The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling
Abstract
1. Introduction
2. Materials and Methods
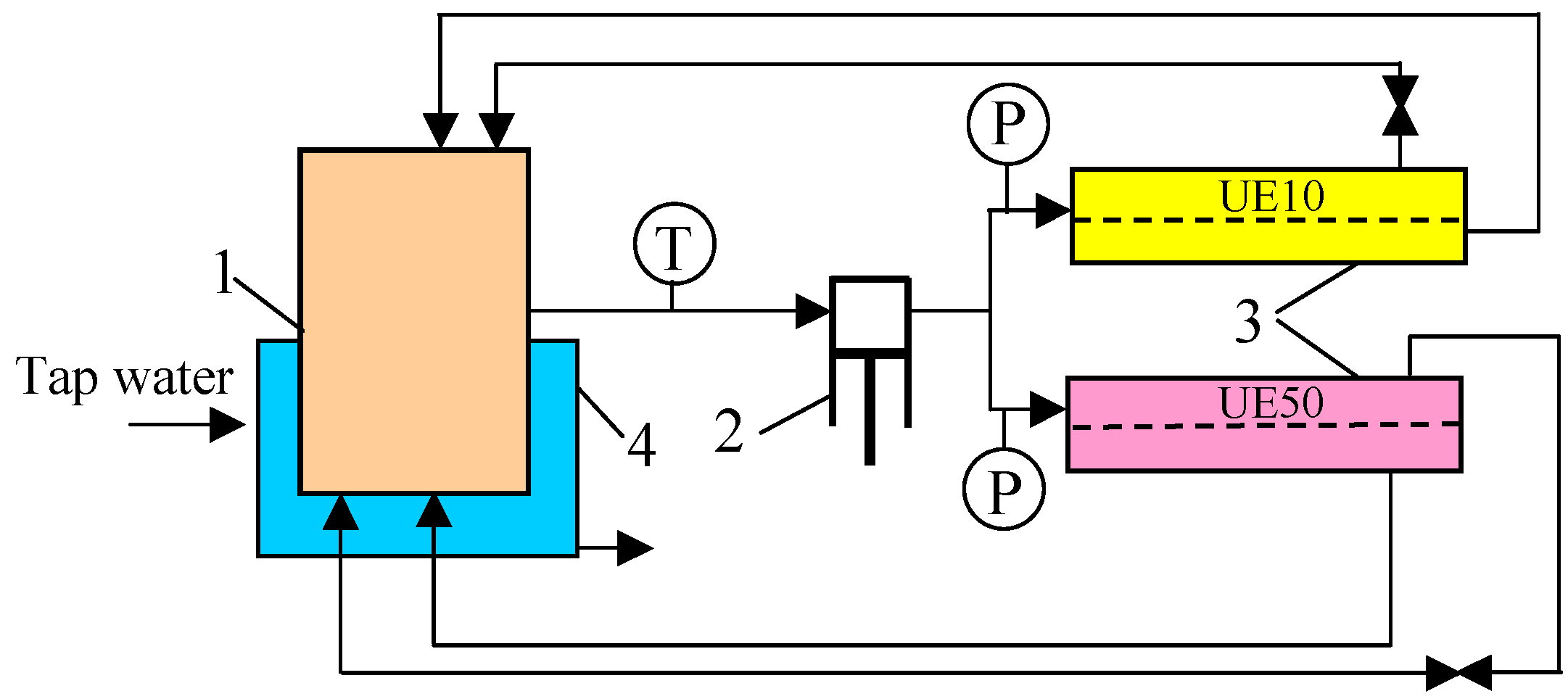
2.1. Ultrafiltration Unit
2.2. Feed Solutions
2.3. Analytical Methods
2.4. Modeling
3. Results and Discussion
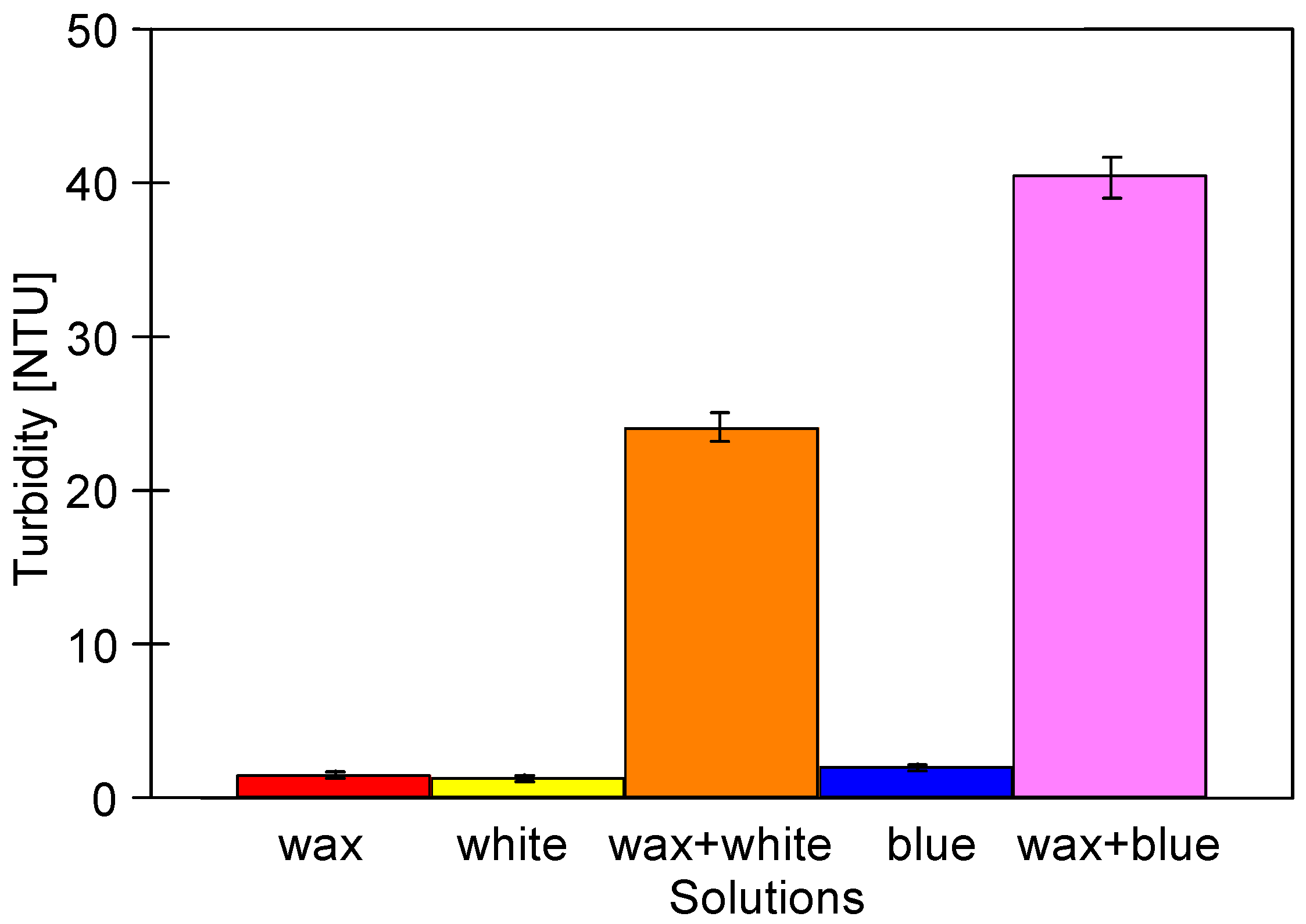
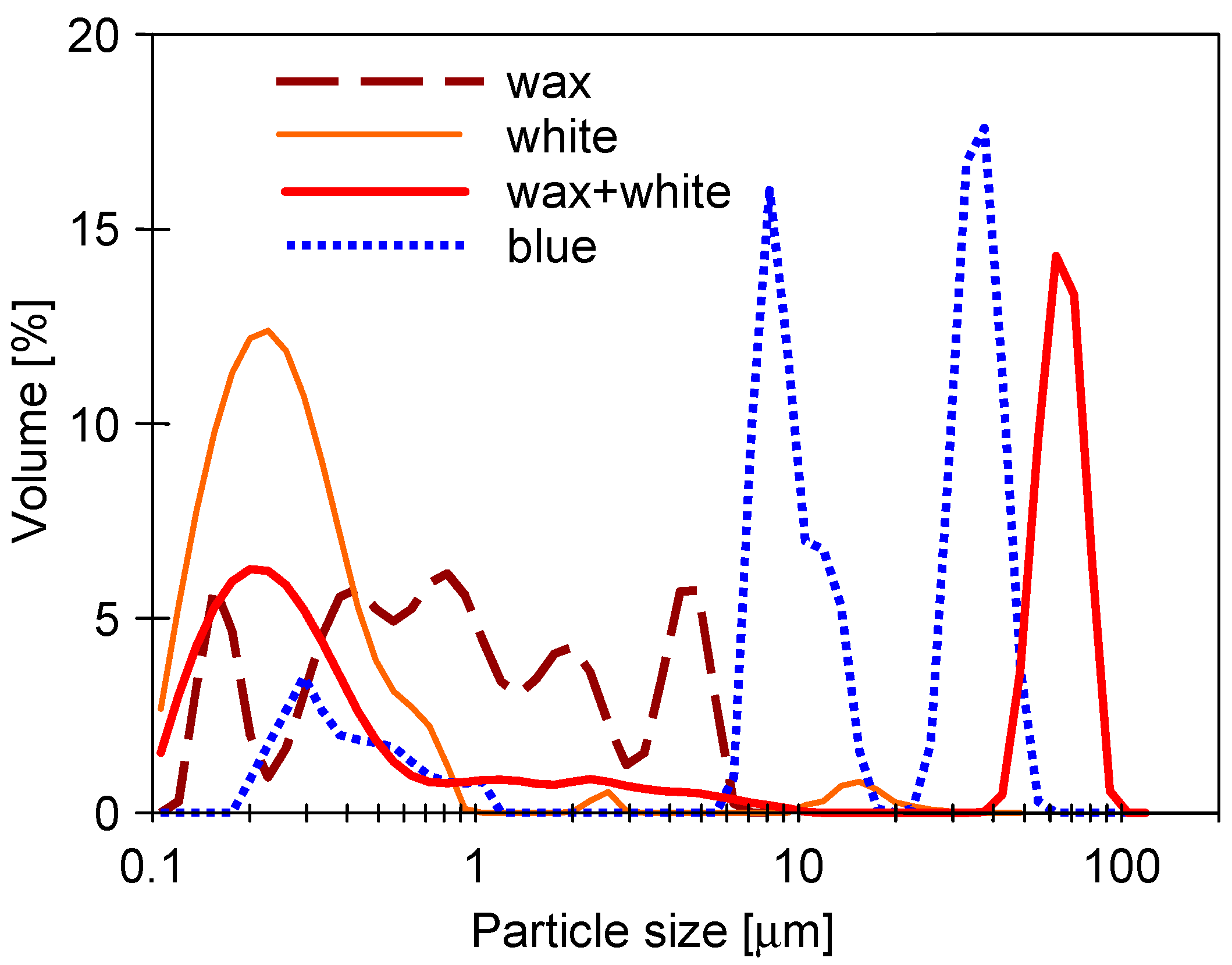
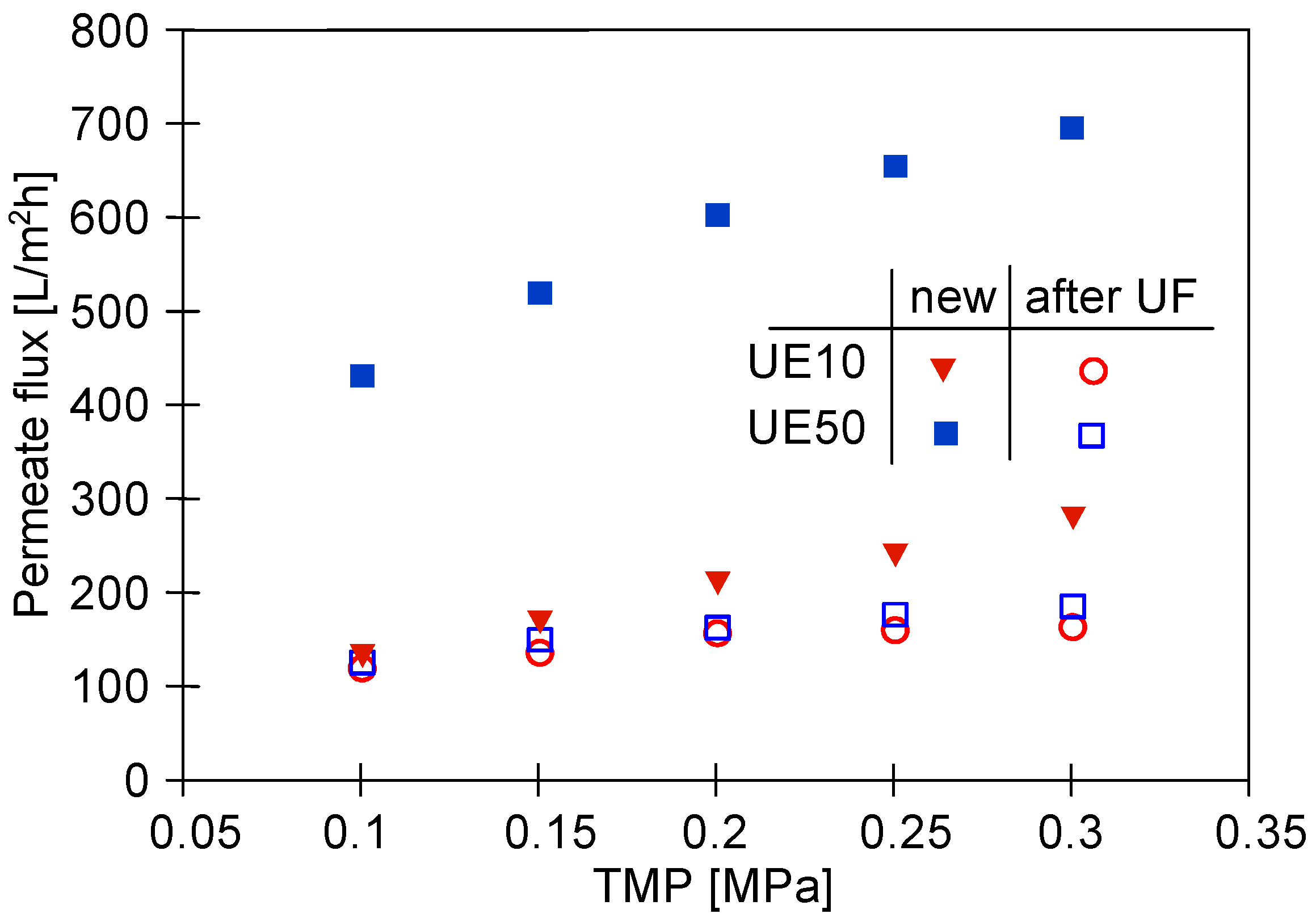
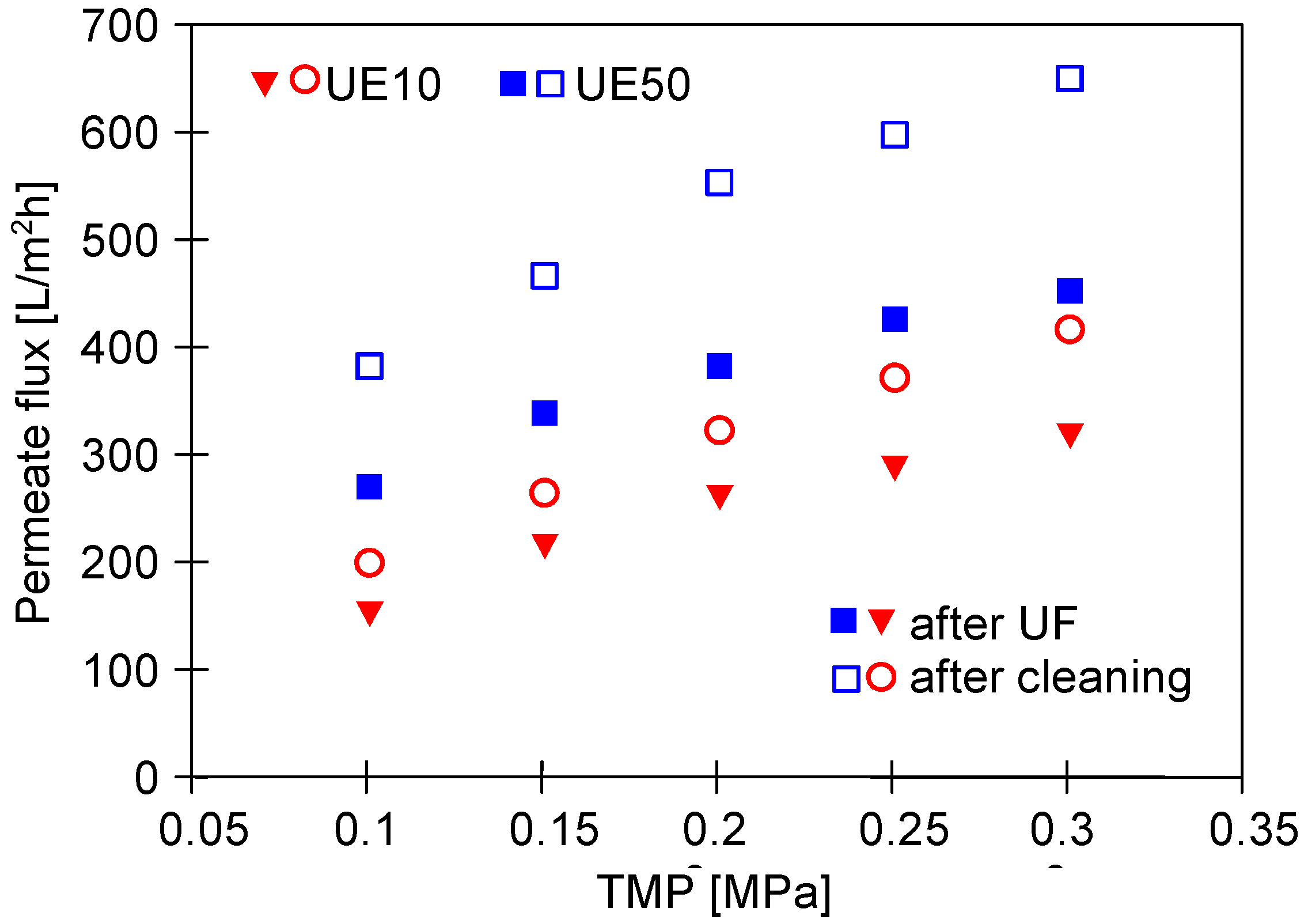
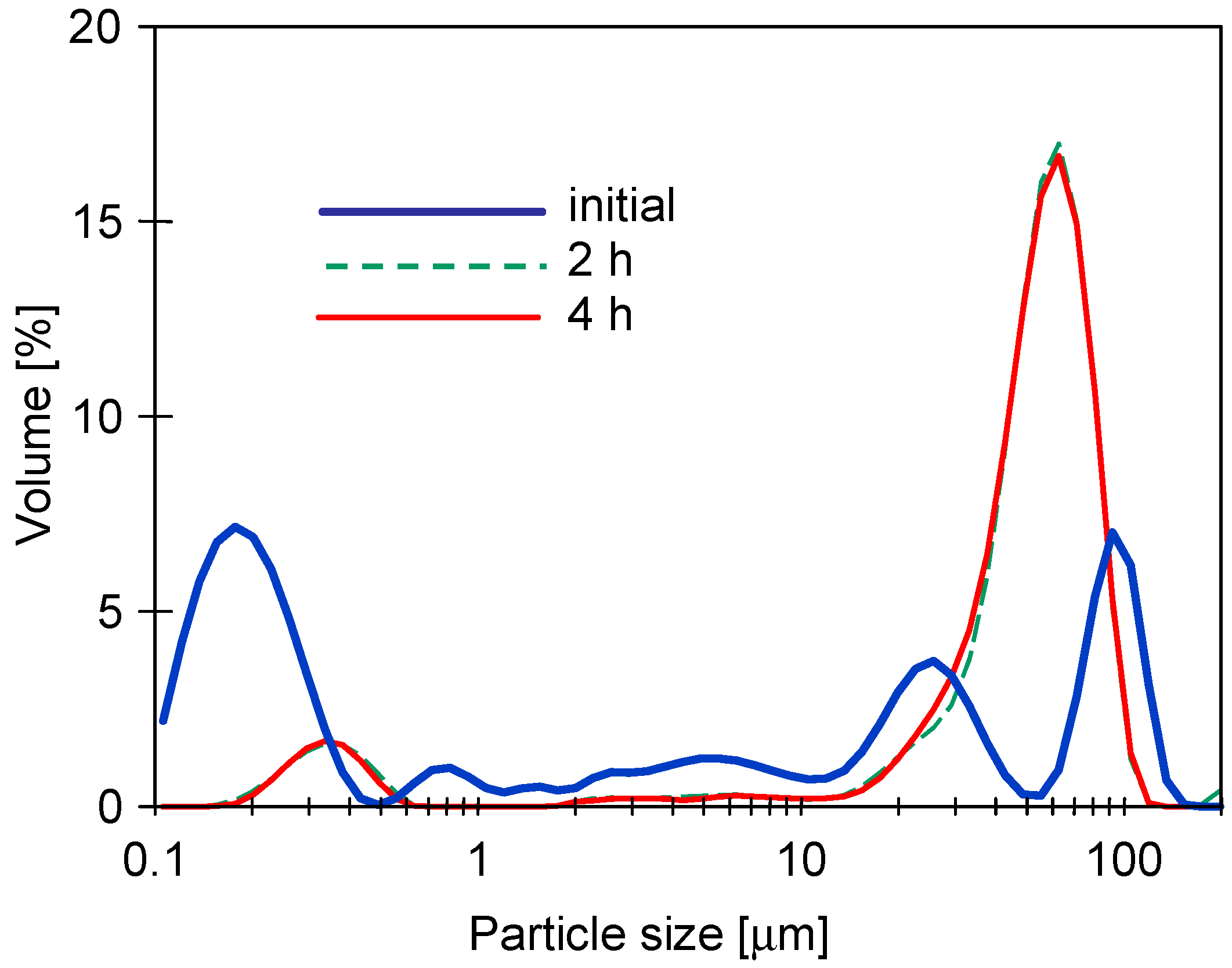
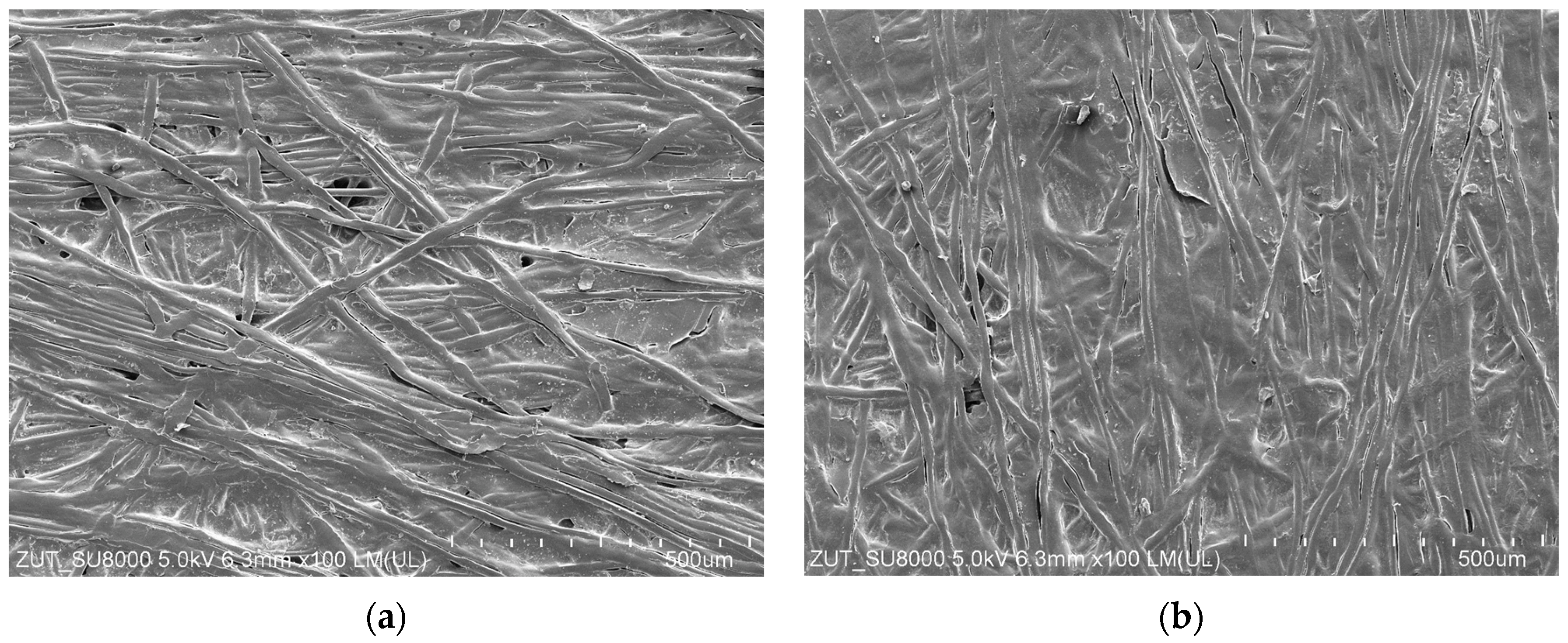
3.1. Properties of Washing Agents and Membranes Characteristics
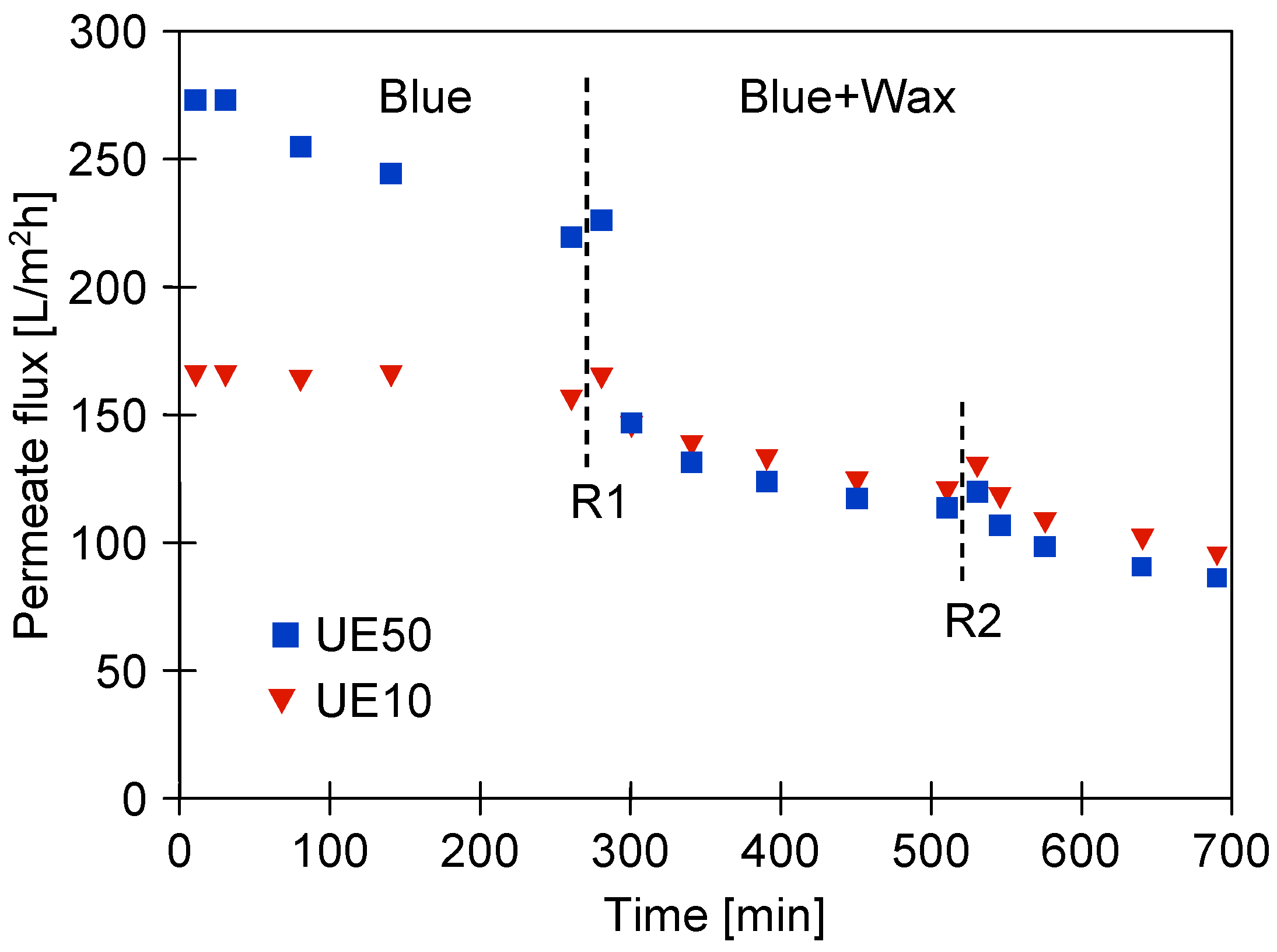
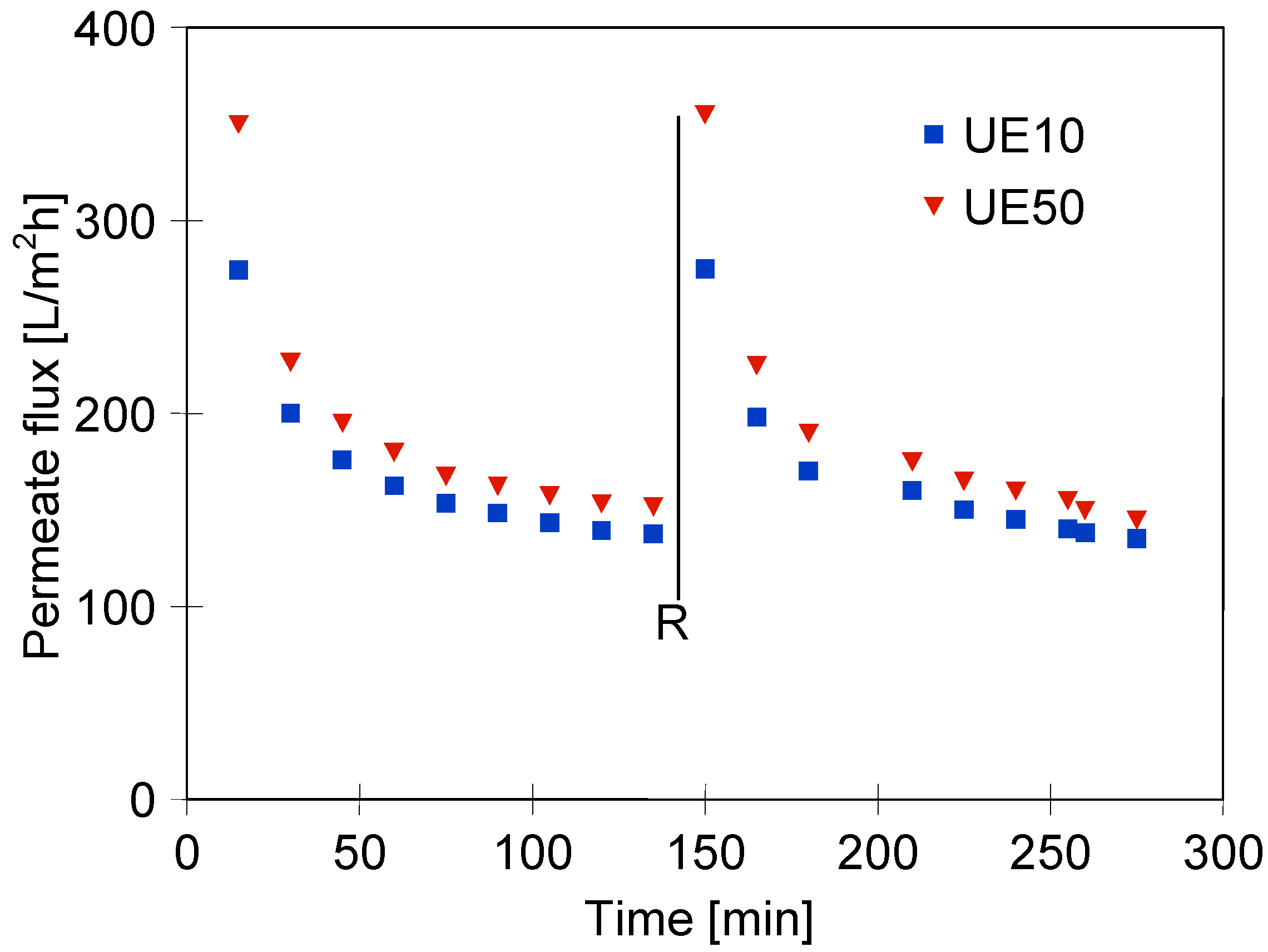
3.2. UF Process of Synthetic Wastewaters
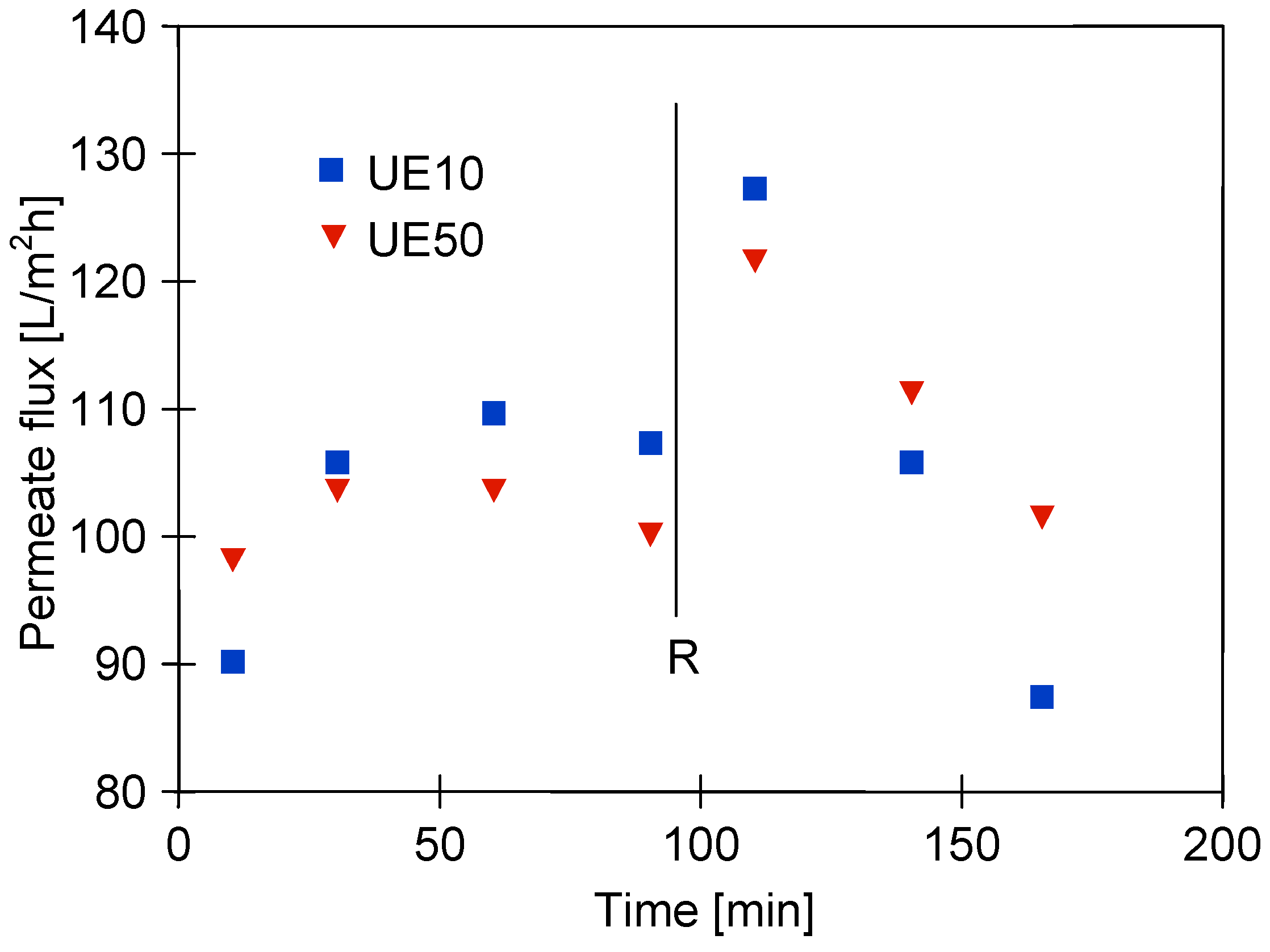
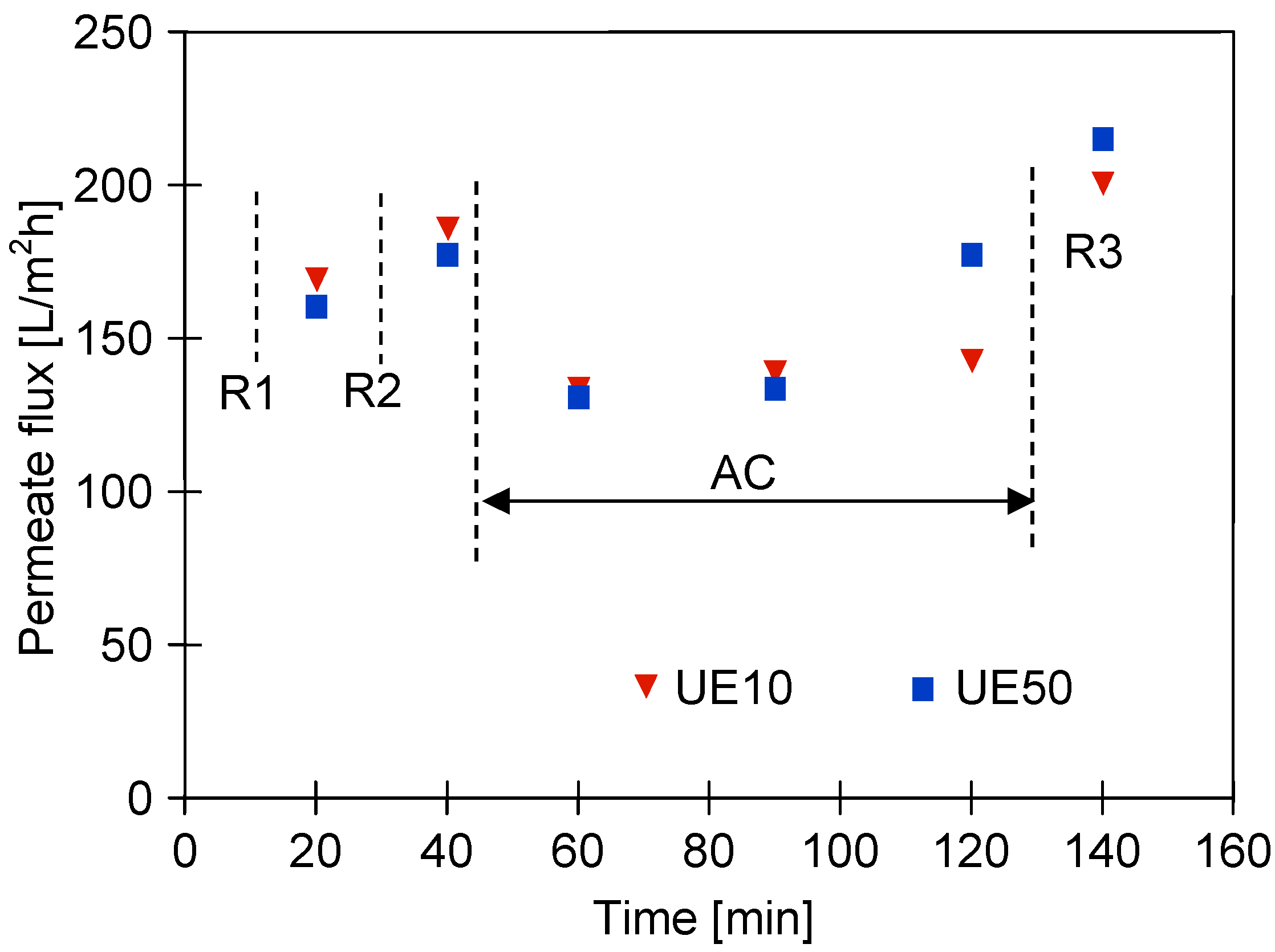
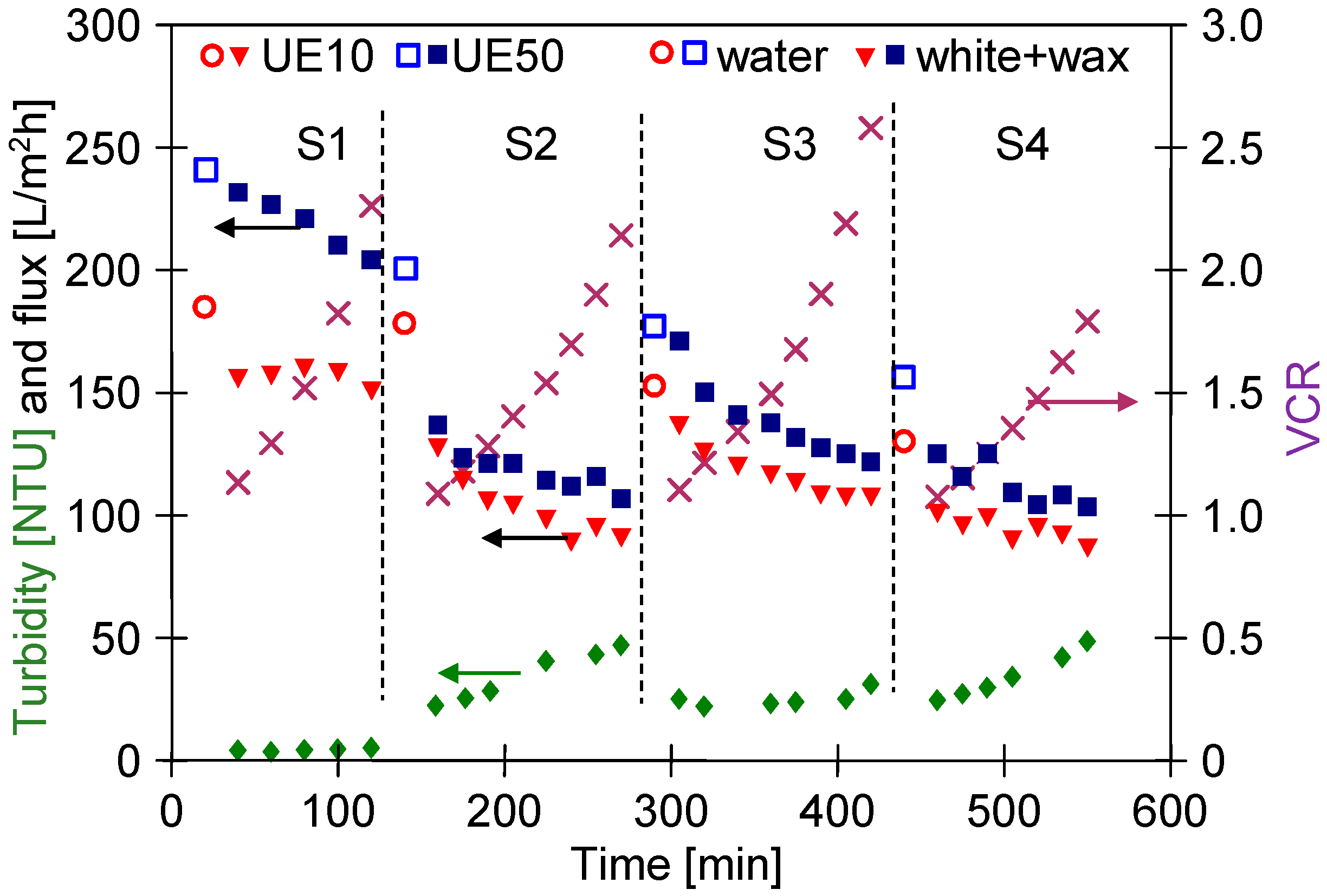
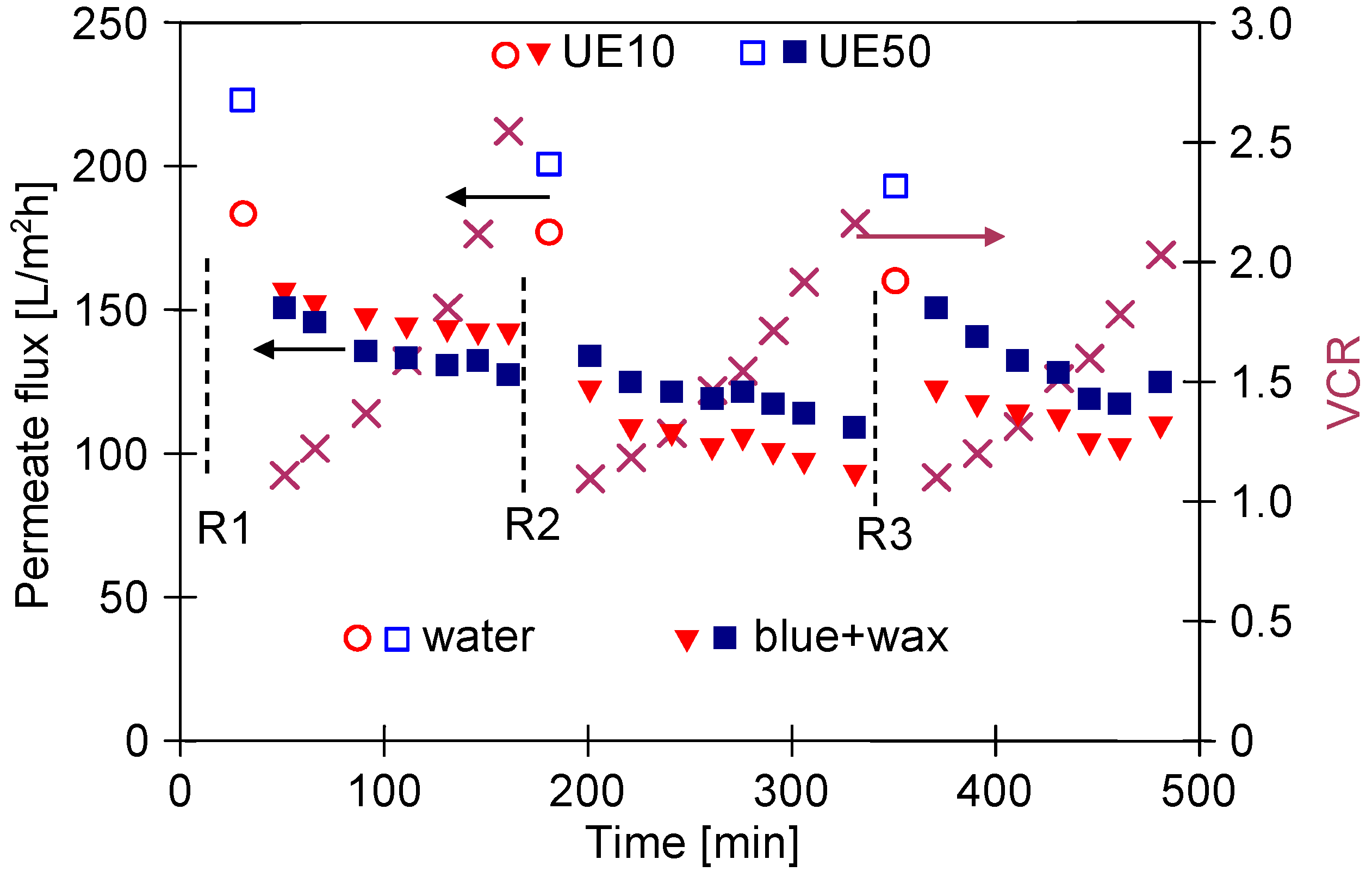
3.3. Wastewaters Concentration by UF process
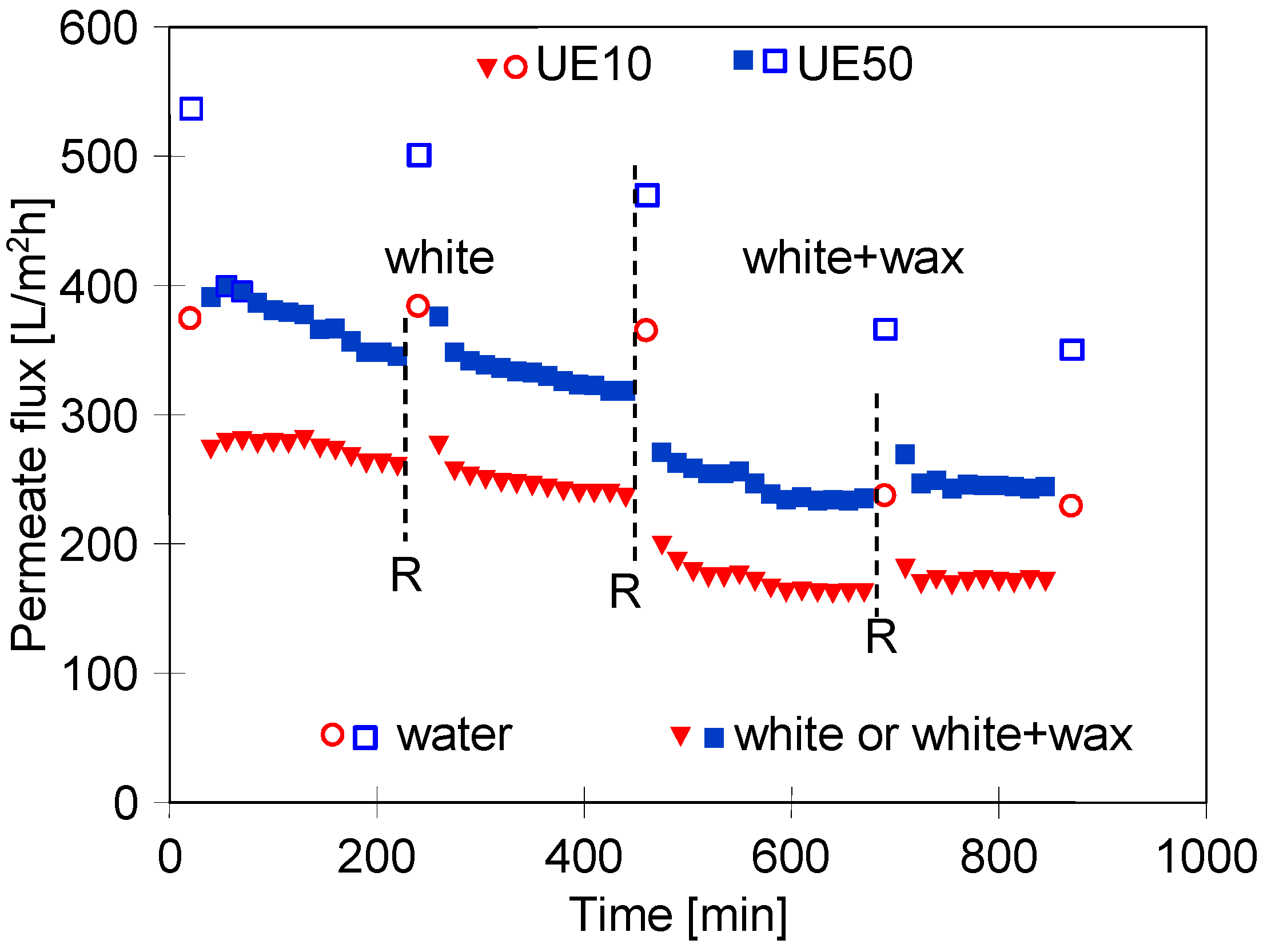
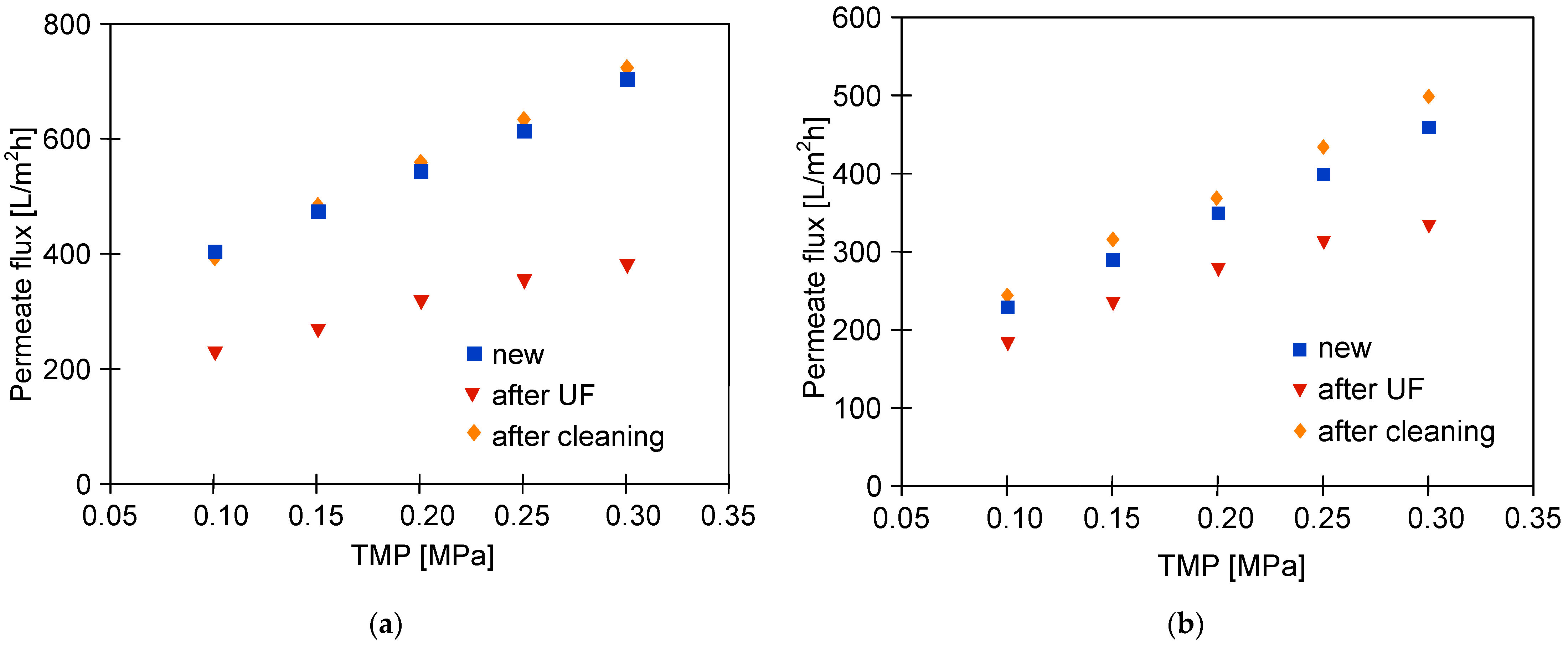
3.4. UF Process of Real Wastewaters
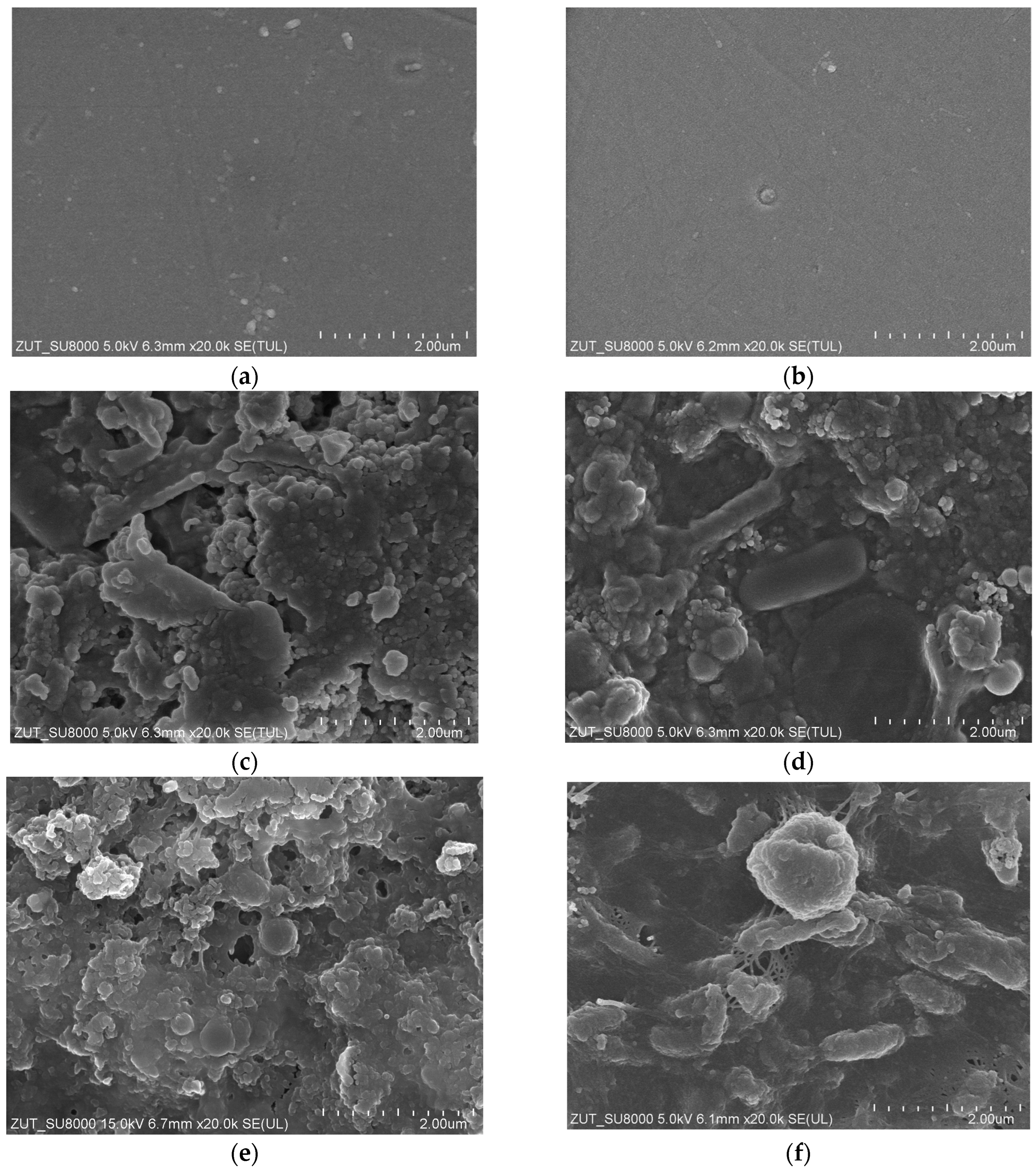
3.5. Membranes Fouling
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Feed | UF Time [min] | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation | ||||
|---|---|---|---|---|---|---|---|---|---|
| UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | ||
| blue | 0–260 | 0.3472 | 0.3815 | 0.3592 | 0.3436 | 0.3719 | 0.6210 * | 0.3987 | 0.4923 |
| blue + wax | 280–690 | 0.9118 | 0.7862 | 0.8915 | 0.8359 | 0.8723 | 0.9085 | 0.9030 | 0.9121 * |
| Feed | UF Time [min] | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation | ||||
|---|---|---|---|---|---|---|---|---|---|
| UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | ||
| white | 0–220 | 0.4949 | 0.7047 | 0.5117 | 0.7354 | 0.5290 | 0.7652 | 0.5644 * | 0.8200 * |
| white | 240–440 | 0.5275 | 0.4782 | 0.5045 | 0.5548 | 0.5316 | 0.5827 | 0.5871 * | 0.6390 * |
| white + wax | 460–670 | 0.4255 | 0.4330 | 0.4699 | 0.4733 | 0.5166 | 0.5158 | 0.6028 * | 0.6144 * |
| white + wax | 690–870 | 0.3553 | 0.4416 | 0.3580 | 0.4524 | 0.3606 | 0.4634 | 0.3654 * | 0.4856 * |
| Feed | UF Time [min] | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation | ||||
|---|---|---|---|---|---|---|---|---|---|
| UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | ||
| white + wax | 0–120 | 0.5099 | 0.9873 * | 0.5128 | 0.9856 | 0.5156 | 0.9838 | 0.5212 * | 0.9793 |
| 140–270 | 0.7803 | 0.6464 | 0.8144 | 0.6803 | 0.8443 | 0.7136 | 0.8883 * | 0.7753 * | |
| 290–420 | 0.8898 | 0.7218 | 0.9041 | 0.7666 | 0.9172 | 0.8090 | 0.9395 * | 0.8826 * | |
| 440–550 | 0.6723 | 0.7682 | 0.6896 | 0.7836 | 0.7062 | 0.7975 | 0.7360 * | 0.8205 * | |
