1. Introduction
The consumption of meat has greatly increased along with the rapid growth in the human population. Compared to other meats such as beef or pork, the daily intake of poultry (particularly chicken) is increasing much more rapidly [
1]. In 1996, the consumption rate of poultry meat was 9 million tonnes per year (Mt/year) which increased to 133 Mt/year in 2020 [
2]. The poultry industry uses an average of 15–20 L/bird of fresh water, and 80 to 90% of this process water is discharged as poultry slaughterhouse wastewater (PSWW) [
3]. Freshwater resources and municipal wastewater treatment facilities are under significant strain due to the rising demand for poultry meat in the United States and worldwide. In addition, the conventional PSWW methods and even advanced oxidation processes that are physiochemical and biological processes do not purify the water to the quality level of potable water. Therefore, reusing wastewater is an excellent approach to the sustainability of water resources [
4].
Pressure-driven membrane technology is one of the solutions for the treatment and reuse of PSWW [
5]. The four types of pressure-driven membranes are microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO). RO membrane has been widely used for water reclamation because of its ability to separate tiny particles and monovalent ions. For example, a RO membrane can remove sodium and chloride ions with up to 99.5% efficiency. Moreover, the MF, UF, and NF usually serve as pretreatment steps for RO. The pressure-drive membrane has made water recovery from wastewater a suitable option. However, the energy requirement remains a significant challenge [
6].
In addition to the pressure-driven membrane, the forward osmosis (FO) membrane is also used for wastewater treatment. FO is an advanced technology that provides several advantages over pressure-driven membrane processes, such as lower energy input, decreased fouling tendency, easier fouling removal, and high-water recovery [
7]. However, the main disadvantage of FO is that the product water of FO is a diluted draw solution, so a post-treatment, e.g., RO, is required to produce clean water. Another challenge that FO faces is that it is difficult to find a suitable draw solute that can generate high osmotic pressure and is simple to recover or regenerate with a very low cost [
8]. The FO membrane fouling is lesser than pressure-driven membranes, but it still affects membrane performance. One commonly used antifouling method for FO membranes is surface modification [
7]. The surface modification also improves the water flux by lowering the membrane fouling. The surface modification process can be carried out in various ways, such as physical adsorption, surface coating, and chemical vapor deposition [
9]. In recent times zwitterion materials have been studied for their antifouling properties because of their high electroneutrality and hydration capacity. Zwitterion materials have a strong ability to resist bacterial adhesion and biofilm development [
10];
L-DOPA is one such zwitterionic polymer that has been used to enhance membrane surface antifouling capabilities.
Compared to conventional wastewater treatment methods, the FO process has several advantages and exhibits promising outcomes for wastewater treatment. Its high performance in water recovery without highly-driven pressure enables the viability of FO processes. Additionally, FO offers a more sustainable flux and pollutant removal. Since the 18th century, over 1000 FO publications have been reported for FO membranes and their applications. The research on FO has been mainly on municipal wastewater, oily wastewater, tanner wastewater, automobile effluents, dairy streams, and produced wastewater. However, to our knowledge, no research has been conducted on PSWW using FO [
11,
12]. This study explores the performance of FO for the first time for the treatment and reusing PSWW treatment. It will also provide ideas to other scientists for future research on FO membrane challenges for meat slaughterhouse wastewater treatment and reuse.
