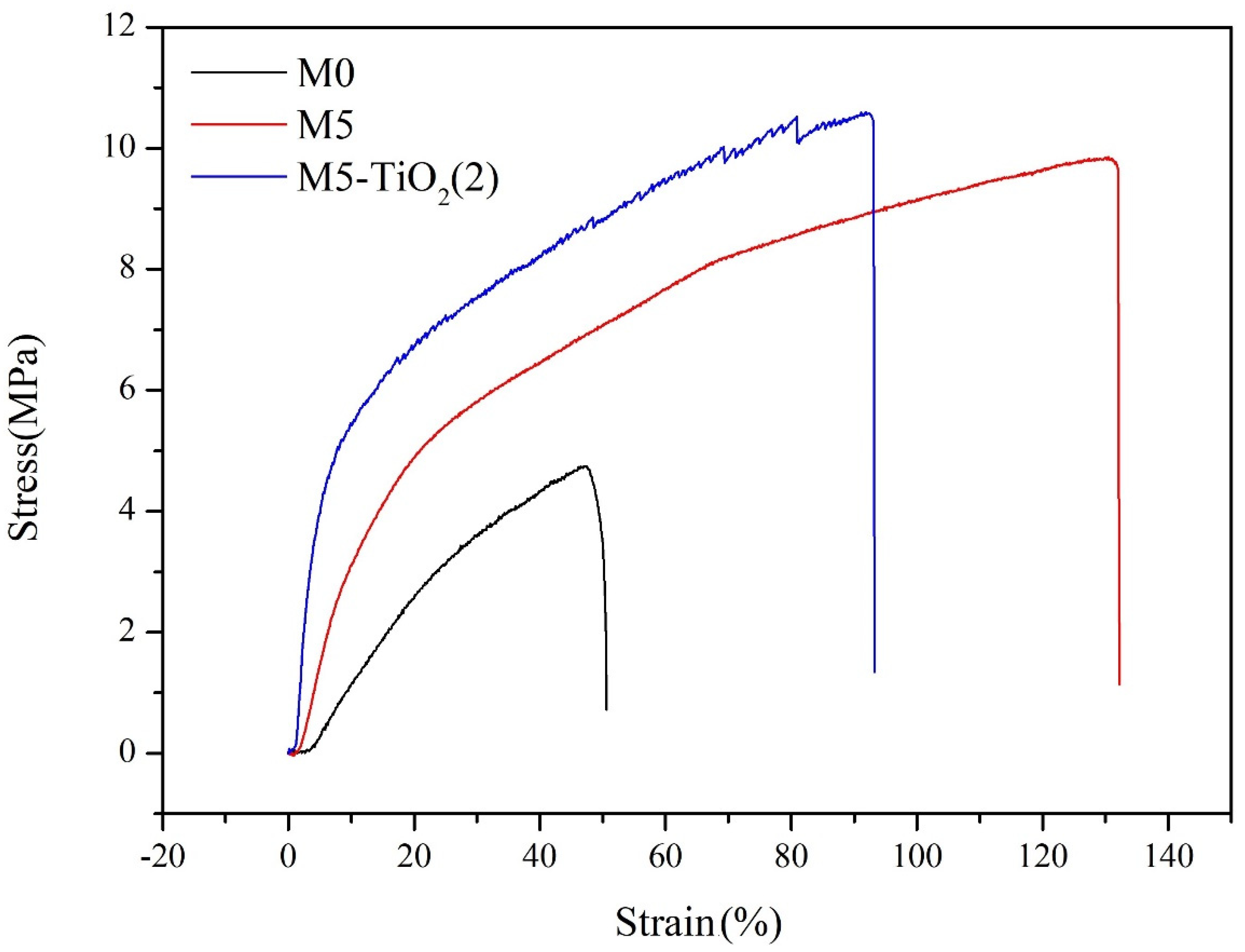
3.5. Mechanical Properties of Membrane
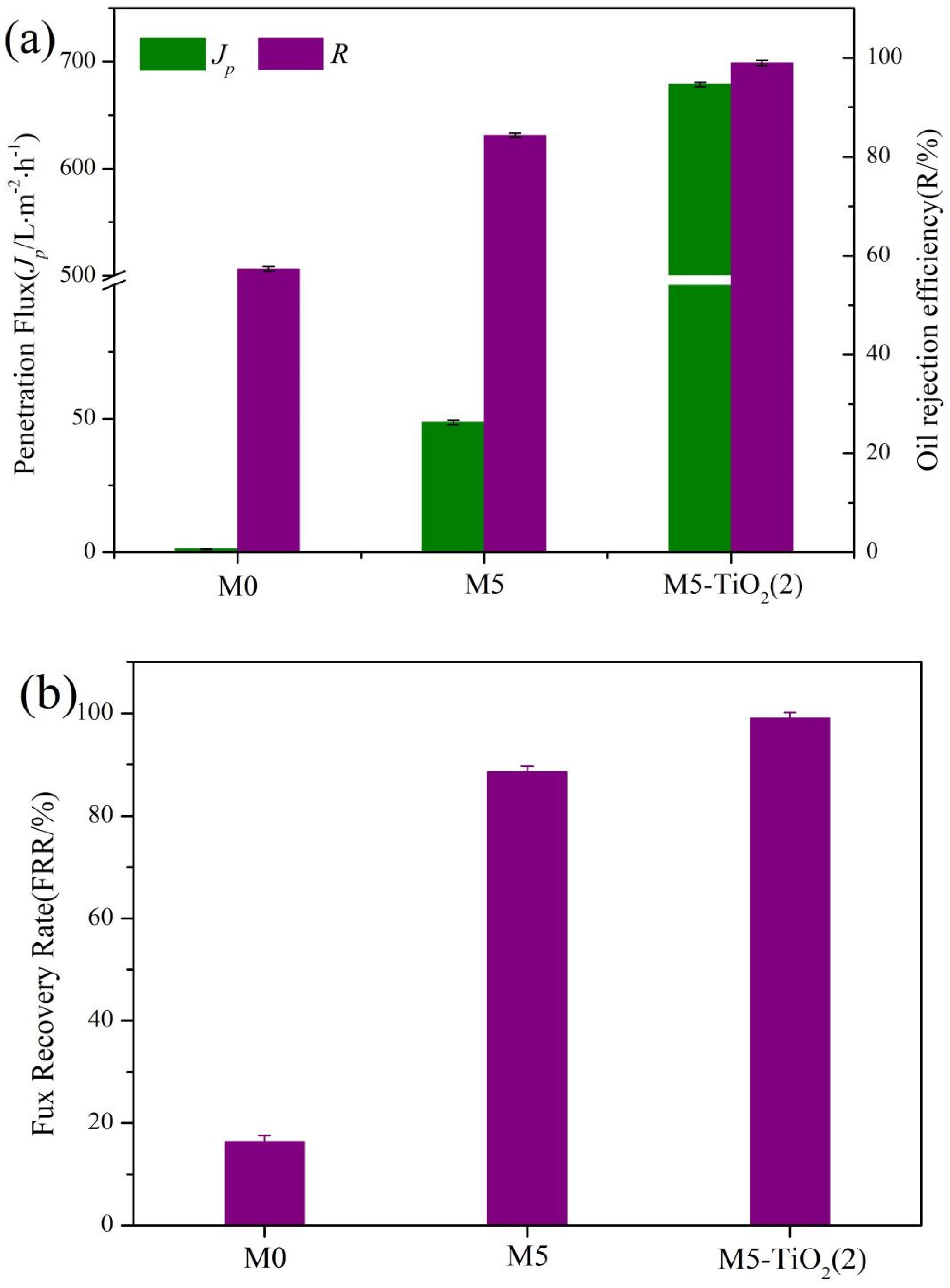
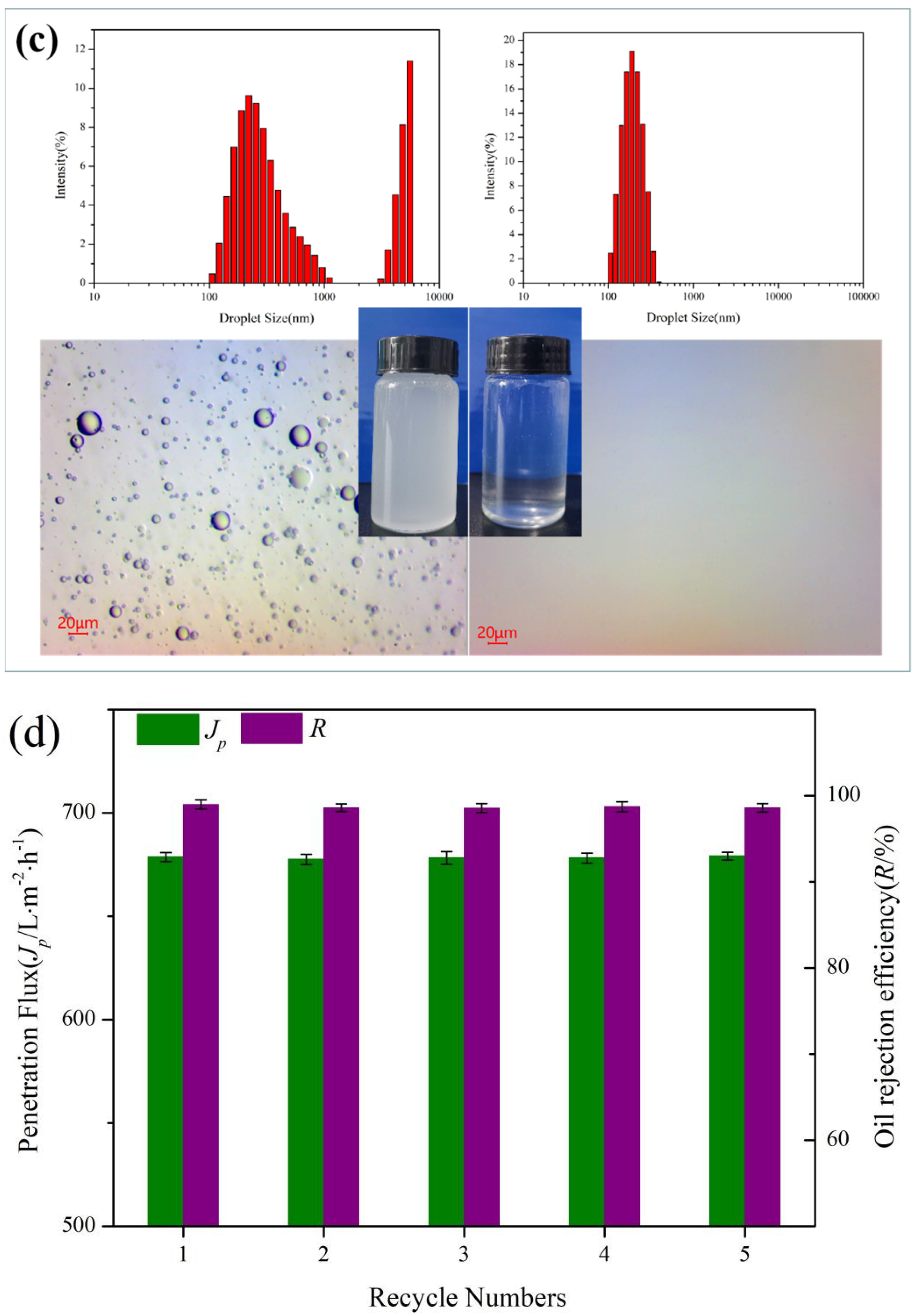
3.6. Separation Performance of the Membrane
3.7. Separation Mechanism of Oil–Water Emulsion
3.8. Photocatalytic Degradation of Organic Dyes
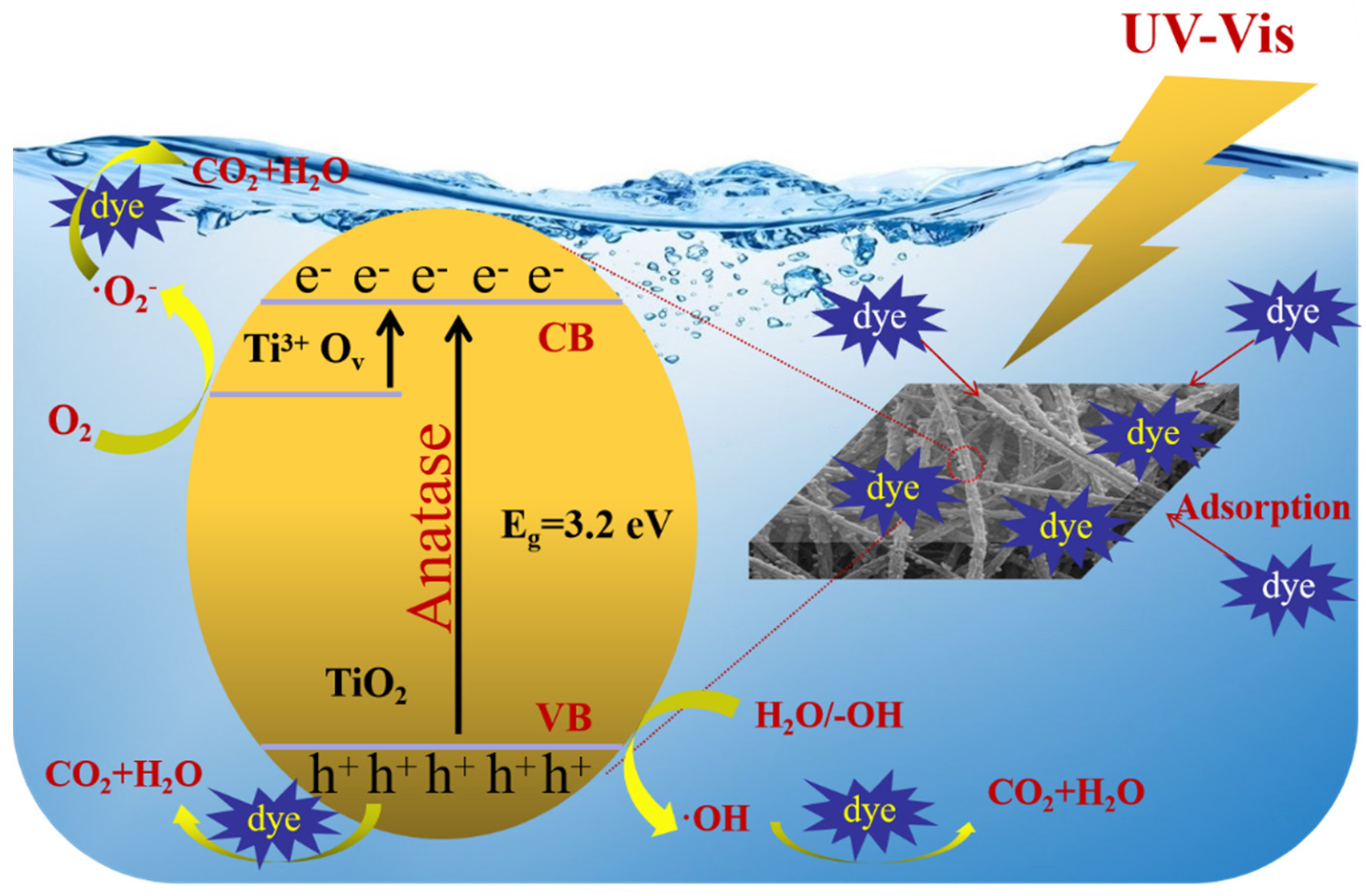
3.9. Mechanism of Photocatalysis of Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pourmortazavi, S.M.; Sahebi, H.; Zandavar, H.; Mirsadeghi, S. Fabrication of Fe3O4 nanoparticles coated by extracted shrimp peels chitosan as sustainable adsorbents for removal of chromium contaminates from wastewater: The design of experiment. Compos. Part B Eng. 2019, 175, 107130. [Google Scholar] [CrossRef]
- Wei, F.; Li, J.; Dong, C.; Bi, Y.; Han, X. Plasmonic Ag decorated graphitic carbon nitride sheets with enhanced visible-light response for photocatalytic water disinfection and organic pollutant removal. Chemosphere 2020, 242, 125201. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Z.; Wang, F.; Zhu, H.; Guo, Y.; Chen, M. Laccase-catalyzed homo-polymer of GAL and cross-linking with PEI to enhance hydrophilicity and antifouling property of PTFE flat membrane. Prog. Org. Coat. 2019, 132, 429–439. [Google Scholar] [CrossRef]
- Bhoi, Y.; Fang, F.; Zhou, X.; Li, Y.; Sun, X.; Wang, J.; Huang, W. Single step combustion synthesis of novel Fe2TiO5/α-Fe2O3/TiO2 ternary photocatalyst with combined double type-II cascade charge migration processes and efficient photocatalytic activity. Appl. Surf. Sci. 2020, 525, 146571. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Yuan, X.; Ding, W.; Li, Y.; Wang, J. Amino-functionalized mesoporous PVA/SiO2 hybrids coated membrane for simultaneous removal of oils and water-soluble contaminants from emulsion. Chem. Eng. J. 2019, 374, 1394–1402. