Membranes for Osmotic Power Generation by Reverse Electrodialysis
Abstract
1. Introduction
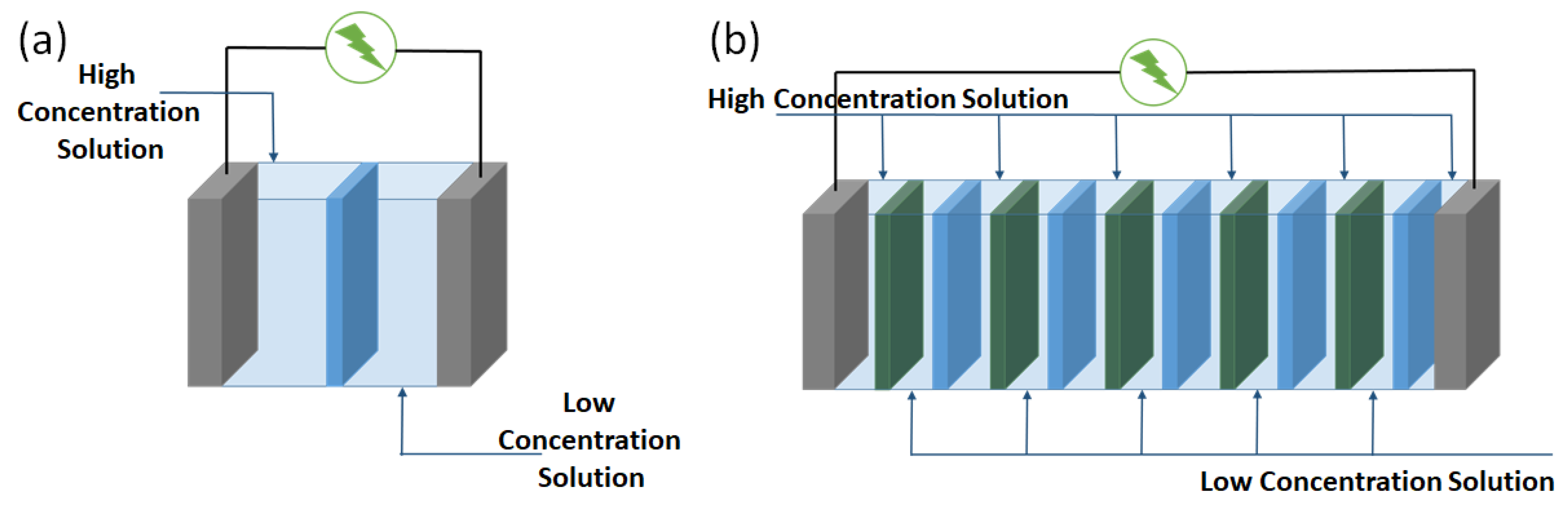
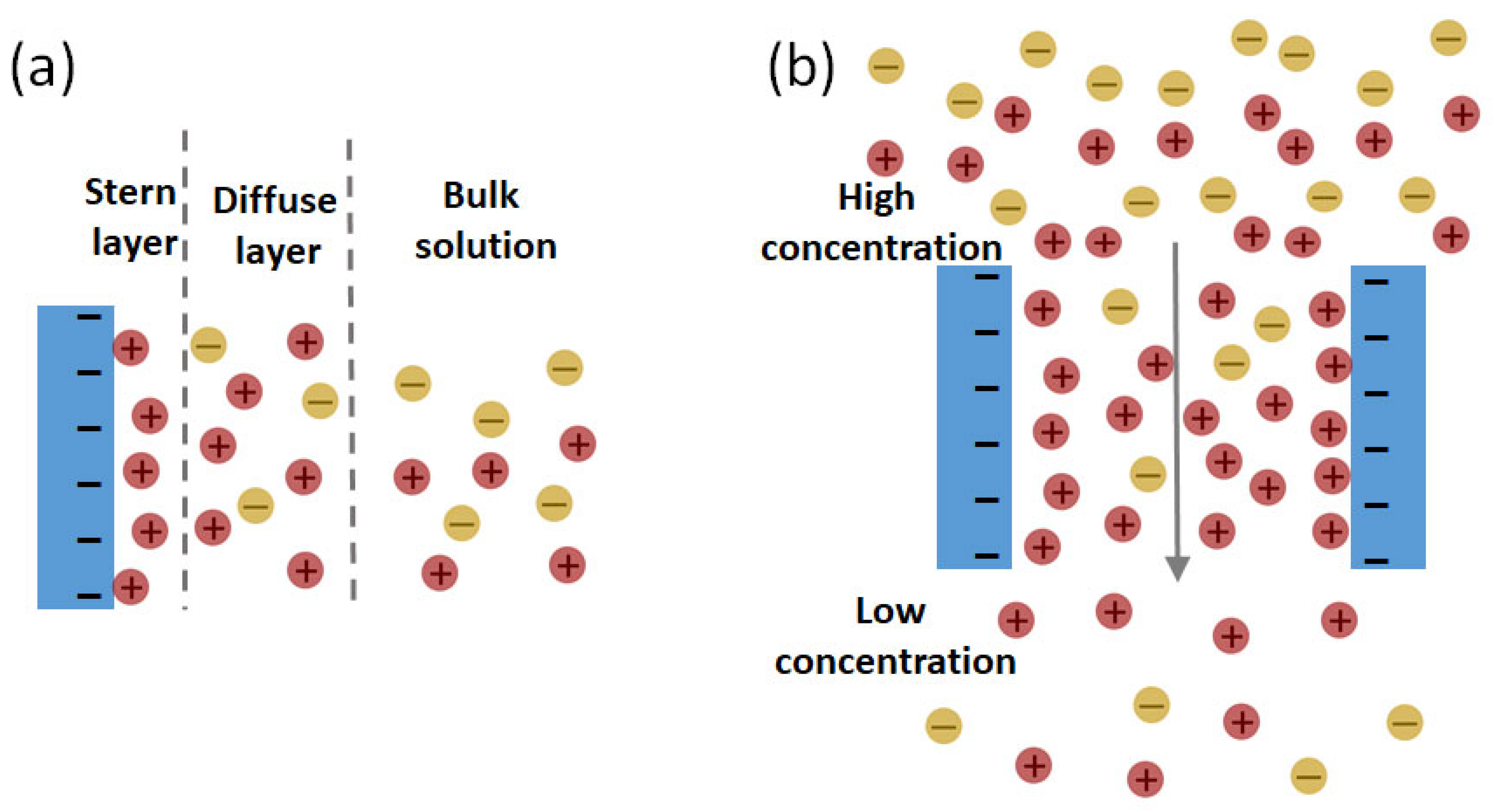
2. Principles of Reverse Electrodialysis
3. Conventional Nonporous Membranes
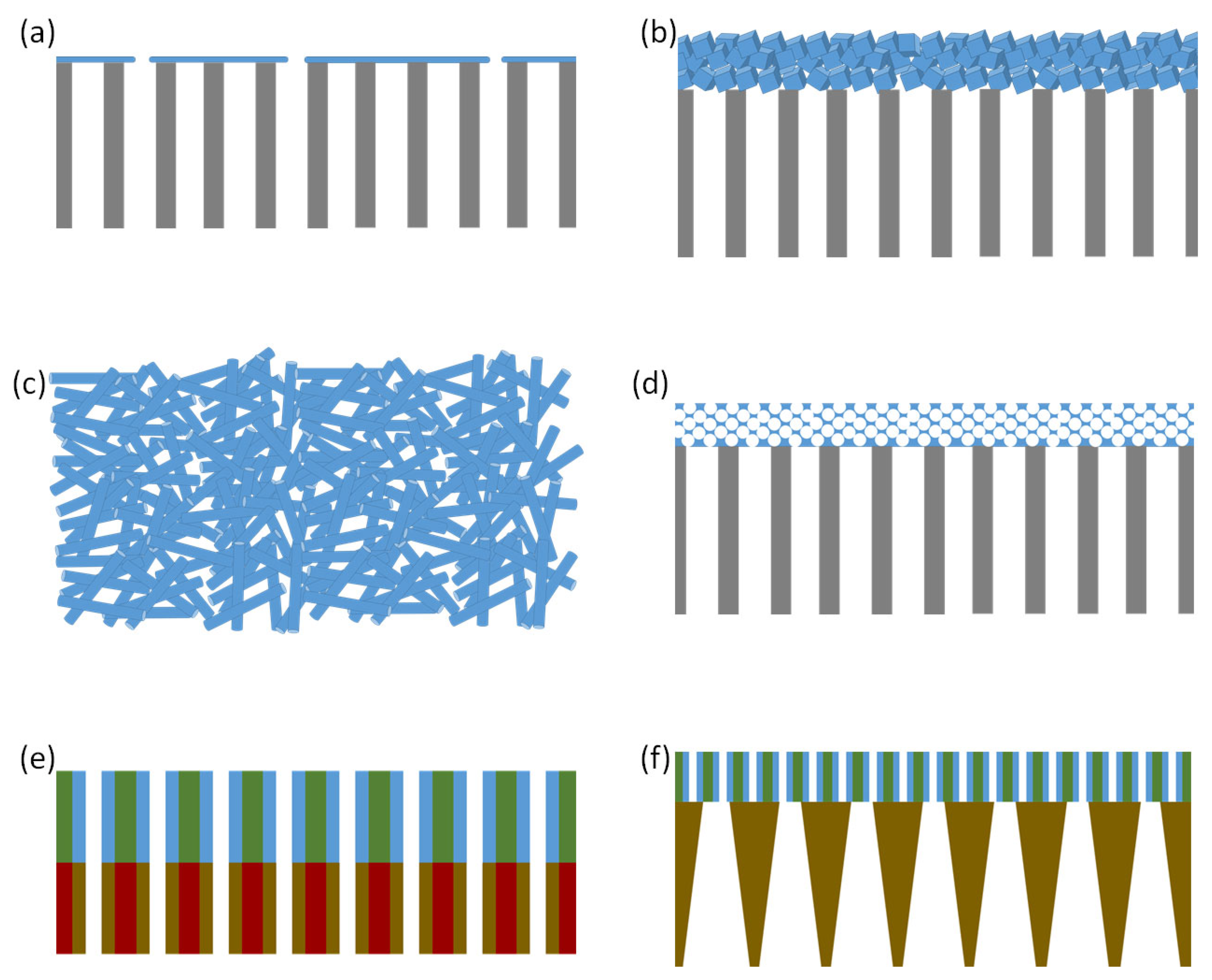
4. Emerging Porous Membranes
4.1. Selective Ion Transport through Porous Membranes
4.2. Track-Etched Polymer Membranes with 1D Pores
4.3. Porous Membranes with Atomic- and Molecular-Scale Thickness
4.4. Nanofluidic Membranes Having 2D Pores
4.5. Nanofiber-Based 3D Porous Membranes
4.6. Metal Organic Frameworks (MOF) Containing Membranes
4.7. Membranes Containing Mesoporous Carbon and a Silica Layer
4.8. Porous Block Copolymer Membranes
4.9. Other Porous Membranes
5. Summary and Outlook
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, X.; Liu, S.; Crittenden, J.; Chen, Y. Nanofluidic membranes to address the challenges of salinity gradient power harvesting. ACS Nano 2021, 15, 5838–5860. [Google Scholar] [CrossRef] [PubMed]
- Ramon, G.Z.; Feinberg, B.J.; Hoek, E.M.V. Membrane-based production of salinity-gradient power. Energy Environ. Sci. 2011, 4, 4423–4434. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Goulão Crespo, J.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Brauns, E. Towards a worldwide sustainable and simultaneous large-scale production of renewable energy and potable water through salinity gradient power by combining reversed electrodialysis and solar power. Desalination 2008, 219, 312–323. [Google Scholar] [CrossRef]
- Brauns, E. Salinity gradient power by reverse electrodialysis: Effect of model parameters on electrical power output. Desalination 2009, 237, 378–391. [Google Scholar] [CrossRef]
- Tristán, C.; Rumayor, M.; Dominguez-Ramos, A.; Fallanza, M.; Ibáñez, R.; Ortiz, I. Life cycle assessment of salinity gradient energy recovery by reverse electrodialysis in a seawater reverse osmosis desalination plant. Sustain. Energy Fuels 2020, 4, 4273–4284. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S.-M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Voutchkov, N. Energy use for membrane seawater desalination—Current status and trends. Desalination 2018, 431, 2–14. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Sorci, M.; Gu, M.; Heldt, C.L.; Grafeld, E.; Belfort, G. A multi-dimensional approach for fractionating proteins using charged membranes. Biotechnol. Bioeng. 2013, 110, 1704–1713. [Google Scholar] [CrossRef]
- Li, W.; Krantz, W.B.; Cornelissen, E.R.; Post, J.W.; Verliefde, A.R.D.; Tang, C.Y. A novel hybrid process of reverse electrodialysis and reverse osmosis for low energy seawater desalination and brine management. Appl. Energy 2013, 104, 592–602. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Pei, Y.; Crittenden, J.C. Unique applications and improvements of reverse electrodialysis: A review and outlook. Appl. Energy 2020, 262, 114482. [Google Scholar] [CrossRef]
- Mercer, E.; Davey, C.J.; Azzini, D.; Eusebi, A.L.; Tierney, R.; Williams, L.; Jiang, Y.; Parker, A.; Kolios, A.; Tyrrel, S.; et al. Hybrid membrane distillation reverse electrodialysis configuration for water and energy recovery from human urine: An opportunity for off-grid decentralised sanitation. J. Membr. Sci. 2019, 584, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Daniilidis, A.; Vermaas, D.A.; Herber, R.; Nijmeijer, K. Experimentally obtainable energy from mixing river water, seawater or brines with reverse electrodialysis. Renew. Energy 2014, 64, 123–131. [Google Scholar] [CrossRef]
