Bilayer Heterogeneous Cation Exchange Membrane with Polyaniline Modified Homogeneous Layer: Preparation and Electrotransport Properties
Abstract
:1. Introduction
2. Materials and Methods
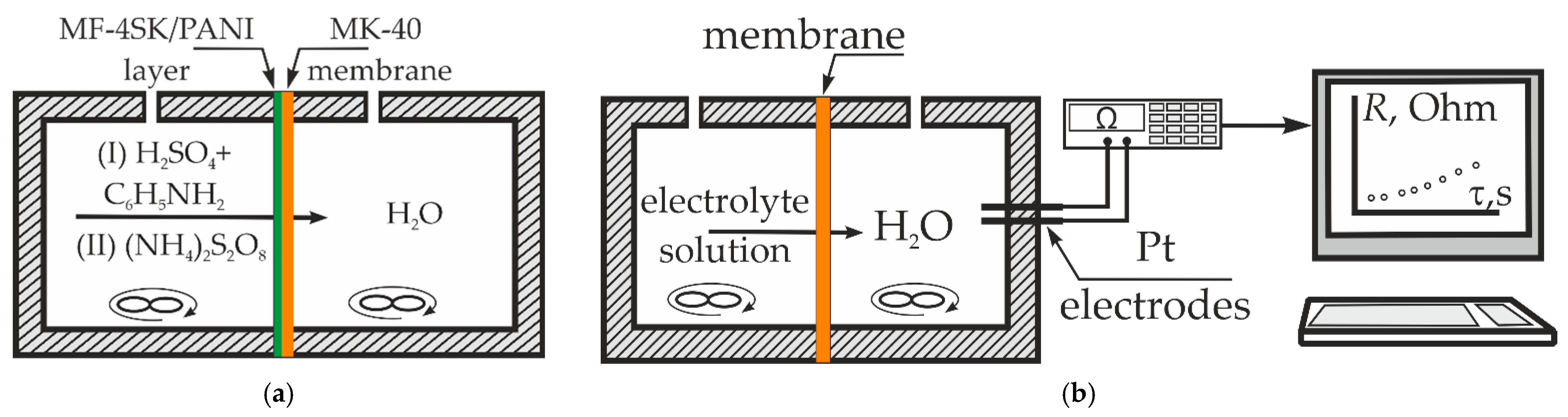
