The Performance of Ultrafiltration Process to Further Refine Lactic Acid from the Pre-Microfiltered Broth of Kitchen Waste Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermented Broth
2.1.1. The Pre-Microfiltered Broth
2.1.2. The Simulative Broth
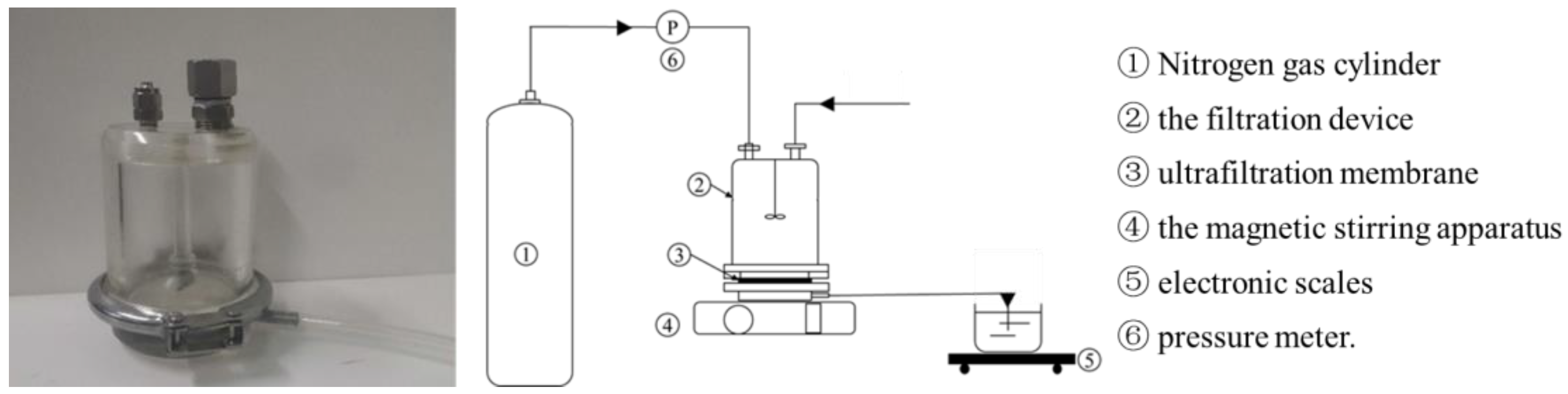
2.2. Experimental Apparatus
2.3. Analysis Items and Methods
2.3.1. Turbidity and Chroma
2.3.2. The Mass of LA
2.3.3. The Mass of Proteins
2.3.4. The Mass of Total Sugars
2.3.5. Membrane Flux
2.3.6. The Membrane Cleaning Performance
3. Results
3.1. Comparison and Selection of UF Membranes
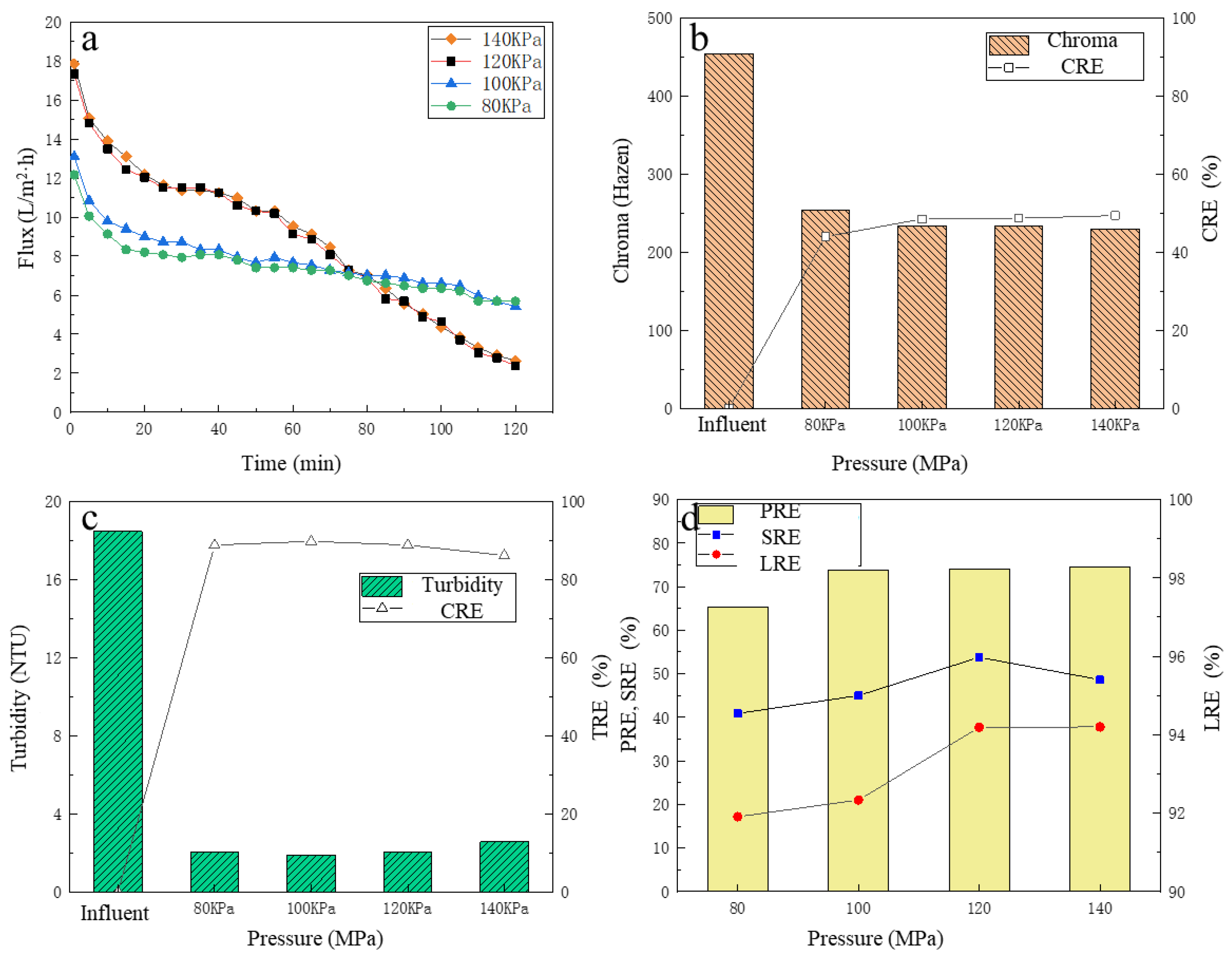
3.2. The Effect of Operating Pressure on LA Refinement Performance
3.2.1. The Change of UF Membrane Flux
3.2.2. The Removal of Chroma and Turbidity
3.2.3. The Removal of Protein, Total Sugar and the LA Recovery
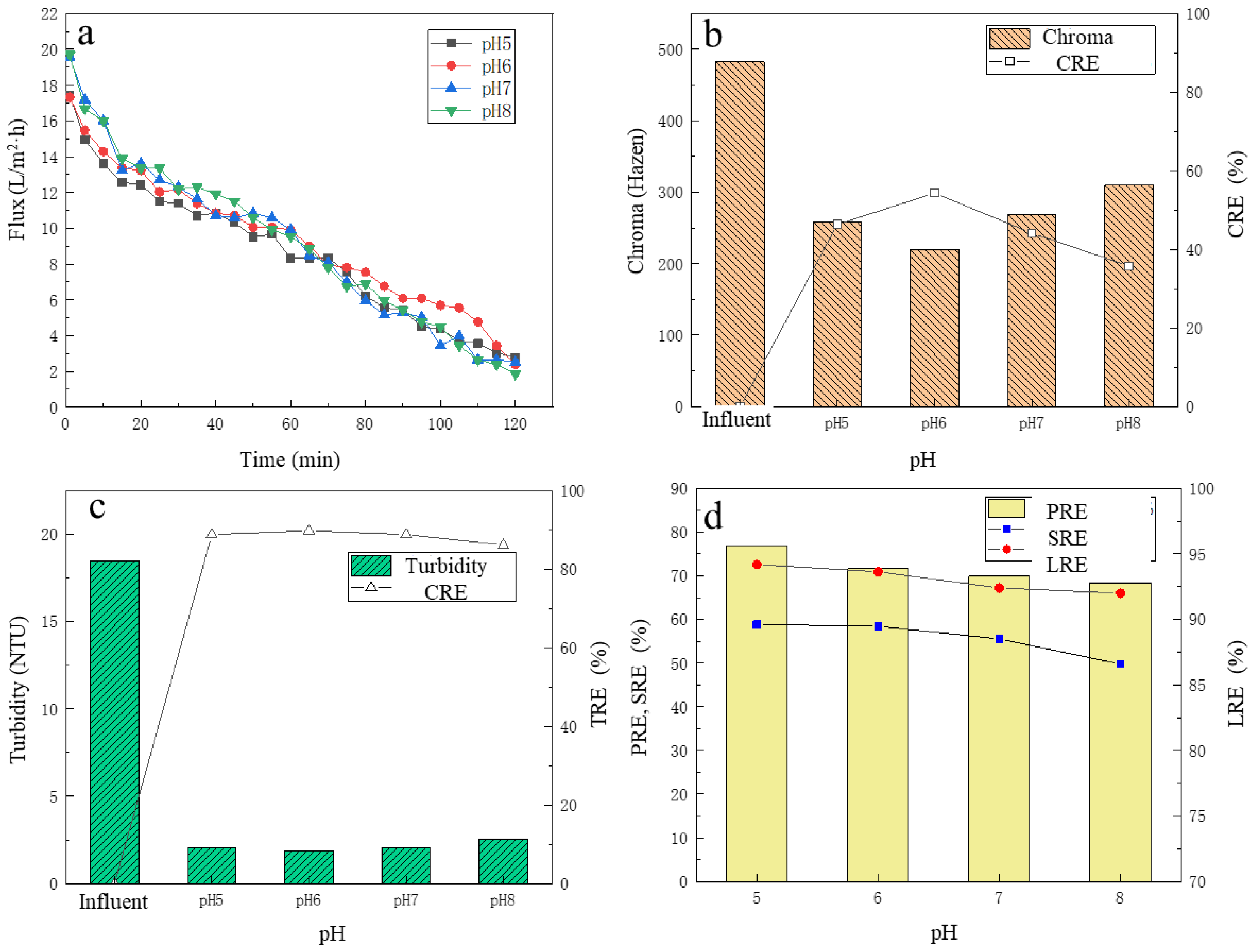
3.3. The Effect of pH on UF Performance
3.3.1. The Change of Membrane Flux
3.3.2. The Removal of Chroma and Turbidity
3.3.3. The Removal of Protein, Total Sugar and the LA Recovery
3.4. The Effect of Cleaning Method on Polluted Membrane Recovery
3.4.1. The Influence of Cleaning Mode on Cleaning Performance
3.4.2. The Influence of Cleaning Agent on Cleaning Performance
3.4.3. The Influence of Cleaning Time on Cleaning Performance
3.4.4. Membrane Surface Morphology Analysis
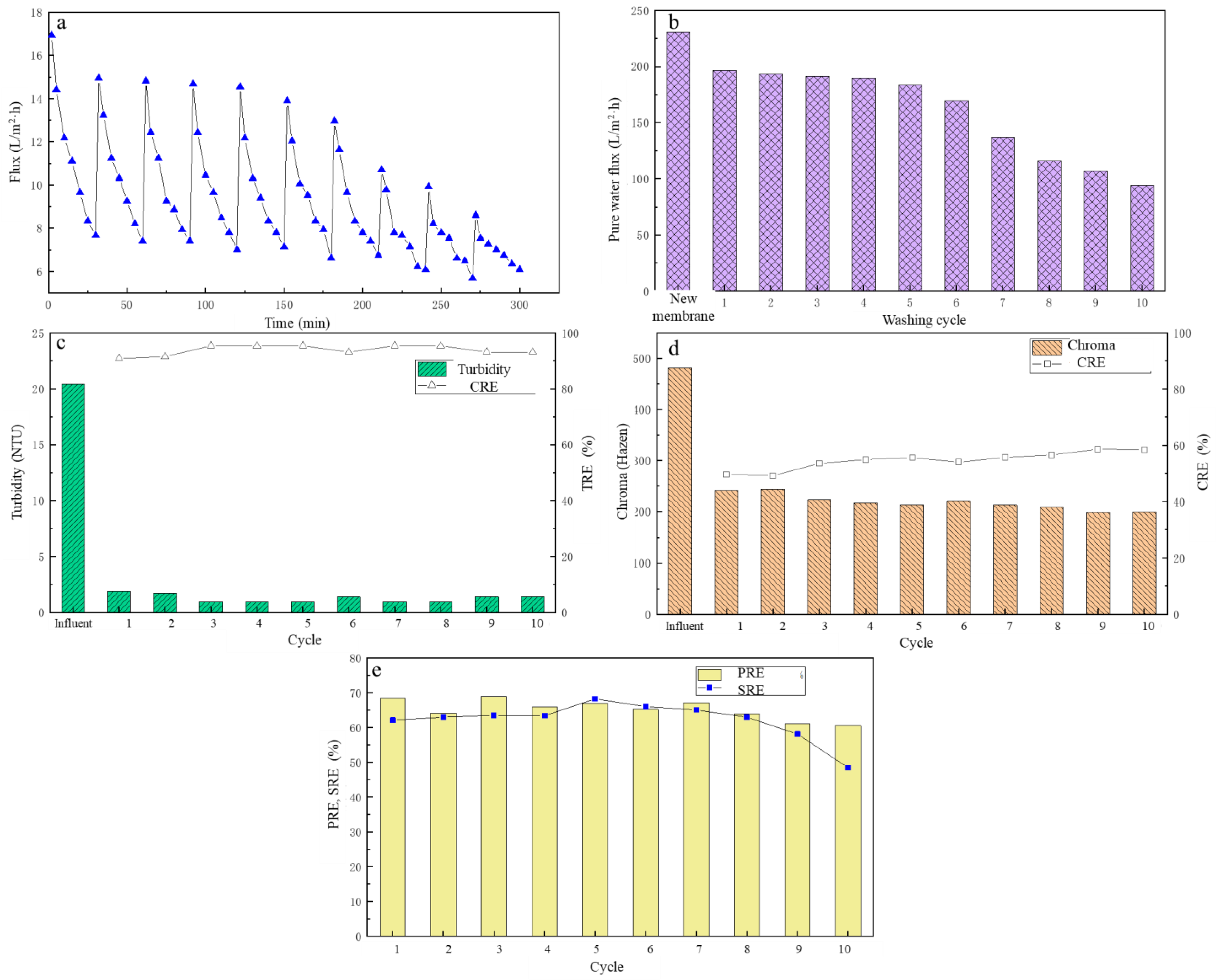
3.5. The Effect of Cleaning Cycle on UF Membrane Performance
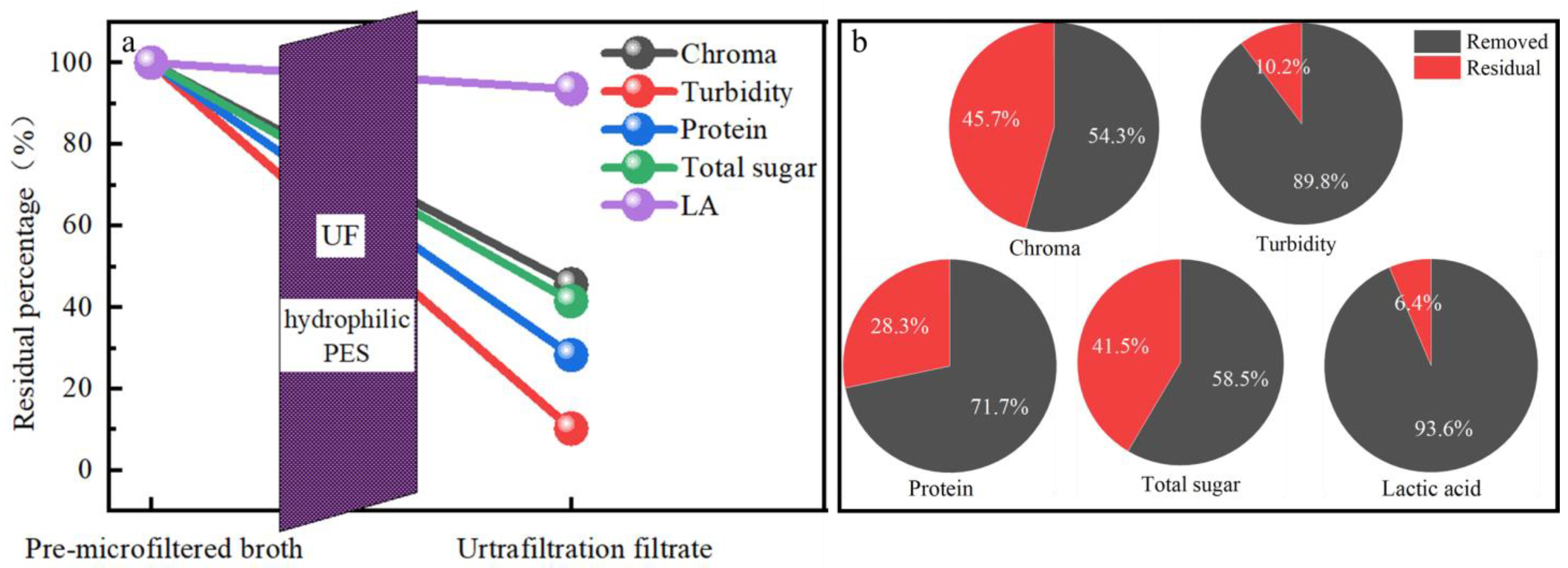