- Guler, E.; Nijmeijer, K. Reverse electrodialysis for salinity gradient power generation: Challenges and future perspectives. J. Membr. Sci. Res. 2018, 4, 108–110. [Google Scholar]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Chen, W.; Xiang, Y.; Kong, X.-Y.; Wen, L. Polymer-based membranes for promoting osmotic energy conversion. Giant 2022, 10, 100094. [Google Scholar] [CrossRef]
- Siria, A.; Poncharal, P.; Biance, A.-L.; Fulcrand, R.; Blase, X.; Purcell, S.T.; Bocquet, L. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 2013, 494, 455–458. [Google Scholar] [CrossRef]
- Mai, V.-P.; Huang, W.-H.; Yang, R.-J. Enhancing ion transport through nanopores in membranes for salinity gradient power generation. ACS EST Eng. 2021, 1, 1725–1752. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Patel, S.K.; Lin, S.; Elimelech, M. Nanopore-based power generation from salinity gradient: Why it is not viable. ACS Nano 2021, 15, 4093–4107. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Elimelech, M. Viability of harvesting salinity gradient (blue) energy by nanopore-based osmotic power generation. Engineering 2022, 9, 51–60. [Google Scholar] [CrossRef]
- Macha, M.; Marion, S.; Nandigana, V.V.R.; Radenovic, A. 2d materials as an emerging platform for nanopore-based power generation. Nat. Rev. Mater. 2019, 4, 588–605. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Besha, A.T.; Tsehaye, M.T.; Aili, D.; Zhang, W.; Tufa, R.A. Design of monovalent ion selective membranes for reducing the impacts of multivalent ions in reverse electrodialysis. Membranes 2020, 10, 7. [Google Scholar] [CrossRef]
- Kim, D.-K.; Duan, C.; Chen, Y.-F.; Majumdar, A. Power generation from concentration gradient by reverse electrodialysis in ion-selective nanochannels. Microfluid. Nanofluid. 2010, 9, 1215–1224. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, Z.; Huang, X.; Hu, Y.; Zhou, T.; Zhu, C.; Kong, X.-Y.; Jiang, L.; Wen, L. High-performance silk-based hybrid membranes employed for osmotic energy conversion. Nat. Commun. 2019, 10, 3876. [Google Scholar] [CrossRef]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reverse electrodialysis: Performance of a stack with 50 cells on the mixing of sea and river water. J. Membr. Sci. 2009, 327, 136–144. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Doubled power density from salinity gradients at reduced intermembrane distance. Environ. Sci. Technol. 2011, 45, 7089–7095. [Google Scholar] [CrossRef] [PubMed]
- Długołęcki, P.; Dąbrowska, J.; Nijmeijer, K.; Wessling, M. Ion conductive spacers for increased power generation in reverse electrodialysis. J. Membr. Sci. 2010, 347, 101–107. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Power generation using profiled membranes in reverse electrodialysis. J. Membr. Sci. 2011, 385–386, 234–242. [Google Scholar] [CrossRef]
- Güler, E.; Elizen, R.; Saakes, M.; Nijmeijer, K. Micro-structured membranes for electricity generation by reverse electrodialysis. J. Membr. Sci. 2014, 458, 136–148. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Enhanced mixing in the diffusive boundary layer for energy generation in reverse electrodialysis. J. Membr. Sci. 2014, 453, 312–319. [Google Scholar] [CrossRef]
- Długołȩcki, P.; Gambier, A.; Nijmeijer, K.; Wessling, M. Practical potential of reverse electrodialysis as process for sustainable energy generation. Environ. Sci. Technol. 2009, 43, 6888–6894. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Early detection of preferential channeling in reverse electrodialysis. Electrochim. Acta 2014, 117, 9–17. [Google Scholar] [CrossRef]
- Długołęcki, P.; Nymeijer, K.; Metz, S.; Wessling, M. Current status of ion exchange membranes for power generation from salinity gradients. J. Membr. Sci. 2008, 319, 214–222. [Google Scholar] [CrossRef]