2.1. Cation Exchange Membrane and Preparation of the Bilayer PANI-Modified Membrane
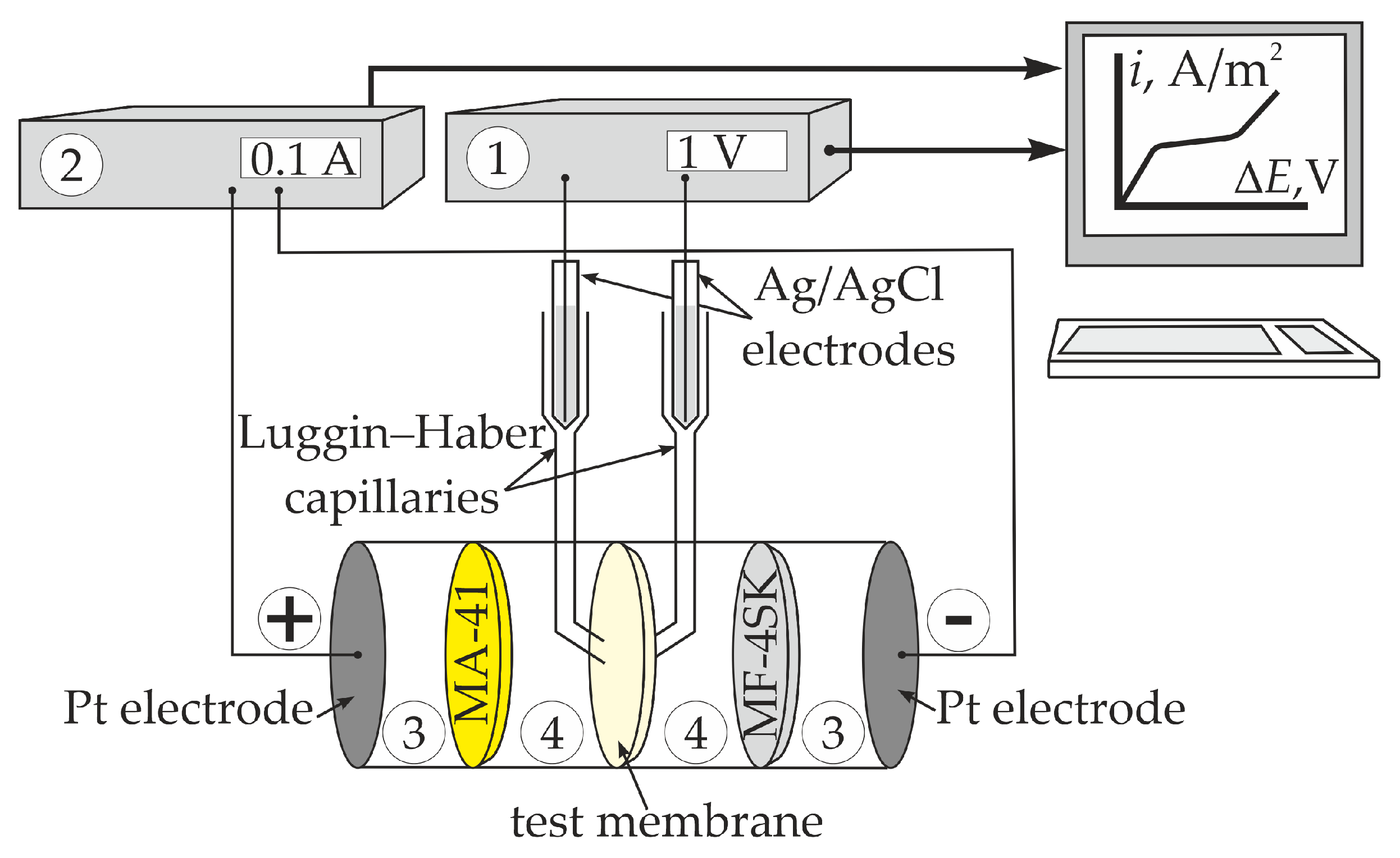
2.2. Diffusion Permeability, Conductivity, and Current–Voltage Curves
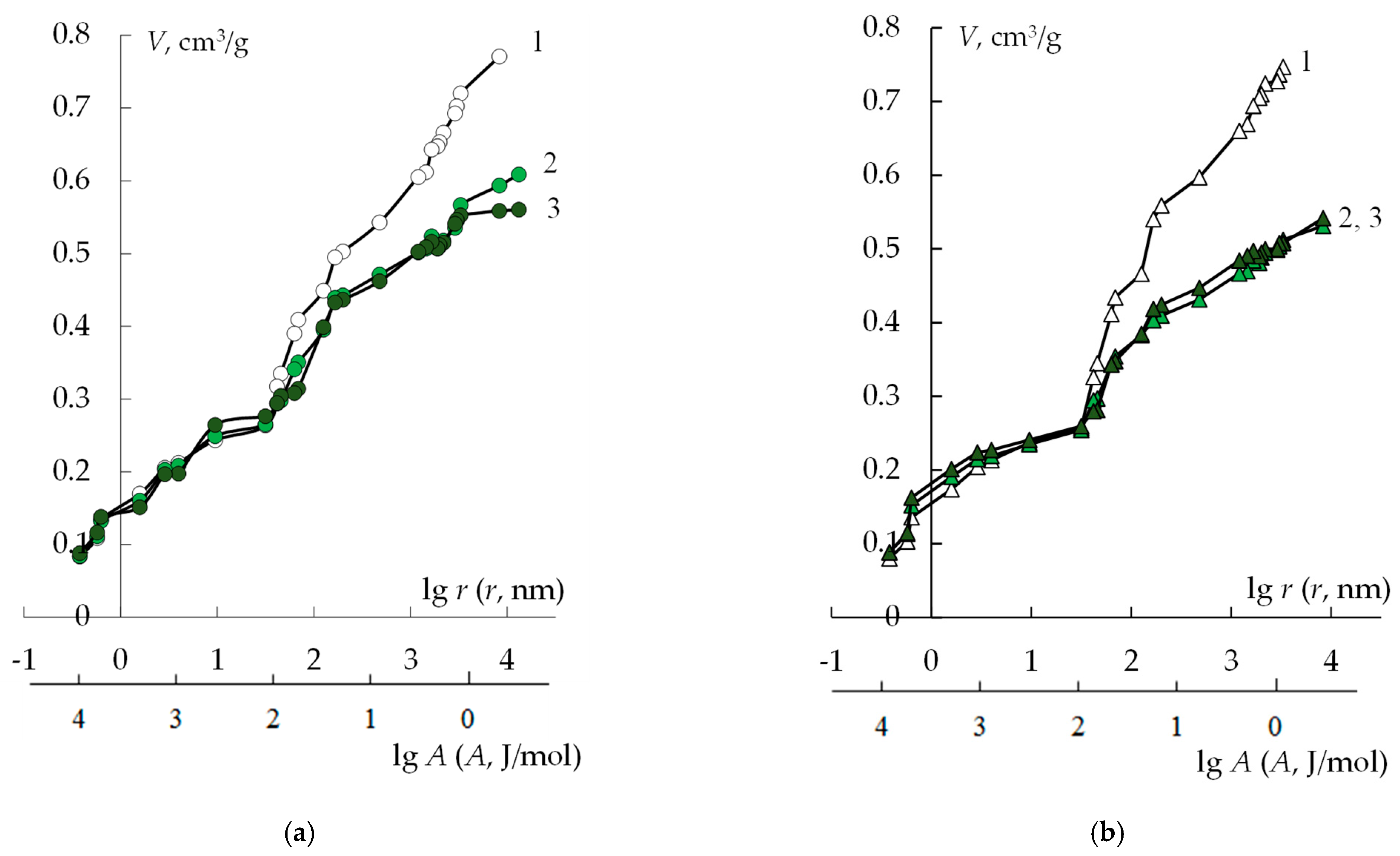