3.6. The Whole Evaluation of the UF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.L.; Wu, Z.; Zhang, L.; Ming Cheung, C.; Yang, S.-T. Production of Carboxylic Acids from Hydrolyzed Corn Meal by Immobilized Cell Fermentation in a Fibrous-Bed Bioreactor. Bioresour. Technol. 2002, 82, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.H.; Yang, H.J.; Jang, M.; Kim, J.R.; Byun, E.-H.; Lee, J.; Na, J.-G.; Kim, S.W.; Park, C. Efficient Simultaneous Production of Biodiesel and Glycerol Carbonate via Statistical Optimization. J. Ind. Eng. Chem. 2017, 51, 49–53. [Google Scholar] [CrossRef]
- Berhanu, M.; Jabasingh, S.A.; Kifile, Z. Expanding Sustenance in Ethiopia Based on Renewable Energy Resources—A Comprehensive Review. Renew. Sustain. Energy Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; Liu, S.; Li, Y.; Sun, X.; Wang, Q. An Innovative Approach for Reducing the Water and Alkali Consumption in the Lactic Acid Fermentation via the Reuse of Pretreated Liquid. Bioresour. Technol. 2022, 352, 127108. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Sun, H.; Yang, M.; Xie, D.; Sun, X.; Meng, J.; Wang, Q.; Wu, C. Biodrying of Biogas Residue through a Thermophilic Bacterial Agent Inoculation: Insights into Dewatering Contribution and Microbial Mechanism. Bioresour. Technol. 2022, 355, 127256. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.-P.; Lovitt, R.W. Recovery of Volatile Fatty Acids (VFA) from Complex Waste Effluents Using Membranes. Water Sci. Technol. 2013, 69, 495–503. [Google Scholar] [CrossRef]