- Geise, G.M.; Curtis, A.J.; Hatzell, M.C.; Hickner, M.A.; Logan, B.E. Salt concentration differences alter membrane resistance in reverse electrodialysis stacks. Environ. Sci. Technol. Lett. 2014, 1, 36–39. [Google Scholar] [CrossRef]
- Geise, G.M.; Hickner, M.A.; Logan, B.E. Ionic resistance and permselectivity tradeoffs in anion exchange membranes. ACS Appl. Mater. Interfaces 2013, 5, 10294–10301. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.H.; Kabay, N.; Guler, E. Principles of reverse electrodialysis and development of integrated-based system for power generation and water treatment: A review. Rev. Chem. Eng. 2022, 38, 921–958. [Google Scholar] [CrossRef]
- Guler, E.; Zhang, Y.; Saakes, M.; Nijmeijer, K. Tailor-made anion-exchange membranes for salinity gradient power generation using reverse electrodialysis. ChemSusChem 2012, 5, 2262–2270. [Google Scholar] [CrossRef]
- Güler, E.; Elizen, R.; Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Performance-determining membrane properties in reverse electrodialysis. J. Membr. Sci. 2013, 446, 266–276. [Google Scholar] [CrossRef]
- Rijnaarts, T.; Huerta, E.; van Baak, W.; Nijmeijer, K. Effect of divalent cations on red performance and cation exchange membrane selection to enhance power densities. Environ. Sci. Technol. 2017, 51, 13028–13035. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Han, E.D.; Kim, B.H.; Seo, Y.H. Ultra-thin ion exchange film on the ceramic supporter for output power improvement of reverse electrodialysis. Sci. Rep. 2019, 9, 17440. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Aguilera, J.A.; Villafaña-López, L.; Rentería-Martínez, E.C.; Anderson, S.M.; Jaime-Ferrer, J.S. Electrospinning of polyepychlorhydrin and polyacrylonitrile anionic exchange membranes for reverse electrodialysis. Membranes 2021, 11, 717. [Google Scholar] [CrossRef]
- Karakoç, E.; Güler, E. Comparison of physicochemical properties of two types of polyepichlorohydrin-based anion exchange membranes for reverse electrodialysis. Membranes 2022, 12, 257. [Google Scholar] [CrossRef]
- Villafaña-López, L.; Reyes-Valadez, D.M.; González-Vargas, O.A.; Suárez-Toriello, V.A.; Jaime-Ferrer, J.S. Custom-made ion exchange membranes at laboratory scale for reverse electrodialysis. Membranes 2019, 9, 145. [Google Scholar] [CrossRef]
- Horkay, F.; McKenna, G.B. Polymer networks and gels. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 497–523. [Google Scholar]
- Mark, J.E. Experimental determinations of crosslink densities. Rubber Chem. Technol. 1982, 55, 762–768. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abetz, V. Tailoring crosslinked polyether networks for separation of CO2 from light gases. Macromol. Rapid Commun. 2021, 42, 2100160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lin, X.; Hu, Y.; Fu, L.; Luo, Q.; Yang, L.; Hou, S.; Kong, X.-Y.; Jiang, L.; Wen, L. Tailoring sulfonated poly(phenyl-alkane)s of intrinsic microporosity membrane for advanced osmotic energy conversion. ACS Mater. Lett. 2022, 4, 1422–1429. [Google Scholar] [CrossRef]
- White, H.S.; Bund, A. Ion current rectification at nanopores in glass membranes. Langmuir 2008, 24, 2212–2218. [Google Scholar] [CrossRef]
- Siwy, Z.S. Ion-current rectification in nanopores and nanotubes with broken symmetry. Adv. Funct. Mater. 2006, 16, 735–746. [Google Scholar] [CrossRef]
- Guo, W.; Cao, L.; Xia, J.; Nie, F.-Q.; Ma, W.; Xue, J.; Song, Y.; Zhu, D.; Wang, Y.; Jiang, L. Energy harvesting with single-ion-selective nanopores: A concentration-gradient-driven nanofluidic power source. Adv. Funct. Mater. 2010, 20, 1339–1344. [Google Scholar] [CrossRef]
- Ma, L.; Lin, K.; Qiu, Y.; Zhuang, J.; An, X.; Yuan, Z.; Huang, C. Significantly enhanced performance of nanofluidic osmotic power generation by slipping surfaces of nanopores. J. Phys. Chem. C 2021, 125, 14195–14203. [Google Scholar] [CrossRef]
