Combining Ultrafiltration and Nanofiltration to Obtain a Concentrated Extract of Purified Polyphenols from Wet Olive Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Raw Material
2.2. Extraction of Polyphenols
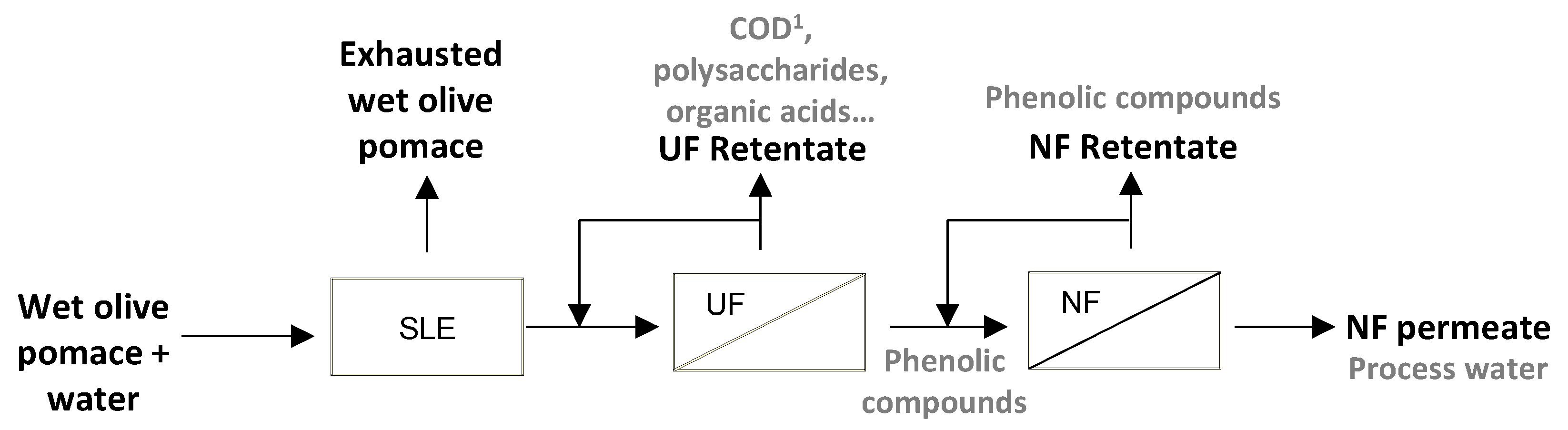
2.3. Membrane Processes
2.3.1. Ultrafiltration Process
2.3.2. Nanofiltration Process
2.3.3. Membrane Cleaning
2.4. Characterization of the Streams
3. Results and Discussion
3.1. Aqueous Extract of Wet Olive Pomace
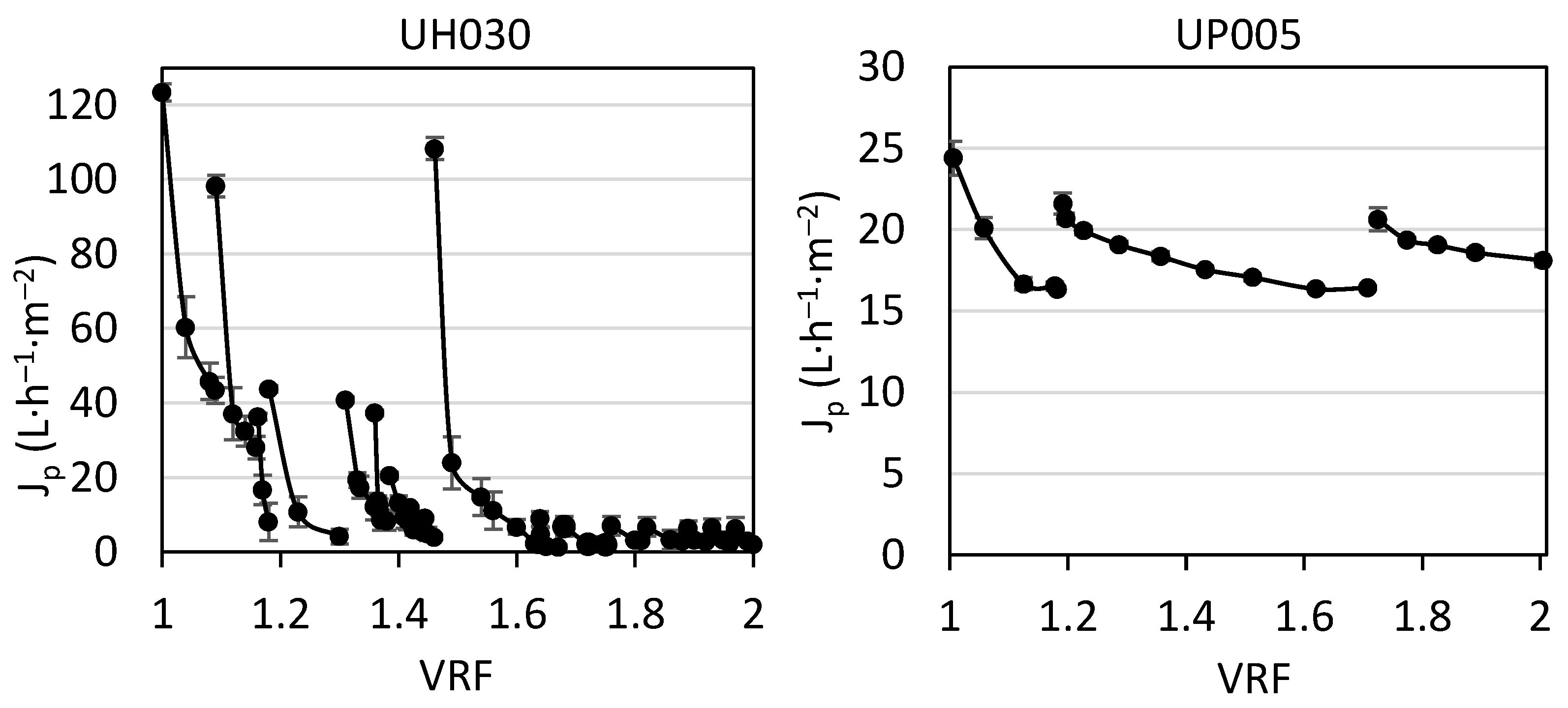
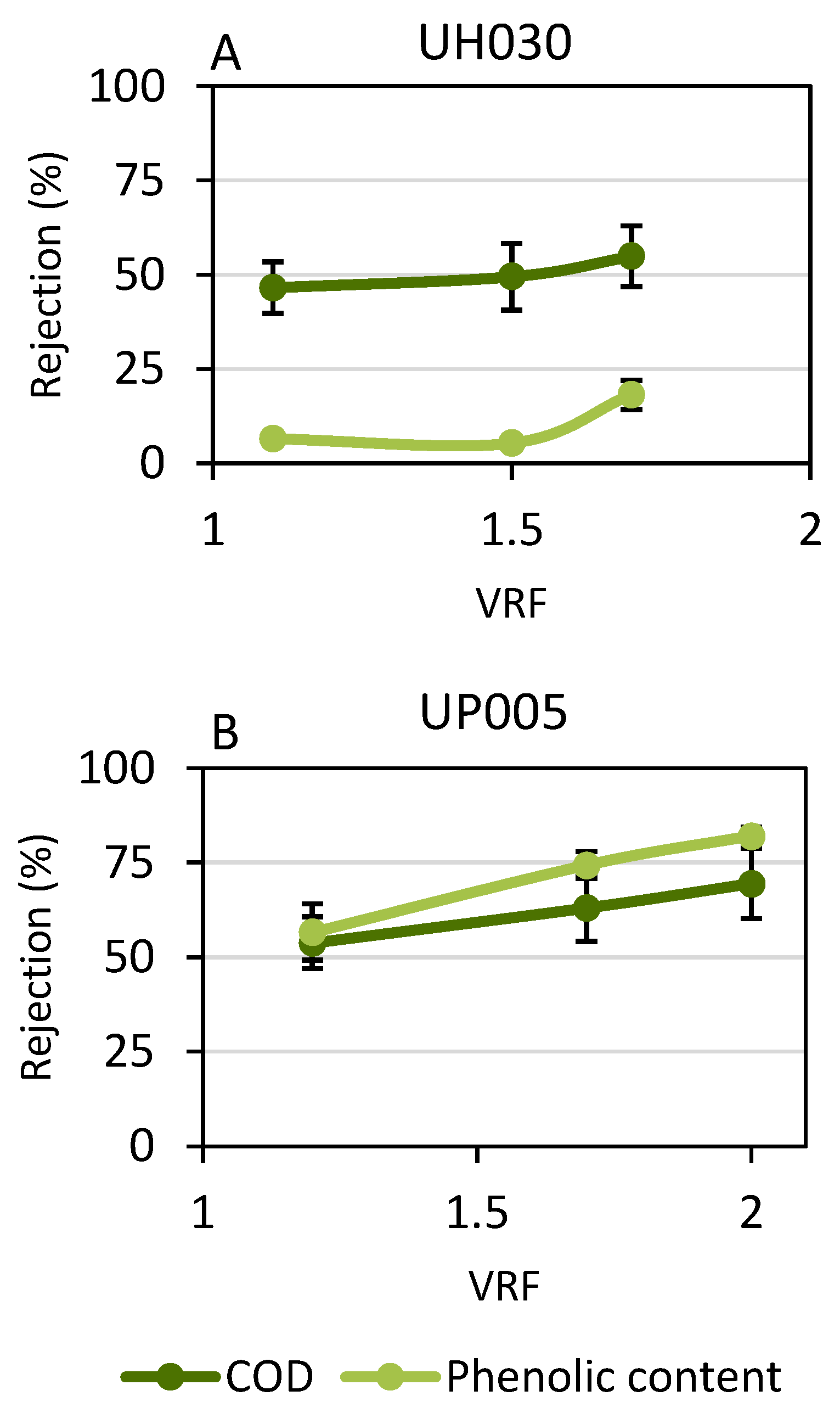
3.2. Performance of Ultrafiltration
3.2.1. Permeate Flux
3.2.2. Rejection Values
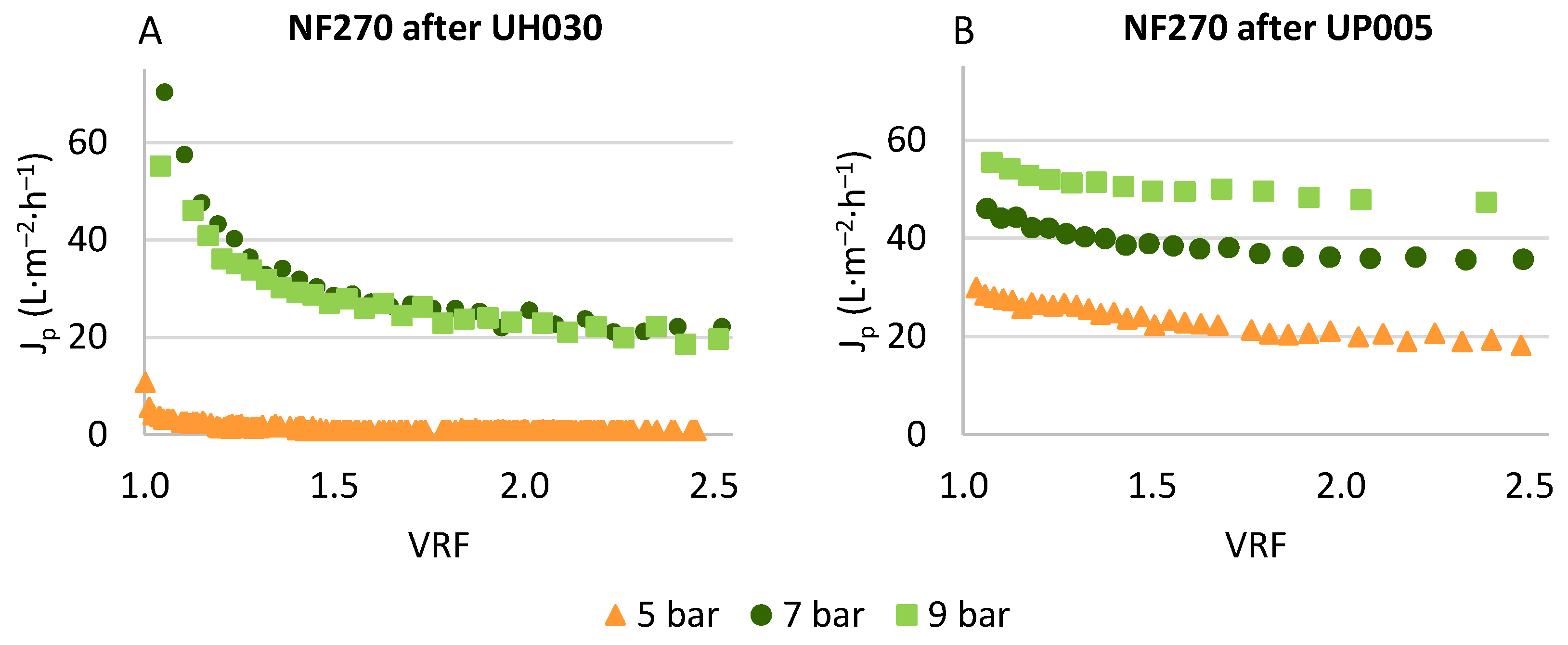
3.3. Concentration of Phenolic Compounds by Means of Nanofiltration
3.3.1. Permeate Flux in the Nanofiltration Step
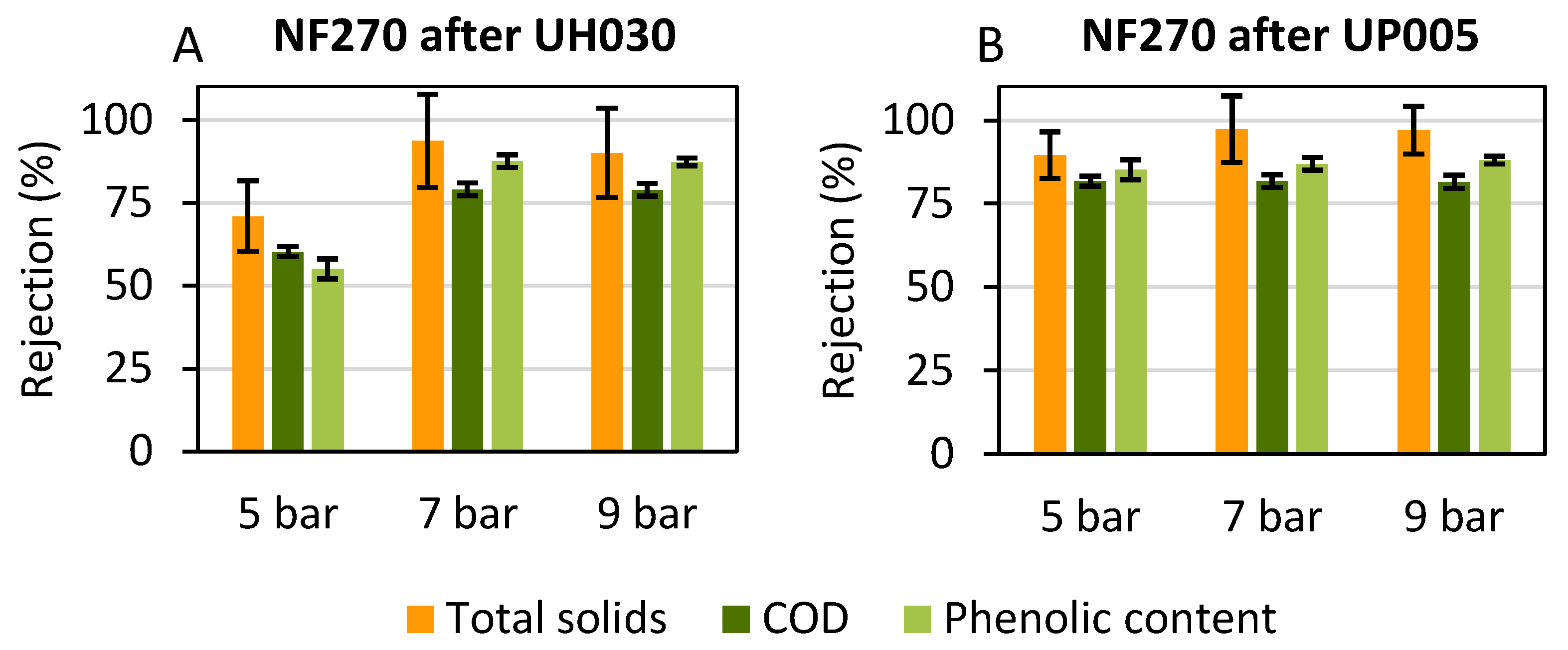
3.3.2. Rejection Values in Nanofiltration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sciubba, F.; Chronopoulou, L.; Pizzichini, D.; Lionetti, V.; Fontana, C.; Aromolo, R.; Socciarelli, S.; Gambelli, L.; Bartolacci, B.; Finotti, E.; et al. Olive Mill Wastes: A Source of Bioactive Molecules for Plant Growth and Protection against Pathogens. Biology 2020, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process. Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Donner, M.; Erraach, Y.; López-I-Gelats, F.; Manuel-I-Martin, J.; Yatribi, T.; Radić, I.; El Hadad-Gauthier, F. Circular bioeconomy for olive oil waste and by-product valorisation: Actors’ strategies and conditions in the Mediterranean area. J. Environ. Manag. 2022, 321, 115836. [Google Scholar] [CrossRef] [PubMed]
- Quero, J.; Ballesteros, L.F.; Ferreira-Santos, P.; Velderrain-Rodriguez, G.R.; Rocha, C.M.R.; Pereira, R.N.; Teixeira, J.A.; Martin-Belloso, O.; Osada, J.; Rodríguez-Yoldi, M.J. Unveiling the Antioxidant Therapeutic Functionality of Sustainable Olive Pomace Active Ingredients. Antioxidants 2022, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Min, J.-S.; Chae, U.; Lee, J.Y.; Song, K.-S.; Lee, H.-S.; Lee, H.J.; Lee, S.-R.; Lee, D.-S. Anti-inflammatory effect of oleuropein on microglia through regulation of Drp1-dependent mitochondrial fission. J. Neuroimmunol. 2017, 306, 46–52. [Google Scholar] [CrossRef]