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Liu, Y.; Xue, C.; Wang, Y.; Dai, J. Preparation of Janus membrane based on biomimetic polydopamine interface regulation and superhydrophobic attapulgite spraying for on-demand oil-water emulsion separation. J. Membr. Sci. 2021, 627, 119242. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Si, Y.; Yu, J.; Ding, B. Electrospun Nanofibrous Membranes: An Effective Arsenal for the Purification of Emulsified Oily Wastewater. Adv. Funct. Mater. 2020, 30, 2002192. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Xu, B.-Q. Preparation and characterization of nanosized anatase TiO2 cuboids for photocatalysis. Appl. Catal. B Environ. 2005, 59, 139–146. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Samoila, P.; Olaru, N.; Bele, A.; Airinei, A. Novel electrospun membranes based on PVDF fibers embedding lanthanide doped ZnO for adsorption and photocatalytic degradation of dye organic pollutants. Mater. Res. Bull. 2021, 141, 111376. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Luo, Y.; Wang, F.; Zhu, H.; Guo, Y. One-bath two step method combined surface micro/nanostructures treatment to enhance antifouling and antibacterial property of PTFE flat membrane. J. Taiwan Inst. Chem. Eng. 2019, 96, 639–651. [Google Scholar] [CrossRef]
- Xue, S.; Li, C.; Li, J.; Zhu, H.; Guo, Y. A catechol-based biomimetic strategy combined with surface mineralization to enhance hydrophilicity and anti-fouling property of PTFE flat membrane. J. Membr. Sci. 2017, 524, 409–418. [Google Scholar] [CrossRef]
