Surface Treatment by Physical Irradiation for Antifouling, Chlorine-Resistant RO Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Vacuum UV (VUV) Treatment and Low-Pressure Plasma
- VUV Activation (AKT)
- 2.
- VUV-PEG
- 3.
- VUV-PDMS
- 4.
- Low-pressure plasma (acryl-plasma)
- 5.
- Low-pressure Plasma (GrowPLAS)
2.2. Membrane Performance
2.3. Membrane Fouling Testing
2.4. Membrane Chlorine Resistance
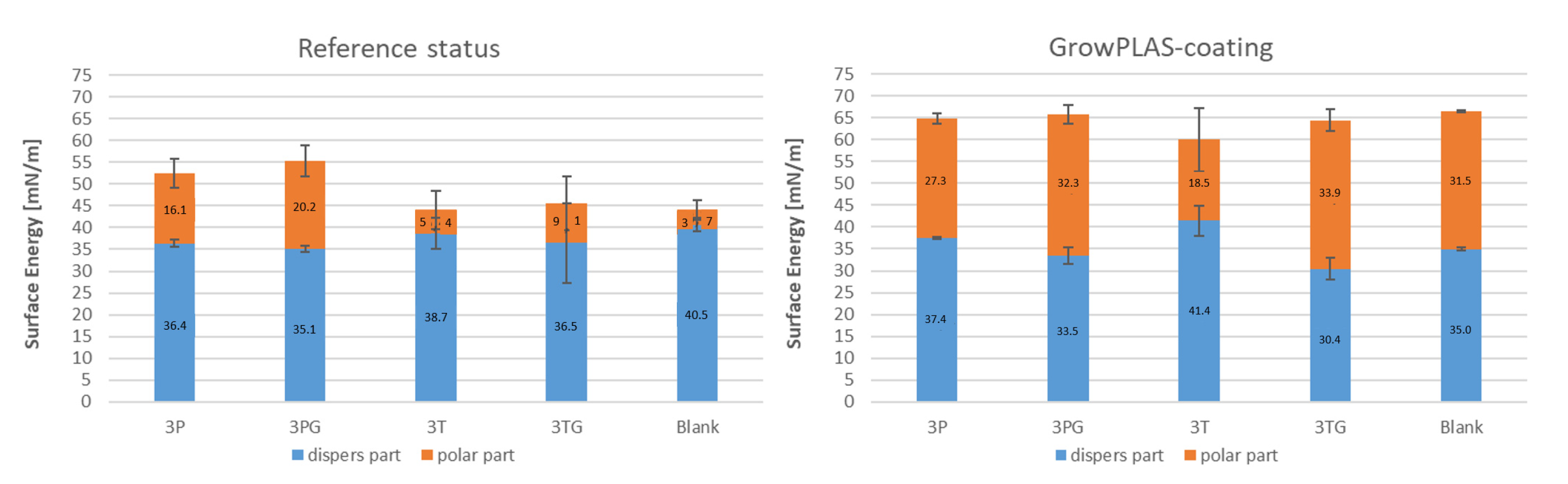
2.5. Analytical Techniques
- X-ray Photoelectron Spectroscopy
- 2.
- Contact angle
- 3.
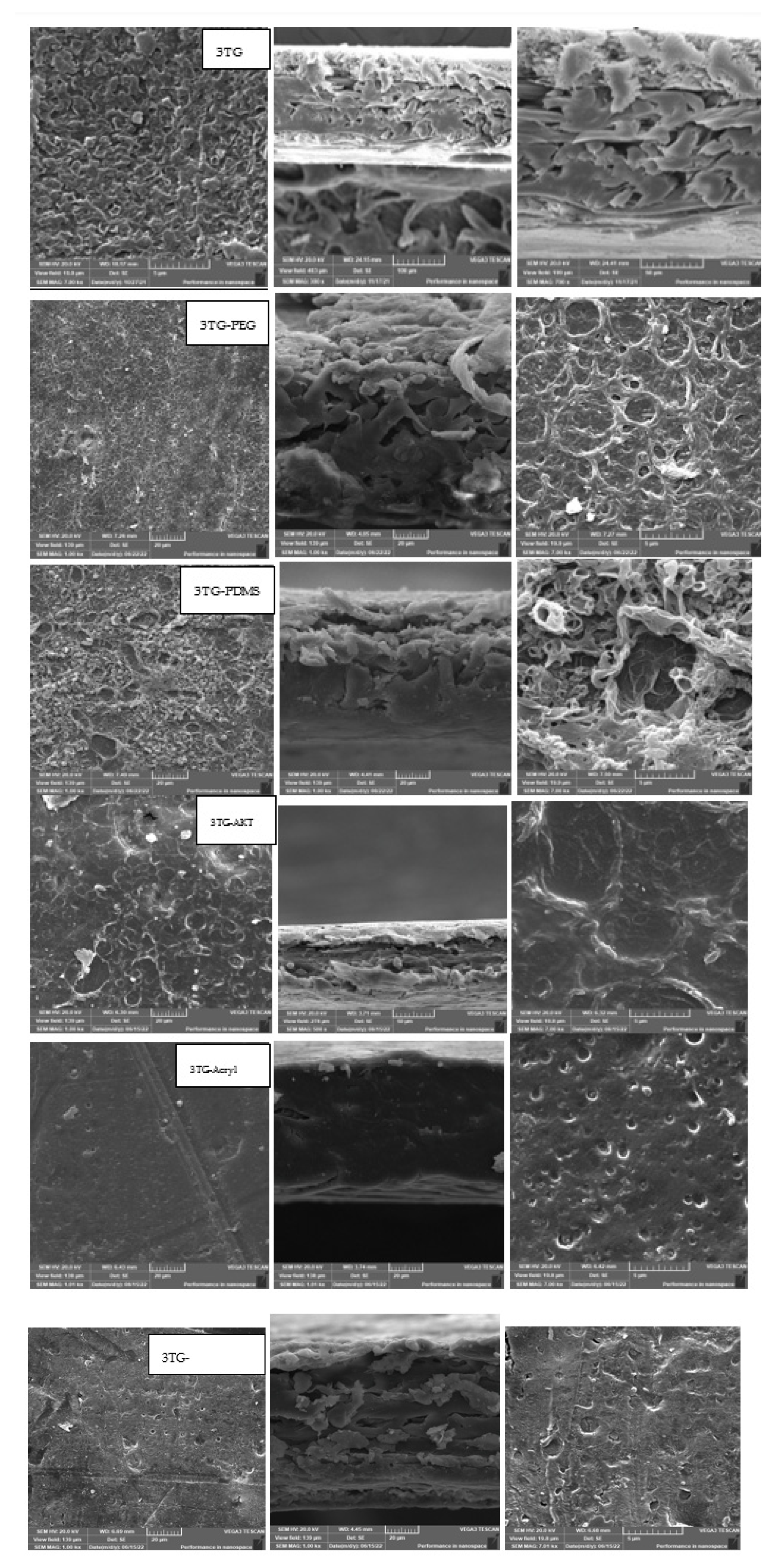
- Scanning Electron Microscope (SEM)
3. Results and Discussions
