Research on Membranes and Their Associated Processes at the Université Paris-Est Créteil: Progress Report, Perspectives, and National and International Collaborations
Abstract
1. Introduction
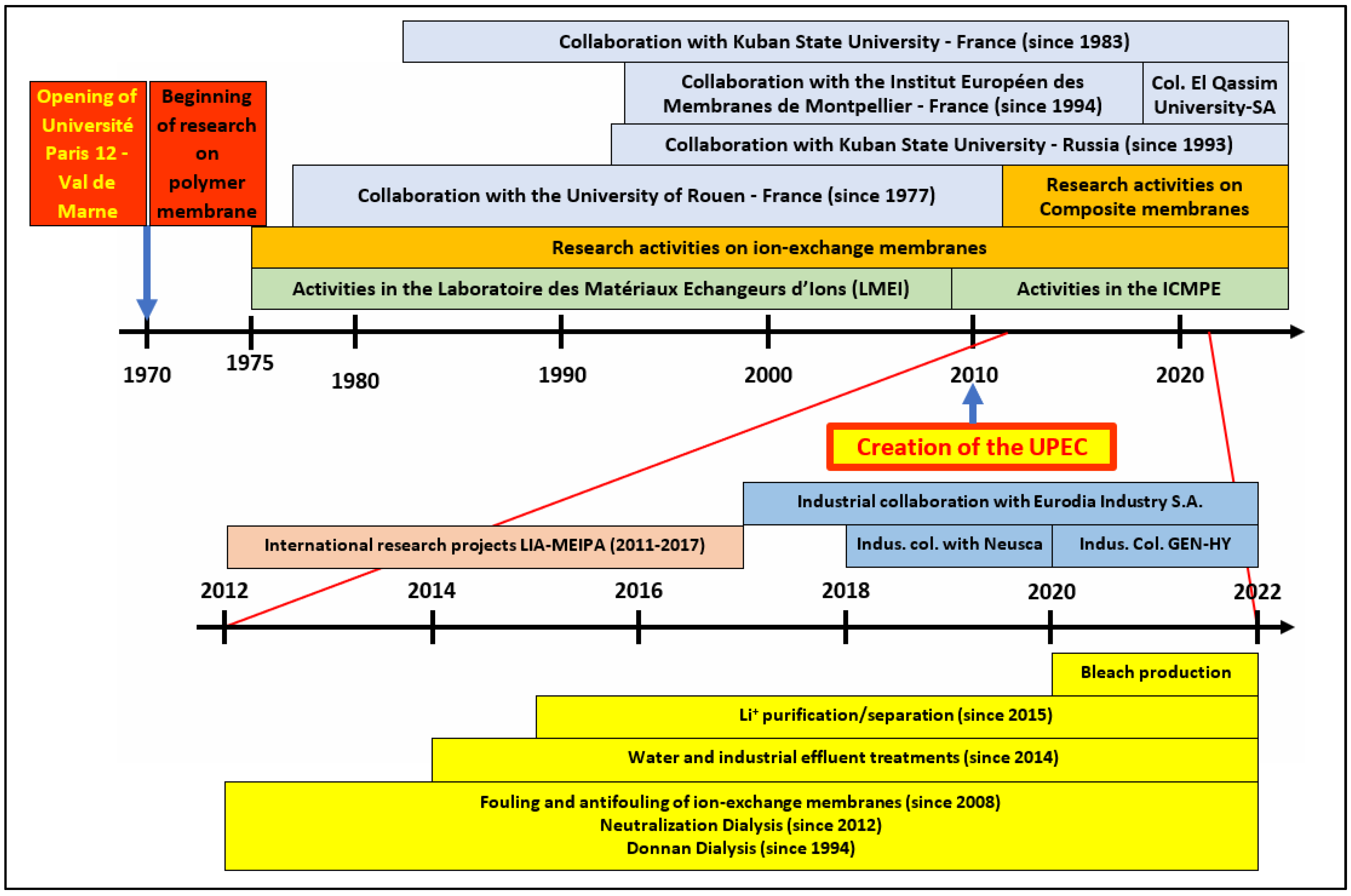
2. Brief History of Polymeric Membrane Research at UPEC
3. Main Results Obtained during the Period 2012–2022
3.1. Composite Membranes: Synthesis, Characterization, and Applications
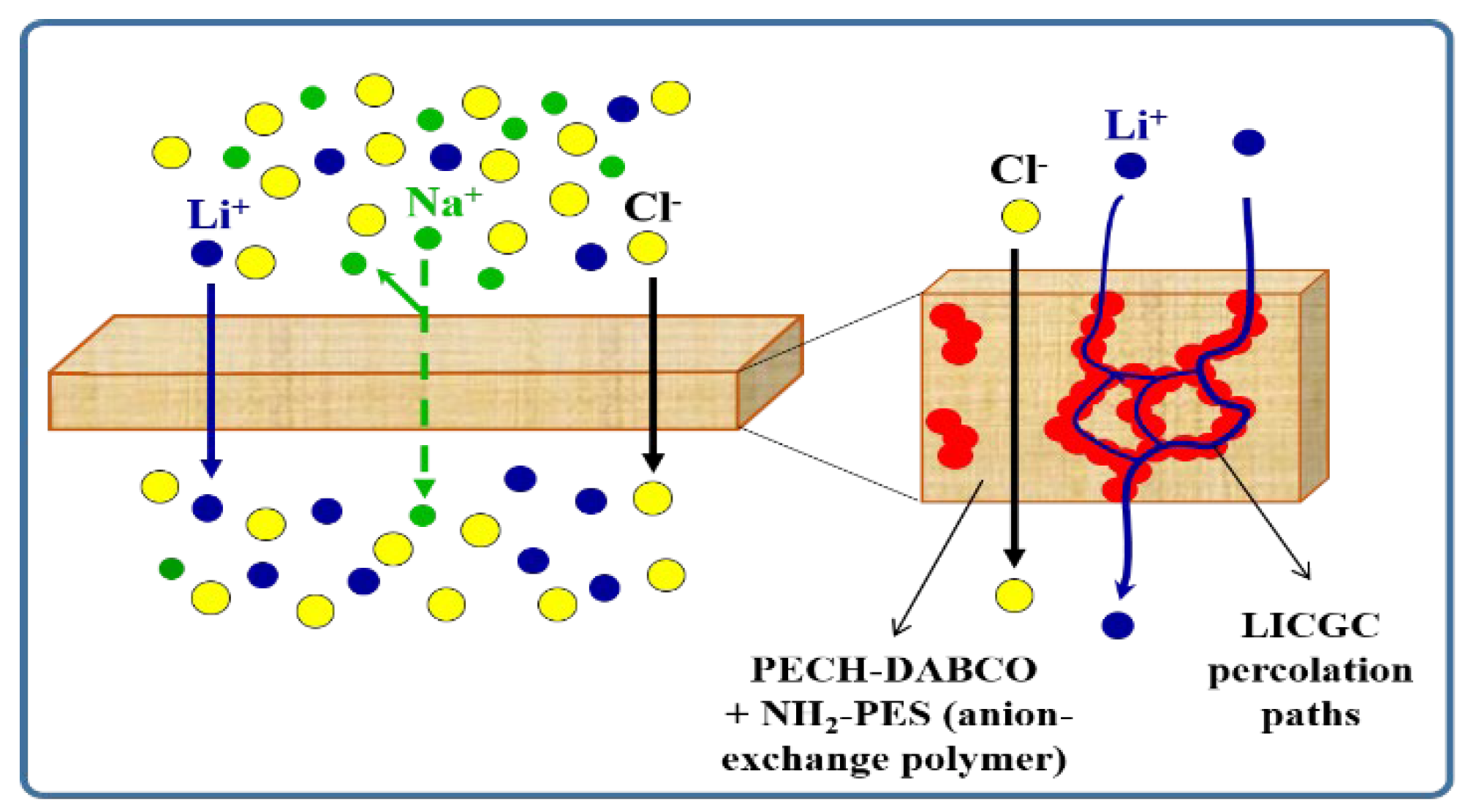
3.1.1. Highly Selective Lithium-Ion Extraction
3.1.2. Bleach Production
3.1.3. Water and Industrial Effluent Treatments
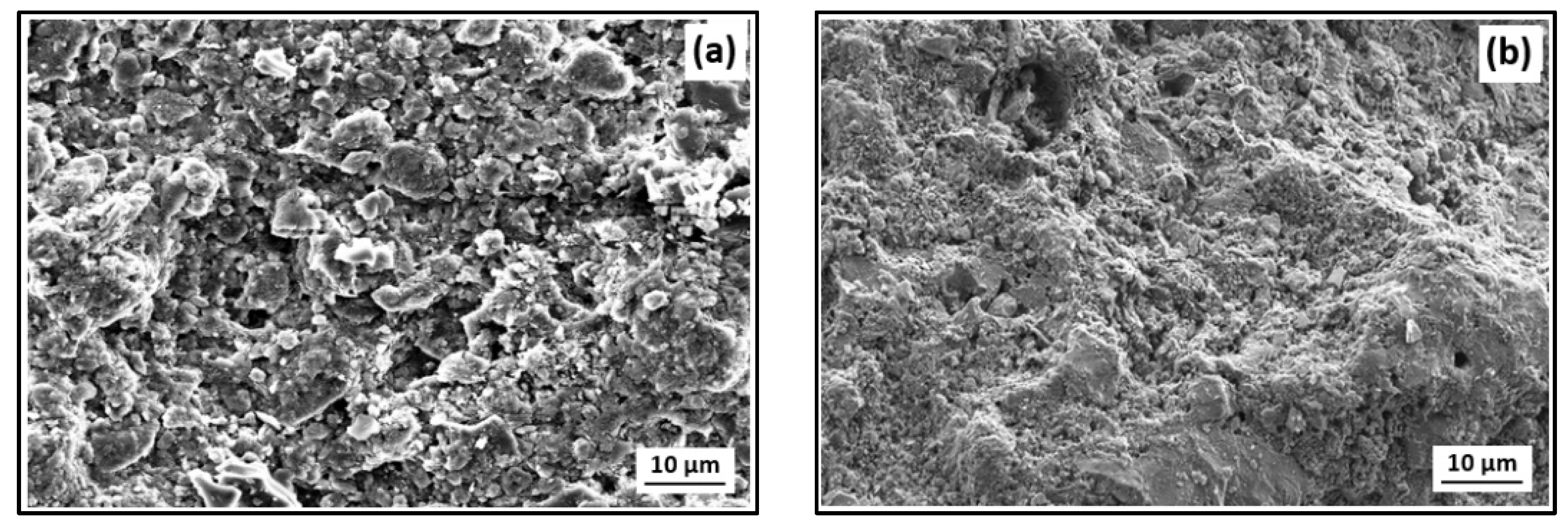
- (a)
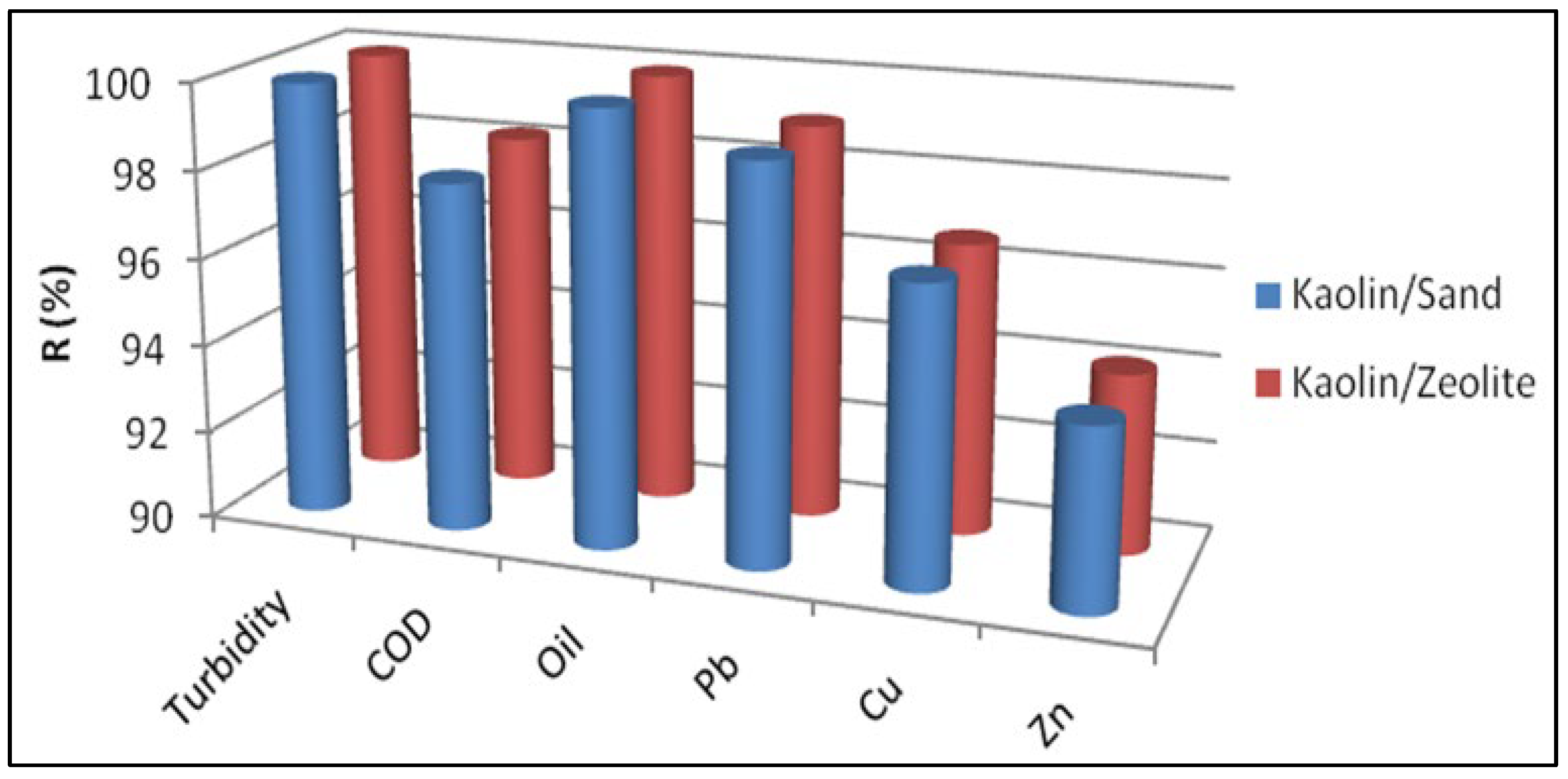
- Development of ultrafiltration Kaolin membranes over Sand and Zeolite supports for the treatment of electroplating wastewater. Collaborations with Qassim University and University of Sfax.
- A total of 8% of Kaolin powder (ϕ < 53 µm) was mixed with 62% of water and 30% of PVA (12 wt% aqueous solution) for Kaolin/Sand membrane.
- A total of 2% of Kaolin powder (ϕ < 53 µm) was mixed with 68% of water and 30% of PVA (12 wt% aqueous solution) for Kaolin/Zeolite membrane.
- (b)
- Polymer inclusion membrane processes for industrial effluent treatment. Collaborations with the University of Gafsa.
- (c)
- Removal of micro-pollutants by Donnan Dialysis. Collaborations with Qassim University and University of Tunis.
3.2. Ion-Exchange Membranes: Characterization, Applications in Dialysis Processes, Fouling and Antifouling Studies
3.2.1. Ion-Exchange Characterization
- To evaluate the individual performance of a new membrane.
- To be able to compare membranes between them.
- To evaluate the effects of a desired modification (surface or mass treatments) or not desired (fouling, scaling) on the performance of a membrane,
- To understand the often-complex relationships between the microstructure and overall performance.