| Feed | UF Time [min] | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation | ||||
|---|---|---|---|---|---|---|---|---|---|
| UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | ||
| blue + wax | 0–270 | 0.4972 | 0.4696 | 0.5160 | 0.4974 | 0.5357 | 0.5270 | 0.5772 * | 0.5881 * |
| 300–610 | 0.4903 | 0.5143 | 0.5176 | 0.5634 | 0.5462 | 0.6146 | 0.7150 * | 0.6066 * | |
| 640–850 | 0.4774 | 0.4211 | 0.4977 | 0.4418 | 0.5193 | 0.4644 | 0.5650 * | 0.5120 * | |
| 900–1095 | 0.6771 | 0.6248 | 0.7211 | 0.7199 | 0.7632 | 0.7900 | 0.8373 * | 0.8893 * | |
| Feed | UF Time [min] | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation | ||||
|---|---|---|---|---|---|---|---|---|---|
| UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | ||
| blue + wax | 0–160 | 0.7045 | 0.6240 | 0.7197 | 0.6516 | 0.7349 | 0.6822 | 0.7425 * | 0.7646 * |
| 180–330 | 0.6900 | 0.6211 | 0.7276 | 0.6552 | 0.7643 | 0.6897 | 0.8306 * | 0.7568 * | |
| 350–480 | 0.6749 | 0.7756 | 0.6922 | 0.7935 | 0.7080 | 0.8088 | 0.7336 * | 0.8308 * | |
| Feed | UF Time [min] | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation | ||||
|---|---|---|---|---|---|---|---|---|---|
| UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | UE10 | UE50 | ||
| real wastewaters | 0–135 | 0.7932 | 0.7349 | 0.8310 | 0.7827 | 0.8655 | 0.8267 | 0.9220 * | 0.8989 * |
References
- Sarmadi, M.; Foroughi, M.; Najafi Saleh, H.; Sanaei, D.; Zarei, A.A.; Ghahrchi, M.; Bazrafshan, E. Efficient Technologies for Carwash Wastewater Treatment: A Systematic Review. Environ. Sci. Pollut. Res. 2020, 27, 34823–34839. [Google Scholar] [CrossRef] [PubMed]
- Boluarte, I.A.R.; Andersen, M.; Pramanik, B.K.; Chang, C.-Y.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L. Reuse of Car Wash Wastewater by Chemical Coagulation and Membrane Bioreactor Treatment Processes. Int. Biodeterior. Biodegrad. 2016, 113, 44–48. [Google Scholar] [CrossRef]
- Li, T.; Xue-jun, T.; Fu-yi, C.; Qi, Z.; Jun, Y. Reuse of Carwash Wastewater with Hollow Fiber Membrane Aided by Enhanced Coagulation and Activated Carbon Treatments. Water Sci. Technol. 2007, 56, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Emamjomeh, M.M.; Jamali, H.A.; Naghdali, Z.; Mousazadeh, M. Carwash Wastewater Treatment by the Application of an Environmentally Friendly Hybrid System: An Experimental Design Approach. Desalin. Water Treat. 2019, 160, 171–177. [Google Scholar] [CrossRef]
- Kiran, S.A.; Arthanareeswaran, G.; Thuyavan, Y.L.; Ismail, A.F. Influence of Bentonite in Polymer Membranes for Effective Treatment of Car Wash Effluent to Protect the Ecosystem. Ecotoxicol. Environ. Saf. 2015, 121, 186–192. [Google Scholar] [CrossRef]