- Cao, L.; Xiao, F.; Feng, Y.; Zhu, W.; Geng, W.; Yang, J.; Zhang, X.; Li, N.; Guo, W.; Jiang, L. Anomalous channel-length dependence in nanofluidic osmotic energy conversion. Adv. Funct. Mater. 2017, 27, 1604302. [Google Scholar] [CrossRef]
- Su, Y.-S.; Hsu, S.-C.; Peng, P.-H.; Yang, J.-Y.; Gao, M.; Yeh, L.-H. Unraveling the anomalous channel-length-dependent blue energy conversion using engineered alumina nanochannels. Nano Energy 2021, 84, 105930. [Google Scholar] [CrossRef]
- Kim, D.-K. Numerical study of power generation by reverse electrodialysis in ion-selective nanochannels. J. Mech. Sci. Technol. 2011, 25, 5–10. [Google Scholar] [CrossRef]
- Rankin, D.J.; Huang, D.M. The effect of hydrodynamic slip on membrane-based salinity-gradient-driven energy harvesting. Langmuir 2016, 32, 3420–3432. [Google Scholar] [CrossRef]
- Bocquet, L.; Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 2010, 39, 1073–1095. [Google Scholar] [CrossRef]
- Li, L.; Su, Y.; Wang, H.; Sheng, G.; Wang, W. A new slip length model for enhanced water flow coupling molecular interaction, pore dimension, wall roughness, and temperature. Adv. Polym. Technol. 2019, 2019, 6424012. [Google Scholar] [CrossRef]
- Williams, I.; Lee, S.; Apriceno, A.; Sear, R.P.; Battaglia, G. Diffusioosmotic and convective flows induced by a nonelectrolyte concentration gradient. Proc. Natl. Acad. Sci. USA 2020, 117, 25263–25271. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Jin, J.; Li, L.; Miller, J.D. Afm slip length measurements for water at selected phyllosilicate surfaces. Coll. Interfaces 2021, 5, 44. [Google Scholar] [CrossRef]
- Laucirica, G.; Albesa, A.G.; Toimil-Molares, M.E.; Trautmann, C.; Marmisollé, W.A.; Azzaroni, O. Shape matters: Enhanced osmotic energy harvesting in bullet-shaped nanochannels. Nano Energy 2020, 71, 104612. [Google Scholar] [CrossRef]
- Pérez-Mitta, G.; Albesa, A.; Gilles, F.M.; Toimil-Molares, M.E.; Trautmann, C.; Azzaroni, O. Noncovalent approach toward the construction of nanofluidic diodes with ph-reversible rectifying properties: Insights from theory and experiment. J. Phys. Chem. C 2017, 121, 9070–9076. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Combs, C.; Su, Y.-S.; Yeh, L.-H.; Siwy, Z.S. Rectification of concentration polarization in mesopores leads to high conductance ionic diodes and high performance osmotic power. J. Am. Chem. Soc. 2019, 141, 3691–3698. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Yameen, B.; Cervera, J.; Ramírez, P.; Neumann, R.; Ensinger, W.; Knoll, W.; Azzaroni, O. Layer-by-layer assembly of polyelectrolytes into ionic current rectifying solid-state nanopores: Insights from theory and experiment. J. Am. Chem. Soc. 2010, 132, 8338–8348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Janot, J.-M.; Balanzat, E.; Balme, S. Mimicking ph-gated ionic channels by polyelectrolyte complex confinement inside a single nanopore. Langmuir 2017, 33, 3484–3490. [Google Scholar] [CrossRef] [PubMed]
- Lepoitevin, M.; Jamilloux, B.; Bechelany, M.; Balanzat, E.; Janot, J.-M.; Balme, S. Fast and reversible functionalization of a single nanopore based on layer-by-layer polyelectrolyte self-assembly for tuning current rectification and designing sensors. RSC Adv. 2016, 6, 32228–32233. [Google Scholar] [CrossRef]
- Balme, S.; Ma, T.; Balanzat, E.; Janot, J.-M. Large osmotic energy harvesting from functionalized conical nanopore suitable for membrane applications. J. Membr. Sci. 2017, 544, 18–24. [Google Scholar] [CrossRef]
- Ma, T.; Balanzat, E.; Janot, J.-M.; Balme, S. Nanopore functionalized by highly charged hydrogels for osmotic energy harvesting. ACS Appl. Mater. Interfaces 2019, 11, 12578–12585. [Google Scholar] [CrossRef]