- Malekjani, N.; Jafari, S.M. Valorization of olive processing by-products via drying technologies: A case study on the recovery of bioactive phenolic compounds from olive leaves, pomace, and wastewater. Crit. Rev. Food Sci. Nutr. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arévalo, C.M.; Iborra-Clar, A.; Vincent-Vela, M.C.; Álvarez-Blanco, S. Exploring the extraction of the bioactive content from the two-phase olive mill waste and further purification by ultrafiltration. LWT 2022, 165, 113742. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ibrahim, S.A.; Koca, I.; Galanakis, C.M. Green and highly extraction of phenolic compounds and antioxidant capacity from kinkeliba (Combretum micranthum G. Don) by natural deep eutectic solvents (NADESs) using maceration, ultrasound-assisted extraction and homogenate-assisted extraction. Arab. J. Chem. 2022, 15, 103752. [Google Scholar] [CrossRef]
- Pavez, I.C.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Ultra- and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and advanced membrane technology for wastewater treatment: A review. J. Basic Microbiol. 2022, 62, 245–259. [Google Scholar] [CrossRef]
- Sikdar, S.K.; Criscuoli, A. Sustainability and How Membrane Technologies in Water Treatment Can Be a Contributor. In Sustainable Membrane Technology for Water and Wastewater Treatment; Springer: Singapore, 2017; pp. 1–21. [Google Scholar] [CrossRef]
- Pekgenc, E.; Gul, B.Y.; Vatanpour, V.; Koyuncu, I. Biocatalytic membranes in anti-fouling and emerging pollutant degradation applications: Current state and perspectives. Sep. Purif. Technol. 2022, 282, 120098. [Google Scholar] [CrossRef]
- Pinto, P.R.; Mota, I.F.; Pereira, C.M.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Separation and recovery of polyphenols and carbohydrates from Eucalyptus bark extract by ultrafiltration/diafiltration and adsorption processes. Sep. Purif. Technol. 2017, 183, 96–105. [Google Scholar] [CrossRef]
