Evaluation of Organic and Inorganic Foulant Interaction Using Modified Fouling Models in Constant Flux Dead-End Operation with Microfiltration Membranes
Abstract
:1. Introduction
2. Model Development
2.1. Complete Fouling Model
2.2. Intermediate Fouling Model
2.3. Standard Fouling Model
2.4. Cake Layer Fouling Model
3. Materials and Methods
3.1. Materials
3.2. Analytical Techniques
3.3. Membrane Exposure and Fouling Analysis
4. Results and Discussion
4.1. Membrane Constant Flux Dead-End Fouling Experiment
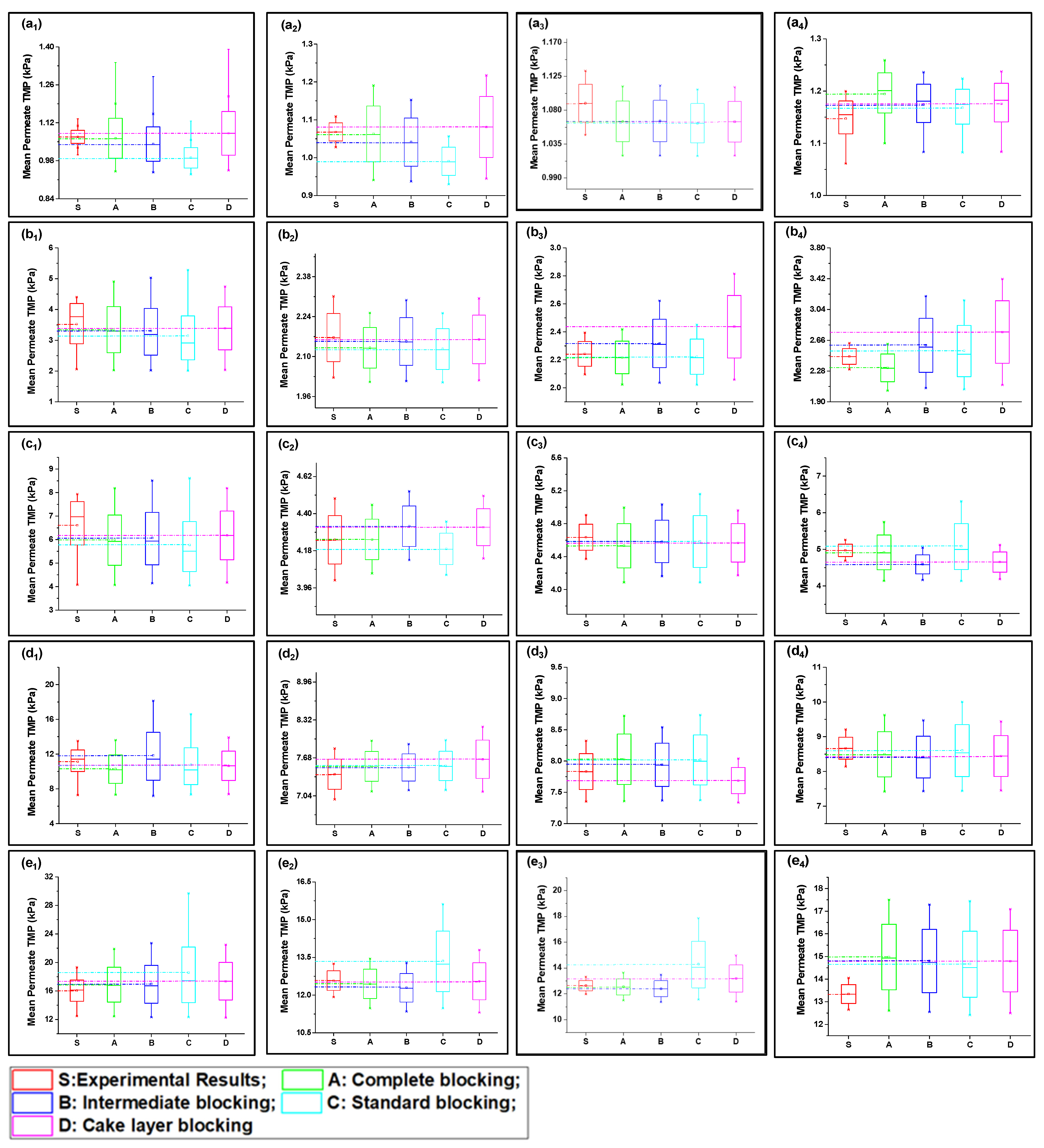
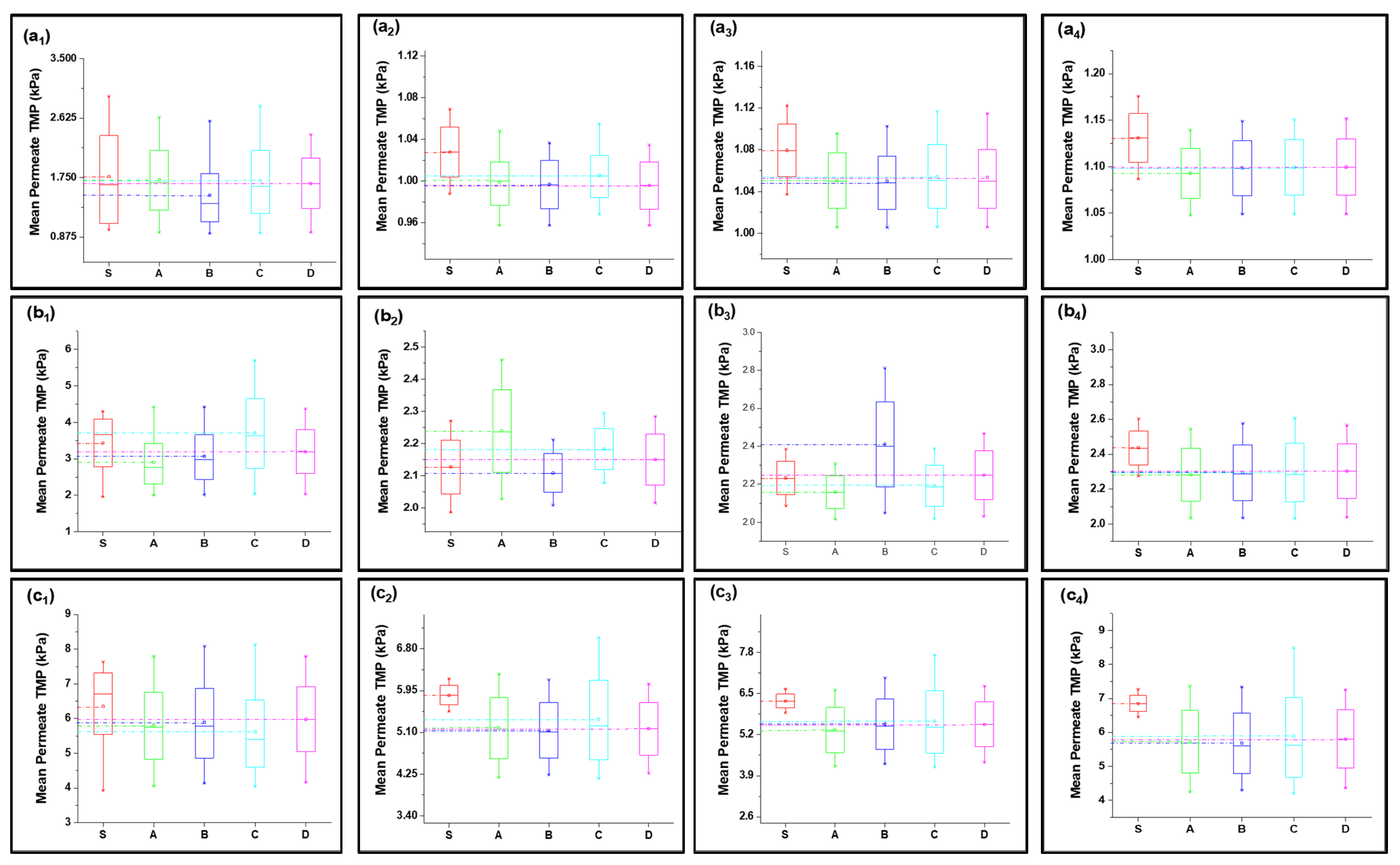
4.2. Verification of Fouling Model and Analysis of Fouling Behavior
4.2.1. Fouling Solution S1 (HA + Ca2+)
4.2.2. Fouling Solution S2 (Inorganic Foulants + Turbidity + Ca2+)
4.2.3. Fouling Solution S3 (HA + Inorganic Foulants + Turbidity + Ca2+)
4.3. Statistical Analysis of Fouling Model Participation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bacchin, P.; Aimar, P.; Sanchez, V. Model for colloidal fouling of membranes. AIChE J. 1995, 41, 368–376. [Google Scholar] [CrossRef]
- Hermia, J. Constant Pressure Blocking Filtration Laws—Application Topower-Law Non-Newtonian Fluids. Trans. Inst. Chem. Eng. 1982, 60, 183–187. [Google Scholar]
- Khan, I.A.; Lee, Y.S.; Kim, J.-O. Chemically enhanced pretreatment (CEPT) to reduce irreversible fouling during the clean-in-place process for membranes operating under constant flux and constant pressure filtration. Desalination 2023, 549, 116313. [Google Scholar] [CrossRef]
- Khan, I.A.; Lee, Y.-S.; Kim, J.-O. A comparison of variations in blocking mechanisms of membrane-fouling models for estimating flux during water treatment. Chemosphere 2020, 259, 127328. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.R.; Calvo, J.I.; Hernández, A. Steps of membrane blocking in flux decline during protein microfiltration. J. Membr. Sci. 1995, 101, 153–165. [Google Scholar] [CrossRef]
- Ho, C.-C.; Zydney, A.L. A Combined Pore Blockage and Cake Filtration Model for Protein Fouling during Microfiltration. J. Colloid Interface Sci. 2000, 232, 389–399. [Google Scholar] [CrossRef]
- Chellam, S.; Xu, W. Blocking laws analysis of dead-end constant flux microfiltration of compressible cakes. J. Colloid Interface Sci. 2006, 301, 248–257. [Google Scholar] [CrossRef]
- Iritani, E. A Review on Modeling of Pore-Blocking Behaviors of Membranes During Pressurized Membrane Filtration. Dry. Technol. 2013, 31, 146–162. [Google Scholar] [CrossRef]
- Kirschner, A.Y.; Cheng, Y.-H.; Paul, D.R.; Field, R.W.; Freeman, B.D. Fouling mechanisms in constant flux crossflow ultrafiltration. J. Membr. Sci. 2019, 574, 65–75. [Google Scholar] [CrossRef]
