Electrodialysis Processes an Answer to Industrial Sustainability: Toward the Concept of Eco-Circular Economy?—A Review
Abstract
1. Introduction
2. Definitions
2.1. Sustainable Development
2.2. Eco-Efficiency
| Basis of the Eco-Efficiency Score | Product | Eco-Efficiency Score | Components of the Eco- Efficiency Score | Rationals and Parameters | Ref. |
|---|---|---|---|---|---|
| Health benefits | Cranberry juice | Value of the product = | Cranberry juice’s health benefits are associated with its high polyphenol content. Hence, the value was related to the polyphenol content of the product and calculated as the sum of the price of 1000 kg of cranberry juice and the price of the polyphenolic compounds present in the deacidified juice. | [33] | |
| Sum of environmental impacts: based on an LCA | The life-cycle assessment was conducted according to ISO 14044. | ||||
| Functional value = content of functional nutrients | Polyphenol content was selected as a functional value parameter due to its beneficial health effects. | [32] | |||
| Environmental impact cost: based on an LCA | The life-cycle assessment was conducted according to ISO 14044. | ||||
| Functional value = abatement of harmful substances | The concentration of organic acids may induce gastrointestinal disturbances. The percentage of removed harmful acid content was chosen as a functional value parameter. | [32] | |||
| Environmental impact cost: based on an LCA | The life-cycle assessment was conducted according to ISO 14044. | ||||
| Sweet whey and whey protein concentrate (WPC) | Phospholipids (PLs) have a positive impact on the brain, aging, and neurodegenerative diseases, as well as on cell growth and the prevention of colon cancer. The ratio of PLs quantity recovered (in g) per quantity of crude protein treated (in g) was chosen as a value parameter to compare sweet whey and WPC on the same basis. | [42] | |||
| Environmental impacts: Volume of water used and effluent produced | Volume of water and effluents involved in the process (in L) as an environmental parameter. | ||||
| As high PL content is desired in the final fraction obtained by precipitation, the ratio of PLs in the precipitated fraction over protein recovered in this same fraction was used as a value parameter. | [42] | ||||
| Environmental impacts: Volume of water used and effluent produced | Volume of water and effluents involved in the process (in L) as an environmental parameter. | ||||
| Value product as a combination of both previous ratios to take into account PL yield in terms of protein treated and the purity of the final fraction. | [42] | ||||
| Environmental impacts: Volume of water used and effluent produced | Volume of water and effluents involved in the process (in L) as an environmental parameter. | ||||
| Health benefits | Whey protein hydrolysate (WPH) | IC50 ACE fraction was normalized using the IC50 ACE of Captopril as an ACE inhibitor standard. Factoring the market cost per kg of this standard and the mass of fraction recovered after 1000 EDUF runs. | [40] | ||
| Environmental impact cost was calculated by summing the energy cost and the chemical cost for 1000 EDUF runs. | |||||
| Process added-value | Beta-lactoglobulin | Degree of hydrolysis (DH) was determined by the ophthaladehyde (OPA) method. | [41] | ||
| Energy consumption (EC) was the specific energy input in MJ/kg for treatments of protein solution with Q, the total energy input, to pasteurize 1 kg of β-lg solution (MJ/kg); W (0.1 MJ/kg), the total work input to the electrical motors to pasteurize 1 kg milk; N, the number of pulses; m, the mass (kg) of the treated protein solution; and Pi, the energy of one electric pulse. | |||||
| Δ∆Cpeptide = 1 × 106 () f | ΔCpeptide (in μM) represents the improvement in the relative concentration of bioactive peptides identified in pretreated β-lg hydrolysates in relation to their concentration in native ones. | [41] | |||
| Energy consumption was the specific energy input in MJ/kg for treatments of protein solution with Q, the total energy input to pasteurize 1 kg of β-lg solution (MJ/kg); W (0.1 MJ/kg), the total work input to the electrical motors to pasteurize 1 kg milk; N, the number of pulses; m, the mass (kg) of the treated protein solution; and Pi, the energy of one electric pulse. | |||||
| Cranberry juice | Value of the product = | The value of the product was linked to the amount of organic acids removed (citric and malic) and available for other industrial applications; the value was calculated as the sum of the price of 1000 kg of cranberry juice and the price of the amounts of citric and malic acids removed and usable after the deacidification treatment. | [33] | ||
| Sum of environmental impacts: based on an LCA | The life-cycle assessment was conducted according to ISO 14044. | ||||
| Process added-value | Cranberry juice | Value of the product = | The value of the product was calculated as the price of cranberry juice with an adjustment when quinic acid was lost during the deacidification treatment: if there was no significant loss of quinic acid in the deacidified juice, the juice was considered non-tampered and its value was 100% of the price. If there was a significant loss of quinic acid, the juice was considered tampered and its value was 80% of the selling price of 1000 kg of cranberry juice (loss of 20% of the regular selling price). | [33] | |