- Cifuentes-Cabezas, M.; Carbonell-Alcaina, C.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Álvarez-Blanco, S. Comparison of different ultrafiltration membranes as first step for the recovery of phenolic compounds from olive-oil washing wastewater. Process. Saf. Environ. Prot. 2021, 149, 724–734. [Google Scholar] [CrossRef]
- Yammine, S.; Rabagliato, R.; Vitrac, X.; Peuchot, M.M.; Ghidossi, R. Selecting ultrafiltration membranes for fractionation of high added value compounds from grape pomace extracts. OENO One 2019, 53, 487–497. [Google Scholar] [CrossRef]
- Alfano, A.; Corsuto, L.; Finamore, R.; Savarese, M.; Ferrara, F.; Falco, S.; Santabarbara, G.; De Rosa, M.; Schiraldi, C. Valorization of Olive Mill Wastewater by Membrane Processes to Recover Natural Antioxidant Compounds for Cosmeceutical and Nutraceutical Applications or Functional Foods. Antioxidants 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.T.V.; Marson, G.V.; Barbero, G.F.; Tarone, A.G.; Cazarin, C.B.B.; Hubinger, M.D.; Martínez, J. Concentration of bioactive compounds from grape marc using pressurized liquid extraction followed by integrated membrane processes. Sep. Purif. Technol. 2020, 250, 117206. [Google Scholar] [CrossRef]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.; Reig, M.; Vecino, X.; Saurina, J.; Granados, M.; Cortina, J. Integration of membrane processes for the recovery and separation of polyphenols from winery and olive mill wastes using green solvent-based processing. J. Environ. Manag. 2022, 307, 114555. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pawlowski, S.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P.; Velizarov, S. Valorization of olive pomace by a green integrated approach applying sustainable extraction and membrane-assisted concentration. Sci. Total. Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef]
