Adsorptive Membranes Incorporating Ionic Liquids (ILs), Deep Eutectic Solvents (DESs) or Graphene Oxide (GO) for Metal Salts Extraction from Aqueous Feed
Abstract
1. Introduction
2. Emerging Materials Utilized in Salts Extraction
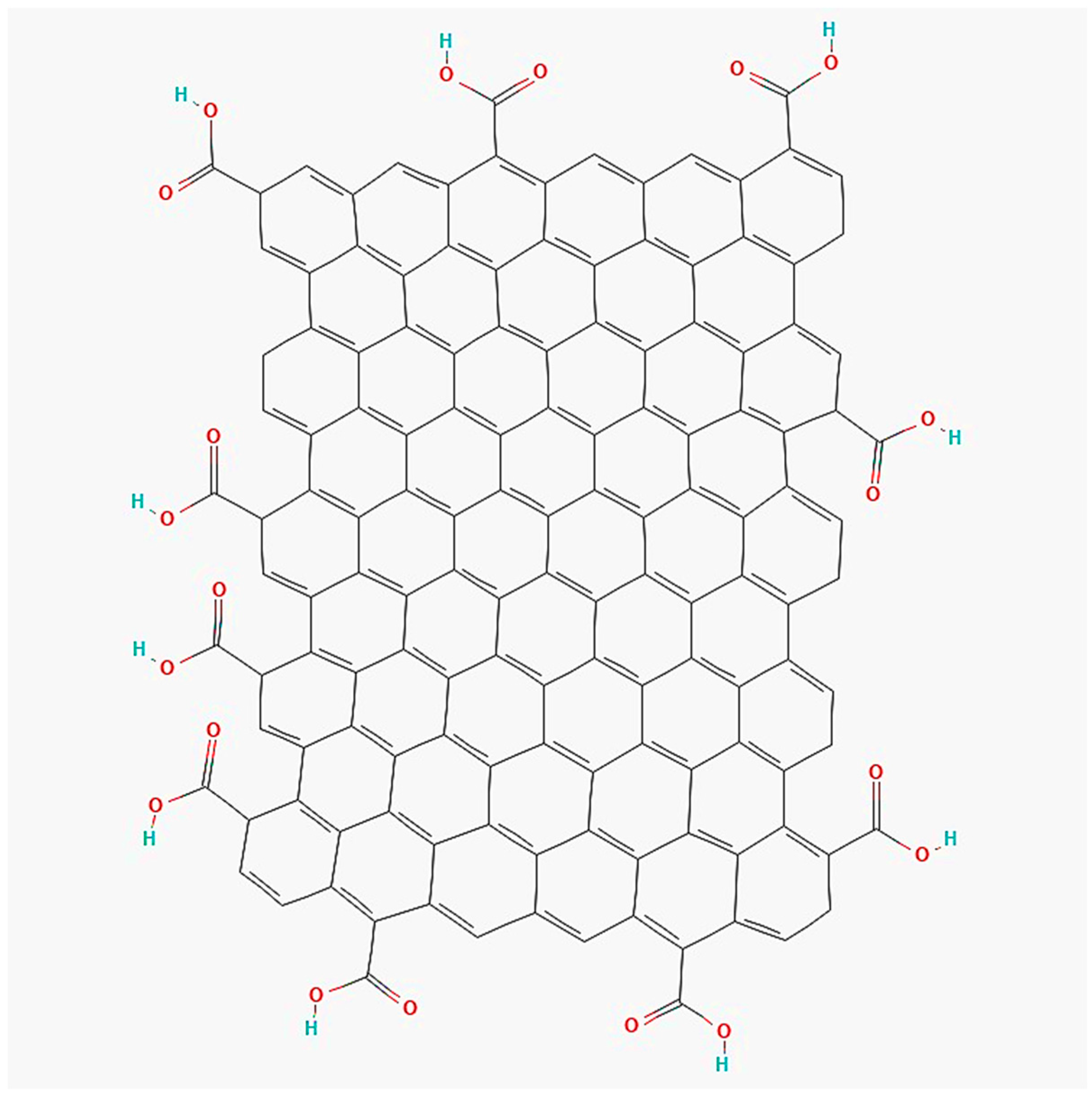
2.1. Graphene Oxide (GO)
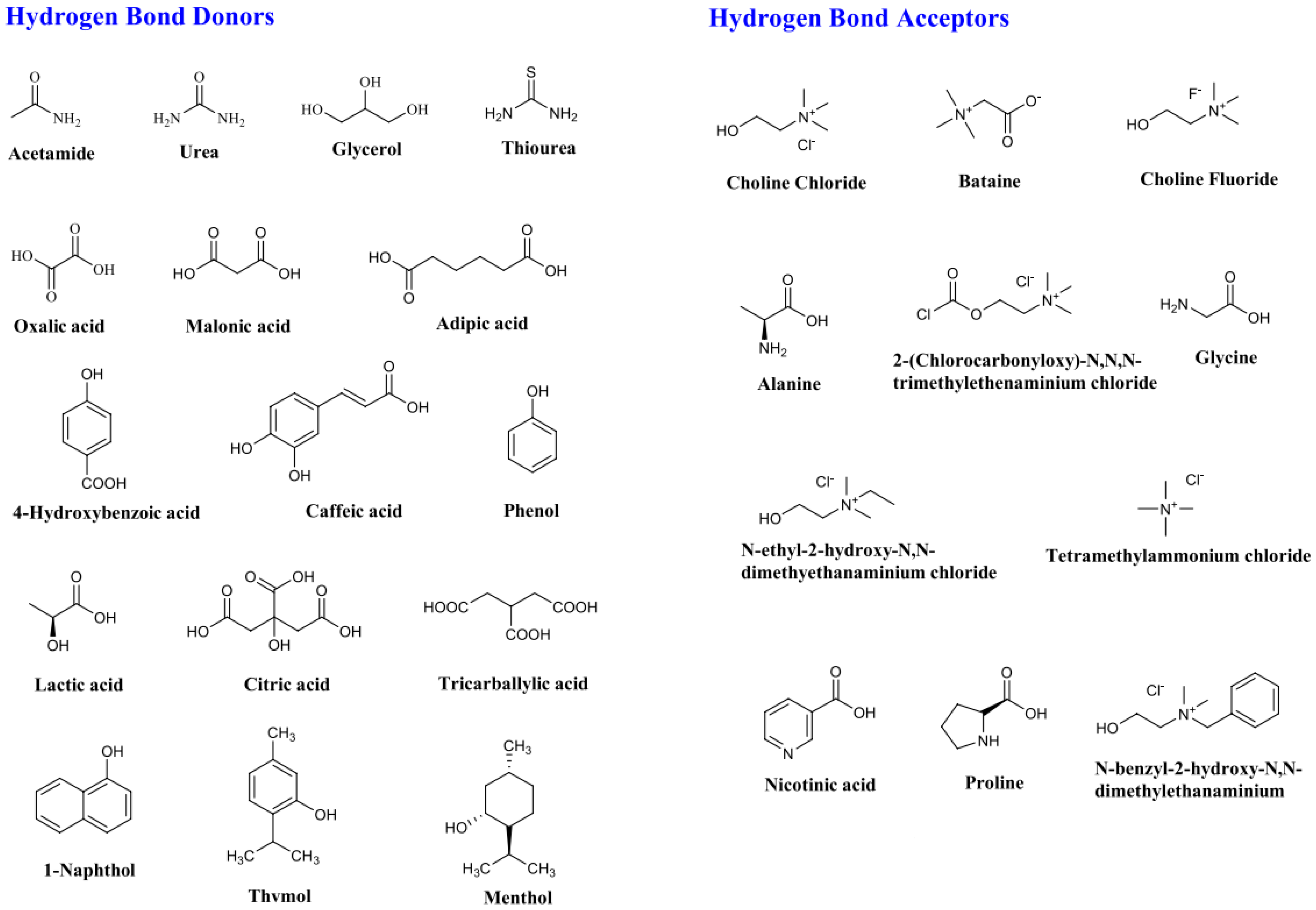
2.2. Ionic Liquids (ILs)
2.3. Deep Eutectic Solvents (DESs)
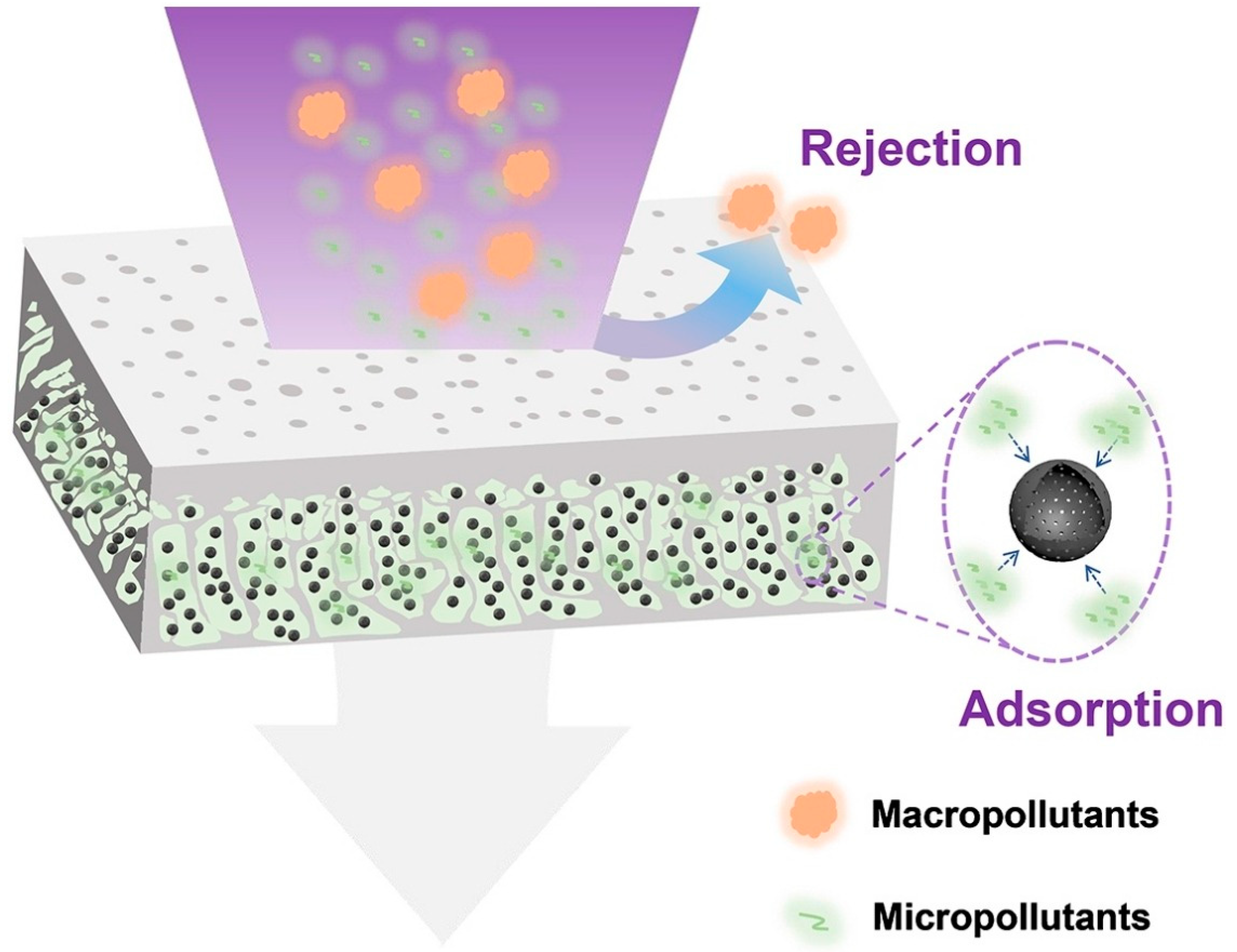
3. Adsorptive Membranes Technology with Emerging Materials
3.1. Incorporation of Graphene Oxide in Membranes
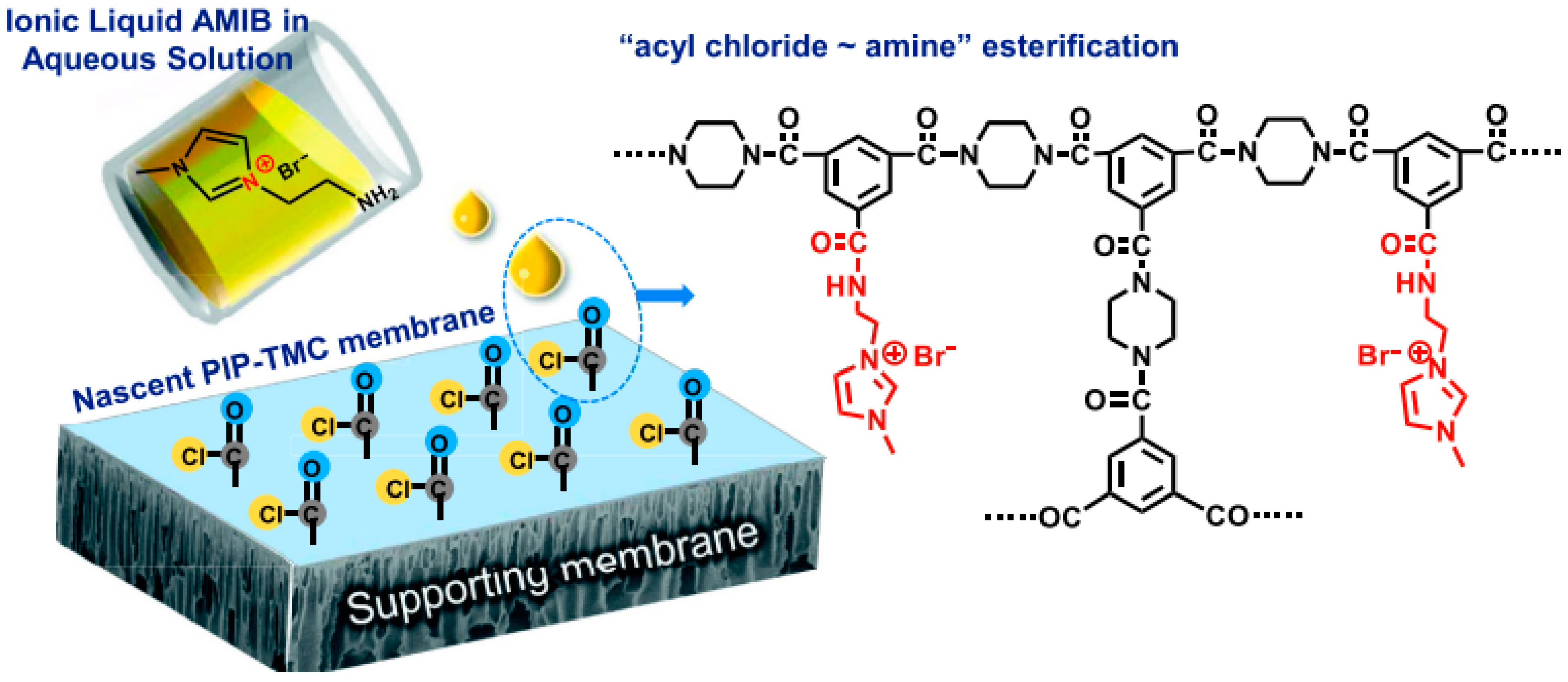
3.2. Incorporation of Ionic Liquids in Membranes
3.3. Incorporation of Deep Eutectic Solvents in Membranes
3.4. Hybrid Membranes
4. Challenges and Future Outlooks
- Understanding the role and mechanisms of these materials and their interactions with membranes: Further research is needed to enhance membrane separation performance by investigating possible downsides such as mechanical instability, durability, nonideal alignment and assembly, surface defects, toxicity, high-pressure resistance, leaching, and deterioration [112].
- Scaling up the fabrication process for commercial production: The ultrathin permeable membranes studied in laboratory environments need to be scaled up for industrial applications. Real-life experiments are necessary to examine different aspects of industrial-scale applications, particularly in synthesizing MMMs and nanocomposite membranes in configurations such as hollow fiber membranes, which provide greater packing density than flat-sheet membranes [146].
- Understanding material–water interactions and conducting toxicity analyses: Some fillers used in these membranes may possess toxic properties, and their application in water purification must be carefully evaluated. ILs and DESs, being diverse classes of materials with various combinations, pose challenges in fully understanding their interactions in water applications.
- Developing predictive models for adsorptive membranes: In-depth research is required to identify the morphology and distinct properties of these membranes. Predictive models need to be developed to anticipate membrane performance, including kinetics and selectivity [147].
- Addressing membrane fouling issues: Increasing the hydrophilicity of membranes can sometimes lead to membrane overload. Although functionalizing polymeric membranes with nanomaterials can reduce biofouling [148], excessive particle loading can result in the formation of macropores and agglomeration, which negatively impact ultrafiltration performance due to particle aggregation [149]. Therefore, careful consideration of the compatibility between the chosen adsorbent and membrane, as well as conducting fouling studies, is essential to minimize fouling.