- Dadebo, D.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Transition towards Sustainable Carwash Wastewater Management: Trends and Enabling Technologies at Global Scale. Sustainability 2022, 14, 5652. [Google Scholar] [CrossRef]
- Etchepare, R.; Zaneti, R.; Azevedo, A.; Rubio, J. Application of Flocculation–Flotation Followed by Ozonation in Vehicle Wash Wastewater Treatment/Disinfection and Water Reclamation. Desalin. Water Treat. 2015, 56, 1728–1736. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Mohamed, R.M.S.R.; Rahman, M.A.A.; Johari, M.R.; Kassim, A.H.M. Treatment of Wastewater from Car Washes Using Natural Coagulation and Filtration System. IOP Conf. Ser. Mater. Sci. Eng. 2016, 136, 012046. [Google Scholar] [CrossRef]
- Awad, E.S.; Abdulla, S.M.; Sabirova, T.M.; Alsalhy, Q.F. Membrane Techniques for Removal of Detergents and Petroleum Products from Carwash Effluents: A Review. Chim. Techno Acta 2023, 10, 202310107. [Google Scholar] [CrossRef]
- Panpanit, S.; Visvanathan, C.; Muttamara, S. Separation of Oil–Water Emulsion from Car Washes. Water Sci. Technol. 2000, 41, 109–116. [Google Scholar] [CrossRef]
- International Organization for Standardization. Available online: https://www.iso.org/standard/67972.html (accessed on 28 January 2023).
- Boussu, K.; Kindts, C.; Vandecasteele, C.; Van der Bruggen, B. Applicability of Nanofiltration in the Carwash Industry. Sep. Purif. Technol. 2007, 54, 139–146. [Google Scholar] [CrossRef]
- Radcliffe, J.C.; Page, D. Water Reuse and Recycling in Australia—History, Current Situation and Future Perspectives. Water Cycle 2020, 1, 19–40. [Google Scholar] [CrossRef]
- Rebelo, A.; Quadrado, M.; Franco, A.; Lacasta, N.; Machado, P. Water Reuse in Portugal: New Legislation Trends to Support the Definition of Water Quality Standards Based on Risk Characterization. Water Cycle 2020, 1, 41–53. [Google Scholar] [CrossRef]
- Do, K.-U.; Kim, J.-H.; Chu, X.-Q. Sludge Characteristics and Performance of a Membrane Bioreactor for Treating Oily Wastewater from a Car Wash Service Station. Desalin. Water Treat. 2018, 120, 166–172. [Google Scholar] [CrossRef]
- Kuan, W.-H.; Hu, C.-Y.; Ke, L.-W.; Wu, J.-M. A Review of On-Site Carwash Wastewater Treatment. Sustainability 2022, 14, 5764. [Google Scholar] [CrossRef]
- Veit, M.T.; Novais, Í.G.V.; Juchen, P.T.; Palácio, S.M.; da Cunha Gonçalves, G.; Zanette, J.C. Automotive Wash Effluent Treatment Using Combined Process of Coagulation/Flocculation/Sedimentation–Adsorption. Water Air Soil Pollut. 2020, 231, 494. [Google Scholar] [CrossRef]
- Zaneti, R.; Etchepare, R.; Rubio, J. Car Wash Wastewater Reclamation. Full-Scale Application and Upcoming Features. Resour. Conserv. Recycl. 2011, 55, 953–959. [Google Scholar] [CrossRef]
