A Recent Review of Electrospun Porous Carbon Nanofiber Mats for Energy Storage and Generation Applications
Abstract
:1. Introduction
2. Manufacturing Technologies and Surface Modification Processes
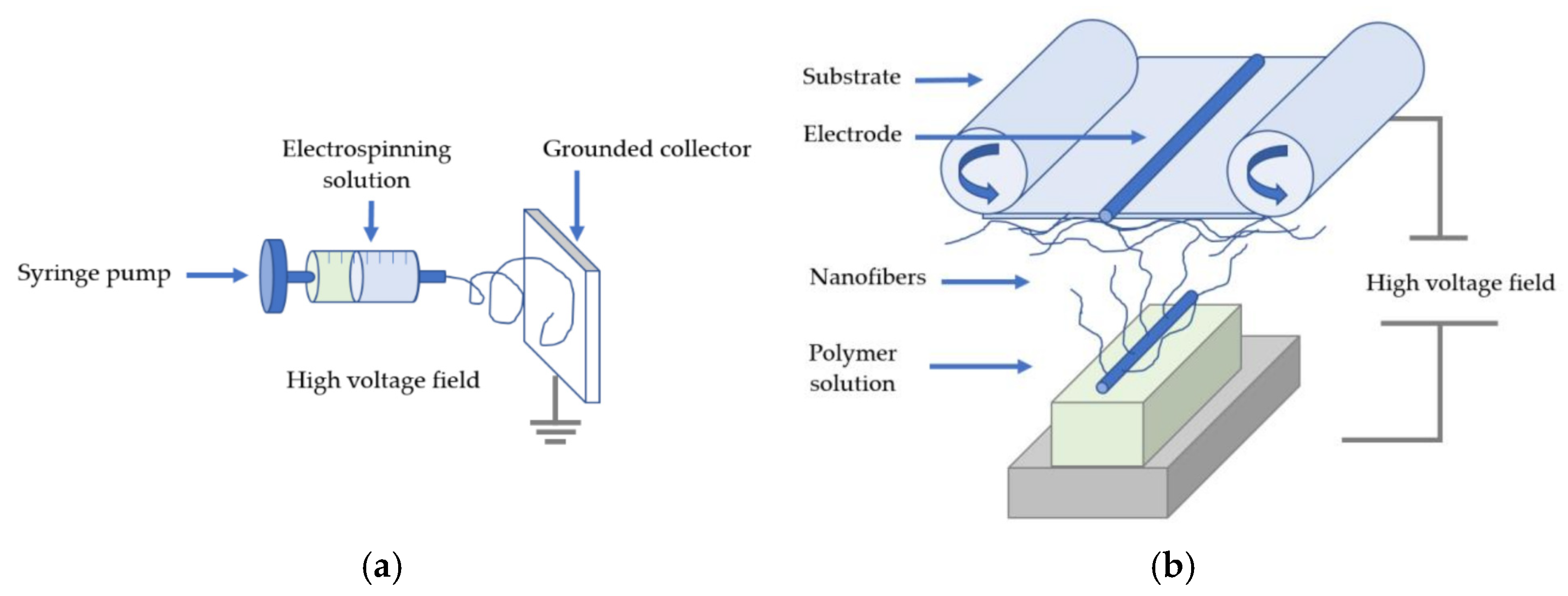
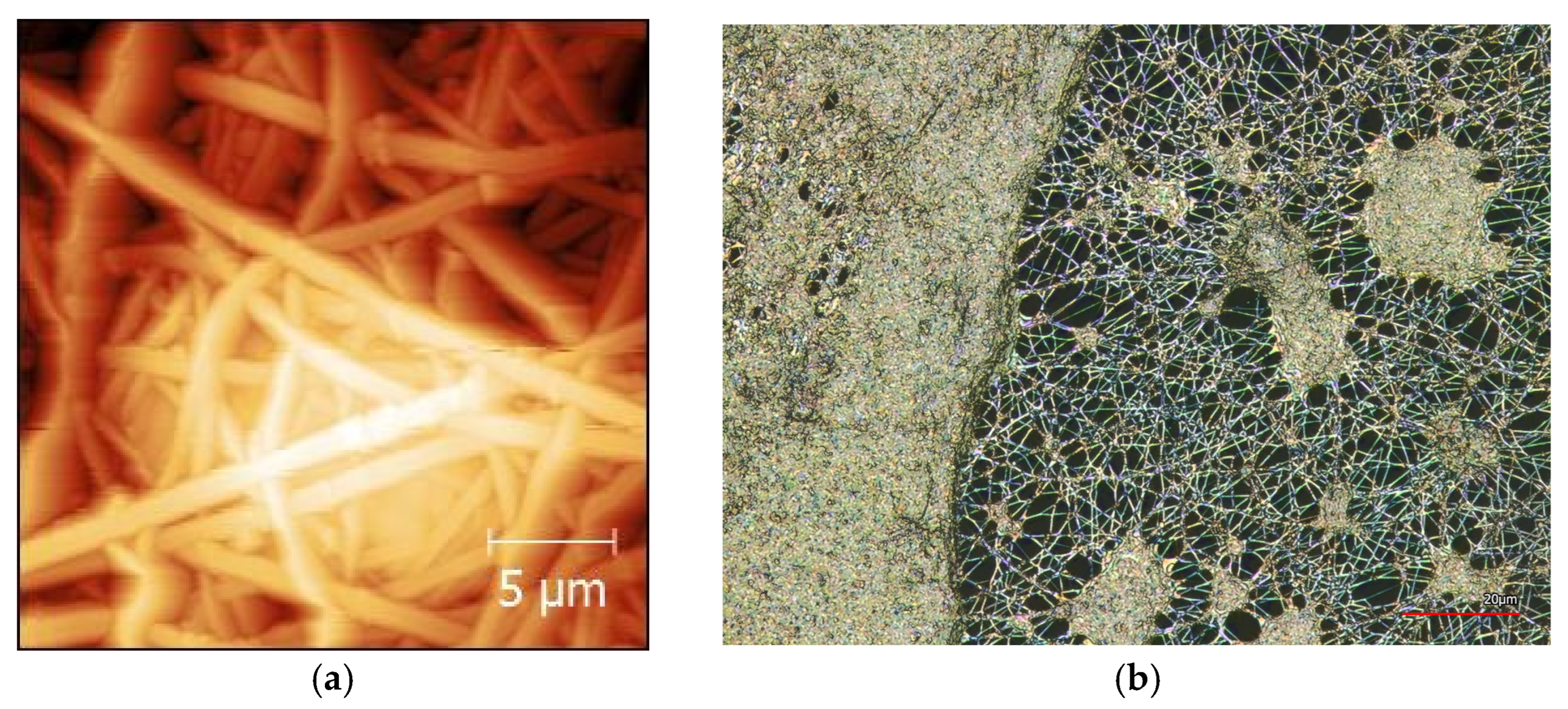
2.1. Electrospinning
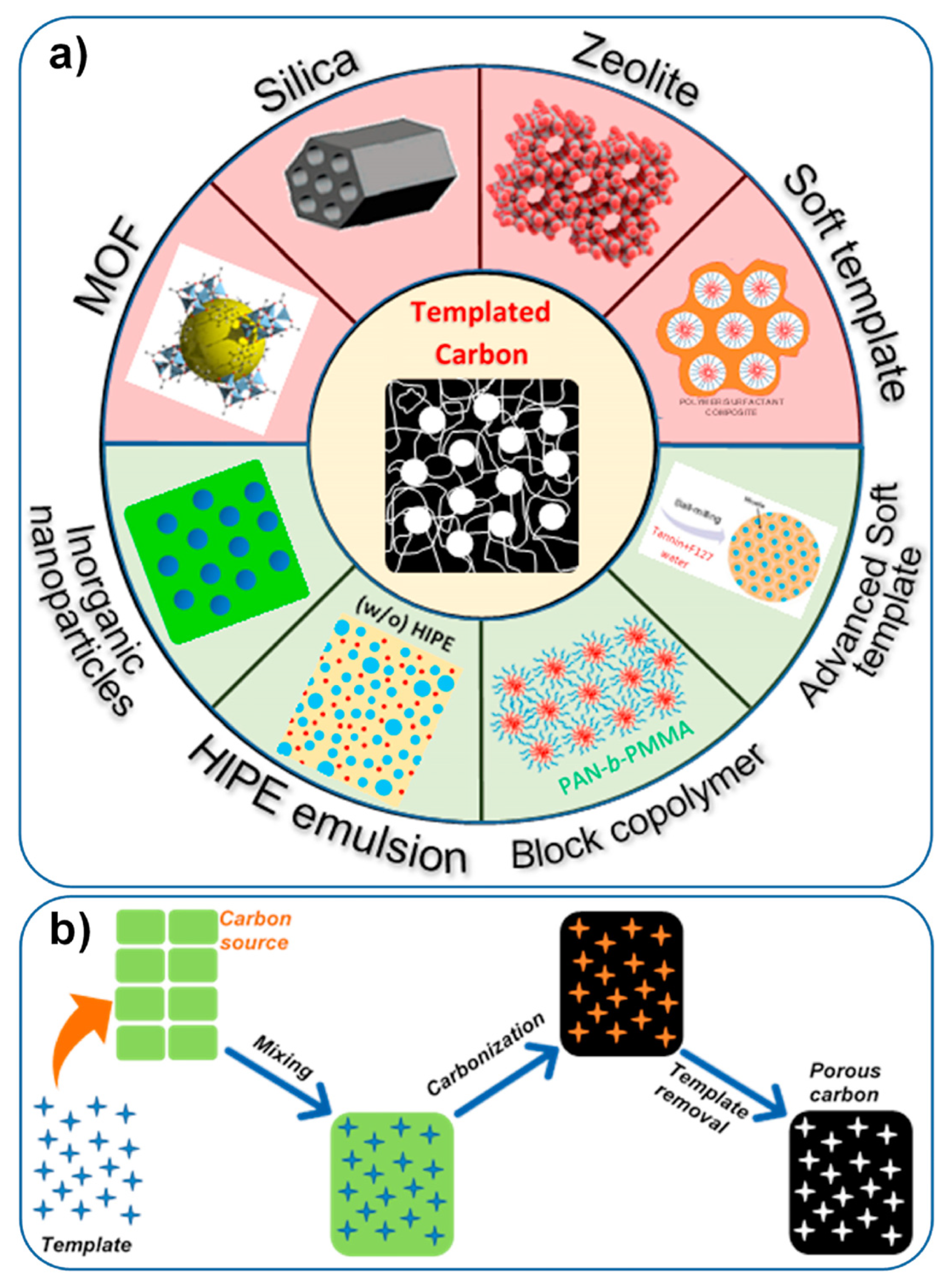
2.2. Template-Based Synthesis
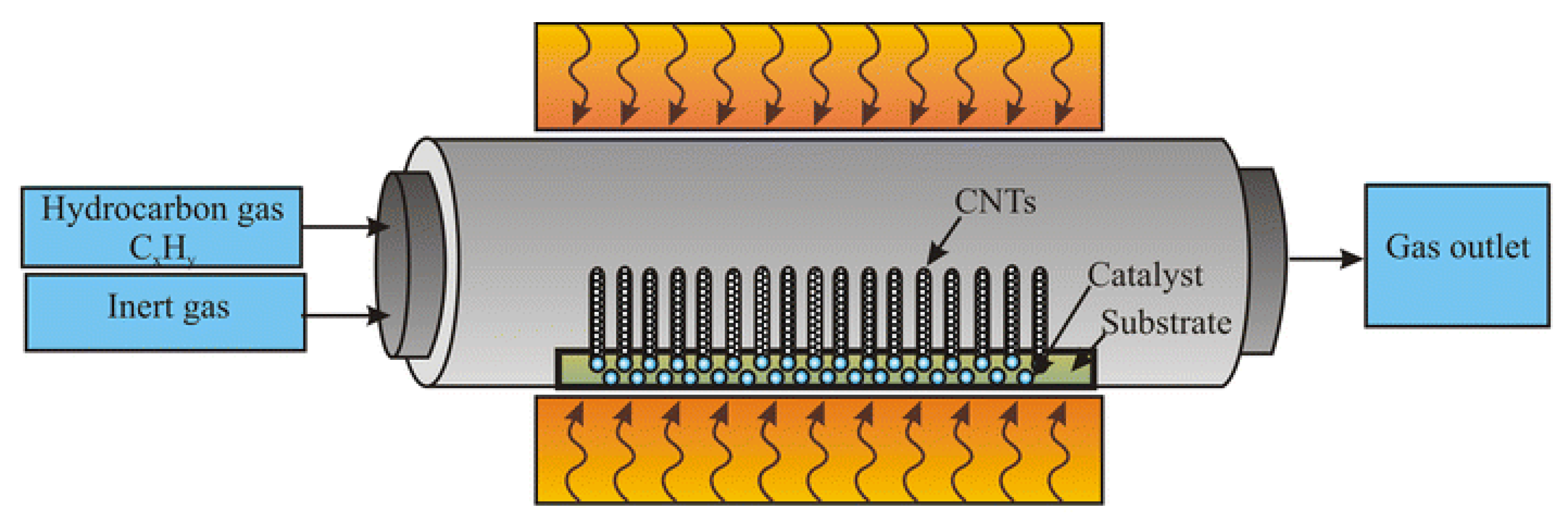
2.3. Chemical Vapor Deposition (CVD)
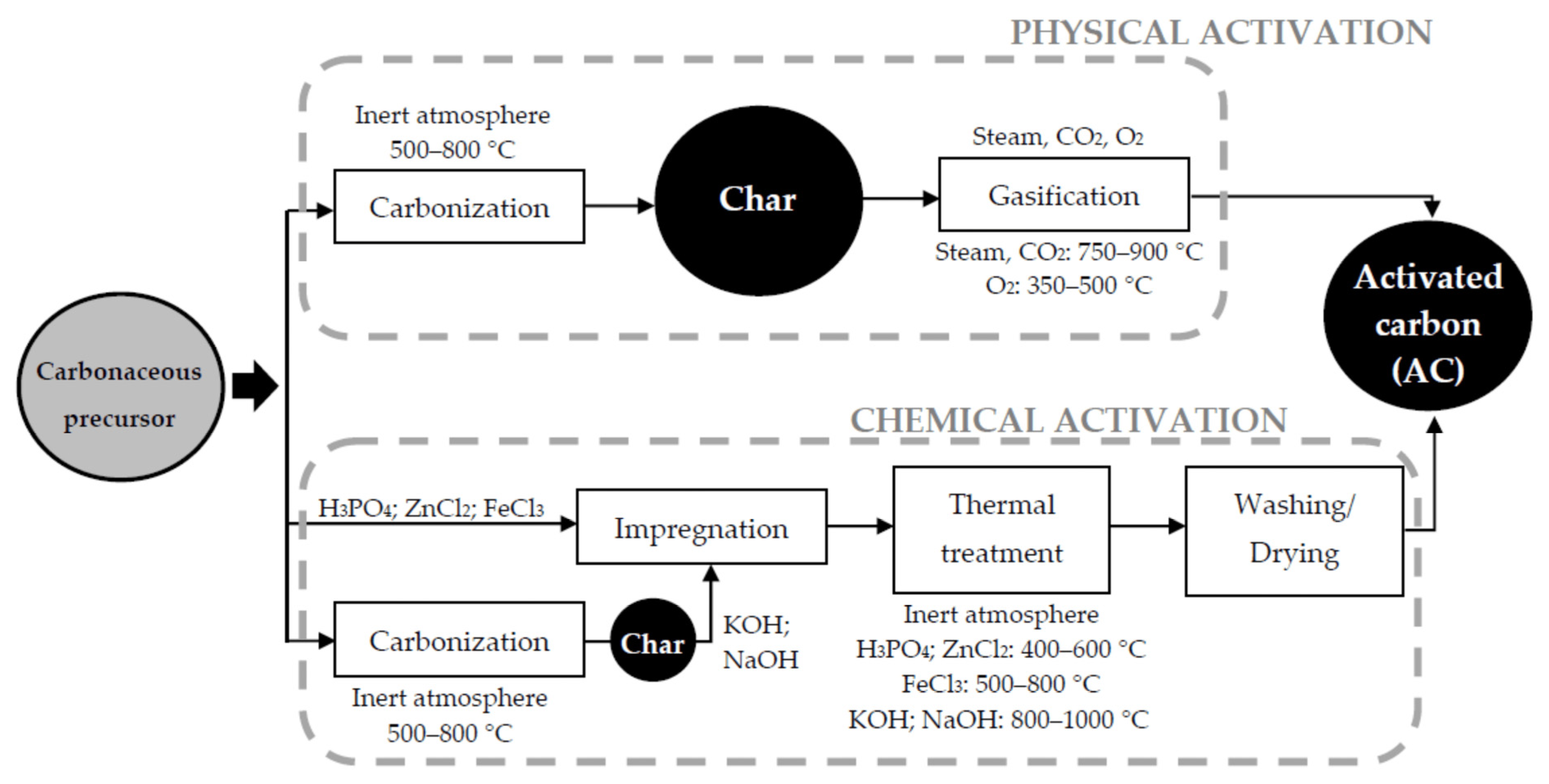
2.4. Activation Processes
2.5. Other Methods
3. Stabilization and Carbonization Process of Nanofibers
4. Energy Storage and Power Generation Applications
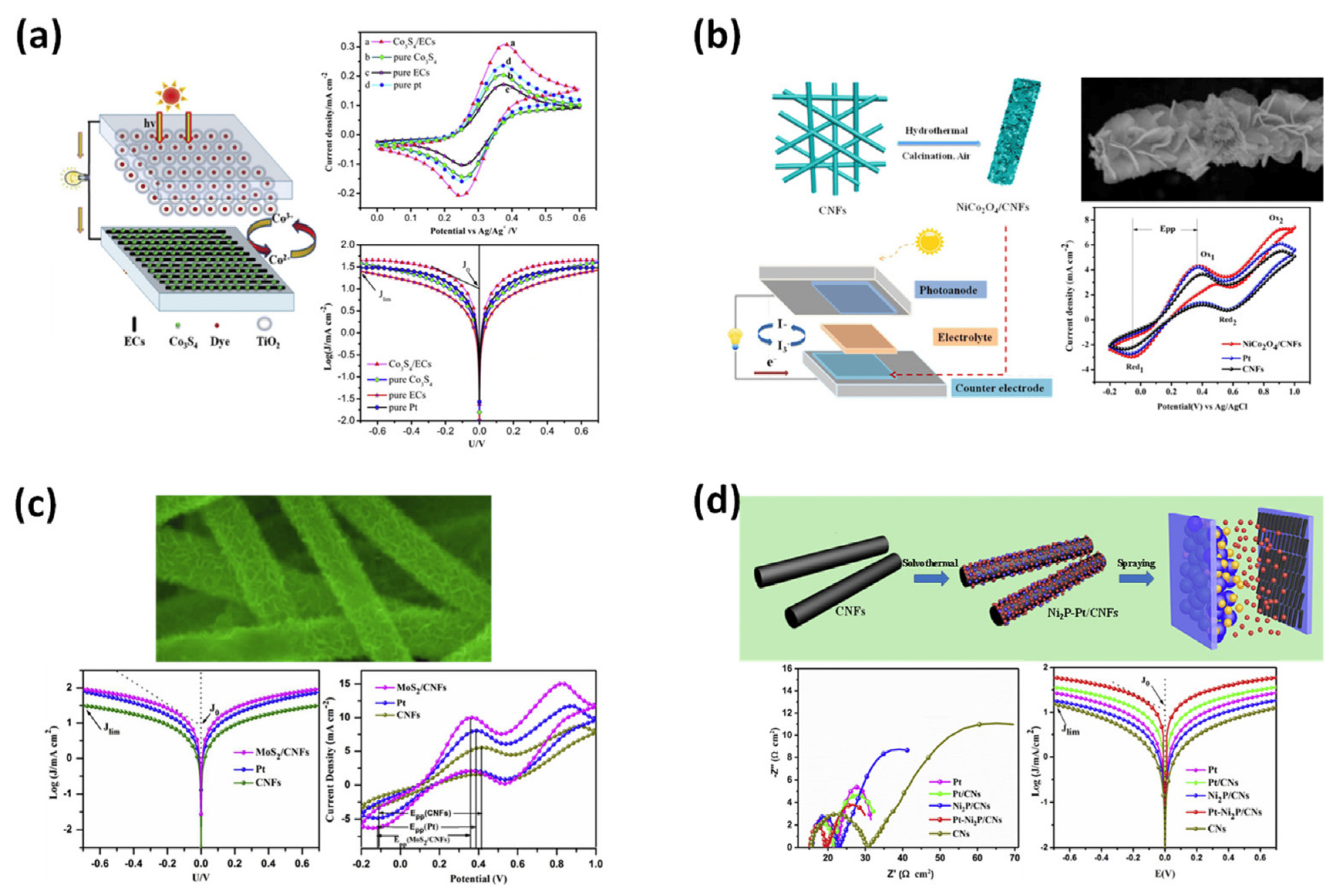
4.1. Dye-Sensitized Solar Cells (DSSCs)
4.2. Fuel Cell Membrane Electrodes
4.3. Catalysts
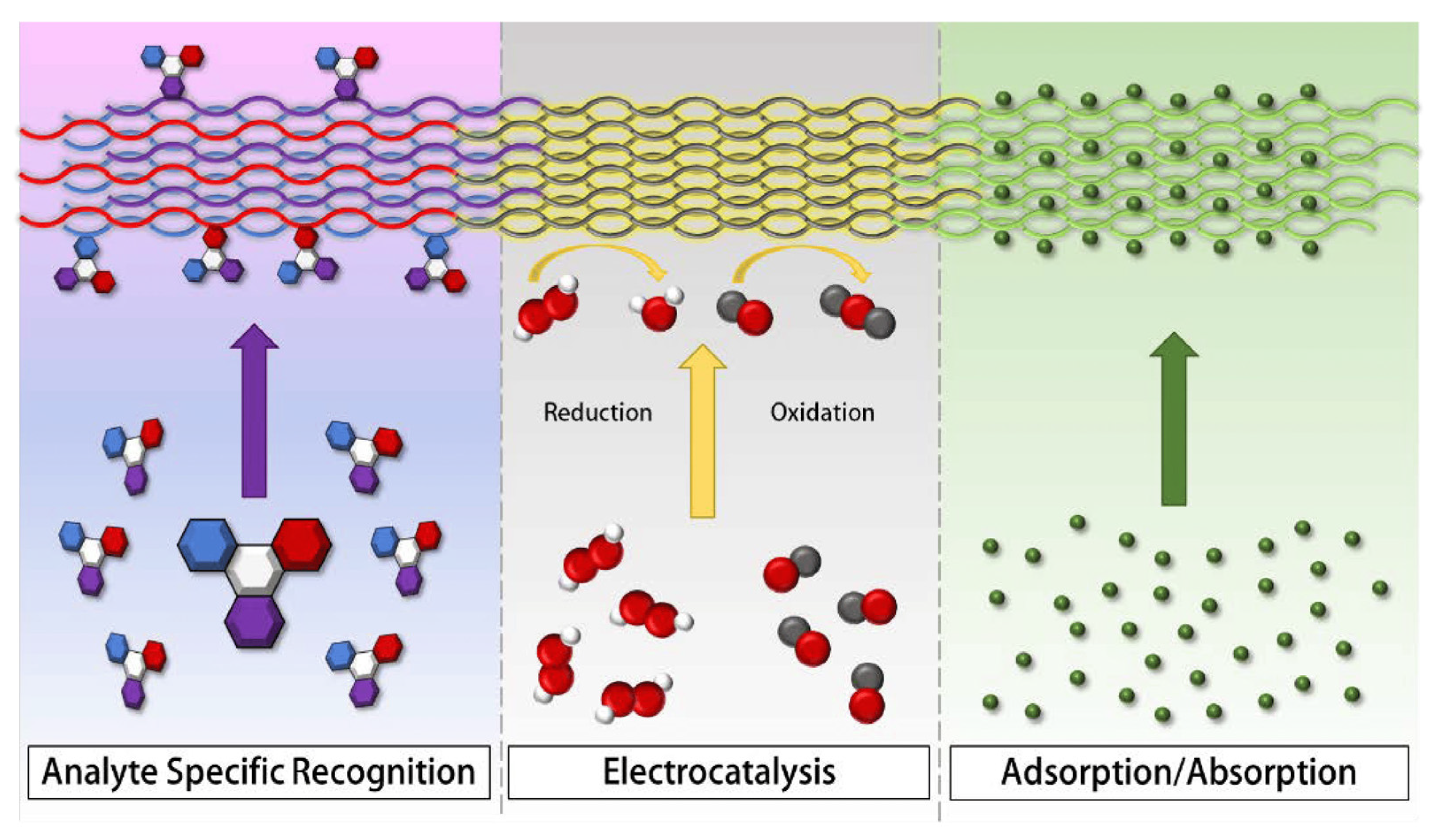
4.4. Sensors Applications
4.5. Biosensors
4.6. Electrochemical and Chemical Sensors
4.7. Batteries
4.8. Supercapacitors
5. Applications for Bio-Based Carbon Nanofibers
6. Challenges and Future Prospects of Research
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moriarty, P.; Honnery, D. Review: Renewable Energy in an Increasingly Uncertain Future. Appl. Sci. 2023, 13, 388. [Google Scholar] [CrossRef]
- Makešová, M.; Valentová, M. The Concept of Multiple Impacts of Renewable Energy Sources: A Critical Review. Energies 2021, 14, 3183. [Google Scholar] [CrossRef]
- Łukasiewicz, K.; Pietrzak, P.; Kraciuk, J.; Kacperska, E.; Cieciora, M. Sustainable Energy Development—A Systematic Literature Review. Energies 2022, 15, 8284. [Google Scholar] [CrossRef]
- Dubal, S.; Chavan, S.; Jadhav, P.; Kadam, S.; Dhotre, S. Effects of stabilization on structures and properties of Electrospun Polyacrylonitrile based carbon nanofibers as a binder free electrode for supercapacitor application. Mater. Today Proc. 2023, 72, 2841–2845. [Google Scholar] [CrossRef]
- Hussain, A.; Bahi, A.; Ko, F.; Abdin, Y. Development of low-cost electrospun carbon nanofibers using asphaltene precursor. Adv. Nat. Sci. Nanosci. Nanotechnol. 2023, 14, 025012. [Google Scholar] [CrossRef]
- Bang, J.; Kim, J.H.; Park, S.W.; Kim, J.; Jung, M.; Jung, S.; Kim, J.C.; Choi, I.G.; Kwak, H.W. Effect of chemically modified lignin addition on the physicochemical properties of PCL nanofibers. Int. J. Biol. Macromol. 2023, 240, 124330. [Google Scholar] [CrossRef]
- Jeon, B.; Ha, T.; Lee, D.Y.; Choi, M.S.; Lee, S.W.; Jung, K.-H. Preparation and Electrochemical Properties of Porous Carbon Nanofiber Electrodes Derived from New Precursor Polymer: 6FDA-TFMB. Polymers 2020, 12, 1851. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, K.; Cao, L.; Huang, X.; Li, Y. Preparation of Reusable Porous Carbon Nanofibers from Oxidized Coal Liquefaction Residue for Efficient Adsorption in Water Treatment. Materials 2023, 16, 3614. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Wang, C.; Chen, D.; Zhang, Q.; Jiang, L.; Zhang, C.; Liud, K.; He, S. Capacitive properties of carbon nanofibers derived from blends of cellulose acetate and polyacrylonitrile. New J. Chem. 2023, 47, 13831–13840. [Google Scholar] [CrossRef]
- Schüth, F. Endo- and Exotemplating to Create High-Surface-Area Inorganic Materials. Angew. Chem. Int. Ed. 2023, 42, 3604–3622. [Google Scholar] [CrossRef]
- Singh, T.; Mutreja, V. Template assisted fabrication of ordered nanoporous carbon materials: A review. AIP Conf. Proc. 2023, 2535, 060002. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Coupling granular activated carbon adsorption with membrane bioreactor treatment for trace organic contaminant removal: Breakthrough behaviour of persistent and hydrophilic compounds. J. Environ. Manag. 2013, 119, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Lee, H.M.; Kang, H.R.; An, K.H.; Kim, H.G.; Kim, B.J. Comparative studies of porous carbon nanofibers by various activation methods. Carbon Lett. 2013, 14, 180–185. [Google Scholar] [CrossRef]
- Sabantina, L.; Wehlage, D.; Klöcker, M.; Mamun, A.; Grothe, T.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T.; Finsterbusch, K.; Ehrmann, A. Stabilization of electrospun PAN/gelatin nanofiber mats for carbonization. Nanomaterials 2018, 2018, 6131085. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Most recent developments in electrospun magnetic nanofibers. J. Eng. Fibers Fabr. 2020, 15, 1558925019900843. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vijayalakshmi, K.; Sangeetha, V.; Radha, E. Electrospinning—Material, Techniques and Biomedical Applications. In Nanobiomaterials, Research Trends and Applications, 1st ed.; Thandapani Gomathi, P.N., Sudha, S.T., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wehlage, D.; Blattner, H.; Mamun, A.; Kutzli, I.; Diestelhorst, E.; Rattenholl, A.; Gudermann, F.; Lütkemeyer, D.; Ehrmann, A. Cell growth on electrospun nanofiber mats from polyacrylonitrile(PAN) blends. AIMS Bioeng. 2019, 7, 43–54. [Google Scholar] [CrossRef]
- Diestelhorst, E.; Mance, F.; Mamun, A.; Ehrmann, A. Chemical and Morphological Modifi cation of PAN Nanofi brous Mats with Addition of Casein after Electrospinning, Stabilisation and Carbonisation. Tekstilec 2020, 63, 38–49. [Google Scholar] [CrossRef]
- Storck, J.L.; Grothe, T.; Mamun, A.; Sabantina, L.; Klöcke, M.; Blachowicz, T.; Ehrmann, A. Orientation of Electro-spun Magnetic Nanofibers Near Conductive Areas. Materials 2020, 13, 47. [Google Scholar] [CrossRef]