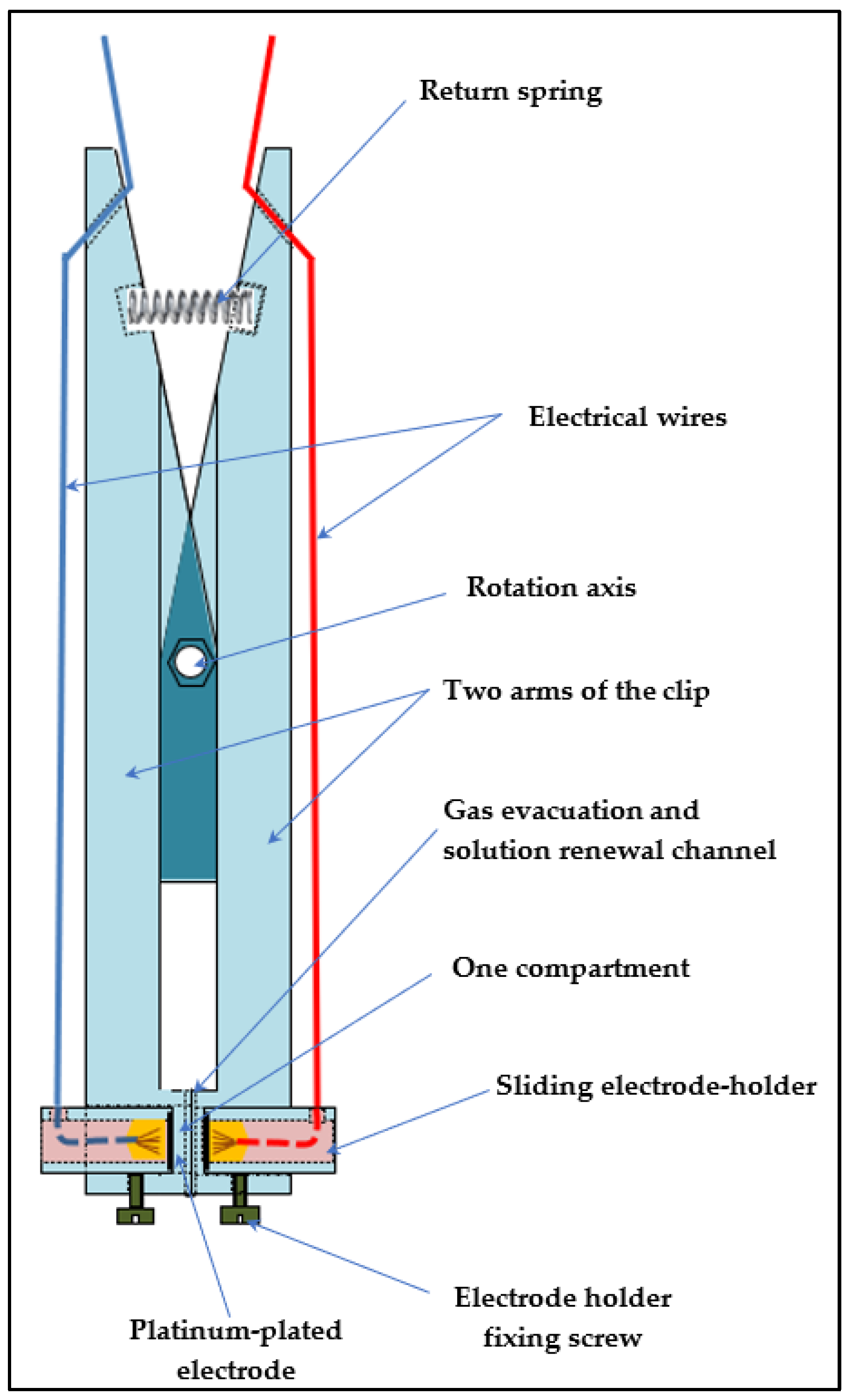
- (a)
- Clip cell for membrane conductivity
- (b)
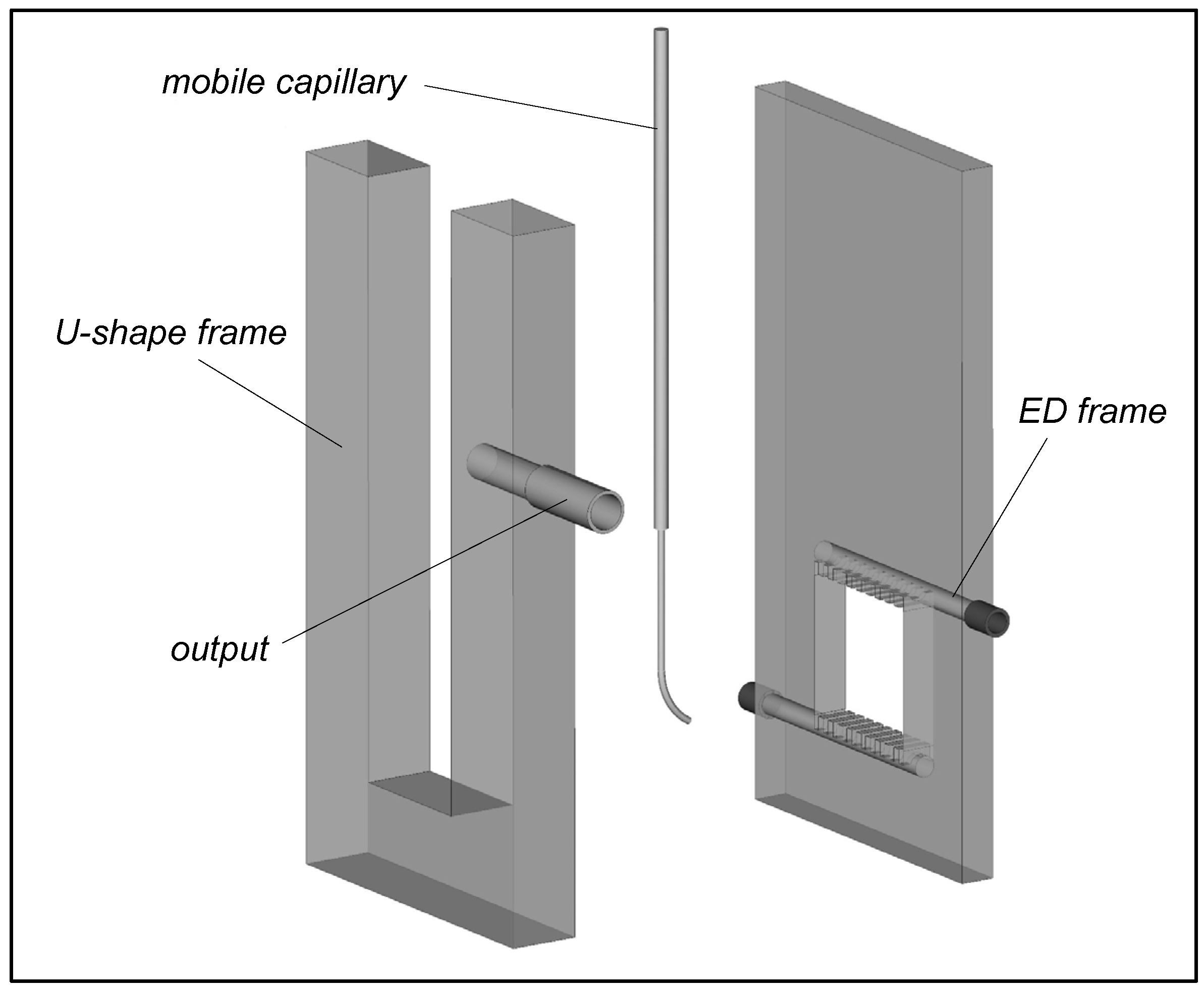
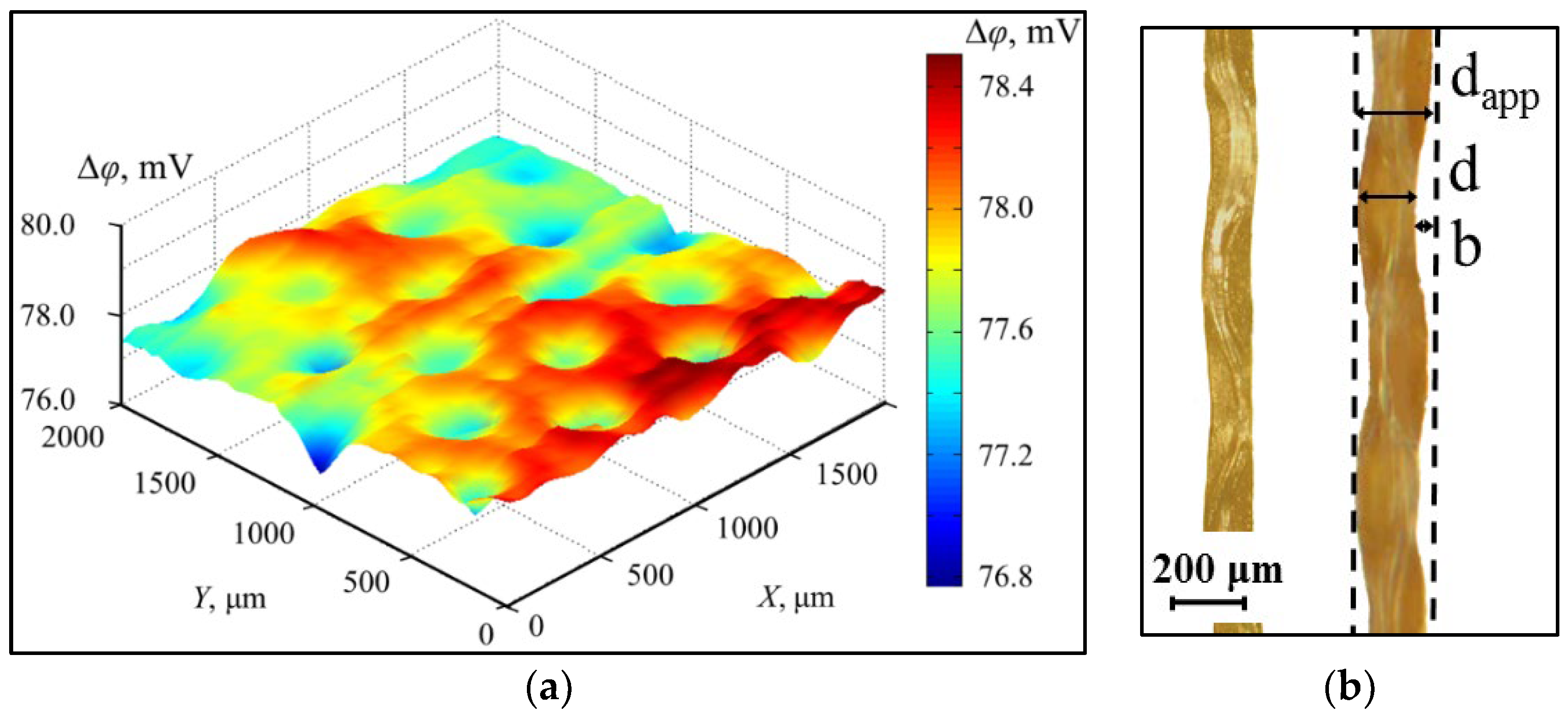
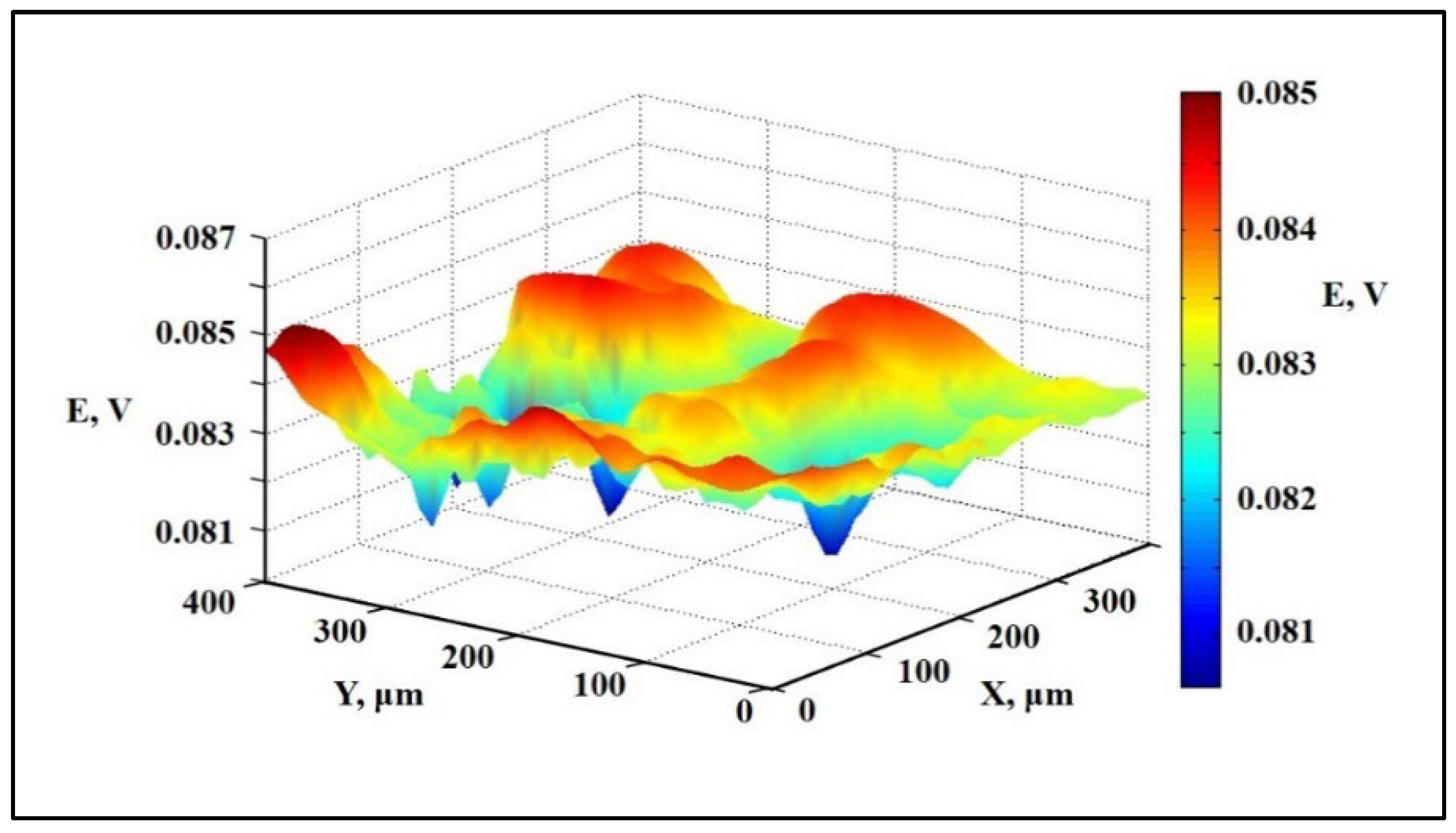
- Scanning Ion Conductance Microscopy. Collaboration with Kuban State University
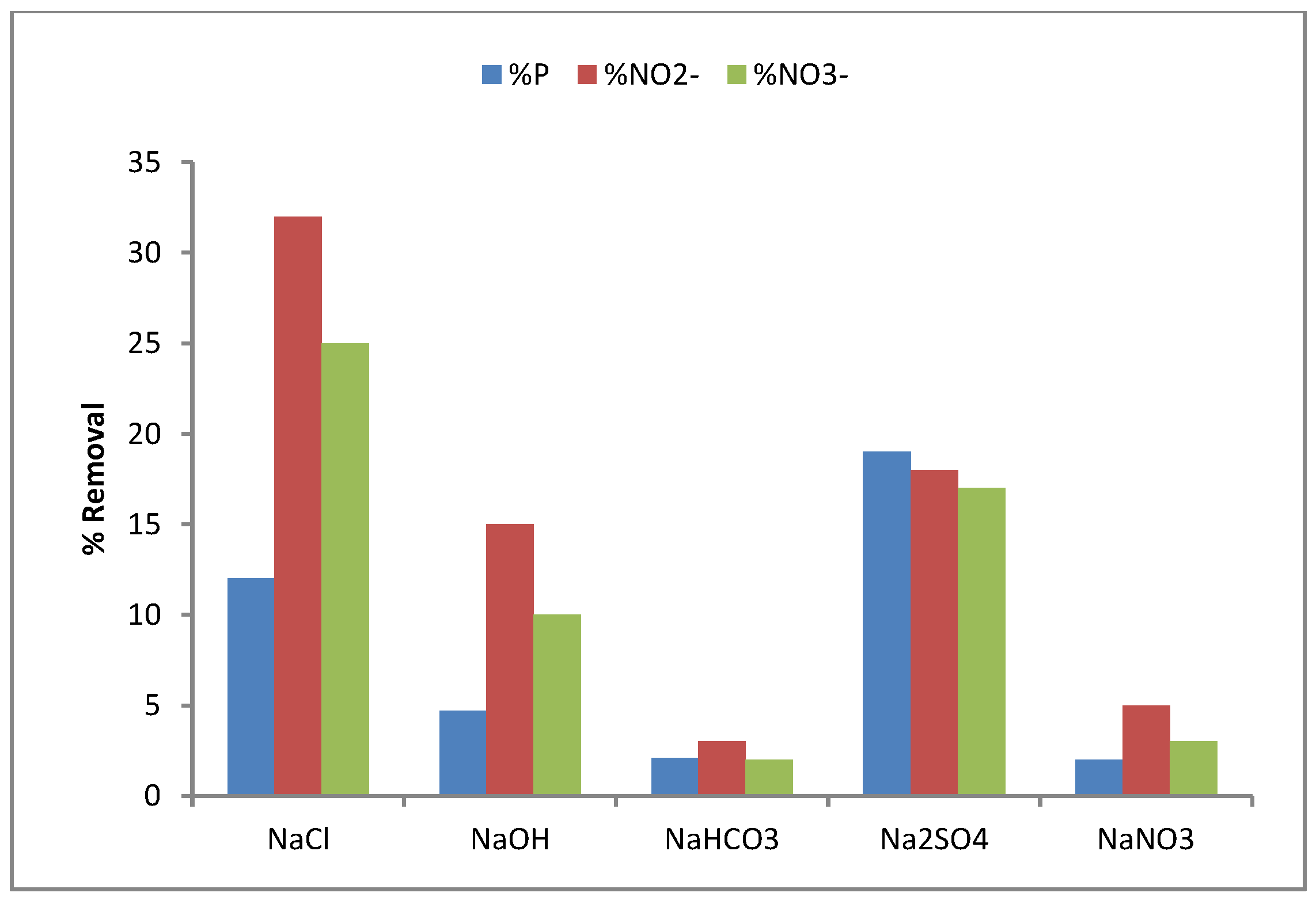
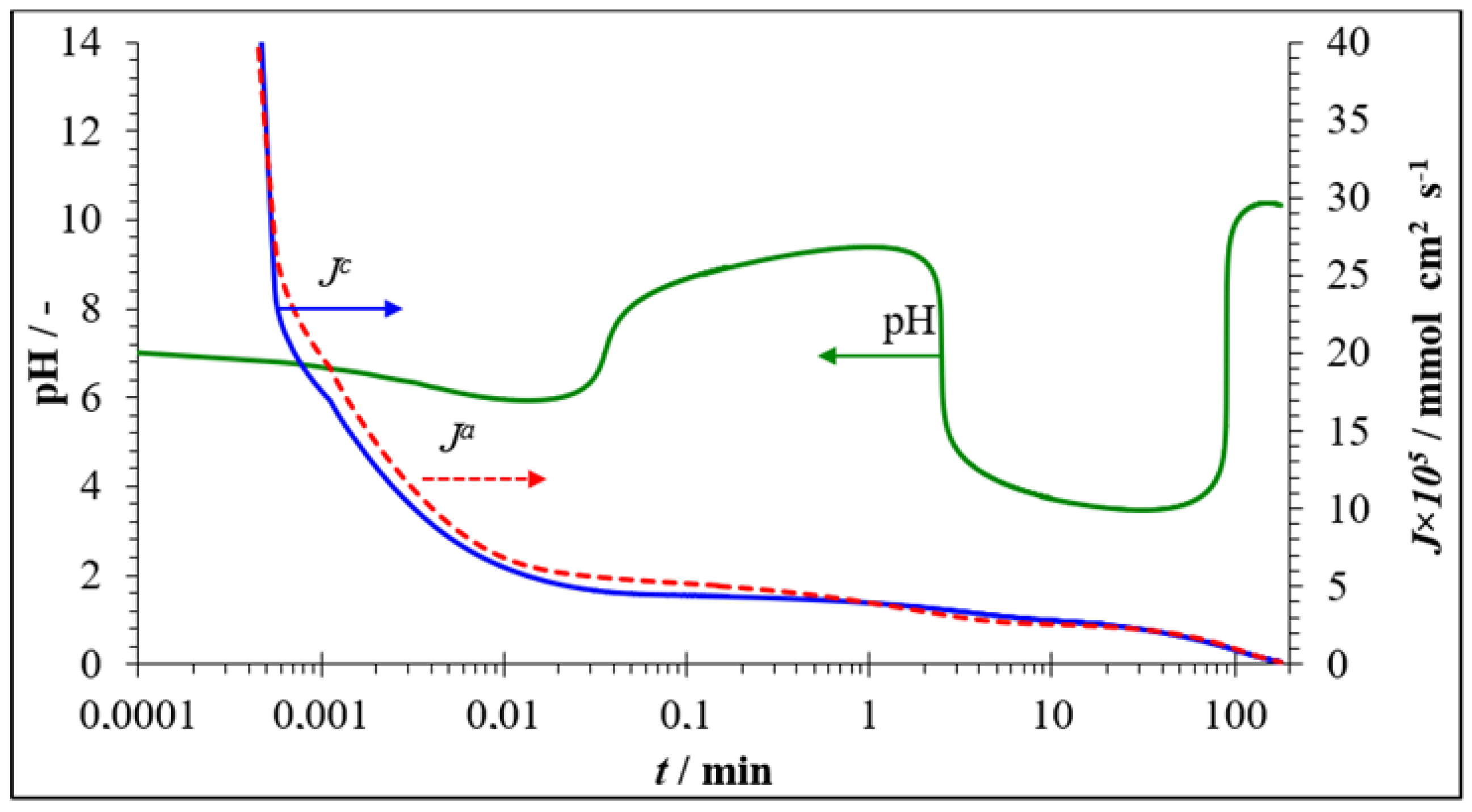
3.2.2. Neutralization Dialysis for Brackish Water Demineralization
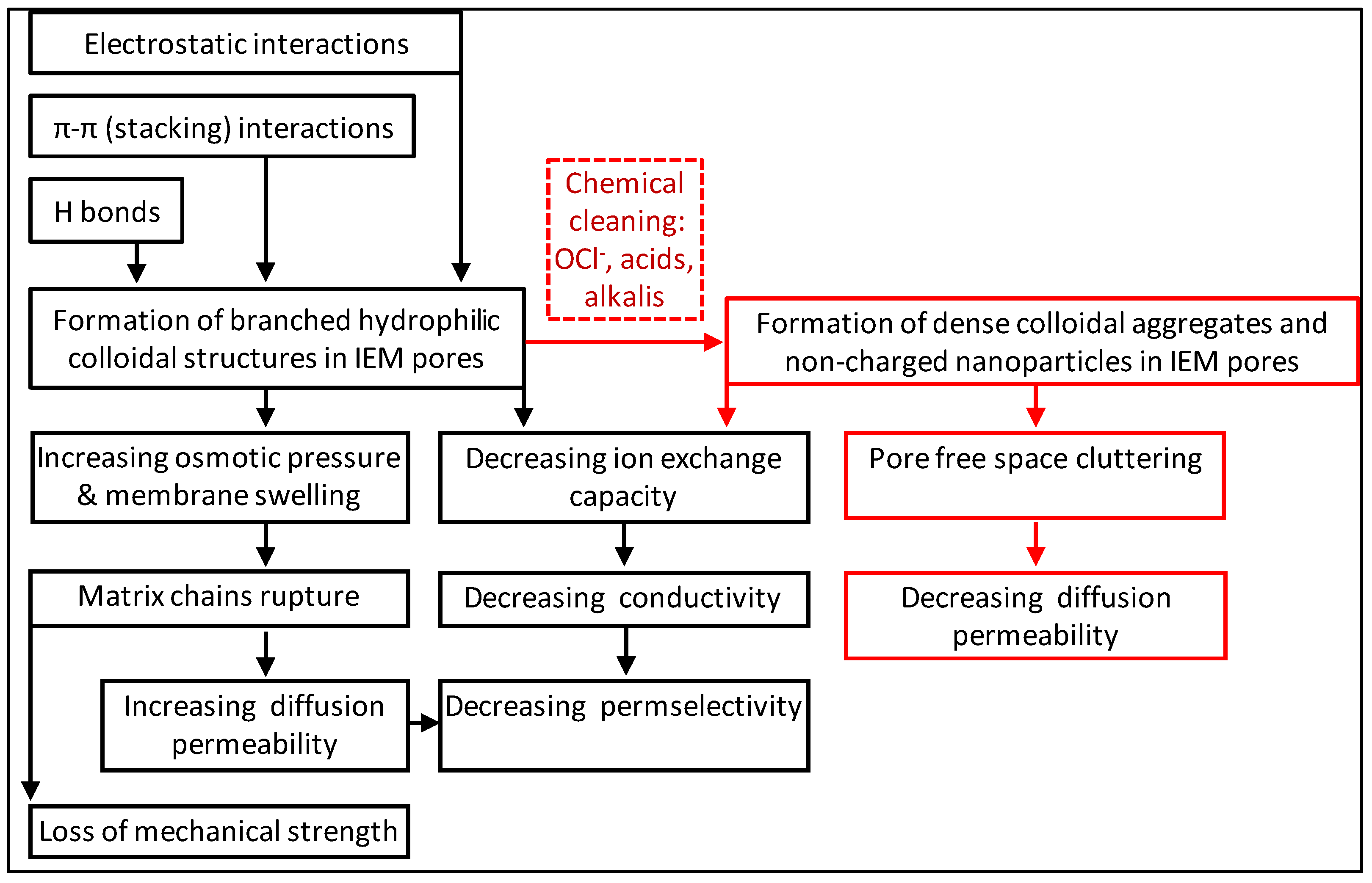
3.2.3. Fouling and Antifouling of Ion-Exchange Membranes
4. Main Collaborations
4.1. National Collaboration
4.2. European Collaboration
4.3. International Collaborations
4.3.1. Collaboration with Kuban State University (KubSU) in Russia
- Implementation of joint research projects;
- Co-supervision of PhD students;
- Mobility of young researchers;
- Joint organization of and participation in scientific conferences;
- Joint participation in juries for the defense of dissertations.
- (a)
- Implementation of joint research projects
- (b)
- Co-supervision of PhD students
- (c)
- Mobility of young researchers
- (d)
- Joint organization of and participation in scientific conferences
- (e)
- Joint participation in juries for the defense of dissertations
4.3.2. Collaboration with the University of El Qassim (Saudi Arabia)
- The central process of Neutralization Dialysis (ND) [181] which is a process developed within the UPEC, and which allows for the substitution of the cations of a charged water with H+, and at the same time, the anions of this water by OH− ions. The latter combines with the H+ to give water. Thus, the water initially charged with mineral salts is demineralized.
- The Bipolar Membrane Electrodialysis (BPED) process [182,183,184], which is used to produce caustic soda and hydrochloric acid by the electrolysis of cooking salt. These two acidic and basic solutions produced will be used to feed the ND process with H+ and OH− ions. The BPED process must be powered by a low-voltage DC power source.
- The production of electricity by Photovoltaic Panels, which allows for the production of the direct current necessary to the operation of the BPED process and the pumps necessary for the circulation of the fluids. These panels will thus ensure the energy autonomy of the whole.
5. Main Perspectives
5.1. Design of Autonomous and Energy Self-Sufficient Processes
- To develop a mobile unit, autonomous in products and energy, to produce drinking water from sea water or brackish water. This unit will be sized for a large family (~10 people) or a village (~100 people).
- To ensure the efficiency of this unit.
- Short term:
- To produce acidic and basic solutions of molarity close to 100 mM from surface or sea water and from the electrical energy produced by photovoltaic panels.
- To study the durability and the robustness of the system.
- To estimate the production cost of these solutions and the carbon impact of this technique.
5.2. Refinement of Characterization by Electrochemical Scanning Microscopy
5.3. Functional Membrane Separators
5.4. Green Membranes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. The List of Joint Projects
- 1.
- INTAS—Kazakhstan 95-0026: Application of electro-membrane technology for the provision of the Aral region population with drinking water (February 1995–September 1998). Coordinator C. Larchet, LMEI—UPEC
- 2.
- The Franco-Russian network program TRAINING—RESEARCH 1997–1999 (ref 1408/AP/LN/MJ, decision n°96P0079; case n°190518K grant n°190528G/bergère). Within the framework of this program, three Russian students completed and defended their theses in France (at the UPEC, the European Membrane Institute, Montpellier, and the University of Rouen): two students from Krasnodar and one student from the State University of Voronezh.
- 3.
- INTAS—Aral Sea 00-1058: Mass transfer phenomena in membrane systems and ion exchangers. Theoretical and experimental research for the improvement of electro-membrane technology for implementing a new technique for quality drinking water produced from the Aral Sea Basin (January 2001–July 2004). Coordination G. Pourcelly, IEM-CNRS Montpellier
- 4.
- PICS 1811: Ion and water transport in membranes and ion exchange materials (2002–2004).
- 5.
- PECO, a series of French–Russian projects between the European Membrane Institute (IEM), Montpellier and the University of Kuban, Krasnodar (2000–2001: PECO Nb 8768; 2002–2003: PECO/CIE Nb 9327; 2004–2005: PECO-NEI Nb 16334). The projects concern studies of ionic transport through ion exchange membranes that take into account the hydrolysis reactions accompanying transfer during the treatment of natural waters.
- 6.
