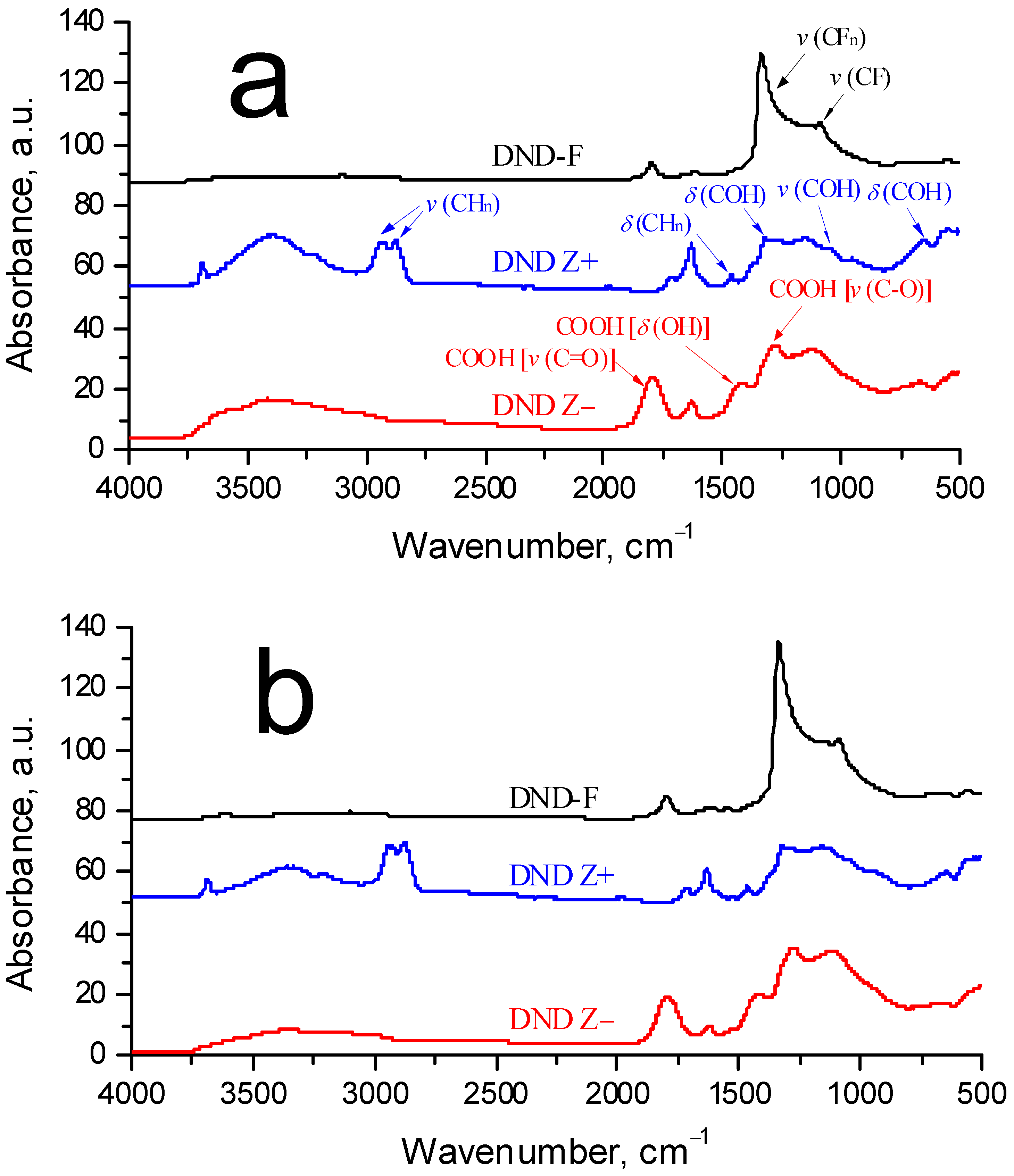
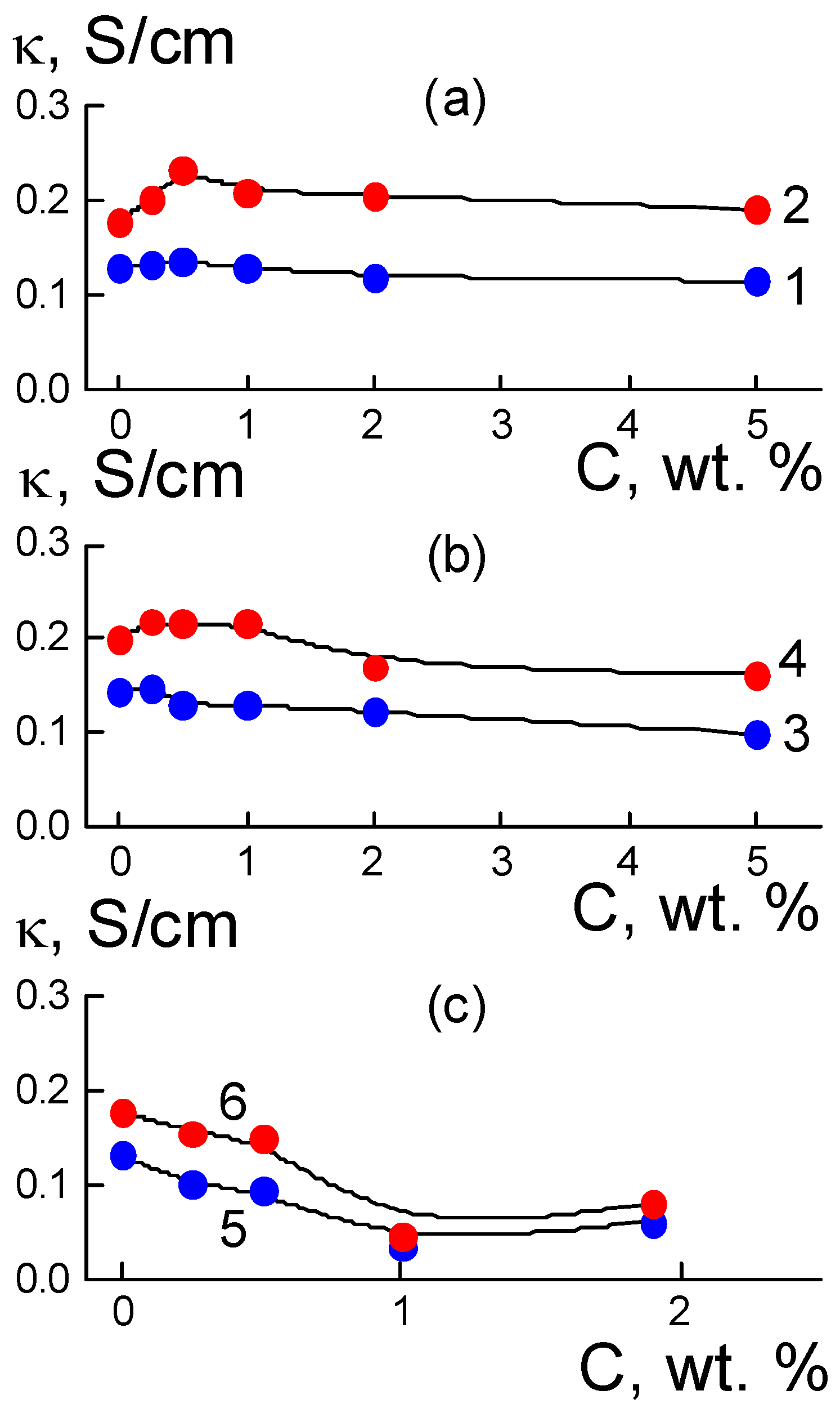
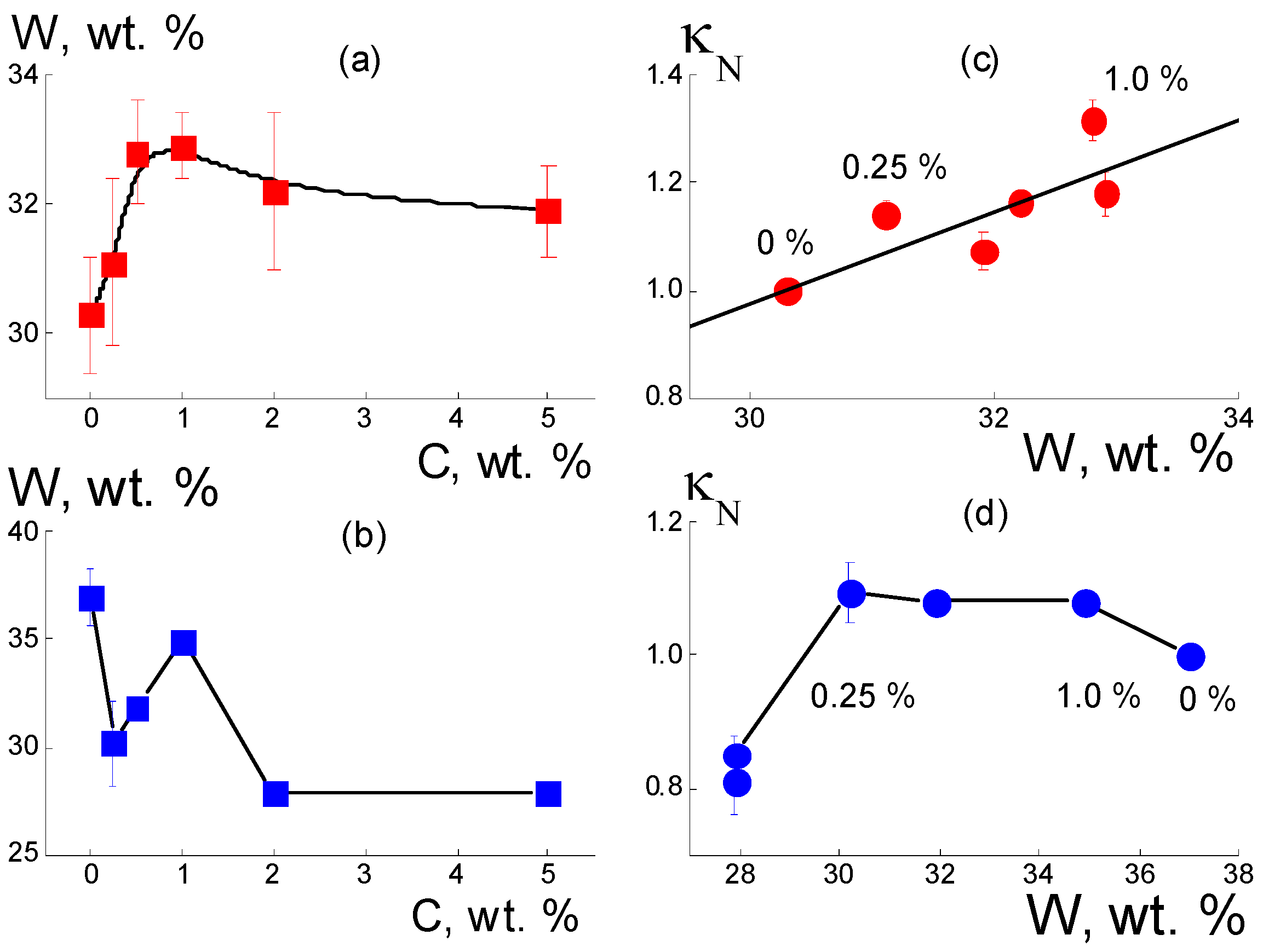
3.2.1. Composites with DND Z+ Diamonds
The mechanism of effect of DND Z+ on the conductivity of composites based on the Aquivion
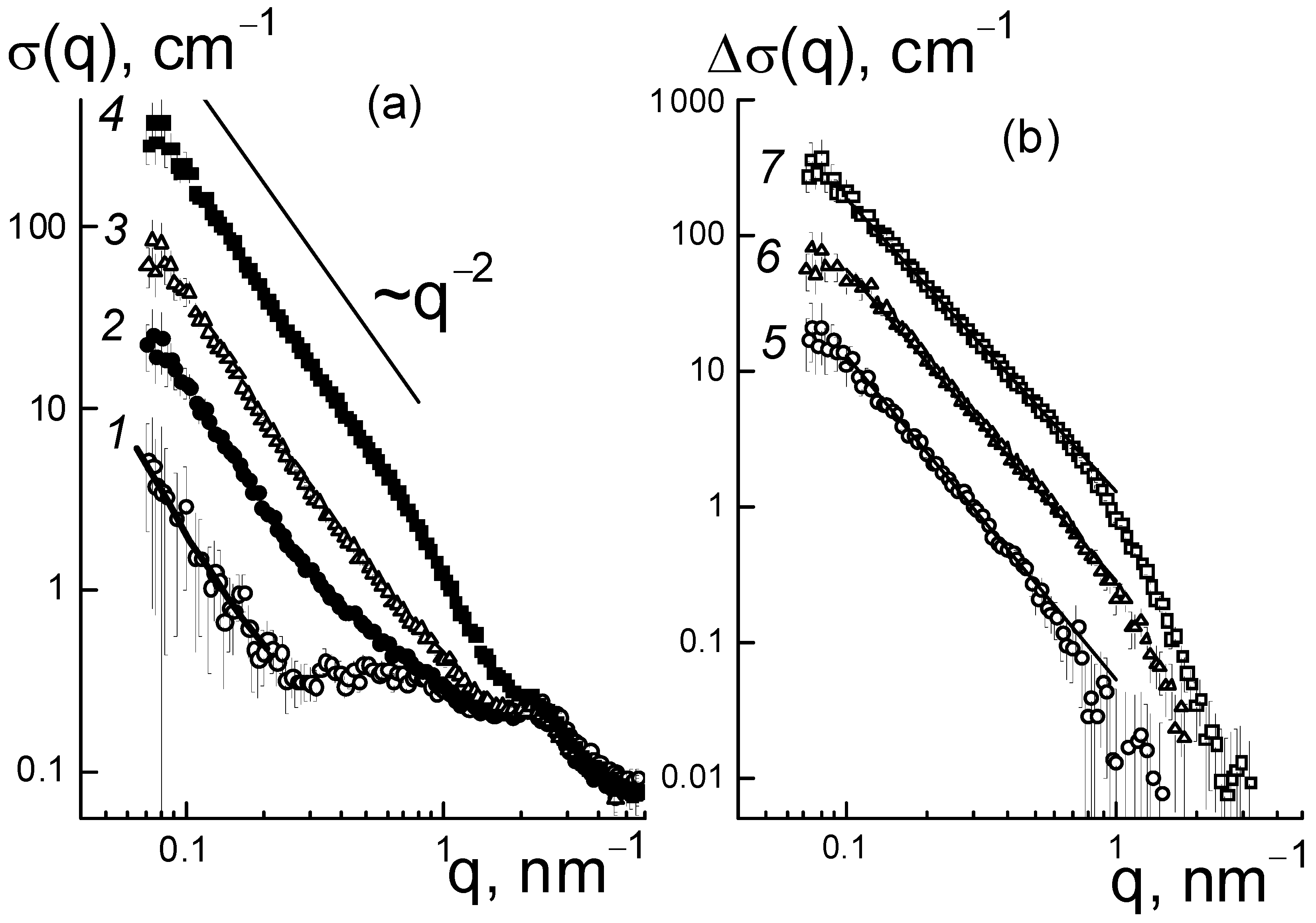
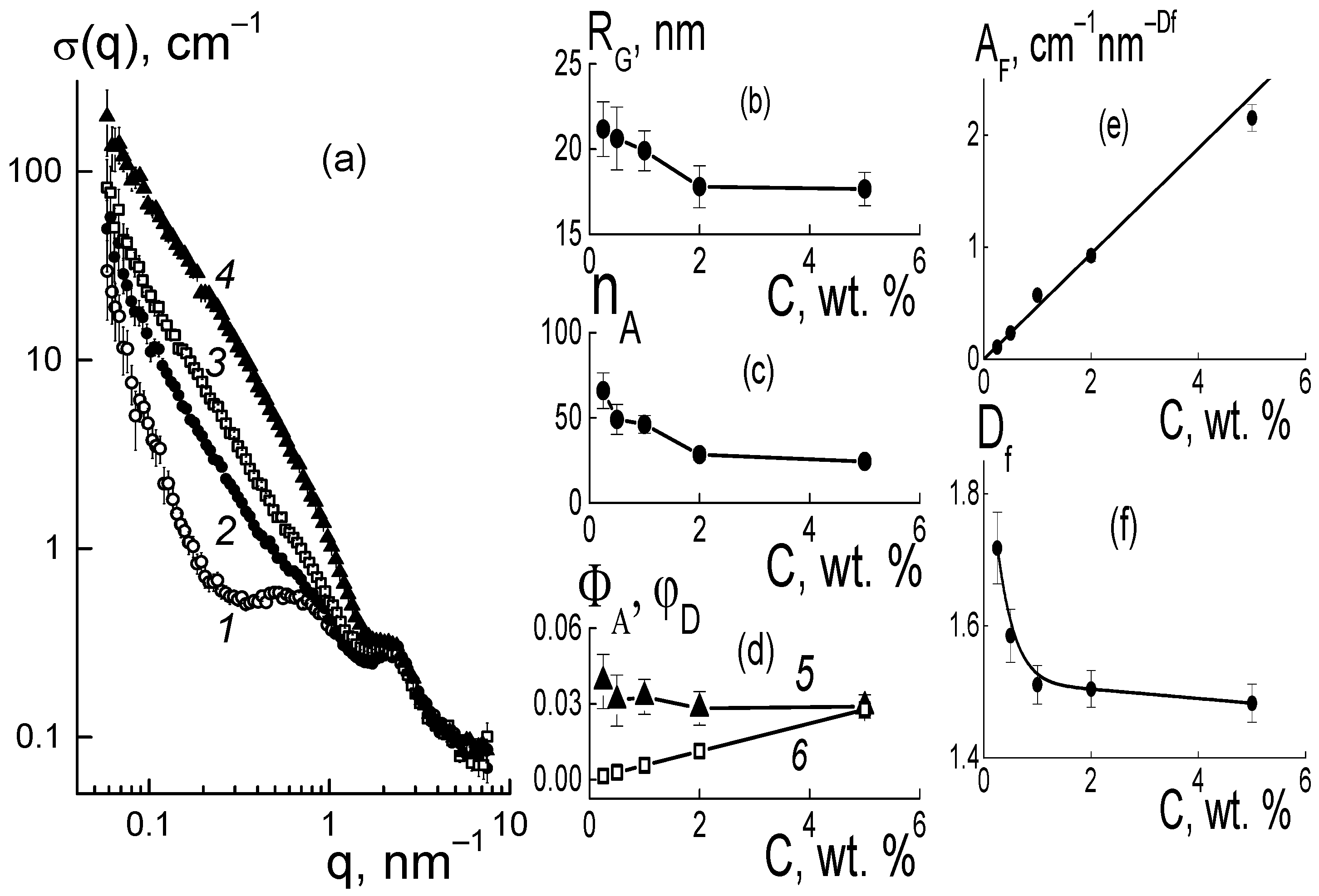
®-type copolymer was elucidated from the analysis of the structural changes in the membranes. In dry composites, with the increase in the amount of diamonds (0.25; 0.5; 1.0; 2.0; 5.0 wt. %), we observed progressive growth in SANS cross section σ(q) at momenta q ~ 0.01–1.0 nm
−1 according to the power-law of σ(q,C) ~ q
−2, which is characteristic of Gaussian polymer chains and chain structures of the particles observed in aqueous diamond dispersions [
14,
15].
This is illustrated in
Figure 6a for the samples with several characteristic modifier concentrations. In this case, because of the high contrast against the copolymer, the diamonds in the membranes, even at a low content (0.25 wt. %), caused scattering of almost an order of magnitude higher than in the original copolymer (
Figure 6a). When increasing the fraction of diamonds up to 5.0 wt. % the scattering raised by two orders of magnitude at a low momenta q ≤ 0.1 nm
−1, while the cross section profiles remained similar (
Figure 6a). Under conditions of intense scattering from diamonds relative to the copolymer, the cross sections associated with them in the first approximation were found through a subtraction of the copolymer contribution, Δσ(q, C) = σ(q, C) − σ(q, C = 0) (
Figure 6b).
In the samples, the data showed fractal behavior, Δσ(q,C) ~ q
−Df (
Figure 6b) with the index D
f(C) ~ 2, which indicated diamond binding into chain aggregates. Similar structures are usually observed in aqueous dispersions of diamonds [
14,
15]. In our case, diamond aggregates formed within the mixed dispersion of diamonds and a copolymer in dimethylformamide (DMF), and were preserved during precipitation of components, removal of the solvent, and heat treatment of the resulting membrane films to achieve an equilibrium structure in the SO
3H form.
Before a detailed analysis of the fractal nature of the organization of diamonds in the composites, the size of the diamond aggregates was estimated from an approximation of the data at a low momentum using the Guinier function,
where R
G(C) is the gyration radius of observed objects, I
o(C) = ΔK
DP2φ
Dv
Pn
A is their cross section in the limit q → 0 dependent on the contrast factor of diamonds against the polymer (ΔK
DP), their volume fraction φD, the volume of a particle (v
P), and the aggregation number (n
A). The radii R
G(C) and the numbers n
A(C) = I
o(C)/ΔK
DP2φ
Dv
P found from the I
o(C) data are shown in
Figure 7a,b.
These parameters made it possible to determine the volume fractions of particles Φ
A = n
Av
P/V
A (
Figure 7c) in aggregates with a volume of V
A = (4π/3)
3/2R
G3 [
14]. It was found that by increasing the amount of modifier (C = 0.25–5.0 wt. %), the volume fraction of diamonds in the aggregates Φ
A(C) ~ 9–10% remained almost constant and was three times higher than the average level (~2.8%) for the sample in the upper limit of concentrations (5 wt. %) (
Figure 7c). Thus, diamonds in the copolymer matrix created a density-stable nano-sized phase similar to that of diamond hydrogels [
15].
Taking into account the fractal behavior of the sections ~q
−2 (
Figure 6b), we considered the correlation between the parameters n
A(C), R
G(C) as in a Gaussian chain of diamonds. Then, the aggregation numbers n
GA = 6(R
G/d
P)
2 were defined by the ratios of gyration radii to diamond size (d
P) (
Figure 7b, data 3). These n
GA magnitudes agreed with the experiment at low diamond fractions C = 0.25–0.5 wt. %, when scattering by individual aggregates predominated.
At C = 0.5 wt. %, the diamond amount N
DND = 6.0 × 10