- Criado-Navarro, I.; Ledesma-Escobar, C.A.; Parrado-Martínez, M.J.; Marchal-López, R.M.; Olmo-Peinado, J.M.; Espejo-Calvo, J.A.; Priego-Capote, F. Monitoring the partition of bioactive compounds in the extraction of extra virgin olive oil. LWT 2022, 162, 113433. [Google Scholar] [CrossRef]
- Xia, L.; Vemuri, B.; Gadhamshetty, V.; Kilduff, J. Poly (ether sulfone) membrane surface modification using norepinephrine to mitigate fouling. J. Membr. Sci. 2020, 598, 117657. [Google Scholar] [CrossRef]
- Xia, L.; Hao, Z.; Vemuri, B.; Zhao, S.; Gadhamshetty, V.; Kilduff, J.E. Improving antifouling properties of poly (ether sulfone) UF membranes with hydrophilic coatings of dopamine and poly(2-dimethylamino) ethyl methacrylate salt to enable water reuse. Sep. Purif. Technol. 2022, 285, 120300. [Google Scholar] [CrossRef]
- Mosikatsi, B.E.; Mabuba, N.; Malinga, S.P. Thin film composite membranes consisting of hyperbranched polyethylenimine (HPEI)-cysteamine layer for cadmium removal in water. J. Water Process. Eng. 2019, 30, 100686. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Nyström, M. Enrichment of α-lactalbumin from diluted whey with polymeric ultrafiltration membranes. J. Membr. Sci. 2009, 337, 248–256. [Google Scholar] [CrossRef]
- Luján-Facundo, M.J.; Mendoza-Roca, J.A.; Cuartas-Uribe, B.; Álvarez-Blanco, S. Evaluation of cleaning efficiency of ultrafiltration membranes fouled by BSA using FTIR–ATR as a tool. J. Food Eng. 2015, 163, 1–8. [Google Scholar] [CrossRef]
- Vieira, G.S.; Moreira, F.K.; Matsumoto, R.L.; Michelon, M.; Filho, F.M.; Hubinger, M.D. Influence of nanofiltration membrane features on enrichment of jussara ethanolic extract (Euterpe edulis) in anthocyanins. J. Food Eng. 2018, 226, 31–41. [Google Scholar] [CrossRef]
- Luján-Facundo, M.; Mendoza-Roca, J.-A.; Cuartas-Uribe, B.; Alvarez-Blanco, S. Ultrasonic cleaning of ultrafiltration membranes fouled with BSA solution. Sep. Purif. Technol. 2013, 120, 275–281. [Google Scholar] [CrossRef]