2.3. Standard Contact Porosimetry and Optical Microscopy
3. Results and Discussion
3.1. Study of the Membrane Structure
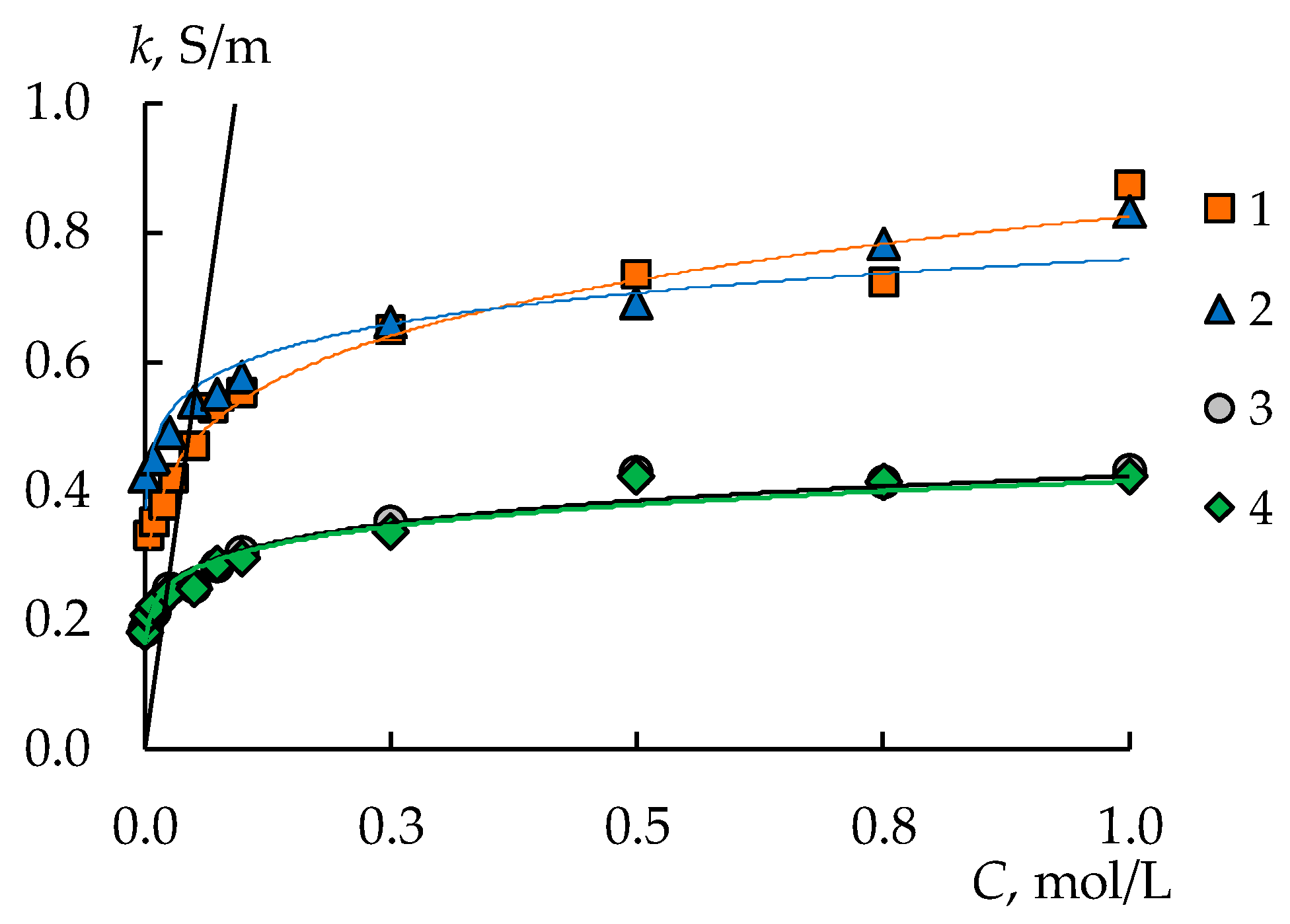
3.2. Diffusion Permeability and Conductivity of the Membranes