- Gao, J.; Chen, L.; Yan, Y.; Lu, J.; Xing, W.; Dai, J.; Meng, M.; Wu, Y. Dotmatrix-initiated molecularly imprinted nanocomposite membranes for selective recognition: A high-efficiency separation system with an anti-oil fouling layer. Environ. Sci.-Nano. 2021, 8, 2932–2949. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Zhou, X.; He, X.; Zhou, M.; Jia, K.; Liu, X. Design of polymer composite-based porous membrane for in-situ photocatalytic degradation of adsorbed organic dyes. J. Phys. Chem. Solids 2021, 154, 110094. [Google Scholar] [CrossRef]
- Tang, T.; Li, C.; He, W.; Hong, W.; Zhu, H.; Liu, G.; Yu, Y.; Lei, C. Preparation of MOF-derived C-ZnO/PVDF composites membrane for the degradation of methylene blue under UV-light irradiation. J. Alloys Compo. 2022, 894, 162559. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, W.; Li, X.; Qu, R.; Zhang, Q.; Wei, Y.; Feng, L.; Jiang, L. Fabrication of robust mesh with anchored Ag nanoparticles for oil removal and in situ catalytic reduction of aromatic dyes. J. Mater. Chem. A 2017, 5, 15822–15827. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Mil-53(Fe)-loaded polyacrylonitrile membrane with superamphiphilicity and double hydrophobicity for effective emulsion separation and photocatalytic dye degradation. Sep. Purif. Technol. 2022, 282, 119910. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Photo-Fenton self-cleaning PVDF/NH2-MIL-88B(Fe) membranes towards highly-efficient oil/water emulsion separation. J. Membr. Sci. 2020, 595, 117499. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Buso, D.; Post, M.; Cantalini, C.; Mulvaney, P.; Martucci, A. Gold Nanoparticle-Doped TiO2 Semiconductor Thin Films: Gas Sensing Properties. Adv. Funct. Mater. 2008, 18, 3843–3849. [Google Scholar] [CrossRef]
- Zhu, T.; Gao, S. The stability, electronic structure, and optical property of TiO2 polymorphs. J. Phys. Chem. C 2014, 118, 11385–11396. [Google Scholar] [CrossRef]