| Sum of environmental impacts: based on an LCA | The life-cycle assessment was conducted according to ISO 14044. | ||||
| Value of the product = | The value of the deacidified juice was the sum of the price of 1000 kg of commercially available cranberry juice with price adjustment considering significant loss or not of quinic acid, the price of the polyphenolic compounds present in the deacidified juice, and the price of the citric and malic acids removed and usable. | [33] | |||
| Sum of environmental impacts: based on an LCA | The life-cycle assessment was conducted according to ISO 14044. | ||||
| Mealworm protein | Value of the product = g Protein x kg product | The protein content of the mealworm extract (or other protein sources) was considered the product value. | [43] | ||
| Global warming potential = kg CO2 éq x kg product | Global warming potential represents the environmental impact. | ||||
| Whey protein hydrolysate (WPH) | The environmental impact cost was calculated based on the energy cost and the chemical cost of 1000 EDUF runs. | [39] | |||
| The recovery of bioactive peptides (in %) was expressed with the area under the curve of bioactive peptide (AUCbioactive peptides) and AUCWPH, added to the areas under the curve (LC–UV data) of individual bioactive peptides in the recovery fraction at t180 min and in the initial WPH at t0min, respectively. | |||||
| Consumers/stakeholders’ criteria | Cranberry juice | Functional value = Taste | The taste, particularly the acidic taste, stops some clients from buying cranberry juice. Hence, taste was selected as a functional value parameter. Taste was determined following an organoleptic test. | [32] | |
| Environmental impact cost: based on an LCA | The life-cycle assessment was conducted according to ISO 14044. | ||||
| Functional value = abatement of harmful substances | The concentration of organic acid impacts the acidic taste of the juice, which stops some clients from buying cranberry juice. The percentage of removed harmful acid content was chosen as a functional value parameter. | [32] | |||
| Environmental impact cost: based on an LCA | The life-cycle assessment was conducted according to ISO 14044. | ||||
| Greek yogurt | Value of the product = Financial profit + Socio-economic value + Functional value | Financial profit is the net income from sales after the deduction of all costs related to production, including material costs, capital costs, labour costs, and taxes. Socio-economic value is composed of the socio-economic value measured by the gross value added (GVA) at the territory level and the socio-economic value measured by the total gross value added (Total GVA) at the society level. Functional value refers to nutritional attributes, health benefits, and sensory characteristics (texture, taste, flavour, etc.) and also encompasses packaging functionalities (shelf life, practicality, lightness, robustness, aesthetics, etc.). Calcium content, probiotic concentration, and typical flavour, representative of the main functions sought by Greek yogurt consumers, were selected as functional indicators. | [34] | ||
| Environmental impact: based on an LCA | The life-cycle assessment was conducted according to ISO 14040 and 14044. |
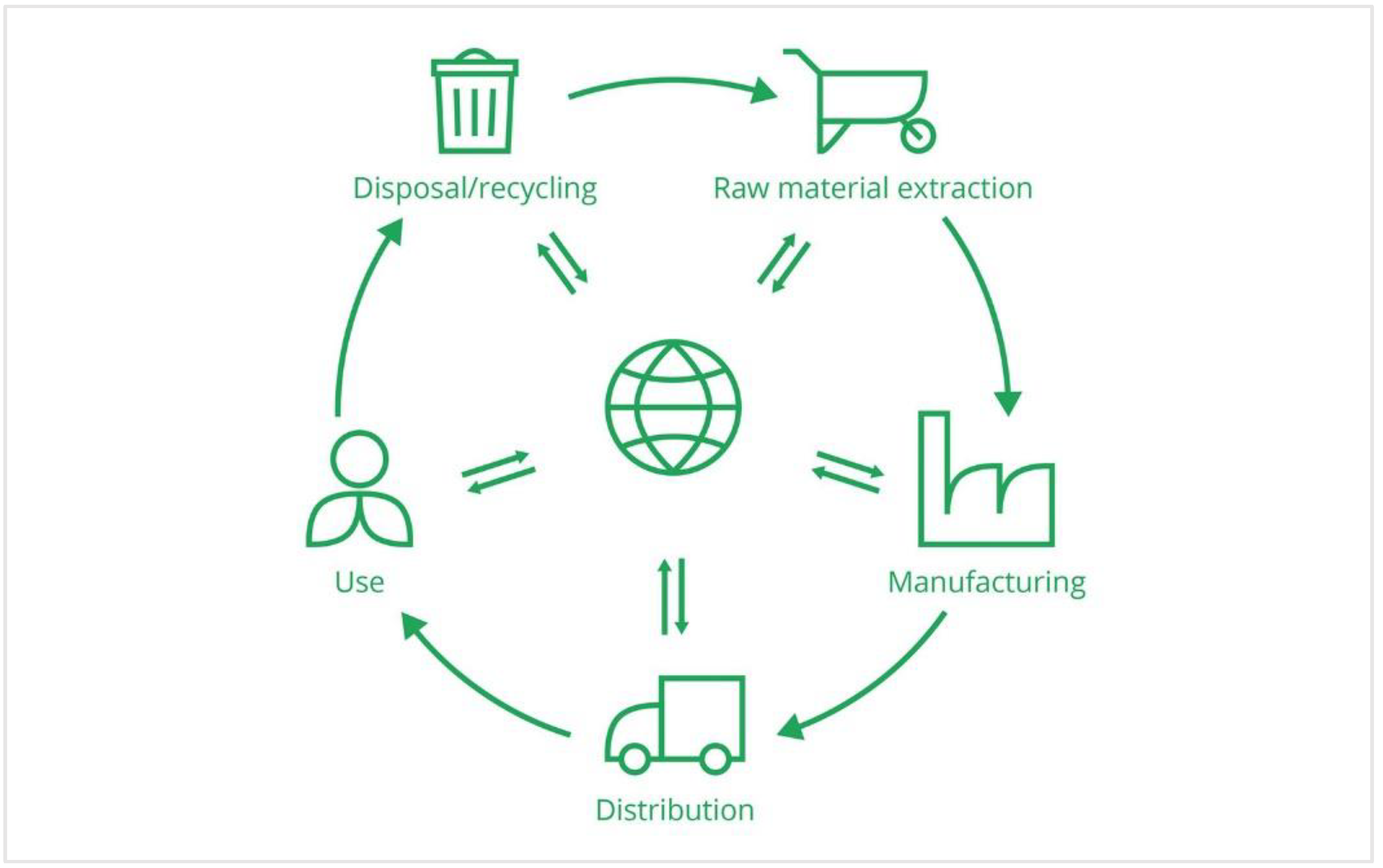
2.3. Circular Economy
3. Electromembrane Processes: General Aspects
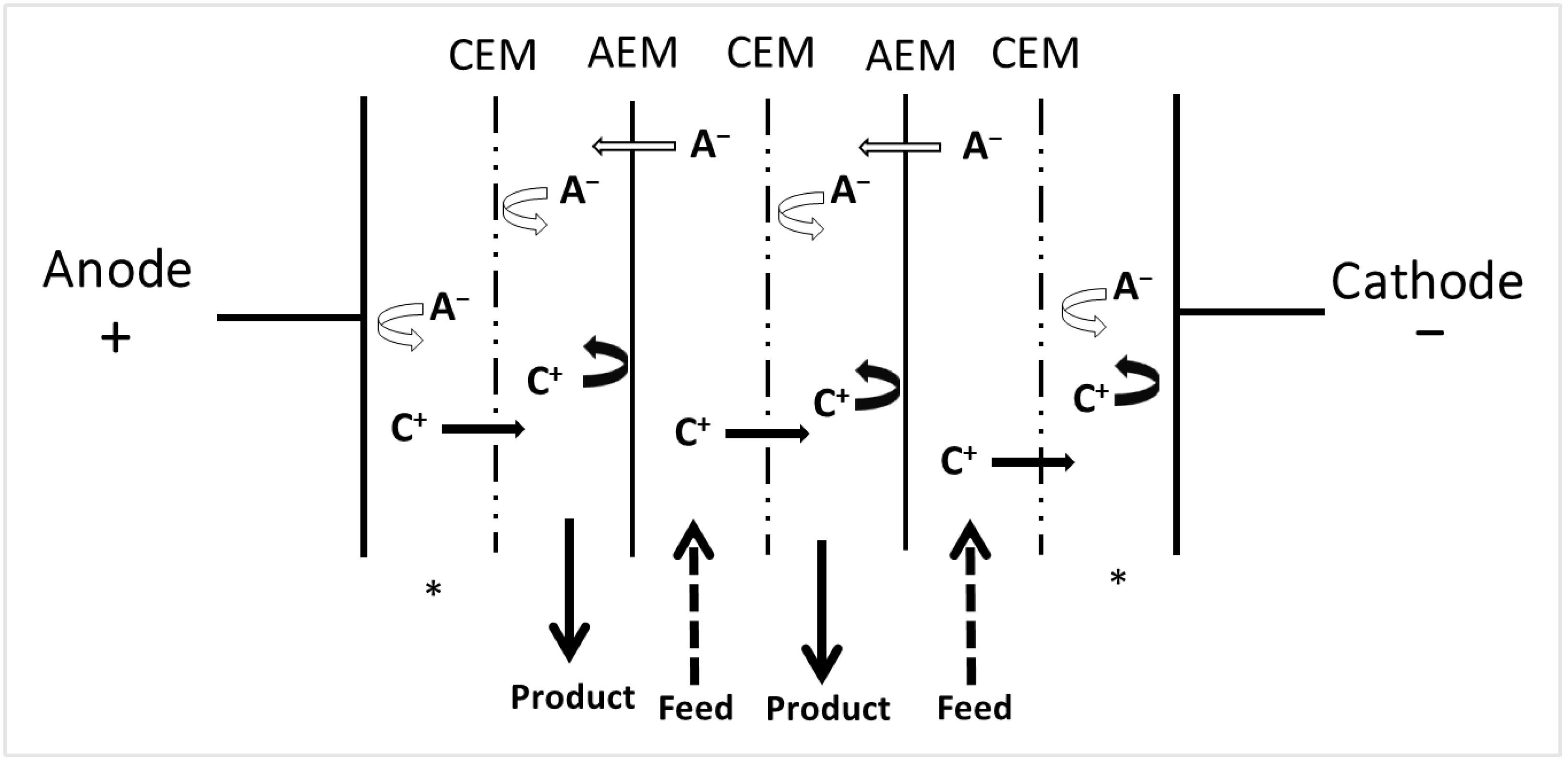
3.1. Electrodialysis with Ion-Exchange Membranes
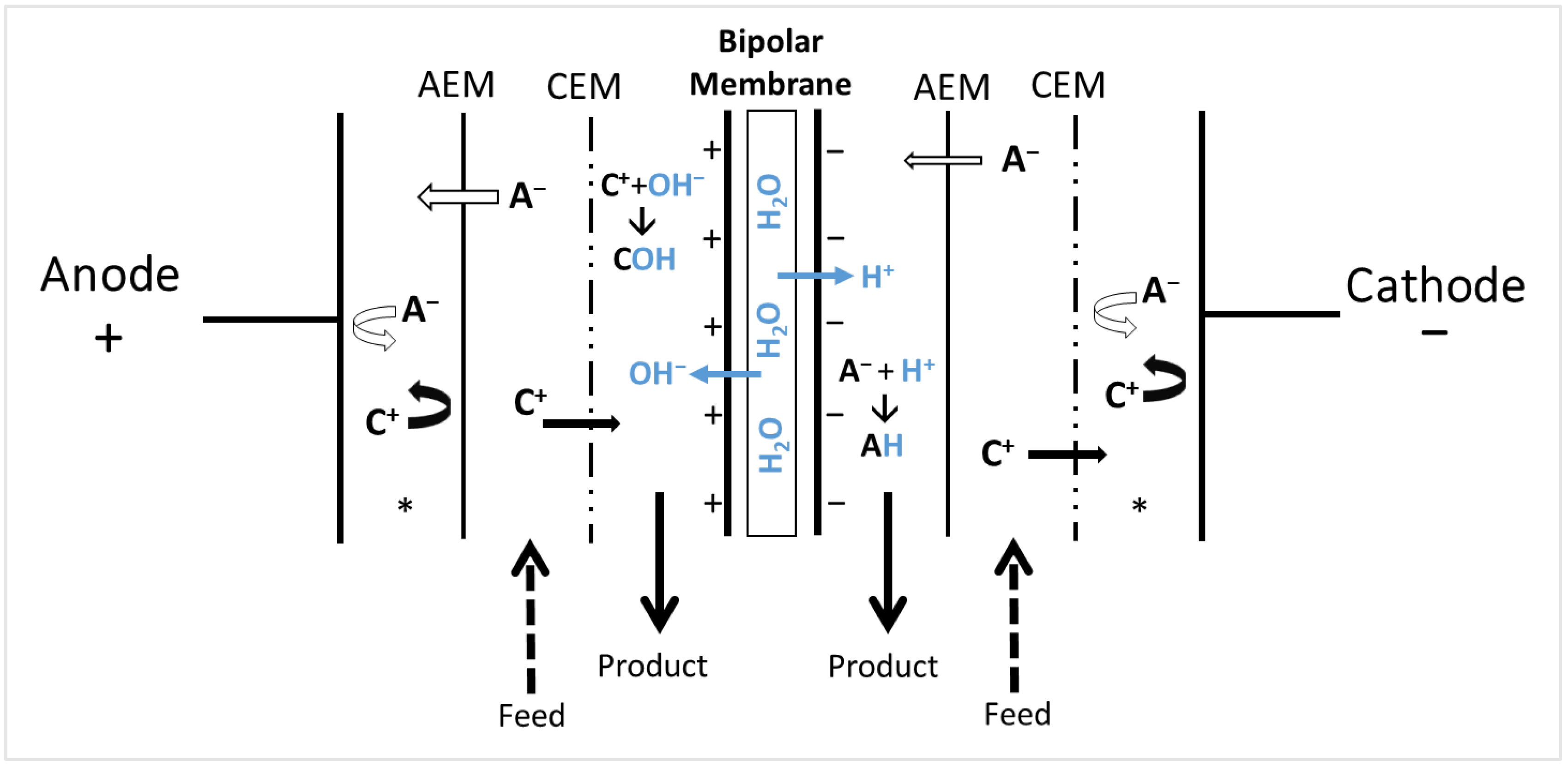
3.2. Electrodialysis with Bipolar Membranes
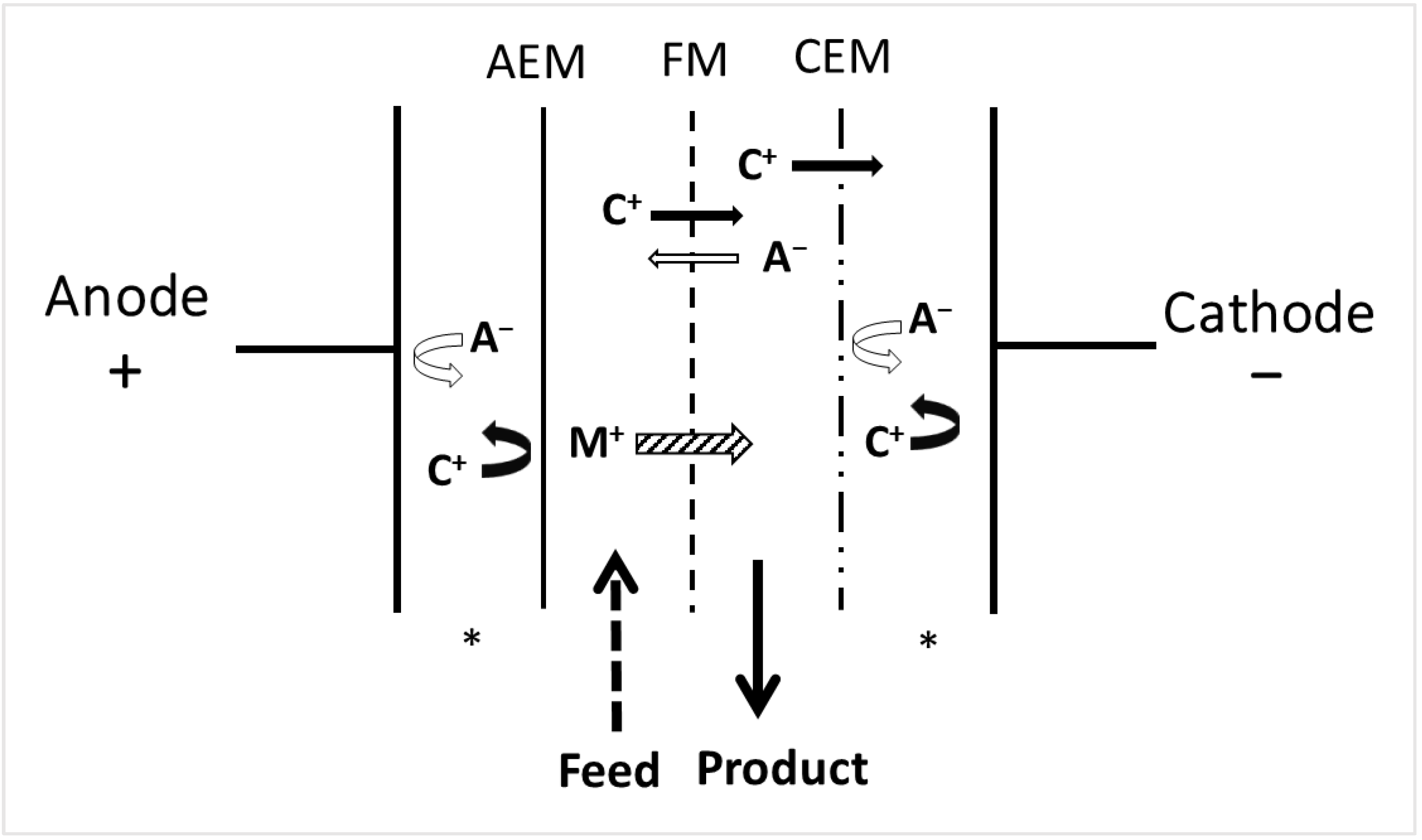
3.3. Electrodialysis with Filtration Membranes
4. Electrodialysis and Eco-Efficiency
4.1. Seawater and Municipal Wastewater
4.2. Food Industry
4.3. Chemical Industry
5. Electrodialysis and Circular Economy
5.1. Desalination of Seawater and Brine
5.2. Chemical, Metallurgical, and Mining Industries
5.2.1. Recovery of Acid and/or Base
5.2.2. Recovery of Valuable Compounds
5.3. Food Industry
5.3.1. Recovery of Acid and Base
5.3.2. Recovery of Protein Compounds
6. Electrodialysis as an Eco-Efficient Process in a Circular Economy
6.1. Cranberry Industry
6.2. Dairy Industry
6.3. Water Treatment Plant
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AEM | Anion-exchange membrane |
| CCS | Carbon Capture and Sequestration |
| CE | Circular economy |
| CEM | Cation-exchange membrane |
| ED | Electrodialysis |
| EE | Eco-efficiency |
| EED | Electro-electrodialysis |
| EDBM | Electrodialysis with bipolar membrane |
| EDFM | Electrodialysis with filtration membrane |
| EDM | Electrodialysis metathesis |
| EDNF | Electrodialysis with nanofiltration membrane |
| EDUF | Electrodialysis with ultrafiltration membrane |
| FM | Filtration membrane |
| IEC | Ion-exchange capacity |
| IEM | Ion-exchange membrane |
| LCA | Life-cycle assessment |
| MSED | Monovalent selective electrodialysis |
| MWCO | Molecular weight cut-off |
| NPG | Neopentyl glycol |
| OECD | Organisation for Economic Co-operation and Development |
| RED | Reverse electrodialysis |
| RO | Reverse osmosis |
| SED | Selectrodialysis |
| WPH | Whey protein hydrolysate |
| WPC | Whey protein concentrate |
References
- United Nations United Nations Conference on the Human Environment. Stockholm 1972. Available online: https://www.un.org/en/conferences/environment/stockholm1972 (accessed on 4 July 2022).