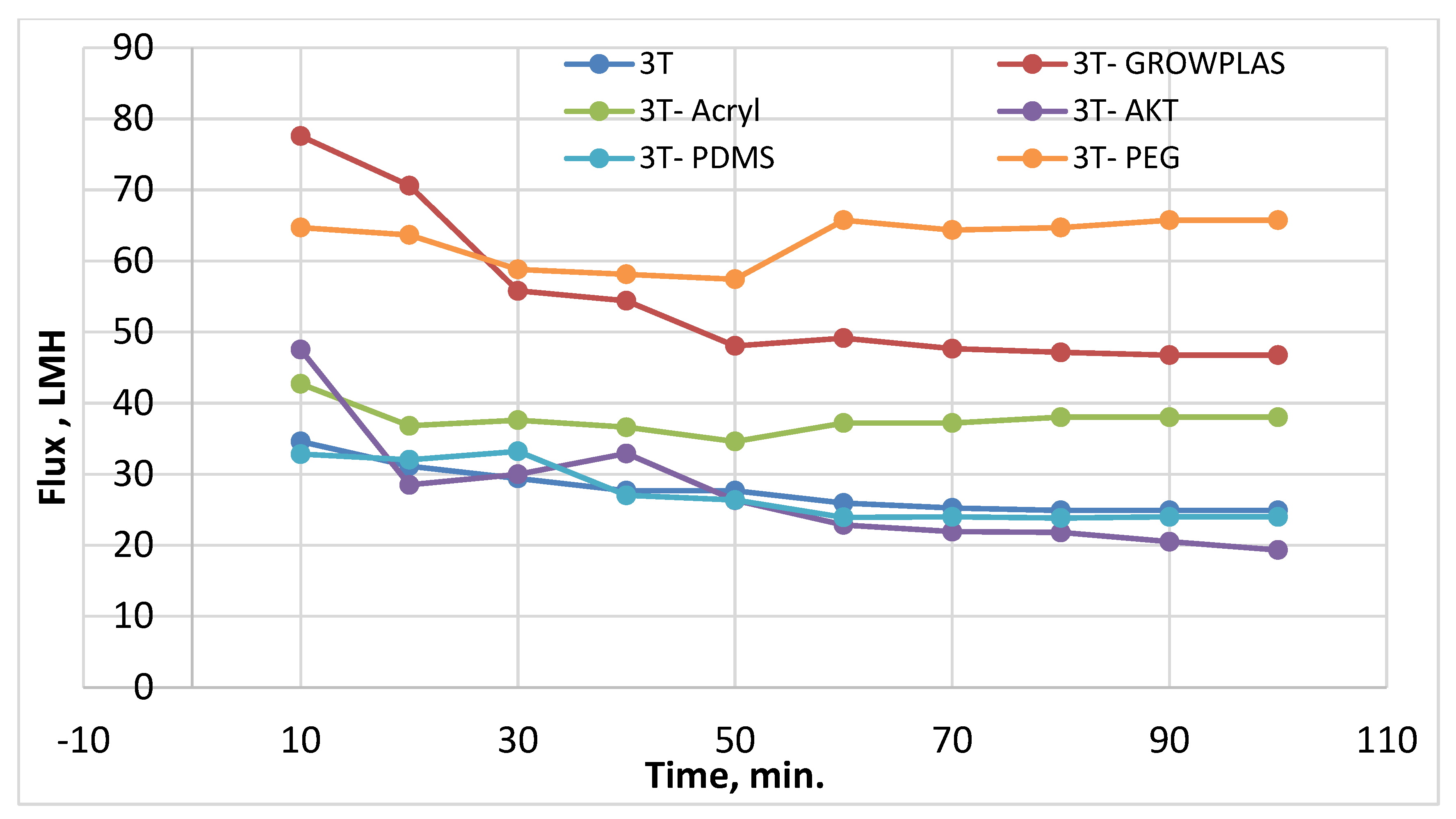
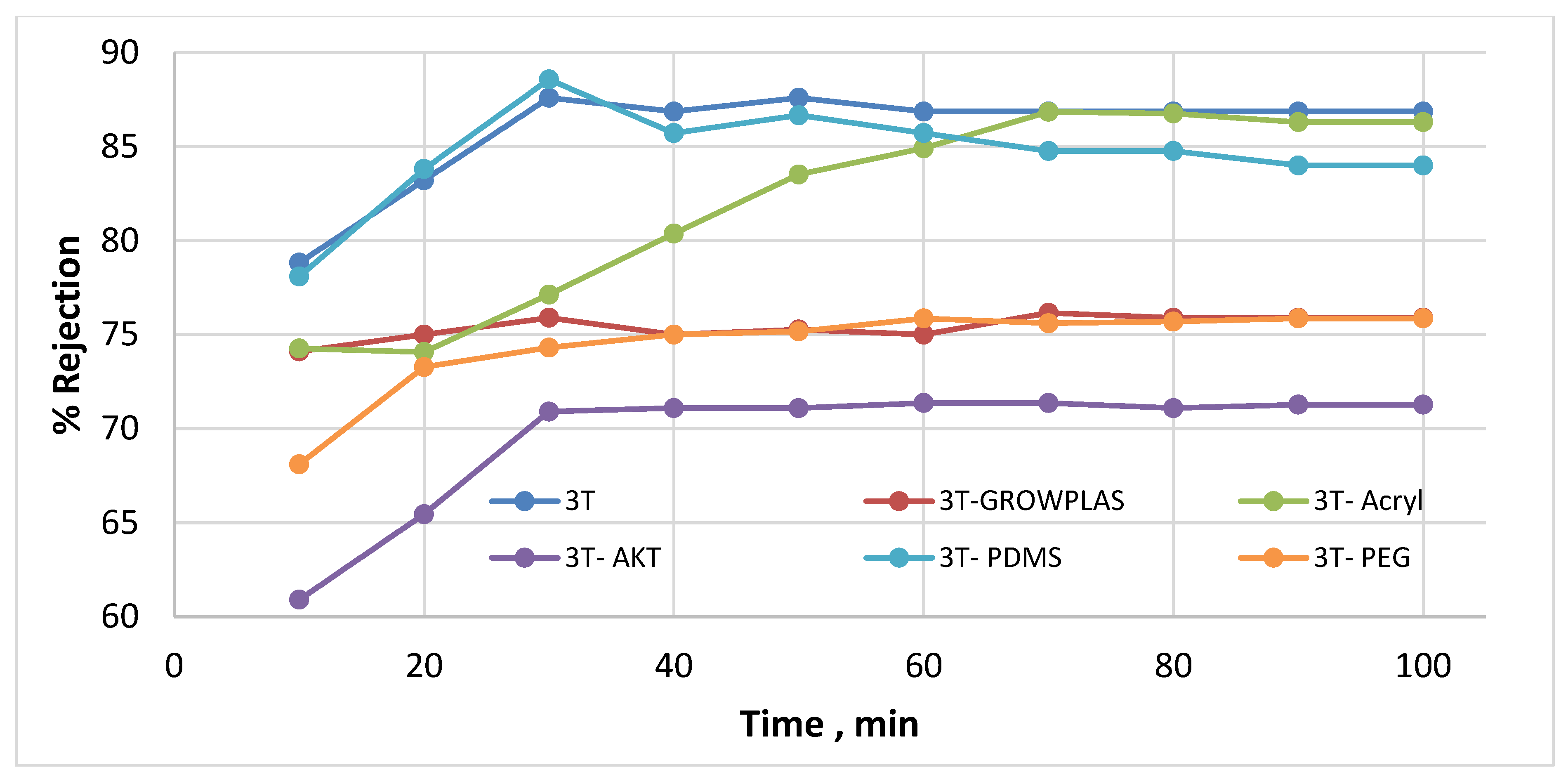
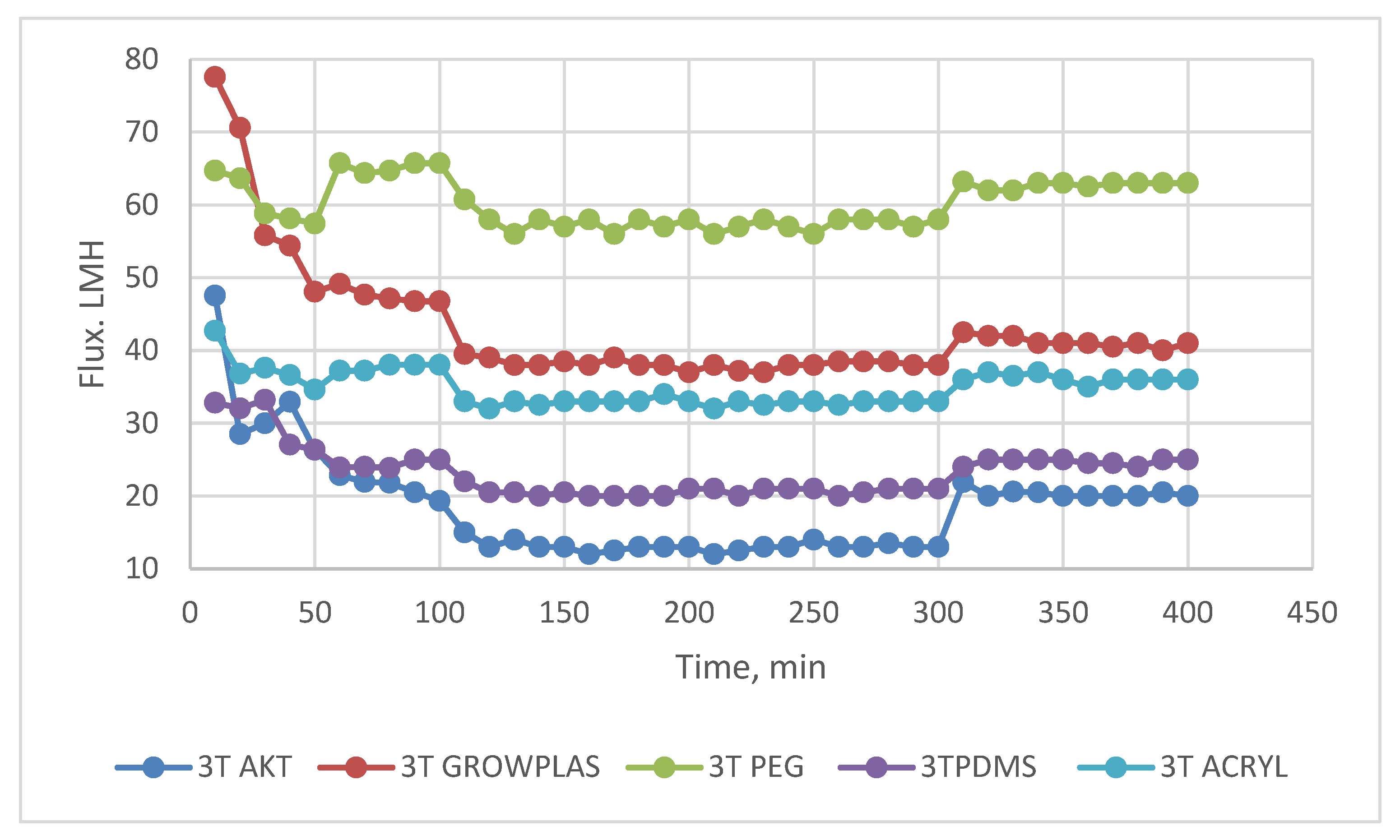
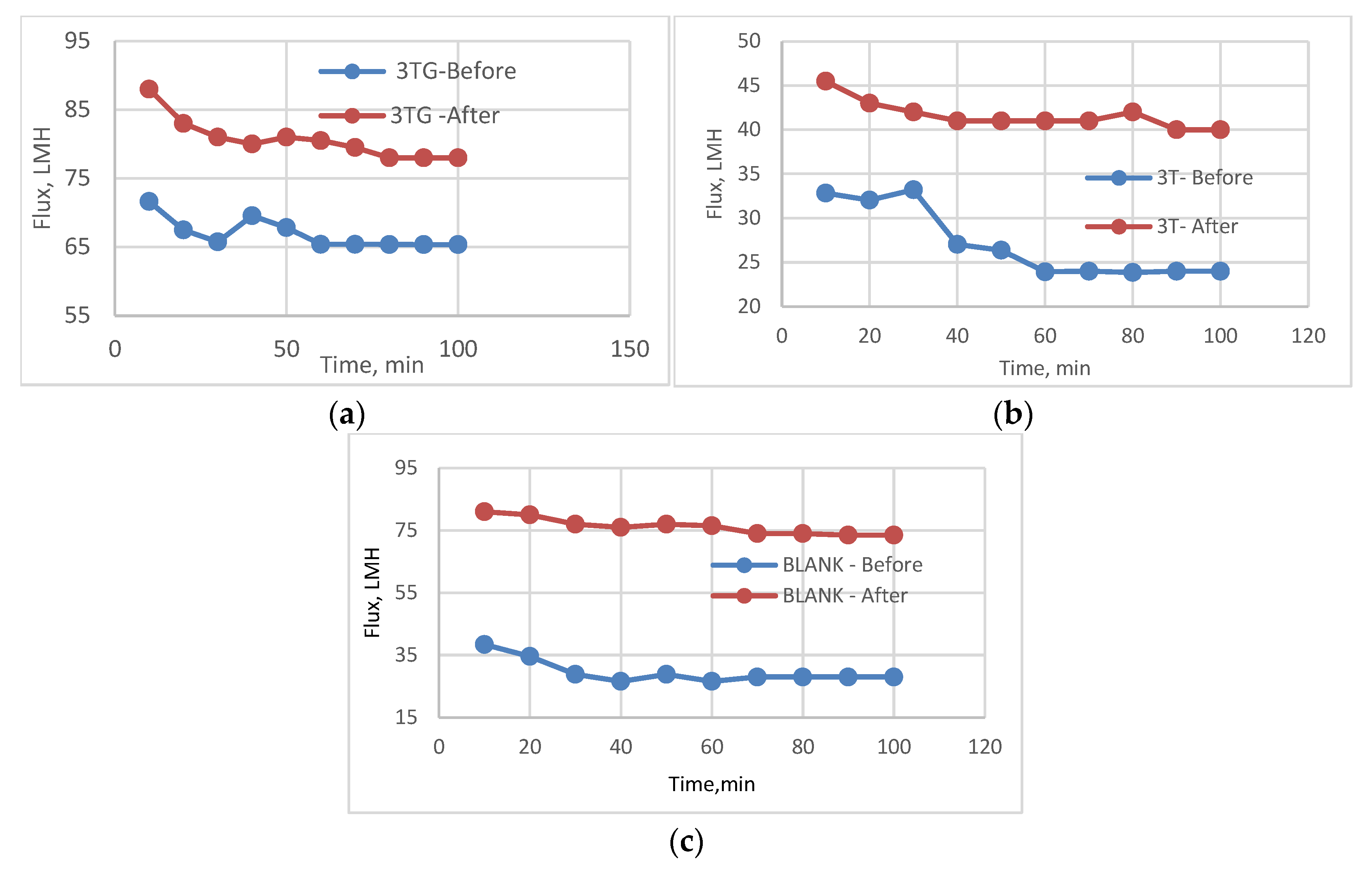
3.1. Effect of Different Physical Irradiations on RO Membranes-3T
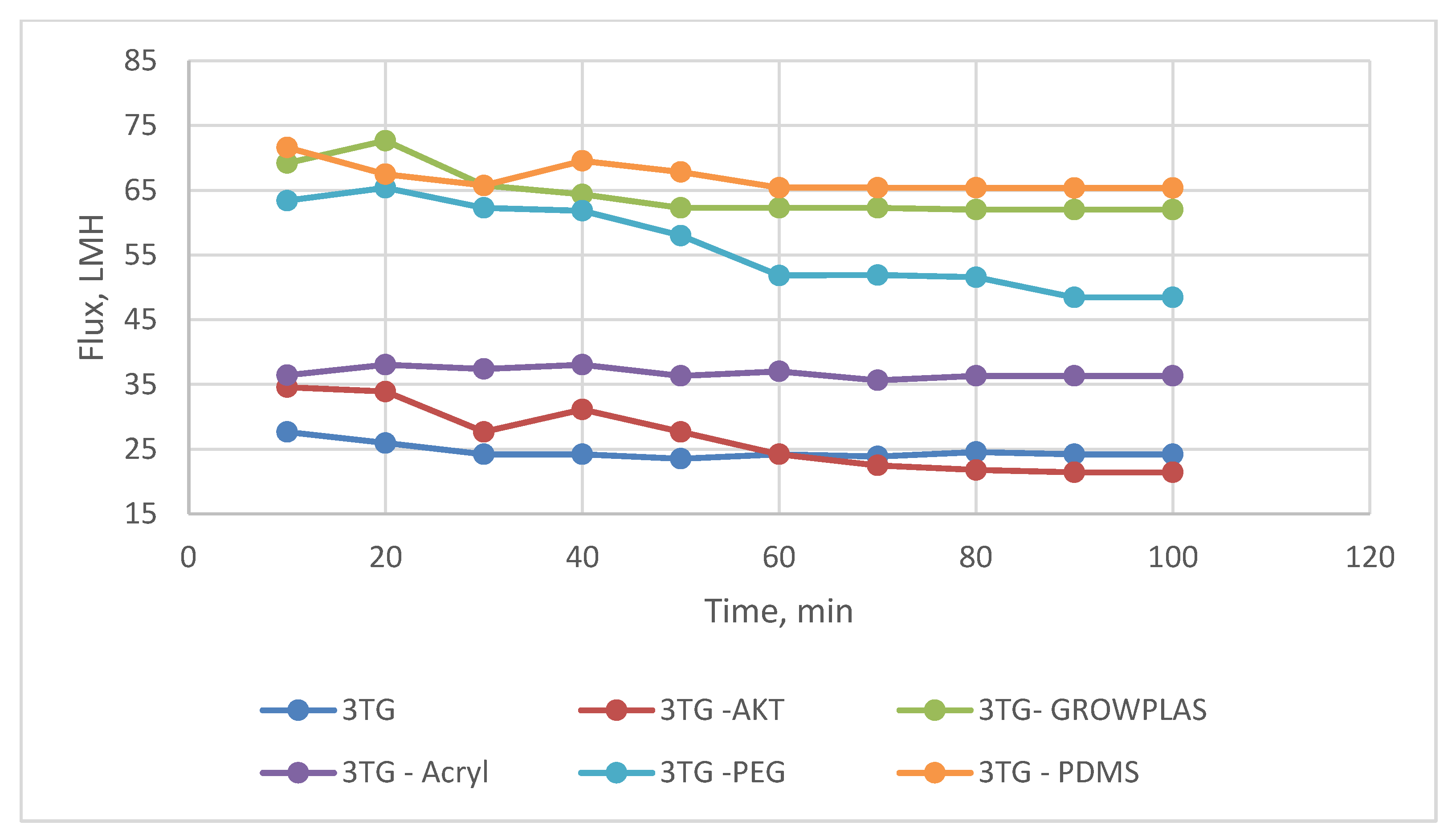
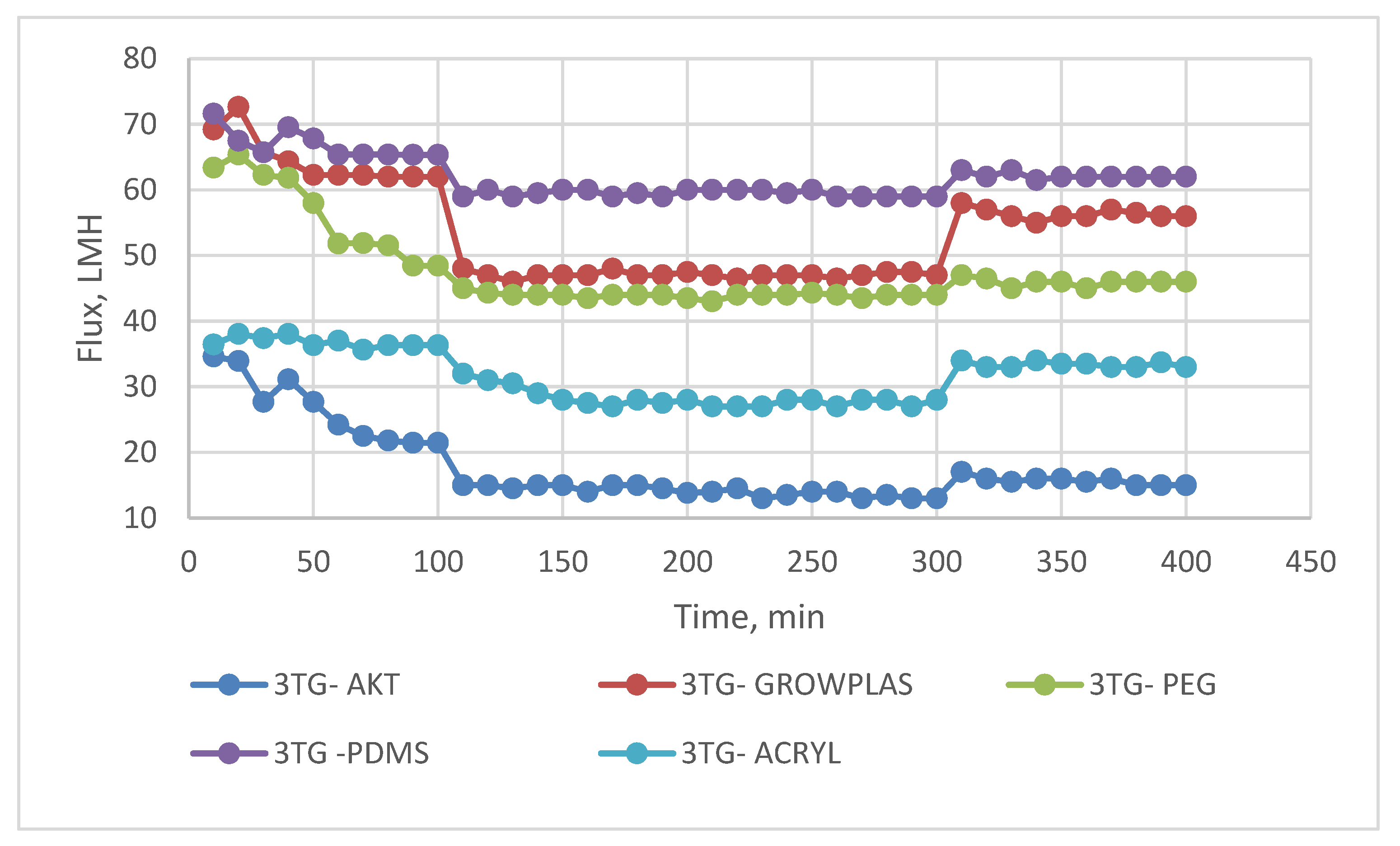