- Laucirica, G.; Toimil-Molares, M.E.; Trautmann, C.; Marmisollé, W.; Azzaroni, O. Polyaniline for improved blue energy harvesting: Highly rectifying nanofluidic diodes operating in hypersaline conditions via one-step functionalization. ACS Appl. Mater. Interfaces 2020, 12, 28148–28157. [Google Scholar] [CrossRef]
- Aliprandi, A.; Pakulski, D.; Ciesielski, A.; Samorì, P. Punctured two-dimensional sheets for harvesting blue energy. ACS Nano 2017, 11, 10654–10658. [Google Scholar] [CrossRef]
- Garaj, S.; Hubbard, W.; Reina, A.; Kong, J.; Branton, D.; Golovchenko, J.A. Graphene as a subnanometre trans-electrode membrane. Nature 2010, 467, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Garaj, S.; Liu, S.; Golovchenko, J.A.; Branton, D. Molecule-hugging graphene nanopores. Proc. Natl. Acad. Sci. USA 2013, 110, 12192–12196. [Google Scholar] [CrossRef]
- Russo, C.J.; Golovchenko, J.A. Atom-by-atom nucleation and growth of graphene nanopores. Proc. Natl. Acad. Sci. USA 2012, 109, 5953–5957. [Google Scholar] [CrossRef] [PubMed]
- O’Hern, S.C.; Boutilier, M.S.H.; Idrobo, J.-C.; Song, Y.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective ionic transport through tunable subnanometer pores in single-layer graphene membranes. Nano Lett. 2014, 14, 1234–1241. [Google Scholar] [CrossRef]
- Feng, J.; Graf, M.; Liu, K.; Ovchinnikov, D.; Dumcenco, D.; Heiranian, M.; Nandigana, V.; Aluru, N.R.; Kis, A.; Radenovic, A. Single-layer mos2 nanopores as nanopower generators. Nature 2016, 536, 197–200. [Google Scholar] [CrossRef]
- Yazda, K.; Bleau, K.; Zhang, Y.; Capaldi, X.; St-Denis, T.; Grutter, P.; Reisner, W.W. High osmotic power generation via nanopore arrays in hybrid hexagonal boron nitride/silicon nitride membranes. Nano Lett. 2021, 21, 4152–4159. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Feng, J.; Kis, A.; Radenovic, A. Atomically thin molybdenum disulfide nanopores with high sensitivity for DNA translocation. ACS Nano 2014, 8, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, K.; Graf, M.; Lihter, M.; Bulushev, R.D.; Dumcenco, D.; Alexander, D.T.L.; Krasnozhon, D.; Vuletic, T.; Kis, A.; et al. Electrochemical reaction in single layer mos2: Nanopores opened atom by atom. Nano Lett. 2015, 15, 3431–3438. [Google Scholar] [CrossRef]
- Wang, H.; Su, L.; Yagmurcukardes, M.; Chen, J.; Jiang, Y.; Li, Z.; Quan, A.; Peeters, F.M.; Wang, C.; Geim, A.K.; et al. Blue energy conversion from holey-graphene-like membranes with a high density of subnanometer pores. Nano Lett. 2020, 20, 8634–8639. [Google Scholar] [CrossRef]
- Liu, X.; He, M.; Calvani, D.; Qi, H.; Gupta, K.B.S.S.; de Groot, H.J.M.; Sevink, G.J.A.; Buda, F.; Kaiser, U.; Schneider, G.F. Power generation by reverse electrodialysis in a single-layer nanoporous membrane made from core–rim polycyclic aromatic hydrocarbons. Nat. Nanotechnol. 2020, 15, 307–312. [Google Scholar] [CrossRef]
- Yang, J.; Tu, B.; Zhang, G.; Liu, P.; Hu, K.; Wang, J.; Yan, Z.; Huang, Z.; Fang, M.; Hou, J.; et al. Advancing osmotic power generation by covalent organic framework monolayer. Nat. Nanotechnol. 2022, 17, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Koltonow, A.R.; Huang, J. Two-dimensional nanofluidics. Science 2016, 351, 1395–1396. [Google Scholar] [CrossRef] [PubMed]
- Raidongia, K.; Huang, J. Nanofluidic ion transport through reconstructed layered materials. J. Am. Chem. Soc. 2012, 134, 16528–16531. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Du, X.; Chen, R.; Zhou, J.; Agostini, M.; Sun, J.; Xiao, L. The combination of 2D layered graphene oxide and 3D porous cellulose heterogeneous membranes for nanofluidic osmotic power generation. Molecules 2021, 26, 5343. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Kang, Q.; Zhou, Y.; Feng, Y.; Chen, X.; Yuan, J.; Guo, W.; Wei, Y.; Jiang, L. Osmotic power generation with positively and negatively charged 2d nanofluidic membrane pairs. Adv. Funct. Mater. 2017, 27, 1603623. [Google Scholar] [CrossRef]