- Pan, L.; Huang, H.; Lim, C.K.; Hong, Q.Y.; Tse, M.S.; Tan, O.K. TiO2 rutile–anatase core–shell nanorod and nanotube arrays for photocatalytic applications. RSC Adv. 2013, 3, 3566–3571. [Google Scholar] [CrossRef]
- Yin, H.; Wada, Y.; Kitamura, T.; Kambe, S.; Murasawa, S.; Mori, H.; Sakata, T.; Yanagida, S. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J. Mater. Chem. 2001, 11, 1694–1703. [Google Scholar] [CrossRef]
- Lu, L.; Wang, G.; Xiong, Z.; Hu, Z.; Liao, Y.; Wang, J.; Li, J. Enhanced photocatalytic activity under visible light by the synergistic effects of plasmonics and Ti3+-doping at the Ag/TiO2-x heterojunction. Ceram. Int. 2020, 46, 10667–10677. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, K.; Qin, X.; Chen, S.; Yu, H.; Quan, X. Treatment of organic wastewater by a synergic electrocatalysis process with Ti3+ self-doped TiO2 nanotube arrays electrode as both cathode and anode. J. Hazard. Mater. 2022, 424, 127747. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhou, Y.; Dong, C.; Xing, M.; Zhang, J. Enhanced photocatalytic activities of vacuum activated TiO2 catalysts with Ti3+ and N co-doped. Catal. Today 2016, 266, 188–196. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, G.; Lu, X.; Tang, C.; Cao, S.; Yu, M. Anatase TiO2 sheet-assisted synthesis of Ti3+ self-doped mixed phase TiO2 sheet with superior visible-light photocatalytic performance: Roles of anatase TiO2 sheet. J. Colloid Interf. Sci. 2017, 490, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Wu, J.; Jia, T.; Peng, C.; Xiao, Y.; Liu, Z.; Liu, Q.; Fan, Y.; Han, J.; Hao, L. In-situ growth of TiO2 phase junction nanorods with Ti3+ and oxygen vacancies to enhance photocatalytic activity. Mater. Res. Bull. 2021, 140, 111291. [Google Scholar] [CrossRef]