- Selecting suitable DESs for optimal desalination performance: Since limited research has been conducted on utilizing DESs for membrane modification in desalination, the wide range of possibilities can make it challenging to choose the most appropriate material. Careful evaluation is necessary to determine the DESs that can achieve the best desalination performance.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chong, W.C.; Choo, Y.L.; Koo, C.H.; Pang, Y.L.; Lai, S.O. Adsorptive membranes for heavy metal removal—A mini review. AIP Conf. Proc. 2019, 2157, 020005. [Google Scholar] [CrossRef]
- Kummu, M.; Guillaume, J.H.A.; de Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The world’s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef] [PubMed]
- Esteves, R.J.A.; Gornick, V.; Alqurwani, D.S.; Koenig-Lovejoy, J.; Abdelrazeq, H.; Khraisheh, M.; Forzano, A.V.; Gad-El-Hak, M.; Tafreshi, H.V.; McLeskey, J.T. Activated carbon-doped polystyrene fibers for direct contact membrane desalination. Emergent Mater. 2020, 3, 807–814. [Google Scholar] [CrossRef]
- Caldera, U.; Bogdanov, D.; Breyer, C. Local cost of seawater RO desalination based on solar PV and wind energy: A global estimate. Desalination 2016, 385, 207–216. [Google Scholar] [CrossRef]
- Liu, C.; Jia, J.; Liu, J.; Liang, X. Hg selective adsorption on polypropylene-based hollow fiber grafted with polyacrylamide. Adsorpt. Sci. Technol. 2018, 36, 287–299. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Seubert, E.L.; Caron, D.A. Impact of Algal Blooms and Their Toxins on Reverse Osmosis Desalination Plant Operations. In Sustainable Desalination Handbook: Plant Selection, Design and Implementation; Butterworth-Heinemann: Oxford, UK, 2018; pp. 489–504. [Google Scholar] [CrossRef]
- Youssef, P.; Al-Dadah, R.; Mahmoud, S. Comparative Analysis of Desalination Technologies. Energy Procedia 2014, 61, 2604–2607. [Google Scholar] [CrossRef]
- Suwaileh, W.; Johnson, D.; Hilal, N. Membrane desalination and water re-use for agriculture: State of the art and future outlook. Desalination 2020, 491, 114559. [Google Scholar] [CrossRef]
- Sheldon, M.; Jingxi, E.Z.; De Jager, D.; Augustine, R.; Korenak, J.; Helix-Nielsen, C.; Petrinic, I. Potential of dyes as draw solutions in forward osmosis for the South African textile industry. Water SA 2018, 44, 258–268. [Google Scholar] [CrossRef]