- INTAS—Kazakhstan 04-81-7318: “Theoretical and experimental research for development of a novel electro-membrane process for deionized water production in order to reduce the environmental pollution” (the project was accepted for funding on 18 January 2005). Seven partners, including two French teams (the IEM, Montpellier, and the LMEI at the UPEC, Créteil), one team from Twente (the University of Twente), two Russian teams (the KubSU, Krasnodar, and the Kurnakov Institute of Inorganic and General Chemistry (ICIG), the Russian Academy of Sciences) and two Kazakh teams (MT Innovative Enterprise and the Scientific Institute of Water and Petroleum, Almaty) are joined for fundamental research aimed at the improvement of the membrane technology of water treatment for the production of ultrapure water for the feeding of steam boilers or pyrogen-free water for medicine.
- 7.
- INTAS Postdoctoral n°04-83-3878: individual grant awarded to a young researcher (Mrs. E. Belova, KubSU, Krasnodar, Russia) whose leaders are Professors V. Nikonenko and C. Larchet, respectively, from Russia and France. The duration of the project was 2 years from March 2005, and two stays of 2 months each were planned at the LMEI.
- 8.
- INTAS n°05-1000007-416: an innovation project: Development of a new electrodialysis module by using profiled ion-exchange membranes in electro-membrane technology applied for drinking water production from brackish waters. The project started on 1 April 2006, and the end date was 30 September 2007. The innovation was the use of ion-exchange membranes with a relief profiled surface.
- 9.
- MemBridge. Project Nb 233253, FP7-NMP-2008-CSA-2, Coordination and support action. This is a project aiming at the creation of a global membrane network, which should combine the European Membranes network NanoMemPro and the Russian network. NanoMemPro brings together 13 leading laboratories in the science and technology of membranes. A similar network exists in Russia. The University of Kuban, where Mr. Nikonenko works, is part of this network and is responsible for the coordination in Russia of work on ion exchange membranes and the development of electro-membrane separation techniques.
- 10.
- Project FP7-Marie Curie, Nb 269135, intitled CoTraPhen—Coupled Ion and Volume Transfer Phenomena in Heterogeneous Systems: Modeling, Experiment and Applications in Clean Energy, Micro-Analysis and Water Treatment. The project provides for the exchange of researchers between the partners, among which are the Ecole Nationale Supérieure de Chimie de Montpellier, the University of Montpellier 2 on the one hand, the UPEC, as well as the KubSU.
- 11.
- Associated International Laboratory (LIA—MEIPA) “Ion Exchange Membranes and Associated Processes”. International laboratory created by the CNRS in September 2010 for a start in January 2011. This project has been very well evaluated by the CNRS, and its duration was extended until 31 December 2018. There were four partners in the LIA: two French partners, representing the CNRS (Ecole Nationale Supérieure de Chimie de Montpellier and the University of Montpellier on the one hand, and the UPEC on the other), and two Russian partners (KubSU, Krasnodar, and the Institute of General and Inorganic Chemistry, Russian Academy of Sciences). Four themes were developed:
- Relating the structure of IEM at nano- and microscopic levels with its transfer properties at the macroscopic level.
- Highlighting the effect of the surface properties of IEM on the global behavior of the separative system. The membrane surface was modified either chemically or structurally.
- Understanding the mechanisms of interfacial phenomena and their role in the behavior of the IEM in operation. Hydrodynamic, physicochemical, or electrical conditions were implemented.
- Modelling all the steps of the electro-separation process to compare them with experimental results and to establish predictive models that can meet the specifications of a given application.
- 12.
- PHC KOLMOGOROV 2017 PROJECT N° 38200SF “Development of new catalytic membrane reactors for hydrogen energy, water treatment and biobased chemistry by physical and chemical modification of the membrane volume and/or surface”. All the groups of the LIA FR MEIPA participated in this project. Apart from the usual separation processes, in this project, the focus was on catalytic membrane reactors. A new group, that of Professor U.-B. Demirci from the University of Montpellier, was involved. The project was based on the development of the scientific basis for new globally competitive technologies to produce high-purity hydrogen. In the field of MEIs, a new element, the generation of H+ and OH− ions with catalytic participation of functional groups, was in the scope of the joint work. The last topic was developed at the UPEC.
References
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2004; ISBN 978-0-470-85445-7. [Google Scholar]
- Seader, J.D.; Henley, E.J.; Roper, D.K. Separation Process Principles; Wiley: New York, NY, USA, 1998; Volume 25. [Google Scholar]
- Reynolds, T.D.; Richards, P.A. Unit Operations and Processes in Environmental Engineering, 2nd ed.; PWS Publishing Campany: Boston, MA, USA, 1996; Available online: https://www.scribd.com/document/407464017/Unit-Operations-and-Processes-in-Environmental-Engineering-Reynolds-2nd-Edition-1996-PWS-pdf (accessed on 9 October 2022).