- Lee, K.H.; Khan, I.A.; Song, L.H.; Kim, J.Y.; Kim, J.-O. Evaluation of structural/performance variation between α-Al2O3 and polyvinylidene fluoride membranes under long-term clean-in-place treatment used for water treatment. Desalination 2022, 538, 115921. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, K.; Wang, X.; Liang, S.; Wei, C.; Wen, X.; Huang, X. Outlining the Roles of Membrane-Foulant and Foulant-Foulant Interactions in Organic Fouling During Microfiltration and Ultrafiltration: A Mini-Review. Front. Chem. 2020, 8, 417. [Google Scholar] [CrossRef]
- Urošević, T.; Trivunac, K. Chapter 3—Achievements in low-pressure membrane processes microfiltration (MF) and ultrafiltration (UF) for wastewater and water treatment. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Ghasemzadeh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–107. [Google Scholar] [CrossRef]
- Tummons, E.; Han, Q.; Tanudjaja, H.J.; Hejase, C.A.; Chew, J.W.; Tarabara, V.V. Membrane fouling by emulsified oil: A review. Sep. Purif. Technol. 2020, 248, 116919. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, Y.; Liu, Y.; Gamal El-Din, M. Effects of ozone pretreatment and operating conditions on membrane fouling behaviors of an anoxic-aerobic membrane bioreactor for oil sands process-affected water (OSPW) treatment. Water Res. 2016, 105, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- He, Z.; Miller, D.J.; Kasemset, S.; Wang, L.; Paul, D.R.; Freeman, B.D. Fouling propensity of a poly(vinylidene fluoride) microfiltration membrane to several model oil/water emulsions. J. Membr. Sci. 2016, 514, 659–670. [Google Scholar] [CrossRef]
- Khan, I.A.; Lee, Y.-S.; Kim, J.-O. Identification of scaling during clean-in-place (CIP) in membrane water treatment process. Chemosphere 2019, 237, 124398. [Google Scholar] [CrossRef]
- Ma, B.; Ding, Y.; Li, W.; Hu, C.; Yang, M.; Liu, H.; Qu, J. Ultrafiltration membrane fouling induced by humic acid with typical inorganic salts. Chemosphere 2018, 197, 793–802. [Google Scholar] [CrossRef]
- Yamamura, H.; Kimura, K.; Higuchi, K.; Watanabe, Y.; Ding, Q.; Hafuka, A. Tracking inorganic foulants irreversibly accumulated on low-pressure membranes for treating surface water. Water Res. 2015, 87, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Li, X.; Wu, Y.; Wang, P.; Wang, L.; Wu, G.; Wang, J.; Lv, Y.; Liu, T. A comparison of the roles of Ca2+ and Mg2+ on membrane fouling with humic acid: Are there any differences or similarities? J. Membr. Sci. 2018, 545, 81–87. [Google Scholar] [CrossRef]
- Lin, T.; Lu, Z.; Chen, W. Interaction mechanisms of humic acid combined with calcium ions on membrane fouling at different conditions in an ultrafiltration system. Desalination 2015, 357, 26–35. [Google Scholar] [CrossRef]
- Li, K.; Huang, T.; Qu, F.; Du, X.; Ding, A.; Li, G.; Liang, H. Performance of adsorption pretreatment in mitigating humic acid fouling of ultrafiltration membrane under environmentally relevant ionic conditions. Desalination 2016, 377, 91–98. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Wang, P.; Liu, Q.; Qiu, W.; Ma, J. Opposite impacts of K+ and Ca2+ on membrane fouling by humic acid and cleaning process: Evaluation and mechanism investigation. Water Res. 2020, 183, 116006. [Google Scholar] [CrossRef]