- Fokin, N.; Grothe, T.; Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Döpke, C.; Blachowicz, T.; Hütten, A.; Ehr-mann, A. Magnetic properties of electrospun magnetic nanofiber mats after stabilization and carbonization. Materials 2020, 13, 1552. [Google Scholar] [CrossRef]
- Mamun, A. Review of Possible Applications of Nanofibrous Mats for Wound Dressings. Tekstilec 2019, 62, 89–100. [Google Scholar] [CrossRef]
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Ehrmann, A. Increased mechanical properties of carbon nanofiber mats for possible medical applications. Fibers 2019, 7, 98. [Google Scholar] [CrossRef]
- Mamun, A.; Sabantina, L. Electrospun Magnetic Nanofiber Mats for Magnetic Hyperthermia in Cancer Treatment Applications—Technology, Mechanism, and Materials. Polymers 2023, 15, 1902. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.; Klöcker, M.; Blachowicz, T.; Sabantina, L. Investigation of the Morphological Structure of Needle-Free Electrospun Magnetic Nanofiber Mats. Magnetochemistry 2022, 8, 25. [Google Scholar] [CrossRef]
- Mamun, A.; Sabantina, L.; Klöcker, M.; Heide, A.; Blachowicz, T.; Ehrmann, A. Electrospinning Nanofiber Mats with Magnetite Nanoparticles Using Various Needle-Based Techniques. Polymers 2022, 14, 533. [Google Scholar] [CrossRef]
- Kozior, T.; Mamun, A.; Trabelsi, M.; Sabantina, L. Comparative Analysis of Polymer Composites Produced by FFF and PJM 3D Printing and Electrospinning Technologies for Possible Filter Applications. Coatings 2022, 12, 48. [Google Scholar] [CrossRef]
- Mamun, A.; Blachowicz, T.; Sabantina, L. Electrospun Nanofiber Mats for Filtering Applications—Technology, Structure and Materials. Polymers 2021, 13, 1368. [Google Scholar] [CrossRef]
- Mamun, A.; Moulefera, I.; Topuz, Y.; Trabelsi, M.; Sabantina, L. The Possibility of Reuse of Nanofiber Mats by Machine Washing at Different Temperatures. Materials 2021, 14, 4788. [Google Scholar] [CrossRef]
- Langwald, S.V.; Ehrmann, A.; Sabantina, L. Measuring Physical Properties of Electrospun Nanofiber Mats for Different Biomedical Applications. Membranes 2023, 13, 488. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Optical Properties of Electrospun Nanofiber Mats. Membranes 2023, 13, 441. [Google Scholar] [CrossRef]
- Zong, H.; Xia, X.; Liang, Y.; Dai, S.; Alsaedi, A.; Hayat, T.; Kong, F.; Pan, J.H. Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. Mater. Sci. Eng. C 2018, 92, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Blachowicz, T.; Hütten, A.; Ehrmann, A. Electromagnetic Interference Shielding with Electrospun Nanofiber Mats—A Review of Production, Physical Properties and Performance. Fibers 2022, 10, 47. [Google Scholar] [CrossRef]
- Kyotani, T. Control of pore structure in carbon. Carbon 2000, 38, 269–286. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered Mesoporous Carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Lee, J.; Han, S.; Hyeon, T. Synthesis of new nanoporous carbon materials using nanostructured silica materials as templates. J. Mater. Chem. 2004, 14, 478–486. [Google Scholar] [CrossRef]
- Hentze, H.; Antonietti, M. Template synthesis of porous organic polymers. Curr. Opin. Solid State Mater. Sci. 2001, 5, 343–353. [Google Scholar] [CrossRef]
- Stein, A. Advances in Microporous and Mesoporous Solids—Highlights of Recent Progress. Adv. Mater. 2003, 15, 763–775. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.Q.; Millar, G.J. Advances in Mesoporous Molecular Sieve MCM-41. Ind. Eng. Chem. Res. 1996, 35, 2075–2090. [Google Scholar] [CrossRef]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and Applications of Supramolecular-Templated Mesoporous Materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Holmberg, K. Surfactant-templated nanomaterials synthesis. J. Colloid Interface Sci. 2004, 274, 355–364. [Google Scholar] [CrossRef]
- Stein, A. Sphere templating methods for periodic porous solids. Microporous Mesoporous Mater. 2001, 44, 227–239. [Google Scholar] [CrossRef]
- Manocha, S.M. Porous carbons. Sadhana 2003, 28, 335–348. [Google Scholar] [CrossRef]
- Menéndez-Díaz, J.A.; Martín-Gullón, I. Chapter 1 Types of carbon adsorbents and their production. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–47. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley Sons: Hoboken, NJ, USA, 2003; ISBN 9780471297413/9780471444091. [Google Scholar] [CrossRef]
- Carel, J.V.O. A review of Active Carbon. J. Dispers. Sci. Technol. 1990, 11, 323. [Google Scholar] [CrossRef]
- Dang, S.; Gabriele, C. A perspective on carbon materials for future energy application. J. Energy Chem. 2013, 22, 151–173. [Google Scholar] [CrossRef]
- Kyotani, T. Synthesis of Various Types of Nano Carbons Using the Template Technique, Award Accounts. Chem. Soc. Jpn. Award. Creat. Work 2004, 79, 1322–1337. [Google Scholar] [CrossRef]
- Pavlenko, V.; Żółtowska, S.; Haruna, A.B.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.I.; Abbas, Q.; Jesionowski, T. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Díez, N.; Sevilla, M.; Fuertes, A.B. Synthesis strategies of templated porous carbons beyond the silica nanocasting technique. Carbon 2021, 178, 451–476. [Google Scholar] [CrossRef]