- Qin, S.; Liu, D.; Wang, G.; Portehault, D.; Garvey, C.J.; Gogotsi, Y.; Lei, W.; Chen, Y. High and stable ionic conductivity in 2D nanofluidic ion channels between boron nitride layers. J. Am. Chem. Soc. 2017, 139, 6314–6320. [Google Scholar] [CrossRef]
- Xiao, K.; Giusto, P.; Wen, L.; Jiang, L.; Antonietti, M. Nanofluidic ion transport and energy conversion through ultrathin free-standing polymeric carbon nitride membranes. Angew. Chem. Int. Ed. 2018, 57, 10123–10126. [Google Scholar] [CrossRef]
- Hong, S.; Ming, F.; Shi, Y.; Li, R.; Kim, I.S.; Tang, C.Y.; Alshareef, H.N.; Wang, P. Two-dimensional ti3c2tx mxene membranes as nanofluidic osmotic power generators. ACS Nano 2019, 13, 8917–8925. [Google Scholar] [CrossRef]
- Ding, L.; Zheng, M.; Xiao, D.; Zhao, Z.; Xue, J.; Zhang, S.; Caro, J.; Wang, H. Bioinspired ti3c2tx mxene-based ionic diode membrane for high-efficient osmotic energy conversion. Angew. Chem. Int. Ed. 2022, 61, e202206152. [Google Scholar]
- Zhang, Z.; Yang, S.; Zhang, P.; Zhang, J.; Chen, G.; Feng, X. Mechanically strong mxene/kevlar nanofiber composite membranes as high-performance nanofluidic osmotic power generators. Nat. Commun. 2019, 10, 2920. [Google Scholar] [CrossRef]
- Ding, L.; Xiao, D.; Zhao, Z.; Wei, Y.; Xue, J.; Wang, H. Ultrathin and ultrastrong kevlar aramid nanofiber membranes for highly stable osmotic energy conversion. Adv. Sci. 2022, 9, 2202869. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wong, E.; Mai, Z.; Van der Bruggen, B. Fabrication of composite polyamide/kevlar aramid nanofiber nanofiltration membranes with high permselectivity in water desalination. J. Membr. Sci. 2019, 592, 117396. [Google Scholar] [CrossRef]
- Yuan, S.; Swartenbroekx, J.; Li, Y.; Zhu, J.; Ceyssens, F.; Zhang, R.; Volodine, A.; Li, J.; Van Puyvelde, P.; Van der Bruggen, B. Facile synthesis of kevlar nanofibrous membranes via regeneration of hydrogen bonds for organic solvent nanofiltration. J. Membr. Sci. 2019, 573, 612–620. [Google Scholar] [CrossRef]
- Zhang, Z.; He, L.; Zhu, C.; Qian, Y.; Wen, L.; Jiang, L. Improved osmotic energy conversion in heterogeneous membrane boosted by three-dimensional hydrogel interface. Nat. Commun. 2020, 11, 875. [Google Scholar] [CrossRef]
- Xie, L.; Zhou, S.; Liu, J.; Qiu, B.; Liu, T.; Liang, Q.; Zheng, X.; Li, B.; Zeng, J.; Yan, M.; et al. Sequential superassembly of nanofiber arrays to carbonaceous ordered mesoporous nanowires and their heterostructure membranes for osmotic energy conversion. J. Am. Chem. Soc. 2021, 143, 6922–6932. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Hou, J.; Li, X.; Hu, X.; Hu, Y.; Easton, C.D.; Li, Q.; Sun, C.; Thornton, A.W.; et al. Efficient metal ion sieving in rectifying subnanochannels enabled by metal–organic frameworks. Nat. Mater. 2020, 19, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Yeh, L.-H.; Zheng, M.-J.; Wu, K.C.-W. Highly selective and high-performance osmotic power generators in subnanochannel membranes enabled by metal-organic frameworks. Sci. Adv. 2021, 7, eabe9924. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Y.; Jiang, J.; Lu, B.; Zhai, J. Sandwich “ion pool”—Structured power gating for salinity gradient generation devices. ACS Appl. Mater. Interfaces 2021, 13, 35197–35206. [Google Scholar] [CrossRef]