- Noronha, L. Why Does Stockholm+50 Matter? What Did It Achieve? What Does It Offer Going Forward? Available online: http://www.stockholm50.global/news-and-stories/why-does-stockholm50-matter-what-did-it-achieve-what-does-it-offer-going-forward (accessed on 4 July 2022).
- Hussain, A.; Yan, H.; Ul Afsar, N.; Jiang, C.; Wang, Y.; Xu, T. Multistage-Batch Bipolar Membrane Electrodialysis for Base Production from High-Salinity Wastewater. Front. Chem. Sci. Eng. 2022, 16, 764–773. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of Organic Pollution in Industrial Saline Wastewater: A Literature Review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada Water Discharge in Mineral Extraction and Thermal-Electric Power Generation Industries, by Type of Final Treatment and Region. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3810008101 (accessed on 5 July 2022).
- Agriculture and Agri-Food Canada Hogs/Pork. Available online: https://agriculture.canada.ca/en/agriculture-and-agri-food-canada/canadas-agriculture-sectors/animal-industry/red-meat-and-livestock-market-information/hogs-pork (accessed on 9 August 2022).
- Llop, A.; Pocurull, E.; Borrull, F. Evaluation of the Removal of Pollutants from Petrochemical Wastewater Using A Membrane Bioreactor Treatment Plant. Water. Air. Soil Pollut. 2009, 197, 349–359. [Google Scholar] [CrossRef]
- Minière, M.; Boutin, O.; Soric, A. Combination of Chemical and Biological Processes to Enhance the Treatment of Hardly Biodegradable Matter in Industrial Wastewater: Selection Parameters and Performances. Can. J. Chem. Eng. 2019, 97, 1361–1370. [Google Scholar] [CrossRef]
- Adin, A.; Asano, T. The Role of Physical-Chemical Treatment in Wastewater Reclamation and Reuse. Water Sci. Technol. 1998, 37, 79–90. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.Q.; Wang, Q.; Xu, T.; Kentish, S.E. Transforming Salty Whey into Cleaning Chemicals Using Electrodialysis with Bipolar Membranes. Desalination 2020, 492, 114598. [Google Scholar] [CrossRef]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef]
- Mensah, J. Sustainable Development: Meaning, History, Principles, Pillars, and Implications for Human Action: Literature Review. Cogent Soc. Sci. 2019, 5, 1653531. [Google Scholar] [CrossRef]
- Wanamaker, C. The Environmental, Economic and Social Components of Sustainability: The Three Spheres of Sustainability; US Army Corps of Engineers: Washington, DC, USA, 2018. [Google Scholar]
- Gray, R. Is Accounting for Sustainability Actually Accounting for Sustainability…and How Would We Know? An Exploration of Narratives of Organisations and the Planet. Account. Organ. Soc. 2010, 35, 47–62. [Google Scholar] [CrossRef]
- Keeble, B.R. The Brundtland report: Our Common Future. Report of the World Commission on Environment and Development. Medicine and War 1988, 4, 17–25. [Google Scholar] [CrossRef]
- Caiado, R.G.G.; de Freitas Dias, R.; Mattos, L.V.; Quelhas, O.L.G.; Leal Filho, W. Towards Sustainable Development through the Perspective of Eco-Efficiency—A Systematic Literature Review. J. Clean. Prod. 2017, 165, 890–904. [Google Scholar] [CrossRef]
- OECD. OECD Eco-Efficiency; OECD: Paris, France, 1998. [Google Scholar]
- Alves, J.L.S.; Dumke de Medeiros, D. Eco-Efficiency in Micro-Enterprises and Small Firms: A Case Study in the Automotive Services Sector. J. Clean. Prod. 2015, 108, 595–602. [Google Scholar] [CrossRef]
- OECD. OECD Eco-Innovation in Industry: Enabling Green Growth; OECD: Paris, France, 2010; ISBN 978-92-64-07721-8. [Google Scholar]
- Tyl, B. Eco-Efficience Industrielle Atteindre l’éco-Efficience à Travers l’éco-Conception et l’écologie Industrielle. Available online: https://docplayer.fr/38909760-Emar-eco-efficience-industrielle-atteindre-l-eco-efficience-a-travers-l-eco-conception-et-l-ecologie-industrielle-guide-pratique-n-5.html (accessed on 10 June 2022).
- Pouliot, Y.; Doyen, A.; Bazinet, L.; Mikhaylin, S.; Benoit, S.; Margni, M. Écoefficience des Procédés de Transformation du Lait: Principes, Méthodologies et Applications. In Science et technologie du lait. 3e édition (éditeur Jean-Christophe Vuillemard); Presses de l’Université Laval: Quebec City, QC, Canada, 2018; pp. 157–176. ISBN 978-2-7637-3633-4. [Google Scholar]
- ISO ISO 14045:2012. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/32/43262.html (accessed on 9 August 2022).
- Charmondusit, K.; Phatarachaisakul, S.; Prasertpong, P. The Quantitative Eco-Efficiency Measurement for Small and Medium Enterprise: A Case Study of Wooden Toy Industry. Clean Technol. Environ. Policy 2014, 16, 935–945. [Google Scholar] [CrossRef]
- Ho, T.Q.; Hoang, V.-N.; Wilson, C.; Nguyen, T.-T. Eco-Efficiency Analysis of Sustainability-Certified Coffee Production in Vietnam. J. Clean. Prod. 2018, 183, 251–260. [Google Scholar] [CrossRef]
- Forleo, M.B.; Palmieri, N.; Suardi, A.; Coaloa, D.; Pari, L. The Eco-Efficiency of Rapeseed and Sunflower Cultivation in Italy. Joining Environmental and Economic Assessment. J. Clean. Prod. 2018, 172, 3138–3153. [Google Scholar] [CrossRef]
- Keating, B.A.; Carberry, P.S.; Bindraban, P.S.; Asseng, S.; Meinke, H.; Dixon, J. Eco-Efficient Agriculture: Concepts, Challenges, and Opportunities. Crop Sci. 2010, 50, S-109–S-119. [Google Scholar] [CrossRef]
- Masuda, K. Measuring Eco-Efficiency of Wheat Production in Japan: A Combined Application of Life Cycle Assessment and Data Envelopment Analysis. J. Clean. Prod. 2016, 126, 373–381. [Google Scholar] [CrossRef]
- Müller, C.; Elliott, J.; Chryssanthacopoulos, J.; Deryng, D.; Folberth, C.; Pugh, T.A.M.; Schmid, E. Implications of Climate Mitigation for Future Agricultural Production. Environ. Res. Lett. 2015, 10, 125004. [Google Scholar] [CrossRef]
- Laso, J.; García-Herrero, I.; Margallo, M.; Vázquez-Rowe, I.; Fullana, P.; Bala, A.; Gazulla, C.; Irabien, Á.; Aldaco, R. Finding an Economic and Environmental Balance in Value Chains Based on Circular Economy Thinking: An Eco-Efficiency Methodology Applied to the Fish Canning Industry. Resour. Conserv. Recycl. 2018, 133, 428–437. [Google Scholar] [CrossRef]
- Ullah, A.; Perret, S.R.; Gheewala, S.H.; Soni, P. Eco-Efficiency of Cotton-Cropping Systems in Pakistan: An Integrated Approach of Life Cycle Assessment and Data Envelopment Analysis. J. Clean. Prod. 2016, 134, 623–632. [Google Scholar] [CrossRef]
- ISO AS ISO 14044:2019 Environmental Management - Life Cycle Assessment—Requirements and Guidelines. Available online: https://infostore.saiglobal.com/en-us/standards/as-iso-14044-2019-1156937_saig_as_as_2747947/?gclid=Cj0KCQiA99ybBhD9ARIsALvZavXquoH5Y4RpHHGoOLvhphptFQx3mUofpiLRBsdYvq9XATreYf3CMi8aAov6EALw_wcB&gclsrc=aw.ds (accessed on 18 November 2022).