- Aghapour Aktij, S.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.J.; Tiraferri, A.; Rahimpour, A. Feasibility of Membrane Processes for the Recovery and Purification of Bio-Based Volatile Fatty Acids: A Comprehensive Review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Cacho, J.A.; Mumme, J.; Orupõld, K.; Esteves, S.; Noguerol-Arias, J.; Picard, S.; Nielfa, A.; Scherer, P.; et al. First International Comparative Study of Volatile Fatty Acids in Aqueous Samples by Chromatographic Techniques: Evaluating Sources of Error. TrAC Trends Anal. Chem. 2013, 51, 127–143. [Google Scholar] [CrossRef]
- Fernández, R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Critical Analysis of Methods for the Measurement of Volatile Fatty Acids. Crit. Rev. Environ. Sci. Technol. 2016, 46, 209–234. [Google Scholar] [CrossRef]
- Masse, L.; Massé, D.I.; Pellerin, Y. The Effect of PH on the Separation of Manure Nutrients with Reverse Osmosis Membranes. J. Memb. Sci. 2008, 325, 914–919. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T.; Zhang, Y.; Xue, Y.; Chen, G. Application of Electrodialysis to the Production of Organic Acids: State-of-the-Art and Recent Developments. J. Memb. Sci. 2007, 288, 1–12. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Bashan, Y. Recent Advances in Removing Phosphorus from Wastewater and Its Future Use as Fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef] [PubMed]
- Gluszcz, P.; Jamroz, T.; Sencio, B.; Ledakowicz, S. Equilibrium and Dynamic Investigations of Organic Acids Adsorption onto Ion-Exchange Resins. Bioprocess. Biosyst. Eng. 2004, 26, 185–190. [Google Scholar] [CrossRef]