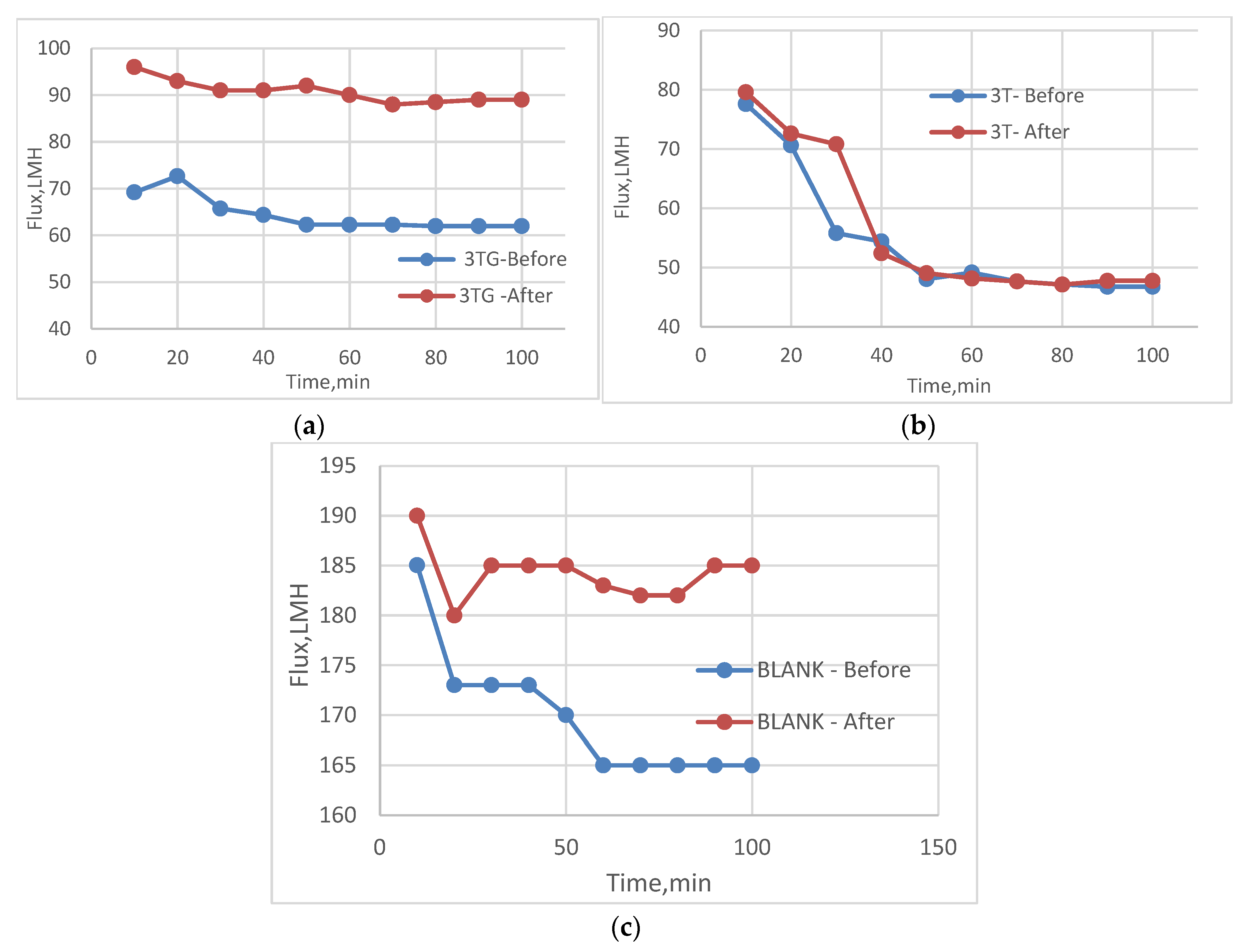
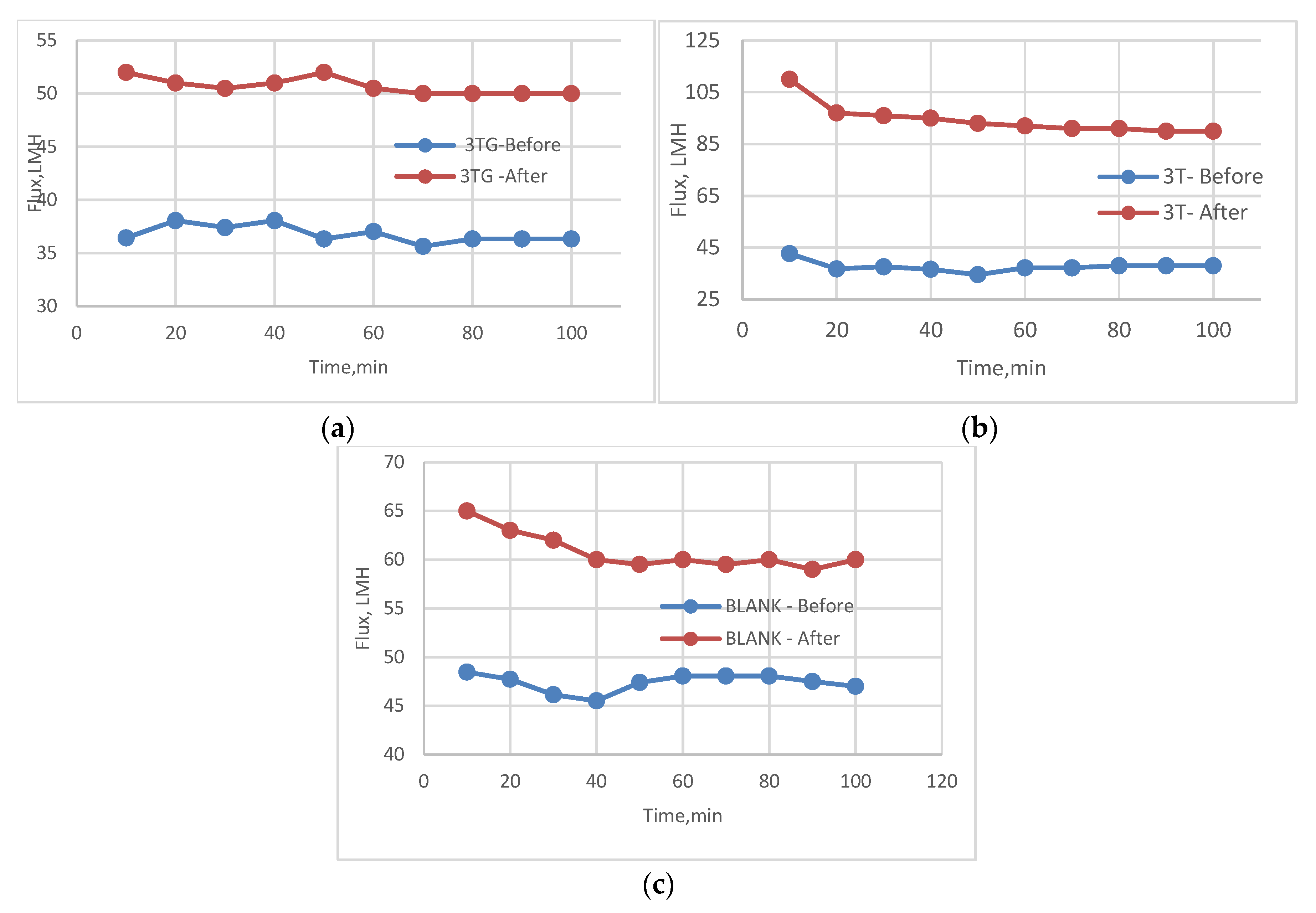
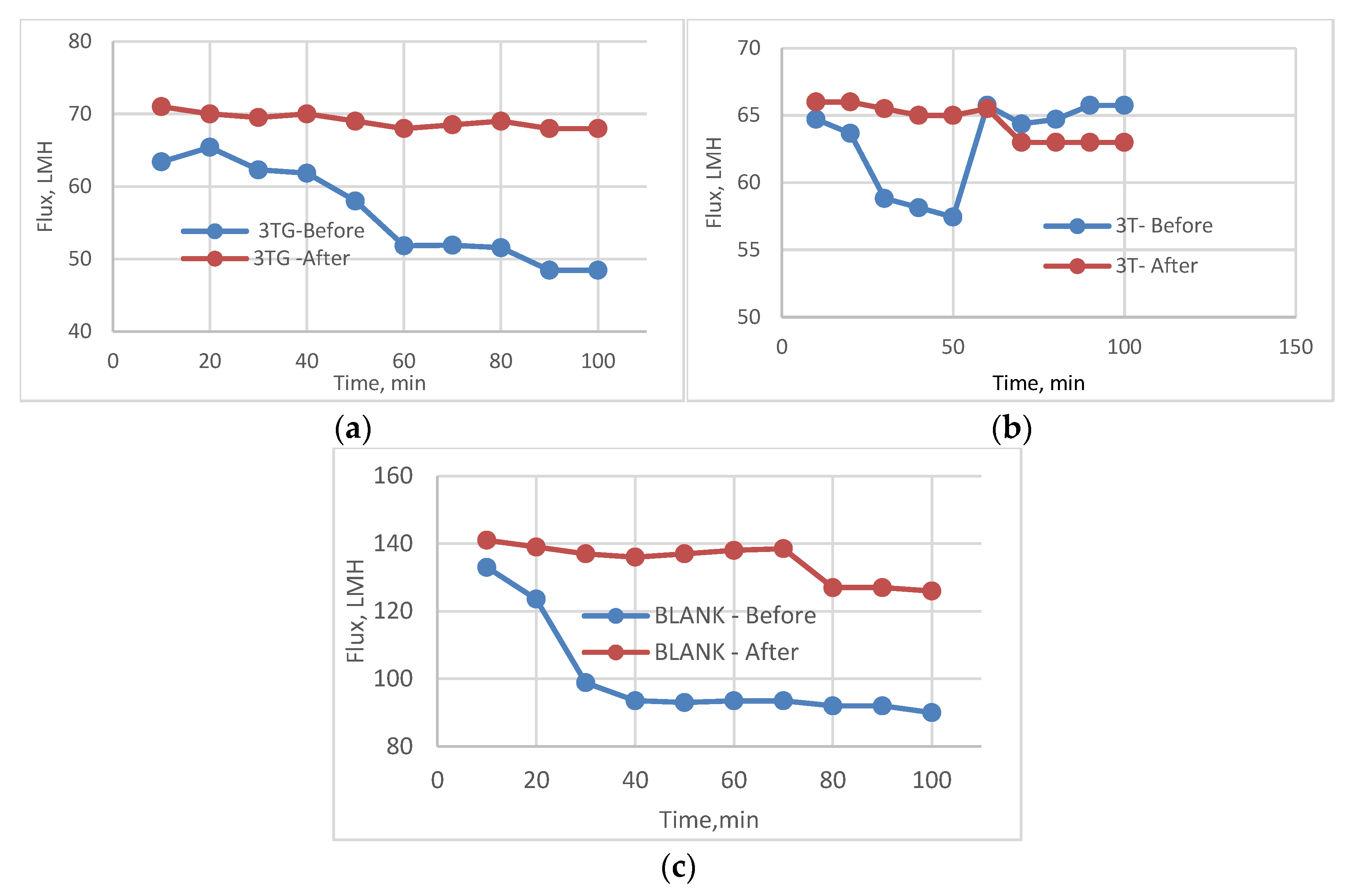
3.2. Effect of Different Irradiations on Chemically Modified Membrane 3TG
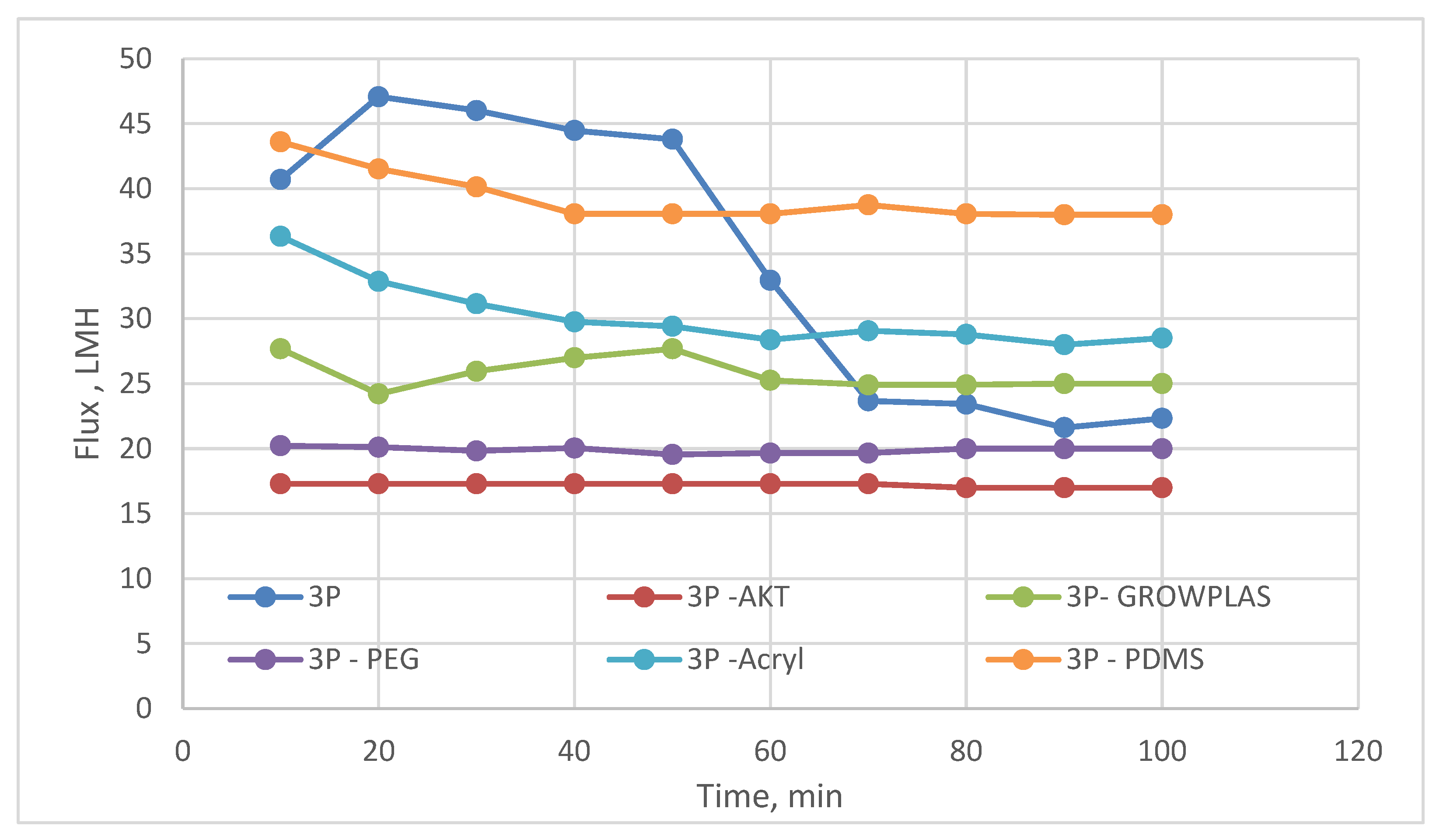
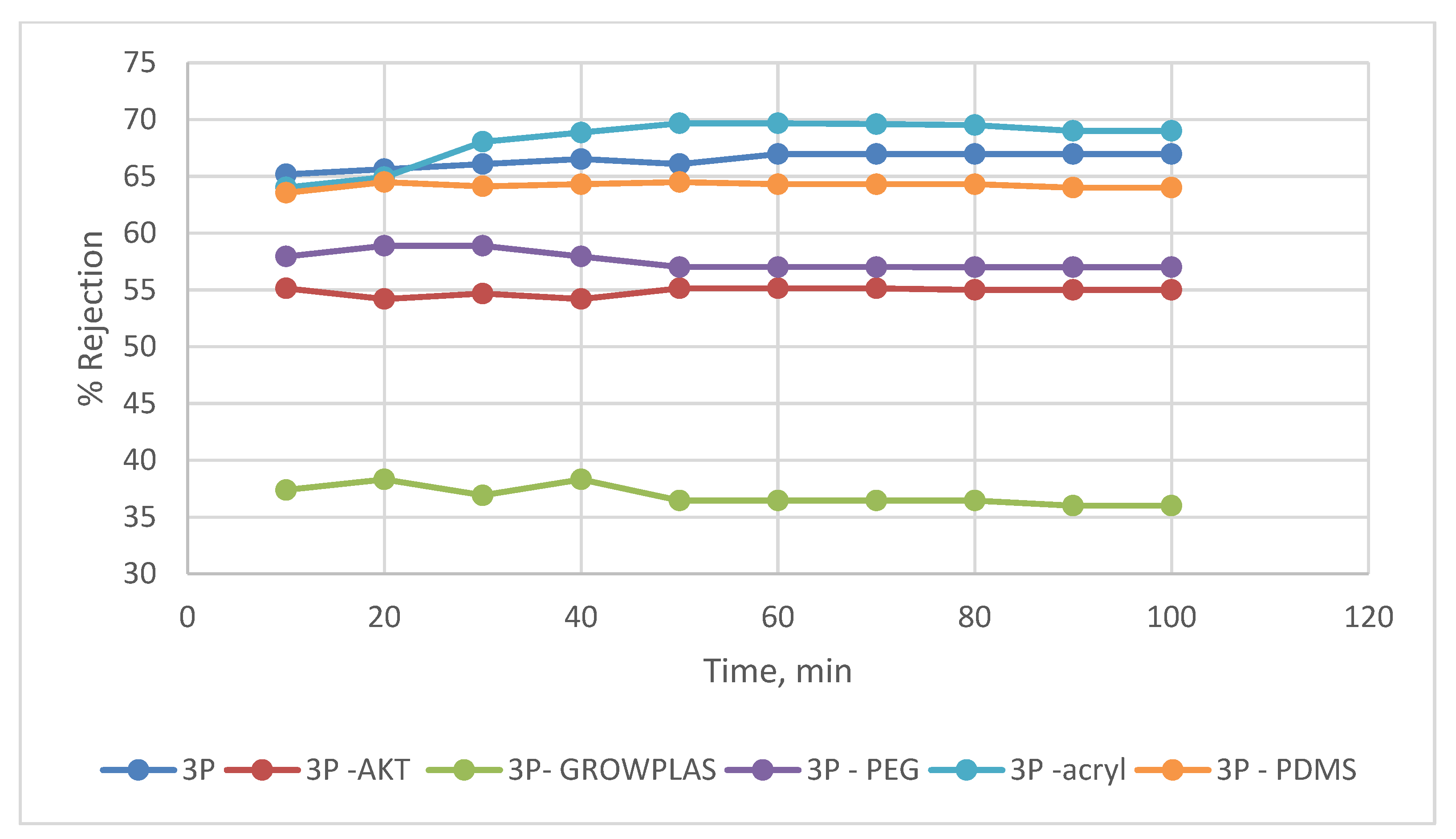
3.3. Effect of Different Irradiation on Blend Membranes with PVA-3P