- Qi, D.; Lu, L.; Xi, Z.; Wang, L.; Zhang, J. Enhanced photocatalytic performance of TiO2 based on synergistic effect of Ti3+ self-doping and slow light effect. Appl. Catal. B Environ. 2014, 160–161, 621–628. [Google Scholar] [CrossRef]
- Yin, J.; Roso, M.; Boaretti, C.; Lorenzetti, A.; Martucci, A.; Modesti, M. PVDF-TiO2 core-shell fibrous membranes by microwave-hydrothermal method: Preparation, characterization, and photocatalytic activity. J. Environ. Chem. Eng. 2021, 9, 106250. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Luo, J.; Wang, F.; Liu, G.; Zhu, H.; Guo, Y. PVDF grafted Gallic acid to enhance the hydrophilicity and antibacterial properties of PVDF composite membrane. Sep. Purif. Technol. 2021, 259, 118127. [Google Scholar] [CrossRef]
- Cao, J.; Su, Y.; Liu, Y.; Guan, J.; He, M.; Zhang, R.; Jiang, Z. Self-assembled MOF membranes with underwater superoleophobicity for oil/water separation. J. Membr. Sci. 2018, 566, 268–277. [Google Scholar] [CrossRef]
- Nworie, F.; Nwabue, F.; Oti, W.; Mbam, E. Removal of methylene blue from aqueous solution using activated rice husk biochar: Adsorption isotherms, kinetics and error analysis. J. Chil. Chem. Soc. 2019, 64, 4365–4376. [Google Scholar] [CrossRef]
- Liu, G.; Pan, X.; Li, J.; Li, C.; Ji, C. Facile preparation and characterization of anatase TiO2/nanocellulose composite for photocatalytic degradation of methyl orange. J. Chil. Chem. Soc. 2021, 25, 101383. [Google Scholar] [CrossRef]
- Tichapondwa, S.; Newman, J.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Choksumlitpol, P.; Mangkornkarn, C.; Sumtong, P.; Onlaor, K.; Eiad-Ua, A. Fabrication of Anodic Titanium Oxide (ATO) for waste water treatment application. Mater. Today: Proc. 2017, 4, 6124–6128. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; An, C.; Feng, R. Interactive toxicity of triclosan and nano-TiO2 to green alga eremosphaera viridis in lake erie: A new perspective based on fourier transform infrared spectromicroscopy and synchrotron-based X-ray fluorescence imaging. Environ. Sci. Technol. 2019, 53, 9884–9894. [Google Scholar] [CrossRef]