- Senol, A.; Dramur, U. Predicting Liquid–Liquid Equilibria of Amine Extraction of Carboxylic Acid Through Solvation Energy Relation. Solvent Extr. Ion Exch. 2004, 22, 865–883. [Google Scholar] [CrossRef]
- Mumtaz, T.; Abd-Aziz, S.; Aini, N.; Rahman, A.; Yee, P.; Shirai, Y.; Hassan, M. Pilot-Scale Recovery of Low Molecular Weight Organic Acids from Anaerobically Treated Palm Oil Mill Effluent (POME) with Energy Integrated System. Afr. J. Biotechnol. 2008, 7, 3900–3905. [Google Scholar]
- Lo, K.-M.; Chien, I.-L. Efficient Separation Method for Tert-Butanol Dehydration via Extractive Distillation. J. Taiwan Inst. Chem. Eng. 2017, 73, 27–36. [Google Scholar] [CrossRef]
- Joglekar, H.G.; Rahman, I.; Babu, S.; Kulkarni, B.D.; Joshi, A. Comparative Assessment of Downstream Processing Options for Lactic. Acid. Sep. Purif. Technol. 2006, 52, 1–17. [Google Scholar] [CrossRef]
- Manzak, A.; Kurşun, C.; Yıldız, Y. Characterization of Humic Acid Extracted from Aqueous Solutions with Polymer Inclusion Membranes. J. Taiwan Inst. Chem. Eng. 2017, 81, 14–20. [Google Scholar] [CrossRef]
- Trad, Z.; Akimbomi, J.; Vial, C.; Larroche, C.; Taherzadeh, M.J.; Fontaine, J.-P. Development of a Submerged Anaerobic Membrane Bioreactor for Concurrent Extraction of Volatile Fatty Acids and Biohydrogen Production. Bioresour. Technol. 2015, 196, 290–300. [Google Scholar] [CrossRef]
- Bastrzyk, J.; Gryta, M.; Karakulski, K. Fouling of Nanofiltration Membranes Used for Separation of Fermented Glycerol Solutions. Chem. Pap. 2014, 68, 757–765. [Google Scholar] [CrossRef]
- Bonnélye, V.; Guey, L.; del Castillo, J. UF/MF as RO Pre-Treatment: The Real Benefit. Desalination 2008, 222, 59–65. [Google Scholar] [CrossRef]