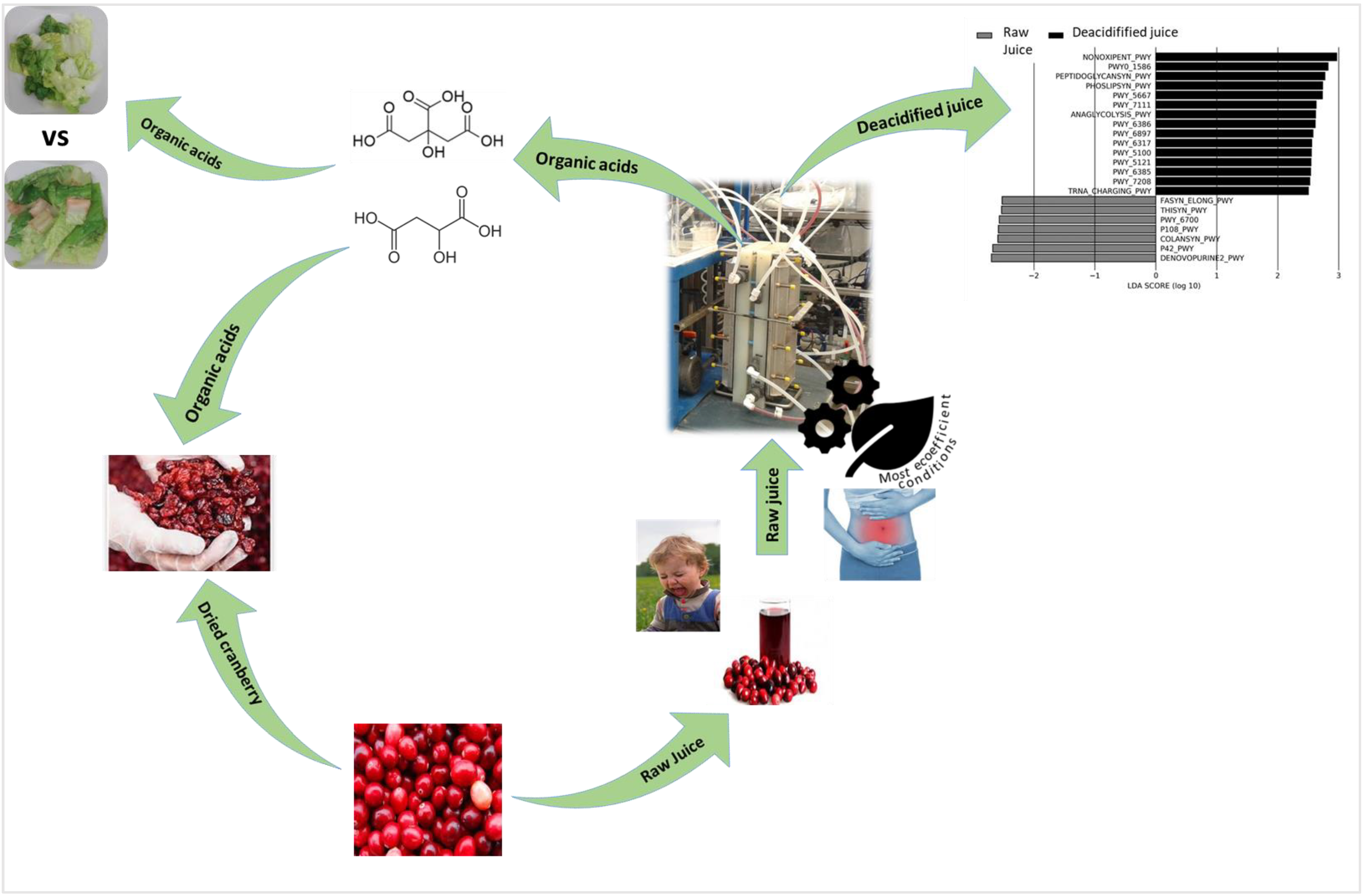
- Chaudron, C.; Faucher, M.; Bazinet, L.; Margni, M. The Cost Is Not Enough—An Alternative Eco-Efficiency Approach Applied to Cranberry de-Acidification. J. Clean. Prod. 2019, 232, 391–399. [Google Scholar] [CrossRef]
- Faucher, M.; Henaux, L.; Chaudron, C.; Mikhaylin, S.; Margni, M.; Bazinet, L. Electromembrane Approach to Substantially Improve the Ecoefficiency of Deacidified Cranberry Juice Production: Physicochemical Properties, Life Cycle Assessment and Ecoefficiency Score. J. Food Eng. 2020, 273, 109802. [Google Scholar] [CrossRef]
- Houssard, C.; Revéret, J.-P.; Maxime, D.; Pouliot, Y.; Margni, M. Measuring Shared Value Creation with Eco-Efficiency: Development of a Multidimensional Value Framework for the Dairy Industry. J. Clean. Prod. 2022, 374, 133840. [Google Scholar] [CrossRef]
- Faucher, M. Valorisation écoefficiente du lactosérum doux par procédés électrodialytiques pour la production de fractions phospholipide et peptidique potentiellement bioactives. Ph.D. Thesis, Laval University, Laval, QC, Canada, 2022. [Google Scholar]
- Jolliet, O.; Saadé, M.; Crettaz, P. Analyse du Cycle de vie: Comprendre et Réaliser un écobilan; PPUR Presses Polytechniques: Lausanne, Switzerland, 2010; ISBN 978-2-88074-886-9. [Google Scholar]
- Muralikrishna, I.V.; Manickam, V. Chapter Five—Life Cycle Assessment. In Environmental Management; Muralikrishna, I.V., Manickam, V., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 57–75. ISBN 978-0-12-811989-1. [Google Scholar]
- Golsteijn, L. Life Cycle Assessment (LCA) Explained. Available online: https://pre-sustainability.com/articles/life-cycle-assessment-lca-basics/ (accessed on 11 July 2022).
- Geoffroy, T.R.; Bernier, M.E.; Thibodeau, J.; Francezon, N.; Beaulieu, L.; Mikhaylin, S.; Langevin, M.E.; Lutin, F.; Bazinet, L. Semi-Industrial Scale-up of EDUF Technology for the Electroseparation of Bioactive Cationic Peptides: Impact of Process Parameters and Cell Configurations on Eco-Efficiency. J. Membr. Sci. 2022, 641, 119856. [Google Scholar] [CrossRef]
- Geoffroy, T.R.; Thibodeau, J.; Faucher, M.; Langevin, M.E.; Lutin, F.; Bazinet, L. Relationship between Feed Concentration and Bioactive Cationic Peptide Recovery: Impact on Ecoefficiency of EDUF at Semi-Industrial Scale. Sep. Purif. Technol. 2022, 286, 120403. [Google Scholar] [CrossRef]
- Agoua, R.-S.; Bazinet, L.; Vorobiev, E.; Grimi, N.; Mikhaylin, S. Substantial Improvement of Tryptic and Chymotryptic Hydrolysis of β-Lactoglobulin Pretreated with High Voltage Electrical Treatments. ACS Sustain. Chem. Eng. 2020, 8, 14775–14785. [Google Scholar] [CrossRef]
- Faucher, M.; Perreault, V.; Ciftci, O.N.; Gaaloul, S.; Bazinet, L. Phospholipid Recovery from Sweet Whey and Whey Protein Concentrate: Use of Electrodialysis with Bipolar Membrane Combined with a Dilution Factor as an Ecoefficient Method. Future Foods 2021, 4, 100052. [Google Scholar] [CrossRef]
- Laroche, M.; Perreault, V.; Marciniak, A.; Mikhaylin, S.; Doyen, A. Eco-Efficiency of Mealworm (Tenebrio Molitor) Protein Extracts. ACS Food Sci. Technol. 2022, 2, 1077–1085. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation Circular Economy Introduction. Available online: https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview (accessed on 11 July 2022).
- What Is a Circular Economy? | Ellen MacArthur Foundation. Available online: https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview (accessed on 11 July 2022).
- Ellen MacArthur Foundation The Butterfly Diagram: Visualising the Circular Economy. Available online: https://ellenmacarthurfoundation.org/circular-economy-diagram (accessed on 11 August 2022).
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Jan Hultink, E. The Circular Economy—A New Sustainability Paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- MacArthur, E. Towards the Circular Economy. J. Ind. Ecol. 2013, 2, 23–44. [Google Scholar]
- Bocken, N.M.P.; Short, S.W.; Rana, P.; Evans, S. A Literature and Practice Review to Develop Sustainable Business Model Archetypes. J. Clean. Prod. 2014, 65, 42–56. [Google Scholar] [CrossRef]
- Weissbrod, I.; Bocken, N.M.P. Developing Sustainable Business Experimentation Capability—A Case Study. J. Clean. Prod. 2017, 142, 2663–2676. [Google Scholar] [CrossRef]
- Koseoglu-Imer, D.Y.; Karagunduz, A. Recent Developments of Electromembrane Desalination Processes. Environ. Technol. Rev. 2018, 7, 199–219. [Google Scholar] [CrossRef]
- Scarazzato, T.; Barros, K.S.; Benvenuti, T.; Rodrigues, M.A.S.; Espinosa, D.C.R.; Bernardes, A.M.B.; Amado, F.D.R.; Pérez-Herranz, V. Chapter 5—Achievements in Electrodialysis Processes for Wastewater and Water Treatment. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Ghasemzadeh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 127–160. ISBN 978-0-12-817378-7. [Google Scholar]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.; Bicer, Y. Integration of Electrodialysis with Renewable Energy Sources for Sustainable Freshwater Production: A Review. J. Environ. Manage. 2021, 289, 112496. [Google Scholar] [CrossRef]
- Arana Juve, J.-M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for Metal Removal and Recovery: A Review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Maigrot, E.; Sabates, J. Apparat Zur Lauterung von Zuckersaften Mittels Elektrizitat. German Patent 50443, 1890. [Google Scholar]
- Cooney, C.L.; Humphrey, A.E. The Principles of Biotechnology: Engineering Considerations; Pergamon Press: Oxford, UK, 1985. [Google Scholar]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for Water Desalination: A Critical Assessment of Recent Developments on Process Fundamentals, Models and Applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Wilson, J.R. Demineralization by Electrodialysis; Butterworths Scientific Publications: London, UK, 1960. [Google Scholar]
- Greiter, M.; Novalin, S.; Wendland, M.; Kulbe, K.-D.; Fischer, J. Desalination of Whey by Electrodialysis and Ion Exchange Resins: Analysis of Both Processes with Regard to Sustainability by Calculating Their Cumulative Energy Demand. J. Membr. Sci. 2002, 210, 91–102. [Google Scholar] [CrossRef]
- Gonçalves, F.; Fernandes, C.; Cameira dos Santos, P.; de Pinho, M.N. Wine Tartaric Stabilization by Electrodialysis and Its Assessment by the Saturation Temperature. J. Food Eng. 2003, 59, 229–235. [Google Scholar] [CrossRef]
- Vera, E.; Sandeaux, J.; Persin, F.; Pourcelly, G.; Dornier, M.; Ruales, J. Deacidification of Clarified Tropical Fruit Juices by Electrodialysis. Part I. Influence of Operating Conditions on the Process Performances. J. Food Eng. 2007, 78, 1427–1438. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis Desalination for Water and Wastewater: A Review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.; Bazinet, L.; Xu, T. Chapter 10—Electrodialysis-Based Separation Technologies in the Food Industry. In Separation of Functional Molecules in Food by Membrane Technology; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 349–381. ISBN 978-0-12-815056-6. [Google Scholar]
- Zhang, Y.; Paepen, S.; Pinoy, L.; Meesschaert, B.; Van der Bruggen, B. Selectrodialysis: Fractionation of Divalent Ions from Monovalent Ions in a Novel Electrodialysis Stack. Sep. Purif. Technol. 2012, 88, 191–201. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T. Electrodialysis with Bipolar Membranes for Sustainable Development. Environ. Sci. Technol. 2006, 40, 5233–5243. [Google Scholar] [CrossRef] [PubMed]
- Frilette, V.J. Preparation and Characterization of Bipolar Ion Exchange Membranes. J. Phys. Chem. 1956, 60, 435–439. [Google Scholar] [CrossRef]
- Bazinet, L.; Castaigne, F. Concepts de Génie Alimentaire - Procédés Associés, Application à La Conservation et Transformation Des Aliments; Presses internationales Polytechnique: Montreal, QC, Canada, 2019; ISBN 978-2-553-01721-6. [Google Scholar]
- Tongwen, X. Electrodialysis Processes with Bipolar Membranes (EDBM) in Environmental Protection—A Review. Resour. Conserv. Recycl. 2002, 37, 1–22. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar Membranes: A Review on Principles, Latest Developments, and Applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Nagasubramanian, K.; Chlanda, F.P.; Liu, K.-J. Use of Bipolar Membranes for Generation of Acid and Base—An Engineering and Economic Analysis. J. Membr. Sci. 1977, 2, 109–124. [Google Scholar] [CrossRef]
- Mani, K.N.; Chlanda, F.P.; Byszewski, C.H. Aquatech Membrane Technology for Recovery of Acid/Base Values for Salt Streams. Desalination 1988, 68, 149–166. [Google Scholar] [CrossRef]
- Bazinet, L.; Lamarche, F.; Ippersiel, D. Bipolar-Membrane Electrodialysis: Applications of Electrodialysis in the Food Industry. Trends Food Sci. Technol. 1998, 9, 107–113. [Google Scholar] [CrossRef]