- Kress, N. Desalination Technologies. In Marine Impacts of Seawater Desalination; Kress, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 11–34. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; McGovern, R.K.; Dave, S.H.; Lienhard, J.H.; Grossman, J.C. Quantifying the potential of ultra-permeable membranes for water desalination. Energy Environ. Sci. 2014, 7, 1134–1141. [Google Scholar] [CrossRef]
- Jacob, J.; Penkova, A.V.; Thomas, S. Special issue: Emerging materials in nanofiltration membranes. Emergent Mater. 2022, 5, 1261–1262. [Google Scholar] [CrossRef]
- Diawara, C.K. Nanofiltration Process Efficiency in Water Desalination. Sep. Purif. Rev. 2008, 37, 302–324. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Nanofiltration. In Encyclopedia of Membrane Science and Technology; Wiley: Hoboken, NJ, USA, 2013; pp. 1–23. [Google Scholar] [CrossRef]
- Yadav, D.; Hazarika, S.; Ingole, P.G. Recent development in nanofiltration (NF) membranes and their diversified applications. Emergent Mater. 2021, 5, 1311–1328. [Google Scholar] [CrossRef]
- Haddad, B.; Heck, N.; Paytan, A.; Potts, D. Social Issues and Public Acceptance of Seawater Desalination Plants. In Sustainable Desalination Handbook: Plant Selection, Design and Implementation; Butterworth-Heinemann: Oxford, UK, 2018; pp. 505–525. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Jia, C.; Wang, Y.; Zhai, L.; Wang, Q.; Zhao, D. Ultrathin mixed matrix membranes containing two-dimensional metal-organic framework nanosheets for efficient CO2/CH4 separation. J. Membr. Sci. 2017, 539, 213–223. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Z.; Liu, Y.; Li, R.; Xu, Y.; Jakaj, G.; Lin, H. Polymeric Membranes Incorporated with ZnO Nanoparticles for Membrane Fouling Mitigation: A Brief Review. Front. Chem. 2020, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Cheng, G.; Lu, T.; Cao, Z.; Wang, L.; Li, Q.; Fan, J. An ionic liquid functionalized polymer for simultaneous removal of four phenolic pollutants in real environmental samples. J. Hazard. Mater. 2019, 373, 347–358. [Google Scholar] [CrossRef]
- Arriaga, S.; Aizpuru, A. Novel NAPL for aqueous two-phase partitioning bioreactor. In Advances in Chemical Engineering; Academic Press: Cambridge, MA, USA, 2019; Volume 54, pp. 299–348. [Google Scholar] [CrossRef]
- Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F. Interfacing Graphene-Based Materials with Neural Cells. Front. Syst. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Lai, W.-F.; Wong, W.-T. Use of graphene-based materials as carriers of bioactive agents. Asian J. Pharm. Sci. 2021, 16, 577–588. [Google Scholar] [CrossRef]
- Raidongia, K.; Tan, A.T.; Huang, J. Graphene Oxide: Some New Insights into an Old Material. In Carbon Nanotubes and Graphene: Edition 2; Elsevier: Amsterdam, The Netherlands, 2014; pp. 341–374. [Google Scholar] [CrossRef]