- Sahu, S.P.; Cates, S.L.; Kim, H.-I.; Kim, J.-H.; Cates, E.L. The Myth of Visible Light Photocatalysis Using Lanthanide Upconversion Materials. Environ. Sci. Technol. 2018, 52, 2973–2980. [Google Scholar] [CrossRef]
- Chen, C.; Bai, H.; Chang, C. Effect of plasma processing gas composition on the nitrogen-doping status and visible light photocatalysis of TiO2. J. Phys. Chem. C 2007, 111, 15228–15235. [Google Scholar] [CrossRef]
- Liao, W.; Yang, J.; Zhou, H.; Murugananthan, M.; Zhang, Y. Electrochemically Self-Doped TiO2 Nanotube Arrays for Efficient Visible Light Photoelectrocatalytic Degradation of Contaminants. Electrochimica Acta 2014, 136, 310–317. [Google Scholar] [CrossRef]
- Liu, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Surface engineering of g-C3N4 by stacked BiOBr sheets rich in oxygen vacancies for boosting photocatalytic performance. Angew. Chem. Int. Ed. Engl. 2020, 59, 4519–4524. [Google Scholar] [CrossRef]
- Seo, J.; Seo, J.-H. Fabrication of an anti-biofouling plasma-filtration membrane by an electrospinning process using photo-cross-linkable zwitterionic phospholipid polymers. ACS Appl. Mater. Inter. 2017, 9, 19591–19600. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Han, N.; Han, C.Y.; Wang, M.D.; Zhang, W.X.; Wang, W.J.; Zhang, Z.X. Design of a janus F-TiO2@PPS porous membrane with asymmetric wettability for switchable oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 22408–22418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, M.; Chen, M.N.; Cui, Y.H.; Chen, L.; Dai, X.H.; Dai, J.D. Fabrication of high flux porphrin-cored with silox-ane-poly(amido amine) dendrimer/PVDF composite membrane for oil/water separation and dye degradation. J. Environ. Chem. Eng 2022, 10, 107634. [Google Scholar] [CrossRef]