- Mittal, M.; Sardar, S.; Jana, A. Chapter 7—Nanofabrication techniques for semiconductor chemical sensors. In Micro and Nano Technologies, Handbook of Nanomaterials for Sensing Applications; Hussain, C.M., Kailasa, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 119–137. ISBN 9780128207833. [Google Scholar] [CrossRef]
- Carlsson, J.O.; Martin, P.M. Chapter 7—Chemical Vapor Deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 314–363. ISBN 9780815520313. [Google Scholar] [CrossRef]
- Shyu, Y.M.; Hong, F.C.N. The effects of pre-treatment and catalyst composition on growth of carbon nanofibers at low temperature. Diam. Relat. Mater. 2001, 10, 1241–1245. [Google Scholar] [CrossRef]
- Ting, J.M.; Huang, N.Z. Thickening of chemical vapor deposited carbon fiber. Carbon 2001, 39, 835–839. [Google Scholar] [CrossRef]
- Manafi, S.; Rahimipour, M.R.; Mobasherpour, I.; Soltanmoradi, A. The Synthesis of Peculiar Structure of Springlike Multiwall Carbon Nanofibers/Nanotubes via Mechanothermal Method, Low-Dimensional Carbon Nanomaterials. J. Nanomater. 2012, 2012, 5–15. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Y.; Wang, Y.; Zhang, G.; Zhao, Y.; Liu, J. Formation of carbon nanofibers/nanotubes by chemical vapor deposition using Al2O3/KOH. Diam. Relat. Mater. 2021, 113, 108265. [Google Scholar] [CrossRef]
- Yarova, S.; Jones, D.; Jaouen, F.; Cavaliere, S. Strategies to Hierarchical Porosity in Carbon Nanofiber Webs for Electrochemical Applications. Surfaces 2019, 2, 159–176. [Google Scholar] [CrossRef]
- Dupuis, A.C. The catalyst in the CCVD of carbon nanotubes—A review. Prog. Mater. Sci. 2005, 50, 929–961. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, B.; Chen, Y.; Guo, L.; Wei, G. Carbon nanofiber-based three-dimensional nanomaterials for energy and environmental applications. Mater. Adv. 2020, 1, 2163–2181. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Gong, C.; Liu, S.; Zhang, M.; Shi, Y.; Zhang, J. Fabrication of TiN/Carbon nanofibers by electrospinning and their electromagnetic wave absorption properties. J. Alloys Compd. 2018, 735, 1488–1493. [Google Scholar] [CrossRef]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger, L.; Meunier, J.L. A simple thermal CVD method for carbon nanotube synthesis on stainless steel 304 without the addition of an external catalyst. Carbon 2009, 47, 313–318. [Google Scholar] [CrossRef]
- Duan, H.; Liang, J.; Xia, Z. Synthetic hierarchical nanostructures: Growth of carbon nanofibers on microfibers by chemical vapor deposition. Mater. Sci. Eng. B 2010, 166, 190–195. [Google Scholar] [CrossRef]
- Carole, E.; Baddour, D.; Upham, C.; Meunier, J.L. Direct and repetitive growth cycles of carbon nanotubes on stainless steel particles by chemical vapor deposition in a fluidized bed. Carbon 2010, 48, 2652–2656. [Google Scholar] [CrossRef]
- Suda, Y.; Maruyama, K.; Iida, T.; Takikawa, H.; Ue, H.; Shimizu, K.; Umeda, Y. High-Yield Synthesis of Helical Carbon Nanofibers Using Iron Oxide Fine Powder as a Catalyst. Crystals 2015, 5, 47–60. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Bais, V.S.S.; Prakash, B.; Verma, N. Multi-scale carbon micro/nanofibers-based adsorbents for protein immobilization. Mater. Sci. Eng. C 2014, 38, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Modi, A.; Verma, N. Enhanced power generation using a novel polymer-coated nanoparticles dispersed-carbon micro-nanofibers-based air-cathode in a membrane-less single chamber microbial fuel cell. Int. J. Hydrogen Energy 2016, 41, 1237–1247. [Google Scholar] [CrossRef]
- Singh, S.; Verma, N. Fabrication of Ni nanoparticles-dispersed carbon micro-nanofibers as the electrodes of a microbial fuel cell for bio-energy production. Int. J. Hydrogen Energy 2015, 40, 1145–1153. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on Activated Carbons by Chemical Activation with FeCl3. C 2020, 6, 21. [Google Scholar] [CrossRef]
- Shariatinia, Z. Chapter 7—Applications of carbon nanotubes. In Micro and Nano Technologies, Handbook of Carbon-Based Nanomaterials; Sabu Thomas, C., Sarathchandran, S.A., Ilangovan, J.C.M.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128219966. [Google Scholar] [CrossRef]
- Hung, N.C.; Anoshkin, I.V.; Rakov, E.G. Chemical activation of carbon nanofibers and nanotubes. Russ. J. Appl. Chem. 2007, 80, 443–447. [Google Scholar] [CrossRef]
- Park, C.; Baker, R.T.K. Catalytic Behavior of Graphite Nanofiber Supported Nickel Particles. 3. The Effect of Chemical Blocking on the Performance of the System. Am. Chem. Soc. 1999, 103, 2453–2459. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite: I. Activation by KOH. Carbon 2001, 39, 741–749. [Google Scholar] [CrossRef]
- Jiménez, V.; Sánchez, P.; Dorado, F.; Valverde, J.L.; Romero, A. Microporosity Development of Herringbone Carbon Nanofibers by RbOH Chemical Activation. J. Nanotechnol. 2009, 2009, 373986. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Lozano-Castell, D.; Cazorla-Amorós, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite: II. Activation by NaOH. Carbon 2001, 39, 751–759. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Z.; Yokogawa, K. Investigation of preparation and structures of activated carbon nanotubes. Mater. Res. Bull. 2006, 41, 1503–1512. [Google Scholar] [CrossRef]