- Carbonell-Alcaina, C.; Soler-Cabezas, J.L.; Bes-Piá, A.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Pastor-Alcañiz, L.; Álvarez-Blanco, S. Integrated Membrane Process for the Treatment and Reuse of Residual Table Olive Fermentation Brine and Anaerobically Digested Sludge Centrate. Membranes 2020, 10, 253. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics Acids with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Carbonell-Alcaina, C.; Álvarez-Blanco, S.; Bes-Piá, M.A.; Mendoza-Roca, J.A.; Pastor-Alcañiz, L. Ultrafiltration of residual fermentation brines from the production of table olives at different operating conditions. J. Clean. Prod. 2018, 189, 662–672. [Google Scholar] [CrossRef]
- Baptista, E.A.; Pinto, P.C.; Mota, I.F.; Loureiro, J.M.; Rodrigues, A.E. Ultrafiltration of ethanol/water extract of Eucalyptus globulus bark: Resistance and cake build up analysis. Sep. Purif. Technol. 2015, 144, 256–266. [Google Scholar] [CrossRef]
- Contreras-Jácquez, V.; Valenzuela-Vázquez, U.; Grajales-Hernández, D.A.; Mateos-Díaz, J.C.; Arrellano-Plaza, M.; Jara-Marini, M.E.; Asaff-Torres, A. Pilot-Scale Integrated Membrane System for the Separation and Concentration of Compounds of Industrial Interest from Tortilla Industry Wastewater (Nejayote). Waste Biomass Valorization 2022, 13, 345–360. [Google Scholar] [CrossRef]
- Cassano, A.; Donato, L.; Conidi, C.; Drioli, E. Recovery of bioactive compounds in kiwifruit juice by ultrafiltration. Innov. Food Sci. Emerg. Technol. 2008, 9, 556–562. [Google Scholar] [CrossRef]
- Corbatón-Báguena, M.-J.; Álvarez-Blanco, S.; Vincent-Vela, M.-C. Cleaning of ultrafiltration membranes fouled with BSA by means of saline solutions. Sep. Purif. Technol. 2014, 125, 1–10. [Google Scholar] [CrossRef]
- Qu, F.; Liang, H.; Zhou, J.; Nan, J.; Shao, S.; Zhang, J.; Li, G. Ultrafiltration membrane fouling caused by extracellular organic matter (EOM) from Microcystis aeruginosa: Effects of membrane pore size and surface hydrophobicity. J. Membr. Sci. 2014, 449, 58–66. [Google Scholar] [CrossRef]
- Hou, D.; Dai, G.; Fan, H.; Wang, J.; Zhao, C.; Huang, H. Effects of calcium carbonate nano-particles on the properties of PVDF/nonwoven fabric flat-sheet composite membranes for direct contact membrane distillation. Desalination 2014, 347, 25–33. [Google Scholar] [CrossRef]