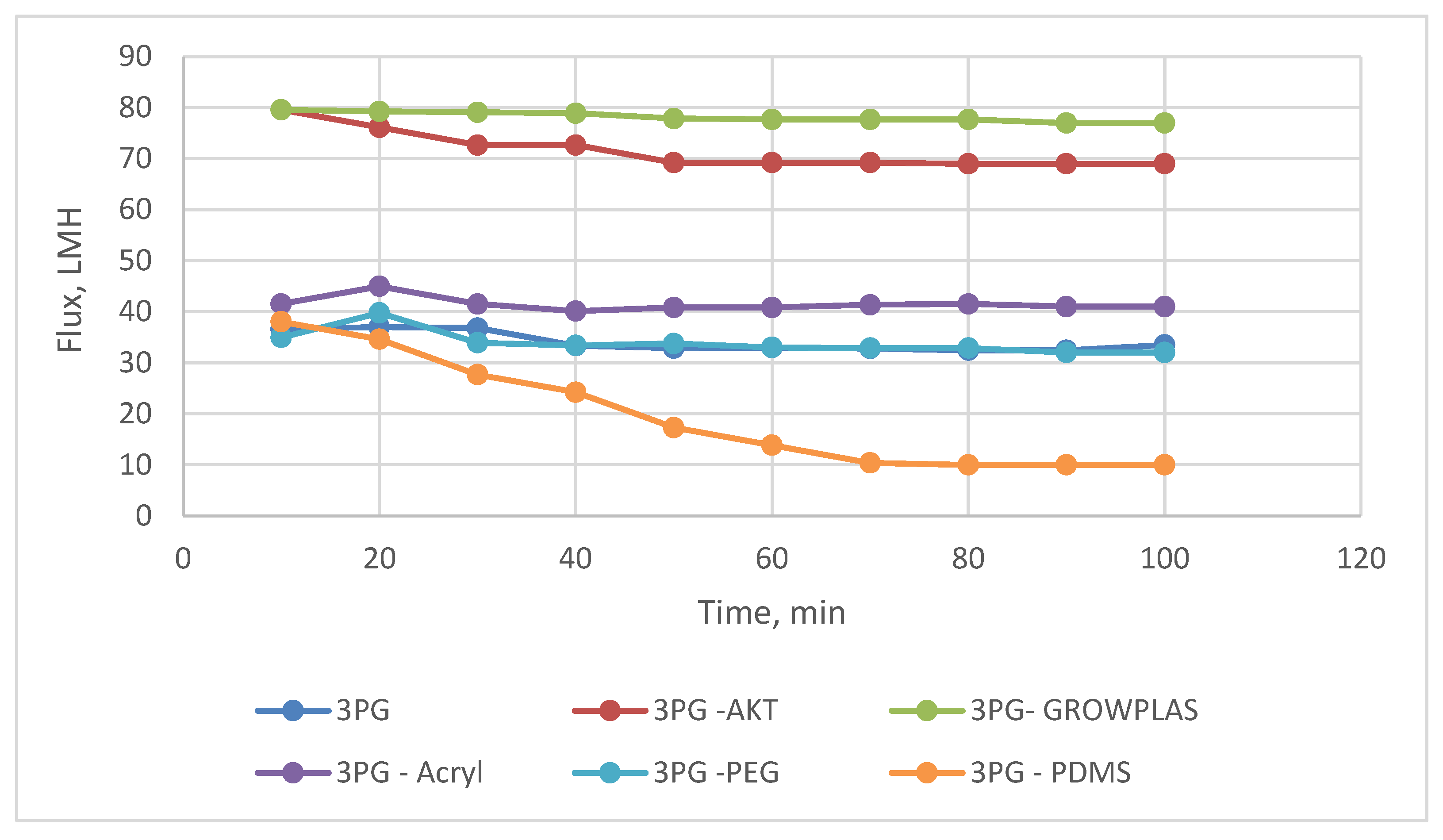
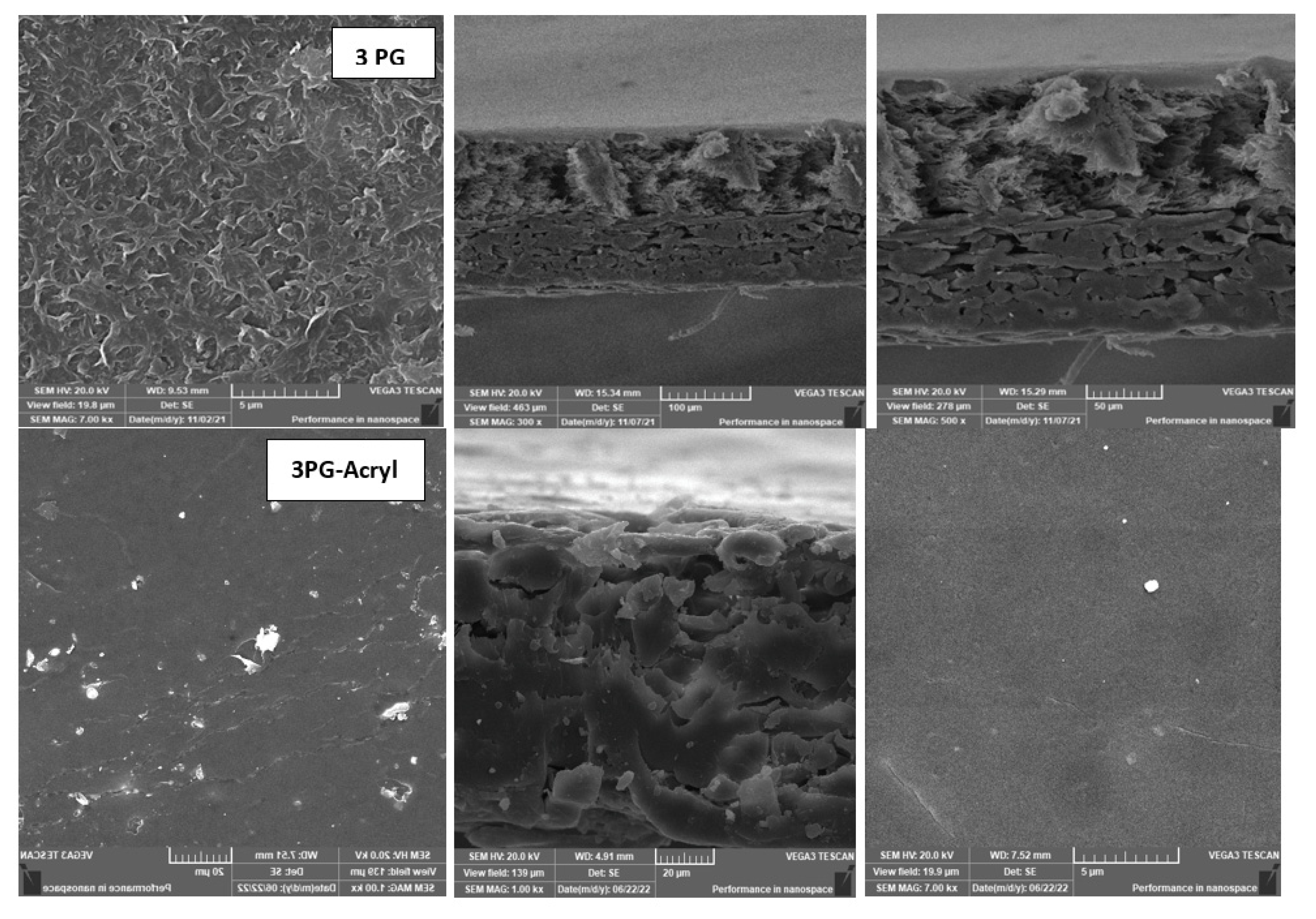
3.4. Effect of Physical Irradiation on Grafted MAA-Blend Membranes/PVA
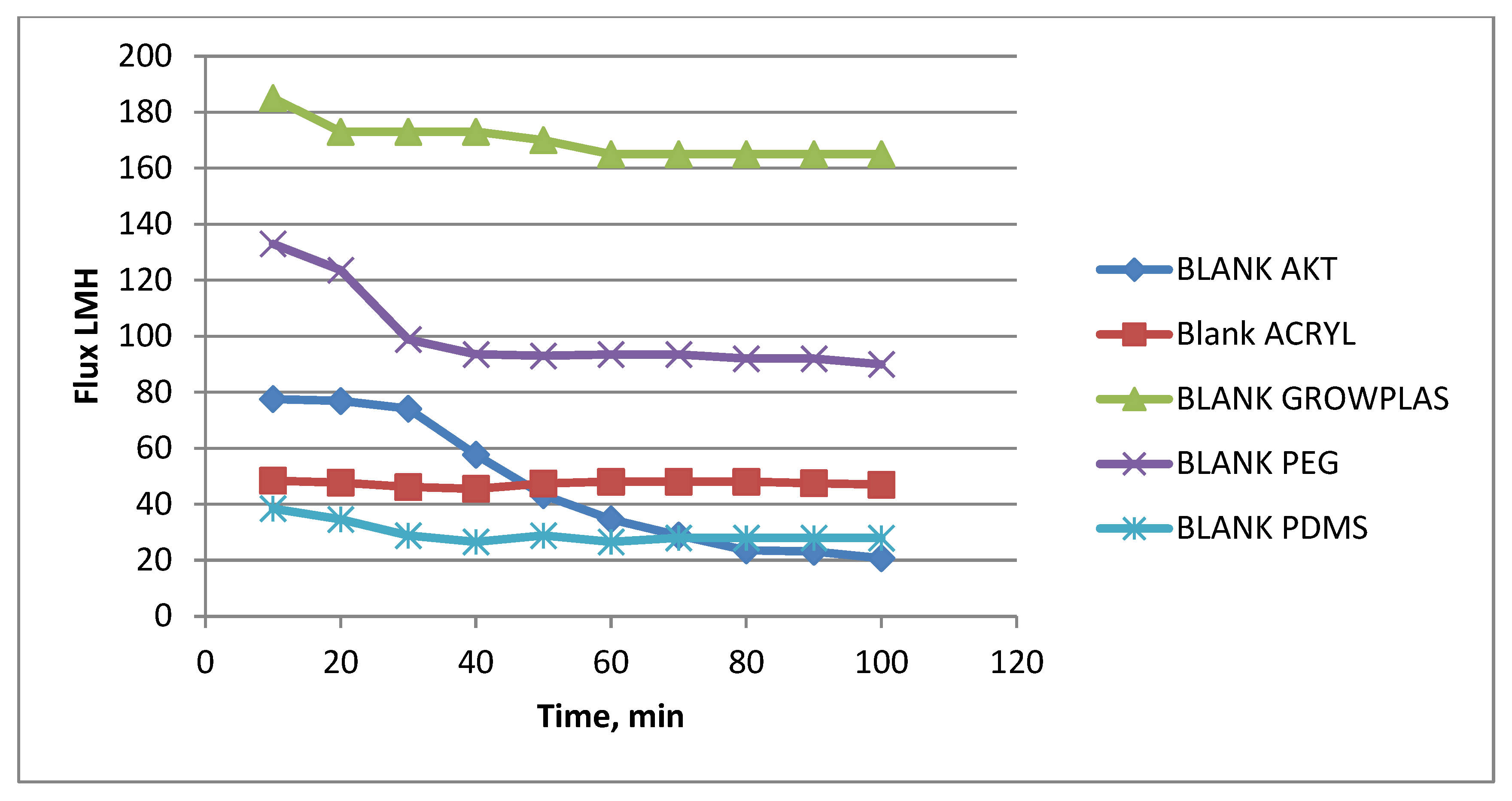
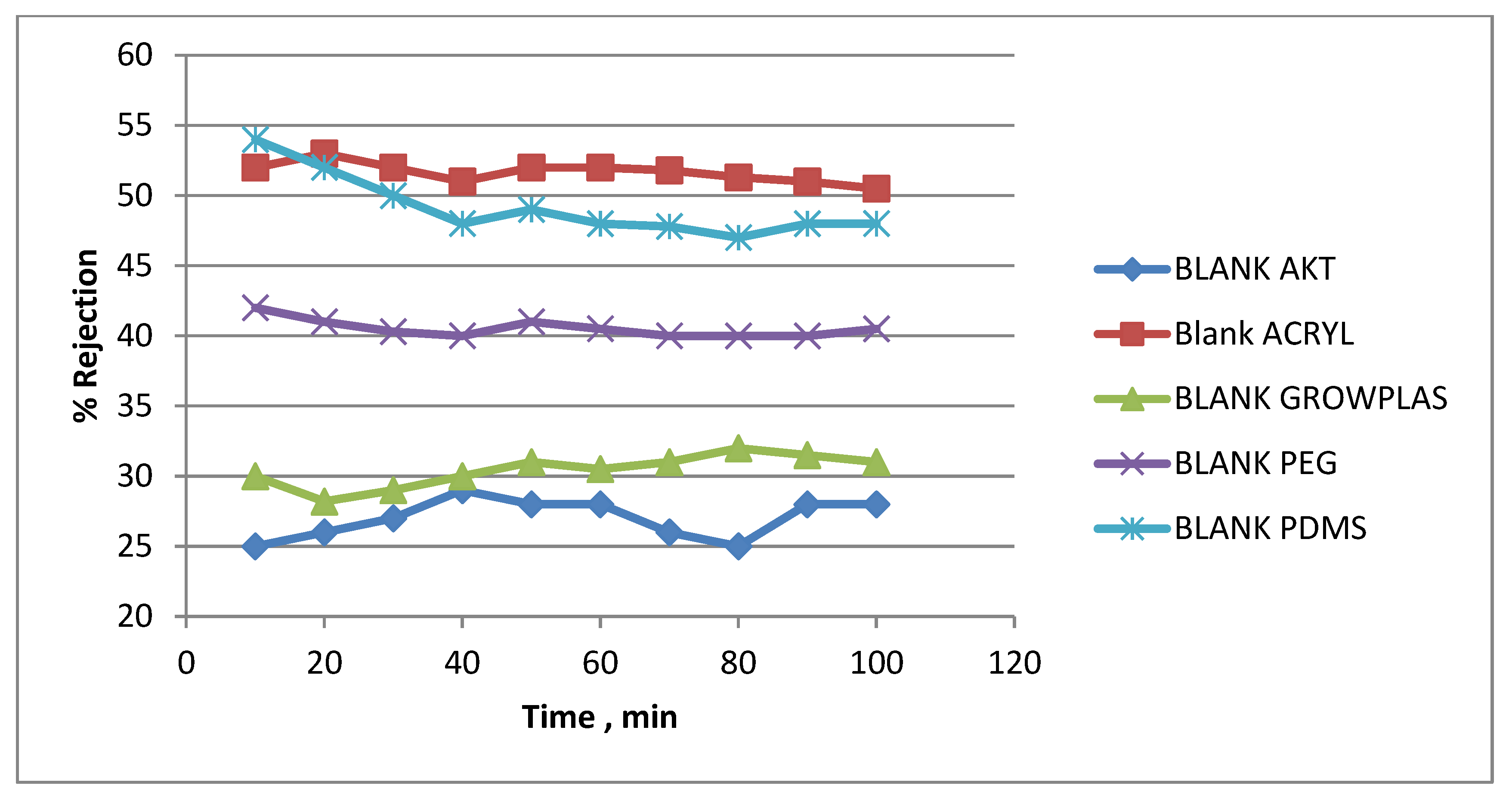
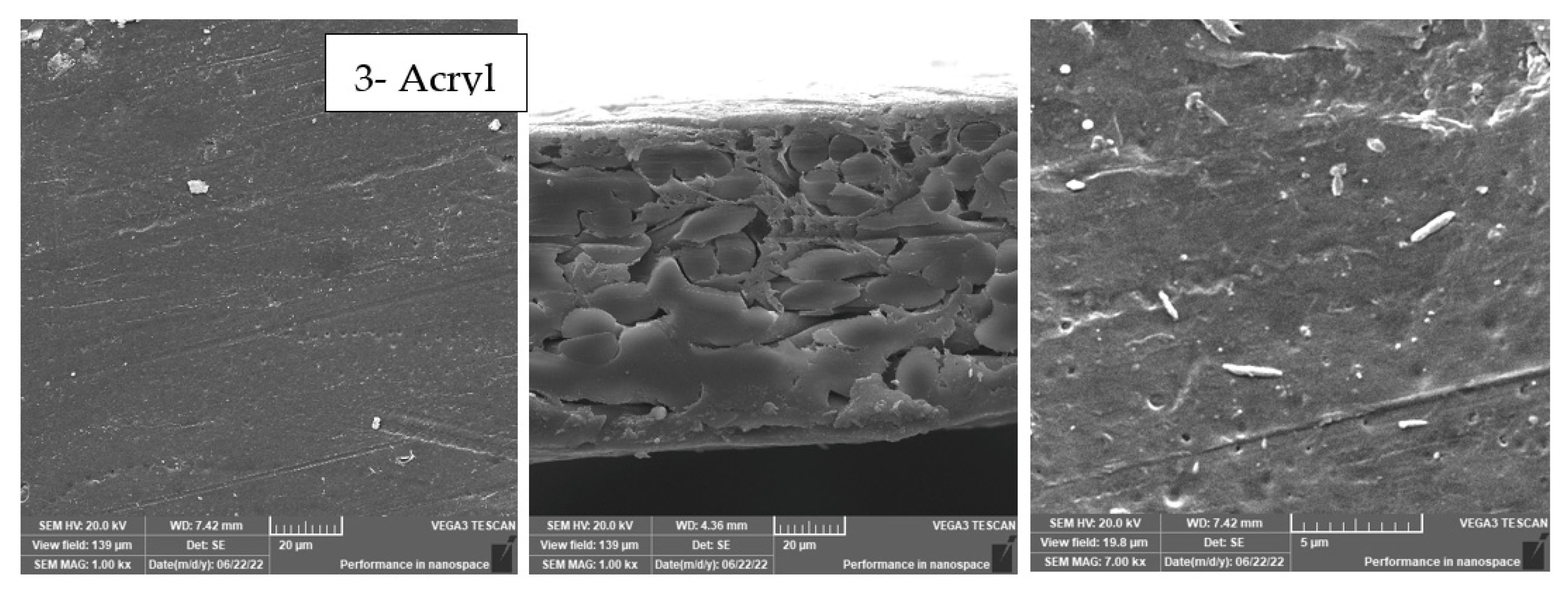
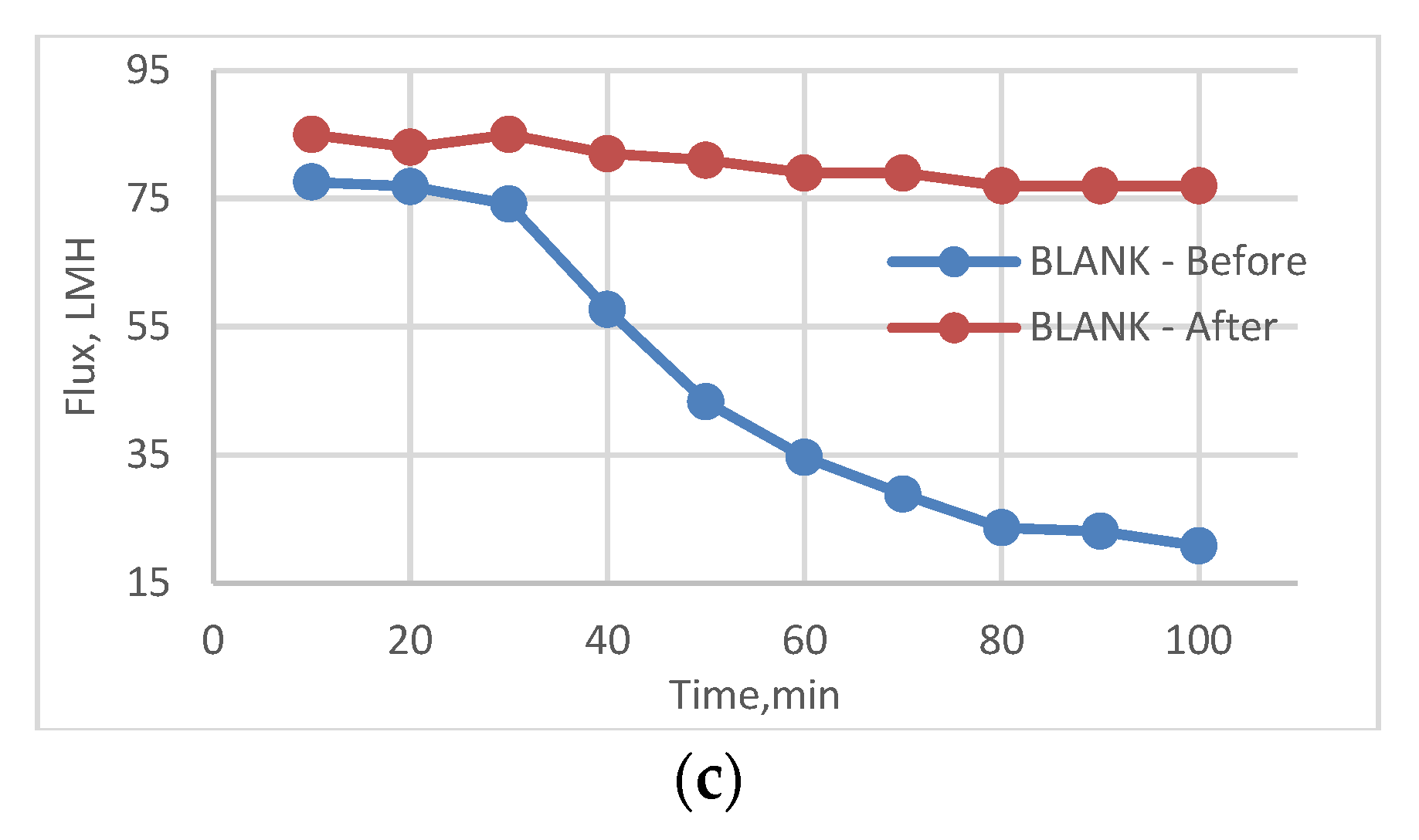