- Karakulski, K.; Gryta, M.; Bastrzyk, J. Treatment of Effluents from a Membrane Bioreactor by Nanofiltration Using Tubular Membranes. Chem. Pap. 2013, 67, 1164–1171. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The Application of Ultrafiltration for Separation of Glycerol Solution Fermented by Bacteria. Pol. J. Chem. Technol. 2013, 15, 115–120. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on Research Achievements of Biogas from Anaerobic Digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Feng, K.; Liu, J. Oriented Fermentation of Food Waste towards High-Value Products: A Review. Energies 2020, 13, 5638. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.v.; Venkata Mohan, S. Acidogenic Fermentation of Food Waste for Volatile Fatty Acid Production with Co-Generation of Biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Gryta, M.; Tomczak, W. Microfiltration of Post-Fermentation Broth with Backflushing Membrane Cleaning. Chem. Pap. 2015, 69, 544–552. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Q.; Zhao, W.; Ma, H.; Sakata, K. Extraction and Purification of Lactic Acid from Fermentation Broth by Esterification and Hydrolysis Method. Sep. Purif. Technol. 2006, 49, 43–48. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, Y.; Liu, Y.; Wang, Q. Waste Cooking Oil Used as Carbon Source for Microbial Lipid Production: Promoter or Inhibitor. Environ. Res. 2022, 203, 111881. [Google Scholar] [CrossRef]