16 cm
−3, the n
A ≈ 80 (
Figure 7b) and the number of aggregates N
AG = N
DND/n
A = 7.1 × 10
14 cm
−3 defined their diameter L
GA = √6 R
G ≈ 40 nm, which was three times lower than the average spacing between them R
INA = (N
DND/n
A)
−1/3 ≈ 110 nm. Hence, an overlapping of aggregates was unlikely. This was also favored by the structure of the Aquivion
®-type copolymer matrix [
11] composed of domains with a radius of ~100 nm. With domains of this size, it is realistic that aggregates can be filled in the gaps between them.
Meanwhile, in the composites with a high filler content, C = 5 wt. %, the numbers of diamonds and aggregates, N
DND = 6.0 × 10
17 cm
−3 and N
AG = 9.8x10
15 cm
−3, were larger by an order of magnitude. On average, the aggregates were spaced at a distance R
INA = N
AG−1/3 ≈ 50 nm approaching a diameter of L
GA ≈ 40 nm. So, it seems their interaction is inevitable, displaying with some trends towards forming bigger and more substantial structures. However, the observed lowering of parameters R
G(C), n
A(C) (
Figure 7a,b) indicated no progress in aggregation, but instead a dominating binding of diamonds with the ionic copolymer groups. This trend was also manifested in the fractal geometry of the chains that formed the aggregates (
Figure 8).
In the range q ~ 0.1–0.6 nm
−1, the differential cross sections (
Figure 6b) obeyed a fractal dependence Δσ(q,C) = A
Fq
−Df with proportionality parameter A
F(C) and index D
f(C) ~ 2.2–2.4 (
Figure 8b), which indicated some branched chain structures. The parameter A
F(C) = α
F·C characterized the chain scattering power and, thus, was proportional to the concentration of diamonds with a factor α
F = 0.24 ± 0.01 cm
−1 nm
−Df (
Figure 8a).
The meaning of the parameter AF became clear when using the model scattering function for the chains, [1 + (qRC)2]−Df/2 ≈ 1/(qRC)Df, at momenta q >> 1/RC above the reciprocal correlation radius of the chain RC. Such a scattering function corresponded to a chain with the aggregation number nC = (RC/rC)Df, given by the ratio of RC to the correlation radius rC of particles forming a chain with the fractal dimension Df. Then, the coefficient, αF = (0.01)(ρP/ρD)(ΔKDP)2vP/rCDf, is a function of rC variable and the following parameters are known: the contrast factor between the diamonds and polymer (ΔKDP), the volume fraction of diamonds (φ), particle volume (vP), and polymer and diamond densities (ρP, ρD). Using the αF value, we found rC ≈ 2.2–2.3 nm, which was comparable to a similar parameter of rC = (2/3)dP ≈ 3 nm for a diamond. Finally, this confirmed the validity of the chain model for diamond assembly.
It is notable that the fractal dimension of the chains decreased logarithmically with the increase in diamond fraction, D
f(C) = D
1 − β
f ln(C), with parameters D
1 = 2.29 ± 0.01, β
f = 0.079 ± 0.006, where D
1 corresponds to the content of diamonds C
1 = 1 wt. %. Such a decrease in the D
f(C) dimension (
Figure 8b) resulted in a transition to less branched structures, in accordance with the weakening of aggregation when the membranes were enriched with a modifier (
Figure 7b) due to progressive binding of the hydrophilic diamonds to the ionic groups of the polymer.
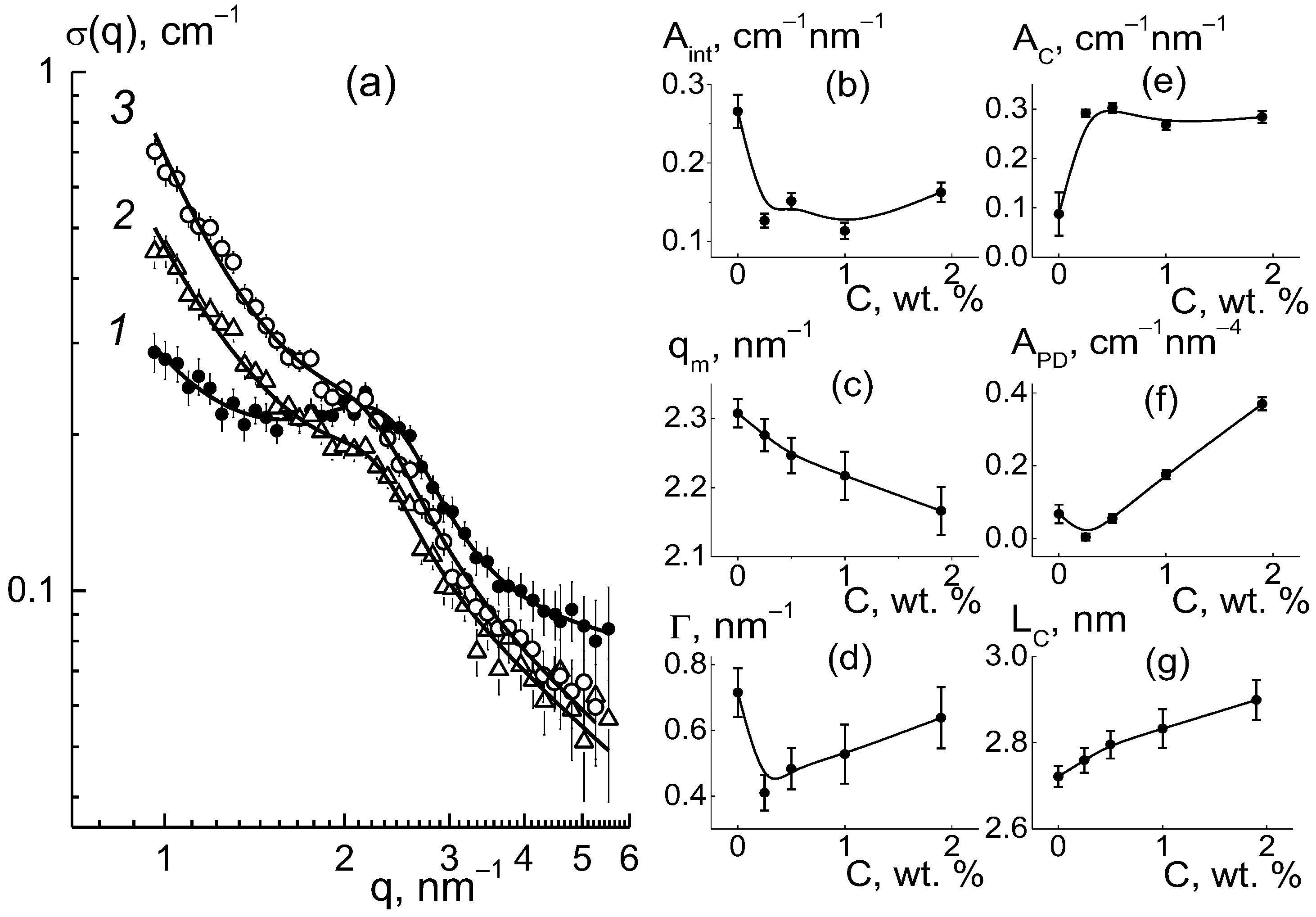
The observed transformation of diamond structures associated with the formation of diamond−polymer interfaces should have affected the network of ion channels in the matrix. However, the scattering patterns showed a relatively stable position for the ionomer peak (q* ~ 2 nm
−1) (
Figure 6a). Hence, the DND Z+ diamonds were compatible with the copolymer and did not interfere with the formation of its inherent supramolecular structure with hydrophobic domains and ion channels during a packing period L
C ~ 2π/q* ~ 3 nm. Meanwhile, the diamonds modulated the characteristics of ion channels and affected their ordering in the polymer matrix.