3.5. Effect of Physical Irradiation on Blank Polymeric Support
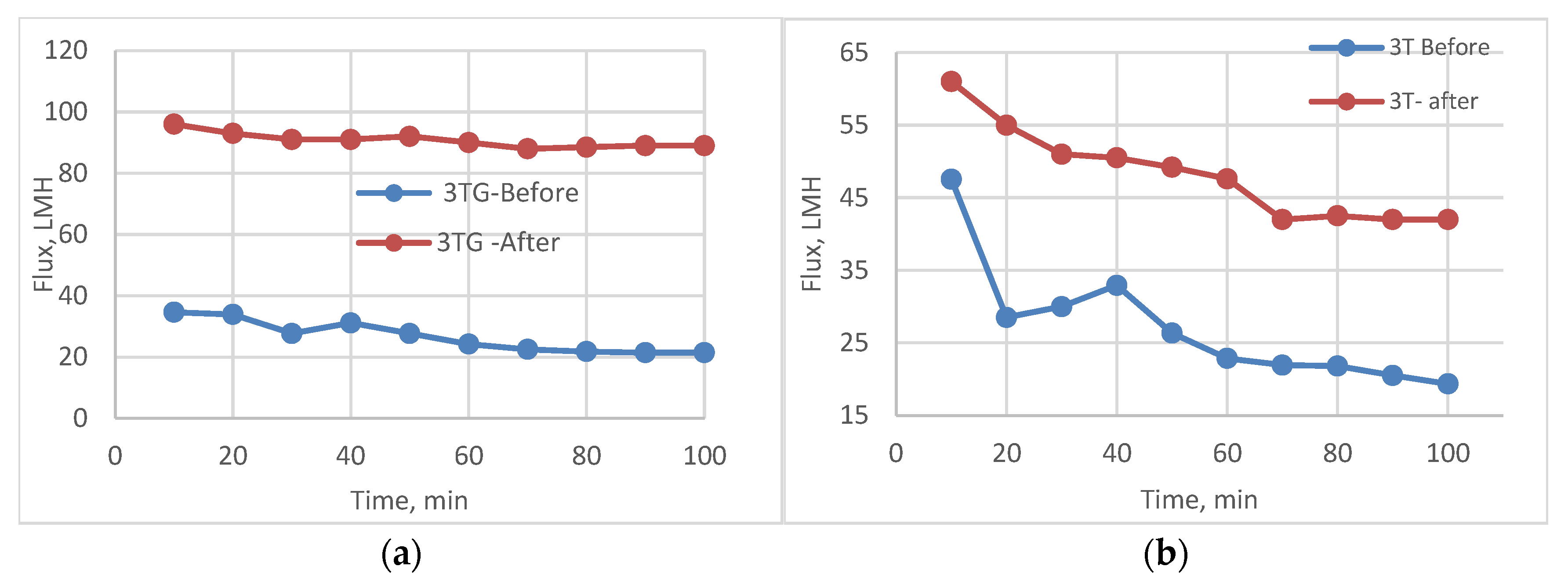
3.6. Antifouling Behavior for 3T, 3TG, and Blank Membranes
3.7. Effect of Chlorination on Different Surface Irradiations
- Effect of Chlorination on VUV Activation (AKT) for different membranes 3T, 3TG, and Blank
- 2.