- Li, Q.; Pan, X.; Qu, Z.; Zhao, X.; Jin, Y.; Dai, H.; Yang, B.; Wang, X. Understanding the dependence of contact angles of commercially RO membranes on external conditions and surface features. Desalination 2013, 309, 38–45. [Google Scholar] [CrossRef]
- Panda, S.R.; Bhandaru, N.; Mukherjee, R.; De, S. Ultrafiltration of oily waste water: Contribution of surface roughness in membrane properties and fouling characteristics of polyacrylonitrile membranes. Can. J. Chem. Eng. 2015, 93, 2031–2042. [Google Scholar] [CrossRef]
- Hobbs, C.; Taylor, J.; Hong, S. Effect of surface roughness on fouling of RO and NF membranes during filtration of a high organic surficial groundwater. J. Water Supply Res. Technol. 2006, 55, 559–570. [Google Scholar] [CrossRef]
- Cai, M.; Lv, Y.; Luo, S.; Liu, Y.; Sun, P. Fouling Behavior of Polyphenols during Model Juice Ultrafiltration: Effect of Membrane Properties. Food Bioprocess Technol. 2018, 11, 1787–1793. [Google Scholar] [CrossRef]
- Li, D.; Yang, X.; Zhou, Z.; Jiang, B.; Tawfik, A.; Zhao, S.; Meng, F. Molecular traits of phenolic moieties in dissolved organic matter: Linkages with membrane fouling development. Environ. Int. 2019, 133, 105202. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Conidi, C.; Drioli, E. Comparison of the performance of UF membranes in olive mill wastewaters treatment. Water Res. 2011, 45, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Madureira, J.; Margaça, F.M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Verde, S.C.; Barros, L. Applications of bioactive compounds extracted from olive industry wastes: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 453–476. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Zhang, J.; Tokuda, H. Potentially Chemopreventive Triterpenoids and Other Secondary Metabolites from Plants and Fungi. Stud. Nat. Prod. Chem. 2016, 51, 1–50. [Google Scholar]
- Tan, X.; Zhu, J.; Wakisaka, M. Effect of phytochemical vanillic acid on the growth and lipid accumulation of freshwater microalga Euglena gracilis. World J. Microbiol. Biotechnol. 2021, 37, 217. [Google Scholar] [CrossRef]