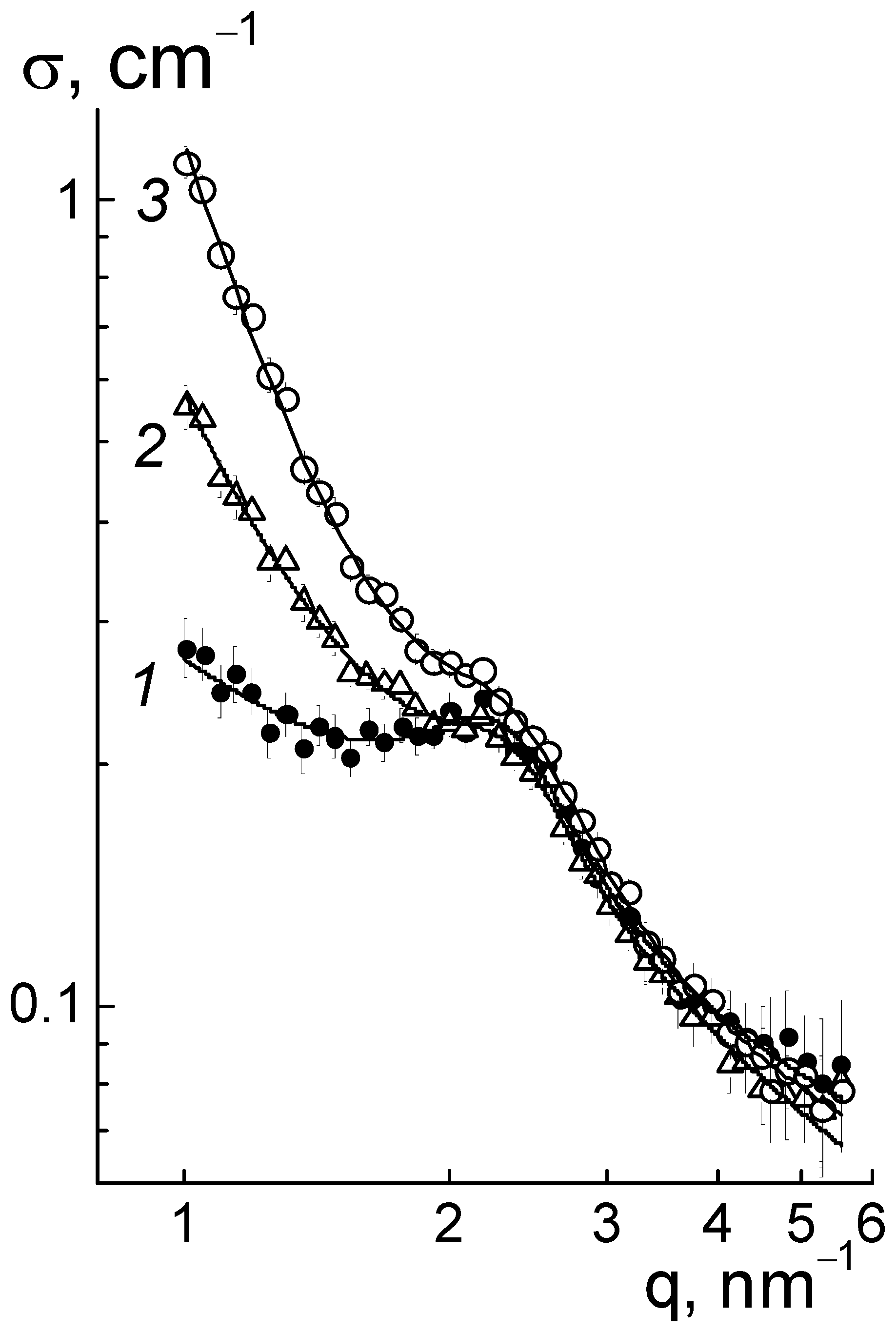
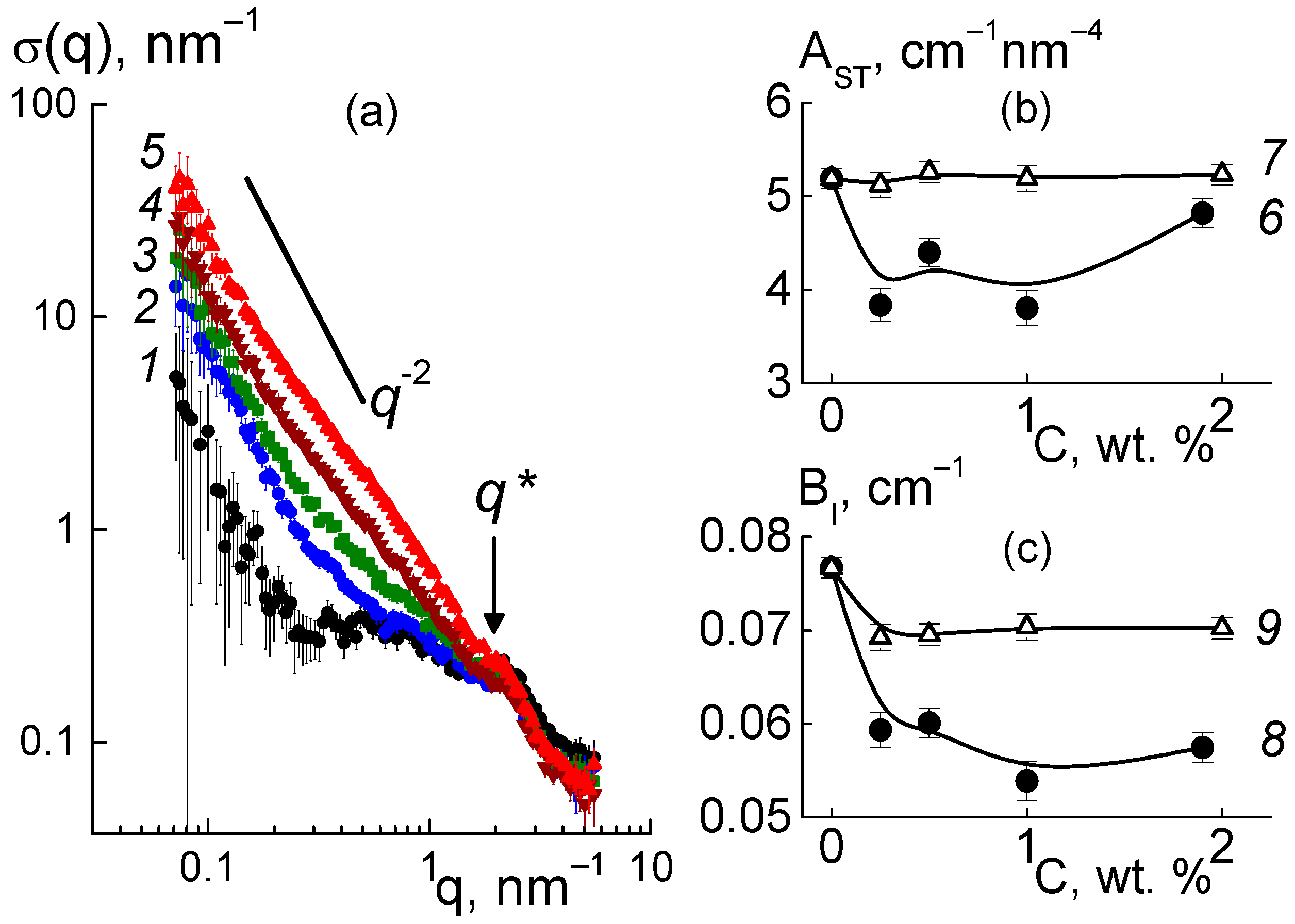
For the composites, the behaviors of cross sections (
Figure 6a) at momenta q ≥ 1 nm
−1 were analyzed using the following function (
Figure 9),
which included the contributions of the ionomer peak (Lorentzian with amplitude A
int, maximum position q
m, half-width at half-maximum Γ), linear fragments of channels (~1/q), diamond surface (~1/q
4), factors A
C, A
P), and background additive (B
g). For all of the membranes, the sections obeyed Function (3), which is shown for several samples (
Figure 9). The approximation parameters are given in
Figure 10.
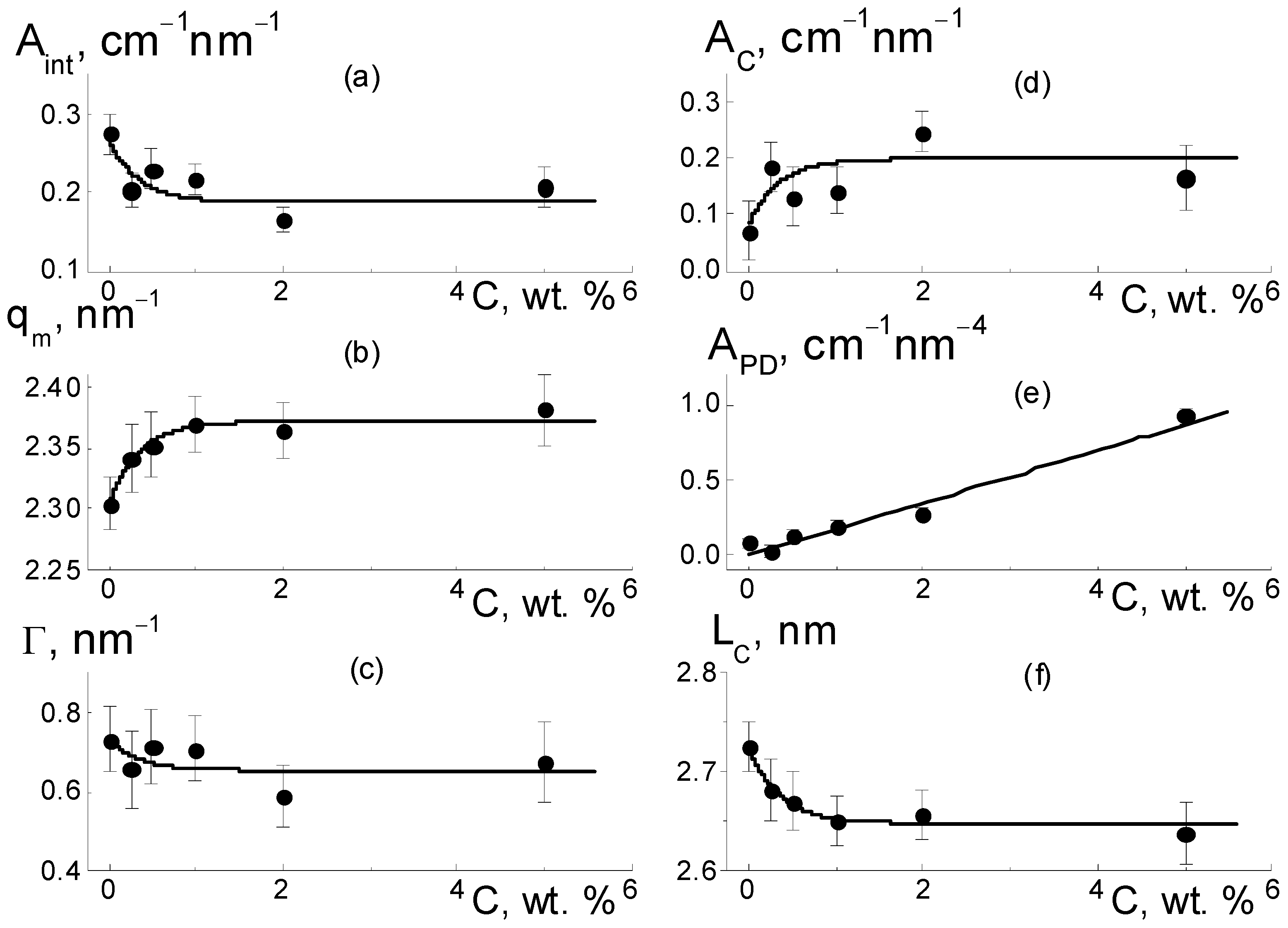
As they are dependent on diamond concentration (
Figure 10), all the parameters (except for the diamond-related A
PD factor) follow a common law
where P
0 = P(C = 0), and ΔP is the initial parameter and its variation at filler content C >> C* = 0.35 ± 0.10 wt. % is far above the critical value found from the approximation of q
m(C) data using Function (4) (
Figure 10b).
As a result of the presence of diamonds, the copolymer structure relaxed from a pristine to modified state when the diamond fraction exceeded a certain level (C*), at which the distance between DND Z+ particles, on average, decreased to ~30 nm. This was comparable to the diameter of bundles (fibrils) composed of ion channels in perfluorinated copolymers [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. Further membrane filling (C > C*) meant the integration of particles into a network of ion channels.
According to the data (
Figure 10a), due to the copolymer with DND Z+ modification, the amplitude of the ionomer peak decreased, ΔA
int(C)/A
int(C = 0) ~ 30%. However, the peak width (Γ) remained practically unchanged (
Figure 10c). Consequently, the diamonds did not disturb the coordination of channels in bundles with a transverse size L
coh = 2π/Γ ~ 10 nm (scattering coherence length). The observed shift in the ionomer peak (q
m) (
Figure 10b) demonstrated a reduction in the period L
C = 2π/q
m ~ 3 nm of channel packing, ΔL
C/L
C ~ 3% (
Figure 10f). This indicated channel compression due to electrostatic attraction between the ionic groups of the diamonds and copolymers. As a result, the interference effect (A
int) was reduced (
Figure 10a).