- Wang, L.-F.; He, D.-Q.; Chen, W.; Yu, H.-Q. Probing the roles of Ca2+ and Mg2+ in humic acids-induced ultrafiltration membrane fouling using an integrated approach. Water Res. 2015, 81, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sillanpää, M.; Gjessing, E.T.; Vogt, R.D. Water quality in the Tibetan Plateau: Major ions and trace elements in the headwaters of four major Asian rivers. Sci. Total Environ. 2009, 407, 6242–6254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-F.; Kim, S.-H. Evaluation of membrane fouling models based on bench-scale experiments: A comparison between constant flowrate blocking laws and artificial neural network (ANNs) model. J. Membr. Sci. 2008, 310, 393–401. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Suarez, J.A.; Veza, J.M. Dead-end microfiltration as advanced treatment for wastewater. Desalination 2000, 127, 47–58. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, J.J. Modelling of permeability loss in membrane filtration: Re-examination of fundamental fouling equations and their link to critical flux. Desalination 2011, 283, 68–74. [Google Scholar] [CrossRef]
- Ye, Y.; Clech, P.L.; Chen, V.; Fane, A.G. Evolution of fouling during crossflow filtration of model EPS solutions. J. Membr. Sci. 2005, 264, 190–199. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Arhin, S.G.; Banadda, N.; Komakech, A.J.; Kabenge, I.; Wanyama, J. Membrane fouling control in low pressure membranes: A review on pretreatment techniques for fouling abatement. Environ. Eng. Res. 2016, 21, 109–120. [Google Scholar] [CrossRef]
- Sioutopoulos, D.C.; Karabelas, A.J. Evolution of organic gel fouling resistance in constant pressure and constant flux dead-end ultrafiltration: Differences and similarities. J. Membr. Sci. 2016, 511, 265–277. [Google Scholar] [CrossRef]
- Chang, H.; Qu, F.; Liu, B.; Yu, H.; Li, K.; Shao, S.; Li, G.; Liang, H. Hydraulic irreversibility of ultrafiltration membrane fouling by humic acid: Effects of membrane properties and backwash water composition. J. Membr. Sci. 2015, 493, 723–733. [Google Scholar] [CrossRef]
- Miao, R.; Wang, L.; Deng, D.; Li, S.; Wang, J.; Liu, T.; Zhu, M.; Lv, Y. Evaluating the effects of sodium and magnesium on the interaction processes of humic acid and ultrafiltration membrane surfaces. J. Membr. Sci. 2017, 526, 131–137. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Organic fouling and chemical cleaning of nanofiltration membranes: Measurements and mechanisms. Environ. Sci. Technol. 2004, 38, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Teychené, J.; Balmann, H.R.-d.; Maron, L.; Galier, S. Investigation of ions hydration using molecular modeling. J. Mol. Liq. 2019, 294, 111394. [Google Scholar] [CrossRef]