- Puro, L.; Kallioinen, M.; Mänttäri, M.; Natarajan, G.; Cameron, D.; Nyström, M. Performance of RC and PES Ultrafiltration Membranes in Filtration of Pulp Mill Process Waters. Desalination 2010, 264, 249–255. [Google Scholar] [CrossRef]
- Charcosset, C. Classical and Recent Applications of Membrane Processes in the Food Industry. Food Eng. Rev. 2021, 13, 322–343. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Díaz-Montes, E.; Cassano, A.; Gontarek, E. Membrane Separation Processes for the Extraction and Purification of Steviol Glycosides: An Overview. Crit. Rev. Food Sci. Nutr. 2021, 61, 2152–2174. [Google Scholar] [CrossRef] [PubMed]
- Adikane, H.; Thakar, D.; Nene, S. Optimisation of Colour and Sugar Rejection of Black Liquor Using Membranes. Sep. Purif. Technol. 2004, 36, 229–234. [Google Scholar] [CrossRef]
- Sadr, S.M.K.; Saroj, D.P. Membrane Technologies for Municipal Wastewater Treatment. In Advances in Membrane Technologies for Water Treatment; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Oxford, UK, 2015; pp. 443–463. ISBN 978-1-78242-121-4. [Google Scholar]
- Li, S.; Chen, H.; Zhao, X.; Lucia, L.A.; Liang, C.; Liu, Y. Impact Factors for Flux Decline in Ultrafiltration of Lignocellulosic Hydrolysis Liquor. Sep. Purif Technol. 2020, 240, 116597. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; Serrano, M.; Rodríguez-Schmidt, R.; Escudero, P.A. Behaviour of Polysulfone Ultrafiltration Membrane for Dyes Removal. Water Sci. Technol. 2018, 77, 2093–2100. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Metabolites Recovery from Fermentation Broths via Pressure-Driven Membrane Processes. Asia-Pac. J. Chem. Eng. 2019, 14, e2332. [Google Scholar] [CrossRef]