- Torkashvand, J.; Pasalari, H.; Gholami, M.; Younesi, S.; Oskoei, V.; Farzadkia, M. On-Site Carwash Wastewater Treatment and Reuse: A Systematic Review. Int. J. Environ. Anal. Chem. 2022, 102, 3613–3627. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Balcıoğlu, G.; Vergili, I.; Kaya, Y. Electrochemical Treatment of Carwash Wastewater Using Fe and Al Electrode: Techno-Economic Analysis and Sludge Characterization. J. Environ. Manag. 2017, 200, 380–390. [Google Scholar] [CrossRef]
- Nadzirah, Z.; Nor Haslina, H.; Rafidah, H. Removal of Important Parameter from Car Wash Wastewater—A Review. Appl. Mech. Mater. 2015, 773–774, 1153–1157. [Google Scholar] [CrossRef]
- Thomas, M.; Drzewicz, P.; Więckol-Ryk, A.; Panneerselvam, B. Effectiveness of Potassium Ferrate (VI) as a Green Agent in the Treatment and Disinfection of Carwash Wastewater. Environ. Sci. Pollut. Res. 2022, 29, 8514–8524. [Google Scholar] [CrossRef] [PubMed]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of Ceramic Ultrafiltration and Reverse Osmosis Membranes in Treating Car Wash Wastewater for Reuse. Environ. Sci. Pollut. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Moazzem, S.; Jegatheesan, V. Treating Car Wash Wastewater by Ceramic Ultrafiltration Membranes for Reuse Purposes. In Water Scarcity and Ways to Reduce the Impact; Pannirselvam, M., Shu, L., Griffin, G., Philip, L., Natarajan, A., Hussain, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–73. ISBN 978-3-319-75198-6. [Google Scholar]
- Pinto, A.C.S.; de Barros Grossi, L.; de Melo, R.A.C.; de Assis, T.M.; Ribeiro, V.M.; Amaral, M.C.S.; de Souza Figueiredo, K.C. Carwash Wastewater Treatment by Micro and Ultrafiltration Membranes: Effects of Geometry, Pore Size, Pressure Difference and Feed Flow Rate in Transport Properties. J. Water Process Eng. 2017, 17, 143–148. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Firdaus, S. Car Wash Industry in Malaysia: Treatment of Car Wash Effluent Using Ultrafiltration and Nanofiltration Membranes. Sep. Purif. Technol. 2013, 104, 26–31. [Google Scholar] [CrossRef]
- Jönsson, C.; Jönsson, A.-S. The Influence of Degreasing Agents Used at Car Washes on the Performance of Ultrafiltration Membranes. Desalination 1995, 100, 115–123. [Google Scholar] [CrossRef]