- Hussain, M.H.; Abu Bakar, N.F.; Chung, M.; Khalid, N.F.N.; Othman, N.H.; Tan, H.L.; Osman, M.S.; Lockman, Z.; Radacsi, N. Electrospun polyetherimide nanofibers with reduced graphene oxide-zeolitic imidazolate framework for conductivity improvement. Emergent Mater. 2022, 6, 271–281. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Chang, C.-T. Preparation and Characterization of Graphene Oxide. J. Nanomater. 2014, 2014, 276143. [Google Scholar] [CrossRef]
- Ray, S.C. Application and Uses of Graphene Oxide and Reduced Graphene Oxide. In Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew Publishing: Amsterdam, The Netherlands, 2015; pp. 39–55. [Google Scholar] [CrossRef]
- Graphene Oxide, Powder, 15–20 Sheets, 4–10% Edge-Oxidized|C140H42O20—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/124202900#section=2D-Structure (accessed on 29 July 2022).
- Schlesinger, O.; Alfonta, L. Encapsulation of Microorganisms, Enzymes, and Redox Mediators in Graphene Oxide and Reduced Graphene Oxide. Methods Enzymol. 2018, 609, 197–219. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.C.; Kim, S.-H.; Shon, H.K.; Tijing, L.D. Introduction: Membrane Desalination Today, Past, and Future. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Curcio, E., Inamuddin, Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. xxv–xlvi. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.; Bang, J.; Lee, J.-H. Layer-by-Layer Assembly of Graphene Oxide Nanosheets on Polyamide Membranes for Durable Reverse-Osmosis Applications. ACS Appl. Mater. Interfaces 2013, 5, 12510–12519. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, Y.; Gao, C. High-Flux Graphene Oxide Nanofiltration Membrane Intercalated by Carbon Nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Mi, B. Layer-by-layer assembly of graphene oxide membranes via electrostatic interaction. J. Membr. Sci. 2014, 469, 80–87. [Google Scholar] [CrossRef]
- Chu, K.H.; Fathizadeh, M.; Yu, M.; Flora, J.R.V.; Jang, A.; Jang, M.; Park, C.M.-C.; Yoo, S.S.; Her, N.G.; Yoon, Y. Evaluation of Removal Mechanisms in a Graphene Oxide-Coated Ceramic Ultrafiltration Membrane for Retention of Natural Organic Matter, Pharmaceuticals, and Inorganic Salts. ACS Appl. Mater. Interfaces 2017, 9, 40369–40377. [Google Scholar] [CrossRef]
- Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 1979 2014, 343, 740–742. [Google Scholar] [CrossRef]
- Staff Writer. Graphene Oxide to ‘Sieve’ Salt from Water. Available online: https://www.thechemicalengineer.com/news/graphene-oxide-to-sieve-salt-from-water/ (accessed on 29 April 2022).