- Tronc, J.-S.; Lamarche, F.; Makhlouf, J. Enzymatic Browning Inhibition in Cloudy Apple Juice by Electrodialysis. J. Food Sci. 1997, 62, 75–78. [Google Scholar] [CrossRef]
- Bazinet, L.; Amiot, J. Poulin, Jean-François; Tremblay, A.; Labbé, D. Process and System for Separation of Organic Charged Compounds. U.S. Patent Application No. 10/591, 238, 24 January 2008. [Google Scholar]
- Henaux, L.; Thibodeau, J.; Pilon, G.; Gill, T.; Marette, A.; Bazinet, L. How Charge and Triple Size-Selective Membrane Separation of Peptides from Salmon Protein Hydrolysate Orientate Their Biological Response on Glucose Uptake. Int. J. Mol. Sci. 2019, 20, 1939. [Google Scholar] [CrossRef] [PubMed]
- Doyen, A.; Saucier, L.; Beaulieu, L.; Pouliot, Y.; Bazinet, L. Electroseparation of an Antibacterial Peptide Fraction from Snow Crab By-Products Hydrolysate by Electrodialysis with Ultrafiltration Membranes. Food Chem. 2012, 132, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Poulin, J.-F.; Amiot, J.; Bazinet, L. Simultaneous Separation of Acid and Basic Bioactive Peptides by Electrodialysis with Ultrafiltration Membrane. J. Biotechnol. 2006, 123, 314–328. [Google Scholar] [CrossRef]
- Firdaous, L.; Dhulster, P.; Amiot, J.; Gaudreau, A.; Lecouturier, D.; Kapel, R.; Lutin, F.; Vézina, L.-P.; Bazinet, L. Concentration and Selective Separation of Bioactive Peptides from an Alfalfa White Protein Hydrolysate by Electrodialysis with Ultrafiltration Membranes. J. Membr. Sci. 2009, 329, 60–67. [Google Scholar] [CrossRef]
- Roblet, C.; Akhtar, M.J.; Mikhaylin, S.; Pilon, G.; Gill, T.; Marette, A.; Bazinet, L. Enhancement of Glucose Uptake in Muscular Cell by Peptide Fractions Separated by Electrodialysis with Filtration Membrane from Salmon Frame Protein Hydrolysate. J. Funct. Foods 2016, 22, 337–346. [Google Scholar] [CrossRef]
- Bazinet, L.; Moalic, M. Coupling of Porous Filtration and Ion-Exchange Membranes in an Electrodialysis Stack and Impact on Cation Selectivity: A Novel Approach for Sea Water Demineralization and the Production of Physiological Water. Desalination 2011, 277, 356–363. [Google Scholar] [CrossRef]
- Lu, H.; Zou, W.; Chai, P.; Wang, J.; Bazinet, L. Feasibility of Antibiotic and Sulfate Ions Separation from Wastewater Using Electrodialysis with Ultrafiltration Membrane. J. Clean. Prod. 2016, 112, 3097–3105. [Google Scholar] [CrossRef]
- Doyen, A.; Husson, E.; Bazinet, L. Use of an Electrodialytic Reactor for the Simultaneous β-Lactoglobulin Enzymatic Hydrolysis and Fractionation of Generated Bioactive Peptides. Food Chem. 2013, 136, 1193–1202. [Google Scholar] [CrossRef]
- Suwal, S.; Rozoy, É.; Manenda, M.; Doyen, A.; Bazinet, L. Comparative Study of in Situ and Ex Situ Enzymatic Hydrolysis of Milk Protein and Separation of Bioactive Peptides in an Electromembrane Reactor. ACS Sustain. Chem. Eng. 2017, 5, 5330–5340. [Google Scholar] [CrossRef]
- Ndiaye, N.; Pouliot, Y.; Saucier, L.; Beaulieu, L.; Bazinet, L. Electroseparation of Bovine Lactoferrin from Model and Whey Solutions. Sep. Purif. Technol. 2010, 74, 93–99. [Google Scholar] [CrossRef]
- Aider, M.; Brunet, S.; Bazinet, L. Electroseparation of Chitosan Oligomers by Electrodialysis with Ultrafiltration Membrane (EDUF) and Impact on Electrodialytic Parameters. J. Membr. Sci. 2008, 309, 222–232. [Google Scholar] [CrossRef]
- Bazinet, L.; Cossec, C.; Gaudreau, H.; Desjardins, Y. Production of a Phenolic Antioxidant Enriched Cranberry Juice by Electrodialysis with Filtration Membrane. J. Agric. Food Chem. 2009, 57, 10245–10251. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Li, Q.; Wang, Y.; Yu, D.; Wu, L.; Pan, J.; Miao, J.; Xu, T. Electrodialysis with Nanofiltration Membrane (EDNF) for High-Efficiency Cations Fractionation. J. Membr. Sci. 2016, 498, 192–200. [Google Scholar] [CrossRef]
- Sheng, F.; Hou, L.; Wang, X.; Irfan, M.; Shehzad, M.A.; Wu, B.; Ren, X.; Ge, L.; Xu, T. Electro-Nanofiltration Membranes with Positively Charged Polyamide Layer for Cations Separation. J. Membr. Sci. 2020, 594, 117453. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Zhang, H.; Sheng, F.; Xu, T.; Zhu, Y.; Zhang, H.; Ge, L.; Xu, T. Polyamide-Based Electronanofiltration Membranes for Efficient Anion Separation. Ind. Eng. Chem. Res. 2022, 61, 9869–9878. [Google Scholar] [CrossRef]
- Dlask, O.; Václavíková, N. Electrodialysis with Ultrafiltration Membranes for Peptide Separation. Chem. Pap. 2018, 72, 261–271. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Q.; Lu, H.; Wang, J.; Zhao, J.; Li, P. Electrodialysis with Porous Membrane for Bioproduct Separation: Technology, Features, and Progress. Food Res. Int. 2020, 137, 109343. [Google Scholar] [CrossRef]
- Macedonio, F.; Curcio, E.; Drioli, E. Integrated Membrane Systems for Seawater Desalination: Energetic and Exergetic Analysis, Economic Evaluation, Experimental Study. Desalination 2007, 203, 260–276. [Google Scholar] [CrossRef]
- Tufa, R.A.; Noviello, Y.; Di Profio, G.; Macedonio, F.; Ali, A.; Drioli, E.; Fontananova, E.; Bouzek, K.; Curcio, E. Integrated Membrane Distillation-Reverse Electrodialysis System for Energy-Efficient Seawater Desalination. Appl. Energy 2019, 253, 113551. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Goulão Crespo, J.; Nijmeijer, K.; Curcio, E. Progress and Prospects in Reverse Electrodialysis for Salinity Gradient Energy Conversion and Storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Zhang, Z.; Xu, T. Electrodialysis of Concentrated Brine from RO Plant to Produce Coarse Salt and Freshwater. J. Membr. Sci. 2014, 450, 323–330. [Google Scholar] [CrossRef]
- Camacho, L.M.; Fox, J.A.; Ajedegba, J.O. Optimization of Electrodialysis Metathesis (EDM) Desalination Using Factorial Design Methodology. Desalination 2017, 403, 136–143. [Google Scholar] [CrossRef]
- Alhéritière, C.; Ernst, W.R.; Davis, T.A. Metathesis of Magnesium and Sodium Salt Systems by Electrodialysis. Desalination 1998, 115, 189–198. [Google Scholar] [CrossRef]
- Mohammadi, R.; Ramasamy, D.L.; Sillanpää, M. Enhancement of Nitrate Removal and Recovery from Municipal Wastewater through Single- and Multi-Batch Electrodialysis: Process Optimisation and Energy Consumption. Desalination 2021, 498, 114726. [Google Scholar] [CrossRef]
- Beery, M.; Wozny, G.; Repke, J.-U. Sustainable Design of Different Seawater Reverse Osmosis Desalination Pretreatment Processes. In Computer Aided Chemical Engineering; Pierucci, S., Ferraris, G.B., Eds.; 20 European Symposium on Computer Aided Process Engineering; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1069–1074. [Google Scholar]
- Beery, M.; Hortop, A.; Wozny, G.; Knops, F.; Repke, J.-U. Carbon Footprint of Seawater Reverse Osmosis Desalination Pre-Treatment: Initial Results from a New Computational Tool. Desalination Water Treat. 2011, 31, 164–171. [Google Scholar] [CrossRef]
- Tarnacki, K.M.; Melin, T.; Jansen, A.E.; van Medevoort, J. Comparison of Environmental Impact and Energy Efficiency of Desalination Processes by LCA. Water Supply 2011, 11, 246–251. [Google Scholar] [CrossRef]
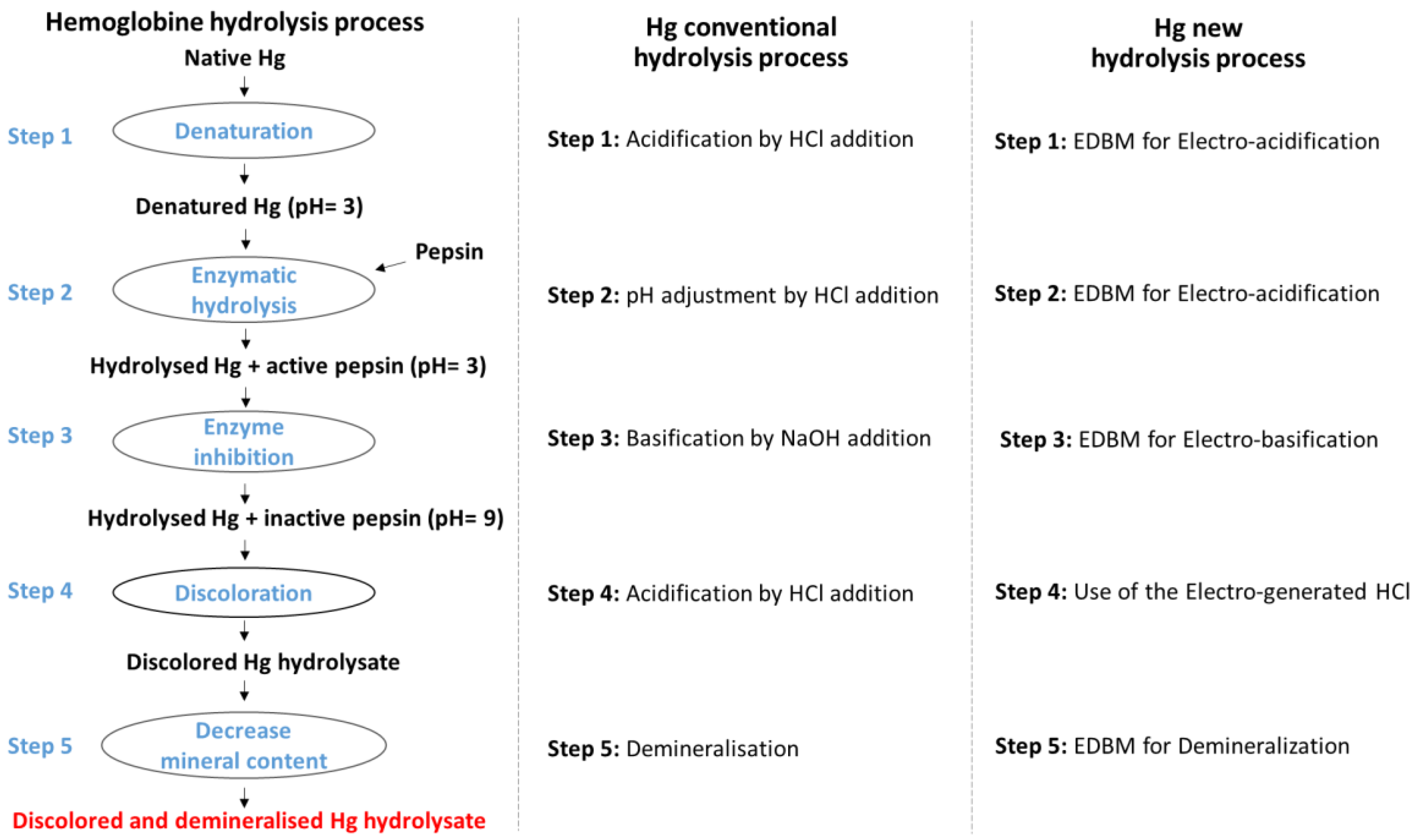
- Abou-Diab, M.; Thibodeau, J.; Deracinois, B.; Flahaut, C.; Fliss, I.; Dhulster, P.; Nedjar, N.; Bazinet, L. Bovine Hemoglobin Enzymatic Hydrolysis by a New Ecoefficient Process—Part I: Feasibility of Electrodialysis with Bipolar Membrane and Production of Neokyotorphin (A137-141). Membranes 2020, 10, 257. [Google Scholar] [CrossRef]
- Abou-Diab, M.; Thibodeau, J.; Fliss, I.; Dhulster, P.; Nedjar, N.; Bazinet, L. Eco-Circular Production of Demineralized Bioactive Peptides from Bovine Hemoglobin by Performing the Necessary Steps Simultaneously Using Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2021, 9, 16905–16917. [Google Scholar] [CrossRef]
- Abou-Diab, M.; Thibodeau, J.; Deracinois, B.; Flahaut, C.; Fliss, I.; Dhulster, P.; Bazinet, L.; Nedjar, N. Bovine Hemoglobin Enzymatic Hydrolysis by a New Eco-Efficient Process-Part II: Production of Bioactive Peptides. Membranes 2020, 10, 268. [Google Scholar] [CrossRef]
- Abou-Diab, M.; Thibodeau, J.; Fliss, I.; Dhulster, P.; Nedjar, N.; Bazinet, L. Impact of Conductivity on the Performances of Electro-Acidification and Enzymatic Hydrolysis Phases of Bovine Hemoglobin by Electrodialysis with Bipolar Membranes for the Production of Bioactive Peptides. Sep. Purif. Technol. 2021, 269, 118650. [Google Scholar] [CrossRef]
- Pereira, A.D.; Gomide, L.A.M.; Cecon, P.R.; Fontes, E.A.F.; Fontes, P.R.; Ramos, E.M.; Vidigal, J.G. Evaluation of Mortadella Formulated with Carbon Monoxide-Treated Porcine Blood. Meat Sci. 2014, 97, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Y.; Zhang, X.; Xu, T. Comparative Study on Regenerating Sodium Hydroxide from the Spent Caustic by Bipolar Membrane Electrodialysis (BMED) and Electro-Electrodialysis (EED). Sep. Purif. Technol. 2013, 118, 1–5. [Google Scholar] [CrossRef]