- Medina, E.; Brenes, M.; Romero, C.; García, A.; de Castro, A. Main Antimicrobial Compounds in Table Olives. J. Agric. Food Chem. 2007, 55, 9817–9823. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; Brenes, M.; García, P.; de Castro, A.; García, A. Profile of anti-lactic acid bacteria compounds during the storage of olives which are not treated with alkali. Eur. Food Res. Technol. 2008, 228, 133–138. [Google Scholar] [CrossRef]
- Zaidi, S.K.; Kumar, A. Effects of ethanol concentration on flux and gel formation in dead end ultrafiltration of PEG and dextran. J. Membr. Sci. 2004, 237, 189–197. [Google Scholar] [CrossRef]
- Tu, S.-C.; Ravindran, V.; Den, W.; Pirbazari, M. Predictive membrane transport model for nanofiltration processes in water treatment. AIChE J. 2001, 47, 1346–1362. [Google Scholar] [CrossRef]
- Avram, A.M.; Morin, P.; Brownmiller, C.; Howard, L.R.; Sengupta, A.; Wickramasinghe, S.R. Concentrations of polyphenols from blueberry pomace extract using nanofiltration. Food Bioprod. Process. 2017, 106, 91–101. [Google Scholar] [CrossRef]
- Erragued, R.; Braga, M.E.; Bouaziz, M.; Gando-Ferreira, L.M. Integration of solvent extraction and membrane processes to produce an oleuropein extract from olive leaves. Sep. Purif. Technol. 2022, 299, 121751. [Google Scholar] [CrossRef]
- Semião, A.J.; Foucher, M.; Schäfer, A.I. Removal of adsorbing estrogenic micropollutants by nanofiltration membranes: Part B—Modeldevelopment. J. Membr. Sci. 2013, 431, 257–266. [Google Scholar] [CrossRef]
- Imbrogno, A.; Schäfer, A.I. Micropollutants breakthrough curve phenomena in nanofiltration: Impact of operational parameters. Sep. Purif. Technol. 2021, 267, 118406. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Fan, Z.; Yang, X.; Wang, J.; Wang, S. Experimental study on treatment of electroplating wastewater by nanofiltration. J. Membr. Sci. 2007, 305, 185–195. [Google Scholar] [CrossRef]

| Process | Working Mode | Permeate Flux (L·h−1·m−2) | Polyphenols Concentration | Reference |