- Sasono, S.R.; Rois, M.F.; Widiyastuti, W.; Nurtono, T.; Setyawan, H. Nanofiber-enrich dispersed activated carbon derived from coconut shell for supercapacitor material. Results Eng. 2023, 18, 101070. [Google Scholar] [CrossRef]
- Yang, J.E.; Jang, I.; Kim, M.; Baeck, S.H.; Hwang, S.; Shim, S.E. Electrochemically polymerized vine-like nanostructured polyaniline on activated carbon nanofibers for supercapacitor. Electrochim. Acta 2013, 111, 136–143. [Google Scholar] [CrossRef]
- Presser, V.; Zhang, L.; Niu, J.J.; McDonough, J.; Perez, C.; Fong, H.; Gogotsi, Y. Flexible Nano-felts of Carbide-Derived Carbon with Ultra-high Power Handling Capability. Adv. Energy Mater. 2011, 1, 423–430. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge. A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Lu, M.; Tao, P.Y.; Zhang, Y.J.; Gong, X.T.; Yang, Z.; Zhang, G.Q.; Li, H.L. Hierarchically porous and heteroatom doped carbon derived from tobacco rods for supercapacitors. J. Power Sources 2016, 307, 391–400. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Mubarak, N.M.; Tiripathi, M.; Jayakumar, N.S.; Sahu, J.N.; Ganesan, P. Chemical, dielectric and structural characterization of optimized hydrochar produced from hydrothermal carbonization of palm shell. Fuel 2016, 163, 88–97. [Google Scholar] [CrossRef]
- Tradler, S.B.; Mayr, S.; Himmelsbach, M.; Priewasser, R.; Baumgartner, W.; Stadler, A.T. Hydrothermal carbonization as an all-inclusive process for food-waste conversion. Bioresour. Technol. Rep. 2018, 2, 77–83. [Google Scholar] [CrossRef]
- Veltri, F.; Alessandro, F.; Scarcello, A.; Beneduci, A.; Arias Polanco, M.; Cid Perez, D.; Vacacela Gomez, C.; Tavolaro, A.; Giordano, G.; Caputi, L.S. Porous Carbon Materials Obtained by the Hydrothermal Carbonization of Orange Juice. Nanomaterials 2020, 10, 655. [Google Scholar] [CrossRef]
- Gergin, İ.; Ismar, E.; Sarac, A.S. Oxidative stabilization of polyacrylonitrile nanofibers and carbon nanofibers containing graphene oxide (GO): A spectroscopic and electrochemical study. Beilstein J. Nanotechnol. 2017, 8, 1616–1628. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Moulefera, I.; Pljonkin, A.; Elleuch, K.; Sabantina, L. Magnetic Carbon Nanofiber Mats for Prospective Single Photon Avalanche Diode (SPAD) Sensing Applications. Sensors 2021, 21, 7873. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, E.; Zanetti, M.; Bracco, P.; Brunella, V.; Luda, M.P.; Costa, L. Crosslinking and carbonization processes in PAN films and nanofibers. Polym. Degrad. Stab. 2016, 123, 178–188. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Cho, M.; Karaaslan, M.; Chowdhury, S.; Ko, F.; Renneckar, S. Skipping Oxidative Thermal Stabilization for Lignin-Based Carbon Nanofibers. ACS Sustain. Chem. Eng. 2018, 6, 6434–6444. [Google Scholar] [CrossRef]
- Sabantina, L.; Rodríguez-Cano, M.A.; Klöcker, M.; García-Mateos, F.J.; Ternero-Hidalgo, J.J.; Mamun, A.; Beermann, F.; Schwakenberg, M.; Voigt, A.-L.; Mirasol, J.R.; et al. Fixing PAN nanofiber mats during stabilization for carbonization and creating novel metal/carbon composites. Polymers 2018, 10, 735. [Google Scholar] [CrossRef]
- Markowski, J.; Zambrzycki, M.; Smolka, W.; Panek, A.; Gubernat, M.; Czaja, P.; Marzec, M.; Fraczek-Szczypta, A. Influence of Heat Treatment of Electrospun Carbon Nanofibers on Biological Response. Int. J. Mol. Sci. 2022, 23, 6278. [Google Scholar] [CrossRef]
- Sazali, N. The Influence of Carbonization Temperature and Heating Rate towards Carbon Membrane Performance: A Review. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 62, 151–158. [Google Scholar]
- Shokrani Havigh, R.; Mahmoudi Chenari, H. A comprehensive study on the effect of carbonization temperature on the physical and chemical properties of carbon fibers. Sci. Rep. 2022, 12, 10704. [Google Scholar] [CrossRef]
- Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Grötsch, G.; Cornelißen, C.; Strei-tenberger, A.; Ehrmann, A. Electrospun nanofiber mats with embedded non-sintered TiO2 for dye-sensitized solar cells (DSSCs). Fibers 2019, 7, 60. [Google Scholar] [CrossRef]
- Wen, J.; Chen, S.; Xu, Y.; Guan, T.; Zhang, X.; Bao, N. Synthesis of Single Crystal 2D Cu2FeSnS4 Nanosheets with High-Energy Facets (111) as a Pt-Free Counter Electrode for Dye-Sensitized Solar Cells. Materials 2023, 16, 4743. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Lee, J.S. Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications. Appl. Sci. 2022, 12, 6048. [Google Scholar] [CrossRef]
- Kou, Y.; Oya, T. Unique Dye-Sensitized Solar Cell Using Carbon Nanotube Composite Papers with Gel Electrolyte. J. Compos. Sci. 2023, 7, 232. [Google Scholar] [CrossRef]
- Montagni, T.; Rodríguez Chialanza, M.; Cerdá, M.F. Blueberries as a Source of Energy: Physical Chemistry Characterization of Their Anthocyanins as Dye-Sensitized Solar Cells’ Sensitizers. Solar 2023, 3, 283–297. [Google Scholar] [CrossRef]
- Wu, T.C.; Huang, W.-M.; Meen, T.H.; Tsai, J.K. Performance Improvement of Dye-Sensitized Solar Cells with Pressed TiO2 Nanoparticles Layer. Coatings 2023, 13, 907. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, X.; Yang, W.; Gao, Y.; Zhang, R.; Liu, J.; Wang, H.; Guo, Y.; Wang, Z. High Performance Dye-Sensitized Solar Cells Based on Electrospun MoS2-Carbon Nanofiber Composite Counter Electrode. Front. Mater. 2020, 7, 593345. [Google Scholar] [CrossRef]