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 5th ed.; McGraw-Hill: New York, NY, USA, 1993; ISBN 978-0-07-112738-7. [Google Scholar]
- Fane, A.G.; Schäfer, A.; Waite, T.D. Nanofiltration: Principles and Applications; Elsevier Science: Amsterdam, The Netherlands, 2004; ISBN 978-1-4933-0382-3. [Google Scholar]
- Lau, W.-J.; Ismail, A.F. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling control—A review. Desalination 2009, 245, 321–348. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Noel, M. Membrane Processes for Dye Wastewater Treatment: Recent Progress in Fouling Control. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1007–1040. [Google Scholar] [CrossRef]
- Mo, J.H.; Lee, Y.H.; Kim, J.; Jeong, J.Y.; Jegal, J. Treatment of dye aqueous solutions using nanofiltration polyamide composite membranes for the dye wastewater reuse. Dye. Pigment. 2008, 76, 429–434. [Google Scholar] [CrossRef]
- Bdiri, M.; Perreault, V.; Mikhaylin, S.; Larchet, C.; Hellal, F.; Bazinet, L.; Dammak, L. Identification of phenolic compounds and their fouling mechanisms in ion-exchange membranes used at an industrial scale for wine tartaric stabilization by electrodialysis. Sep. Purif. Technol. 2019, 233, 115995. [Google Scholar] [CrossRef]
- Catarino, M.; Mendes, A. Dealcoholizing wine by membrane separation processes. Innov. Food Sci. Emerg. Technol. 2011, 12, 330–337. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Blackman, J.W.; Agboola, S.O. Production Technologies for Reduced Alcoholic Wines. J. Food Sci. 2012, 77, R25–R41. [Google Scholar] [CrossRef] [PubMed]
- Charcosset, C. Classical and Recent Applications of Membrane Processes in the Food Industry. Food Eng. Rev. 2021, 13, 322–343. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Marzouk, I.; Chaabane, L.; Dammak, L.; Hamrouni, B. Application of Donnan Dialysis Coupled to Adsorption onto Activated Alumina for Chromium (VI) Removal. AJAC 2013, 04, 420–425. [Google Scholar] [CrossRef]
- Kozmai, A.; Chérif, M.; Dammak, L.; Bdiri, M.; Larchet, C.; Nikonenko, V. Modelling non-stationary ion transfer in neutralization dialysis. J. Membr. Sci. 2017, 540, 60–70. [Google Scholar] [CrossRef]
- Trifi, I.M.; Chaabane, L.; Dammak, L.; Baklouti, L.; Hamrouni, B. Response Surface Methodology for Boron Removal by Donnan Dialysis: Doehlert Experimental Design. Membranes 2021, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Kobuchi, Y.; Motomura, H.; Noma, Y.; Hanada, F. Application of ion exchange membranes to the recovery of acids by diffusion dialysis. J. Membr. Sci. 1986, 27, 173–179. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Lopez, J.; Gueccia, R.; Randazzo, S.; Cipollina, A.; Cortina, J.L.; Micale, G. Diffusion dialysis for the treatment of H2SO4-CuSO4 solutions from electroplating plants: Ions membrane transport characterization and modelling. Sep. Purif. Technol. 2021, 266, 118215. [Google Scholar] [CrossRef]
- Nouri, S.; Dammak, L.; Bulvestre, G.; Auclair, B. Studies of the crossed ionic fluxes through a cation-exchange membrane in the case of Donnan dialysis. Desalination 2002, 148, 383–388. [Google Scholar] [CrossRef]
- Tourreuil, V.; Dammak, L.; Bulvestre, G.; Auclair, B. Dialysis applied to the selectivity study of cation exchange membranes in contact with strong electrolytes. New J. Chem. 1998, 22, 1463–1468. [Google Scholar] [CrossRef]
- Marzouk, I.; Dammak, L.; Chaabane, L.; Hamrouni, B. Optimization of Chromium (Vi) Removal by Donnan Dialysis. AJAC 2013, 4, 306–313. [Google Scholar] [CrossRef]
- Chérif, M.; Mkacher, I.; Ghalloussi, R.; Chaabane, L.; Ben Salah, A.; Walha, K.; Dammak, L.; Grande, D. Experimental investigation of neutralization dialysis in three-compartment membrane stack. Desalination Water Treat. 2015, 56, 2567–2575. [Google Scholar] [CrossRef]
- Chérif, M.; Mkacher, I.; Dammak, L.; Ben Salah, A.; Walha, K.; Grande, D.; Nikonenko, V. Water desalination by neutralization dialysis with ion-exchange membranes: Flow rate and acid/alkali concentration effects. Desalination 2015, 361, 13–24. [Google Scholar] [CrossRef]
- Chérif, M.; Mkacher, I.; Dammak, L.; Ben Salah, A.; Walha, K.; Nikonenko, V.; Korchane, S.; Grande, D. Fractional factorial design of water desalination by neutralization dialysis process: Concentration, flow rate, and volume effects. Desalination Water Treat. 2016, 57, 14403–14413. [Google Scholar] [CrossRef]
- Chérif, M.; Korchane, S.; Chaabane, L.; Dammak, L.; Ben Salah, A.; Walha, K.; Kozmai, A. Reconstituted and brackish waters desalination by neutralization dialysis process with ion-exchange membranes. DWT 2017, 65, 52–59. [Google Scholar] [CrossRef]
- Kozmai, A.; Nikonenko, V.; Pismenskaya, N.; Dammak, L.; Baklouti, L.; Yutskevich, Y. Effect of anion exchange membrane capacity loss on pH and electric conductivity of saline solution during neutralization dialysis. J. Membr. Sci. 2020, 595, 117573. [Google Scholar] [CrossRef]
- Ould Tfeil, H.; Mar-Diop, C.; Sambé, F.; Sock, O.; Dammak, L.; Auclair, B. Defluoruration des eaux de la région de Diourbel (Sénégal) par dialyse ionique croisé. Phys. Chem. News 2005, 25, 118–126. [Google Scholar]
- Ould Tfeil, H.; Mar-Diop, C.; Sambé, F.; Dammak, L.; Chaabane, L.; Auclair, B. A simple steady state model for ion-exchange membranes applied to defluoridation of drinking water by crossed-fluxes dialysis. J. Sci. Ingénieur. 2007, 8, 37–49. [Google Scholar]
- Šímová, H.; Kysela, V.; Černín, A. Demineralization of natural sweet whey by electrodialysis at pilot-plant scale. Desalination Water Treat. 2010, 14, 170–173. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Abu Hasan, H. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Galama, A.H.; Saakes, M.; Bruning, H.; Rijnaarts, H.H.M.; Post, J.W. Seawater predesalination with electrodialysis. Desalination 2014, 342, 61–69. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Dammak, L.; Fouilloux, J.; Bdiri, M.; Larchet, C.; Renard, E.; Baklouti, L.; Sarapulova, V.; Kozmai, A.; Pismenskaya, N. A Review on Ion-Exchange Membrane Fouling during the Electrodialysis Process in the Food Industry, Part 1: Types, Effects, Characterization Methods, Fouling Mechanisms and Interactions. Membranes 2021, 11, 789. [Google Scholar] [CrossRef]
- Lindstrand, V.; Sundström, G.; Jönsson, A.-S. Fouling of electrodialysis membranes by organic substances. Desalination 2000, 128, 91–102. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Grossman, G.; Sonin, A.A. Membrane fouling in electrodialysis: A model and experiments. Desalination 1973, 12, 107–125. [Google Scholar] [CrossRef]
- Belashova, E.; Mikhaylin, S.; Pismenskaya, N.; Nikonenko, V.; Bazinet, L. Impact of cation-exchange membrane scaling nature on the electrochemical characteristics of membrane system. Sep. Purif. Technol. 2017, 189, 441–448. [Google Scholar] [CrossRef]
- Corbatón-Báguena, M.-J.; Álvarez-Blanco, S.; Vincent-Vela, M.-C. Cleaning of ultrafiltration membranes fouled with BSA by means of saline solutions. Sep. Purif. Technol. 2014, 125, 1–10. [Google Scholar] [CrossRef]
- Whittaker, C.; Ridgway, H.; Olson, B.H. Evaluation of Cleaning Strategies for Removal of Biofilms from Reverse-Osmosis Membranes. Appl. Environ. Microbiol. 1984, 48, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Bdiri, M.; Dammak, L.; Larchet, C.; Hellal, F.; Porozhnyy, M.; Nevakshenova, E.; Pismenskaya, N.; Nikonenko, V. Characterization and cleaning of anion-exchange membranes used in electrodialysis of polyphenol-containing food industry solutions; comparison with cation-exchange membranes. Sep. Purif. Technol. 2019, 210, 636–650. [Google Scholar] [CrossRef]
- Larchet, C.; Dammak, L.; Auclair, B.; Parchikov, S.; Nikonenko, V. A simplified procedure for ion-exchange membrane characterisation. New J. Chem. 2004, 28, 1260. [Google Scholar] [CrossRef]
- Ncib, S.; Barhoumi, A.; Bouguerra, W.; Larchet, C.; Dammak, L.; Hamrouni, B.; Elaloui, E. Preparation and characterization of cellulose triacetate polymer inclusion membrane blended with acetylated kraft lignin: Effect of AKL. DWT 2018, 104, 263–272. [Google Scholar] [CrossRef]
- Lteif, R.; Dammak, L.; Larchet, C.; Auclair, B. Détermination du nombre de transport d’un contre-ion dans une membrane échangeuse d’ions en utilisant la méthode de pile de concentration. Eur. Polym. J. 2001, 37, 627–639. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Lebedev, K.A.; Zabolotsky, V.I.; Dammak, L.; Larchet, C.; Auclair, B. A mathematical model for the bi-ionic potential. Eur. Polym. J. 1997, 33, 1057–1059. [Google Scholar] [CrossRef]
- Belaid, N.N.; Ngom, B.; Dammak, L.; Larchet, C.; Auclair, B. Conductivité membranaire: Interprétation et exploitation selon le modèle à solution interstitielle hétérogène. Eur. Polym. J. 1999, 35, 879–897. [Google Scholar] [CrossRef]
- Dammak, L.; Larchet, C.; Auclair, B. Theoretical study of the bi-ionic potential and confrontation with experimental results. J. Membr. Sci. 1999, 155, 193–207. [Google Scholar] [CrossRef]
- Mokrani, S.; Dammak, L.; Larchet, C.; Auclair, B. Bi-ionic system: Theoretical investigation on the ionic fluxes through an ion-exchange membrane. New J. Chem. 1999, 23, 375–380. [Google Scholar] [CrossRef]
- Dammak, L.; Lteif, R.; Bulvestre, G.; Pourcelly, G.; Auclair, B. Determination of the diffusion coefficients of ions in cation-exchange membranes, supposed to be homogeneous, from the electrical membrane conductivity and the equilibrium quantity of absorbed electrolyte. Electrochim. Acta 2001, 47, 451–457. [Google Scholar] [CrossRef]
- Auclair, B.; Nikonenko, V.; Larchet, C.; Métayer, M.; Dammak, L. Correlation between transport parameters of ion-exchange membranes. J. Membr. Sci. 2002, 195, 89–102. [Google Scholar] [CrossRef]
- Nouri, S.; Dammak, L.; Bulvestre, G.; Auclair, B. Comparison of three methods for the determination of the electrical conductivity of ion-exchange polymers. Eur. Polym. J. 2002, 38, 1907–1913. [Google Scholar] [CrossRef]
- Nouri, S.; Dammak, L.; Larchet, C.; Auclair, B. Correlation between ion-exchange membranes characteristics for evaluation of the permselectivity and the diffusion coefficients. Desalination 2002, 147, 363–368. [Google Scholar] [CrossRef]
- Poilbout, K.; Mokrani, S.; Dammak, L.; Larchet, C.; Auclair, B. Détermination du coefficient d’affinité d’une membrane échangeuse de cations pour différentes forces ioniques. Eur. Polym. J. 2000, 36, 1555–1561. [Google Scholar] [CrossRef]
- Kozmai, A.E.; Nikonenko, V.V.; Zyryanova, S.; Pismenskaya, N.D.; Dammak, L. A simple model for the response of an anion-exchange membrane to variation in concentration and pH of bathing solution. J. Membr. Sci. 2018, 567, 127–138. [Google Scholar] [CrossRef]
- Kozmai, A.E.; Nikonenko, V.V.; Zyryanova, S.; Pismenskaya, N.D.; Dammak, L.; Baklouti, L. Modelling of anion-exchange membrane transport properties with taking into account the change in exchange capacity and swelling when varying bathing solution concentration and pH. J. Membr. Sci. 2019, 590, 117291. [Google Scholar] [CrossRef]
- Mareev, S.; Butylskii, D.Y.; Kovalenko, A.V.; Dammak, L.; Pismenskaya, N.; Larchet, C.; Nikonenko, V. Accounting for the concentration dependence of electrolyte diffusion coefficient in the Sand equations. Electrochim. Acta 2016, 52, 996–1000. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Loza, S.A.; Sharafan, M.V. Physicochemical Properties of Profiled Heterogeneous Ion-Exchange Membranes. Russ. J. Electrochem. 2005, 41, 1053–1060. [Google Scholar] [CrossRef]
- Porozhnyy, M.; Huguet, P.; Cretin, M.; Safronova, E.; Nikonenko, V. Mathematical modeling of transport properties of proton-exchange membranes containing immobilized nanoparticles. Int. J. Hydrog. Energy 2016, 41, 15605–15614. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of structural membrane inhomogeneity on transport properties. J. Membr. Sci. 1993, 79, 181–198. [Google Scholar] [CrossRef]
- Guillou, M. Répartitions Couplées du Potentiel et des Concentrations dans les Cellules Électrochimiques; Féculté des Sciences de l’Univesrité de Paris: Paris, France, 1968. [Google Scholar]
- Heredia, F.; Martinez, A.L.; Urtubey, V.S. The importance of lithium for achieving a low-carbon future: Overview of the lithium extraction in the ‘Lithium Triangle’. J. Energy Nat. Resour. Law 2020, 38, 213–236. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2021; p. 200. [Google Scholar]
- Choubey, P.K.; Kim, M.; Srivastava, R.R.; Lee, J.; Lee, J.-Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Meng, F.; McNeice, J.; Zadeh, S.S.; Ghahreman, A. Review of Lithium Production and Recovery from Minerals, Brines, and Lithium-Ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 123–141. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, L.; Sun, W. Systematic review of lithium extraction from salt-lake brines via precipitation approaches. Miner. Eng. 2019, 139, 105868. [Google Scholar] [CrossRef]
- Lu, J.; Stevens, G.W.; Mumford, K.A. Development of heterogeneous equilibrium model for lithium solvent extraction using organophosphinic acid. Sep. Purif. Technol. 2021, 276, 119307. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Functionalized titanate nanotubes for efficient lithium adsorption and recovery from aqueous media. J. Solid State Chem. 2020, 283, 121157. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yang, Y.; Lin, S.; Li, P. Preparation of granular titanium-type lithium-ion sieves and recyclability assessment for lithium recovery from brines with different pH value. Sep. Purif. Technol. 2021, 267, 118613. [Google Scholar] [CrossRef]
- Swain, B. Separation and purification of lithium by solvent extraction and supported liquid membrane, analysis of their mechanism: A review: Separation and purification of lithium. J. Chem. Technol. Biotechnol. 2016, 91, 2549–2562. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Wu, K.-J.; He, C.-H. Tailoring hydrophobic deep eutectic solvent for selective lithium recovery from dilute aqueous solutions. Sep. Purif. Technol. 2022, 281, 119928. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Roobavannan, S.; Vigneswaran, S.; Naidu, G. Enhancing the performance of membrane distillation and ion-exchange manganese oxide for recovery of water and lithium from seawater. Chem. Eng. J. 2020, 396, 125386. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Wang, X.; Li, P.; Ying, W.; Chen, D.; Ma, X.; Deng, Z.; Peng, X. Simultaneous Recovery of Metal Ions and Electricity Harvesting via K-Carrageenan@ZIF-8 Membrane. ACS Appl. Mater. Interfaces 2019, 11, 34039–34045. [Google Scholar] [CrossRef] [PubMed]
- Ounissi, T.; Dammak, L.; Larchet, C.; Fauvarque, J.-F.; Selmane Bel Hadj Hmida, E. Novel lithium selective composite membranes: Synthesis, characterization and validation tests in dialysis. J. Mater. Sci. 2020, 55, 16111–16128. [Google Scholar] [CrossRef]
- Ounissi, T.; Dammak, L.; Fauvarque, J.-F.; Selmane Bel Hadj Hmida, E. Ecofriendly lithium-sodium separation by diffusion processes using lithium composite membrane. Sep. Purif. Technol. 2021, 275, 119134. [Google Scholar] [CrossRef]
- Ounissi, T.; Belhadj Ammar, R.; Larchet, C.; Chaabane, L.; Baklouti, L.; Dammak, L.; Selmane Bel Hadj Hmida, E. Lithium-Sodium Separation by a Lithium Composite Membrane Used in Electrodialysis Process: Concept Validation. Membranes 2022, 12, 244. [Google Scholar] [CrossRef]
- Butylskii, D.; Dammak, L.; Larchet, C.; Pismenskaya, N.; Nikonenko, V. Selective recovery and re-utilization of lithium: Prospects for the use of membrane methods. Russ. Chem. Rev. 2023, 92, RCR5074. [Google Scholar]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Zsirai, T.; Qiblawey, H.; Buzatu, P.; Al-Marri, M.; Judd, S.J. Cleaning of ceramic membranes for produced water filtration. J. Pet. Sci. Eng. 2018, 166, 283–289. [Google Scholar] [CrossRef]
- Chen, R.; Liao, X.; Ge, Q. A novel multinuclear zinc complex Zn-Bet-Tf2N for electroplating wastewater treatment using forward osmosis technique. Chem. Eng. J. 2021, 404, 126569. [Google Scholar] [CrossRef]
- Yan, F.-L.; Wang, Y.; Wang, W.-H.; Zhao, J.-X.; Feng, L.-L.; Li, J.-J.; Zhao, J.-C. Application of biochars obtained through the pyrolysis of Lemna minor in the treatment of Ni-electroplating wastewater. J. Water Process Eng. 2020, 37, 101464. [Google Scholar] [CrossRef]
- Malik, N.; Bulasara, V.K.; Basu, S. Preparation of novel porous ceramic microfiltration membranes from fly ash, kaolin and dolomite mixtures. Ceram. Int. 2020, 46, 6889–6898. [Google Scholar] [CrossRef]
- Mgbemena, C.O.; Ibekwe, N.O.; Sukumar, R.; Menon, A.R.R. Characterization of kaolin intercalates of oleochemicals derived from rubber seed (Hevea brasiliensis) and tea seed (Camelia sinensis) oils. J. King Saud Univ. Sci. 2013, 25, 149–155. [Google Scholar] [CrossRef]
- Bousbih, S.; Errais, E.; Darragi, F.; Duplay, J.; Trabelsi-Ayadi, M.; Daramola, M.O.; Ben Amar, R. Treatment of textile wastewater using monolayered ultrafiltation ceramic membrane fabricated from natural kaolin clay. Environ. Technol. 2021, 42, 3348–3359. [Google Scholar] [CrossRef] [PubMed]
- Aloulou, H.; Bouhamed, H.; Ghorbel, A.; Ben Amar, R.; Khemakhem, S. Elaboration and characterization of ceramic microfiltration membranes from natural zeolite: Application to the treatment of cuttlefish effluents. DWT 2017, 95, 9–17. [Google Scholar] [CrossRef]
- Aloulou, H.; Bouhamed, H.; Amar, R.B.; Khemakhem, S. New ceramic microfiltration membrane from Tunisian natural sand: Application for tangential waste water treatment. DWT 2017, 78, 41–48. [Google Scholar] [CrossRef]
- Aloulou, H.; Bouhamed, H.; Daramola, M.O.; Khemakhem, S.; Amar, R.B. Fabrication of asymmetric ultrafiltration membranes from natural zeolite and their application in industrial wastewater treatment. Euro-Mediterr. J. Environ. Integr. 2020, 5, 36. [Google Scholar] [CrossRef]
- Aloulou, W.; Hamza, W.; Aloulou, H.; Oun, A.; Khemakhem, S.; Jada, A.; Chakraborty, S.; Curcio, S.; Amar, R.B. Developing of titania-smectite nanocomposites UF membrane over zeolite based ceramic support. Appl. Clay Sci. 2018, 155, 20–29. [Google Scholar] [CrossRef]