- Bazinet, L.; Lamarche, F.; Labrecque, R.; Toupin, R.; Boulet, M.; Ippersiel, D. Electroacidification of Soybean Proteins for Production of Isolate. Food Technol. Chic. 1997, 51, 52–60. [Google Scholar]
- Alvarez, F.; Alvarez, R.; Coca, J.; Sandeaux, J.; Sandeaux, R.; Gavach, C. Salicylic Acid Production by Electrodialysis with Bipolar Membranes. J. Membr. Sci. 1997, 123, 61–69. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Jiang, C.; Lin, X.; Xu, T. Bipolar Membrane Electrodialysis in Aqua–Ethanol Medium: Production of Salicylic Acid. J. Membr. Sci. 2015, 482, 76–82. [Google Scholar] [CrossRef]
- Eisaman, M.; Alvarado, L.; Larner, D.; Wang, P.; Garg, B.; Littau, K. CO2 Separation Using Bipolar Membrane Electrodialysis. Energy Environ. Sci. 2011, 4. [Google Scholar] [CrossRef]
- EPA Emission Facts: Average Carbon Dioxide Emissions Resulting from Gasoline and Diesel Fuel. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P1001YTF.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2000+Thru+2005&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C00thru05%5CTxt%5C00000017%5CP1001YTF.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 2 December 2022).
- Eisaman, M.; Alvarado, L.; Larner, D.; Wang, P.; Littau, K. CO2 Desorption Using High-Pressure Bipolar Membrane Electrodialysis. Energy Environ. Sci. 2011, 4. [Google Scholar] [CrossRef]
- Schaffner, F.; Pontalier, P.-Y.; Sanchez, V.; Lutin, F. Bipolar Electrodialysis for Glycerin Production from Diester Wastes. Filtr. Sep. 2003, 40, 35–39. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Irabien, A.; Ibañez, R. Highly Concentrated HCl and NaOH from Brines Using Electrodialysis with Bipolar Membranes. Sep. Purif. Technol. 2020, 242, 116785. [Google Scholar] [CrossRef]
- Gazigil, L.; Er, E.; Kestioğlu, O.E.; Yonar, T. Pilot-Scale Test Results of Electrodialysis Bipolar Membrane for Reverse-Osmosis Concentrate Recovery. Membranes 2022, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.I.; Yuzer, B.; Hasancebi, B.; Selcuk, H. Application of Electrodialysis Membrane Process to Recovery Sulfuric Acid and Wastewater in the Chalcopyrite Mining Industry. Desalination Water Treat. 2019, 172, 206–211. [Google Scholar] [CrossRef]
- Yuzer, B.; Aydin, M.I.; Yildiz, H.; Hasançebi, B.; Selcuk, H.; Kadmi, Y. Optimal Performance of Electrodialysis Process for the Recovery of Acid Wastes in Wastewater: Practicing Circular Economy in Aluminum Finishing Industry. Chem. Eng. J. 2022, 434, 134755. [Google Scholar] [CrossRef]
- Paleologou, M.; Thibault, A.; Wong, P.-Y.; Thompson, R.; Berry, R.M. Enhancement of the Current Efficiency for Sodium Hydroxide Production from Sodium Sulphate in a Two-Compartment Bipolar Membrane Electrodialysis System. Sep. Purif. Technol. 1997, 11, 159–171. [Google Scholar] [CrossRef]
- Ghyselbrecht, K.; Huygebaert, M.; Van der Bruggen, B.; Ballet, R.; Meesschaert, B.; Pinoy, L. Desalination of an Industrial Saline Water with Conventional and Bipolar Membrane Electrodialysis. Desalination 2013, 318, 9–18. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Valderrama, C.; Gibert, O.; Cortina, J.L. Integration of Monopolar and Bipolar Electrodialysis for Valorization of Seawater Reverse Osmosis Desalination Brines: Production of Strong Acid and Base. Desalination 2016, 398, 87–97. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Photovoltaic Solar Electrodialysis with Bipolar Membranes. Desalination 2018, 433, 155–163. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Peng, Z.; Liu, Y. Treatment of High Salinity Sulfanilic Acid Wastewater by Bipolar Membrane Electrodialysis. Sep. Purif. Technol. 2022, 281, 119842. [Google Scholar] [CrossRef]
- Reig, M.; Valderrama, C.; Gibert, O.; Cortina, J.L. Selectrodialysis and Bipolar Membrane Electrodialysis Combination for Industrial Process Brines Treatment: Monovalent-Divalent Ions Separation and Acid and Base Production. Desalination 2016, 399, 88–95. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Yan, H.; Jiang, C.; Xu, T. A Sustainable Valorization of Neopentyl Glycol Salt Waste Containing Sodium Formate via Bipolar Membrane Electrodialysis. Sep. Purif. Technol. 2021, 254, 117563. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, H.; Zhang, Y.; Feng, H.; Shehzad, M.A.; Wang, Y.; Xu, T. Complexation Electrodialysis as a General Method to Simultaneously Treat Wastewaters with Metal and Organic Matter. Chem. Eng. J. 2018, 348, 952–959. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, H.; Liu, Y.; Chen, R.; Qian, Q.; Van der Bruggen, B. Cr(III) Recovery in Form of Na2CrO4 from Aqueous Solution Using Improved Bipolar Membrane Electrodialysis. J. Membr. Sci. 2020, 604, 118097. [Google Scholar] [CrossRef]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A Critical Assessment of Chromium in the Environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Y.; Xie, S.; Wu, G.; Hu, Z.; Zhan, X. Recovery of Nutrients and Volatile Fatty Acids from Pig Manure Hydrolysate Using Two-Stage Bipolar Membrane Electrodialysis. Chem. Eng. J. 2018, 334, 134–142. [Google Scholar] [CrossRef]
- Li, C.; Ramasamy, D.L.; Sillanpää, M.; Repo, E. Separation and Concentration of Rare Earth Elements from Wastewater Using Electrodialysis Technology. Sep. Purif. Technol. 2021, 254, 117442. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Puhakka, V.; Doshi, B.; Iftekhar, S.; Sillanpää, M. Fabrication of Carbon Nanotubes Reinforced Silica Composites with Improved Rare Earth Elements Adsorption Performance. Chem. Eng. J. 2019, 365, 291–304. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Q.; Huang, X.; Yan, Z.; Lin, X.; Ye, W.; Arcadio, S.; Luis, P.; Bi, J.; Van der Bruggen, B.; et al. Integrated Loose Nanofiltration-Electrodialysis Process for Sustainable Resource Extraction from High-Salinity Textile Wastewater. J. Hazard. Mater. 2021, 419, 126505. [Google Scholar] [CrossRef]
- Benvenuti, T.; Siqueira Rodrigues, M.A.; Bernardes, A.M.; Zoppas-Ferreira, J. Closing the Loop in the Electroplating Industry by Electrodialysis. J. Clean. Prod. 2017, 155, 130–138. [Google Scholar] [CrossRef]
- Lopes, A.C.A.; Eda, S.H.; Andrade, R.P.; Amorim, J.C.; Duarte, W.F. 14—New Alcoholic Fermented Beverages—Potentials and Challenges. In Fermented Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 577–603. ISBN 978-0-12-815271-3. [Google Scholar]
- Saffari, M.; Langrish, T. Effect of Lactic Acid In-Process Crystallization of Lactose/Protein Powders during Spray Drying. J. Food Eng. 2014, 137, 88–94. [Google Scholar] [CrossRef]
- Chandrapala, J.; Vasiljevic, T. Properties of Spray Dried Lactose Powders Influenced by Presence of Lactic Acid and Calcium. J. Food Eng. 2017, 198, 63–71. [Google Scholar] [CrossRef]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. Positive Impact of Pulsed Electric Field on Lactic Acid Removal, Demineralization and Membrane Scaling during Acid Whey Electrodialysis. Int. J. Mol. Sci. 2019, 20, 797. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Garthe, M.; Roghmans, F.; Chen, G.Q.; Kentish, S.E. Lactic Acid and Salt Separation Using Membrane Technology. Membranes 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Suarez, F.; Chen, G.Q.; Chen, X.; Bathurst, K.; Kentish, S.E. Pilot Study on the Removal of Lactic Acid and Minerals from Acid Whey Using Membrane Technology. ACS Sustain. Chem. Eng. 2020, 8, 2742–2752. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of Monopolar and Bipolar Electrodialysis Processes for Tartaric Acid Recovery from Residues of the Winery Industry. ACS Sustain. Chem. Eng. 2020, 8, 13387–13399. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.Q.; Lin, L.; Li, X.; Kentish, S.E. Purification of Organic Acids Using Electrodialysis with Bipolar Membranes (EDBM) Combined with Monovalent Anion Selective Membranes. Sep. Purif. Technol. 2021, 279, 119739. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.Q.; Kentish, S.E. Isolation of Lactoferrin and Immunoglobulins from Dairy Whey by an Electrodialysis with Filtration Membrane Process. Sep. Purif. Technol. 2020, 233, 115987. [Google Scholar] [CrossRef]
- Kadel, S.; Thibodeau, J.; Parjikolaei, B.R.; Bazinet, L. How PH Conditions Impact the Production of Growth Factors Enriched Fractions by Electro-Based Membrane Process. 2023. in submission. [Google Scholar]
- Faucher, M.; Geoffroy, T.R.; Thibodeau, J.; Gaaloul, S.; Bazinet, L. Semi-Industrial Production of a DPP-IV and ACE Inhibitory Peptide Fraction from Whey Protein Concentrate Hydrolysate by Electrodialysis with Ultrafiltration Membrane. Membranes 2022, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Agriculture and Agri-Food Canada. Red Meat and Livestock Slaughter Reports 2019. Available online: https://agriculture.canada.ca/en/sector/animal-industry/red-meat-and-livestock-market-information/slaughter-and-carcass-weights (accessed on 10 January 2023).