- Joshi, P.; Zhang, L.; Chen, Q.; Galipeau, D.; Fong, F.; Qiao, Q. Electrospun Carbon Nanofibers as Low-Cost Counter Electrode for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2010, 2, 3572–3577. [Google Scholar] [CrossRef]
- López-Covarrubias, J.G.; Soto-Muñoz, L.; Iglesias, A.L.; Villarreal-Gómez, L.J. Electrospun Nanofibers Applied to Dye Solar Sensitive Cells: A Review. Materials 2019, 12, 3190. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, X.; Wang, M.; Zhang, W.; Li, X.; Wang, H.; Li, L. Electrospun carbon nanofibers decorated with Pt-Ni2P nanoparticles as high efficiency counter electrode for dye-sensitized solar cells. J. Alloys Compd. 2019, 786, 50–55. [Google Scholar] [CrossRef]
- Aboagye, A.; Elbohy, H.; Kelkar, A.D.; Qiao, Q.; Zai, J.; Qian, X.; Zhang, X. Electrospun carbon nanofibers with surface-attached platinum nanoparticles as cost-effective and efficient counter electrode for dye-sensitized solar cells. Nano Energy 2015, 11, 550–556. [Google Scholar] [CrossRef]
- Skupov, K.M.; Ponomarev, I.I.; Vtyurina, E.S.; Volkova, Y.A.; Ponomarev, I.I.; Zhigalina, O.M.; Khmelenin, D.N.; Cherkovskiy, E.N.; Modestov, A.D. Proton-Conducting Polymer-Coated Carbon Nanofiber Mats for Pt-Anodes of High-Temperature Polymer-Electrolyte Membrane Fuel Cell. Membranes 2023, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Banitaba, S.N.; Ehrmann, A. Application of Electrospun Nanofibers for Fabrication of Versatile and Highly Efficient Electrochemical Devices: A Review. Polymers 2021, 13, 1741. [Google Scholar] [CrossRef] [PubMed]
- Chandra Kishore, S.; Perumal, S.; Atchudan, R.; Alagan, M.; Wadaan, M.A.; Baabbad, A.; Manoj, D. Recent Advanced Synthesis Strategies for the Nanomaterial-Modified Proton Exchange Membrane in Fuel Cells. Membranes 2023, 13, 590. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, K.; Wycisk, R.; Pintauro, P.N. Application of electrospinning for the fabrication of proton-exchange membrane fuel cell electrodes. Curr. Opin. Electrochem. 2020, 21, 257–264. [Google Scholar] [CrossRef]
- Delikaya, Ö.; Bevilacqua, N.; Eifert, L.; Kunz, U.; Zeis, R.; Roth, C. Porous electrospun carbon nanofibers network as an integrated electrode@gas diffusion layer for high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2020, 345, 136192. [Google Scholar] [CrossRef]
- Iskandarani, B.; Mojarrad, N.R.; Yürüm, A.; Gürsel, S.K.; Kaplan, B.Y. Electrospun Nanofiber Electrodes for Boosted Performance and Durability at Lower Humidity Operation of PEM Fuel Cells. Energy Fuels 2022, 36, 9282–9294. [Google Scholar] [CrossRef]
- Yusoff, Y.N.; Shaari, N. An overview on the development of nanofiber-based as polymer electrolyte membrane and electrocatalyst in fuel cell application. Int. J. Energy Res. 2021, 45, 18441–18472. [Google Scholar] [CrossRef]
- Waldrop, K.; Slack, J.J.; Gumeci, C.; Parrondo, J.; Dale, N.; Reeves, K.S.; Cullen, D.A.; More, K.L.; Pintauro, P.N. Electrospun Nanofiber Electrodes for High and Low Humidity PEMFC Operation. J. Electrochem. Soc. 2023, 170, 024507. [Google Scholar] [CrossRef]
- Beltrán-Gastélum, M.; Portillo-Fuentes, S.G.; Flores-Hernández, J.R.; Salazar-Gastélum, M.I.; Trujillo-Navarrete, B.; Romero-Castañón, T.; Silva-Carrillo, C.; Reynoso-Soto, E.A.; Félix-Navarro, R.M. Ag-Cu Nanoparticles as Cathodic Catalysts for an Anion Exchange Membrane Fuel Cell. Catalysts 2023, 13, 1050. [Google Scholar] [CrossRef]
- Maafa, I.M.; Zouli, N.; Abutaleb, A.; Yousef, A.; Qudsieh, I.Y.; Matar, S.M.; Adam, A.S.M.; El-Halwany, M.M. Synthesis of Ilmenite Nickel Titanite-Supported Carbon Nanofibers Derived from Polyvinylpyrrolidone as Photocatalyst for H2 Production from Ammonia Borane Photohydrolysis. Polymers 2023, 15, 3262. [Google Scholar] [CrossRef]
- Schossig, J.; Gandotra, A.; Arizapana, K.; Weber, D.; Wildy, M.; Wei, W.; Xu, K.; Yu, L.; Chimenti, R.; Mantawy, I.; et al. CO2 to Value-Added Chemicals: Synthesis and Performance of Mono- and Bimetallic Nickel–Cobalt Nanofiber Catalysts. Catalysts 2023, 13, 1017. [Google Scholar] [CrossRef]
- Stonkus, O.; Kibis, L.; Slavinskaya, E.; Zadesenets, A.; Garkul, I.; Kardash, T.; Stadnichenko, A.; Korenev, S.; Podyacheva, O.; Boronin, A. Pd-Ceria/CNMs Composites as Catalysts for CO and CH4 Oxidation. Materials 2023, 16, 4257. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, M.M.; Gomaa, H.E.; Abdu, H.M.; Almasri, R.A.; Irfan, O.M.; Barakat, N.A.M. Molybdenum Carbide/Ni Nanoparticles Embedded into Carbon Nanofibers as an Effective Non-Precious Catalyst for Green Hydrogen Production from Methanol Electrooxidation. Polymers 2023, 15, 2430. [Google Scholar] [CrossRef]
- Ghosh, S.; Courthéoux, L.; Brunet, S.; Lacroix-Desmazes, P.; Pradel, A.; Girard, E.; Uzio, D. Effect of the Microstructure of Composite CoMoS/Carbon Catalysts on Hydrotreatment Performances. Catalysts 2023, 13, 862. [Google Scholar] [CrossRef]
- Popov, A.A.; Afonnikova, S.D.; Varygin, A.D.; Bauman, Y.I.; Trenikhin, M.V.; Plyusnin, P.E.; Shubin, Y.V.; Vedyagin, A.A.; Mishakov, I.V. Pt1−xNix Alloy Nanoparticles Embedded in Self-Grown Carbon Nanofibers: Synthesis, Properties and Catalytic Activity in HER. Catalysts 2023, 13, 599. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O. Research Progress on the Applications of Electrospun Nanofibers in Catalysis. Catalysts 2022, 12, 9. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Cao, X.; Liu, J.; Zhang, J.; Jia, J.; Wang, F.; Pan, K. Electrospun Fe2C-loaded carbon nanofibers as efficient electrocatalysts for oxygen reduction reaction. Nanotechnology 2019, 30, 32. [Google Scholar] [CrossRef]