- Israelachvili, J.N. 4—Interactions Involving Polar Molecules. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 71–90. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Chapter 13—Van der Waals Forces between Particles and Surfaces. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: Boston, MA, USA, 2011; pp. 253–289. [Google Scholar] [CrossRef]
- Miao, R.; Wang, L.; Zhu, M.; Deng, D.; Li, S.; Wang, J.; Liu, T.; Lv, Y. Effect of Hydration Forces on Protein Fouling of Ultrafiltration Membranes: The Role of Protein Charge, Hydrated Ion Species, and Membrane Hydrophilicity. Environ. Sci. Technol. 2017, 51, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.-Y.; Kalinichev, A.G.; Clark, M.M. Effects of background cations on the fouling of polyethersulfone membranes by natural organic matter: Experimental and molecular modeling study. J. Membr. Sci. 2008, 309, 128–140. [Google Scholar] [CrossRef]
- Tanneru, C.T.; Chellam, S. Mechanisms of virus control during iron electrocoagulation—Microfiltration of surface water. Water Res. 2012, 46, 2111–2120. [Google Scholar] [CrossRef]
- Yamamura, H.; Kimura, K.; Watanabe, Y. Mechanism Involved in the Evolution of Physically Irreversible Fouling in Microfiltration and Ultrafiltration Membranes Used for Drinking Water Treatment. Environ. Sci. Technol. 2007, 41, 6789–6794. [Google Scholar] [CrossRef]
- Mustafa, G.; Wyns, K.; Buekenhoudt, A.; Meynen, V. New insights into the fouling mechanism of dissolved organic matter applying nanofiltration membranes with a variety of surface chemistries. Water Res. 2016, 93, 195–204. [Google Scholar] [CrossRef]
- Shao, S.; Cai, L.; Li, K.; Li, J.; Du, X.; Li, G.; Liang, H. Deposition of powdered activated carbon (PAC) on ultrafiltration (UF) membrane surface: Influencing factors and mechanisms. J. Membr. Sci. 2017, 530, 104–111. [Google Scholar] [CrossRef]
- Shao, S.; Liang, H.; Qu, F.; Li, K.; Chang, H.; Yu, H.; Li, G. Combined influence by humic acid (HA) and powdered activated carbon (PAC) particles on ultrafiltration membrane fouling. J. Membr. Sci. 2016, 500, 99–105. [Google Scholar] [CrossRef]







| Models | Fitting Parameters | S1 Solution | S2 Solution | S3 Solution | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete Blocking (Kb) × 10−4 | Intermediate Blocking (Ki) | Standard Blocking (Ks) | Cake Layer Blocking (Kgl) | Complete Blocking (Kb) | Intermediate Blocking (Ki) | Standard Blocking (Ks) | Cake Layer Blocking (Kgl) | Complete Blocking (Kb) | Intermediate Blocking (Ki) | Standard Blocking (Ks) | Cake Layer Blocking (Kgl) | ||
| 40 lmh | |||||||||||||
| 1 h continuous filtration | K R2 | 7.52 0.9868 | 31.3 0.9708 | 95.4 0.9276 | 44.9 0.9146 | 7.40 0.9392 | 30.9 0.9658 | 94.9 0.9534 | 44.1 0.8966 | 11.9 0.7217 | 42.7 0.6596 | 107.7 0.6133 | 80.3 0.8593 |