- Silva, W.; Zanatta, M.; Ferreira, A.S.; Corvo, M.C.; Cabrita, E.J. Revisiting Ionic Liquid Structure-Property Relationship: A Critical Analysis. Int. J. Mol. Sci. 2020, 21, 7745. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Ratti, R. Ionic Liquids: Synthesis and Applications in Catalysis. Adv. Chem. 2014, 2014, 729842. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.P.; Mezzetta, A.; Marrucho, I.M.; Guazzelli, L. History repeats itself again: Will the mistakes of the past for ILs be repeated for DESs? From being considered ionic liquids to becoming their alternative: The unbalanced turn of deep eutectic solvents. Green Chem. 2023, 25, 59–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Han, B. Catalytic Transformation of Lignocellulose into Chemicals and Fuel Products in Ionic Liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Phenolic Acids from Plants: Extraction and Application to Human Health. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 389–417. [Google Scholar] [CrossRef]
- Chiappe, C.; Douton, M.J.R.; Mezzetta, A.; Guazzelli, L.; Pomelli, C.S.; Assanelli, G.; de Angelis, A.R. Exploring and exploiting different catalytic systems for the direct conversion of cellulose into levulinic acid. New J. Chem. 2018, 42, 1845–1852. [Google Scholar] [CrossRef]
- Mezzetta, A.; Guazzelli, L.; Chiappe, C. Access to cross-linked chitosans by exploiting CO2 and the double solvent-catalytic effect of ionic liquids. Green Chem. 2017, 19, 1235–1239. [Google Scholar] [CrossRef]
- Endres, F.; El Abedin, S.Z. Air and water stable ionic liquids in physical chemistry. Phys. Chem. Chem. Phys. 2006, 8, 2101–2116. [Google Scholar] [CrossRef]
- Wei, L.; Wang, L.; Cui, Z.; Liu, Y.; Du, A. Multifunctional Applications of Ionic Liquids in Polymer Materials: A Brief Review. Molecules 2023, 28, 3836. [Google Scholar] [CrossRef]
- Bakshi, K.; Mitra, S.; Sharma, V.K.; Jayadev, M.S.K.; Sakai, V.G.; Mukhopadhyay, R.; Gupta, A.; Ghosh, S.K. Imidazolium-based ionic liquids cause mammalian cell death due to modulated structures and dynamics of cellular membrane. Biochim. Biophys. Acta BBA-Biomembr. 2020, 1862, 183103. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Blanchard, G.J. Ionic Liquids Exhibit the Piezoelectric Effect. J. Phys. Chem. Lett. 2023, 14, 2731–2735. [Google Scholar] [CrossRef] [PubMed]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tucker, Z.D.; Wang, Y.; Ashfeld, B.L.; Luo, T. Ionic liquid enables highly efficient low temperature desalination by directional solvent extraction. Nat. Commun. 2021, 12, 437. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef]
- Sun, X.; Luo, H.; Dai, S. Ionic Liquids-Based Extraction: A Promising Strategy for the Advanced Nuclear Fuel Cycle. Chem. Rev. 2012, 112, 2100–2128. [Google Scholar] [CrossRef]
- Veríssimo, N.V.; Vicente, F.A.; de Oliveira, R.C.; Likozar, B.; Oliveira, R.P.d.S.; Pereira, J.F.B. Ionic liquids as protein stabilizers for biological and biomedical applications: A review. Biotechnol. Adv. 2022, 61, 108055. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mero, A.; Mezzetta, A.; Tofani, G.; D’Andrea, F.; Pomelli, C.; Guazzelli, L. Novel access to ionic liquids based on trivalent metal–EDTA complexes and their thermal and electrochemical characterization. J. Mol. Liq. 2021, 340, 117210. [Google Scholar] [CrossRef]
- Bara, J.E.; Carlisle, T.K.; Gabriel, C.J.; Camper, D.; Finotello, A.; Gin, D.L.; Noble, R.D. Guide to CO2 Separations in Imidazolium-Based Room-Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2009, 48, 2739–2751. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J., Jr.; Rogers, R.D. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem. Commun. 2001, 1, 135–136. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Bagh, F.S.G.; Mjalli, F.S.; Hashim, M.A.; Hadj-Kali, M.K.O.; AlNashef, I.M. Solubility of Sodium Chloride in Ionic Liquids. Ind. Eng. Chem. Res. 2013, 52, 11488–11493. [Google Scholar] [CrossRef]
- Abu Khalifeh, H.; AlNashef, I.; Nazir, A.; Al Mansoori, N.; Yousef, I.; Sharhahabiel, A. Investigating the Potential Use of Ionic Liquids in Pre-Treatment Application for Water Desalination. MATEC Web Conf. 2018, 187, 01003. [Google Scholar] [CrossRef][Green Version]
- Parmentier, D. Selective Recovery of Metal Salts from Aqueous Streams Using Ionic Liquids; Eindhoven University of Technology: Eindhoven, The Netherlands, 2015. [Google Scholar]
- Chen, Q.; Ge, Q.; Xu, W.; Pan, W. Functionalized imidazolium ionic liquids promote seawater desalination through forward osmosis. J. Membr. Sci. 2019, 574, 10–16. [Google Scholar] [CrossRef]
- Cho, C.-W.; Pham, T.P.T.; Zhao, Y.; Stolte, S.; Yun, Y.-S. Review of the toxic effects of ionic liquids. Sci. Total Environ. 2021, 786, 147309. [Google Scholar] [CrossRef]
- Kohli, R. Removal of Surface Contaminants Using Ionic Liquids. In Developments in Surface Contamination and Cleaning; William Andrew Publishing: Amsterdam, The Netherlands, 2013; Volume 6, pp. 1–63. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.-K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef]
- Erdocia, X.; Hernández-Ramos, F.; Morales, A.; Izaguirre, N.; de Hoyos-Martínez, P.L.; Labidi, J. Lignin extraction and isolation methods. In Lignin-Based Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 61–104. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Martínez, G.M.; Townley, G.G.; Martínez-Espinosa, R.M. Controversy on the toxic nature of deep eutectic solvents and their potential contribution to environmental pollution. Heliyon 2022, 8, e12567. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Kohli, R. Applications of Ionic Liquids in Removal of Surface Contaminants. In Developments in Surface Contamination and Cleaning: Applications of Cleaning Techniques; Elsevier: Amsterdam, The Netherlands, 2019; Volume 11, pp. 619–680. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, P.; Li, Q.; Xia, H. Recent Advances in the Catalytic Conversion of Biomass to Furfural in Deep Eutectic Solvents. Front. Chem. 2022, 10, 911674. [Google Scholar] [CrossRef] [PubMed]
- Sunol, A.K.; Sunol, S.G.; Cogswell, K. Substitution of Solvents by Safer Products. In Handbook of Solvents; ChemTec Publishing: Scarborough, ON, Canada, 2019; pp. 1455–1634. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Q.; Sun, X.; Wu, H.; Hao, J.; Wei, L.; Zhai, S.; Xiao, Z.; An, Q. Dual-active-sites deep eutectic solvents based on imidazole and resorcinol for efficient capture of NH3. Chem. Eng. J. 2021, 416, 129114. [Google Scholar] [CrossRef]
- Abbott, A.P. Deep eutectic solvents and their application in electrochemistry. Curr. Opin. Green Sustain. Chem. 2022, 36, 100649. [Google Scholar] [CrossRef]