- Aloulou, H.; Attia, A.; Aloulou, W.; Chakraborty, S.; Baklouti, L.; Dammak, L.; Amar, R.B. Statistical Simulation, a Tool for the Process Optimization of Oily Wastewater by Crossflow Ultrafiltration. Membranes 2022, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Keskin, B.; Zeytuncu-Gökoğlu, B.; Koyuncu, I. Polymer inclusion membrane applications for transport of metal ions: A critical review. Chemosphere 2021, 279, 130604. [Google Scholar] [CrossRef] [PubMed]
- Bożejewicz, D.; Witt, K.; Kaczorowska, M.A. The comparison of the removal of copper(II) and zinc(II) ions from aqueous solution using 2,6-diaminopyridine in a polymer inclusion membrane and in a classic solvent extraction. DWT 2021, 214, 194–202. [Google Scholar] [CrossRef]
- Cachet, N.; Camy, S.; Benjelloun-Mlayah, B.; Condoret, J.-S.; Delmas, M. Esterification of organosolv lignin under supercritical conditions. Ind. Crop. Prod. 2014, 58, 287–297. [Google Scholar] [CrossRef]
- Azizitorghabeh, A.; Rashchi, F.; Babakhani, A. Stoichiometry and structural studies of Fe(III) and Zn(II) solvent extraction using D2EHPA/TBP. Sep. Purif. Technol. 2016, 171, 197–205. [Google Scholar] [CrossRef]
- Kebiche-Senhadji, O.; Mansouri, L.; Benamor, M. Consideration of polymer inclusion membranes containing D2EHPA for toxic metallic ion (Pb2+) extraction recovery. Int. Proc. Chem. Biol. Environ. Eng. 2015, 83, 169. [Google Scholar]
- Ghaderi, N.; Dolatyari, L.; Kazemi, D.; Sharafi, H.R.; Shayani-Jam, H.; Yaftian, M.R. Application of a polymer inclusion membrane made of cellulose triacetate base polymer and trioctylamine for the selective extraction of bismuth( III ) from chloride solutions. J. Appl. Polym. Sci. 2022, 139, 51480. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P. Preparation and characterization of polymeric plasticized membranes (PPM) embedding a crown ether carrier application to copper ions transport. Mater. Sci. Eng. C 2005, 25, 436–443. [Google Scholar] [CrossRef]
- Baczyńska, M.; Waszak, M.; Nowicki, M.; Prządka, D.; Borysiak, S.; Regel-Rosocka, M. Characterization of Polymer Inclusion Membranes (PIMs) Containing Phosphonium Ionic Liquids as Zn(II) Carriers. Ind. Eng. Chem. Res. 2018, 57, 5070–5082. [Google Scholar] [CrossRef]
- Arabi, H.R.; Milani, S.A.; Abolghasemi, H.; Zahakifar, F. Recovery and transport of thorium(IV) through polymer inclusion membrane with D2EHPA from nitric acid solutions. J. Radioanal. Nucl. Chem. 2021, 327, 653–665. [Google Scholar] [CrossRef]
- Rajewski, J. Transport of chromium(III) from mixtures of chromium ions by CTA- and PVC-based inclusion membranes. Water Sci. Technol. 2018, 78, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Turkman, A. Nitrate and pesticides removal from contaminated water using biodenitrification reactor. Process Biochem. 2006, 41, 882–886. [Google Scholar] [CrossRef]
- Ward, M.H.; Kilfoy, B.A.; Weyer, P.J.; Anderson, K.E.; Folsom, A.R.; Cerhan, J.R. Nitrate Intake and the Risk of Thyroid Cancer and Thyroid Disease. Epidemiology 2010, 21, 389–395. [Google Scholar] [CrossRef]
- WHO. Health Hazards from Nitrate in Drinking-Water, Report on a WHO Meeting, Copenhagen, 5–9 March 1984; Regional Office for Europe: Copenhagen, Denmark, 1985. [Google Scholar]
- Altintas, O.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Removal of nitrate from the aqueous phase by Donnan dialysis. Desalination 2009, 239, 276–282. [Google Scholar] [CrossRef]
- Ben Hamouda, S.; Touati, K.; Ben Amor, M. Donnan dialysis as membrane process for nitrate removal from drinking water: Membrane structure effect. Arab. J. Chem. 2017, 10, S287–S292. [Google Scholar] [CrossRef]
- Turki, T.; Hamdi, R.; Tlili, M.; Ben Amor, M. Donnan Dialysis Removal of Nitrate from Water: Effects of Process Parameters. AJAC 2015, 6, 569–576. [Google Scholar] [CrossRef]
- Turki, T.; Ben Amor, M. Nitrate removal from natural water by coupling adsorption and Donnan dialysis. Water Supply 2017, 17, 771–779. [Google Scholar] [CrossRef]
- Trifi, I.M.; Trifi, B.; Ben Ayed, S.; Hamrouni, B. Removal of phosphate by Donnan dialysis coupled with adsorption onto calcium alginate beads. Water Sci. Technol. 2019, 80, 117–125. [Google Scholar] [CrossRef]
- Trifi, I.M.; Trifi, B.; Djemal, A.; Hamrouni, B. Simultaneous removal of nitrates and nitrites from water by DONNAN dialysis using DOEHLERT design. Environ. Eng. Manag. J. 2021, 20, 973–983. [Google Scholar] [CrossRef]
- French Standard NF X 45-200; Séparation d’Ions en Phase Liquides—Membranes Polymères Echangeuses d’Ions—Caractéristiques et Méthodes d’Essais des Membranes Homopolaires. AFNOR: Paris, France, 1995.
- Lteif, R.; Dammak, L.; Larchet, C.; Auclair, B. Conductivitéélectrique membranaire: Étude de l’effet de la concentration, de la nature de l’électrolyte et de la structure membranaire. Eur. Polym. J. 1999, 35, 1187–1195. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Nikonenko, V.V.; Melnik, N.A.; Shevtsova, K.A.; Belova, E.I.; Pourcelly, G.; Cot, D.; Dammak, L.; Larchet, C. Evolution with time of hydrophobicity and microrelief of a cation-exchange membrane surface and its impact on overlimiting mass transfer. J. Phys. Chem. B 2012, 116, 2145–2161. [Google Scholar] [CrossRef] [PubMed]
- Nebavskaya, K.A.; Butylskii, D.Y.; Moroz, I.A.; Nebavsky, A.V.; Pismenskaya, N.D.; Nikonenko, V.V. Enhancement of Mass Transfer Through a Homogeneous Anion-Exchange Membrane in Limiting and Overlimiting Current Regimes by Screening Part of Its Surface with Nonconductive Strips. Pet. Chem. 2018, 58, 780–789. [Google Scholar] [CrossRef]
- Davidson, S.M.; Wessling, M.; Mani, A. On the Dynamical Regimes of Pattern-Accelerated Electroconvection. Sci. Rep. 2016, 6, 22505. [Google Scholar] [CrossRef] [PubMed]
- Zabolotsky, V.I.; Novak, L.; Kovalenko, A.V.; Nikonenko, V.V.; Urtenov, M.H.; Lebedev, K.A.; But, A.Y. Electroconvection in systems with heterogeneous ion-exchange membranes. Pet. Chem. 2017, 57, 779–789. [Google Scholar] [CrossRef]
- Pawlowski, S.; Crespo, J.G.; Velizarov, S. Profiled ion exchange membranes: A comprehensible review. Int. J. Mol. Sci. 2019, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Hansma, P.; Drake, B.; Marti, O.; Gould, S.; Prater, C. The scanning ion-conductance microscope. Science 1989, 243, 641–643. [Google Scholar] [CrossRef]
- Korchev, Y.E.; Milovanovic, M.; Bashford, C.L.; Bennett, D.C.; Sviderskaya, E.V.; Vodyanoy, I.; Lab, M.J. Specialized scanning ion-conductance microscope for imaging of living cells. J. Microsc. 1997, 188, 17–23. [Google Scholar] [CrossRef]
- Butylskii, D.Y.; Mareev, S.A.; Porozhnyy, M.A.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Dammak, L. Electrodialyzer RU197029U1. Available online: https://patents.google.com/patent/RU197029U1/en (accessed on 10 November 2020).
- Mareev, S.A.; Butylskii, D.Y.; Pismenskaya, N.D.; Larchet, C.; Dammak, L.; Nikonenko, V.V. Geometric heterogeneity of homogeneous ion-exchange Neosepta membranes. J. Membr. Sci. 2018, 563, 768–776. [Google Scholar] [CrossRef]
- Sarapulova, V.; Nevakshenova, E.; Pismenskaya, N.; Dammak, L.; Nikonenko, V. Unusual concentration dependence of ion-exchange membrane conductivity in ampholyte-containing solutions: Effect of ampholyte nature. J. Membr. Sci. 2015, 479, 28–38. [Google Scholar] [CrossRef]
- Sedkaoui, Y.; Szymczyk, A.; Lounici, H.; Arous, O. A new lateral method for characterizing the electrical conductivity of ion-exchange membranes. J. Membr. Sci. 2016, 507, 34–42. [Google Scholar] [CrossRef]
- Butylskii, D.Y.; Mareev, S.A.; Nikonenko, V.V.; Pismenskaya, N.D.; Larchet, C.; Dammak, L.; Grande, D.; Apel, P.Y. In situ investigation of electrical inhomogeneity of ion exchange membrane surface using scanning electrochemical microscopy. Pet. Chem. 2016, 56, 1006–1013. [Google Scholar] [CrossRef]
- Volodina, E.; Pismenskaya, N.; Nikonenko, V.; Larchet, C.; Pourcelly, G. Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J. Colloid Interface Sci. 2005, 285, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Igawa, M.; Echizenya, K.; Hayashita, T.; Seno, M. Neutralization Dialysis for Deionization. BCSJ 1987, 60, 381–383. [Google Scholar] [CrossRef]
- Igawa, M.; Tanabe, H.; Ida, T.; Yamamoto, F.; Okochi, H. Separation of Weak Acids and Bases by Neutralization Dialysis. Chem. Lett. 1993, 22, 1591–1594. [Google Scholar] [CrossRef]
- Igawa, M.; Mikami, K.; Okochi, H. Transport Characteristics of Neutralization Dialysis and Desalination of Tap Water. BCSJ 2003, 76, 437–441. [Google Scholar] [CrossRef]
- Bleha, M.; Tishchenko, G.A. Neutralization dialysis for desalination. J. Membr. Sci. 1992, 73, 305–311. [Google Scholar] [CrossRef]
- Wang, M.; Hou, S.; Liu, Y.; Xu, X.; Lu, T.; Zhao, R.; Pan, L. Capacitive neutralization deionization with flow electrodes. Electrochim. Acta 2016, 216, 211–218. [Google Scholar] [CrossRef]
- Goleva, E.A.; Vasileva, V.I.; Solomina, E. Separation of Amino Acid and Mineral Salt by Neutralization Dialysis; Voronej University: Voronej, Russia, 2016; pp. 102–104. [Google Scholar]
- Wang, G.; Tanabe, H.; Igawa, M. Transport of glycine by neutralization dialysis. J. Membr. Sci. 1995, 106, 207–211. [Google Scholar] [CrossRef]
- Denisov, G.A.; Tishchenko, G.A.; Bleha, M.; Shataeva, L.K. Theoretical analysis of neutralization dialysis in the three-compartment membrane cell. J. Membr. Sci. 1995, 98, 13–25. [Google Scholar] [CrossRef]
- PCCell GmbH, Pccell—Model ED 64 004—Lab-Scale Salt Metathesis and Bipolar Electrodialysis Cell. Heusweiler, Germany. Available online: https://www.chemeurope.com/en/companies/22566/pccell-gmbh.html (accessed on 16 February 2023).
- Pismenskaya, N.; Bdiri, M.; Sarapulova, V.; Kozmai, A.; Fouilloux, J.; Baklouti, L.; Larchet, C.; Renard, E.; Dammak, L. A Review on Ion-Exchange Membranes Fouling during Electrodialysis Process in Food Industry, Part 2: Influence on Transport Properties and Electrochemical Characteristics, Cleaning and Its Consequences. Membranes 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Teorell, T. Studies on the “Diffusion Effect” upon Ionic Distribution. Some Theoretical Considerations. Proc. Natl. Acad. Sci. USA 1935, 21, 152–161. [Google Scholar] [CrossRef]
- Meyer, K.H.; Sievers, J.-F. La perméabilité des membranes I. Théorie de la perméabilité ionique. HCA 1936, 19, 649–664. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Gierke, T.D.; Munn, G.E.; Wilson, F.C. The morphology in nafion perfluorinated membrane products, as determined by wide- and small-angle x-ray studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Freeman, B.D. Equilibrium ion partitioning between aqueous salt solutions and inhomogeneous ion exchange membranes. Desalination 2018, 446, 31–41. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, H.; Geise, G.M. Specific co-ion sorption and diffusion properties influence membrane permselectivity. J. Membr. Sci. 2018, 563, 492–504. [Google Scholar] [CrossRef]
- Fridman-Bishop, N.; Freger, V. What makes aromatic polyamide membranes superior: New insights into ion transport and membrane structure. J. Membr. Sci. 2017, 540, 120–128. [Google Scholar] [CrossRef]
- Filippov, A.N.; Kononenko, N.A.; Demina, O.A. Diffusion of electrolytes of different natures through the cation-exchange membrane. Colloid. J. 2017, 79, 556–566. [Google Scholar] [CrossRef]
- Gnusin, N.P.; Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A. Transport structural parameters to characterize ion exchange membranes. J. Membr. Sci. 2004, 243, 301–310. [Google Scholar] [CrossRef]
- Doi, S.; Yasukawa, M.; Kakihana, Y.; Higa, M. Alkali attack on anion exchange membranes with PVC backing and binder: Effect on performance and correlation between them. J. Membr. Sci. 2019, 573, 85–96. [Google Scholar] [CrossRef]
- Chandra, A.; Bhuvanesh, E.; Chattopadhyay, S. Physicochemical interactions of organic acids influencing microstructure and permselectivity of anion exchange membrane. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 260–269. [Google Scholar] [CrossRef]
- Davydov, D.; Nosova, E.; Loza, S.; Achoh, A.; Korzhov, A.; Sharafan, M.; Melnikov, S. Use of the Microheterogeneous Model to Assess the Applicability of Ion-Exchange Membranes in the Process of Generating Electricity from a Concentration Gradient. Membranes 2021, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Tuan, L.X.; Mertens, D.; Buess-Herman, C. The two-phase model of structure microheterogeneity revisited by the study of the CMS cation exchange membrane. Desalination 2009, 240, 351–357. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Safronova, E.Y.; Ilyin, A.B.; Shevlyakov, N.V.; Tverskoi, V.A.; Pourcelly, G.; Yaroslavtsev, A.B. Water state and ionic conductivity of grafted ion exchange membranes based on polyethylene and sulfonated polystyrene. Mendeleev Commun. 2017, 27, 380–381. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Freeman, B.D. Effect of fixed charge group concentration on equilibrium ion sorption in ion exchange membranes. J. Mater. Chem. A 2017, 5, 4638–4650. [Google Scholar] [CrossRef]
- Kamcev, J.; Sujanani, R.; Jang, E.-S.; Yan, N.; Moe, N.; Paul, D.R.; Freeman, B.D. Salt concentration dependence of ionic conductivity in ion exchange membranes. J. Membr. Sci. 2018, 547, 123–133. [Google Scholar] [CrossRef]
- Khoiruddin, K.; Ariono, D.; Subagjo, S.; Wenten, I. Structure and transport properties of polyvinyl chloride-based heterogeneous cation-exchange membrane modified by additive blending and sulfonation. J. Electroanal. Chem. 2020, 873, 114304. [Google Scholar] [CrossRef]
- Niftaliev, S.I.; Kozaderova, O.A.; Kim, K.B. Electroconductance of heterogeneous ion-exchange membranes in aqueous salt solutions. J. Electroanal. Chem. 2017, 794, 58–63. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Akberova, E.M.; Kostylev, D.V.; Tzkhai, A.A. Diagnostics of the Structural and Transport Properties of an Anion-Exchange Membrane MA-40 after Use in Electrodialysis of Mineralized Natural Waters. Membr. Membr. Technol. 2019, 1, 153–167. [Google Scholar] [CrossRef]
- Choy, T.C. Effective Medium Theory: Principles and Applications; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-870509-3. [Google Scholar]
- Yaroslavtsev, A.B.; Nikonenko, V.V. Ion-exchange membrane materials: Properties, modification, and practical application. Nanotechnol Russ. 2009, 4, 137–159. [Google Scholar]
- Larchet, C.; Nouri, S.; Auclair, B.; Dammak, L.; Nikonenko, V. Application of chronopotentiometry to determine the thickness of diffusion layer adjacent to an ion-exchange membrane under natural convection. Adv. Colloid Interface Sci. 2008, 139, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Porozhnyy, M.V.; Sarapulova, V.V.; Pismenskaya, N.D.; Huguet, P.; Deabate, S.; Nikonenko, V.V. Mathematical modeling of concentration dependences of electric conductivity and diffusion permeability of anion-exchange membranes soaked in wine. Pet. Chem. 2017, 57, 511–517. [Google Scholar] [CrossRef]
- Perreault, V.; Sarapulova, V.; Tsygurina, K.; Pismenskaya, N.; Bazinet, L. Understanding of Adsorption and Desorption Mechanisms of Anthocyanins and Proanthocyanidins on Heterogeneous and Homogeneous Cation-Exchange Membranes. Membranes 2021, 11, 136. [Google Scholar] [CrossRef]
- Bukhovets, A.; Eliseeva, T.; Oren, Y. Fouling of anion-exchange membranes in electrodialysis of aromatic amino acid solution. J. Membr. Sci. 2010, 364, 339–343. [Google Scholar] [CrossRef]
- Nevakshenova, E.E.; Sarapulova, V.V.; Nikonenko, V.V.; Pismenskaya, N.D. Application of Sodium Chloride Solutions to Regeneration of Anion-Exchange Membranes Used for Improving Grape Juices and Wines. Membr. Membr. Technol. 2019, 1, 14–22. [Google Scholar] [CrossRef]
- Ghalloussi, R.; Garcia-Vasquez, W.; Chaabane, L.; Dammak, L.; Larchet, C.; Deabate, S.V.; Nevakshenova, E.; Nikonenko, V.; Grande, D. Ageing of ion-exchange membranes in electrodialysis: A structural and physicochemical investigation. J. Membr. Sci. 2013, 436, 68–78. [Google Scholar] [CrossRef]
- Bdiri, M.; Dammak, L.; Chaabane, L.; Larchet, C.; Hellal, F.; Nikonenko, V.; Pismenskaya, N.D. Cleaning of cation-exchange membranes used in electrodialysis for food industry by chemical solutions. Sep. Purif. Technol. 2018, 199, 114–123. [Google Scholar] [CrossRef]
- Guo, H.; You, F.; Yu, S.; Li, L.; Zhao, D. Mechanisms of chemical cleaning of ion exchange membranes: A case study of plant-scale electrodialysis for oily wastewater treatment. J. Membr. Sci. 2015, 496, 310–317. [Google Scholar] [CrossRef]
- Shi, L.; Xie, S.; Hu, Z.; Wu, G.; Morrison, L.; Croot, P.; Hu, H.; Zhan, X. Nutrient recovery from pig manure digestate using electrodialysis reversal: Membrane fouling and feasibility of long-term operation. J. Membr. Sci. 2019, 573, 560–569. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, Z.; Yan, H.; Irfan, M.; Xu, Y.; Li, W.; Wang, H.; Wang, Y. Electrodialytic Desalination of Tobacco Sheet Extract: Membrane Fouling Mechanism and Mitigation Strategies. Membranes 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Pismenskaya, N.; Sarapulova, V.; Nevakshenova, E.; Kononenko, N.; Fomenko, M.; Nikonenko, V. Concentration Dependencies of Diffusion Permeability of Anion-Exchange Membranes in Sodium Hydrogen Carbonate, Monosodium Phosphate, and Potassium Hydrogen Tartrate Solutions. Membranes 2019, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Labbé, D.; Bazinet, L. Effect of membrane type on cation migration during green tea electromigration and equivalent mass transported calculation. J. Membr. Sci. 2006, 275, 220–228. [Google Scholar] [CrossRef]
- Labbé, D.; Araya-Farias, M.; Tremblay, A.; Bazinet, L. Electromigration feasibility of green tea catechins. J. Membr. Sci. 2005, 254, 101–109. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 978-0-470-01039-6. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 978-0-12-373646-8. [Google Scholar]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Histoire du Laboratoire. Available online: https://www.pbs.cnrs.fr/laboratoire/histoire/ (accessed on 30 November 2022).