Such interference (A
int) weakening (
Figure 10a) was caused by the formation of diamond-polymer interface enclosed ionic groups for both components. Such a process meant building additional ion channels, which is evident from the increase in the total scattering ability of channels (A
C) (
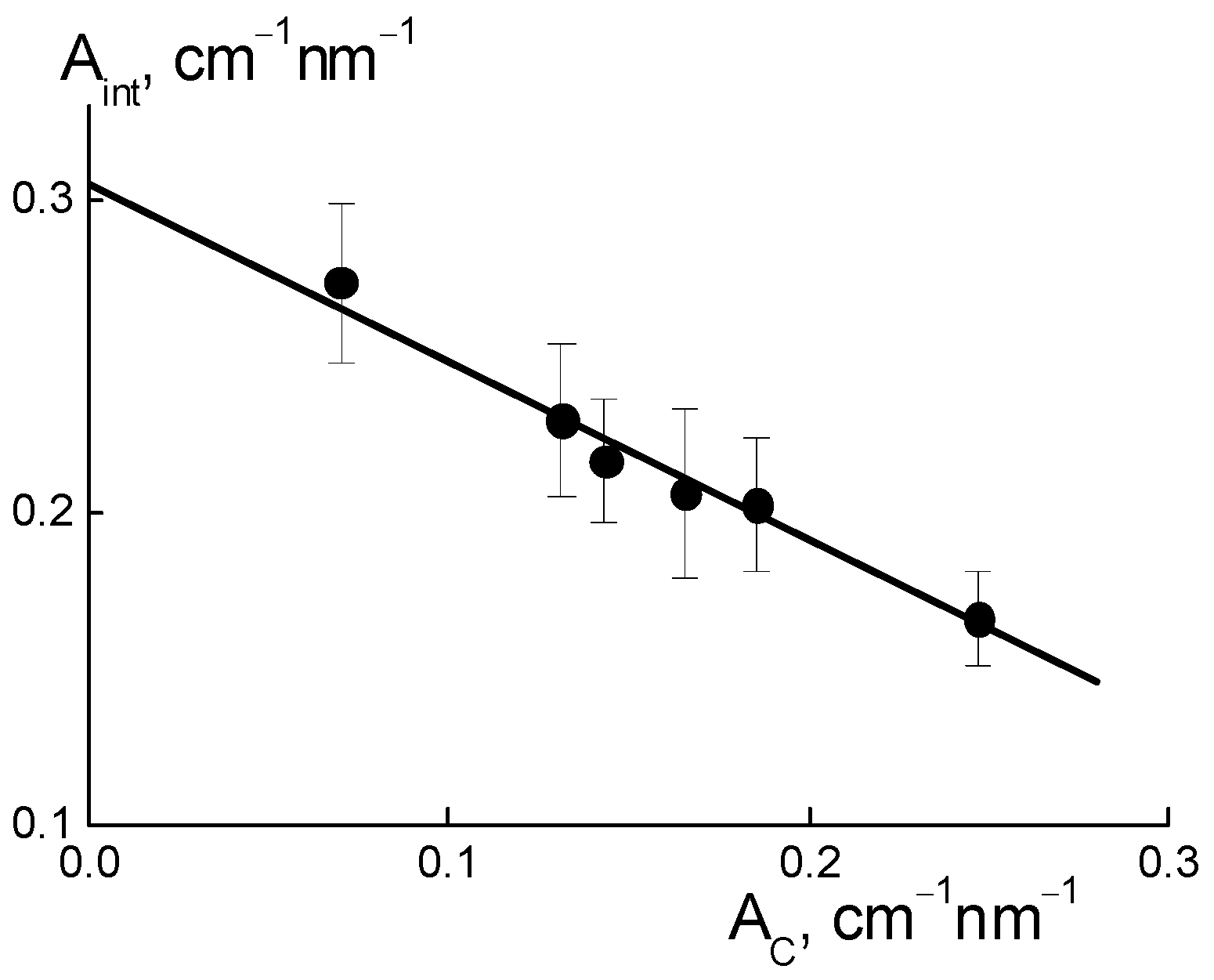
Figure 10d). Along with this, we discovered a linear correlation between the parameters (
Figure 11),
The excess in scattering from channels (A
C), together with interference damping (A
int) (
Figure 11), should be treated as a result of diamond-polymer interface formation when increased ionic groups in the copolymers do not create their own channels. A lowered interference effect from channel packs (bundles) was not compensated for by scattering from the interface, with different scattering abilities than that for the ionic channels in the matrix. The observed correlation between parameters A
int(C) and A
C(C) can be explained by a linear increase in the values A
C(C) = A
C0 + β
DC with the concentration of diamonds. They created additional ion channels with a certain ability (factor β
D) through the integration of ionic copolymer groups. As a result, the quantity of polymer channels also decreased linearly.
As a result of the decrease in total volume of polymer channels, the amplitude of the ionomer peak was weakened, A
int(C) = (ν
C − 1)(A
C0 − α
IG·C), where ν
C is the number of channels collected in a bundle and α
IG is the coefficient that determines the rate of decrease in the volume fraction of polymer channels. This implies a linear relationship between the parameters A
int(C) and A
C(C) (
Figure 11),
From Equation (6) with the A
I = ν
CA
C0(1 + α
IG/β
D), B
I = ν
C(α
I/β
D) parameters found above, we computed the ratio (α
IG/β
D) ≈ 0.15 and channel number in a bundle ν
C = B
I/(α
I/β
D) + 1 ≈ 5. A low magnitude of (α
IG/β
D) ≈ 0.15, the formation of hybrid channels was shown through the diamond-polymer interface. This was seven times more active than the growth of the polymer channel’s lack. Owing to the substitution of polymeric ion channels by hybrid planar ones, there was a good profit in the water adsorption and proton conductivity in the membranes when modified with DND Z+ (
Figure 4a,b).
With the concentration of diamonds (
Figure 10e), the interface area (S
DP) increased linearly according to the dependence A
PD = 2π(ΔK
DP)
2S
DPφ
F = k
P·C, where the coefficient k
P = 0.17 ±0.01 cm
−1 nm
−4 is proportional to the fraction φ
F of free surface in the total area of the diamond interface. Using the k
P value, we estimated the magnitude of φ
F ≈ 2/3. We found that a third of the diamond surface was not manifested in scattering due to joint crystal facets in chains when the fraction of the open surface was almost the same as for the contacts of the cubic particles.
In general, our analysis showed that in the copolymer matrix, when modified with diamonds (C = 0.25–5.0 wt. %), the channel bundles were retained like in the pure copolymer, and these packages with a transverse size of ~10 nm were composed of ~5 channels. However, in the composites, the channel packing density increased by ~6% as a result of a reduction in the spacing between channels, LC ~ 2.6–2.7 nm.
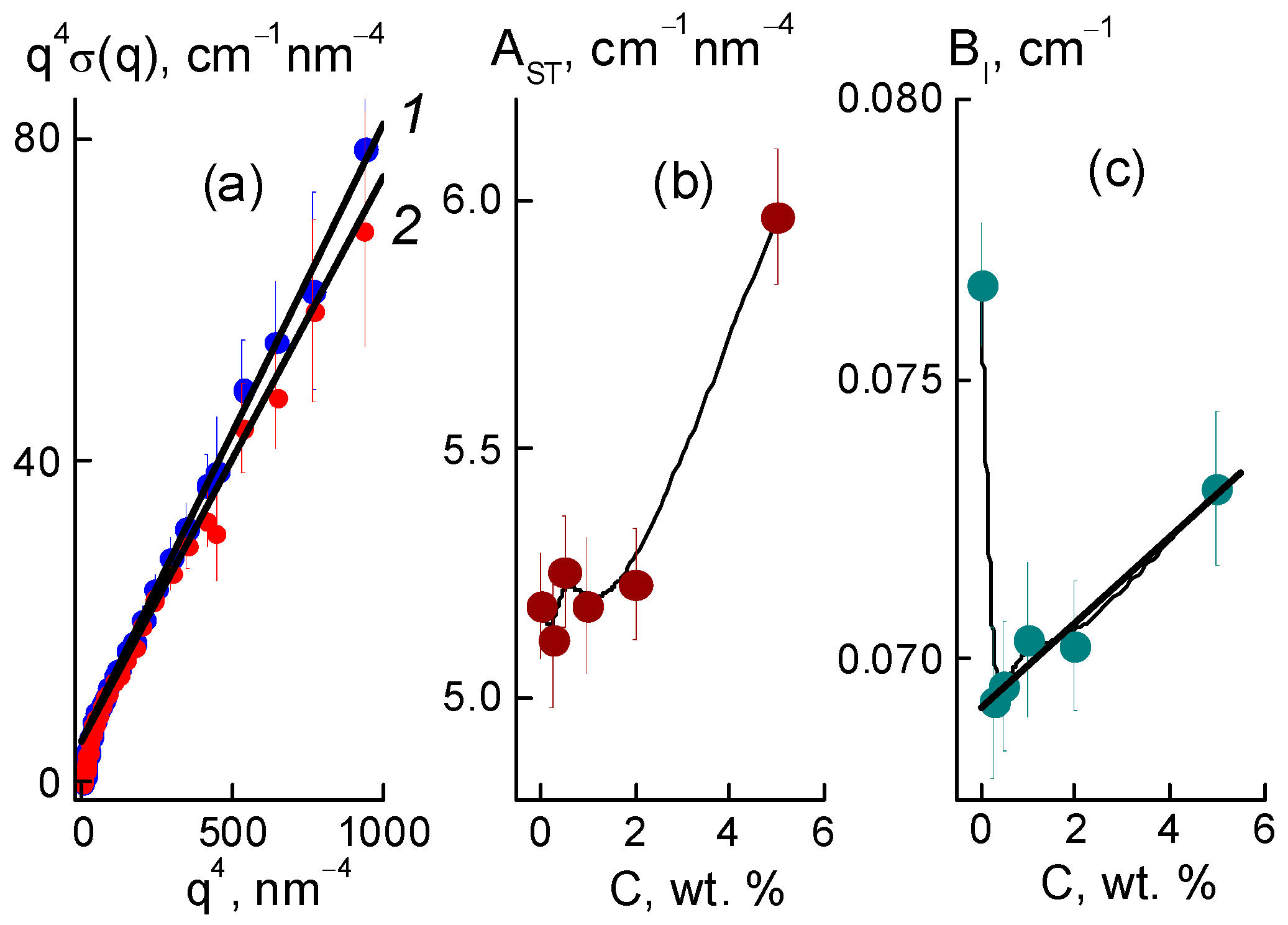
Further, we found a narrowing of ion channels from the estimates of their total area. Direct information about the surface of the ion channels in the membranes was obtained from SANS at a high momenta q ≥ 3 nm
−1, when channel boundaries and diamond facets predominantly induced scattering according to Porod’s law, σ(q) ~ q
−4. For the samples, the modified sections q
4σ(q) dependent on the argument q
4 demonstrated linear behaviors (
Figure 12a),
where parameter A
ST = 2π[(ΔK
P2)S
TP + (ΔK
DP2)S
TD] includes the contributions proportional to the areas S
TP, S
TD of the polymer channel surface, and the diamond-polymer interface. The A
ST depends on the contrast factors for channel walls and diamond-polymer interfaces, ΔK
P = 4.3 × 10
10 cm
−2 and ΔK
DP = K
D − K
P = 7.4 × 10
10 cm
−2, where the scattering length densities for the diamond and polymer were K
D = 11.7 × 10
10 cm
−2, K
P = 4.3 × 10
10 cm
−2, respectively. The B
I coefficient is the cross section for scattering from individual atoms in the membranes. A
ST, B
I are plotted vs. the diamond fraction in the membranes (
Figure 12b,c).
Filling with diamonds up to 5 wt. % increased the area of internal boundaries in the membranes according to the growth of the A
ST parameter (
Figure 12b). Here, the increase ΔA
ST ≈ 0.8 cm
−1 nm
−4 corresponded approximately to the gain in similar characteristic A
PD(C) found for the diamonds at momenta q ≥ 1 nm
−1 (
Figure 10e). Note, A
ST(C) included contributions from both the polymer and diamonds (
Figure 12b). Therefore, A
ST(C) deviation from a linear dependence was associated with a reduction in the polymer channel surface in the composites.