- Nolan, M.D.; Mezzetta, A.; Guazzelli, L.; Scanlan, E.M. Radical-mediated thiol–ene ‘click’ reactions in deep eutectic solvents for bioconjugation. Green Chem. 2022, 24, 1456–1462. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Mezzetta, A.; Grecchi, S.; Longhi, M.; Emanuele, E.; Rizzo, S.; Arduini, F.; Micheli, L.; Guazzelli, L.; Mussini, P.R. Natural-based chiral task-specific deep eutectic solvents: A novel, effective tool for enantiodiscrimination in electroanalysis. Electrochim. Acta 2021, 380, 138189. [Google Scholar] [CrossRef]
- Morais, E.S.; Lopes, A.M.d.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Li, H. Green Extraction of Polyphenols via Deep Eutectic Solvents and Assisted Technologies from Agri-Food By-Products. Molecules 2023, 28, 6852. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, J.; Wang, W.; Yang, Q.; Yang, G. Deep eutectic solvents: Recent advances in fabrication approaches and pharmaceutical applications. Int. J. Pharm. 2022, 622, 121811. [Google Scholar] [CrossRef]
- Ren, H.; Lian, S.; Wang, X.; Zhang, Y.; Duan, E. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture. J. Clean. Prod. 2018, 193, 802–810. [Google Scholar] [CrossRef]
- Zhang, K.; Ren, S.; Yang, X.; Hou, Y.; Wu, W.; Bao, Y. Efficient absorption of low-concentration SO2 in simulated flue gas by functional deep eutectic solvents based on imidazole and its derivatives. Chem. Eng. J. 2017, 327, 128–134. [Google Scholar] [CrossRef]
- Liu, F.; Chen, W.; Mi, J.; Zhang, J.; Kan, X.; Zhong, F.; Huang, K.; Zheng, A.; Jiang, L. Thermodynamic and molecular insights into the absorption of H2S, CO2, and CH4 in choline chloride plus urea mixtures. AIChE J. 2019, 65, e16574. [Google Scholar] [CrossRef]
- Chen, Y.; Han, X.; Liu, Z.; Yu, D.; Guo, W.; Mu, T. Capture of Toxic Gases by Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 5410–5430. [Google Scholar] [CrossRef]
- Bagh, F.S.G.; Mjalli, F.S.; Hashim, M.A.; Hadj-Kali, M.K.O.; AlNashef, I.M. Solubility of Sodium Salts in Ammonium-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2013, 58, 2154–2162. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Adam, M.R.; Hubadillah, S.K.; Esham, M.I.M.; Othman, M.H.D.; Rahman, M.A.; Ismail, A.F.; Jaafar, J. Adsorptive Membranes for Heavy Metals Removal from Water. In Membrane Separation Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 361–400. [Google Scholar] [CrossRef]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S. Recent progress and challenges on adsorptive membranes for the removal of pollutants from wastewater. Part I: Fundamentals and classification of membranes. Case Stud. Chem. Environ. Eng. 2021, 3, 100086. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, Z. Recent advances in adsorptive membranes for removal of harmful cations. J. Appl. Polym. Sci. 2020, 137, 48579. [Google Scholar] [CrossRef]
- Liao, Z.; Nguyen, M.N.; Wan, G.; Xie, J.; Ni, L.; Qi, J.; Li, J.; Schäfer, A.I. Low pressure operated ultrafiltration membrane with integration of hollow mesoporous carbon nanospheres for effective removal of micropollutants. J. Hazard. Mater. 2020, 397, 122779. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Voicu, S.I.; Thakur, V.K. Graphene-based composite membranes for nanofiltration: Performances and future perspectives. Emergent Mater. 2021, 5, 1429–1441. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater—A review. Desalin. Water Treat. 2016, 57, 27573–27586. [Google Scholar] [CrossRef]
- Ogunleye, D.T.; Akpotu, S.O.; Moodley, B. Adsorption of sulfamethoxazole and reactive blue 19 using graphene oxide modified with imidazolium based ionic liquid. Environ. Technol. Innov. 2020, 17, 100616. [Google Scholar] [CrossRef]
- Lawal, I.A.; Lawal, M.M.; Akpotu, S.O.; Okoro, H.K.; Klink, M.J.; Ndungu, P. Noncovalent Graphene Oxide Functionalized with Ionic Liquid: Theoretical, Isotherm, Kinetics, and Regeneration Studies on the Adsorption of Pharmaceuticals. Ind. Eng. Chem. Res. 2020, 59, 4945–4957. [Google Scholar] [CrossRef]
- Salihi, E.; Wang, J.; Kabacaoğlu, G.; Kırkulak, S.; Šiller, L. Graphene oxide as a new generation adsorbent for the removal of antibiotics from waters. Sep. Sci. Technol. 2020, 56, 453–461. [Google Scholar] [CrossRef]
- Allgayer, R.; Yousefi, N.; Tufenkji, N. Graphene oxide sponge as adsorbent for organic contaminants: Comparison with granular activated carbon and influence of water chemistry. Environ. Sci. Nano 2020, 7, 2669–2680. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2009, 39, 228–240. [Google Scholar] [CrossRef]
- Cao, K.; Jiang, Z.; Zhao, J.; Zhao, C.; Gao, C.; Pan, F.; Wang, B.; Cao, X.; Yang, J. Enhanced water permeation through sodium alginate membranes by incorporating graphene oxides. J. Membr. Sci. 2014, 469, 272–283. [Google Scholar] [CrossRef]
- Hussain, A.; Janson, A.; Matar, J.M.; Adham, S. Membrane distillation: Recent technological developments and advancements in membrane materials. Emergent Mater. 2021, 5, 347–367. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Water Desalination across Nanoporous Graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhu, M.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Xu, Z.; Zhu, H. Selective Ion Penetration of Graphene Oxide Membranes. ACS Nano 2013, 7, 428–437. [Google Scholar] [CrossRef]
- Nicolaï, A.; Sumpter, B.G.; Meunier, V. Tunable water desalination across graphene oxide framework membranes. Phys. Chem. Chem. Phys. 2014, 16, 8646–8654. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Hegab, H.M.; Zou, L. Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification. J. Membr. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Kim, S.G.; Hyeon, D.H.; Chun, J.H.; Chun, B.-H.; Kim, S.H. Novel thin nanocomposite RO membranes for chlorine resistance. Desalin. Water Treat. 2013, 51, 6338–6345. [Google Scholar] [CrossRef]
- Lai, G.; Lau, W.; Goh, P.; Ismail, A.; Yusof, N.; Tan, Y. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Zhang, B.; Liu, J.; Zhang, H.; Song, C. Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Membr. Sci. 2013, 447, 452–462. [Google Scholar] [CrossRef]
- Nan, Q.; Li, P.; Cao, B. Fabrication of positively charged nanofiltration membrane via the layer-by-layer assembly of graphene oxide and polyethylenimine for desalination. Appl. Surf. Sci. 2016, 387, 521–528. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N.; et al. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Membr. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Enhanced Hydrophilicity and Salt Rejection Study of Graphene Oxide-Polysulfone Mixed Matrix Membrane|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0011916412006455?token=F54D2F6A0CBD7979A7CC7674D225B714A3C3CE76EAC5527E6D69FA07ED5BBB3A5937C739E9CD791220350F8447CCA58E&originRegion=eu-west-1&originCreation=20220216145106 (accessed on 16 February 2022).