- Institut Européen des Membranes (IEM). Available online: https://iem.umontpellier.fr/ (accessed on 16 February 2023).
- Eurodia Industrie (Pertuis) Chiffre D’affaires, Résultat, Bilans sur SOCIETE.COM-390953545. Available online: https://www.societe.com/societe/eurodia-industrie-390953545.html (accessed on 30 November 2022).
- Neusca (Buthiers) Chiffre D’affaires, Résultat, Bilans sur SOCIETE.COM-802280669. Available online: https://www.societe.com/societe/neusca-802280669.html (accessed on 30 November 2022).
- Efficient Hydrogen Solutions–GEN-HY. Available online: https://gen-hy.com/ (accessed on 30 November 2022).
- Nikonenko, V.V.; Pismenskaya, N.D.; Belova, E.I.; Sistat, P.; Huguet, P.; Pourcelly, G.; Larchet, C. Intensive current transfer in membrane systems: Modelling, mechanisms and application in electrodialysis. Adv. ColloidInterface Sci. 2010, 160, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.V.; Andreeva, M.A.; Jansezian, L.; Han, J.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Dammak, L. Impact of heterogeneous cation-exchange membrane surface modification on chronopotentiometric and current–voltage characteristics in NaCl, CaCl2 and MgCl2 solutions. Electrochim. Acta 2018, 281, 472–485. [Google Scholar] [CrossRef]
- Mannai, S.; Dammak, L.; Baklouti, L.; Hamdi, A. Synthesis and cation complexation of p-tert-butyl-calix[4]arene bearing two 8-hydroxyquinoline units. J. Incl. Phenom. Macrocycl. Chem. 2019, 94, 257–261. [Google Scholar] [CrossRef]
- Cherif, M. Etude Théorique et Expérimentale de Dessalement des Eaux de Surface par Dialyse de Neutralisation. Ph.D. Thesis, Université Paris-Est Créteil (UPEC): Val de Marne, France, 2015. [Google Scholar]
- Wilhelm, F.G.; van der Vegt, N.F.A.; Wessling, M.; Strathmann, H. Bipolar membrane preparation. In Handbook Bipolar Membrane Technology; Kemperman, A., Ed.; Twente University Press (TUP): Enschede, The Netherlands, 2000; pp. 79–108. [Google Scholar]
- Jiang, C.; Wang, Y.; Wang, Q.; Feng, H.; Xu, T. Production of Lithium Hydroxide from Lake Brines through Electro–Electrodialysis with Bipolar Membranes (EEDBM). Ind. Eng. Chem. Res. 2014, 53, 6103–6112. [Google Scholar] [CrossRef]
- Pelletier, S.; Serre, É.; Mikhaylin, S.; Bazinet, L. Optimization of cranberry juice deacidification by electrodialysis with bipolar membrane: Impact of pulsed electric field conditions. Sep. Purif. Technol. 2017, 186, 106–116. [Google Scholar] [CrossRef]
- Bousbih, S.; Belhadj Ammar, R.; Ben Amar, R.; Dammak, L.; Darragi, F.; Selmane, E. Synthesis and Evaluation of Asymmetric Mesoporous PTFE/Clay Composite Membranes for Textile Wastewater Treatment. Membranes 2021, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; First Publ. New as Paperback; Oxford University Press: Oxford, UK, 2000; ISBN 978-0-19-850698-0. [Google Scholar]
- Clark, J.H. Green chemistry: Challenges and opportunities. Green Chem. 1999, 1, 1–8. [Google Scholar] [CrossRef]
- Ivanković, A. Review of 12 Principles of Green Chemistry in Practice. IJSGE 2017, 6, 39. [Google Scholar] [CrossRef]
- Stein, R.S. Polymer Science and Engineering: The Shifting Research Frontiers; National Academies Press: Washington, DC, USA, 1994; p. 2307. ISBN 978-0-309-04998-6. [Google Scholar]
- Contreras-Martínez, J.; Sanmartino, J.A.; Khayet, M.; García-Payo, M.C. Reuse and recycling of end-of-life reverse osmosis membranes. In Advancement in Polymer-Based Membranes for Water Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 381–417. ISBN 978-0-323-88514-0. [Google Scholar]
- Coutinho de Paula, E.; Santos Amaral, M.C. Environmental and economic evaluation of end-of-life reverse osmosis membranes recycling by means of chemical conversion. J. Clean. Prod. 2018, 194, 85–93. [Google Scholar] [CrossRef]
- Wang, X.; Han, H.; Zhou, H.; Wang, T.; Dai, R.; Wang, Z. Rapid Upcycling of End-of-Life Microfiltration Membrane Mediated by the Healing of Metal–Organic Complex. ACS Sustain. Chem. Eng. 2022, 10, 9841–9849. [Google Scholar] [CrossRef]