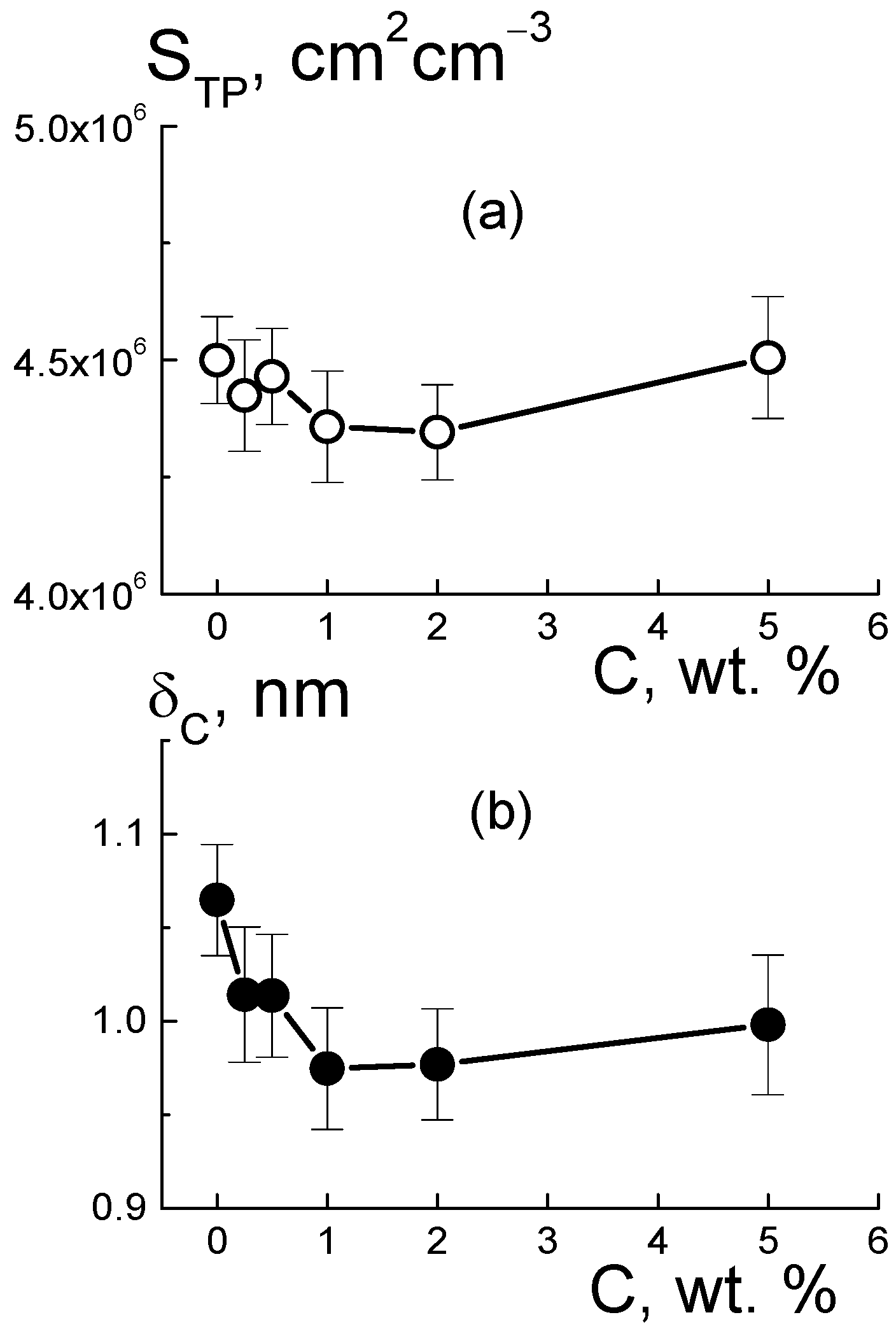
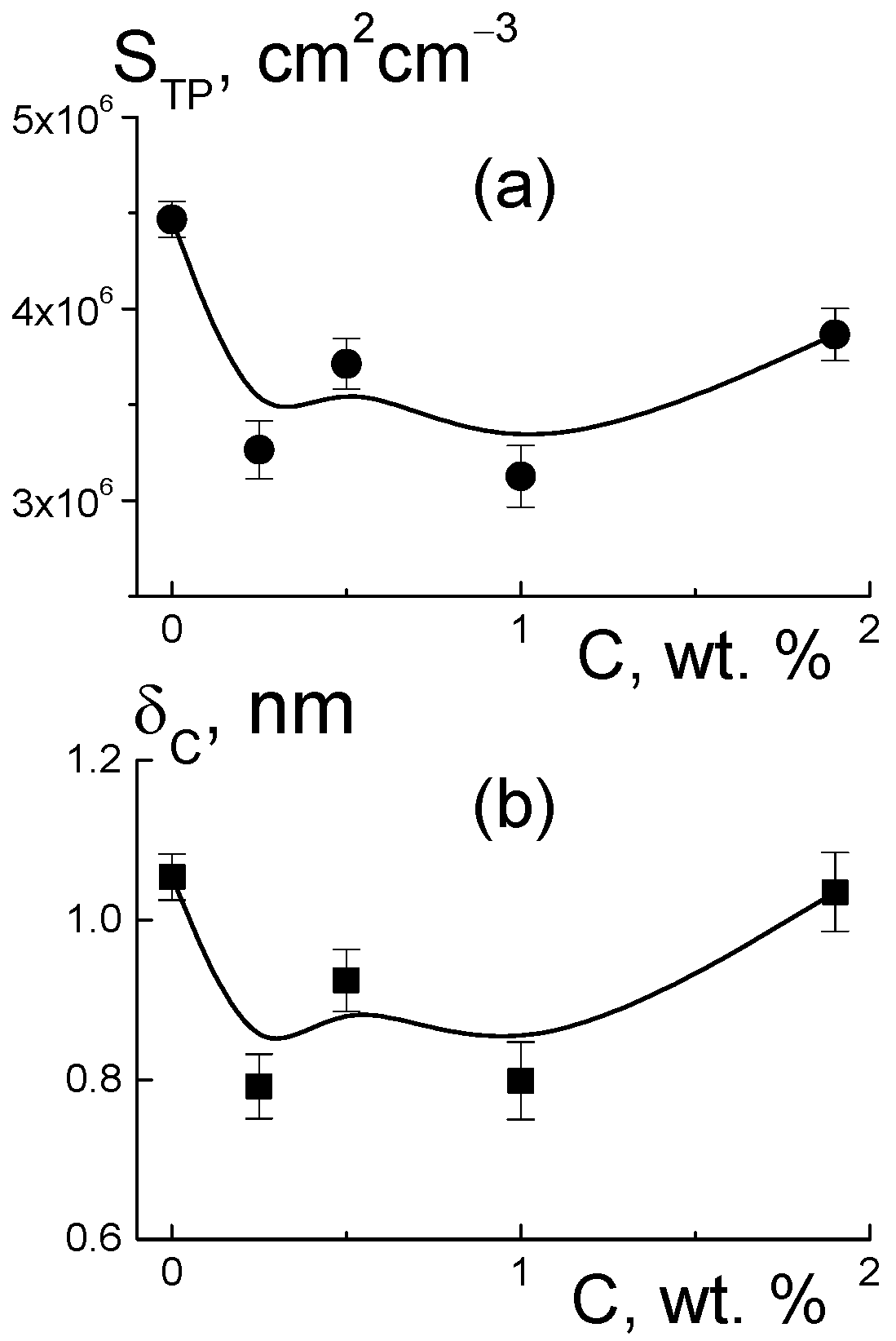
Based on the value of AST for the pure copolymer, we found the integral area of channels STP = AST/2π[(ΔKP2) ≈ 4.5 × 106 cm2/cm3. Because of the large number of polymer ionic groups, NGR = 1.35 × 1021 cm−3, which created channels, area STP was greater by an order of magnitude than the one for the free borders of diamonds StD = 3.9·106 cm2/cm3, even at a diamond content of 5.0 wt. %. In the polymer channel, there was a small area per a group, S1 = STP/NGR ≈ 0.3 nm2. On average, the distance between groups √S1 ≈ 0.5 nm was close to the length of the polymeric side chains with the terminal groups. This ensured their overlap when they covered channel surfaces.
For the pure membrane from the integral area S
TP and the packing period of channels L
C (
Figure 10f), we estimated their diameter, δ
C ≈ S
TPL
C2/π ≈ 1 nm. Locally, the channels were linear within the distance limited by the size of the polymer domains, L
P ~ 2π/q
mp ~ 12 nm, in accordance with the position of wide scattering peak q
mp ≈ 0.6 nm
−1, which reflected the packing of domains in the matrix (
Figure 6a). This size was consistent with the scale of the coherence region in scattering from channel bundles, L
coh = 2π/Γ ~ 10 nm, estimated using the width of the ionomer peak (
Figure 10c). In the copolymer matrix, the channel units with a length of ~ L
P formed branched structures with a fractal dimension D
cf = 2.6 ± 0.3. This parameter was found from an approximation of SANS data in the interval q = 0.07–0.3 nm
–1 using the function σ(q) = A
cf/q
Dcf + B
cf with the parameters A
cf = 0.0049 ± 0.0034 cm
−1 nm
−Dcf, B
cf = 0.17 ± 0.06 cm
−1 (
Figure 6a, data 1).
Then, we determined the characteristics of the polymeric channels in the composites using the A
ST(C) parameter (
Figure 12b) corrected for the contribution of diamond borders, A
PD(C). In this way, we evaluated the surface area S
TP(C) = [A
ST − A
PD]/2π(ΔK
P2) and polymer channel diameter δ
C = S
TPL
C2/π in the membranes with various proportions of diamonds (
Figure 13). The addition of diamonds up to 1 wt. % into the membranes caused a reduction in the total area of the polymer channels (S
TP) by ~3% and a narrowing in their diameter (δ
C) by ~8% (
Figure 13). The enrichment of membranes with diamonds (5 wt. %) did not affect the channel diameters (δ
C ≈ 1 nm) (
Figure 13b), as their area was restored to the initial level (
Figure 13a). The behaviors of these characteristics allowed us to state that the increase in total channel length was ~10%. This effect could be attributed to the partial segregation of excess diamond when it disturbed the copolymer structure less.
The data in
Figure 13b explain the shortening in the packing period of channels (L
C) (
Figure 10f) as a consequence of the compression of the channels themselves at a stable shell thickness when the copolymer was modified with DND Z+. Despite channel squeezing, the membranes obtained an enhanced ability to absorb water. This was revealed in the behavior of the B
I(C) parameter (
Figure 12c). Although the magnitude of B
I(C) decreased sharply when a small fraction of diamonds (0.25 wt. %) was introduced into the copolymer, the enrichment of the samples with diamonds caused linear growth of the parameter (
Figure 12c). In following analysis, we compared these results with the data for the nuclei of the elements (C, F, O, S, and H) in the samples, taking into account the nuclear coherent lengths b
ci (i = 1, 2, …, 5) and the incoherent cross sections σ
INi/4π,
where N
i is the numerical concentration of atoms.
Using the data [
49] and Equation (8), we calculated B
Iest ≈ 0.037 cm
–1 for the dry copolymer (density ρ = 2.2 g/cm
3) with the equivalent weight EW = 890 g-eq/mol. The copolymer was composed of fragments -[CF
2-CF
2]
n-[CF
2-CFG
S]- consisting of tetrafluoroethylene units (n ≈ 6) and a unit carrying the side group G
S (-O-CF
2-CF
2-SO
3H).
The experimental value of B
I = 0.077 ± 0.001 cm
−1 exceeded the estimate, as the sample contained bound water, similar to the usual amount in films of Aquivion
®-type copolymers from the mechanical tests (~6 wt. %) [
50]. The difference B
I-B
Iest = 2(σ
H/4π)N
W ≈ 0.04 cm
−1 was determined by the proton incoherent scattering cross section, σ
H ≈ 80.3 × 10
−24 cm
2 [
49] and water molecule concentration N
W. As a result, we found that each SO
3H group was linked to λ
W ≈ 2.3 water molecules on average. This corresponded to water mass and volume fractions of Wm = 4.7 wt. %, Wv = 8.8 vol. %, respectively.
Such treatment of B
I(C) data for membranes with a variation in diamond fraction showed a significant influence of the DND Z+ filler on the adsorption properties of the membranes. Even a small filler amount (0.25 wt. %) caused a substantial decrease in the parameter, δB
I ~ 10% (
Figure 12c), and lack of water in the composite, ΔWv = 1.8% (20% reduction). The diamonds, which occupied 0.17% of the membrane volume, could not substitute an amount of water an order of magnitude greater. However, due to electrostatic attraction to the ionic groups of the copolymer, these diamonds could block some of the channels and reduce the degree of hydration of the SO
3H groups. With further modification, the adsorption capacity of the membranes was restored, as the diamonds with hydrogen atoms and hydroxyls at the surface formed hydrogen bonds with water molecules and the hydrophilic diamond-copolymer interface attached well to water. The estimates below endorse this mechanism.
The saturation of membranes with diamonds (5 wt. %, volume content of 3.4 vol. %), when their numerical concentration reached a high magnitude, N
DND ≈ 6 × 10
17 cm
−3, led to linear growth of the B
I parameter (
Figure 12c). The gain δB
I~5.5% corresponded to the relative additive water volume ΔWv = 0.84%. If we associate this amount with diamonds, then each DND Z+ particle could accept ~470 water molecules, covering the entire available diamond surface, S
D1 ≈ πd
P2(2/3) ≈ 42 nm
2, where the factor (2/3) was a part of the open surface in diamond aggregates, as estimated above from the data in
Figure 10e.
In fact [
13,
35], the number of functional groups (H, OH) grafted to a diamond was an order of magnitude less than the actual amount of water molecules. Hence, high adsorption occurred mainly due to polymeric ionic groups forming hydrophilic layers around the diamonds, and this assumption was confirmed by the conductivity effect (
Figure 3a,b). For a particle, we obtained a number of polymeric ionic groups in the interface, n
ig = S
D1/S
1 ≈ 140, where S
1 ≈ 0.3 nm
2 is the area per group on the surface of the channels. The SO
3H group was attached to approximately 2.3 water molecules and the number per diamond particles was ~320; as a result of diamond group hydration, the total amount of water molecules was ~400, which was in agreement with the experimental quantity (~470).
As stated above, the developed a diamond-copolymer interface stimulated water adsorption (
Figure 4a), and the hybrid diamond-polymer channels helped increase conductivity (
Figure 4c). In the composite with a high modifier fraction (5 wt. %), the numerical concentration of particles N
DND = 6.0 × 10
17 cm
−3 presented a minimal gap between the diamond particles, ΔR
D ≈ (N
DND−1/3 − d
P) ~ 10 nm. As it was comparable to a transverse diameter of channel bundles, the formation of hybrid channels seemed probable as a result of the association of diamonds carrying ionic groups that were positively charged with a copolymer bearing negatively charged groups. This was consistent with the enhancement of scattering from the linear channel fragments with the diamond concentration (parameter A
C) (
Figure 10d), which indicated the growth of the total volume fraction of channels in the membranes.
When the surface of the DND Z+ diamond framework became conductive, the membrane was transformed into a hybrid form; the polymeric ion channel bundles between the diamond chains also created a conductive interface with the copolymer. The results were achieved through the use of DND Z+ particles with a positive potential as modifiers of perfluorinated copolymers. Conversely, when we modified the same copolymer with negatively charged DND Z− diamonds carrying carboxyl groups, it resulted in a different structure of the composite, where the diamonds contributed minimally to the increase in conductivity of membranes (
Figure 3c,d).
3.2.2. Membranes with DND Z− Diamonds
The SANS data for dry composites with DND Z− (
Figure 14a) were similar to those for the membranes with DND Z+ (
Figure 6a). In both cases, the initial copolymers and composites were prepared in similar ways, although the cross-sectional profiles of the initial samples in the low-momentum range (q ≤ 0.1 nm
−1) were slightly different (
Figure 6a and
Figure 14a); this is related to spatial scales of tens or more nanometers and was not as significant in our work. We focused on the nature and mechanisms of channel formation and ordering in the composites at scales of ~10
0–10
1 nm, which has not been studied much to date.
According to the data in
Figure 14a, the cross sections of composites with DND Z− at momenta q ≤ 0.1 nm
−1 were approximately two times less than those of the samples with DNDZ+ (
Figure 6a). This could be attributed to the mutual repulsion of the copolymeric and diamond ionic groups carrying negative charges. Such interactions are less conducive to extended chain assembly, but stimulate the segregation of DND Z− particles into submicron(micron)-sized globules detected on the surface of composite films by atomic force microscopy (AFM) [
31]. In our neutron studies, such large aggregates were beyond experimental resolution, which was limited by the observation of structures with scales ~2π/q ~ 10
0–10
2 nm.
Similar to what has been shown above, when analyzing diamond structures in composites, the contribution of the polymer matrix was subtracted from the cross sections, Δσ(q,C) = σ(q,C) − σ(q,C=0). At small momenta q < 0.1 nm
−1, we used the Guinier approximation to treat the different data and to determine the gyration radii R
G and aggregation numbers n
A of the diamond aggregates (
Figure 14b–d). These structures had gyration radii R
G ~ 20 nm (
Figure 14b) comparable to the sizes of the DND Z+ aggregates (
Figure 7a).
A comparison of the data (
Figure 7a,b and
Figure 14b,c) for the composites with DND Z− and DND Z+ showed that in the samples with DND Z− particles, both the size and mass of aggregates (R
G, n
A) decreased more strongly than in samples with DND Z+. In the DND Z− aggregates, the volume content of diamonds, Φ
A~3–4%, dropped to the level of the total fraction of diamonds φ
D in the sample with 5 wt. % modifier (
Figure 14c). This fact was associated with the low fractal dimension of chains in the aggregates found from the approximation data at q~0.1–0.4 nm
−1 using the function Δσ(q,C) = A
Fq
−Df with parameters A
F(C), D
f(C) (
Figure 14e,f).
Similar to the samples with DND Z+ (
Figure 7a), the parameter A
F(C) = α
F·C was found to be proportional to the modifier content (
Figure 14f). However, factor α
F = 0.47 ± 0.02 cm
−1 nm
−Df was twice as high as α
F = 0.24 ± 0.01 cm
−1 nm
−Df in the membranes with DND Z+. A difference resulted from a lowering of the fractal dimension of DND Z− chains. This affected the coefficient α
F ~ 1/r
CDf, which depended on the correlation radius of particles (r
C). Thus, DND Z− diamonds formed chains with smaller indices D
f(C) ~1.5–1.7 (
Figure 14f), comparative to D
f(C) ~ 2.2–2.4 in the DND Z+ structures (
Figure 8b).