- Huang, S.; Ras, R.H.A.; Tian, X. Antifouling Membranes for Oily Wastewater Treatment: Interplay between Wetting and Membrane Fouling. Curr. Opin. Colloid Interface Sci. 2018, 36, 90–109. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Sharifnia, S.; Moradi, G. Chemical Cleaning of Microfiltration Membranes Fouled by Whey. J. Chin. Chem. Soc. 2001, 48, 179–191. [Google Scholar] [CrossRef]
- Chandrapala, J.; Duke, M.C.; Gray, S.R.; Weeks, M.; Palmer, M.; Vasiljevic, T. Strategies for Maximizing Removal of Lactic Acid from Acid Whey—Addressing the Un-Processability Issue. Sep. Purif. Technol. 2017, 172, 489–497. [Google Scholar] [CrossRef]
- Koschuh, W.; Thang, V.H.; Krasteva, S.; Novalin, S.; Kulbe, K.D. Flux and Retention Behaviour of Nanofiltration and Fine Ultrafiltration Membranes in Filtrating Juice from a Green Biorefinery: A Membrane Screening. J. Memb. Sci. 2005, 261, 121–128. [Google Scholar] [CrossRef]
- Paugam, L.; Delaunay, D.; Diagne, N.W.; Rabiller-Baudry, M. Cleaning of Skim Milk PES Ultrafiltration Membrane: On the Real Effect of Nitric Acid Step. J. Memb. Sci. 2013, 428, 275–280. [Google Scholar] [CrossRef]
- Hou, L.; Gao, K.; Li, P.; Zhang, X.; Wang, Z.; Song, P.; Yao, W. A Kinetic Model for Calculating Total Membrane Fouling Resistance in Chemical Cleaning Process. Chem. Eng. Res. Des. 2017, 128, 59–72. [Google Scholar] [CrossRef]
- Bird, M.R.; Bartlett, M. Measuring and Modelling Flux Recovery during the Chemical Cleaning of MF Membranes for the Processing of Whey Protein Concentrate. J. Food Eng. 2002, 53, 143–152. [Google Scholar] [CrossRef]