- Woo, S.; Lee, J.; Lee, D.S.; Kim, J.K.; Lim, B. Electrospun Carbon Nanofibers with Embedded Co-Ceria Nanoparticles for Efficient Hydrogen Evolution and Overall Water Splitting. Materials 2020, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, I.I.; Zhigalina, M.O.; Skupov, M.K.; Modestov, D.A.; Basu, G.V.; Sufiyanova, E.A.; Ponomareva, I.I.; Dmitry, Y. Preparation and thermal treatment influence on Pt-decorated electrospun carbon nanofiber electrocatalysts. RSC Adv. 2019, 9, 27406–27418. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Ghaferi, A.A. Graphene Incorporated Electrospun Nanofiber for Electrochemical Sensing and Biomedical Applications: A Critical Review. Sensors 2022, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Feeney, S.G.; LaFreniere, J.M.J.; Halpern, J.M. Perspective on Nanofiber Electro-chemi-cal Sensors: Design of Relative Selectivity Experiments. Polymers 2021, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, S.; Sundaresan, R.; Chen, S.-M.; Devi, J.M.; Chandrasekar, N.; Ramachandran, B. Fabrication of an Electrocatalyst Based on Rare Earth Manganites Incorporated with Carbon Nanofiber Hybrids: An Efficient Electrochemical Biosensor for the Detection of Anti-Inflammatory Drug Mefenamic Acid. C 2023, 9, 47. [Google Scholar] [CrossRef]
- Rathinasamy, S.K.; Maheswar, R.; Lorincz, J. Silk Fibroin-Based Piezoelectric Sensor with Carbon Nanofibers for Wearable Health Monitoring Applications. Sensors 2023, 23, 1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Yan, C.; Ma, Q.; Yang, X.; Peng, H.; Wang, H.; Du, J.; Zheng, B.; Guo, Y. Bismuth-Decorated Honeycomb-like Carbon Nanofibers: An Active Electrocatalyst for the Construction of a Sensitive Nitrite Sensor. Molecules 2023, 28, 3881. [Google Scholar] [CrossRef]
- Karlapudi, M.C.; Vahdani, M.; Bandari, S.M.; Peng, S.; Wu, S. A Comparative Study on the Effects of Spray Coating Methods and Substrates on Polyurethane/Carbon Nanofiber Sensors. Sensors 2023, 23, 3245. [Google Scholar] [CrossRef]
- Ketmen, S.; Er Zeybekler, S.; Gelen, S.S.; Odaci, D. Graphene Oxide-Magnetic Nanoparticles Loaded Polystyrene-Polydopamine Electrospun Nanofibers Based Nanocomposites for Immunosensing Application of C-Reactive Protein. Biosensors 2022, 12, 1175. [Google Scholar] [CrossRef]
- Li, M.; Dong, J.; Deng, D.; Ouyang, X.; Yan, X.; Liu, S.; Luo, L. Mn3O4/NiO Nanoparticles Decorated on Carbon Nanofibers as an Enzyme-Free Electrochemical Sensor for Glucose Detection. Biosensors 2023, 13, 264. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, Y.; Ma, Z.; Xu, Y.; Zhang, D. Bio-inspired Flexible Lateral Line Sensor Based on P(VDF-TrFE)/BTO Nanofiber Mat for Hydrodynamic Perception. Sensors 2019, 19, 5384. [Google Scholar] [CrossRef]
- Sengupta, D.; Trap, D.; Kottapalli, A.G.P. Piezoresistive Carbon Nanofiber-Based Cilia-Inspired Flow Sensor. Nanomaterials 2020, 10, 211. [Google Scholar] [CrossRef]
- Kim, M.; Kaliannagounder, V.K.; Unnithan, A.R.; Park, C.H.; Kim, C.S.; Ramachandra Kurup Sasikala, A. Development of In-Situ Poled Nanofiber Based Flexible Piezoelectric Nanogenerators for Self-Powered Motion Monitoring. Appl. Sci. 2020, 10, 3493. [Google Scholar] [CrossRef]
- Devaraj, V.; Han, J.; Kim, C.; Kang, Y.C.; Oh, J.W. Self-Assembled Nanoporous Biofilms from Functionalized Nanofibrous M13 Bacteriophage. Viruses 2018, 10, 322. [Google Scholar] [CrossRef]
- Zheng, C.; Yue, Y.; Gan, L.; Xu, X.; Mei, C.; Han, J. Highly Stretchable and Self-Healing Strain Sensors Based on Nanocellulose-Supported Graphene Dispersed in Electro-Conductive Hydrogels. Nanomaterials 2019, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Alamry, K.A.; Althomali, R.H. Chemically Modified Carbon Nanotubes for Electrochemical Sensors. In Chemically Modified Carbon Nanotubes for Commercial Applications; Aslam, J., Hussain, C.M., Aslam, R., Eds.; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Scroccarello, A.; Pelle, F.D.; Bukhari, Q.U.A.; Silveri, F.; Zappi, D.; Cozzoni, E.; Compagnone, D. Eucalyptus Biochar as a Sustainable Nanomaterial for Electrochemical Sensors. Chem. Proc. 2021, 5, 13. [Google Scholar] [CrossRef]
- da Silva, V.A.O.P.; Stefano, J.S.; Kalinke, C.; Bonacin, J.A.; Janegitz, B.C. Additive Manufacturing Sensor for Stress Biomarker Detection. Chemosensors 2023, 11, 306. [Google Scholar] [CrossRef]
- Galvão, J.C.R.; Araujo, M.d.S.; Prete, M.C.; Neto, V.L.; Dall’Antonia, L.H.; Matos, R.; Tarley, C.R.T.; Medeiros, R.A. Electrochemical Determination of 17-β-Estradiol Using a Glassy Carbon Electrode Modified with α-Fe2O3 Nanoparticles Supported on Carbon Nanotubes. Molecules 2023, 28, 6372. [Google Scholar] [CrossRef]