In the composites, DND Z− particles formed linear, but not branched, chains with an excluded volume conformation, with a fractal exponent of ~ 5/3 [
51]; these chains created nano-sized rarefied aggregates that coexisted together with dense submicron(micron) globular formations that were detected earlier by AFM at the surfaces of the membranes with DND Z− (1; 2 wt. %) [
31]. Altogether, the neutron and AFM data [
31] showed the whole structural organization of diamonds in the copolymer matrix in a wide range of spatial scales ~10
0–10
3 nm.
The bimodal character of the segregation of DND Z− diamonds limited their influence on the structuring of the copolymer, as evidenced by the behavior of the scattering cross sections σ(q,C) around the ionomer peak. Its profile obeyed Function (3) (
Figure 15a), but the related structural parameters depended less on the diamond content (
Figure 15b–g) than that in the samples with DND Z+ (
Figure 10).
In the samples with different fractions of DND Z− diamonds, the ionomer peak retained its amplitude A
int(C) (
Figure 15b) near the average level A
INTA = 0.35 ± 0.01 cm
−1 nm
−1. This reflected the weak influence of DND Z− particles on the polymer channel packing compared with the strong effect of the DND Z+ modifier. Meanwhile, the ionomer peak changed position q
m ~ 2.3–2.4 nm
−1 (
Figure 15c). The curve q
m(C) demonstrated a minimum at point C = 1 wt. % (
Figure 15c), which corresponded a transition to more straightened diamond chains with a lower fractal dimension, D
f(C) ~ 1.7 → D
f(C) ~ 1.5 (
Figure 14f). Such changes only slightly disturbed channel packing. This can be seen from the stable peak width Γ(C) near the average level Γ
A = 0.71 ± 0.04 nm
−1 (
Figure 15d).
Parameters A
int(C) and A
C(C), characterizing the interference in scattering from the channels in bundles and the scattering intensity from the linear fragments of channels, also did not show significant variations (
Figure 15b,e). It indicated the conservation of channel coordination in the bundles, with only weak changes in their packing period, L
C = 2π/q
m ~ 2.6–2.7 nm (
Figure 15g), when a number of channels ν
C in a bundle and the transverse size of bundle L
B ≈ √ν
C·L
C were quite stable. Then, we used the average magnitudes of the parameters, A
INTA = 0.35 ± 0.01 cm
−1 nm
−1, A
CA = 0.10 ± 0.02 cm
−1 nm
−1 (
Figure 15b,e) to find the number of channels ν
C = (A
INTA/A
CA) + 1 = 4.4 ± 0.2, and the transverse size of a bundle L
B ≈ √ν
C·L
C ≈ 6 nm.
We found that both hydrophilic diamonds (DND Z+ and DND Z−), forming branched or linear chains in the copolymer matrix, did not change the character of the copolymer chain assembly, where ion channels were packed into bundles consisting of the same number of coordinated channels, ν
C ~ 4–5, like in the pristine copolymer. The developed diamond-copolymer interface had an area proportional to the content of diamonds according to a variation in the A
PD parameter (
Figure 10e and
Figure 15f). Comparative with DND Z+ diamonds, the particles of DND Z− were less able to form additional ion channels with copolymer participation. This led to a significant difference in the conductivity of the compositional membranes with DND Z+ and DND Z− (
Figure 2 and
Figure 3).
It should be noted that, until now, there have been no attempts to compare the action of the detonation diamonds with different signs of the surface potential on the structure and conductivity of Aquivion
®-type membranes. Here, we can mention only the article [
52], where the authors detected substantial growth in the conductivity of Nafion
®- and Aquivion
®-type materials as a result of embedding of diamonds (0.26–4.0 wt. %) like our DND Z− particles. However, such an effect was observed only at a low humidity of composites (RH~10%), while it disappeared completely at higher water contents. These authors did not present any structural data to explain the conductivity changes in the composites.
For a complete understanding of the mechanisms influencing the different types of diamonds on the membrane conductivity, the results of the composites with hydrophilic fillers (DND Z+ and DND Z−) were compared with the data for samples with hydrophobic fluorinated DND-F diamonds.
3.2.4. Micellar Models for Membranes
The results obtained provide grounds to consider cylindrical inverted micelles with a shell of hydrophobic chain fragments around the ion channel as the key element of the membrane structure. As a result of micelle packing, a matrix with a system of channels was created, the polar surface of which formed the boundaries of water inclusions in the surrounding hydrophobic polymer matrix.
In the composites with hydrophilic diamonds (DND Z+ and DND Z−), we considered such micelles as being in contact with crystal facets when the fragments of macromolecules achieved both micellar and lamellar conformation, and the facets served to connect ionic channels (
Figure 19).
As it is known, in solutions with low molecular weight surfactants, the size and geometry of micelles are determined by the length of the surfactant molecules and their concentration [
55,
56,
57]. Similar to this, a membrane-forming copolymer can be considered as a polymerized surfactant composed of a sequence of fragments. Each fragment includes a short hydrophobic chain with a hydrophilic unit carrying a side chain with a terminal ionic group. This specifies the equivalent weight parameter (EW), e.g., for Aquivion
®, it is usually EW ~ 500–1000 g-eq/mol. Such a molecular structure has all of the necessary preconditions for the conformation of inverted cylindric micelle in a nonpolar surrounding, which is, in our case, a bulk copolymer matrix.
We used a copolymer of Aquivion®-type with an equivalent weight EW = 890 g-eq/mol. This chain fragment included a number of fluorocarbon units (on average ≈ 6.12) and one unit with the ionic group. The total number of units nU ≈ 7.12 corresponded to a chain with a contour length Lm = nUlU ≈ 1.8 nm composed of units with a size lU = 0.25 nm, similar to that generally found in carbon chain polymers.
Here, we assumed that such chains were folded when forming cylindric micelle (
Figure 19) with a diameter L
C ≈ 2.7 nm, according to neutron scattering data for the channel packing period in the membranes (
Figure 10f). Then, the difference δ
C = L
C − L
m ≈ 0.9 nm provided the estimate of the diameter of channels covered with ionic groups with a size D
IG ≈ 0.3 nm. This implieds the number of groups along the perimeter in the cross section of the channel, n
TR = π(δ
C − D
IG)/D
IG ≈ 6.
To achieve the minimum energy for the dipole interactions, the electric dipoles of the groups were oriented “head-to-tail” and formed a spiral “winding” in the form of a sequence of dipoles and a helix pitch of about twice the chain diameter (~1 nm).
This corresponded to hollow polymerized micelle, formed by joining initially flat multiplets (~6 groups) into a cylindrical entity. Here, an analogy can be drawn between such micelles and structures synthesized from low-molecular-weight surfactants that form cylindrical micelles in solutions, which are stabilized by chemically cross-linking surfactant molecules [
58,
59,
60].
This also resembled a coil-globule transition observed for sulfonated polystyrene in chloroform as result of the electric dipole interaction of SO
3Na ionogenic groups in chains (1.35 mol. %). Increasing the number of ionic groups to 2.6 mol. % led to packing the ionomer chain into a vesicular structure with a solvent inside (vesicle) [
61].
The ordering process for perfluorinated copolymers at the initial stage can be represented as a result of the segregation of several neighboring ionic groups interacting through electric dipole moments and hydrogen bonds, accompanied by the docking of their hydrophobic tails, radially diverging from the multiplet. A cylindrical polymer layer with a thickness of Lm/2 and a width corresponding to double the diameter of the chain was created around it. Adjacent sections of the chain were attached to such a structure according to the same principle of segregation of ionic groups and sequential stacking of hydrophobic chain fragments.
As a result, the copolymer turned into a cylindrical micelle with a cavity covered with ionic groups and a polymer shell with a local orientational order of chain fragments. The relationship between the inner and outer diameters of the micelle was determined using the equivalent weight of the copolymer. Because EW was fixed, the micellar structure remained stable under various conditions (interaction with solvents and modifier particles, mechanical deformations, etc.), while the ion channels underwent an expansion by membranes hydration [
62]. As shown in [
63,
64], for Aquivion
®-type membranes (EW = 790; 870 g-eq/mol) without nanoparticle fillers, through uniaxial stretching of the membranes, their structure was rearranged, but the position of the ionomer peak did not change, which indicated high stability of the channel structures.
As for the structural changes, the following question arises: how can diamond particles (~4.5 nm) significantly larger than the diameter of the ion channels be incorporated into the copolymer matrix? Obviously, a hydrophilic surface of DND Z+ or DND Z− crystals with grafted ionic groups (H, OH or COOH) is capable of adsorbing polymeric acid groups SO3H due to dipole interactions and hydrogen bonds. Hence, the chain fragments with the associated SO3H groups should be stacked perpendicular to the diamond surface and form a flat lamella, but outside the crystal facet, the chain takes the conformation of a cylindrical micelle. If the fragments of several chains condense on a diamond facet, a planar conduction channel linking ion channels can be created using different chains.
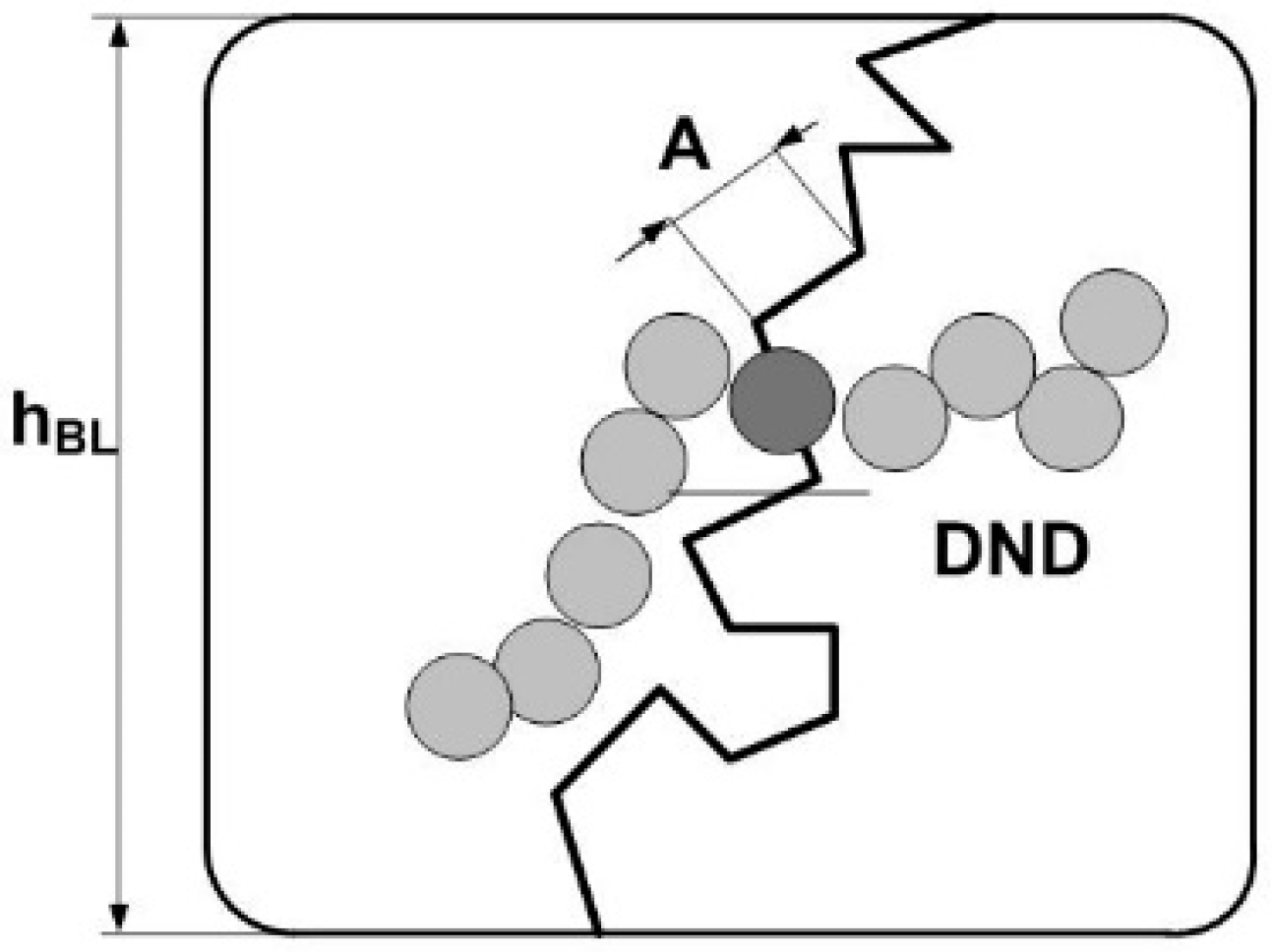
A positive effect of polymer modification with diamonds was expressed not only in the appearance of additional sites for protons due to ionic groups on diamonds, but also in the formation of links for ion channels (
Figure 19). It is worth paying attention to the fact that the diamonds were organized in the chains that adsorbed SO
3H groups, which made an adjacent lamellar layer from the polymer. In this way, hybrid micelles formed on the diamond core (diameter ~ d
P ~ 5 nm) (
Figure 20) when conducting diamond-polymer interface and lamellar polymer shell had the same thickness, Δ
DP ~ L
m ~ 1 nm, and the outer diameter of such micelle was twice the size of the diamond, D
DM = (d
P + Δ
DP + L
m) ~ 9 nm.
Packing the micelles in a matrix suggested their local orientational order with a gap between the diamond surfaces 2(ΔDP + Lm) of ~ 4 nm. This length determined the upper limit for the concentration of diamond particles in membranes, NP* ~ 1/(dPDDM2) ~ 2.7 × 1018 cm−3, when the entire polymer was completely consumed to coat the diamonds with a mass fraction of ~23% (volume content ~13%).
Although both hydrophilic fillers (DND Z+ and DND Z−) had a trend to form conducting interfaces adjacent to the diamond chains, this is actually preferable for positively charged DND Z+ particles.