- Przybylski, R.; Firdaous, L.; Châtaigné, G.; Dhulster, P.; Nedjar, N. Production of an Antimicrobial Peptide Derived from Slaughterhouse By-Product and Its Potential Application on Meat as Preservative. Food Chem. 2016, 211, 306–313. [Google Scholar] [CrossRef]
- Bauer, A.K.; Dwyer-Nield, L.D.; Hankin, J.A.; Murphy, R.C.; Malkinson, A.M. The Lung Tumor Promoter, Butylated Hydroxytoluene (BHT), Causes Chronic Inflammation in Promotion-Sensitive BALB/CByJ Mice but Not in Promotion-Resistant CXB4 Mice (Vol 169, Pg 1, 2001). Toxicology 2002, 176, 159–161. [Google Scholar] [CrossRef]
- Lanigan, R.S.; Yamarik, T.A. Final Report on the Safety Assessment of BHT (1). Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar] [PubMed]
- Przybylski, R.; Bazinet, L.; Firdaous, L.; Kouach, M.; Goossens, J.-F.; Dhulster, P.; Nedjar, N. Harnessing Slaughterhouse By-Products: From Wastes to High-Added Value Natural Food Preservative. Food Chem. 2020, 304, 125448. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, R.; Bazinet, L.; Firdaous, L.; Kouach, M.; Goossens, J.-F.; Dhulster, P.; Nedjar-Arroume, N. Electroseparation of Slaughterhouse By-Product: Antimicrobial Peptide Enrichment by PH Modification. Membranes 2020, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Vanhoute, M.; Firdaous, L.; Bazinet, L.; Froidevaux, R.; Lecouturier, D.; Guillochon, D.; Dhulster, P. Effect of Haem on the Fractionation of Bovine Haemoglobin Peptic Hydrolysate by Electrodialysis with Ultrafiltration Membranes. J. Membr. Sci. 2010, 365, 16–24. [Google Scholar] [CrossRef]
- Sanchez-Reinoso, Z.; Cournoyer, A.; Thibodeau, J.; Said, L.B.; Fliss, I.; Bazinet, L.; Mikhaylin, S. Effect of PH on the Antimicrobial Activity and Peptide Population of Pepsin Hydrolysates Derived from Bovine and Porcine Hemoglobins. ACS Food Sci. Technol. 2021, 1, 1687–1701. [Google Scholar] [CrossRef]
- Cournoyer, A.; Thibodeau, J.; Ben Said, L.; Sanchez-Reinoso, Z.; Mikhaylin, S.; Fliss, I.; Bazinet, L. How Discoloration of Porcine Cruor Hydrolysate Allowed the Identification of New Antifungal Peptides. Foods 2022, 11, 4035. [Google Scholar] [CrossRef]
- Henaux, L.; Pereira, K.D.; Thibodeau, J.; Pilon, G.; Gill, T.; Marette, A.; Bazinet, L. Glucoregulatory and Anti-Inflammatory Activities of Peptide Fractions Separated by Electrodialysis with Ultrafiltration Membranes from Salmon Protein Hydrolysate and Identification of Four Novel Glucoregulatory Peptides. Membranes 2021, 11, 528. [Google Scholar] [CrossRef]
- Renaud, V.; Houde, V.P.; Pilon, G.; Varin, T.V.; Roblet, C.; Marette, A.; Boutin, Y.; Bazinet, L. The Concentration of Organic Acids in Cranberry Juice Modulates the Gut Microbiota in Mice. Int. J. Mol. Sci. 2021, 22, 11537. [Google Scholar] [CrossRef]
- McMurdo, M.E.T.; Bissett, L.Y.; Price, R.J.G.; Phillips, G.; Crombie, I.K. Does Ingestion of Cranberry Juice Reduce Symptomatic Urinary Tract Infections in Older People in Hospital? A Double-Blind, Placebo-Controlled Trial. Age Ageing 2005, 34, 256–261. [Google Scholar] [CrossRef]
- Wing, D.A.; Rumney, P.J.; Preslicka, C.W.; Chung, J.H. Daily Cranberry Juice for the Prevention of Asymptomatic Bacteriuria in Pregnancy: A Randomized, Controlled Pilot Study. J. Urol. 2008, 180, 1367–1372. [Google Scholar] [CrossRef]
- Serre, E.; Boutin, Y.; Langevin, M.-E.; Lutin, F.; Pedneault, K.; Lacour, S.; Bazinet, L. Deacidification of Cranberry Juice Protects against Disruption of In-Vitro Intestinal Cell Barrier Integrity. J. Funct. Foods 2016, 26, 208–216. [Google Scholar] [CrossRef]
- Renaud, V.; Faucher, M.; Perreault, V.; Serre, E.; Dubé, P.; Boutin, Y.; Bazinet, L. Evolution of Cranberry Juice Compounds during in Vitro Digestion and Identification of the Organic Acid Responsible for the Disruption of in Vitro Intestinal Cell Barrier Integrity. J. Food Sci. Technol. 2020, 57, 2329–2342. [Google Scholar] [CrossRef] [PubMed]
- Serre, E.; Rozoy, E.; Pedneault, K.; Lacour, S.; Bazinet, L. Deacidification of Cranberry Juice by Electrodialysis: Impact of Membrane Types and Configurations on Acid Migration and Juice Physicochemical Characteristics (Vol 163, Pg 228, 2016). Sep. Purif. Technol. 2016, 165, 222–223. [Google Scholar] [CrossRef]
- Shui, G.; Leong, L.P. Separation and Determination of Organic Acids and Phenolic Compounds in Fruit Juices and Drinks by High-Performance Liquid Chromatography. J. Chromatogr. A 2002, 977, 89–96. [Google Scholar] [CrossRef]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of Organic Acids in Fruits and Vegetables by Liquid Chromatography with Tandem-Mass Spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Faucher, M.; Serre, É.; Langevin, M.-È.; Mikhaylin, S.; Lutin, F.; Bazinet, L. Drastic Energy Consumption Reduction and Ecoefficiency Improvement of Cranberry Juice Deacidification by Electrodialysis with Bipolar Membranes at Semi-Industrial Scale: Reuse of the Recovery Solution. J. Membr. Sci. 2018, 555, 105–114. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Ölmez, H. Inactivation of Escherichia Coli and Listeria Monocytogenes on Iceberg Lettuce by Dip Wash Treatments with Organic Acids. Lett. Appl. Microbiol. 2007, 44, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Castañer, M.; Gil, M.I.; Artes, F.; Tomas-Barberan, F.A. Inhibition of Browning of Harvested Head Lettuce. J. Food Sci. 1996, 61, 314–316. [Google Scholar] [CrossRef]
- Castañer, M.; Gil, M.I.; Artés, F. Organic Acids as Browning Inhibitors on Harvested “Baby” Lettuce and Endive. Z. Leb. Forsch. A 1997, 205, 375–379. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Identification of a Novel Angiotensin-I-Converting Enzyme Inhibitory Peptide Corresponding to a Tryptic Fragment of Bovine β-Lactoglobulin. FEBS Lett. 1997, 402, 99–101. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mazzocchi, C.; Paolella, S.; FitzGerald, R.J. Release of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides from Milk Protein Isolate (MPI) during Enzymatic Hydrolysis. Food Res. Int. 2017, 94, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Morr, C.V.; Ha, E.Y.W. Off-Flavors of Whey Protein Concentrates: A Literature Review. Int. Dairy J. 1991, 1, 1–11. [Google Scholar] [CrossRef]
- Hwang, D.-C.; Damodaran, S. Selective Precipitation and Removal of Lipids from Cheese Whey Using Chitosan. J. Agric. Food Chem. 1995, 43, 33–37. [Google Scholar] [CrossRef]
- Damodaran, S. Straightforward Process for Removal of Milk Fat Globule Membranes and Production of Fat-Free Whey Protein Concentrate from Cheese Whey. J. Agric. Food Chem. 2011, 59, 10271–10276. [Google Scholar] [CrossRef] [PubMed]
- Adje, E.Y.; Balti, R.; Kouach, M.; Dhulster, P.; Guillochon, D.; Nedjar-Arroume, N. Obtaining Antimicrobial Peptides by Controlled Peptic Hydrolysis of Bovine Hemoglobin. Int. J. Biol. Macromol. 2011, 49, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health Effects of Dietary Phospholipids. Lipids Health Dis. 2012, 11, 1–16. [Google Scholar] [CrossRef]