- Robertson, M.; Zagho, M.M.; Nazarenko, S.; Qiang, Z. Mesoporous carbons from self-assembled polymers. J. Polym. Sci. 2022, 60, 2015–2042. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Stein, A.; et al. A family of highly ordered mesoporous polymer resin and carbon structures from organic−organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, L.; Zhang, L.; Wen, L.; Tang, J.; Zeng, J.; Liu, T.; Peng, D.; Yan, M.; Qiu, B.; et al. Interfacial super-assembly of ordered mesoporous silica–alumina heterostructure membranes with ph-sensitive properties for osmotic energy harvesting. ACS Appl. Mater. Interfaces 2021, 13, 8782–8793. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shi, Y.; Wan, Y.; Meng, Y.; Zhang, F.; Gu, D.; Chen, Z.; Tu, B.; Zhao, D. Triconstituent co-assembly to ordered mesostructured polymer−silica and carbon-silica nanocomposites and large-pore mesoporous carbons with high surface areas. J. Am. Chem. Soc. 2006, 128, 11652–11662. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, W.; Feng, D.; Wang, H.; Zhao, D.; Jiang, L. High-performance ionic diode membrane for salinity gradient power generation. J. Am. Chem. Soc. 2014, 136, 12265–12272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xie, L.; Li, X.; Huang, Y.; Zhang, L.; Liang, Q.; Yan, M.; Zeng, J.; Qiu, B.; Liu, T.; et al. Interfacial super-assembly of ordered mesoporous carbon-silica/aao hybrid membrane with enhanced permselectivity for temperature- and ph-sensitive smart ion transport. Angew. Chem. Int. Ed. 2021, 60, 26167–26176. [Google Scholar] [CrossRef]
- Abetz, V. Isoporous block copolymer membranes. Macromol. Rapid Commun. 2015, 36, 10–22. [Google Scholar] [CrossRef]
- Radjabian, M.; Abetz, V. Advanced porous polymer membranes from self-assembling block copolymers. Prog. Polym. Sci. 2020, 102, 101219. [Google Scholar] [CrossRef]
- Rahman, M.M. Selective swelling and functionalization of integral asymmetric isoporous block copolymer membranes. Macromol. Rapid Commun. 2021, 42, 2100235. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, X.-Y.; Xiao, K.; Liu, Q.; Xie, G.; Li, P.; Ma, J.; Tian, Y.; Wen, L.; Jiang, L. Engineered asymmetric heterogeneous membrane: A concentration-gradient-driven energy harvesting device. J. Am. Chem. Soc. 2015, 137, 14765–14772. [Google Scholar] [CrossRef]
- Zhang, Z.; Sui, X.; Li, P.; Xie, G.; Kong, X.-Y.; Xiao, K.; Gao, L.; Wen, L.; Jiang, L. Ultrathin and ion-selective janus membranes for high-performance osmotic energy conversion. J. Am. Chem. Soc. 2017, 139, 8905–8914. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zhai, J. Gap confinement effect of a tandem nanochannel system and its application in salinity gradient power generation. ACS Appl. Mater. Interfaces 2021, 13, 41159–41168. [Google Scholar] [CrossRef]
- Yeh, L.-H.; Huang, Z.-Y.; Liu, Y.-C.; Deng, M.-J.; Chou, T.-H.; Ou Yang, H.-C.; Ahamad, T.; Saad, M.A.; Wu, K.C.-W. A nanofluidic osmotic power generator demonstrated in polymer gel electrolytes with substantially enhanced performance. J. Mater. Chem. A 2019, 7, 26791–26796. [Google Scholar] [CrossRef]
- Li, R.; Zhai, J.; Jiang, J.; Wang, Q.; Wang, S. Improved interfacial ion transport through nanofluidic hybrid membranes based on covalent organic frameworks for osmotic energy generation. ACS Appl. Energy Mater. 2022, 5, 7176–7184. [Google Scholar] [CrossRef]
- Tedesco, M.; Cipollina, A.; Tamburini, A.; Micale, G. Towards 1kw power production in a reverse electrodialysis pilot plant with saline waters and concentrated brines. J. Membr. Sci. 2017, 522, 226–236. [Google Scholar] [CrossRef]
- Tedesco, M.; Scalici, C.; Vaccari, D.; Cipollina, A.; Tamburini, A.; Micale, G. Performance of the first reverse electrodialysis pilot plant for power production from saline waters and concentrated brines. J. Membr. Sci. 2016, 500, 33–45. [Google Scholar] [CrossRef]
- Available online: www.reapower.eu/news.html (accessed on 27 January 2023).