- Chen, J.; Rong, F.; Xie, Y. Fabrication, Microstructures and Sensor Applications of Highly Ordered Electrospun Nanofibers: A Review. Materials 2023, 16, 3310. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.; You, Y.; Wang, M.; Huang, Y.; Li, Y.; Li, K.; Yang, Y.; Feng, W.; Liu, Q.; et al. Fabrication of High-Performance Colorimetric Membrane by Incorporation of Polydiacetylene into Polyarylene Ether Nitriles Electrospinning Nanofibrous Membranes. Nanomaterials 2022, 12, 4379. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Kalimuthu, P.; Veerakumar, P.; Lin, K.C.; Chen, S.M.; Ramachandran, R.; Mariyappan, V.; Chitra, S. Recent Developments in Carbon-Based Nanocomposites for Fuel Cell Applications: A Review. Molecules 2022, 27, 761. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, D.; Feng, J.; Tanga, T.; Cheng, H. Rapid quantitative detection of luteolin using an electrochemical sensor based on electrospinning of carbon nanofibers doped with single-walled carbon nanoangles. Anal. Methods 2023, 15, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.U.; Ali, M.; Sarker, M.R.; Md Ali, S.H.; Akhtar, N.; Khan, N.A.; Asif, M.; Shah, S. Synthesis, Characterization, and Applications of Silver Nano Fibers in Humidity, Ammonia, and Temperature Sensing. Micromachines 2021, 12, 682. [Google Scholar] [CrossRef]
- Yoo, S.H.; Yoon, H.-S.; Han, H.; Na, K.H.; Choi, W.Y. Fabrications of Electrospun Mesoporous TiO2 Nanofibers with Various Amounts of PVP and Photocatalytic Properties on Methylene Blue (MB) Photodegradation. Polymers 2023, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, P.; Hui, J.; Janfaza, S.; O’Brien, A.; Tasnim, N.; Najjaran, H.; Hoorfar, M. Fabrication of SnO2 Composite Nanofiber-Based Gas Sensor Using the Electrospinning Method for Tetrahydrocannabinol (THC) Detection. Micromachines 2020, 11, 190. [Google Scholar] [CrossRef]
- Liu, P.; Wu, S.; Zhang, Y.; Zhang, H.; Qin, X. A Fast Response Ammonia Sensor Based on Coaxial PPy–PAN Nanofiber Yarn. Nanomaterials 2016, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Sun, J.; Li, K.; Zhao, S.; Zhao, D.; Wang, J.; Yang, C.; Wang, X.; Cao, B. Corncob-derived hierarchical porous activated carbon for high-performance lithium-ion capacitors. Energy Fuels 2020, 34, 16885–16892. [Google Scholar] [CrossRef]
- Sun, J.; Yang, S.; Yang, C.; Jia, Q.; Yang, X.; Cao, B. Corncob-derived hierarchical porous carbons constructed by re-activation for high-rate lithium-ion capacitors. New J. Chem. 2019, 43, 10103–10108. [Google Scholar] [CrossRef]
- Yarin, A.L.; Zussman, E.; Wendorff, J.H.; Greiner, A. Material encapsulation and transport in core-shell micro/nanofibers, polymer and carbon nanotubes and micro/nanochannels. J. Mater. Chem. 2007, 17, 2585–2599. [Google Scholar] [CrossRef]
- Stenina, I.; Minakova, P.; Kulova, T.; Yaroslavtsev, A. Electrochemical Properties of LiFePO4 Cathodes: The Effect of Carbon Additives. Batteries 2022, 8, 111. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Fabrication of porous carbon nanofibers and their application as anode materials for rechargeable lithium-ion batteries. Nanotechnology 2009, 20, 155705. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond—A 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.B.; Motola, M.; Rauf, S.; Li, C.J.; Li, C.X. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Cao, T.; Kwon, O.; Gorte, R.J.; Vohs, J.M. Metal exsolution to enhance the catalytic activity of electrodes in solid oxide fuel cells. Nanomaterials 2020, 10, 2445. [Google Scholar] [CrossRef]
- Qiu, P.; Yang, X.; Wang, W.; Wei, T.; Lu, Y.; Lin, J.; Yuan, Z.; Jia, L.; Li, J.; Chen, F. Redox-reversible electrode material for direct hydrocarbon solid oxide fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 13988–13995. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; ul Abadeen, Z.; Khan, K.; Tayyab, Z.; Qayyum, S.; Mosiałek, M.; Shao, Z.; Li, C.X.; Motola, M. Proton-conducting solid oxide electrolysis cells: Relationship of composition-structure-property, their challenges, and prospects. Matter 2023, 6, 1782–1830. [Google Scholar] [CrossRef]
- Kang, Y.; Deng, C.; Chen, Y.; Liu, X.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y. Binder-Free Electrodes and Their Application for Li-Ion Batteries. Nanoscale Res. Lett. 2020, 15, 112. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Xia, M.; Wei, K.; Zhang, L.; Zhang, X.; Yang, C.C.; Jose, R. Issues and challenges of layered lithium nickel cobalt manganese oxides for lithium-ion batteries. J. Electroanal. Chem. 2021, 895, 115412. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Fu, W.; Cui, Y.; Wang, J.; Zhao, D.; Yang, C.; Wang, X.; Cao, B. Plasma-induced ε-MnO2 based aqueous zinc-ion batteries and their dissolution-deposition mechanism. J. Mater. Sci. Technol. 2022, 127, 206–213. [Google Scholar] [CrossRef]
- Han, S.D.; Kim, S.; Li, D.; Petkov, V.; Yoo, H.D.; Phillips, P.J.; Wang, H.; Kim, J.J.; More, K.L.; Key, B.; et al. Mechanism of Zn insertion into nanostructured δ-MnO2: A nonaqueous rechargeable Zn metal battery. Chem. Mater. 2017, 29, 4874–4884. [Google Scholar] [CrossRef]
- Herbert, W.; Kappauf, H.W. Metadata of the Chapter That Will Be Visualized Online Spontanremissionen; Springer: Cham, Switzerland, 2021; pp. 1–12. [Google Scholar] [CrossRef]
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Burke, A. R & D considerations for the performance and application of electrochemical capacitors. Electrochim. Acta 2007, 53, 1083–1091. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Yang, S.; Cui, Y.; Yang, G.; Zhao, S.; Wang, J.; Zhao, D.; Yang, C.; Wang, X.; Cao, B. ZnCl2 induced hierarchical porous carbon for zinc-ion hybrid supercapacitors. J. Power Sources 2023, 554, 232347. [Google Scholar] [CrossRef]