|---|---|---|---|---|
| UF 1-NF 2-RO 3 | Concentration | n.d. 4 | 200 mg GAE 5/L | [20] |
| UF-NF-RO | Concentration | n.d. | 32.9 mg/L flavonoids | [21] |
| NF | Concentration | 15 (20 bar) | 1234.3 ± 54.0 mg GAE/L | [22] |
| UF-NF | Concentration | UF: 18 (2.5 bar); NF: 47 (9 bar) | 882 mg TY 6/L | This work |
| Parameter | UH030 | UP005 | NF270 |
|---|---|---|---|
| MWCO (kDa) 1 | 30 | 5 | 0.3–0.4 |
| Material | HPES 2 | PES 3 | Polyamide |
| Contact angle | 56 ± 3° [27] | 54.27 ± 3.48° [28] | 15.9 ± 1.3° [29] |
| Manufacturer | Mycrodin Nadir | Mycrodin Nadir | DuPont |
| Process | UF | UF | NF |
| Parameter | Concentration |
|---|---|
| COD 1 (mg/L) | 8290 ± 548 |
| Total solids (g/L) | 9.05 ± 0.05 |
| Total phenolic content (mg tyrosol/kg) | 3970 ± 80 |
| pH | 5.4 ± 0.1 |
| Conductivity (µS/cm) | 1642 ± 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Arévalo, C.M.; Pérez García-Serrano, A.; Vincent-Vela, M.C.; Álvarez-Blanco, S. Combining Ultrafiltration and Nanofiltration to Obtain a Concentrated Extract of Purified Polyphenols from Wet Olive Pomace. Membranes 2023, 13, 119. https://doi.org/10.3390/membranes13020119
Sánchez-Arévalo CM, Pérez García-Serrano A, Vincent-Vela MC, Álvarez-Blanco S. Combining Ultrafiltration and Nanofiltration to Obtain a Concentrated Extract of Purified Polyphenols from Wet Olive Pomace. Membranes. 2023; 13(2):119. https://doi.org/10.3390/membranes13020119
Chicago/Turabian StyleSánchez-Arévalo, Carmen M., Ane Pérez García-Serrano, María Cinta Vincent-Vela, and Silvia Álvarez-Blanco. 2023. "Combining Ultrafiltration and Nanofiltration to Obtain a Concentrated Extract of Purified Polyphenols from Wet Olive Pomace" Membranes 13, no. 2: 119. https://doi.org/10.3390/membranes13020119
APA StyleSánchez-Arévalo, C. M., Pérez García-Serrano, A., Vincent-Vela, M. C., & Álvarez-Blanco, S. (2023). Combining Ultrafiltration and Nanofiltration to Obtain a Concentrated Extract of Purified Polyphenols from Wet Olive Pomace. Membranes, 13(2), 119. https://doi.org/10.3390/membranes13020119