- Mamba, F.B.; Mbuli, B.S.; Ramontja, J. Recent Advances in Biopolymeric Membranes towards the Removal of Emerging Organic Pollutants from Water. Membranes 2021, 11, 798. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Tomietto, P.; Loulergue, P.; Paugam, L.; Audic, J.-L. Biobased polyhydroxyalkanoate (PHA) membranes: Structure/performances relationship. Sep. Purif. Technol. 2020, 252, 117419. [Google Scholar] [CrossRef]
- Bdiri, M.; Larchet, C.; Dammak, L. A Review on Ion-exchange Membranes Fouling and Antifouling During Electrodialysis Used in Food Industry: Cleanings and Strategies of Prevention. Chem. Afr. 2020, 3, 609–633. [Google Scholar] [CrossRef]
- Bdiri, M.; Bensghaier, A.; Chaabane, L.; Kozmai, A.; Baklouti, L.; Larchet, C. Preliminary Study on Enzymatic-Based Cleaning of Cation-Exchange Membranes Used in Electrodialysis System in Red Wine Production. Membranes 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Ghalloussi, R.; Chaabane, L.; Larchet, C.; Dammak, L.; Grande, D. Structural and physicochemical investigation of ageing of ion-exchange membranes in electrodialysis for food industry. Sep. Purif. Technol. 2014, 123, 229–234. [Google Scholar] [CrossRef]
- Ghalloussi, R.; Garcia-Vasquez, W.; Chaabane, L.; Dammak, L.; Larchet, C.; Bellakhal, N. Decline of ion-exchange membranes after utilization in electrodialysis for food applications. Phys. Chem. News 2012, 65, 66–72. [Google Scholar]
- Garcia-Vasquez, W.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Effects of acid–base cleaning procedure on structure and properties of anion-exchange membranes used in electrodialysis. J. Membr. Sci. 2016, 507, 12–23. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Dammak, L.; Larchet, C.; Nikonenko, V.; Pismenskaya, N.; Grande, D. Evolution of anion-exchange membrane properties in a full scale electrodialysis stack. J. Membr. Sci. 2013, 446, 255–265. [Google Scholar] [CrossRef]
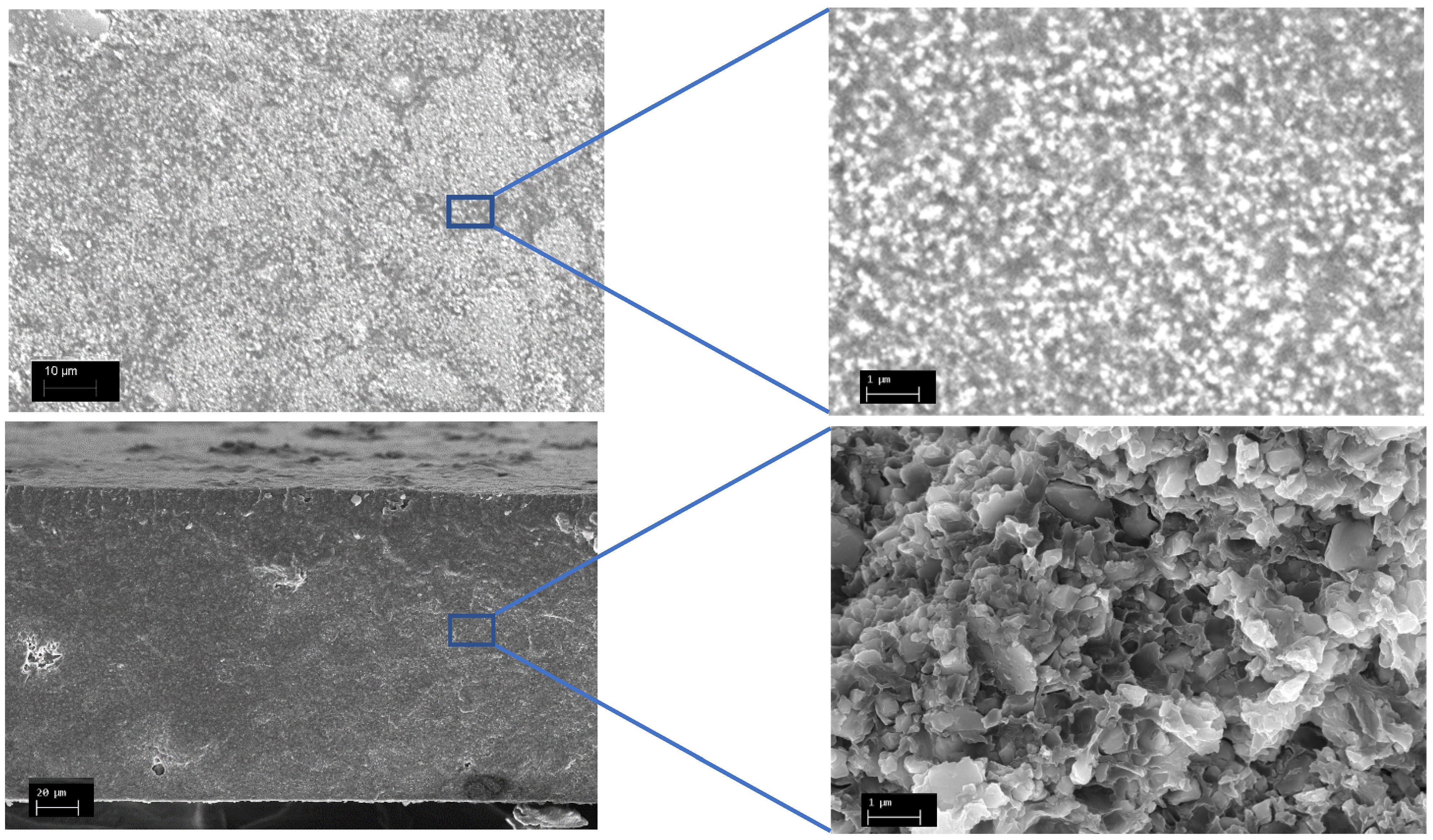
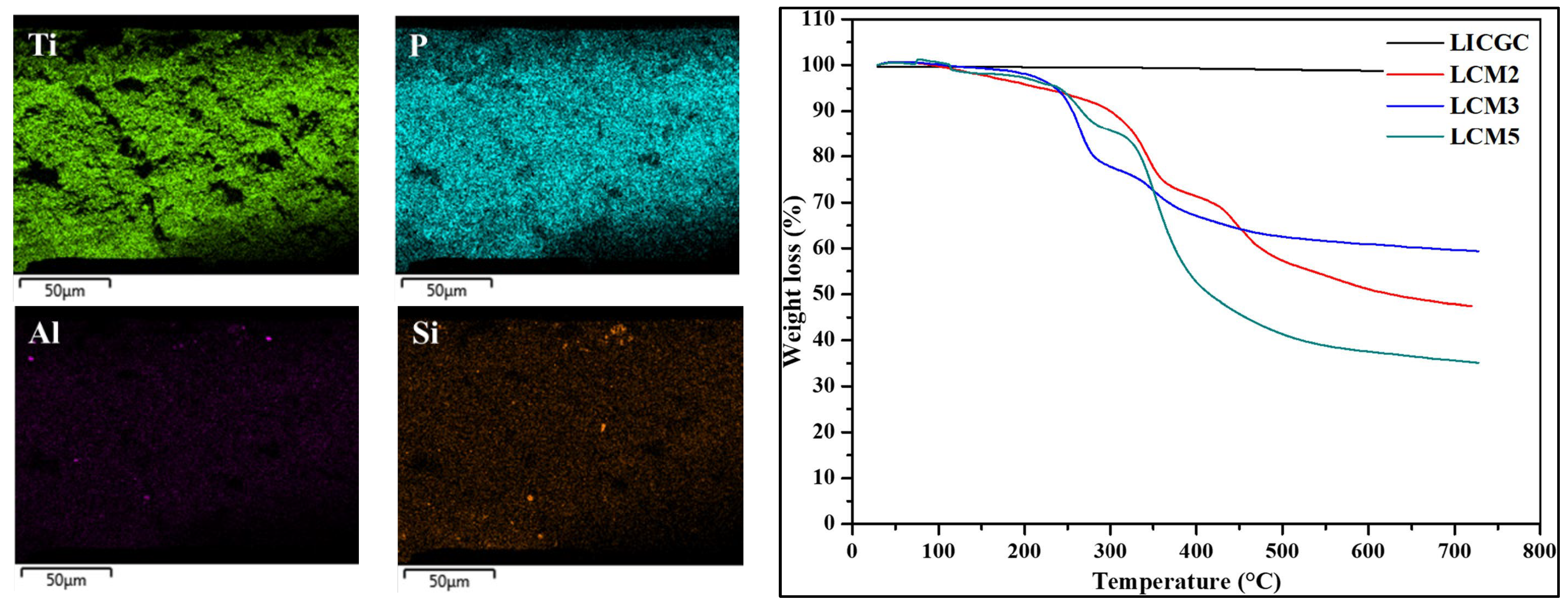
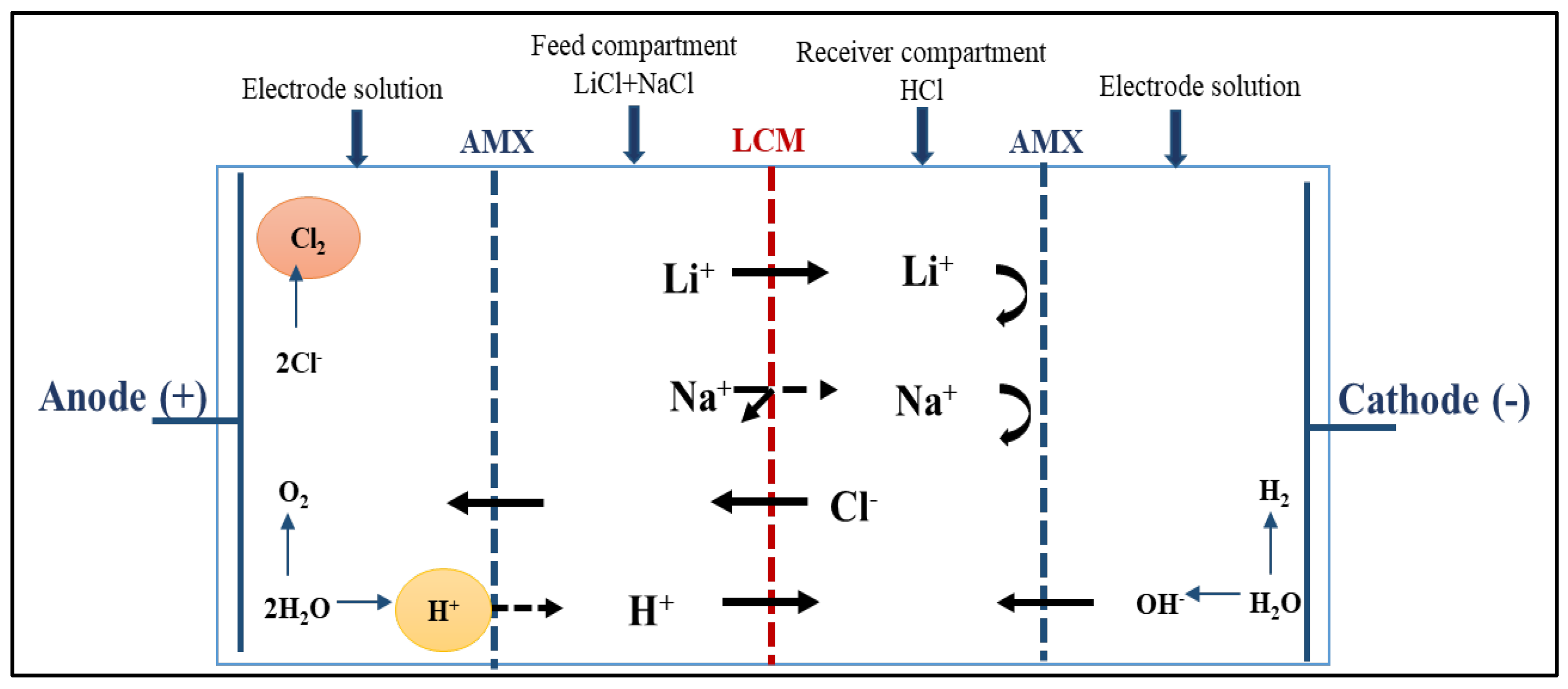
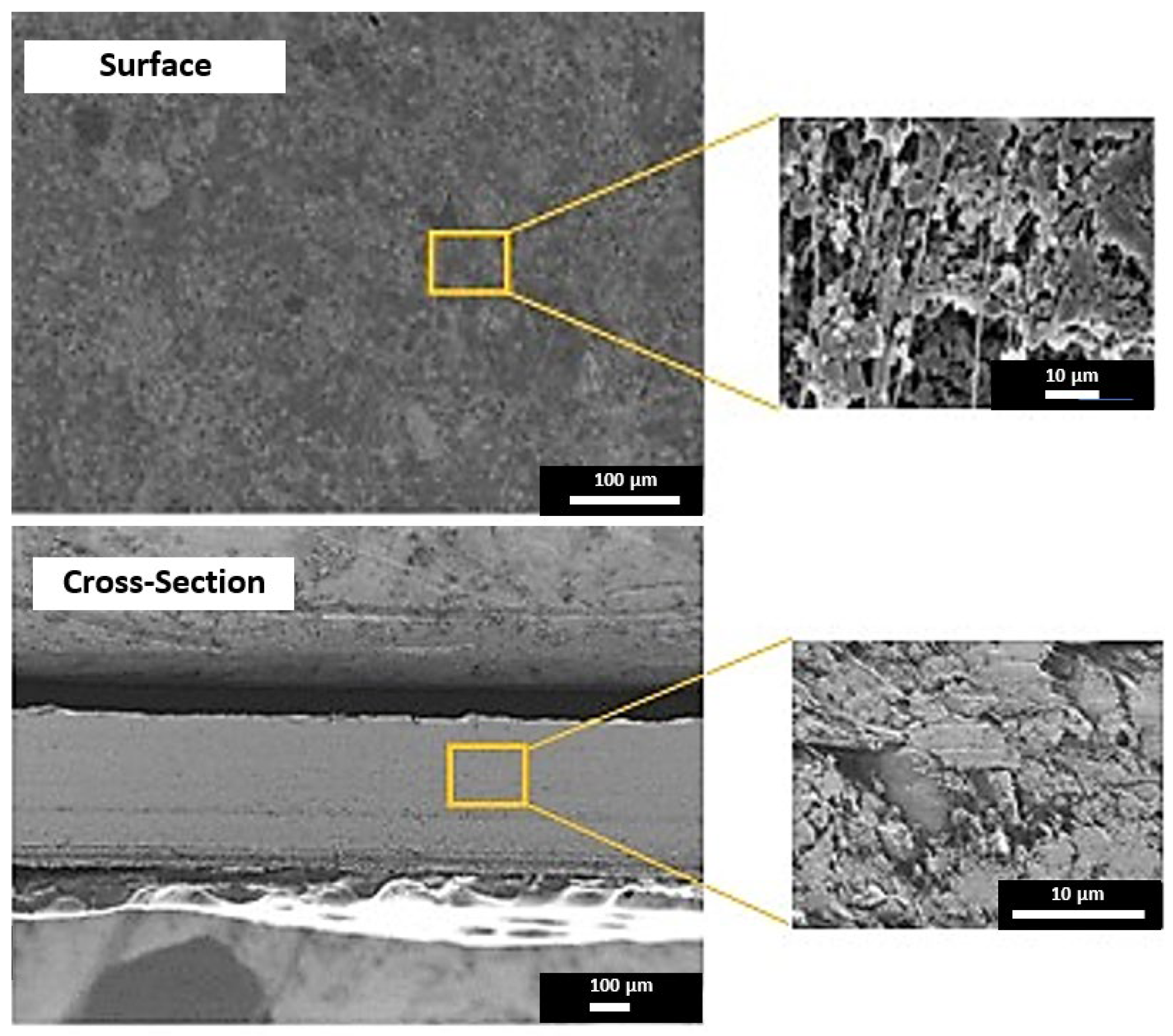
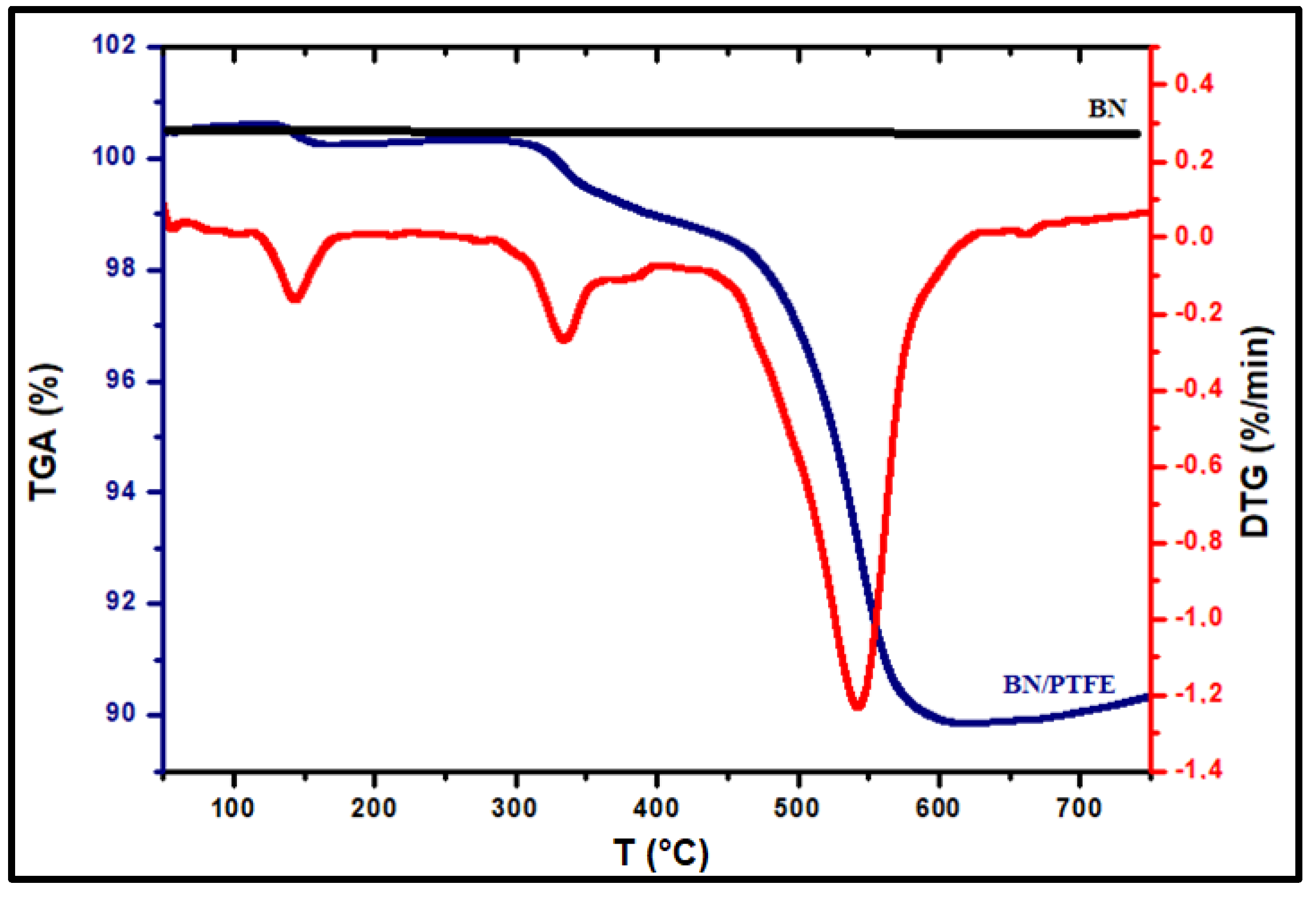
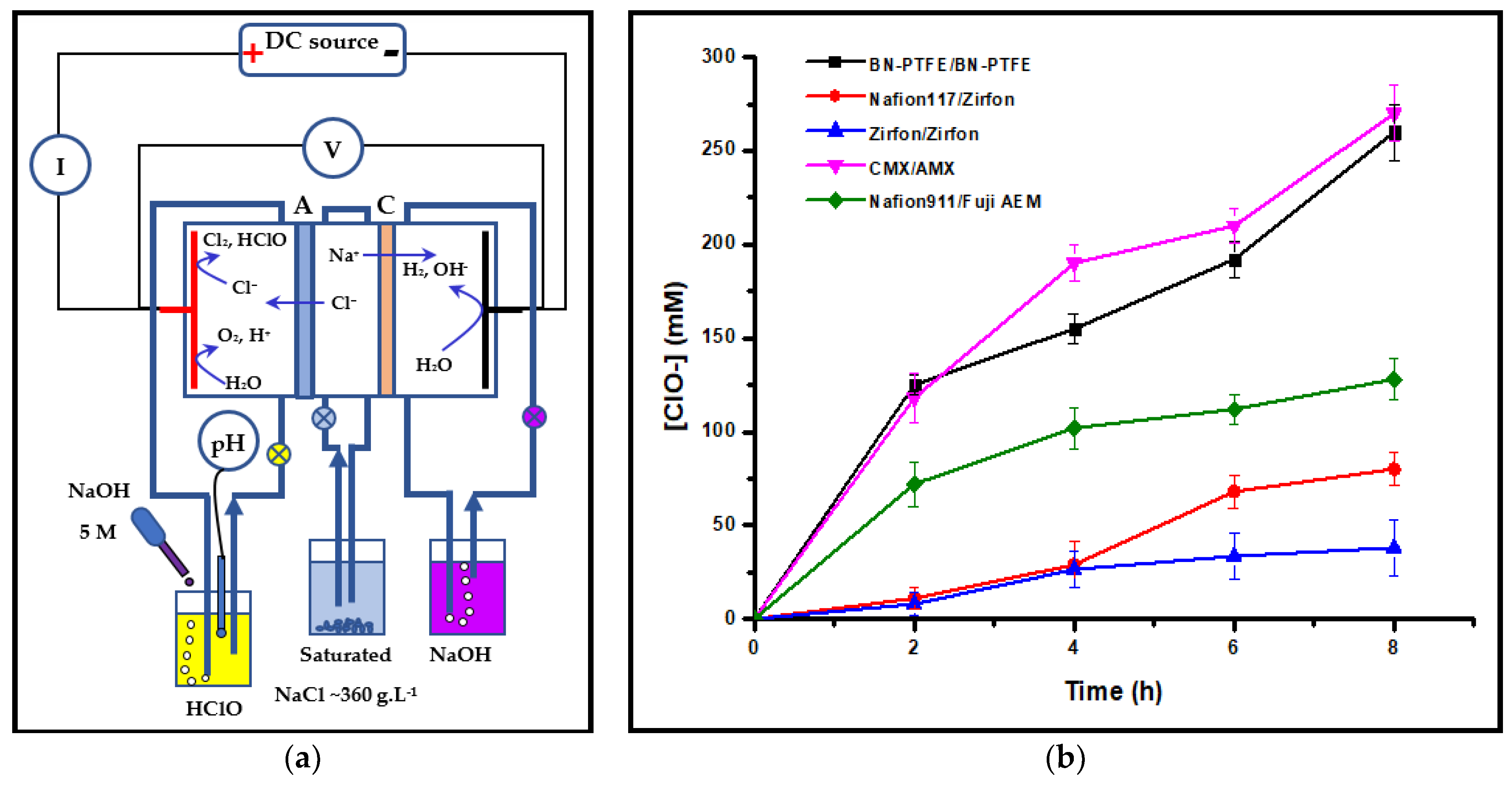




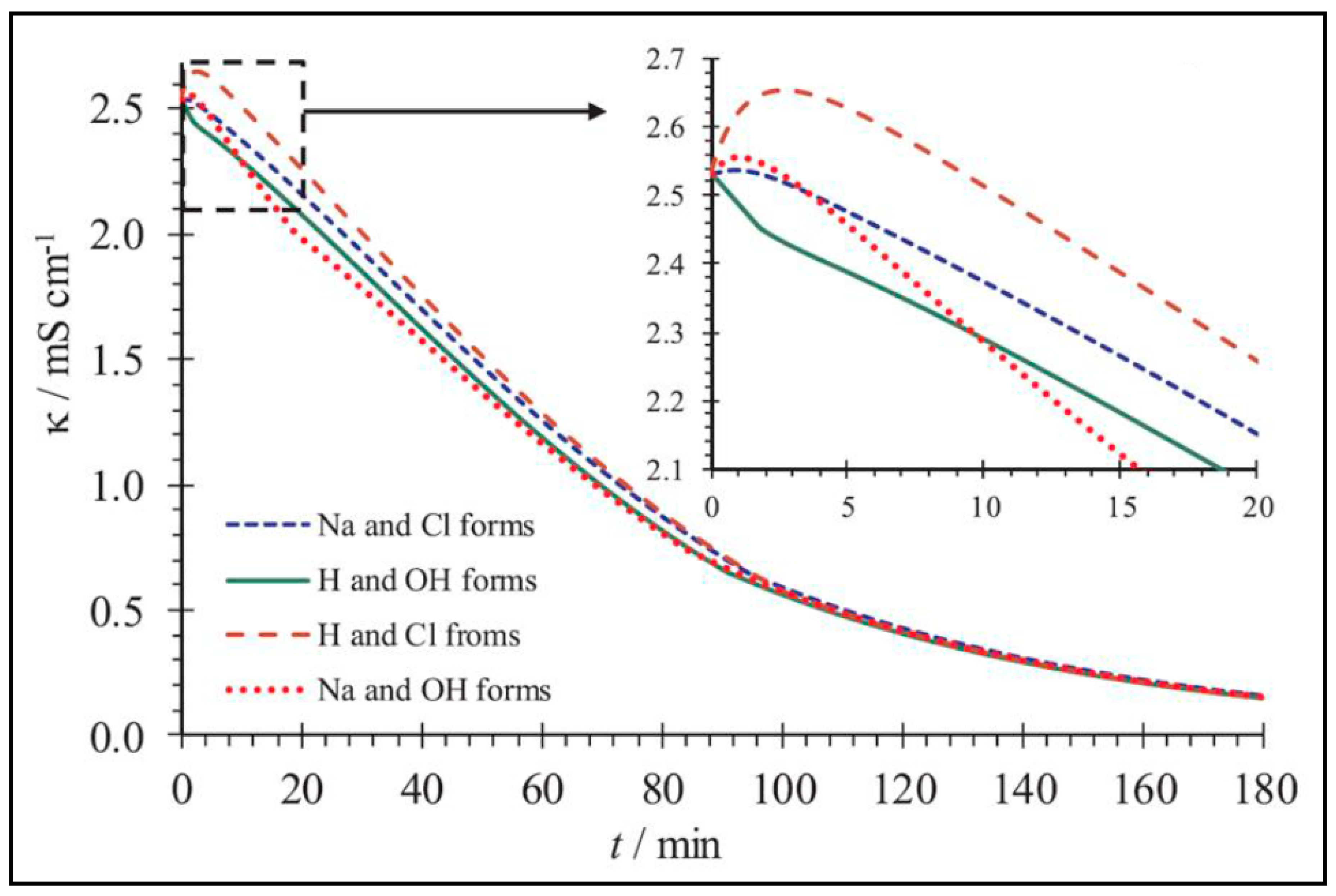

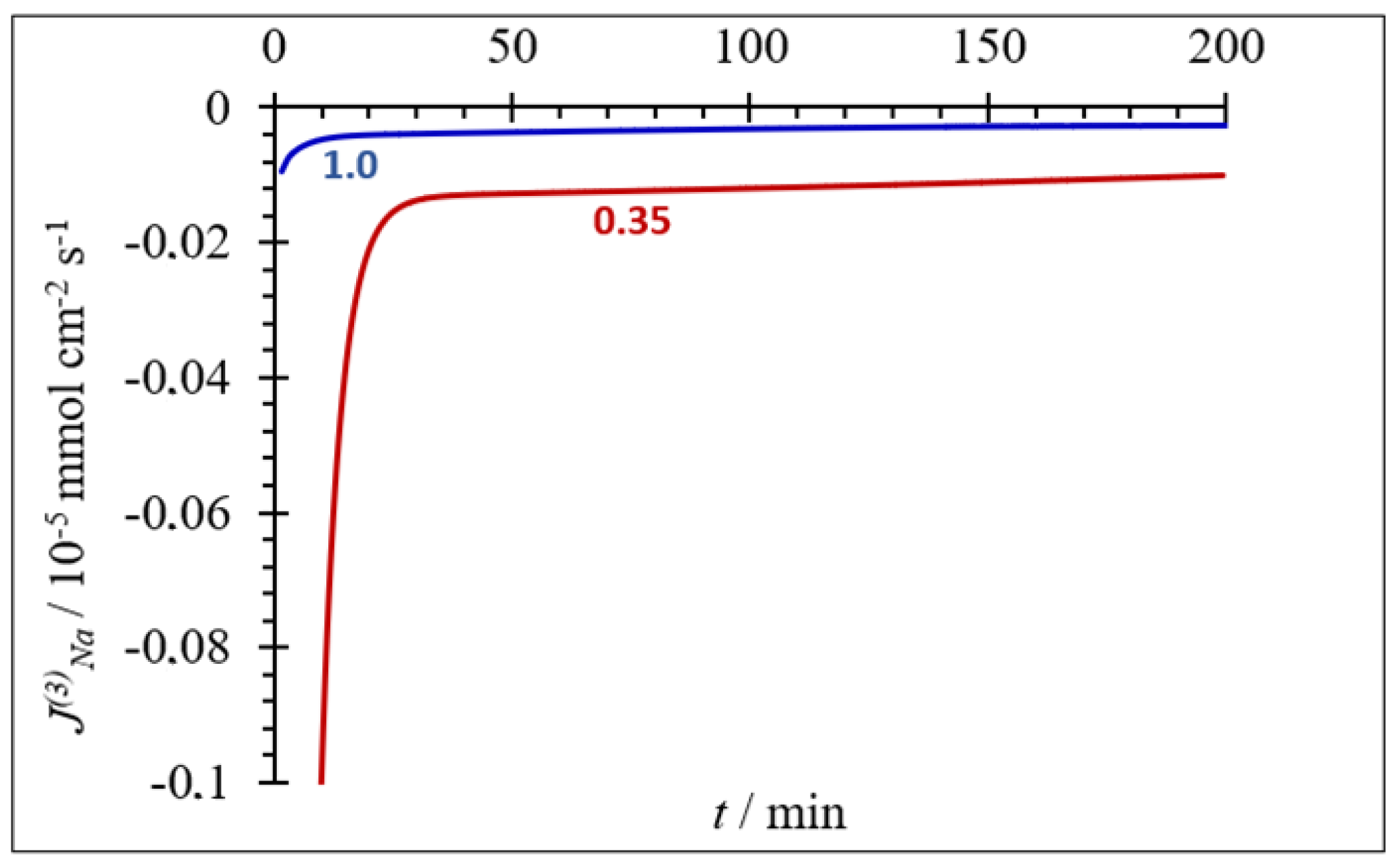
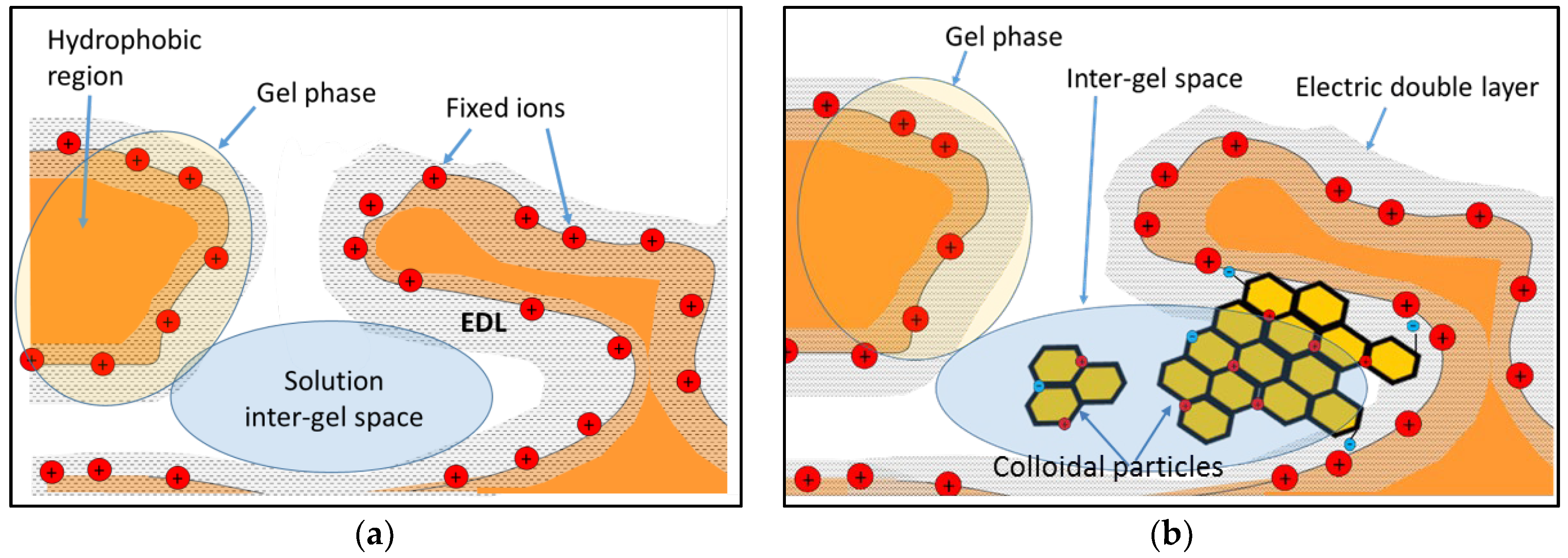
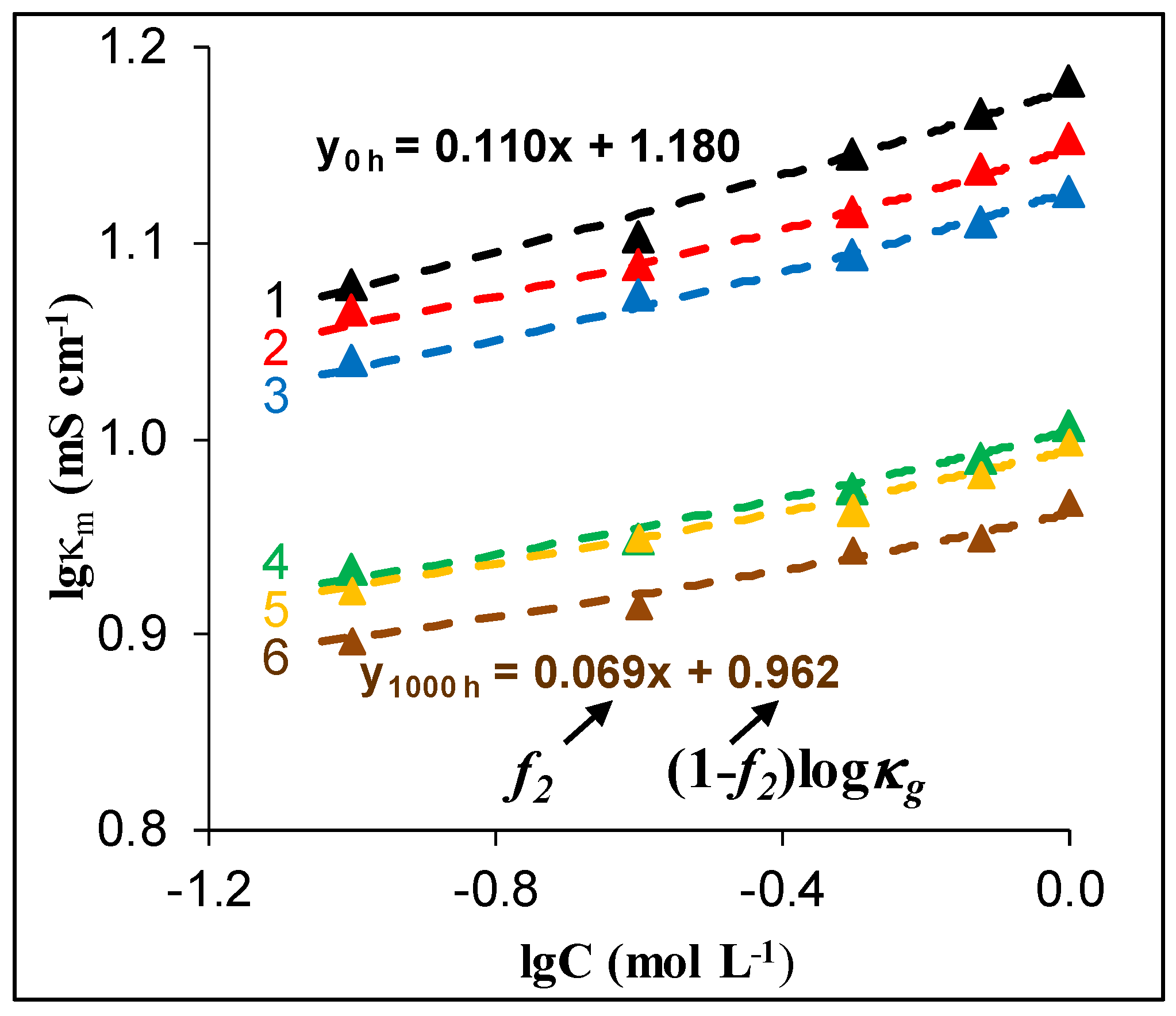

| Composition (% wt.) | Wu (%) | θ (°) | Km (10−4 S·cm−1) | |||||
|---|---|---|---|---|---|---|---|---|
| LICGC | PECH-DABCO | NH2-PES | BRIJ76 | LiCl 0.1 M | NaCl 0.1 M | |||
| LCM1 | 38 | 28 | 28 | 6 | 8.5 | 52.8 | 0.62 | 0.39 |
| LCM2 | 43 | 25.5 | 25.5 | 6 | 8.3 | 58.7 | 1.40 | 0.85 |
| LCM3 | 48 | 23 | 23 | 6 | 8.1 | 62.6 | 1.86 | 1.26 |
| LCM4 | 53 | 20.5 | 20.5 | 6 | 6.6 | 65.3 | 2.18 | 1.32 |
| LCM5 | 50.5 | 25.5 | 18 | 6 | 11.3 | 61.3 | 7.50 | 3.20 |
| LCM6 | 50.5 | 18 | 25.5 | 6 | 5.7 | 70.8 | 0.40 | 0.28 |
| Parameter | Sand Support | Zeolite Support |
|---|---|---|
| Sintering temperature (°C) | 1250 | 900 |
| Pore size (µm) | 10.36 | 0.55 |
| Mechanical strength (MPa) | 15.1 | 12.6 |
| Water permeability (L·h−1·m−2·bar−1) | 3611 | 1218 |
| Element | Cu | Ni | Cr |
|---|---|---|---|
| RF (%) | 74.75 | 20.52 | 55.54 |
| J0 (10−5 mol·m−2·s−1) | 8.62 | 1.14 | 5.72 |
| Membranes | Neosepta® AFN | Neosepta® AMX | Neosepta® ACS |
|---|---|---|---|
| Ion-exchange capacity (mmol/g) | 3.00 | 1.30 | 1.30 |
| Water content % | 47.8 | 26.0 | 26.0 |
| Thickness (mm) | 0.12 | 0.13 | 0.13 |
| Technique | Application | Device Complexity | Interpretation Complexity | Use Frequency |
|---|---|---|---|---|
| 2D fluorescence/Fourier transform infrared correlation spectroscopy | V, I | H | H | L |
| 31P nuclear magnetic resonance spectroscopy | I | H | M | L |
| Atomic force microscopy (AFM) | H | L | M | |
| Classical optical microscopy | V | L | M | H |