- Istirokhatun, T.; Destianti, P.; Hargianintya, A.; Oktiawan, W.; Susanto, H. Treatment of Car Wash Wastewater by UF Membranes; AIP Publishing LLC: Semarang, Indonesia, 2015; p. 060025. [Google Scholar]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Based Membranes for Water and Wastewater Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Amin, S.K.; Abdallah, H.A.M.; Roushdy, M.H.; El-Sherbiny, S.A. An Overview of Production and Development of Ceramic Membranes. Int. J. Appl. Eng. Res. 2016, 11, 15. [Google Scholar]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces Cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef]
- Zrelli, A.; Bessadok, A.; Alsalhy, Q. Important Parameters of Ceramic Membranes Derived from Oasis Waste and Its Application for Car Wash Wastewater Treatment. JMSR 2021, 8, 413. [Google Scholar] [CrossRef]
- Susanto, H.; Robbani, M.H.; Istirokhatun, T.; Firmansyah, A.A.; Rhamadhan, R.N. Preparation of Low-Fouling Polyethersulfone Ultrafiltration Membranes by Incorporating High-Molecular-Weight Chitosan with the Help of a Surfactant. S. Afr. J. Chem. Eng. 2020, 33, 133–140. [Google Scholar] [CrossRef]
- Xin, R.; Ma, H.; Venkateswaran, S.; Hsiao, B.S. Electrospun Nanofibrous Adsorption Membranes for Wastewater Treatment: Mechanical Strength Enhancement. Chem. Res. Chin. Univ. 2021, 37, 355–365. [Google Scholar] [CrossRef]
- Kallem, P.; Ibrahim, Y.; Hasan, S.W.; Show, P.L.; Banat, F. Fabrication of Novel Polyethersulfone (PES) Hybrid Ultrafiltration Membranes with Superior Permeability and Antifouling Properties Using Environmentally Friendly Sulfonated Functionalized Polydopamine Nanofillers. Sep. Purif. Technol. 2021, 261, 118311. [Google Scholar] [CrossRef]
- Rezania, H.; Vatanpour, V.; Arabpour, A.; Shockravi, A.; Ehsani, M. Structural Manipulation of PES Constituents to Prepare Advanced Alternative Polymer for Ultrafiltration Membrane. J. Appl. Polym. Sci. 2020, 137, 48690. [Google Scholar] [CrossRef]
- Antón, E.; Álvarez, J.R.; Palacio, L.; Prádanos, P.; Hernández, A.; Pihlajamäki, A.; Luque, S. Ageing of Polyethersulfone Ultrafiltration Membranes under Long-Term Exposures to Alkaline and Acidic Cleaning Solutions. Chem. Eng. Sci. 2015, 134, 178–195. [Google Scholar] [CrossRef]
- Hermia, J. Constant Pressure Blocking Filtration Laws-Application to Power-Law Non-Newtonian Fluids. Trans. Inst. Chem. Eng. 1982, 60, 183–187. [Google Scholar]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane Fouling in Photocatalytic Membrane Reactors (PMRs) for Water and Wastewater Treatment: A Critical Review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of Natural Organic Matter in Membrane Filtration for Surface Water Treatment—A Review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mehejabin, F.; Momtahin, A.; Tasannum, N.; Faria, N.T.; Mofijur, M.; Hoang, A.T.; Vo, D.-V.N.; Mahlia, T.M.I. Strategies to Improve Membrane Performance in Wastewater Treatment. Chemosphere 2022, 306, 135527. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Zhao, X. Treatment of Surfactants with Concentrations below Critical Micelle Concentration by Ultrafiltration: A Mini-Review. Water Cycle 2022, 3, 50–55. [Google Scholar] [CrossRef]