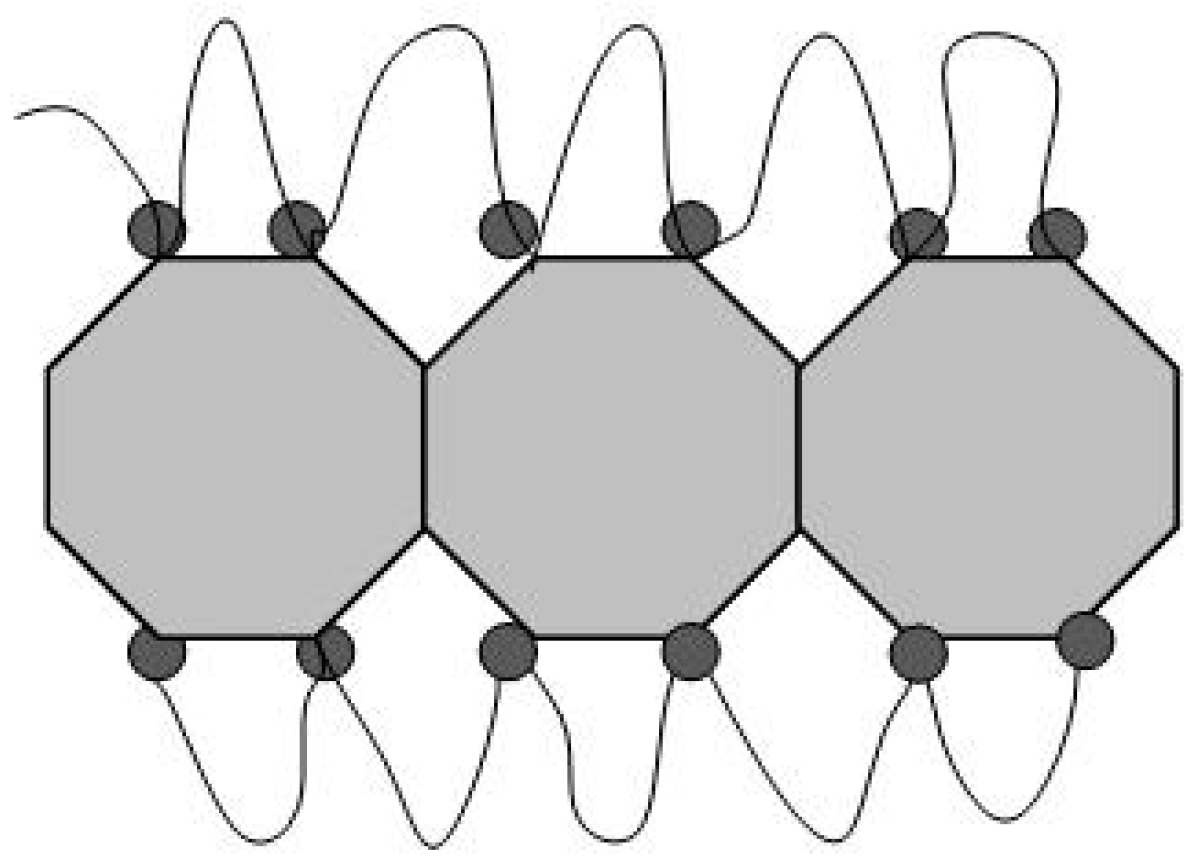
In contrast with this case, predominantly hydrophobic DND-F particles did not bond to polymeric ion channels, but rather interrupted them because of the adsorption of backbone chains on crystal facets, when their ionic groups could not participate in conducting an interface (
Figure 21).
We should note, because of the very long total channel length per membrane’s unit volume Lct ≈ 2.3 × 1013 cm/cm3, fluorinated diamonds (1 wt. %) could reduce it only slightly, ΔLct/Lct ≈ 0.24%. Here, we calculated Lct assuming that every six ionic groups formed a multiplet, which provided a contribution of ~2dpol ~1 nm to the channel length, and the fluorinated diamonds destroyed small channel fragments with a particle diameter size of dP ≈ 4.5 nm. Therefore, the diamond’s effect on membrane conductivity could be a consequence of channel disconnection, but not shortening.
To assess the probability of channel fragmentation in the membrane, we compared the number (n
BL) of channels penetrating a polymer block (size h
BL ~ 150 nm) in the membrane and the number of diamond particles (concentration N
DND) in a block, N
DBL = N
DNDh
BL3 (
Figure 22).
A channel in the form of a Gaussian chain composed of nA segments (A ~ 10 nm in size) had a contour length L1 = A·nA and squared chain diameter hBL2 = nA·A2. With the total length of channels in the block LtBL = nBL·L1 = hBL3·Lct, the number of channels, nBL = hBL·Lct·A ≈ 340 does not exceed the number of diamond particles, NDBL ≈ 400, in each polymer block at the filler fraction C = 1 wt. %. Thus, most channels should be destroyed by diamonds, and membrane conductivity is expected to be decreased in proportion to exp(-NDBL/nBL) ≈ 0.30, which is a probability of a particle not entering a channel. This estimate agreed with a low conductivity level for such a modified membrane, ~26% of the conductivity of the pristine membrane.
Our studies of Aquivion®-type composites concerned the problems regarding membrane modification with diamonds carrying hydrogen or fluorine atoms, hydroxyl, or carboxyl groups at the surface. However, there are other methods of improving membrane functional properties, for example, using diamonds with sulfonic acid groups similar to those in copolymer side chains.
In a recent work [
11], an Aquivion
®-type material was filled with diamonds with grafted sulfonic acid groups (0.5–2.0 wt. %) to stimulate proton conductivity, while maintaining good mechanical properties for the membranes; furthermore, their structure was not noticeably disturbed by such a modifier. Meanwhile, DND Z+ diamonds provide an increase in conductivity through heating the from 20 to 50 °C (
Figure 3), approximately twice as much as that observed under the same conditions for membranes with sulfonated diamonds [
11].
In connection with the obtained results, we have to stress the general problems of comprehension for the structuring mechanism of membranes from perfluorinated copolymers with nonpolar fluorocarbon backbones and side ionic groups. Attempts have been made to understand the patterns of formation of membrane morphology based on studies of the behaviors of perfluorinated copolymers in solutions [
65,
66,
67,
68].
In polar solvents (water-alcohol mixtures), the structuring of perfluorinated copolymers (Nafion
®) occurs by combining fluorocarbon backbones into fibrils [
65,
66,
67,
68]. Neutron experiments [
68] have established that in solutions and gels, Nafion
®- and Aquivion
®-type macromolecules form highly elongated fibrils with hexagonal ordering transversely to their axis. Fibrils with a perfluorinated core and charged ionic groups on the surface had a length of ~1000 nm and a diameter of 4–6 nm when the transverse size of the aggregates depended more on the surface tension than on the dielectric constant of the solvent.
The fibrillar organization of copolymers in solutions, as in the formation of cylindrical surfactant micelles [
69], does not provide compelling reasons to expect that fibrils with a hydrophobic core and ionic groups on the surface will predetermine the morphology of a dry membrane, implying only the transformation of original fibrils into an inverted form during the deposition of the copolymer from the solution onto solid substrates, the removal of the solvent, and the subsequent heat treatment in order to achieve an equilibrium structure.
To create and stabilize a network of ion channels, the membranes were heated above the glass transition region and were annealed (T ~ 120 °C > T
g ~ 100 °C for Nafion
®) [
70]. The achieved equilibrium structure of Nafion
® membranes was dominated by cylindrical (curved) fibrils with a diameter of ~3 nm [
70]. During annealing, the fibrils were oriented and straightened in an electric field to increase the proton conductivity of the material. Transmission electron microscopy data [
70] did not show obvious accumulations of ionic groups with a high contrast (lead modification) on the surface of the fibrils. This indicated a structural transformation of the copolymer during deposition from the solution, formation, and annealing of the membrane film.
In fact, heat treatment provided high segmental mobility to the copolymer chains, facilitating the transition of macromolecules to an energetically favorable (equilibrium) conformation, as determined, first of all, by dipole interactions of the ionic groups neighboring in the chain with the formation of multiplets with the participation of hydrogen bonds [
71]. In turn, sequential linear binding of multiplets transformed macromolecules into cylindrical fibrils with a central ion channel (~1 nm in diameter) and a non-polar shell (outer diameter ~3 nm) in the form of folded packing chain fragments between groups (
Figure 19 and
Figure 21). In this case, the size of the copolymer’s statistical segment did not exceed the length of the chain fragment between the ionic groups (~2 nm with an equivalent weight of ~1000 g-eq/mol), which were defects in the linear chain and contributed to its fracture. Thus, the persistent chain length of ~1 nm (half the segment length) determined the thickness of the polymer shell, with segments radially arranged from the central channel in the cross section of the micelle, and the outer diameter of the micelle being ~3 nm, which was the sum of the channel diameter and twice the shell thickness.
It should be noted that another (spherically symmetric) option for the formation of a micelle from a high-molecular copolymer would be geometrically impossible, as then the size of the central area (~10 nm) filled with ionic groups would be larger than the length of the chain fragment between the groups (~2 nm). Although the formation of an inverse micelle with a central cavity is possible according to Gierke [
12], this model has not been confirmed experimentally [
5,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30].
The formation of monomolecular cylindrical micelles is favored by segmental diffusion inside polymer coils, when contacts of neighboring and more distant ionic groups occur, and their binding by dipole forces and hydrogen bonds leads to segregation of groups from non-polar fragments. As a result of primary self-organization at the molecular level and transformation into micelles, macromolecules create packings with short-range orientational orders (bundles).
In contrast with such a sequential formation of membrane morphology, the authors [
27] proposed an alternative version of supramolecular ordering, when ion channels form in the gaps between parallel polymer chains, which serve as a stabilizing framework and are combined into bundles (fibrils). This model [
27] is valid for rigid chains in a strained conformation, when the association of ionic groups due to dipole forces entails the assembly of chains along the perimeter of the channel. The formation of such structures in membranes obtained by the precipitation of a copolymer from a solution would mean the transformation of already existing fibrils (~1000 nm long, ~4–6 nm in diameter) [
68] into inverse micelles as a result of the rotation of the chains around the fibril axis and the movement of ionic groups into the channel surrounded by fluorocarbon chains.
However, such large linear micelles bound into bundles have not been observed in membranes. In contrast, transmission electron microscopy (TEM) demonstrated curved micelles with a diameter of ~3 nm and a length no more than an order of magnitude greater than the diameter [
70], consistent with the model of monomolecular cylindrical micelles (
Figure 19 and
Figure 21). This was also supported by atomic force microscopy data (measurements of topography and local current from the surface) [
70]. The authors of [
72] identified conducting structures on the surface of a moistened Aquivion
®-type membrane at a scale of tens of nanometers as rolled up conductive lamellae with water conduction channels several nanometers thick enclosed between them, but the mechanism of the appearance of channels with shells of rolled up lamellae was not discussed.
The idea of folding lamellas was used in this work. We assumed that the macromolecule from the flat lamella conformation was twisted into a cylindrical helix with an ion channel along the axis (
Figure 19 and
Figure 21). Thus, a copolymer with an equivalent weight of ~1000 g-eq/mol and molecular weight ~10
6 Da was converted into a micelle with a length of ~100 nm, when ~10 ionic groups were placed on the transverse perimeter of a channel with a diameter of ~1 nm. When micelles were packed in parallel with a period around their diameter of ~3 nm, bundles were formed; from this scattering, a corresponding ionomer peak was observed (
Figure 9,
Figure 15a and
Figure 17a). The transverse and longitudinal dimensions of the micelles were in good agreement with the neutron and synchrotron scattering data, as well as transmission electron and atomic force microscopy [
5].
Hypothetically, the assembly of a micelle with a central ion channel is possible as a result of the association of ~10 macromolecules in a flat lamella conformation, when the number of ionic groups on the transverse perimeter of the channel is also ~10. The length of the assembled micelle will reach ~10
3 nm, and it will be a rigid rod. In this case, the polymer matrix will consist of micron-sized domains in the form of parallel-packed micelles. However, such large micelles and domains have not been observed in membranes [
5]. Thus, the copolymer ordering model, based on the mechanism of conformational rearrangement of a flat macromolecular lamella into a cylindrical micelle, allowed us to combine the ideas of the lamellar model [
73], which specifies the packing period of ion channels by the chain length between the ion groups and the channel size, and the model [
27] of bundles of cylindrical nano-sized water channels with stabilizing polymer frames.
It is important to note that it is energetically favorable in a polymer matrix to form precisely cylindrical monomolecular micelles with ionic groups almost completely shielded from the weakly polar polymer medium. In this case, the assembly of groups into sequentially connected multiplets (channels) has no steric restrictions and provides a high density of group placement on the channel surface. At the same time, narrowing of the channels is achieved, when, with a channel diameter of ~ 1 nm, an increase in the diffusion and proton conductivity of water is observed by several orders of magnitude in comparison with bulk water [
74]. The introduction of diamonds into the copolymer creates conditions for the formation of ionic conduction channels along the crystal facets, when adjacent fragments of the copolymer transform into a flat lamellar conformation (
Figure 19,
Figure 20 and
Figure 21). Thus, the copolymer matrix combines different packaging options for the copolymer to form ion channels.
It is of interest to compare the results obtained for composite membranes based on perfluorinated copolymers with short chains with data for perfluorinated sulfonic acid membranes of the Nafion
®-type with long side chains when these materials are modified with various nanoparticles in connection with the prospects for the development of hydrogen energy and the tasks of creating materials for the entire technological chain, including hydrogen production, purification, storage, and electricity generation [
75]. Ion exchange membranes [
75] based on perfluorosulfonic acids are characterized by a high conductivity and selectivity, especially when using copolymers with a short side chain. However, perfluorinated membranes are very expensive and can only operate effectively at high humidity and temperatures up to 100 °C; this introduces the problem of poisoning of catalysts with carbon monoxide, the sorption of which at temperatures up to 120 °C is virtually irreversible. Hence, there is a need to develop and modify membrane materials by introducing nanoparticles to improve proton conductivity and selectivity during proton transfer.
The authors of [
76] studied the mechanism of the effect of functionalization of the surface of Nafion
®-type membranes and their modifications containing SiO
2 nanoparticles (3 wt. %), propyl, 3-aminopropyl, and 3,3,3-trifluoropropyl, on the physicochemical and electrochemical properties of membranes. It has been shown that filling Nafion membranes with negatively charged nanoparticles (3 wt. % SiO
2; 5 mol. % 3,3,3-trifluoropropyl) led to an increase in their conductivity by 20% in all cases, except when 3-aminopropyl was used, which imparted nanoparticles with a positive charge, and the selectivity of membranes increased in all composites, which was explained by the transformation of the mesoporous structure of the membrane into a microporous one. The effect of nanoparticles on membrane conductivity was mainly caused by the additional (positive) spatial charge introduced into the pore solution and onto the membrane surface by the electrical double layer surrounding the nanoparticles. The greater the surface charge density of nanoparticles and the smaller their size, the stronger the effect. Therefore, the sample doped with SiO
2 and 3,3,3-trifluoropropyl showed the highest conductivity and current density at a low fixed voltage.
The possibilities for improving the hydrophilic and conductive properties of perfluorinated membranes, in particular Nafion
® 117 and Nafion
® 115 materials in comparison with CMX (Neosepta) and Fuji-CEM 80050 (Fujifilm) membranes used in RED (reverse electrodialysis) installations, were discussed in the literature [
77]. The inclusion of inorganic nanoparticles (hydroxides of polyvalent elements of silicon, zirconium, etc.) into their structure led to a decrease in the gas permeability and methanol permeability of hybrid membranes for a variety of applications, including metal-ion batteries and redox cells. There have been known attempts to use nanoporous membranes with a high selectivity and permeability for the RED process through the use of silicon oxide nanotubes and anodic aluminum oxide.