- Laucirica, G.; Toimil-Molares, M.E.; Trautmann, C.; Marmisollé, W.; Azzaroni, O. Nanofluidic osmotic power generators–advanced nanoporous membranes and nanochannels for blue energy harvesting. Chem. Sci. 2021, 12, 12874–12910. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, J.; Fang, M.; Wang, X.; Liu, Y.; Li, S.; Wen, L.; Zhu, Y.; Jiang, L. Large-scale, ultrastrong cu2+ cross-linked sodium alginate membrane for effective salinity gradient power conversion. ACS Appl. Polym. Mater. 2021, 3, 3902–3910. [Google Scholar] [CrossRef]


| Membrane Type | Membrane Description | Concentration Gradient | Maximum Power Density | Reference |
|---|---|---|---|---|
| Atomic and molecularly thin porous membranes | Multi-porous hexagonal boron nitride membrane | 1000-fold (KCl) | 15 Wm−2 | [77] |
| Multi-porous graphene sheets containing –NH2 groups at the pore edges | 100-fold (KCl) | 35 Wm−2 | [80] | |
| Crosslinked core–rim structure polycyclic aromatic hydrocarbon monomer hexa(2,2′-dipyridylamino)hexabenzocoronene | 50-fold (NaCl) | 67 Wm−2 | [81] | |
| metal tetraphenylporphyrin COF (MTPP-COF) monolayer | 50-fold (NaCl) 50-fold (MgCl2) 50-fold (CaCl2) | 135.8 Wm−2 317.5 Wm−2 267.7 Wm−2 | [82] | |
| Nanofluidic membranes with 2D pores | Layered carbon nitride membrane | 1000-fold (KCl) | 0.21 Wm−2 | [88] |
| Free-standing Ti3C2Tx, MXene membrane | 1000-fold (KCl) | 21 Wm−2 | [89] | |
| Diode-type membrane containing negative Ti3C2Tx, MXene nanosheets and polydiallyl dimethyl ammonium-adsorbed positive MXene nanosheets | 50-fold (NaCl) 500-fold (NaCl) | 8.6 Wm−2 17.8 Wm−2 | [90] | |
| Aramid nanofiber intercalated Ti3C2Tx, MXene nanosheets | 50-fold (NaCl) | 3.7 Wm−2 | [91] | |
| Nanofiber-based 3D porous membranes | Free-standing aramid nanofiber membrane | 50-fold (NaCl) 500-fold (NaCl) | 4.8 Wm−2 15 Wm−2 | [92] |
| Double-layer membrane containing one layer of aramid nanofiber and one layer of polyelectrolyte hydrogel | 50-fold (NaCl) | 5.06 Wm−2 | [95] | |
| Double-layer membrane containing nanowires deposited on a porous anodic alumina oxide layer | 50-fold (NaCl) | 2.78 Wm−2 | [96] | |
| Double-layer membrane containing a silk nanofibril layer and a porous anodic aluminum oxide layer | 50-fold (NaCl) | 2.86 Wm−2 | [26] | |
| Metal organic frameworks (MOF) containing membranes | Double-layer membrane containing an amino-substituted UiO-66 layer on a porous alumina layer | 5-fold (KCl) 50-fold (KCl) 500-fold (KCl) | 2.19 Wm−2 4.93 Wm−2 7.12 Wm−2 | [99] |
| “Ion Pool” membrane containing a sandwiched anodic aluminum oxide (AAO) layer between a tungsten oxide (WO3) layer and a ZIF-8 layer (WO3-AAO-ZIF-8) | 50-fold (NaCl) | 1.93 Wm−2 | [100] | |
| Membranes containing a mesoporous carbon and silica layer | Double-layer membrane with a mesoporous carbon layer on a porous alumina layer | 50-fold (NaCl) | 3.46 Wm−2 | [105] |
| Double-layer membrane with a mesoporous silica layer on a porous alumina layer | 50-fold (NaCl) | 4.5 Wm−2 | [103] | |
| Double-layer membrane with a mesoporous carbon–silica hybrid layer on a porous alumina layer | 50-fold (NaCl) 200-fold (NaCl) | 5.04 Wm−2 10.75 Wm−2 | [106] | |
| Porous block copolymer membranes | Double-layer membrane containing a spin-coated polystyrene–block–poly (4vinylpyridine) (PS-b-P4VP) layer on top of a track-etched poly(ethylene terephthalate) layer | 50-fold (NaCl) | 0.35 Wm−2 | [110] |
| Janus type membrane having a porous PS-b-P4VP layer and a porous crosslinked block copolymer substrate containing a poly (ethyelene oxide) minor block | 50-fold (NaCl) | 2.04 Wm−2 | [111] | |
| Covalent organic framework (COF)-containing membrane | Hybrid membrane with COF-LZU1 on a cellulose nanofiber support with a carbon nanotube intermediate layer | 50-fold (NaCl) | 4.26 Wm−2 | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M. Membranes for Osmotic Power Generation by Reverse Electrodialysis. Membranes 2023, 13, 164. https://doi.org/10.3390/membranes13020164
Rahman MM. Membranes for Osmotic Power Generation by Reverse Electrodialysis. Membranes. 2023; 13(2):164. https://doi.org/10.3390/membranes13020164
Chicago/Turabian StyleRahman, Md. Mushfequr. 2023. "Membranes for Osmotic Power Generation by Reverse Electrodialysis" Membranes 13, no. 2: 164. https://doi.org/10.3390/membranes13020164
APA StyleRahman, M. M. (2023). Membranes for Osmotic Power Generation by Reverse Electrodialysis. Membranes, 13(2), 164. https://doi.org/10.3390/membranes13020164