| Participation equation | TMP = 3.09TMPb − 1.75TMPi + 2.01TMPs − 2.93TMPgl | TMP = 8.19TMPb − 2.27TMPi + 2.05TMPs − 3.34TMPgl | TMP = 5.31TMPb − 6.14TMPi + 4.19 TMPs + 4.31TMPgl | ||||||||||
| p-value | 5.74 × 10−13 | 1.74 × 10−07 | 0.018 | 3.25 × 10−17 | 1.07 × 10−12 | 2.47 × 10−7 | 0.002 | 5.25 × 10−17 | 0.089 | 0.097 | 0.10 | 0.091 | |
| Cycle 1 | K R2 | 3.77 0.5496 | 19.5 0.3928 | 88.5 0.5325 | 18.4 0.5453 | 2.25 0.9935 | 12.1 0.9494 | 56.8 0.9987 | 11.4 0.9295 | 6.5 0.9182 | 32.4 0.8893 | 83.2 0.7692 | 52.4 0.9709 |
| Participation equation | TMP = −6.51TMPb +6.49TMPi − 1.49TMPs + 1.85TMPgl | TMP = −0.74TMPb + 5.50TMPi + 0.98TMPs − 2.28TMPgl | TMP = 1.86TMPb − 2.41TMPi + 3.15TMPs + 1.31TMPgl | ||||||||||
| p-value | 9 × 10−4 | 0.057 | 0.069 | 1.5 × 10−4 | 0.060 | 0.054 | 0.052 | 0.0597 | 0.031 | 0.036 | 0.039 | 0.034 | |
| Cycle 2 | K R2 | 4.56 0.6248 | 23.4 0.5367 | 89.6 0.5468 | 22.8 0.5127 | 4.22 0.7832 | 21.6 0.7138 | 85.3 0.7687 | 21.0 0.7418 | 9.7 0.9142 | 35.3 0.8654 | 94.4 0.6734 | 67.3 0.9527 |
| Participation equation | TMP = 0.47TMPb − 0.19TMPi − 0.18TMPs + 0.25TMPgl | TMP = 0.55TMPb − 0.23TMPi + 1.18TMPs − 2.26TMPgl | TMP = 3.31TMPb − 3.21TMPi + 3.19TMPs + 2.42TMPgl | ||||||||||
| p-value | 0.85 | 0.92 | 0.91 | 0.046 | 0.013 | 0.96 | 0.019 | 0.046 | 0.012 | 0.013 | 0.013 | 0.010 | |
| Cycle 3 | K R2 | 6.98 0.6327 | 29.8 0.5108 | 92.5 0.6987 | 34.8 0.5837 | 6.13 0.6132 | 30.6 0.6944 | 89.3 0.6237 | 30.7 0.9872 | 10.1 0.9806 | 41.6 0.9586 | 102.3 0.8456 | 72.5 0.9834 |
| Participation equation | TMP = 0.61TMPb − 0.01TMPi + 0.06TMPs + 0.20TMPgl | TMP = −1.55TMPb + 2.21TMPi + 1.15TMPs − 2.67TMPgl | TMP = 4.74TMPb − 4.21TMPi + 3.24TMPs + 3.07TMPgl | ||||||||||
| p-value | 9.4 × 10−3 | 0.012 | 0.014 | 4.1 × 10−3 | 0.14 | 0.11 | 0.115 | 0.021 | 6.6 × 10−3 | 4.9 × 10−3 | 4.3 × 10−3 | 6.1 × 10−3 | |
| 80 lmh | |||||||||||||
| 1 h continuous filtration | K R2 | 6.08 0.8564 | 11.5 0.7836 | 41.4 0.7034 | 17.3 0.9294 | 5.82 0.8874 | 11.1 0.8294 | 40.5 0.672 | 16.6 0.9426 | 10.2 0.6738 | 13.9 0.6678 | 50.5 0.7328 | 32.0 0.8226 |
| Participation equation | TMP = 4.34TMPb − 0.15TMPi + 2.87TMPs − 1.99TMPgl | TMP = 6.91TMPb − 4.62TMPi + 6.24TMPs + 1.34TMPgl | TMP = 1.87TMPb − 7.25TMPi + 6.34TMPs + 5.39TMPgl | ||||||||||
| p-value | 1.2 × 10−18 | 4.17 × 10−20 | 1.85 × 10−17 | 1.2 × 10−10 | 6.7 × 10−10 | 8.9 × 10−21 | 1.4 × 10−18 | 1.5 × 10−10 | 0.037 | 9.6 × 10−7 | 1.9 × 10−6 | 5.5 × 10−12 | |
| Cycle 1 | K R2 | 3.52 0.9768 | 6.99 0.9346 | 35.6 0.9413 | 7.28 0.9264 | 2.77 0.8637 | 5.35 0.7768 | 27.6 0.7843 | 5.5 0.7689 | 15.8 0.9785 | 11.3 0.9782 | 39.4 0.9828 | 29.6 0.9537 |
| Participation equation | TMP = 0.32TMPb +0.84TMPi − 0.22TMPs + 0.07TMPgl | TMP = −0.31TMPb + 2.46TMPi − 0.39TMPs − 0.28TMPgl | TMP = 0.81TMPb − 2.76TMPi + 3.51TMPs + 1.36TMPgl | ||||||||||
| p-value | 0.92 | 0.74 | 0.71 | 0.51 | 0.96 | 0.85 | 0.86 | 0.93 | 0.026 | 0.034 | 0.046 | 0.016 | |