- Goh, K.; Setiawan, L.; Wei, L.; Si, R.; Fane, A.G.; Wang, R.; Chen, Y. Graphene oxide as effective selective barriers on a hollow fiber membrane for water treatment process. J. Membr. Sci. 2015, 474, 244–253. [Google Scholar] [CrossRef]
- Perreault, F.; Tousley, M.E.; Elimelech, M. Thin-Film Composite Polyamide Membranes Functionalized with Biocidal Graphene Oxide Nanosheets. Environ. Sci. Technol. Lett. 2013, 1, 71–76. [Google Scholar] [CrossRef]
- Pyridinic Nitrogen Doped Nanoporous Graphene as Desalination Membrane_Molecular Simulation Study|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0376738815301484?token=A4ED191141B84FAEA21FD5024F3800F85D89417D552138AE4E8F61E78DA9AD6B5BD3B91E75458825DC47F327B07B690D&originRegion=us-east-1&originCreation=20220216132441 (accessed on 16 February 2022).
- Sun, M.; Li, J. Graphene oxide membranes: Functional structures, preparation and environmental applications. Nano Today 2018, 20, 121–137. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, Y.-S.; Lim, M.-Y.; Jung, K.H.; Kim, D.-G.; Kim, J.-J.; Kang, H.; Lee, J.-C. Reverse osmosis nanocomposite membranes containing graphene oxides coated by tannic acid with chlorine-tolerant and antimicrobial properties. J. Membr. Sci. 2016, 514, 25–34. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.; Rahbari-Sisakht, M.; Daneshfar, A.; Ghanbari, M.; Mayahi, A.; Matsuura, T.; Ismail, A. A novel thin film nanocomposite reverse osmosis membrane with superior anti-organic fouling affinity for water desalination. Desalination 2015, 368, 106–113. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A.; Zarrabi, H.; Gholami, P.; Yekavalangi, M.E. High flux and fouling resistant reverse osmosis membrane modified with plasma treated natural zeolite. Desalination 2017, 411, 89–100. [Google Scholar] [CrossRef]
- Raval, H.D.; Rana, P.S.; Maiti, S. A novel high-flux, thin-film composite reverse osmosis membrane modified by chitosan for advanced water treatment. RSC Adv. 2014, 5, 6687–6694. [Google Scholar] [CrossRef]
- Kulkarni, A.; Mukherjee, D.; Gill, W.N. Flux enhancement by hydrophilization of thin film composite reverse osmosis membranes. J. Membr. Sci. 1996, 114, 39–50. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Filho, R.M. Are ionic liquids eco-friendly? Renew. Sustain. Energy Rev. 2022, 157, 112039. [Google Scholar] [CrossRef]
- Sasikumar, B.; Arthanareeswaran, G. Advances in the integration of ionic liquids with the membrane technology for gas separation. In Ionic Liquid-Based Technologies for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 167–187. [Google Scholar] [CrossRef]
- Xiao, H.-F.; Chu, C.-H.; Xu, W.-T.; Chen, B.-Z.; Ju, X.-H.; Xing, W.; Sun, S.-P. Amphibian-inspired amino acid ionic liquid functionalized nanofiltration membranes with high water permeability and ion selectivity for pigment wastewater treatment. J. Membr. Sci. 2019, 586, 44–52. [Google Scholar] [CrossRef]
- Meng, H.; Gong, B.; Geng, T.; Li, C. Thinning of reverse osmosis membranes by ionic liquids. Appl. Surf. Sci. 2014, 292, 638–644. [Google Scholar] [CrossRef]
- Zheng, D.; Hua, D.; Hong, Y.; Ibrahim, A.-R.; Yao, A.; Pan, J.; Zhan, G. Functions of Ionic Liquids in Preparing Membranes for Liquid Separations: A Review. Membranes 2020, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Gao, H.; Bai, L.; Han, J.; Yang, B.; Zhang, S.; Zhang, X. Functionalized ionic liquid membranes for CO2 separation. Chem. Commun. 2018, 54, 12671–12685. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Peng, H.; Chen, Y.; Zhao, Q. High performance polyamide nanofiltration membranes enabled by surface modification of imidazolium ionic liquid. J. Membr. Sci. 2020, 608, 118202. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Z.; Yang, L.; Guo, H.; Han, S. Activation promoted ionic liquid modification of reverse osmosis membrane towards enhanced permeability for desalination. J. Taiwan Inst. Chem. Eng. 2017, 80, 25–33. [Google Scholar] [CrossRef]
- Yang, X.; Qiao, C.; Li, Y.; Li, T. Dissolution and resourcfulization of biopolymers in ionic liquids. React. Funct. Polym. 2016, 100, 181–190. [Google Scholar] [CrossRef]
- Raval, H.; Mehta, B.; Joshi, R.; Kumar, A. A novel thin film composite reverse osmosis membrane modified by ionic liquid. Braz. J. Chem. Eng. 2018, 35, 1249–1256. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Z.; Zhang, Y.; Wang, T.; Zhao, P.; Zhang, Z.; Yuan, J.; Wang, H. All-Poly(ionic liquid) Membrane-Derived Porous Carbon Membranes: Scalable Synthesis and Application for Photothermal Conversion in Seawater Desalination. ACS Nano 2018, 12, 11704–11710. [Google Scholar] [CrossRef]
- Farooq, M.Q.; Abbasi, N.M.; Anderson, J.L. Deep eutectic solvents in separations: Methods of preparation, polarity, and applications in extractions and capillary electrochromatography. J. Chromatogr. A 2020, 1633, 461613. [Google Scholar] [CrossRef]
- Ismail, N.; Pan, J.; Rahmati, M.; Wang, Q.; Bouyer, D.; Khayet, M.; Cui, Z.; Tavajohi, N. Non-ionic deep eutectic solvents for membrane formation. J. Membr. Sci. 2022, 646, 120238. [Google Scholar] [CrossRef]
- What Is a Hybrid Technology Membrane, and Why Use It—LiqTech. Available online: https://liqtech.com/ceramic-membranes-dpf/silicon-carbide-ceramic-membrane/what-is-a-hybrid-technology-membrane-and-why-use-it/ (accessed on 1 August 2022).