- Zhou, C.; Cheng, J.; Hou, K.; Zhu, Z.; Zheng, Y. Preparation of CuWO4@Cu2O film on copper mesh by anodization for oil/water separation and aqueous pollutant degradation. Chem. Eng. J. 2017, 307, 803–811. [Google Scholar] [CrossRef]
- Si, Y.; Dong, Z.; Jiang, L. Bioinspired designs of superhydrophobic and superhydrophilic materials. ACS Central Sci. 2018, 4, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, M.; Heng, L.; Jiang, L. Underwater mechanically robust oil-repellent materials: Combining conflicting properties using a heterostructure. Adv. Mater. 2018, 30, 1706634–1706641. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kim, S.; Kallem, P.; Choi, H. Capillary effect in Janus electrospun nanofiber membrane for oil/water emulsion separation. Chemosphere 2019, 221, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Namazian, M.; Halvani, S. Calculations of pKa values of carboxylic acids in aqueous solution using density functional theory. J. Chem. Thermodyn. 2006, 38, 1495–1502. [Google Scholar] [CrossRef]
- Sadegh, F.; Politakos, N.; Roman, E.G.D.S.; Sanz, O.; Modarresi-Alam, A.R.; Tomovska, R. Toward enhanced catalytic activity of magnetic nanoparticles integrated into 3D reduced graphene oxide for heterogeneous Fenton organic dye degradation. Sci. Rep. 2021, 11, 18343. [Google Scholar] [CrossRef]
- Rayne, S.; Forest, K.; Friesen, K. Extending the semi-empirical PM6 method for carbon oxyacid pKa prediction to sulfonic acids: Application towards congener-specific estimates for the environmentally and toxicologically relevant C1 through C8 perfluoroalkyl derivatives. Nat. Prec. 2009, 2922, 1–13. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Z.; Yuan, Y.; Liu, L.; Ma, S.; Wang, W.; Hu, Y.; Hu, W.; Gui, Z. Nanosized bimetal-organic frameworks as robust coating for multi-functional flexible polyurethane foam: Rapid oil-absorption and excellent fire safety. Compos. Sci. Technol. 2019, 177, 66–72. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]

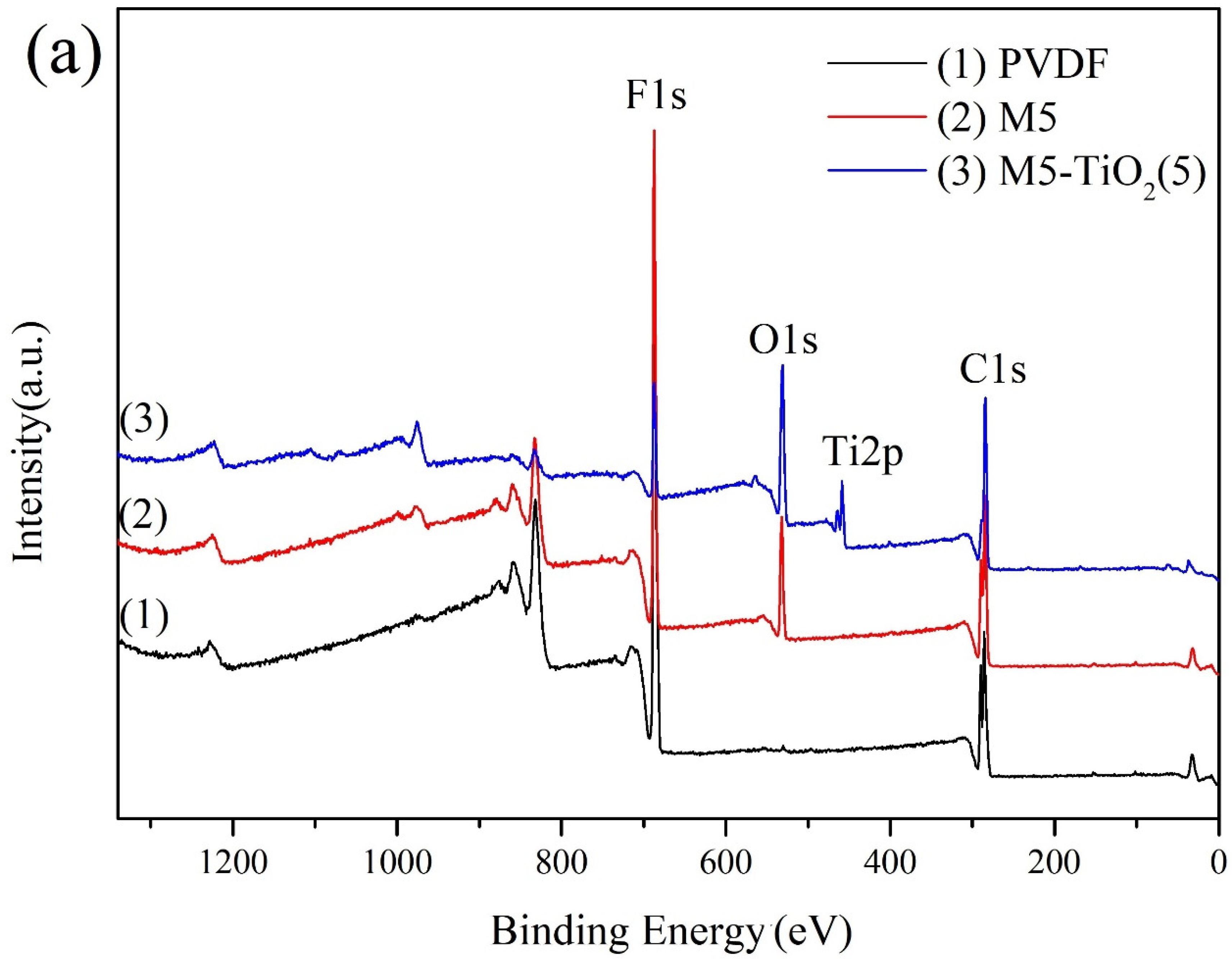
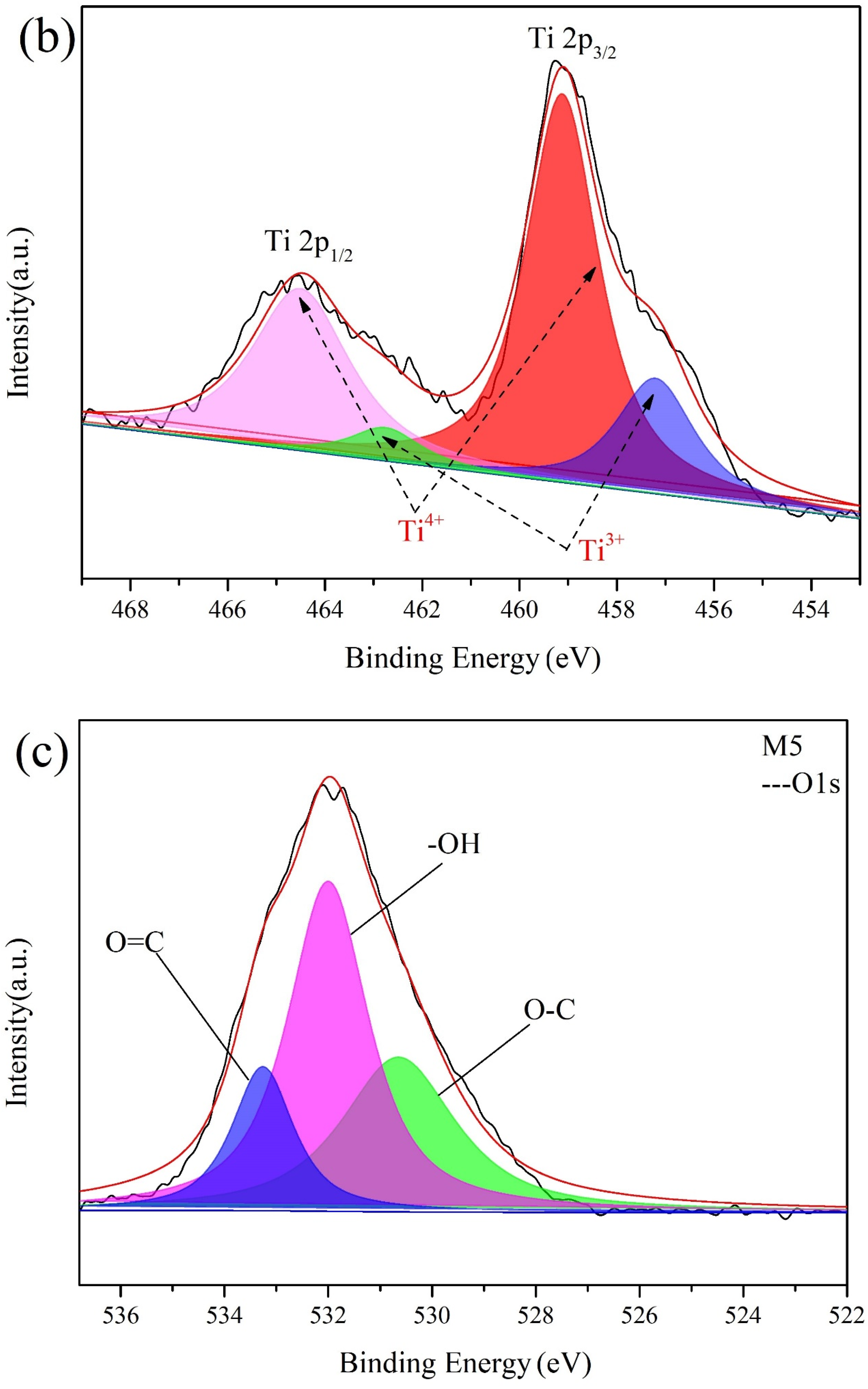


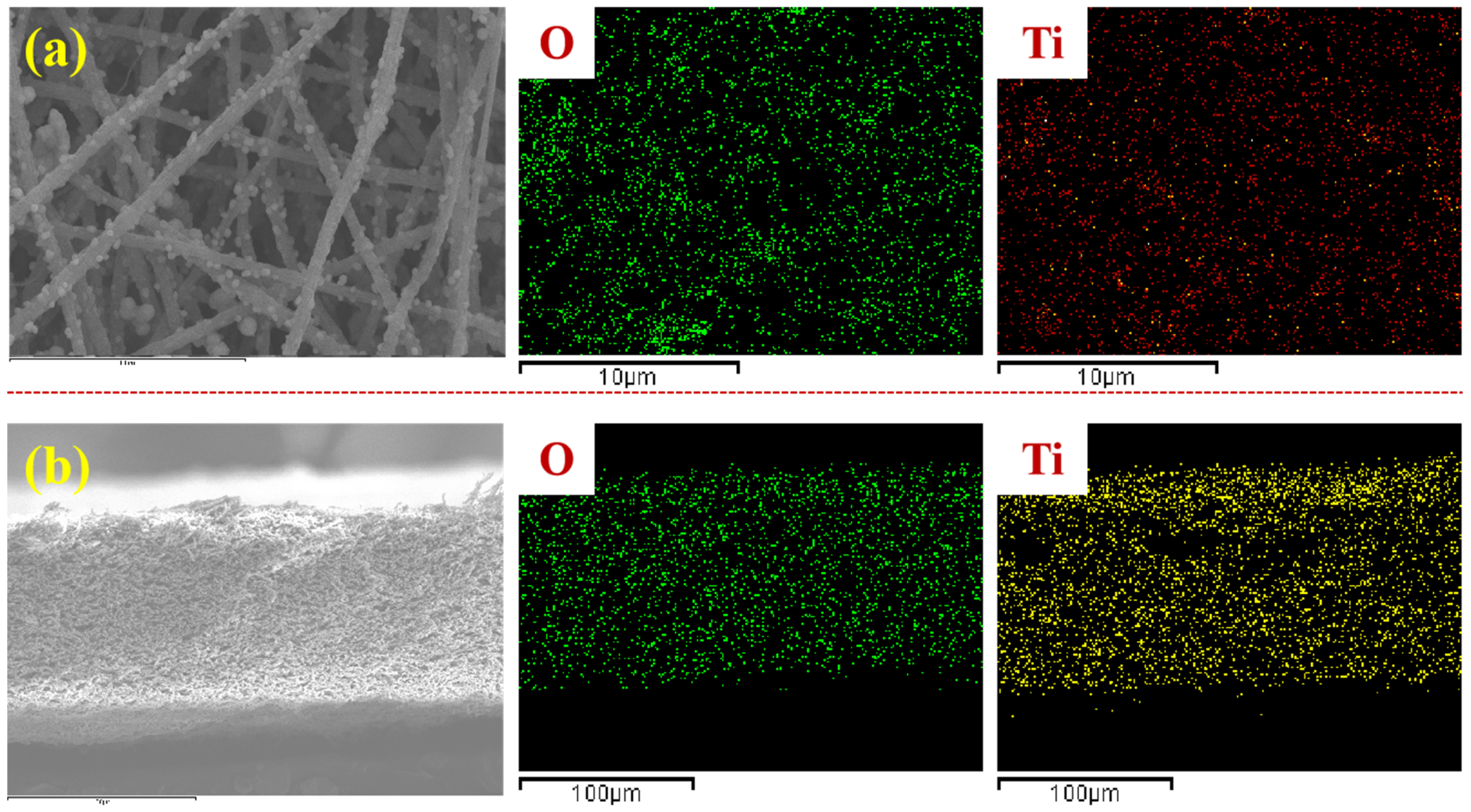



| Membrane | Composition (at%) | |||
|---|---|---|---|---|
| C | F | O | Ti | |
| PVDF | 51.23 | 48.77 | / | / |
| M5 | 54.7 | 34.29 | 11.01 | / |
| M5-TiO2 (2) | 59.28 | 12.21 | 24.15 | 4.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yu, H.; Huang, B.; Liu, G.; Guo, Y.; Zhu, H.; Yu, B. Fabrication of Anatase TiO2/PVDF Composite Membrane for Oil-in-Water Emulsion Separation and Dye Photocatalytic Degradation. Membranes 2023, 13, 364. https://doi.org/10.3390/membranes13030364
Li C, Yu H, Huang B, Liu G, Guo Y, Zhu H, Yu B. Fabrication of Anatase TiO2/PVDF Composite Membrane for Oil-in-Water Emulsion Separation and Dye Photocatalytic Degradation. Membranes. 2023; 13(3):364. https://doi.org/10.3390/membranes13030364
Chicago/Turabian StyleLi, Chengcai, Hewei Yu, Biao Huang, Guojin Liu, Yuhai Guo, Hailin Zhu, and Bin Yu. 2023. "Fabrication of Anatase TiO2/PVDF Composite Membrane for Oil-in-Water Emulsion Separation and Dye Photocatalytic Degradation" Membranes 13, no. 3: 364. https://doi.org/10.3390/membranes13030364
APA StyleLi, C., Yu, H., Huang, B., Liu, G., Guo, Y., Zhu, H., & Yu, B. (2023). Fabrication of Anatase TiO2/PVDF Composite Membrane for Oil-in-Water Emulsion Separation and Dye Photocatalytic Degradation. Membranes, 13(3), 364. https://doi.org/10.3390/membranes13030364