| Cycle 2 | K R2 | 4.94 0.9583 | 8.17 0.9871 | 38.2 0.9748 | 10.7 0.9943 | 4.76 0.9775 | 9.71 0.9267 | 35.6 0.9927 | 10.3 0.9185 | 19.8 0.9775 | 12.8 0.9832 | 44.6 0.9267 | 30.5 0.9915 |
| Participation equation | TMP = 1.02TMPb − 0.23TMPi + 0.09TMPs + 0.02TMPgl | TMP = 1.56TMPb − 0.15TMPi + 0.19TMPs + 0.24TMPgl | TMP = 0.91TMPb − 3.42TMPi + 4.24TMPs + 2.21TMPgl | ||||||||||
| p-value | 0.03 | 0.27 | 0.24 | 0.04 | 0.01 | 0.029 | 0.036 | 0.02 | 0.014 | 0.056 | 0.017 | 0.14 | |
| Cycle 3 | K R2 | 5.83 0.7156 | 10.9 0.6348 | 39.1 0.8735 | 15.37 0.8687 | 5.28 0.8483 | 9.86 0.9997 | 42.87 0.7778 | 14.6 0.7612 | 21.68 0.4863 | 13.4 0.6489 | 48.04 0.7164 | 31.6 0.8076 |
| Participation equation | TMP = 1.29TMPb − 0.62TMPi + 0.11TMPs − 0.04TMPgl | TMP = 2.91TMPb − 1.69TMPi + 3.14TMPs + 0.64TMPgl | TMP = 1.27TMPb − 5.61TMPi + 6.61TMPs + 2.91TMPgl | ||||||||||
| p-value | 9 × 10−4 | 0.86 | 0.96 | 4.9 × 10−5 | 0.018 | 0.034 | 0.019 | 1.9 × 10−3 | 4.7 × 10−6 | 0.02 | 0.018 | 2.6 × 10−13 | |
| 120 lmh | |||||||||||||
| 1 h continuous filtration | K R2 | 4.58 0.8393 | 6.11 0.78323 | 23.63 0.5971 | 8.303 0.9039 | 4.21 0.8717 | 5.67 0.8307 | 22.41 0.6939 | 7.52 0.9175 | 7.62 0.6202 | 9.23 0.6228 | 30.41 0.6823 | 15.35 0.7638 |
| Participation equation | TMP = 4.55TMPb − 6.62TMPi + 2.52TMPs + 4.64TMPgl | TMP = 4.31TMPb − 4.75TMPi + 7.61TMPs + 4.57TMPgl | TMP = −1.80TMPb − 7.35TMPi + 7.24TMPs + 5.51TMPgl | ||||||||||
| p-value | 0.002 | 3.2 × 10−3 | 1.6 × 10−4 | 1.3 × 10−4 | 0.12 | 2.4 × 10−3 | 1.4 × 10−4 | 2.7 × 10−3 | 2.63 × 10−21 | 2.51 × 10−11 | 8.76 × 10−5 | 1.09 × 10−34 | |
| Cycle 1 | K R2 | 2.96 0.9672 | 4.28 0.9562 | 10.44 0.9331 | 4.12 0.9197 | 2.58 0.9061 | 3.72 0.8884 | 17.07 0.8494 | 3.53 0.8349 | 5.59 0.6309 | 7.11 0.8467 | 25.00 0.9966 | 5.16 0.9742 |
| Participation equation | TMP = 2.91TMPb − 1.12TMPi + 1.77TMPs − 1.04TMPgl | TMP = 2.86TMPb − 0.82TMPi + 0.52TMPs − 1.24TMPgl | TMP = −0.86TMPb − 0.05TMPi +0.06TMPs + 0.26TMPgl | ||||||||||
| p-value | 0.14 | 0.24 | 0.25 | 0.12 | 0.13 | 0.25 | 0.26 | 0.09 | 1.24 × 10−4 | 0.63 | 0.45 | 1.45 × 10−12 | |
| Cycle 2 | K R2 | 4.35 0.8720 | 5.66 0.9937 | 15.33 0.7593 | 6.76 0.9614 | 4.11 0.8906 | 5.54 0.9972 | 18.36 0.7299 | 6.62 0.9712 | 6.23 0.8475 | 8.32 0.8345 | 26.32 0.80667 | 9.75 0.9805 |
| Participation equation | TMP = 3.52TMPb − 2.91TMPi + 1.93TMPs + 0.31TMPgl | TMP = 3.45TMPb − 2.12TMPi + 3.23TMPs + 0.28TMPgl | TMP = −1.24TMPb − 1.22TMPi + 2.11TMPs + 1.35TMPgl | ||||||||||
| p-value | 0.017 | 0.041 | 0.023 | 7.6 × 10−6 | 0.026 | 0.047 | 0.012 | 2.9 × 10−4 | 3.37 × 10−8 | 0.019 | 0.28 | 2.87 × 10−15 | |
| Cycle 3 | K R2 | 4.68 0.8978 | 6.74 0.9068 | 27.64 0.8152 | 8.376 0.9278 | 4.433 0.9378 | 6.43 0.9424 | 23.89 0.8703 | 7.65 0.8651 | 7.14 0.9271 | 8.33 0.9190 | 22.64 0.9541 | 12.03 0.5126 |
| Participation equation | TMP = 4.09TMPb − 3.12TMPi + 2.14TMPs + 2.14TMPgl | TMP = 4.21TMPb − 2.78TMPi + 4.11TMPs + 0.19TMPgl | TMP = −1.29TMPb − 2.71TMPi + 3.64TMPs + 3.14TMPgl | ||||||||||