- Effect of Chlorination on Plasma treatment with GrowPLAS for different membranes 3T, 3TG, and Blank
- 3.
- Effect of Chlorination on Plasma treatment with Polyacrylic acid (Acryl) for different membranes 3T, 3TG, and Blank
- 4.
- Effect of Chlorination on VUV with Polyethylene glycol (PEG) for different membranes 3T, 3TG, and Blank
- 5.
- Effect of Chlorination on VUV with Poly Di-Methyl Siloxane (PDMS) for different membranes 3T, 3TG, and Blank
4. Conclusions
- The mixed-polymeric substrate based upon PSU/PAN/GO showed a great influence on RO membrane surface hydrophilicity, which was greatly affected by the deposition of a plasma polymeric acrylic acid coating (acryl).
- The enhancement in membrane performance by the plasma polymeric acrylic acid coating in terms of permeate flux and salt rejection was measured at a constant pressure of 20 bar and was proven for blank samples.
- Additional surface treatments of 3TG membranes by VUV and plasma processes showed a large change in performance. The best was 3TG-PEG, and this confirms the interaction of the top layer with radiation.
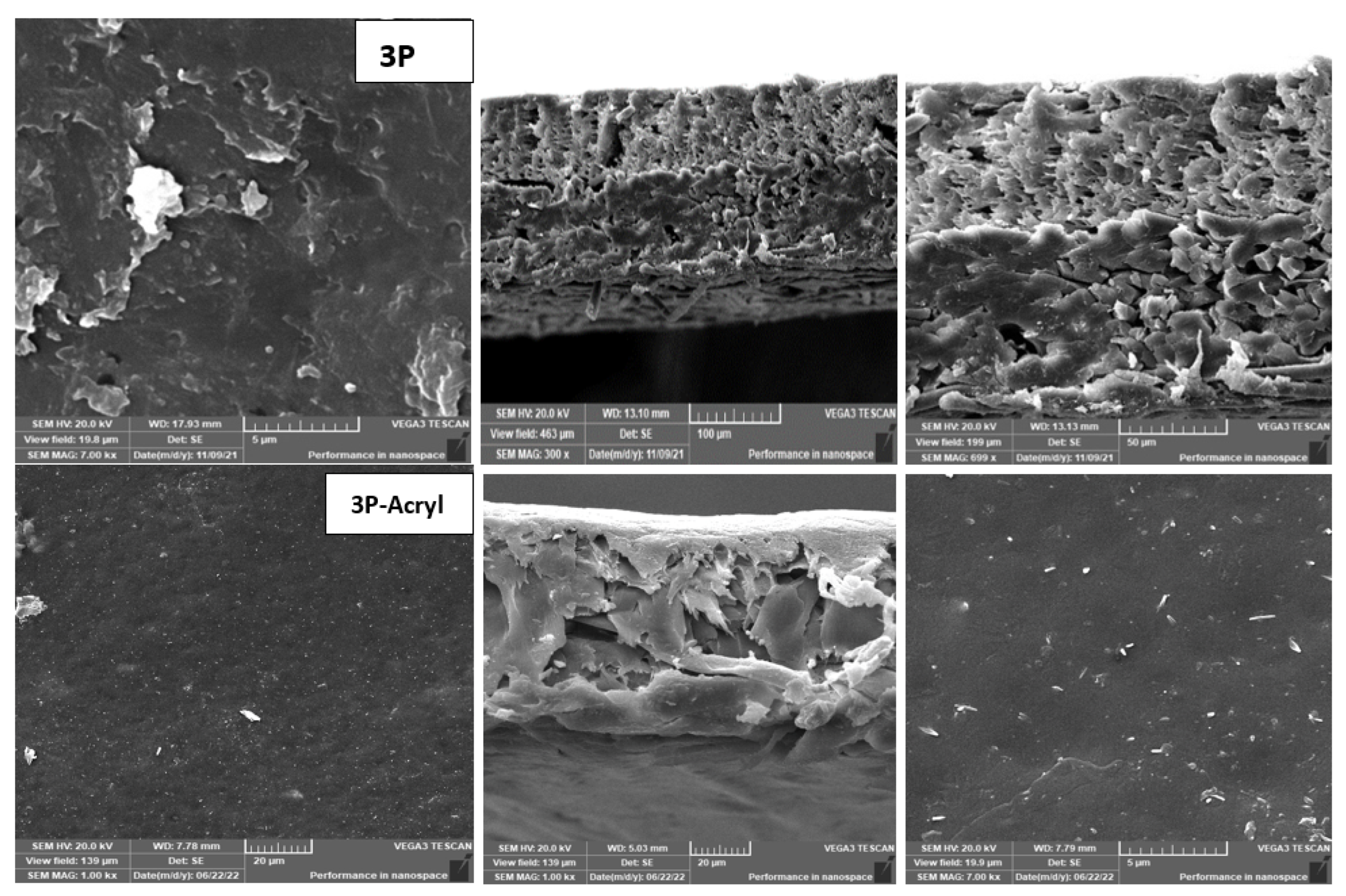
- The achieved performance was confirmed by XPS, SEM, and contact angle analyses, which also confirmed chemical and morphological changes on the membrane surface before and after modification.
- Antifouling behavior was improved for 3T-PEG, where % FRR was found to be 96% and so minimum irreversible fouling was 3%.
- In the case of the 3TG and blank membranes, the best % FRR was achieved with VUV-PDMS to obtain 96% and 92% respectively. This means that VUV treatment results in good antifouling effects.
- Chlorine resistance was enhanced and presented with changes in flux and salt rejection before and after chlorine exposure with high doses. It was found that each membrane showed some better behavior with VUV polymeric grafting with PEG and with polymeric plasma irradiation with either acryl or grow plasma, but for VUV activation, it was the worst.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, G.-R.; Wang, J.-N.; Li, C.-J. Polyamide nanofilm composite membranes (NCMs) supported by chitosan coated elec-trospun nanofibrous membranes: Preparation and separation performance research. Desalination 2013, 328, 31–41. [Google Scholar] [CrossRef]
- Fujioka, T.; Oshima, N.; Suzuki, R.; Price, W.E.; Nghiem, L.D. Probing the internal structure of reverse osmosis membranes by positron annihilation spectroscopy: Gaining more insight into the transport of water and sma. J. Membr. Sci. 2015, 486, 106–118. [Google Scholar] [CrossRef]
- Kang, G.-D.; Gao, C.-J.; Chen, W.-D.; Jie, X.-M.; Cao, Y.-M.; Yuan, Q. Study on hypochlorite degradation of aromatic polyamide reverse osmosis membrane. J. Membr. Sci. 2007, 300, 165–171. [Google Scholar] [CrossRef]
- Shinohara, H.; Hori, T.; Umeda, S.; Takahashi, Y.; Shoji, S.; Ohara, O.; Mizuno, J. Integrated hydrophilic surface treatment system of vapor deposition polymerization and vacuum ultraviolet irradiation for chemical/biochemical microchips. Microelectron. Eng. 2011, 88, 2751–2754. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes–A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Shinohara, H.; Mizuno, J.; Shoji, S. Studies on low-temperature direct bonding of VUV, VUV/O3 and O2 plasma pretreated cyclo-olefin polymer. Sensors Actuators A Phys. 2011, 165, 124–131. [Google Scholar] [CrossRef]
- Shinohara, H.; Takahashi, Y.; Mizuno, J.; Shoji, S. Surface hydrophilic treatment of polyurea film realized by vacuum ultraviolet light irradiation and its application for poly(methylmethacrylate) blood analysis chip. Sensors Actuators B Chem. 2008, 132, 374–379. [Google Scholar] [CrossRef]
- Desai, H.; Xiaolu, L.; Entenberg, A.; Kahn, B.; Egitto, F.; Matienzo, L.; Debies, T.; Takacs, G. Adhesion of copper to poly(tetrafluoroethylene) surfaces modified with vacuum UV radiation downstream from He and Ar microwave plasmas. In Polymer Surface Modification: Relevance to Adhesion; Mittal, K.L., Ed.; CRC Press: Boca Raton, FL, USA, 2004; Volume 3, pp. 139–157. [Google Scholar]
- Stewart, R.; Goodship, V.; Guild, F.; Green, M.; Farrow, J. Investigation and demonstration of the durability of air plasma pre-treatment on polypropylene automotive bumpers. Int. J. Adhes. Adhes. 2005, 25, 93–99. [Google Scholar] [CrossRef]
- Chashmejahanbin, M.; Salimi, A.; Langroudi, A.E. The study of the coating adhesion on PP surface modified in different plasma/acrylic acid solution. Int. J. Adhes. Adhes. 2014, 49, 44–50. [Google Scholar] [CrossRef]
- Kwon, O.-J.; Myung, S.-W.; Lee, C.-S.; Choi, H.-S. Comparison of the surface characteristics of polypropylene films treated by Ar and mixed gas (Ar/O2) atmospheric pressure plasma. J. Colloid Interface Sci. 2006, 295, 409–416. [Google Scholar] [CrossRef]
- Bhattacharya, A. Grafting: A versatile means to modify polymersTechniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, J.; Wang, S. Improving the water flux and bio-fouling resistance of reverse osmosis (RO) membrane through surface modification by zwitterionic polymer. J. Membr. Sci. 2015, 493, 188–199. [Google Scholar] [CrossRef]
- Kim, M.-M.; Lin, N.H.; Lewis, G.T.; Cohen, Y. Surface nano-structuring of reverse osmosis membranes via atmospheric pressure plasma-induced graft polymerization for reduction of mineral scaling propensity. J. Membr. Sci. 2010, 354, 142–149. [Google Scholar] [CrossRef]
- Tirado, M.L.M.; Bass, M.; Piatkovsky, M.; Ulbricht, M.; Herzberg, M.; Freger, V. Assessing biofouling resistance of a polyamide reverse osmosis membrane surface-modified with a zwitterionic polymer. J. Membr. Sci. 2016, 520, 490–498. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Iborra-Clar, M.-I.; Alcaina-Miranda, M.-I.; Mendoza-Roca, J.-A.; Pastor-Alcañiz, L. Development of fouling-resistant polyethersulfone ultrafiltration membranes via surface UV photografting with polyethylene glycol/aluminum oxide nanoparticles. Sep. Purif. Technol. 2014, 135, 88–99. [Google Scholar] [CrossRef]
- McVerry, B.T.; Wong, M.C.Y.; Marsh, K.L.; Temple, J.A.T.; Marambio-Jones, C.; Hoek, E.M.V.; Kaner, R.B. Scalable Antifouling Reverse Osmosis Membranes Utilizing Perfluorophenyl Azide Photochemistry. Macromol. Rapid Commun. 2014, 35, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fina, A.; Venturello, A.; Geobaldo, F. Effects of gas atmospheres on poly(lactic acid) film in acrylic acid plasma treatment. Appl. Surf. Sci. 2013, 283, 181–187. [Google Scholar] [CrossRef]
- Shalaby, M.S.; Abdallah, H.; Wilken, R.; Christoph, S.; Shaban, A.; Gaber, M.; Sołowski, G. Effect graphene oxide nanostructure/tannic acid on mixed polymeric substrate-surface modified RO membranes. J. Appl. Polym. Sci. 2022, 139, e53195. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Boddu, V.; Kumar, M.; Shalaby, M.; Abdallah, H.; Chetty, R. Effect of ZnO morphology on GO-ZnO modified polyamide reverse osmosis membranes for desalination. Desalination 2019, 467, 245–256. [Google Scholar] [CrossRef]