- Yadav, R.; Zabihi, O.; Fakhrhoseini, S.; Nazarloo, H.A.; Kiziltas, A.; Blanchard, P.; Naebe, M. Lignin derived carbon fiber and nanofiber: Manufacturing and applications. Compos. Part B Eng. 2023, 255, 110613. [Google Scholar] [CrossRef]
- Guilminot, E.; Gavillon, R.; Chatenet, M.; Berthon-Fabry, S.; Rigacci, A.; Budtova, T. New nanostructured carbons based on porous cellulose: Elaboration, pyrolysis and use as platinum nanoparticles substrate for oxygen reduction electrocatalysis. J. Power Sources 2008, 185, 717–726. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Graphitic carbon nanostructures from cellulose. Chem. Phys. Lett. 2010, 490, 63–68. [Google Scholar] [CrossRef]
- Yalcinkaya, F. A review on advanced nanofiber technology for membrane distillation. J. Eng. Fibers Fabr. 2019, 14, 1558925018824901. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Jing, X.; Zhao, R.; Zhu, G.; Wang, C. Bio-inspired functionalization of electrospun nanofibers with anti-biofouling property for efficient uranium extraction from seawater. Chem. Eng. J. 2023, 465, 142844. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Tan, X.; Wang, H.; Holt, C.M.B.; Stephenson, T.; Olsen, B.C.; Mitlin, D. Mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors. Energy Environ. Sci. 2013, 6, 871–878. [Google Scholar] [CrossRef]
- Khan, A.; Senthil, R.A.; Pan, J.; Osman, S.; Sun, Y.; Shu, X. A new biomass derived rod-like porous carbon from tea-waste as inexpensive and sustainable energy material for advanced supercapacitor application. Electrochim. Acta 2020, 335, 135588. [Google Scholar] [CrossRef]
- Mousa, A.O.; Chuang, C.-H.; Kuo, S.-W.; Mohamed, M.G. Strategic Design and Synthesis of Ferrocene Linked Porous Organic Frameworks toward Tunable CO2 Capture and Energy Storage. Int. J. Mol. Sci. 2023, 24, 12371. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.J.; Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Chaiya, C.; Jantasee, S. Bio-based Carbon Materials for Catalysis. In High-Performance Materials from Bio-Based Feedstocks; Hunt, A.J., Supanchaiyamat, N., Jetsrisuparb, K., Knijnenburg, J.T.N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- González, J.; Ghaffarinejad, A.; Ivanov, M.; Ferreira, P.; Vilarinho, P.M.; Borrás, A.; Amorín, H.; Wicklein, B. Advanced Cellulose–Nanocarbon Composite Films for High-Performance Triboelectric and Piezoelectric Nanogenerators. Nanomaterials 2023, 13, 1206. [Google Scholar] [CrossRef] [PubMed]
- Gandavadi, D.; Sundarrajan, S.; Ramakrishna, S. Bio-Based Nanofibers Involved in Wastewater Treatment. Macromol. Mater. Eng. 2019, 304, 1900345. [Google Scholar] [CrossRef]
- Li, S.; Cai, G.; Wu, S.; Raut, A.; Borges, W.; Sharma, P.R.; Sharma, S.K.; Hsiao, B.S.; Rafailovich, M. Sustainable Plant-Based Biopolymer Membranes for PEM Fuel Cells. Int. J. Mol. Sci. 2022, 23, 15245. [Google Scholar] [CrossRef]
- Gaur, A.; Tiwari, S.; Kumar, C.; Maiti, A. Biobased Piezoelectric Nanogenerator for Mechanical Energy Harvesting using Nanohybrid of Poly(vinylidene fluoride). Nanoscale Adv. 2019, 1, 3200–3211. [Google Scholar] [CrossRef]
- He, M.M.; Wang, D.; Shiigi, H.; Liu, C.H.; Wang, W.C.; Shan, X.L.; Chen, Z.D. Black phosphorous dots phosphatized bio-based carbon nanofibers/bimetallic organic framework as catalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 17194–17203. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, Y.; Chen, J.; Zhu, M.; Yang, C.; Guo, H.; Song, Y.; Li, Y.; Zhou, J. Electrospun biomass based carbon nanofibers as high-performance supercapacitors. Ind. Crop. Prod. 2020, 148, 112181. [Google Scholar] [CrossRef]
| Precursors | Applications | References |
|---|---|---|
| Lignin | Electrodes | [175] |
| Cellulose, carboxycellulose, plant-based, bio-inspired zwitterionic phosphate groups | Fuel cells | [175,176,177,178] |
| Protein | Battery anodes and supercapacitors | [179] |
| Food and bio-based waste | Advanced supercapacitors | [180] |
| Porous organic polymers | High-performance supercapacitors | [181] |
| Biomass, chitosan/PEO, chitosan, cellulose, protein | Catalysts | [182,183,184] |
| Cellulose–nanocarbon composite films | High-performance triboelectric and piezoelectric nanogenerators | [185] |
| Eggshell | Mechanical energy harvesting | [186] |
| Lignin, cellulose, and chitin | Energy storage | [187] |
| Biomass | Supercapacitors | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamun, A.; Kiari, M.; Sabantina, L. A Recent Review of Electrospun Porous Carbon Nanofiber Mats for Energy Storage and Generation Applications. Membranes 2023, 13, 830. https://doi.org/10.3390/membranes13100830
Mamun A, Kiari M, Sabantina L. A Recent Review of Electrospun Porous Carbon Nanofiber Mats for Energy Storage and Generation Applications. Membranes. 2023; 13(10):830. https://doi.org/10.3390/membranes13100830
Chicago/Turabian StyleMamun, Al, Mohamed Kiari, and Lilia Sabantina. 2023. "A Recent Review of Electrospun Porous Carbon Nanofiber Mats for Energy Storage and Generation Applications" Membranes 13, no. 10: 830. https://doi.org/10.3390/membranes13100830
APA StyleMamun, A., Kiari, M., & Sabantina, L. (2023). A Recent Review of Electrospun Porous Carbon Nanofiber Mats for Energy Storage and Generation Applications. Membranes, 13(10), 830. https://doi.org/10.3390/membranes13100830