- Madaeni, S.; Tavakolian, H.; Rahimpour, F. Cleaning Optimization of Microfiltration Membrane Employed for Milk Sterilization. Sep. Sci. Technol. 2011, 46, 571–580. [Google Scholar] [CrossRef]
- Naim, R.; Levitsky, I.; Gitis, V. Surfactant Cleaning of UF Membranes Fouled by Proteins. Sep. Purif. Technol. 2012, 94, 39–43. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Li, C.; Zhao, H.; Wang, X.; Gao, M.; Sun, X.; Wang, Q. The Performance of Ultrafiltration Process to Further Refine Lactic Acid from the Pre-Microfiltered Broth of Kitchen Waste Fermentation. Membranes 2023, 13, 330. https://doi.org/10.3390/membranes13030330
Guo Y, Li C, Zhao H, Wang X, Gao M, Sun X, Wang Q. The Performance of Ultrafiltration Process to Further Refine Lactic Acid from the Pre-Microfiltered Broth of Kitchen Waste Fermentation. Membranes. 2023; 13(3):330. https://doi.org/10.3390/membranes13030330
Chicago/Turabian StyleGuo, Yan, Chenglong Li, Hongjun Zhao, Xiaona Wang, Ming Gao, Xiaohong Sun, and Qunhui Wang. 2023. "The Performance of Ultrafiltration Process to Further Refine Lactic Acid from the Pre-Microfiltered Broth of Kitchen Waste Fermentation" Membranes 13, no. 3: 330. https://doi.org/10.3390/membranes13030330
APA StyleGuo, Y., Li, C., Zhao, H., Wang, X., Gao, M., Sun, X., & Wang, Q. (2023). The Performance of Ultrafiltration Process to Further Refine Lactic Acid from the Pre-Microfiltered Broth of Kitchen Waste Fermentation. Membranes, 13(3), 330. https://doi.org/10.3390/membranes13030330