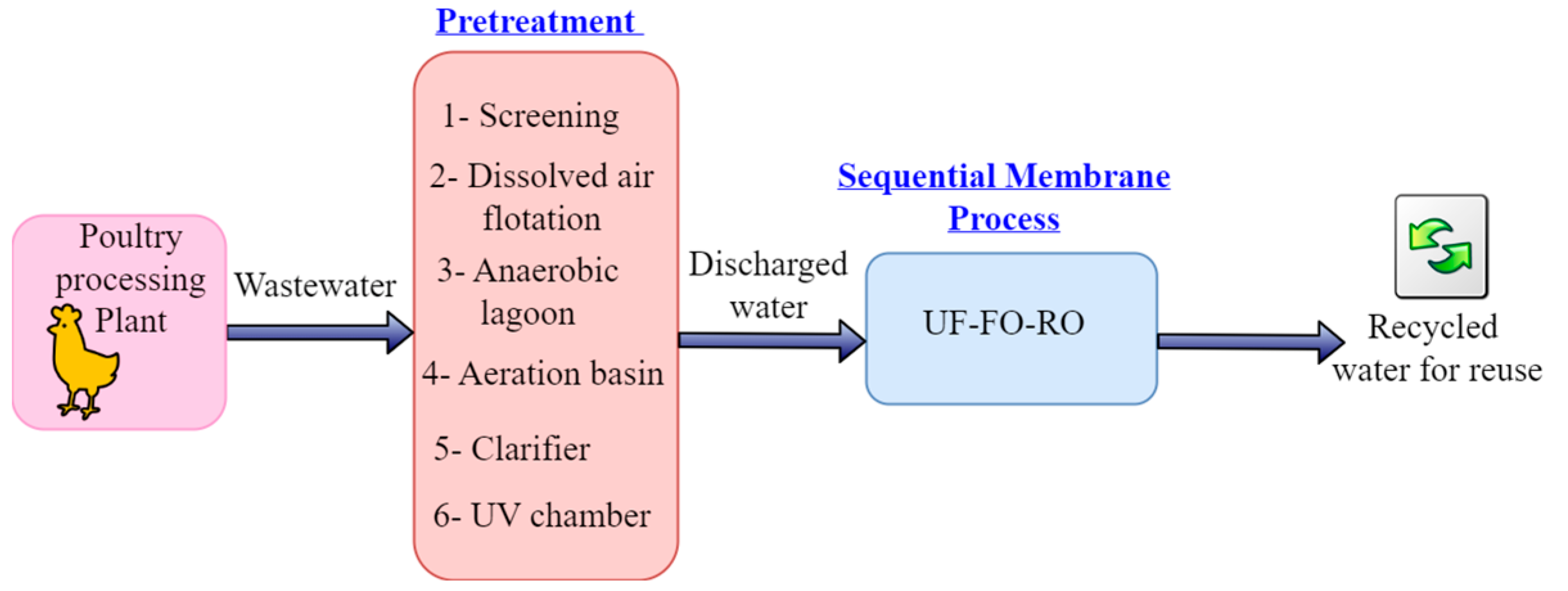
The sequential membrane process (UF-FO-RO) included in the entire process of PSWW treatment, as shown in
Figure 1, will address these challenges of food security and sustainability of the poultry processing industry by removing all the contaminants from wastewater and recycling water in the poultry industry. The proposed sequential membrane processes (UF-FO-RO) will improve food safety and sustainability during poultry processing. It will also resolve ecological and environmental problems, such as depleting freshwater resources, spreading foodborne contaminants via inefficiently treated wastewater, rising operating costs of poultry processing plants, and growing nutrient pollution in watersheds.