| p-value | 5.5 × 10−11 | 4.7 × 10−12 | 1.6 × 10−14 | 2.3 × 10−9 | 5.5 × 10−4 | 0.16 | 0.011 | 4.7 × 10−7 | 2.41 × 10−6 | 0.025 | 0.014 | 1.12 × 10−14 | |
| 160 lmh | |||||||||||||
| 1 h continuous filtration | K R2 | 3.97 0.6833 | 6.89 0.6032 | 18.64 0.6162 | 5.71 0.9031 | 8.41 0.8031 | 5.83 0.7546 | 17.40 0.5264 | 4.86 0.9446 | 8.37 0.6683 | 5.32 0.5746 | 17.31 0.5264 | 5.06 0.8970 |
| Participation equation | TMP = 6.73TMPb + 0.01TMPi + 1.23TMPs + 5.93TMPgl | TMP = 3.14TMPb − 5.67TMPi + 7.92TMPs + 4.82TMPgl | TMP = −4.61TMPb − 8.12TMPi + 7.94TMPs + 6.14TMPgl | ||||||||||
| p-value | 4.69 × 10−28 | 2.59 × 10−22 | 2.48 × 10−15 | 3.64 × 10−32 | 3.88 × 10−27 | 3.32 × 10−19 | 4.56 × 10−15 | 6.99 × 10−48 | 8.11 × 10−39 | 3.98 × 10−32 | 2.63 × 10−20 | 1.32 × 10−51 | |
| Cycle 1 | K R2 | 3.514 0.9992 | 3.52 0.9820 | 16.92 0.9962 | 7.32 0.5253 | 3.29 0.9905 | 3.28 0.9577 | 17.71 0.9821 | 7.07 0.6163 | 1.02 0.6635 | 3.19 0.9707 | 15.71 0.9671 | 3.48 0.9987 |
| Participation equation | TMP = 2.61TMPb − 1.43TMPi + 0.31TMPs + 2.14TMPgl | TMP = 0.96TMPb − 1.20TMPi + 0.55TMPs + 0.23TMPgl | TMP = −0.11TMPb − 0.08TMPi + 0.01TMPs + 0.28TMPgl | ||||||||||
| p-value | 0.22 | 0.57 | 0.56 | 0.47 | 0.019 | 0.046 | 0.45 | 0.016 | 2.01 × 10−6 | 2.73 × 10−4 | 4.54 × 10−5 | 1.77 × 10−33 | |
| Cycle 2 | K R2 | 5.86 0.6943 | 5.03 0.9586 | 27.82 0.8381 | 3.31 0.9123 | 5.81 0.7116 | 4.98 0.9632 | 27.57 0.8515 | 3.25 0.9020 | 4.97 0.8361 | 4.41 0.9746 | 21.77 0.9727 | 3.80 0.9997 |
| Participation equation | TMP = 3.41TMPb − 0.94TMPi + 1.04TMPs + 3.16TMPgl | TMP = 1.57TMPb − 1.34TMPi + 1.74TMPs + 1.16TMPgl | TMP = −2.45TMPb − 3.45TMPi + 2.31TMPs + 1.34TMPgl | ||||||||||
| p-value | 3.57 × 10−3 | 0.065 | 0.055 | 5.64 × 10−6 | 1.35 × 10−3 | 0.049 | 0.041 | 8.73 × 10−6 | 3.75 × 10−7 | 0.021 | 0.029 | 2.42 × 10−17 | |
| Cycle 3 | K R2 | 8.32 0.9321 | 6.27 0.9653 | 29.32 0.9657 | 3.32 0.9996 | 3.50 0.9906 | 4.15 0.9992 | 23.48 0.9774 | 3.78 0.9632 | 5.32 0.9789 | 4.26 0.9863 | 33.13 0.7032 | 3.92 0.9979 |
| Participation equation | TMP = 3.96TMPb − 0.04TMPi + 1.96TMPs + 4.02TMPgl | TMP = 2.25TMPb − 2.94TMPi + 3.25TMPs + 1.98TMPgl | TMP = −4.12TMPb − 5.94TMPi + 4.04TMPs + 2.16TMPgl | ||||||||||
| p-value | 2.15 × 10−4 | 0.085 | 0.049 | 3.39 × 10−4 | 0.073 | 0.013 | 6.61 × 10−9 | 1.48 × 10−7 | 0.012 | 0.046 | 5.15 × 10−15 | ||
| 200 lmh | |||||||||||||
| 1 h continuous filtration | K R2 | 3.05 0.9262 | 2.45 0.9355 | 10.97 0.8234 | 3.61 0.9834 | 2.53 0.9903 | 2.04 0.9804 | 9.851 0.9532 | 2.88 0.9932 | 5.31 0.7322 | 2.67 0.9028 | 11.23 0.8153 | 3.26 0.9676 |
| Participation equation | TMP = 7.95TMPb + 0.65TMPi − 0.21TMPs + 7.83TMPgl | TMP = 2.09TMPb − 6.87TMPi + 8.34TMPs + 4.97TMPgl | TMP = −7.64TMPb − 9.12TMPi + 9.89TMPs + 8.64TMPgl | ||||||||||
| p-value | 8.53 × 10−13 | 1.28 × 10−9 | 0.014 | 9.95 × 10−19 | 1.93 × 10−10 | 7.89 × 10−5 | 7.4 × 10−4 | 2.6 × 10−12 | 6.81 × 10−29 | 1.75 × 10−22 | 3.52 × 10−13 | 2.08 × 10−36 | |