| Combined with energy dispersive X-ray spectrometry (EDS) | I | H | H | L |
| Confocal laser scanning microscopy (CLSM) | V | H | M | L |
| Contact angle | Q | M | L | H |
| Fluorescence excitation-emission matrix (EEM) | Q, I | H | H | L |
| Fluorescence spectroscopy | I | H | M | L |
| Fourier transform-ion cyclotron resonance-mass spectrometry (FT-ICR-MS) | I | H | H | L |
| High-liquid performance chromatography (HPLC) | Q | M | L | M |
| High-resolution optical microscopy | V | M | M | M |
| Inductively coupled plasma optical emission spectrometry | I | M | H | L |
| Mass spectrometry (MS) coupled | I | H | M | L |
| Molybdate colorimetry inductively coupled plasma optical emission | I | M | M | L |
| Optical coherence tomography (OCT) | I | H | M | L |
| Optical microscopy combined with a color scale for pH indication | V | L | L | L |
| Raman spectroscopy | I | H | M | M |
| Reflectance–Fourier-transform infrared (ATR—FTIR) | I | M | M | H |
| Rutherford backscattering spectroscopy (RBS) | I | H | H | L |
| Scanning electrochemical microscopy (SECM) | V | M | L | M |
| Scanning electron microscopy (SEM) | V | H | L | H |
| Scanning ion conductance microscopy (SICM) | V | H | L | L |
| Size-exclusion (SEC) | I | M | L | M |
| Smear-prints | V | M | M | L |
| Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) | I | M | M | L |
| Standard contact porosimetry method | Q | L | M | L |
| Surface plasmon resonance (SPR) | I | H | H | L |
| Surface-enhanced Raman spectroscopy (SERS) | V, I | H | H | L |
| Synchrotron Fourier transform infrared mapping | V, I | H | H | L |
| Tip-enhanced Raman spectroscopy (TERS) | V, I | H | H | L |
| Total nitrogen content analysis Dumas method | Q | M | M | L |
| Total nitrogen content analysis LECO nitrogen quantification | Q | M | M | L |
| Ultra-high-liquid performance chromatography (UPLC) | Q | M | L | L |
| X-ray absorption fine structure (EXAFS) | I | H | M | L |
| X-ray diffraction (XRD) | I | H | L | L |
| X-ray photoelectron spectroscopy (XPS) | V, I | H | M | M |
| Zeta (the electrokinetic) potential | Q | H | M | L |
| AEM Soaking Duration, h | f2app | f2 | f2s | fcp | d/d(h = 0) | IECsw (mmol cm−3) | |
|---|---|---|---|---|---|---|---|
| 0 | 0.11 | 0.09 | 0.09 | 0 | 1.00 | 1.00 | 2.30 |
| 24 | 0.087 | 0.09 | 0.07 | 0.02 | 1.26 | 1.02 | 1.95 |
| 100 | 0.084 | 0.12 | 0.07 | 0.05 | 1.44 | 1.05 | 1.84 |
| 500 | 0.073 | 0.16 | 0.06 | 0.1 | 1.47 | 1.08 | 1.77 |
| 750 | 0.071 | 0.18 | 0.05 | 0.13 | 1.74 | 1.08 | 1.72 |
| 1000 | 0.069 | 0.20 | 0.045 | 0.155 | 1.88 | 1.08 | 1.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baklouti, L.; Larchet, C.; Hamdi, A.; Hamdi, N.; Baraket, L.; Dammak, L. Research on Membranes and Their Associated Processes at the Université Paris-Est Créteil: Progress Report, Perspectives, and National and International Collaborations. Membranes 2023, 13, 252. https://doi.org/10.3390/membranes13020252
Baklouti L, Larchet C, Hamdi A, Hamdi N, Baraket L, Dammak L. Research on Membranes and Their Associated Processes at the Université Paris-Est Créteil: Progress Report, Perspectives, and National and International Collaborations. Membranes. 2023; 13(2):252. https://doi.org/10.3390/membranes13020252
Chicago/Turabian StyleBaklouti, Lassaad, Christian Larchet, Abdelwaheb Hamdi, Naceur Hamdi, Leila Baraket, and Lasâad Dammak. 2023. "Research on Membranes and Their Associated Processes at the Université Paris-Est Créteil: Progress Report, Perspectives, and National and International Collaborations" Membranes 13, no. 2: 252. https://doi.org/10.3390/membranes13020252
APA StyleBaklouti, L., Larchet, C., Hamdi, A., Hamdi, N., Baraket, L., & Dammak, L. (2023). Research on Membranes and Their Associated Processes at the Université Paris-Est Créteil: Progress Report, Perspectives, and National and International Collaborations. Membranes, 13(2), 252. https://doi.org/10.3390/membranes13020252