- Liu, L.; Xie, X.; Zambare, R.S.; Selvaraj, A.P.J.; Sowrirajalu, B.N.; Song, X.; Tang, C.Y.; Gao, C. Functionalized Graphene Oxide Modified Polyethersulfone Membranes for Low-Pressure Anionic Dye/Salt Fractionation. Polymers 2018, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, S.S.; Azizi, N.; Vatanpour, V. Tuning thin-film composite reverse osmosis membranes using deep eutectic solvents and ionic liquids toward enhanced water permeation. J. Membr. Sci. 2020, 610, 118267. [Google Scholar] [CrossRef]
- Mehrabi, N.; Lin, H.; Aich, N. Deep eutectic solvent functionalized graphene oxide nanofiltration membranes with superior water permeance and dye desalination performance. Chem. Eng. J. 2021, 412, 128577. [Google Scholar] [CrossRef]
- Sutrisna, P.D. Challenges in commercialization of sustainable membranes with FNMs. In Membranes with Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–353. [Google Scholar] [CrossRef]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S. Recent progress and challenges of adsorptive membranes for the removal of pollutants from wastewater. Part II: Environmental applications. Case Stud. Chem. Environ. Eng. 2021, 3, 100102. [Google Scholar] [CrossRef]
- El Allaoui, B.; Chakhtouna, H.; Zari, N.; Bouhfid, R.; Qaiss, A.e.K. Recent developments in functionalized polymer NF membranes for biofouling control. Emergent Mater. 2022, 5, 1345–1371. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, J.; Liu, F.; Xu, B.-M.; Pan, Y. Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review. Membranes 2022, 12, 519. [Google Scholar] [CrossRef]

| Solubility of Metal Salts in ILs | Main Findings | Reference |
|---|---|---|
| NaCl, MgCl2, and CaCl2 solubility in 1,3-Dimethylimidazolium dimethyl phosphate was studied | Highest solubility achieved is with MgCl2 with a maximum concentration of 15,115 ppm at 100 °C. In contrast, it was 6639 ppm for NaCl at 60 °C and 1393 ppm for CaCl2 at 25 °C. | [65] |
| Hydrophobic tetraalkylammonium oleate and linoleate ILs were tested for different metal salts removal |
| [66] |
| 1-sodium acetate-2,3-dimethyl imidazolium iodide ionic liquid (IL-1) and 1-sodium propionate-2,3-dimethyl imidazolium iodide for the removal of NaCl |
| [67] |
| GO and Membranes in Desalination Studies | Main Findings | Reference |
| GO multilayers were coated on polyamide TFC membrane using layer-by-layer deposition method of the oppositely charged GO nanosheets |
| [33] |
| Nanofiltration polyethylenimine membrane modified with GO via layer-by-layer assembly used for water softening |
| [116] |
| Modified GO with 3-(aminopropyl)triethoxysilane (APTS) embedded in PVDF membrane for salt rejection |
| [117] |
| Graphene oxide dispersed polysulfone MMM prepared via wet phase inversion method |
| [118] |
| GO surface-deposited poly(amide-imide)–polyethyleneimine (PAI–PEI) hollow fiber membrane for MgCl2, CaCl2, and NaCl salt solutions filtration |
| [119] |
| Thin-film composite polyamide membranes surface-functionalized by GO covalently bonded to the surface |
| [120] |
| Functionalized graphene membrane with limited hydroxyl group addition for NaCl removal from aqueous solutions |
| [121] |
| GO deposition in polymeric membranes such as polycarbonate, polyvinylidene fluoride, polysulfone and poly(acrylonitrile) |
| [122] |
| Graphene oxide coated with tannic acid (GOT) and incorporated as filler material in polyamide RO membrane |
| [123] |
| Hybrid Membranes | Main Findings | Reference |
| Polyamide RO membrane was modified with choline chloride–urea-based DES and commercial ILs (1-hexyl-3-methyl-imidazolium chloride, 3-methyl-1-octyl-imidazolium tetra fluoroborate, N-butyl pyridinium and betaine monohydrate) |
| [144] |
| GO membrane was improved with choline chloride- and ethylene glycol-based novel DESs for dye/salt filtration |
| [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qalyoubi, L.; Zuburtikudis, I.; Abu Khalifeh, H.; Nashef, E. Adsorptive Membranes Incorporating Ionic Liquids (ILs), Deep Eutectic Solvents (DESs) or Graphene Oxide (GO) for Metal Salts Extraction from Aqueous Feed. Membranes 2023, 13, 874. https://doi.org/10.3390/membranes13110874
Qalyoubi L, Zuburtikudis I, Abu Khalifeh H, Nashef E. Adsorptive Membranes Incorporating Ionic Liquids (ILs), Deep Eutectic Solvents (DESs) or Graphene Oxide (GO) for Metal Salts Extraction from Aqueous Feed. Membranes. 2023; 13(11):874. https://doi.org/10.3390/membranes13110874
Chicago/Turabian StyleQalyoubi, Liyan, Ioannis Zuburtikudis, Hadil Abu Khalifeh, and Enas Nashef. 2023. "Adsorptive Membranes Incorporating Ionic Liquids (ILs), Deep Eutectic Solvents (DESs) or Graphene Oxide (GO) for Metal Salts Extraction from Aqueous Feed" Membranes 13, no. 11: 874. https://doi.org/10.3390/membranes13110874
APA StyleQalyoubi, L., Zuburtikudis, I., Abu Khalifeh, H., & Nashef, E. (2023). Adsorptive Membranes Incorporating Ionic Liquids (ILs), Deep Eutectic Solvents (DESs) or Graphene Oxide (GO) for Metal Salts Extraction from Aqueous Feed. Membranes, 13(11), 874. https://doi.org/10.3390/membranes13110874