The authors of [
78] studied the effect of ultrasonic treatment for Nafion
® copolymer solutions in the presence of SiO
2 nanoparticles regarding the characteristics of Nafion+SiO
2 cast hybrid membranes. Ultrasonic treatment of polymer solutions reduced the length of the macromolecules and the number of sulfonic groups. When the polymer solutions were treated with ultrasound in the presence of SiO
2, additional chain cross-linking occurred when SiO
2 interacted with the sulfonic groups of the copolymer. As a result, up to 20% of –SO
3H groups were excluded from the ion-exchange mechanism, and the temperature of the destabilization of ion clusters decreased; but, at the same time, the hydrophilic filler was included in the pores, the overall water absorption of the hybrid membranes increased, larger pores formed in them, and the connecting channels became wider, which facilitated the transport of protons and led to greater proton conductivity. Thus, ultrasonic treatment when dispersing filler nanoparticles in Nafion
® solutions made it possible to obtain hybrid membranes with improved transport characteristics.
In membrane technologies, the most important task remains to increase the chemical resistance of proton exchange membranes [
79]. Membrane life limitations arise from free radicals in membrane processes. Nanoparticles can serve as free radical scavengers. The authors of [
79] described a one-step method for preparing in situ hybrid membranes based on the copolymer Nafion
® 117 and sulfonic or phosphoric acid functionalized with cerium oxide. The conductivity of membranes containing sulfonic acid modified with cerium exceeded that of the original Nafion
® 117 membrane at a relative humidity RH = 30% [
79]. In this regard, it is known that membranes initially treated with an oxidizing agent have a better conductivity with a lower permeability to water [
80]. The effect of modifying Nafion 117 membranes with 3,4-ethylenedioxythiophene (EDOT) and hot pressing of hydrogen-oxygen membrane-electrode assemblies increased the efficiency of proton exchange membrane fuel cells [
80].
Another way to improve the electrical transport characteristics of membranes was to plasticize the polymer electrolyte with high-boiling bipolar aprotic solvents, as shown by the example of the lithiated Nafion
® 115 membrane plasticized with sulfolane (SL), ethylene carbonate (EC), and diglyme (G2) [
81]. A Nafion
® 115 membrane in lithiated form (Li-Nafion) was plasticized with a mixture of ethylene carbonate (EC) and sulfolane (SL) to obtain a polymer electrolyte with single-lithium conductivity and increased stability [
82]. The electrochemical properties of swollen Li-Nafion with non-volatile binary plasticizer EC/SL were found to be suitable for practical applications in lithium batteries [
82].
The authors of [
83] introduced ferrocyanide-ferrocyanide Fc (II)–Fc (III) particles into proton exchange membranes (PEM), perfluorosulfonic acid (PFSA), and sulfonated hydrocarbon membranes. The particles participated in the redox cycle and scavenged radicals. Composite membranes based on Nafion
® and Aquivion
®, as well as hydrocarbon SPEEK, SPSf, SPS, and SPN, had increased chemical stability and durability for operation in fuel cells compared with the original membranes and were superior to the composite membranes with cerium Ce
3+ ions traditionally used as antioxidants. The proposed strategy is considered to be a universal one for improving the chemical oxidation resistance of hydrocarbon-based PFSA and PEM membranes.
In connection with the operation of fuel cells, attention has been paid to the problem of hydrogen crossover, which affects the durability of hydrogen fuel cells [
84]. The authors [
84] discussed the effect of hydrogen crossover on the components and characteristics of fuel cells; analyzed the factors of structural permeability and the reasons for the destruction of the membrane, as well as ways of increasing its durability, including through chemical cross-linking, but this led to a loss of conductivity. It was also proposed to add stable porous materials to the membrane to reinforce it. To improve the chemical stability and durability of the membrane and reduce hydrogen crossover, methods were considered in order to change the chemical structure of the membrane and reduce the quantity of free radicals by introducing scavengers (inhibitors), such as metal oxides and their complexes (CeO
2, ZrO
2, MnO
2, etc.).
Our analysis of works in the field of ion-exchange membranes [
75,
76,
77,
78,
79,
80,
81,
82,
83,
84] showed high research activity and achievements in the field of improving the functional properties of membranes in various ways, including using nano-sized modifiers, but without the use of nanodiamonds, despite the beneficial properties of such crystalline particles. These particles are chemically inert and heat-resistant, and are capable of carrying grafted ionic groups that create a charge on the surface of the particles, the sign and magnitude of which can be set during the modification process. Hence, the novelty and significance of the results obtained in our work on the modification of diamonds and the preparation of composite membranes with different concentrations of diamonds is obvious.
The membrane modification methods developed in our work can be compared, in particular, with the introduction of nanofibers into proton exchange membranes (PEM) [
85], where the fibers are able to form a stabilizing framework and long-range channels for proton transport, while reducing fuel crossover. This kind of structural design using functionalized fibers that create interconnected channels (networks) for proton transfer is attractive for membrane technologies, but requires significant improvements in the methods for obtaining fibers with desired structural and physicochemical characteristics. A related area can be considered as the development of methods for modifying membranes with polymers. The authors of [
86] synthesized sulfonated poly(indene) (SPInd, degree of sulfonation 35%; 45%) and mixed it with the Nafion
® material, obtaining composites (10, 15, and 20 wt. % of modifier)—the proton conductivity of which reached twice the value relative to the base material. At the same time, the thermal resistance of the composite remained at the level of the indicator for Nafion, with a slight increase in water absorption. This approach is convenient in that it allows one to adjust the characteristics of membranes without affecting the mechanism of formation of the conducting channels.
In this regard, diamond frameworks in membranes with variations in diamond functionalization methods provide greater opportunities for targeted modification of the structural and conductive properties of proton exchange membranes through the organization of hybrid conductive channels involving the diamond surface and through the transport of protons along diamond chains at the diamond-polymer interface. In our work, it was possible to modify the mechanism of proton transport in order to improve the conductivity and mechanical properties, and increase the strength of the membrane material due to the special qualities of diamonds (chemically inert, durable, thermally stable, and resistant to ionizing radiation, as a result of its developed surface with a controlled composition and number of functional groups) compared with other nano-sized objects.
Due to their beneficial properties, nanodiamonds are also used in filtration membranes for nuclear technologies with requirements for radiation resistance in the materials [
87]. Carboxylated nanodiamonds (CND) were used to modify polysulfone (Psf), which had limited radiation resistance (up to 100 kGy). Membranes with a CND fraction of 0.5% wt. withstood doses an order of magnitude higher (1000 kGy) [
87], as the diamond particles adsorbed chemically aggressive products of water radiolysis [
88]. The prospects for using diamonds to increase the thermal and radiation resistance and service life of filtration polymer membranes were discussed in review [
89], in connection with the problems of modifying the surface of diamonds. Nanodiamond particles were silanized [
90] to be incorporated into Psf membranes (up to 1 wt. %) to increase the hydrophilicity, water permeability, and porosity of the material with smaller void sizes. Through interfacial polymerization, a polyamide composite was obtained with diamonds that had reactive functional groups and a hydrophilic surface for binding to the matrix, increasing the wettability of the membrane surface and increasing the thermal stability [
91].
When discussing the possibilities of using diamonds in membrane technologies, it should be noted that not only nanodiamonds, but also other carbon particles with a high specific surface area and acid groups, have been introduced into Nafion
® and Aquivion
® materials [
92,
93,
94]. Multiwalled nanotubes (CNTs), deagglomerated detonation diamonds (DNDs), and nanocharcoal enhanced the conductivity of the Aquivion
® material [
93]. CNTs (diameter 8–10 nm, specific surface area 276 m
2/g) grown from the gas phase and treated with concentrated nitric acid were used. The synthesis of Aquivion
® composite membranes with oxidized carbon nanotubes was also performed [
95]. The increase in proton conductivity was observed in such membranes, especially at a low humidity, which was also confirmed by experiments [
93].
To modify the membranes, nanocharcoal (particle size 30–40 nm, specific surface area 1000 m
2/g), obtained by igniting methane in a chlorine atmosphere, followed by purification in a nitrogen flow (1000 °C) and oxidation in air (300 °C), was used [
93]. Sulfonated graphene oxide was incorporated into Nafion
® to improve the conductivity over a wide temperature range by reorganizing the conductive channels and increasing the proportion of bound water [
94]. In samples of CNTs, nanocharcoal, and DND, the concentrations of acid sites on the particle surface were 0.75, 3.5, and 0.4 mmol/g [
93]. Despite the low content of acid groups, diamonds provided the greatest effect for increasing conductivity at a low humidity [
93]. At 12% humidity, the conductivity of the Aquivion material increased four times as a result of the addition of 0.4% DND. This was explained by the small size (5 nm) of particles which could enter membrane channels, changing their geometry and improving conductivity [
93], according to the theory of [
96].
These data, in connection with the results of our work, seem important, as they show that diamonds modify membrane structure due to their ability to self-organize in low-molecular and polymeric media. Suspensions of nanodiamonds in liquid polydimethylsiloxane were tested using broadband dielectric spectroscopy and X-ray scattering [
97]. The conductivity and dielectric constant of the suspensions strongly depended on the chemical composition of the surface of the particles; polarization processes with temperature changes were of an activation nature, which indicated the structuring of diamonds [
97]. The conclusion was confirmed by rheological and structural data for DND hydrosols and gels (particles 4–5 nm, 1–7 wt. %) with negative (ζ < 0) and positive (ζ > 0) electrokinetic potentials [
98]. At DND fractions of 4–6 wt. % in experiments with rotational viscometry, X-ray scattering, NMR, and cryo-electron tomography, a sol-gel transition with viscosity hysteresis and a thixotropic effect was discovered, which was explained by the formation of a network during the interaction of diamonds [
98]. The aggregation of diamonds was enhanced as a result of the grafting of lanthanide atoms (Eu and Gd) to the surface of particles, which led to the formation of branched fractal structures with a dimension of 2.4 and a scale of 40–1500 nm, according to neutron scattering data in aqueous dispersions of modified diamonds [
99].
As our results have shown, fractal patterns of ordering for ensembles of diamond particles are especially important for the formation of composite membranes with a diamond modifier. In this case, it is necessary to regulate the state of the surface of diamond particles, as well as the number and type of functional groups (proton donors) in order to set the particle potentials that determine their interaction and ordering in membranes. The results of quantum chemical modeling of charges on hydrogen atoms of groups (H, OH, and COOH) on the surface of diamonds showed that a hydrogen atom in the OH group had the highest charge [
1,
100,
101,
102,
103,
104]. The same methods were used to calculate the electronic structure of SO
3H groups in Nafion
® and Aquivion
® monomers in order to evaluate the influence of the charge states of atoms in ionic groups on the proton mobility [
101].
The developed modeling approaches [
100,
101] allowed for optimizing the strategy for creating composite membranes with nanodiamonds and other forms of nanocarbon in connection with various applications (chemical energy sources—fuel cells, redox batteries, reverse electrodialysis devices, and lithium-ion batteries) [
1]. Although the possibilities of perfluorinated membrane technologies have not been exhausted, composites based on other polymers, in particular polybenzimidazole, are being created. Such composites showed the highest conductivity with a significant proportion of silicon dioxide in propylimidazoline groups (10 wt. %), as at low concentrations of the modifier, the permeability of the membranes to hydrogen decreased [
102].
Along with this, interest remains in the development of membranes made from perfluorinated sulfocationic polymers (thermally initiated polymerization at high pressure) [
103]. The resulting samples, tested at temperatures of 21 and 79 °C, had conductivities of 57 and 114 mS/cm, respectively, which is higher than the commercial Nafion
® material, reespectively. Research is being developed [
104] in the field of perfluorinated sulfonated cation-exchange membranes, differing in the length of the side chain and the proportion of fragments with ether groups. The material MF-4SK (Plastpolymer, St. Petersburg, Russia) in protic and potassium formed when modified with membrane foil (Mega, Czech Republic) and phosphate-modified zirconium dioxide [
105] demonstrated a strong increase in conductivity and selectivity. Recently created [
106] polymer membranes (Nepem-117) in Li+ form, when saturated with polar aprotic solvents (dimethylformamide, dimethyl sulfoxide, dimethylacetamide, and mixtures of solvents), showed an increase in ion mobility as the degree of membrane solvation increased (NMR data), which exceeded the characteristics of Nafion
® membranes with aprotic solvents [
106].
At the same time, the variety of possibilities for modifying membranes with various nanoparticles is much wider, especially taking into account the options for grafting functional groups onto their surface. Thus, due to phosphonic groups grafted to the polymer in hybrid membranes of N-phosphorylated polybenzimidazole with silica (2–20 wt. %, particles with sizes of 3–5 and 20–60 nm), as a result of additional hydration, the increase in the proton conductivity of the composites occurred at a sufficiently high temperature (130 °C) [
107]. Considerable attention has been paid to studying the influence of the acid-base properties of inorganic particles on the physicochemical and transport properties of ion-exchange membranes [
108]. Particles of Zr, Ti, and Si oxides were synthesized in the pores and channels of the membranes, which, depending on the acid-base properties, increased or decreased water absorption, conductivity, and selectivity. Cross-linking of ion-exchange membranes using ZrO
2 particles made it possible to increase the swelling, conductivity, and selectivity of salt-permeable membranes in the Na
+ form [
108]. The introduction of sulfonated zirconium oxide into perfluorinated cation-exchange membranes MF-4SK made it possible to increase their conductivity at room temperature by four times, while reducing gas permeability to hydrogen by a factor of three [
109]. Hybrid ion-exchange membranes were obtained by directly synthesizing amorphous zirconium phosphate (0.5–24 wt. %) in the pores of the RALEX
® CM matrix, which led to the displacement of water from the pores and an increase in the number of cation transfers, increasing selectivity for monovalent ions [
110].
Thus, oxides of metals and other elements are mainly used in hybrid membranes, where particles are introduced into matrices or synthesized in their pores while carbon nanoparticle applications are just beginning to develop. The results of our work show the abilities and prospects for introducing diamonds into membrane technologies using various methods of modifying the surface of diamonds, regulating the type and number of grafted functional groups, surface potentials of particles, and, ultimately, forms of structuring diamonds in polymer matrices to achieve increased conductivity, moisture absorption, and mechanical and temperature stability for hybrid membranes. The original results obtained can be compared with a detailed analysis of studies in the field of membranes based on perfluorinated copolymers, given in the review of [
5]. The main trends in the development of perfluorinated membrane technologies are presented in a series of original studies and reviews devoted to the improvement of functional properties of these materials through modification with various nanoparticles [
111,
112,
113,
114,
115,
116,
117,
118].