- Abdallah, H.; Shalaby, M.; Shaban, A. Performance and Characterization for Blend Membrane of PES with Manganese(III) Acetylacetonate as Metalorganic Nanoparticles. Int. J. Chem. Eng. 2015, 2015, 896486. [Google Scholar] [CrossRef]












| Membrane | Physical Surface Modification (Irradiation) | ||||
|---|---|---|---|---|---|
| Low-Pressure Plasma | Vacuum Ultraviolet (VUV) | ||||
| Low-Pressure Plasma—GrowPLAS | Low-Pressure Plasma (Polyacrylic Acid)—Acryl | Vacuum UV (Activation)—AKT | Vacuum UV (Polyethylene Glycol)—PEG | Vacuum UV (Poly-SiOx)—PDMS | |
| 3T | GrowPLAS over polyamide layer | Acryl-PLAS over polyamide layer | VUV-AKT over polyamide layer | VUV-PEG over polyamide layer | VUV-PDMS over polyamide layer |
| 3TG | GrowPLAS over polyamide/grafted PMAA | Acryl-PLAS over polyamide/grafted PMAA | VUV-AKT over polyamide/grafted PMAA | VUV-PEG over polyamide/grafted PMAA | VUV-PDMS over polyamide/grafted PMAA |
| 3P | GrowPLAS over PVA/GA layer | Acryl-PLAS over PVA/GA layer | VUV-AKT over PVA/GA layer | VUV-PEG over PVA/GA layer | VUV-PDMS over PVA/GA layer |
| 3PG | GrowPLAS over PVA/GA and PMAA | Acryl-PLAS over PVA/GA and PMAA | VUV-AKT over PVA/GA and PMAA | VUV-PEG over PVA/GA and PMAA | VUV-PDMS over PVA/GA and PMAA |
| Blank | GrowPLAS alone | Acryl-PLAS alone | VUV-AKT alone | VUV-PEG alone | VUV-PDMS alone |
| C (at%) | O (at%) | N (at%) | Si (at%) | Mg (at%) | Cl (at%) | Al (at%) | Na (at%) | Ca (at%) | S (at%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 3TG Ref, Pos1 | 66.3 | 24.1 | 4.0 | 0.3 | 0.4 | 0.9 | 0.2 | 0.8 | 2.6 | 0.4 |
| 3TG Ref, Pos2 | 64.5 | 25.8 | 3.0 | 1.1 | 0.3 | 0.8 | 0.9 | 0.7 | 2.7 | 0.3 |
| 3T Ref, Pos1 | 72.6 | 16.0 | 9.9 | 0.4 | - | 0.8 | - | 0.2 | 0.2 | <0.1 |
| 3T Ref, Pos2 | 71.9 | 16.4 | 10.0 | 0.4 | - | 0.8 | - | 0.2 | 0.2 | <0.1 |
| Akt, Pos1 | 63.3 | 23.6 | 11.1 | <0.1 | 0.2 | 0.7 | - | 0.5 | 0.5 | <0.1 |
| Akt, Pos2 | 62.6 | 24.0 | 11.0 | 0.1 | 0.2 | 0.8 | - | 0.5 | 0.8 | <0.1 |
| Grow, Pos1 | 42.8 | 35.5 | 7.1 | 13.7 | - | 0.3 | - | <0.1 | 0.1 | 0.4 |
| Grow, Pos2 | 47.8 | 31.2 | 8.5 | 11.8 | - | 0.3 | - | <0.1 | 0.2 | 0.2 |
| PDMS, Pos1 | 16.4 | 57.6 | 2.6 | 23.2 | - | <0.1 | - | - | <0.1 | <0.1 |
| PDMS, Pos2 | 10.4 | 60.8 | 1.1 | 27.8 | - | - | - | - | - | - |
| PEG, Pos1 | 60.9 | 26.7 | 9.8 | 0.4 | 0.4 | 0.6 | - | 0.3 | 0.9 | 0.2 |
| PEG, Pos2 | 61.8 | 26.7 | 9.4 | 0.5 | 0.2 | 0.4 | - | 0.3 | 0.5 | 0.3 |
| Acryl, Pos1 | 73.5 | 25.2 | 0.4 | - | - | - | - | 1.0 | - | - |
| Acryl, Pos2 | 74.8 | 24.0 | 0.3 | - | - | - | - | 0.9 | - | - |
| C-C/C-H (%C) | -C-OH/C-O-C or –C-NH (%C) | C=O (%C) | -COOR/-COOH (%C) | π-π* Shake Up (%C) | |
|---|---|---|---|---|---|
| 3TG Ref, Pos1 | 67.0 | 16.4 | 13.7 | 2.8 | - |
| 3TG Ref, Pos2 | 69.9 | 14.9 | 12.8 | 2.2 | 0.2 |
| 3T Ref, Pos1 | 70.5 | 15.5 | 12.2 | 0.2 | 1.5 |
| 3T Ref, Pos2 | 69.5 | 16.2 | 10.9 | 1.3 | 2.1 |
| Akt, Pos1 | 60.5 | 11.9 | 23.2 | 0.4 | 4.1 |
| Akt, Pos2 | 61.4 | 11.2 | 23.1 | 1.1 | 3.3 |
| Grow, Pos1 | 72.1 | 19.0 | 6.4 | 1.2 | 1.4 |
| Grow, Pos2 | 72.3 | 20.7 | 4.9 | 1.6 | 0.6 |
| PDMS, Pos1 | 62.2 | 13.5 | 17.8 | 5.5 | 1.0 |
| PDMS, Pos2 | 62.7 | 16.3 | 12.4 | 7.6 | 1.1 |
| PEG, Pos1 | 52.6 | 21.8 | 19.7 | - | 3.8 |
| PEG, Pos2 | 49.7 | 19.5 | 18.0 | 1.2 | 3.8 |
| Acryl, Pos1 | 67.9 | 14.3 | 6.9 | 11.0 | - |
| Acryl, Pos2 | 69.7 | 13.6 | 6.8 | 9.7 | 0.2 |
| Treatment | C (at%) | O (at%) | Cl (at%) | N (at%) | Si (at%) | Al (at%) | S (at%) | Ca (at%) | Mg (at%) | Na (at%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Blank Reference | 76.0 | 16.5 | 0.15 | 2.5 | 1.4 | 0.25 | 2.6 | 0.25 | 0.15 | - |
| Blank GrowPLAS | 30.4 | 49.5 | - | 1.6 | 16.7 | 0.45 | 1.1 | 0.2 | - | 0.15 |
| Blank Acryl | 74.2 | 25.4 | - | 0.35 | - | - | - | - | - | - |
| Blank Activation | 58.0 | 30.3 | 0.1 | 4.9 | 1.9 | 0.55 | 2.7 | 0.3 | 0.3 | 0.95 |
| Blank Act + PEG | 66.1 | 26.9 | 0.4 | 1.7 | 1.0 | 0.35 | 1.4 | 0.6 | 0.55 | 1.0 |
| Blank Act + PEG + purging | 77.8 | 18.5 | 0.1 | 1.7 | 0.85 | 0.2 | 0.55 | 0.1 | 0.1 | 0.25 |
| Blank Act + PDMS | 11.3 | 60.1 | - | 0.8 | 27.2 | - | 0.65 | - | - | 0.1 |
| Wafer Act | 5.0 | 35.9 | - | - | 59.1 | - | - | - | - | 0.1 |
| Wafer Act + PEG | 84.7 | 13.7 | - | 1.0 | 0.5 | - | 0.1 | - | - | 0.25 |
| Wafer Act + PEG + purging | 70.2 | 21.6 | - | 1.0 | 7.3 | - | - | 0.1 | - | 0.1 |
| Wafer Act + PDMS | 10.5 | 60.7 | - | 0.2 | 28.8 | - | - | - | - | - |
| Treatment | C-C/C-H (%C) | -C-OH/C-O-C or –C-NH (%C) | C=O (%C) | -COOR/-COOH (%C) | π-π* Shake-Up (%C) |
|---|---|---|---|---|---|
| Blank Reference | 74.3 | 21.1 | 0.9 | 0.2 | 3.7 |
| Blank GrowPLAS | 56.9 | 28.0 | 8.2 | 6.2 | 0.75 |
| Blank Acryl | 68.4 | 14.0 | 5.3 | 12.5 | - |
| Blank Activation | 50.9 | 32.0 | 6.2 | 9.1 | 2.0 |
| Blank Act + PEG | 55.5 | 33.9 | 8.3 | 2.3 | 0.3 |
| Blank Act + PEG + purging | 69.2 | 22.6 | 6.9 | 1.0 | 0.4 |
| Blank Act + PDMS | 67.0 | 19.7 | 6.5 | 6.0 | 0.9 |
| Membrane | % Salt Rejection before Chlorination | % Salt Rejection after Chlorination |
|---|---|---|
| 3T | 71.36 | 68 |
| 3TG | 56.54 | 50.13 |
| Blank”3” | 28 | 25 |
| Membrane | % Salt Rejection before Chlorination | % Salt Rejection after Chlorination |
|---|---|---|
| 3T | 75.7 | 74.9 |
| 3TG | 40.17 | 35.72 |
| Blank”3” | 31 | 28.56 |
| Membrane | % Salt Rejection before Chlorination | % Salt Rejection after Chlorination |
|---|---|---|
| 3T | 86.86 | 70.37 |
| 3TG | 82.68 | 80 |
| Blank”3” | 52 | 50.8 |
| Membrane | % Salt Rejection before Chlorination | % Salt Rejection after Chlorination |
|---|---|---|
| 3T | 75.6 | 75 |
| 3TG | 86.17 | 85.54 |
| Blank”3” | 40.5 | 35 |
| Membrane | % Salt Rejection before Chlorination | % Salt Rejection after Chlorination |
|---|---|---|
| 3T | 84.76 | 83.2 |
| 3TG | 73.45 | 70.72 |
| Blank”3” | 48 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalaby, M.S.; Abdallah, H.; Wilken, R.; Christoph, S.; Shaban, A.M. Surface Treatment by Physical Irradiation for Antifouling, Chlorine-Resistant RO Membranes. Membranes 2023, 13, 227. https://doi.org/10.3390/membranes13020227
Shalaby MS, Abdallah H, Wilken R, Christoph S, Shaban AM. Surface Treatment by Physical Irradiation for Antifouling, Chlorine-Resistant RO Membranes. Membranes. 2023; 13(2):227. https://doi.org/10.3390/membranes13020227
Chicago/Turabian StyleShalaby, Marwa S., Heba Abdallah, Ralph Wilken, Schmüser Christoph, and Ahmed M. Shaban. 2023. "Surface Treatment by Physical Irradiation for Antifouling, Chlorine-Resistant RO Membranes" Membranes 13, no. 2: 227. https://doi.org/10.3390/membranes13020227
APA StyleShalaby, M. S., Abdallah, H., Wilken, R., Christoph, S., & Shaban, A. M. (2023). Surface Treatment by Physical Irradiation for Antifouling, Chlorine-Resistant RO Membranes. Membranes, 13(2), 227. https://doi.org/10.3390/membranes13020227