- Kowalska, I.; Kabsch-Korbutowicz, M.; Majewska-Nowak, K.; Winnicki, T. Separation of Anionic Surfactants on Ultrafiltration Membranes. Desalination 2004, 162, 33–40. [Google Scholar] [CrossRef]
- Kowalska, I. Separation of Anionic Surfactants in a Sequential Ultrafiltration—Ion Exchange Purification System. Pol. J. Environ. Stud. 2012, 21, 677–684. [Google Scholar]
- Xu, H.; Xiao, K.; Yu, J.; Huang, B.; Wang, X.; Liang, S.; Wei, C.; Wen, X.; Huang, X. A Simple Method to Identify the Dominant Fouling Mechanisms during Membrane Filtration Based on Piecewise Multiple Linear Regression. Membranes 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Aloulou, H.; Aloulou, W.; Daramola, M.O.; Ben Amar, R. Silane-Grafted Sand Membrane for the Treatment of Oily Wastewater via Air Gap Membrane Distillation: Study of the Efficiency in Comparison with Microfiltration and Ultrafiltration Ceramic Membranes. Mater. Chem. Phys. 2021, 261, 124186. [Google Scholar] [CrossRef]
- Salahi, A.; Abbasi, M.; Mohammadi, T. Permeate Flux Decline during UF of Oily Wastewater: Experimental and Modeling. Desalination 2010, 251, 153–160. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Application of Ultrafiltration Ceramic Membrane for Separation of Oily Wastewater Generated by Maritime Transportation. Sep. Purif. Technol. 2021, 261, 118259. [Google Scholar] [CrossRef]
- Mokarizadeh, H.; Raisi, A. Industrial Wastewater Treatment Using PES UF Membranes Containing Hydrophilic Additives: Experimental and Modeling of Fouling Mechanism. Environ. Technol. Innov. 2021, 23, 101701. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Wang, Y.; Song, G.; Zhang, L. Industrial Application of Ceramic Ultrafiltration Membrane in Cold-Rolling Emulsion Wastewater Treatment. Sep. Purif. Technol. 2022, 289, 120724. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Application of Capillary Polypropylene Membranes for Microfiltration of Oily Wastewaters: Experiments and Modeling. Fibers 2021, 9, 35. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental Risks and Toxicity of Surfactants: Overview of Analysis, Assessment, and Remediation Techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Pashirova, T.N.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef]
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in Aquatic and Terrestrial Environment: Occurrence, Behavior, and Treatment Processes. Environ. Sci. Pollut. Res. 2016, 23, 3195–3216. [Google Scholar] [CrossRef]