- Pereira, C.D.; Diaz, O.; Cobos, A. Valorization of By-Products from Ovine Cheese Manufacture: Clarification by Thermocalcic Precipitation/Microfiltration before Ultrafiltration. Int. Dairy J. 2002, 12, 773–783. [Google Scholar] [CrossRef]
- Price, N.; Wan, Z.; Fei, T.; Clark, S.; Wang, T. Development of Industrially Scalable Method for Phospholipids and Branch-Chain Fatty Acids of Dairy by-Product. J. Am. Oil Chem. Soc. 2020, 97, 1043–1053. [Google Scholar] [CrossRef]
- Damodaran, S. Zinc-Induced Precipitation of Milk Fat Globule Membranes: A Simple Method for the Preparation of Fat-Free Whey Protein Isolate. J. Agric. Food Chem. 2010, 58, 11052–11057. [Google Scholar] [CrossRef]
- Gesan, G.; Daufin, G.; Merin, U.; Labbe, J.-P.; Quemerais, A. Microfiltration Performance: Physicochemical Aspects of Whey Pretreatment. J. Dairy Res. 1995, 62, 269–279. [Google Scholar] [CrossRef]
- Adolphson, S.J.; Ward, L.S. Method for Defatting Whey Protein Concentrate and Producing Whey Protein Isolate. U.S. Patent Application No 15/338,439, 31 October 2016. [Google Scholar]
- Chen, G.Q.; Eschbach, F.I.I.; Weeks, M.; Gras, S.L.; Kentish, S.E. Removal of Lactic Acid from Acid Whey Using Electrodialysis. Sep. Purif. Technol. 2016, 158, 230–237. [Google Scholar] [CrossRef]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. How Electrodialysis Configuration Influences Acid Whey Deacidification and Membrane Scaling. J. Dairy Sci. 2018, 101, 7833–7850. [Google Scholar] [CrossRef]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. Systematic Study of the Impact of Pulsed Electric Field Parameters (Pulse/Pause Duration and Frequency) on ED Performances during Acid Whey Treatment. Membranes 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
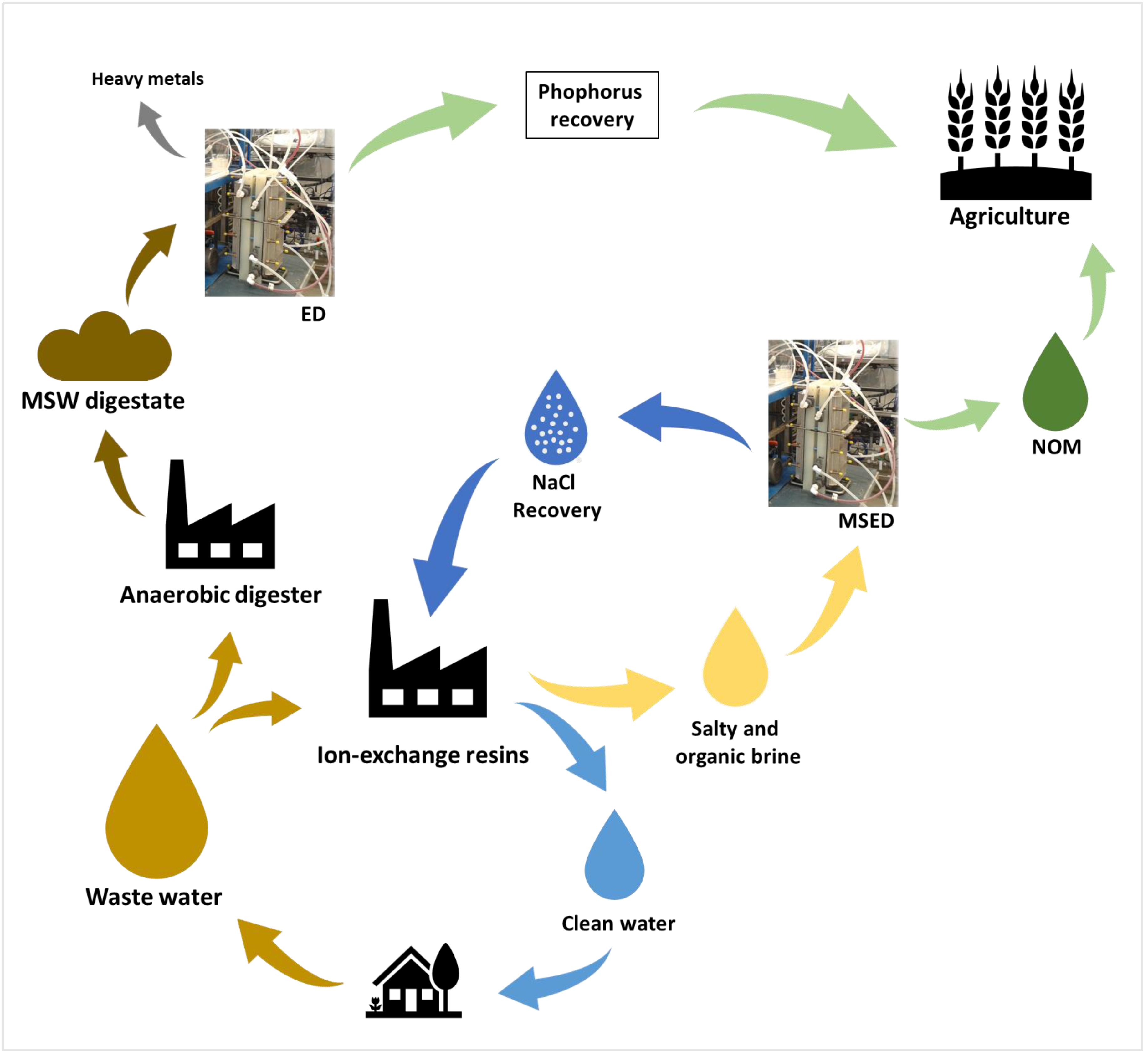
- Haddad, M.; Bazinet, L.; Barbeau, B. Towards Water, Sodium Chloride and Natural Organic Matter Recovery from Ion Exchange Spent Brine. Membranes 2021, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Bazinet, L.; Barbeau, B. Eco-Efficient Treatment of Ion Exchange Spent Brine via Electrodialysis to Recover NaCl and Minimize Waste Disposal. Sci. Total Environ. 2019, 690, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Dias-Ferreira, C.; Labrincha, J.; Rocha, J.L.; Kirkelund, G.M. Testing New Strategies to Improve the Recovery of Phosphorus from Anaerobically Digested Organic Fraction of Municipal Solid Waste. J. Chem. Technol. Biotechnol. 2020, 95, 439–449. [Google Scholar] [CrossRef]
- Oliveira, V.; Kirkelund, G.M.; Horta, C.; Labrincha, J.; Dias-Ferreira, C. Improving the Energy Efficiency of an Electrodialytic Process to Extract Phosphorus from Municipal Solid Waste Digestate through Different Strategies. Appl. Energy 2019, 247, 182–189. [Google Scholar] [CrossRef]
- Ebbers, B.; Ottosen, L.M.; Jensen, P.E. Electrodialytic Treatment of Municipal Wastewater and Sludge for the Removal of Heavy Metals and Recovery of Phosphorus. Electrochimica Acta 2015, 181, 90–99. [Google Scholar] [CrossRef]
- Haddad, M.; Bazinet, L.; Savadogo, O.; Paris, J. Electrochemical Acidification of Kraft Black Liquor: Impacts of Pulsed Electric Field Application on Bipolar Membrane Colloidal Fouling and Process Intensification. J. Membr. Sci. 2017, 524, 482–492. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, H.; Lu, F.; Su, Y.; Li, W.; An, J.; Wang, Y.; Xu, T. Electrodialytic Concentration of Landfill Leachate Effluent: Lab- and Pilot-Scale Test, and Assessment. Sep. Purif. Technol. 2021, 276, 119311. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Navarro, R.; García-Calvo, E. Circular Economy in Membrane Technology: Using End-of-Life Reverse Osmosis Modules for Preparation of Recycled Anion Exchange Membranes and Validation in Electrodialysis. J. Membr. Sci. 2020, 593, 117423. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Riccardelli, G.; García-Calvo, E. Influence of Acid/Base Activation Treatment in the Performance of Recycled Electromembrane for Fresh Water Production by Electrodialysis. Chemosphere 2020, 248, 126027. [Google Scholar] [CrossRef] [PubMed]




| Concept | Field of Application | Applied ED Process | Reference | |
|---|---|---|---|---|
| Eco-efficiency | Water | Seawater desalinization | Reverse ED on RO brine | [94] |
| Conventional ED on RO brine | [96] | |||
| ED metathesis on seawater | [97] | |||
| Water treatment plant | Single- and multi-batch ED | [99] | ||
| Food industry | Meat industry | EDBM | [103,105,106] | |
| Chemical industry | General | EDBM/EED | [108] | |
| Salicylic acid | EDBM | [110,111] | ||
| CO2 | EDBM | [112,114] | ||
| Glycerin | EDBM | [115] | ||
| Circular economy | Water | Seawater desalinization | Conventional ED EDBM | [96] [116] |
| Mining industry | Chalcopyrite | Conventional ED | [118] | |
| General | Multistage-batch EDBM | [3] | ||
| Chemical industry | General | EDBM SED Complexation by ED | [117] [125] [127] | |
| Sulfanilic acid | EDBM | [124] | ||
| Nickel electroplating industry | Conventional ED (Industrial scale) | [134] | ||
| Aluminum finishing industry | Conventional ED | [119] | ||
| Neopentyl Glycol | EDBM | [126] | ||
| Separation of rare earth elements | Conventional ED | [131] | ||
| Textile industry | General | Conventional ED | [133] | |
| Food industry | Dairy industry | EDBM ED with PEF ED EDFM EDUF | [10] [138] [139,140] [85,143,144] [78,83] | |
| Meat industry | EDUF + EDBM | [105,150,151,152] | ||
| Seafood processing | EDUF | [77,155] | ||
| Wine industry | ED + EDBM | [141] | ||
| Fermented broth | EDBM with monovalent selective AEM | [142] | ||
| Combined concepts | Food industry | Cranberry industry | EDBM | [32,33,156,164] |
| Dairy industry | ED EDBM EDUF | [10,39,42,145,180,181,182] | ||
| Water | Water treatment plant | MSED ED (dual-stage extraction) | [184,185,186,187,188,189] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cournoyer, A.; Bazinet, L. Electrodialysis Processes an Answer to Industrial Sustainability: Toward the Concept of Eco-Circular Economy?—A Review. Membranes 2023, 13, 205. https://doi.org/10.3390/membranes13020205
Cournoyer A, Bazinet L. Electrodialysis Processes an Answer to Industrial Sustainability: Toward the Concept of Eco-Circular Economy?—A Review. Membranes. 2023; 13(2):205. https://doi.org/10.3390/membranes13020205
Chicago/Turabian StyleCournoyer, Aurore, and Laurent Bazinet. 2023. "Electrodialysis Processes an Answer to Industrial Sustainability: Toward the Concept of Eco-Circular Economy?—A Review" Membranes 13, no. 2: 205. https://doi.org/10.3390/membranes13020205
APA StyleCournoyer, A., & Bazinet, L. (2023). Electrodialysis Processes an Answer to Industrial Sustainability: Toward the Concept of Eco-Circular Economy?—A Review. Membranes, 13(2), 205. https://doi.org/10.3390/membranes13020205