4. Conclusions
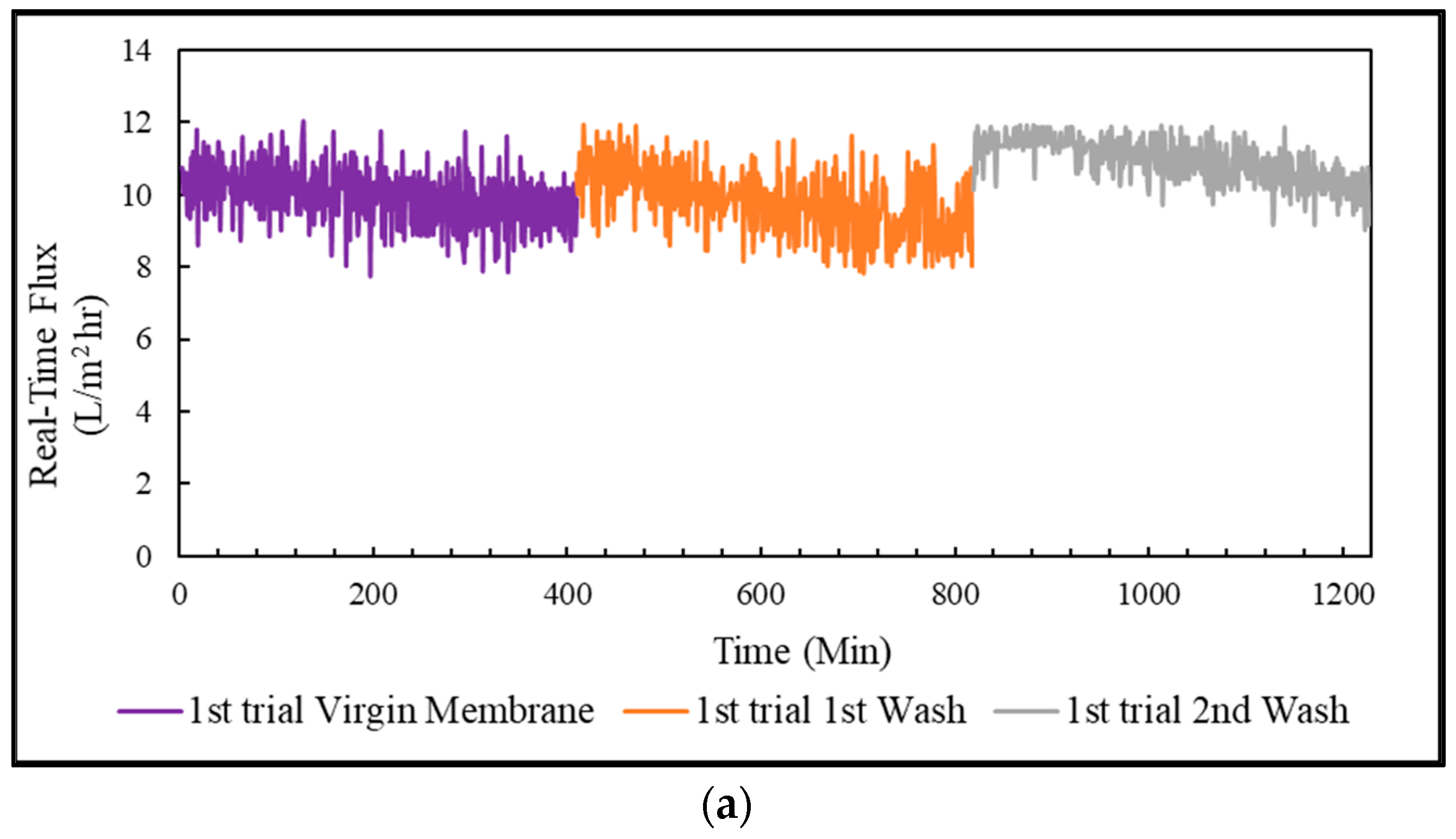
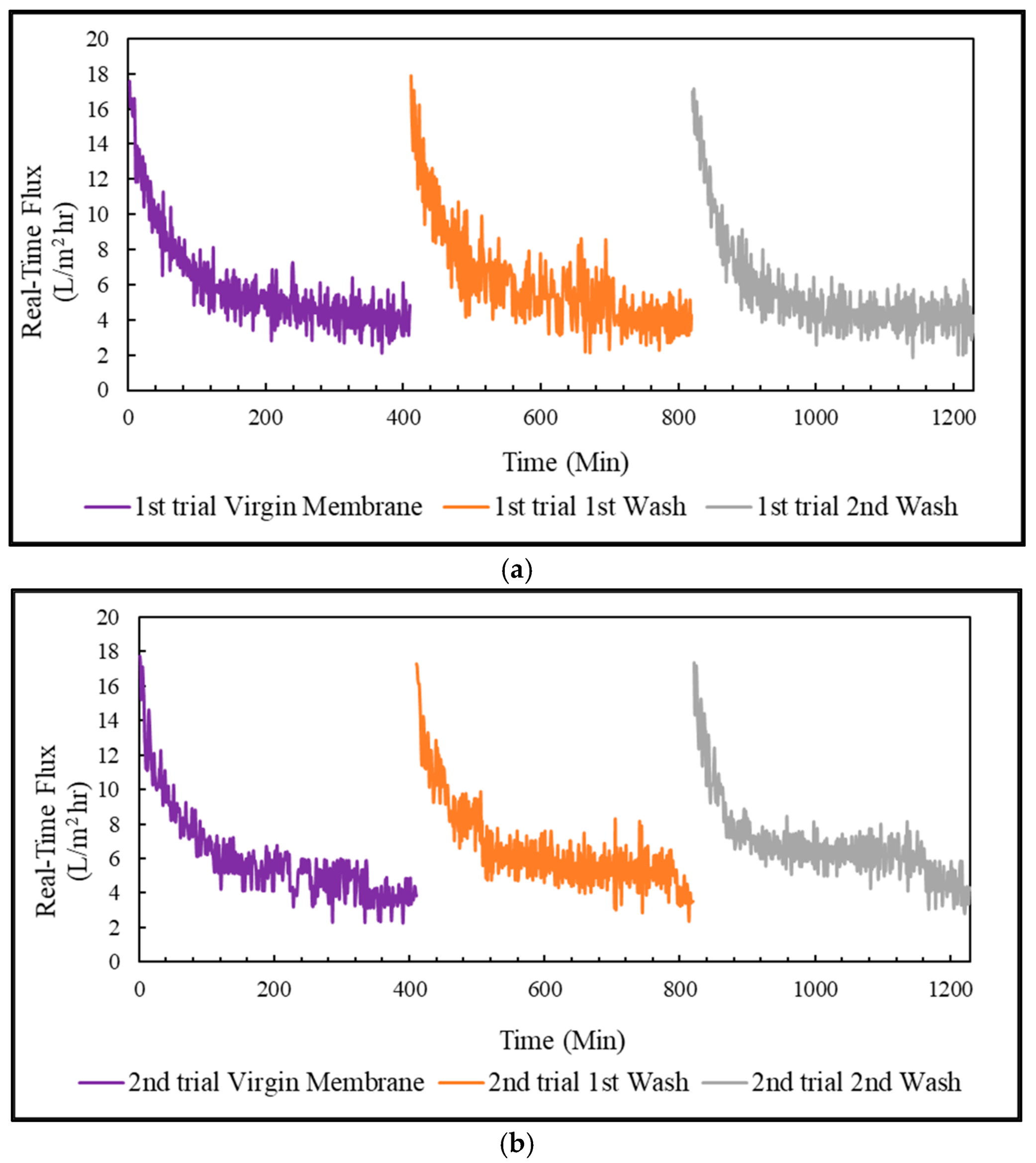
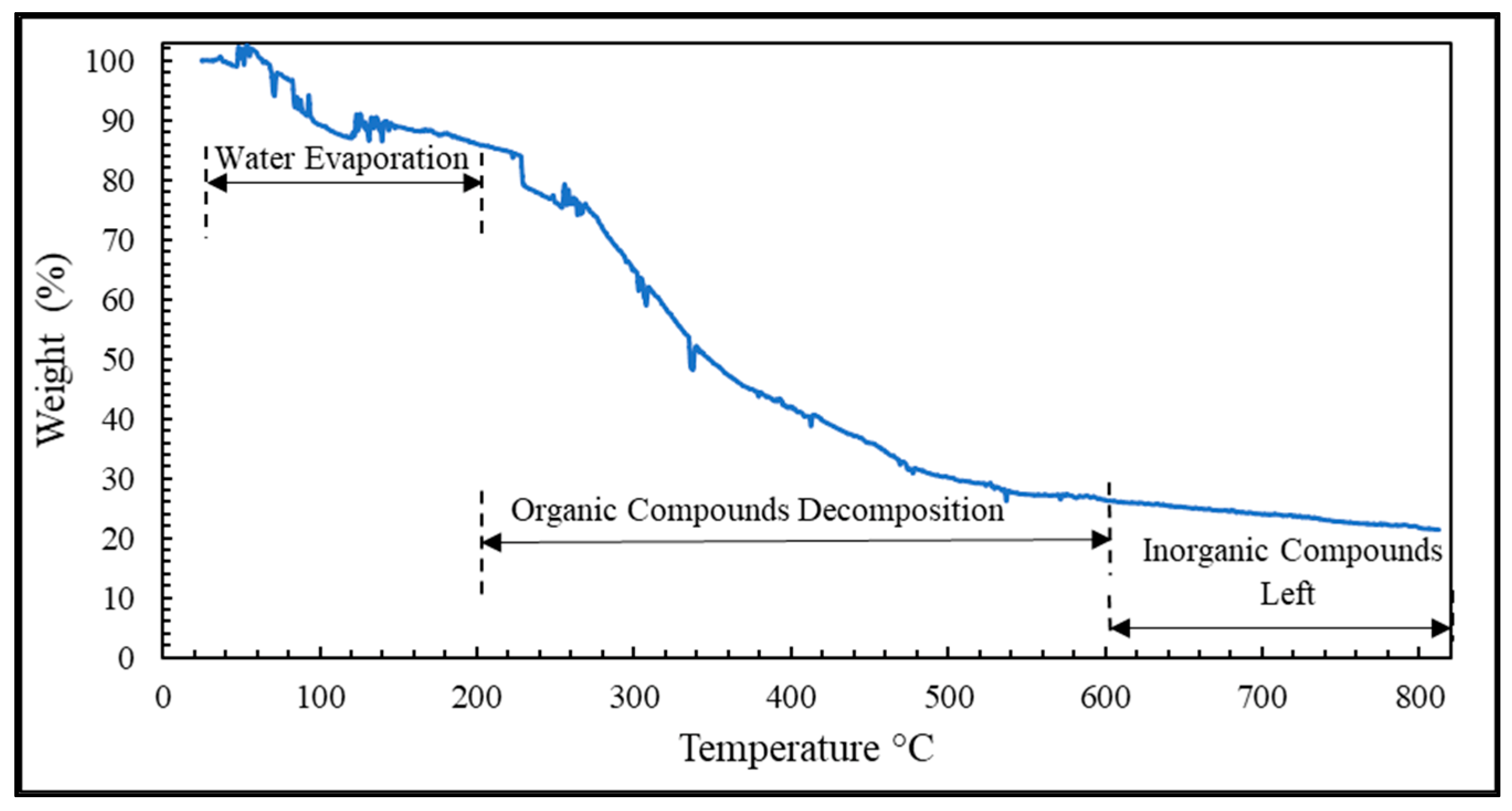
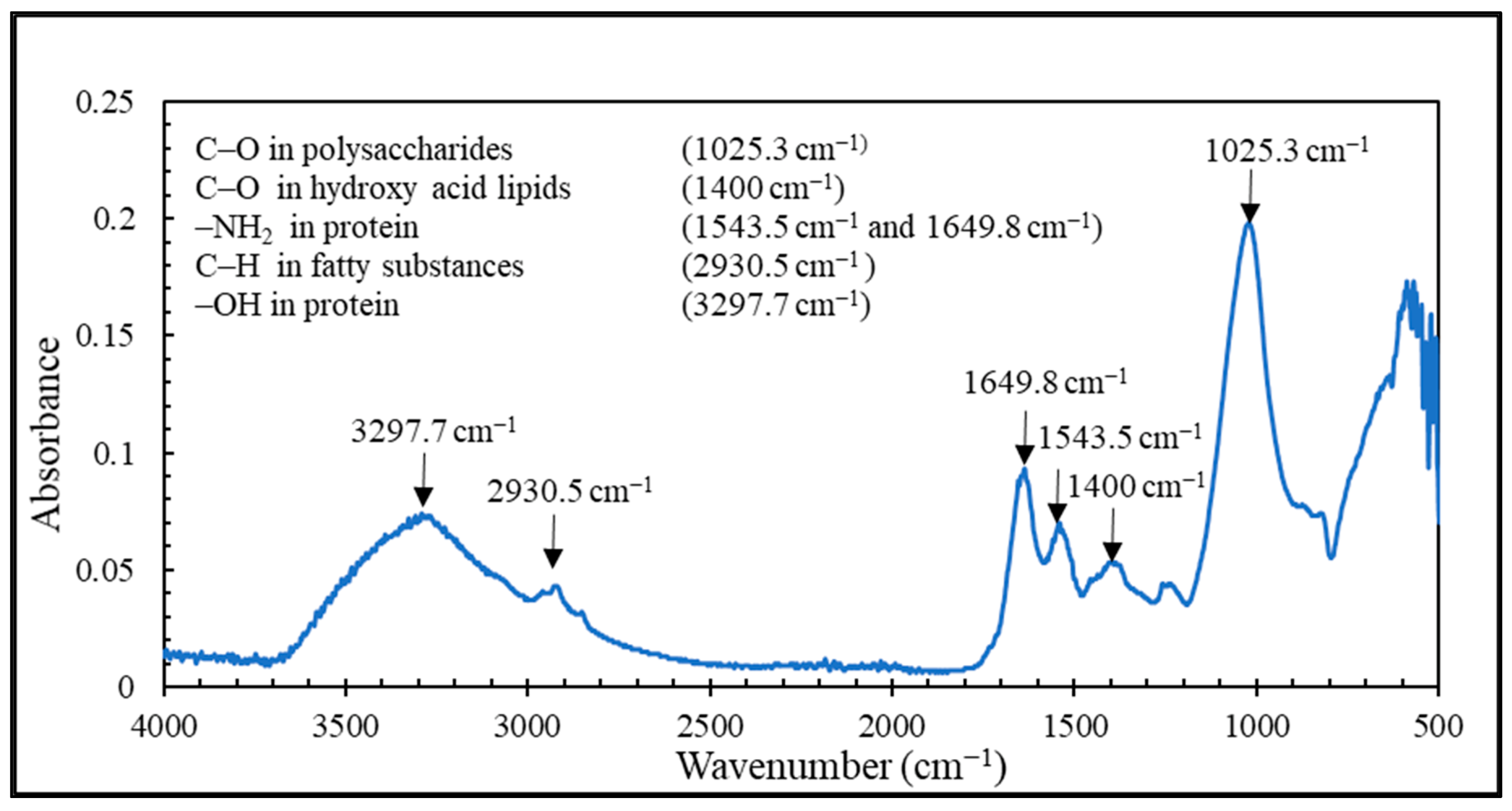
In this research, the PSWW was collected from the discharging stage of the poultry slaughterhouse plant after the conventional treatment and was further purified with a sequential process of UF-FO-RO for water recycling. Our results demonstrate that UF is a promising pretreatment option for FO that can significantly reduce FO fouling and pollutant levels in PSWW. The fouling of UF was irreversible, and the main foulants of UF are protein and carbohydrates. The UF process showed removal efficiency of 36.7% of COD, 38.9% of TP, 12.1% of TN, 24.7% of TS, 14.5% of TVS, and 27.3% of TFS. The FO process is used for further purification of PSWW. Compared to the PRO mode, the FO mode was the most efficient by providing higher removal efficiency and higher average flux. The FO fouling is reversible for all operations, including FO, PRO mode, and coated membrane in PRO mode. The product water quality after RO is almost comparable to potable water except for TN. It is recommended that future studies must be conducted on the removal of TN from high nitrogen concentration draw solution for the FO process. Overall, the results show that a sequential membrane process (UF-FO-RO) is a promising approach for PSWW treatment. It exhibits excellent performance by providing high efficiency for pollutant removal and recovering valuable products. It removes almost all the pollutants and purifies the water as required to reuse for industrial poultry purposes. We expect future research to focus on the cost reduction of RO by selecting a low-energy-demand RO membrane at the end of the sequential UF-FO-RO treatment.