- Xiarchos, I.; Doulia, D.; Gekas, V.; Trägårdh, G. Polymeric Ultrafiltration Membranes and Surfactants. Sep. Purif. Rev. 2003, 32, 215–278. [Google Scholar] [CrossRef]
- Khery, Y.; Daniar, S.E.; Mat Nawi, N.I.; Bilad, M.R.; Wibisono, Y.; Nufida, B.A.; Ahmadi, A.; Jaafar, J.; Huda, N.; Kobun, R. Ultra-Low-Pressure Membrane Filtration for Simultaneous Recovery of Detergent and Water from Laundry Wastewater. Membranes 2022, 12, 591. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, W.; Huang, G.; Wei, J.; Ding, L.; Jaffrin, M.Y. Studies of Membrane Fouling Mechanisms Involved in the Micellar-Enhanced Ultrafiltration Using Blocking Models. RSC Adv. 2015, 5, 48484–48491. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Tang, B.; Ding, J.; Zheng, Y.; Zhang, Z. Membrane Fouling Mechanism of Biofilm-Membrane Bioreactor (BF-MBR): Pore Blocking Model and Membrane Cleaning. Bioresour. Technol. 2018, 250, 398–405. [Google Scholar] [CrossRef]
- Kowalska, I. Dead-End and Cross-Flow Ultrafiltration of Ionic and Non-Ionic Surfactants. Desalin. Water Treat. 2012, 50, 397–410. [Google Scholar] [CrossRef]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.J.; Nigmatullin, R. Methods Employed for Control of Fouling in MF and UF Membranes: A Comprehensive Review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar] [CrossRef]
- Suárez, L.; Diez, M.A.; Riera, F.A. Transport Mechanisms of Detergent Ingredients through Ultrafiltration Membranes. Sep. Purif. Technol. 2014, 136, 115–122. [Google Scholar] [CrossRef]
- Othman, N.; Noah, N.F.M.; Shu, L.Y.; Ooi, Z.-Y.; Jusoh, N.; Idroas, M.; Goto, M. Easy Removing of Phenol from Wastewater Using Vegetable Oil-Based Organic Solvent in Emulsion Liquid Membrane Process. Chin. J. Chem. Eng. 2017, 25, 45–52. [Google Scholar] [CrossRef]
- Mortaheb, H.R.; Amini, M.H.; Sadeghian, F.; Mokhtarani, B.; Daneshyar, H. Study on a New Surfactant for Removal of Phenol from Wastewater by Emulsion Liquid Membrane. J. Hazard. Mater. 2008, 160, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, W.; Liu, Y.; Wang, H.; Zhang, Z.; Fan, W.; Li, L. Treatment of Oily Waste Water by PVP Grafted PVDF Ultrafiltration Membranes. Chem. Eng. J. 2015, 273, 421–429. [Google Scholar] [CrossRef]
- Gong, Y.-W.; Zhang, H.-X.; Cheng, X.-N. Treatment of Dairy Wastewater by Two-Stage Membrane Operation with Ultrafiltration and Nanofiltration. Water Sci. Technol. 2012, 65, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Ghalami Choobar, B.; Alaei Shahmirzadi, M.A.; Kargari, A.; Manouchehri, M. Fouling Mechanism Identification and Analysis in Microfiltration of Laundry Wastewater. J. Environ. Chem. Eng. 2019, 7, 103030. [Google Scholar] [CrossRef]