| Cycle 1 | K R2 | 2.87 0.9832 | 2.55 0.9815 | 17.07 0.9512 | 2.70 0.9921 | 2.75 0.9692 | 2.43 0.9668 | 16.55 0.9832 | 2.57 0.9679 | 5.11 0.6685 | 4.49 0.6621 | 21.87 0.6238 | 3.56 0.9821 |
| Participation equation | TMP = 0.49TMPb − 0.03TMPi + 0.08TMPs +0.16TMPgl | TMP = 0.27TMPb − 0.07TMPi + 0.02TMPs +0.18TMPgl | TMP = 0.09TMPb − 0.04TMPi + 3.98TMPs + 0.29TMPgl | ||||||||||
| p-value | 8.86 × 10−4 | 0.65 | 0.59 | 2.64 × 10−5 | 9.18 × 10−4 | 0.046 | 0.039 | 9.64 × 10−7 | 8.68 × 10−7 | 0.012 | 0.066 | 8.17 × 10−14 | |
| Cycle 2 | K R2 | 3.06 0.9956 | 2.71 0.9945 | 13.53 0.9933 | 1.60 0.7273 | 3.03 0.9937 | 2.69 0.9927 | 13.41 0.9991 | 1.59 0.7158 | 4.34 0.9075 | 3.86 0.9008 | 19.20 0.8782 | 3.44 0.9946 |
| Participation equation | TMP = 0.87TMPb + 0.10TMPi + 0.01TMPs +0.15TMPgl | TMP = 0.98TMPb − 0.98TMPi + 0.87TMPs +0.69TMPgl | TMP = −0.06TMPb − 0.69TMPi + 4.19TMPs + 1.56TMPgl | ||||||||||
| p-value | 1.5 × 10−4 | 0.023 | 0.087 | 5.8 × 10−7 | 4.33 × 10−5 | 0.073 | 0.063 | 2.16 × 10−6 | 1.75 × 10−7 | 0.86 | 0.023 | 1.6 × 10−12 | |
| Cycle 3 | K R2 | 4.02 0.9367 | 3.58 0.9319 | 17.90 0.9157 | 3.08 0.9981 | 3.95 0.9492 | 3.52 0.9450 | 14.90 0.9987 | 3.01 0.9990 | 4.01 0.9591 | 3.33 0.9857 | 20.19 0.8121 | 3.17 0.9997 |
| Participation equation | TMP = 1.81TMPb + 1.24TMPi + 0.84TMPs + 0.21TMPgl | TMP = 2.34TMPb − 2.14TMPi + 1.59TMPs + 1.63TMPgl | TMP = −1.93TMPb − 1.31TMPi + 6.13TMPs +2.31TMPgl | ||||||||||
| p-value | 5.72 × 10−6 | 0.04 | 0.03 | 3.04 × 10−13 | 9.12 × 10−7 | 0.85 | 0.61 | 9.28 × 10−10 | 9.57 × 10−8 | 0.93 | 0.14 | 1.72 × 10−12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qasim, M.; Akbar, A.; Khan, I.A.; Ali, M.; Lee, E.-J.; Lee, K.H. Evaluation of Organic and Inorganic Foulant Interaction Using Modified Fouling Models in Constant Flux Dead-End Operation with Microfiltration Membranes. Membranes 2023, 13, 853. https://doi.org/10.3390/membranes13110853
Qasim M, Akbar A, Khan IA, Ali M, Lee E-J, Lee KH. Evaluation of Organic and Inorganic Foulant Interaction Using Modified Fouling Models in Constant Flux Dead-End Operation with Microfiltration Membranes. Membranes. 2023; 13(11):853. https://doi.org/10.3390/membranes13110853
Chicago/Turabian StyleQasim, Muhammad, Ali Akbar, Imtiaz Afzal Khan, Mumtaz Ali, Eui-Jong Lee, and Kang Hoon Lee. 2023. "Evaluation of Organic and Inorganic Foulant Interaction Using Modified Fouling Models in Constant Flux Dead-End Operation with Microfiltration Membranes" Membranes 13, no. 11: 853. https://doi.org/10.3390/membranes13110853
APA StyleQasim, M., Akbar, A., Khan, I. A., Ali, M., Lee, E.-J., & Lee, K. H. (2023). Evaluation of Organic and Inorganic Foulant Interaction Using Modified Fouling Models in Constant Flux Dead-End Operation with Microfiltration Membranes. Membranes, 13(11), 853. https://doi.org/10.3390/membranes13110853