| Membrane | MWCO [kDa] | pH Range | Hydraulic Permeability [Lm2h/bar] | WCA [°] | Thickness [μm] |
|---|---|---|---|---|---|
| UE10 | 10 | 2–12 | 74 | 73 ± 1.3 | 160–200 |
| UE50 | 100 | 2–12 | 296 | 68 ± 2.2 | 160–200 |
| Trade Name | Name Used in the Present Study | Component | Concentration [%] |
|---|---|---|---|
| Euro Turbo Foam | white | diethylene glycol butyl ether | 1–5 |
| benzenesulfonic acid | 1–5 | ||
| polymers | 1–5 | ||
| Euro Turbo Foam Color Blue | blue | blue dye | |
| diethylene glycol butyl ether | 1–5 | ||
| benzenesulfonic acid | 1–5 | ||
| polymers | 1–5 | ||
| Euro Turbo Active Green | green | green dye | |
| sulfonic acids, C14-17-sec-alkane, sodium salts | 2.5–5.0 | ||
| ethylenediaminetetraacetic Acid Tetrasodium Salt | 2.5–5.0 | ||
| sodium Laureth Sulfate | <2 | ||
| sodium N-(2-carboxyethyl)-N-(2-ethylhexyl)-beta-alaninate | <2 | ||
| amines, C12-14-alkyldimethyl, N-oxides | <2 | ||
| sodium Cumenesulphonate | <2 | ||
| Hydrowax | wax | polymeric waxes | |
| diethylene glycol monobutyl ether polymer | 10–20 |
| Fouling Mechanism | n | Physical Concept | Schematic Description |
|---|---|---|---|
| Cake formation | 0 | solutes diameter is larger than membrane pores size, solutes deposit on the particles that already block the pores |  |
| Intermediate blocking | 1.0 | a single particle can precipitate on other particles to form multi-layers |  |
| Standard blocking | 1.5 | particles are adsorbed and deposited on the internal pore wall |  |
| Complete blocking | 2.0 | solutes diameter is similar to the size of membrane pores, and consequently, the number of open pores is reduced |  |
| Surfactants | White | Blue | Green |
|---|---|---|---|
| non-ionic | 470 ± 10 | 560 ± 24 | 520 ± 18 |
| anionic | 770 ± 4 | 620 ± 8 | 650 ± 5 |
| Membrane | SDS | Triton | White (Anionic) | Waste (Anionic) |
|---|---|---|---|---|
| UE10 | 28 ± 1 | 0 | 40 ± 1 | 60 ± 2 |
| UE50 | 15 ± 2 | 0 | 42 ± 3 | 53 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Gryta, M. The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes 2023, 13, 321. https://doi.org/10.3390/membranes13030321
Tomczak W, Gryta M. The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes. 2023; 13(3):321. https://doi.org/10.3390/membranes13030321
Chicago/Turabian StyleTomczak, Wirginia, and Marek Gryta. 2023. "The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling" Membranes 13, no. 3: 321. https://doi.org/10.3390/membranes13030321
APA StyleTomczak, W., & Gryta, M. (2023). The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes, 13(3), 321. https://doi.org/10.3390/membranes13030321