3.3. Selectivity of the Bilayer Membranes
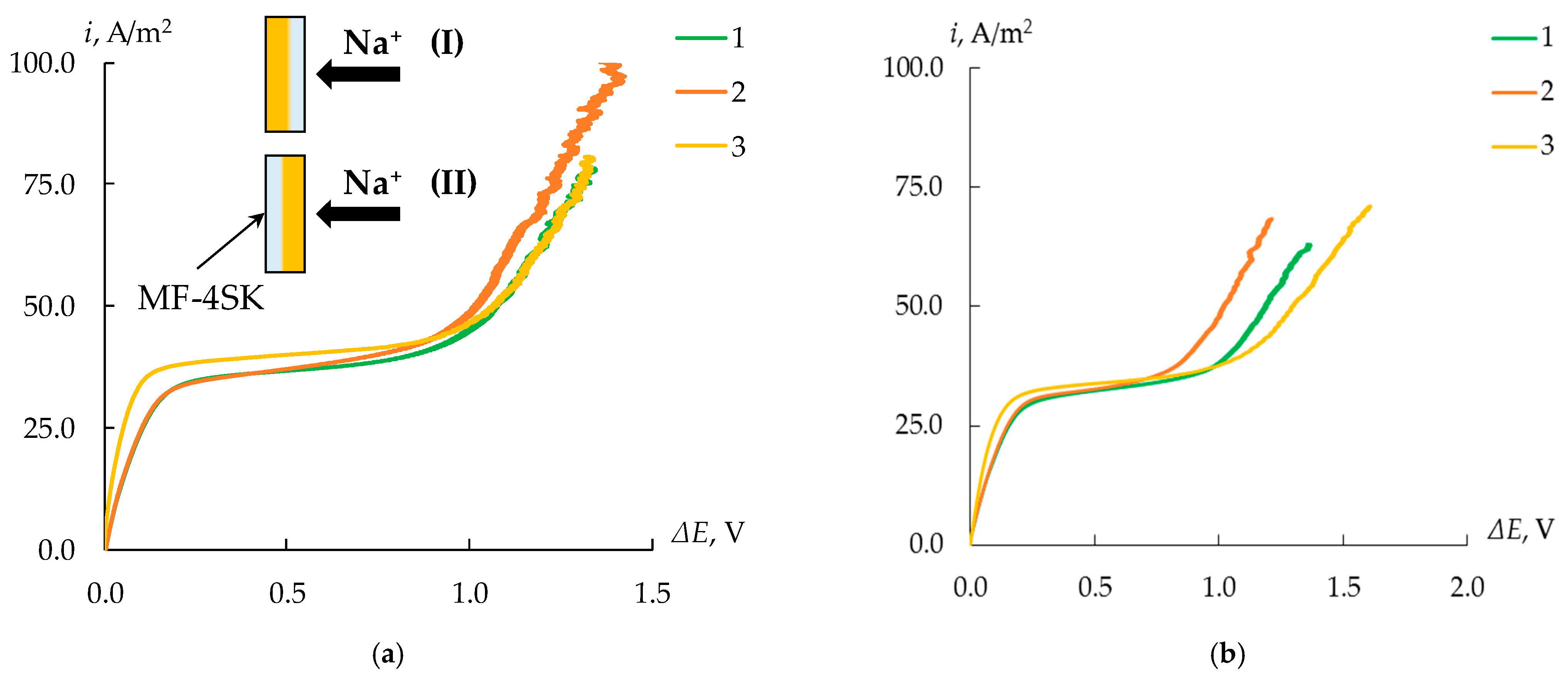
3.4. Current–Voltage Curves of the Bilayer Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Extended Three-Wire Model of Ion Exchange Membrane
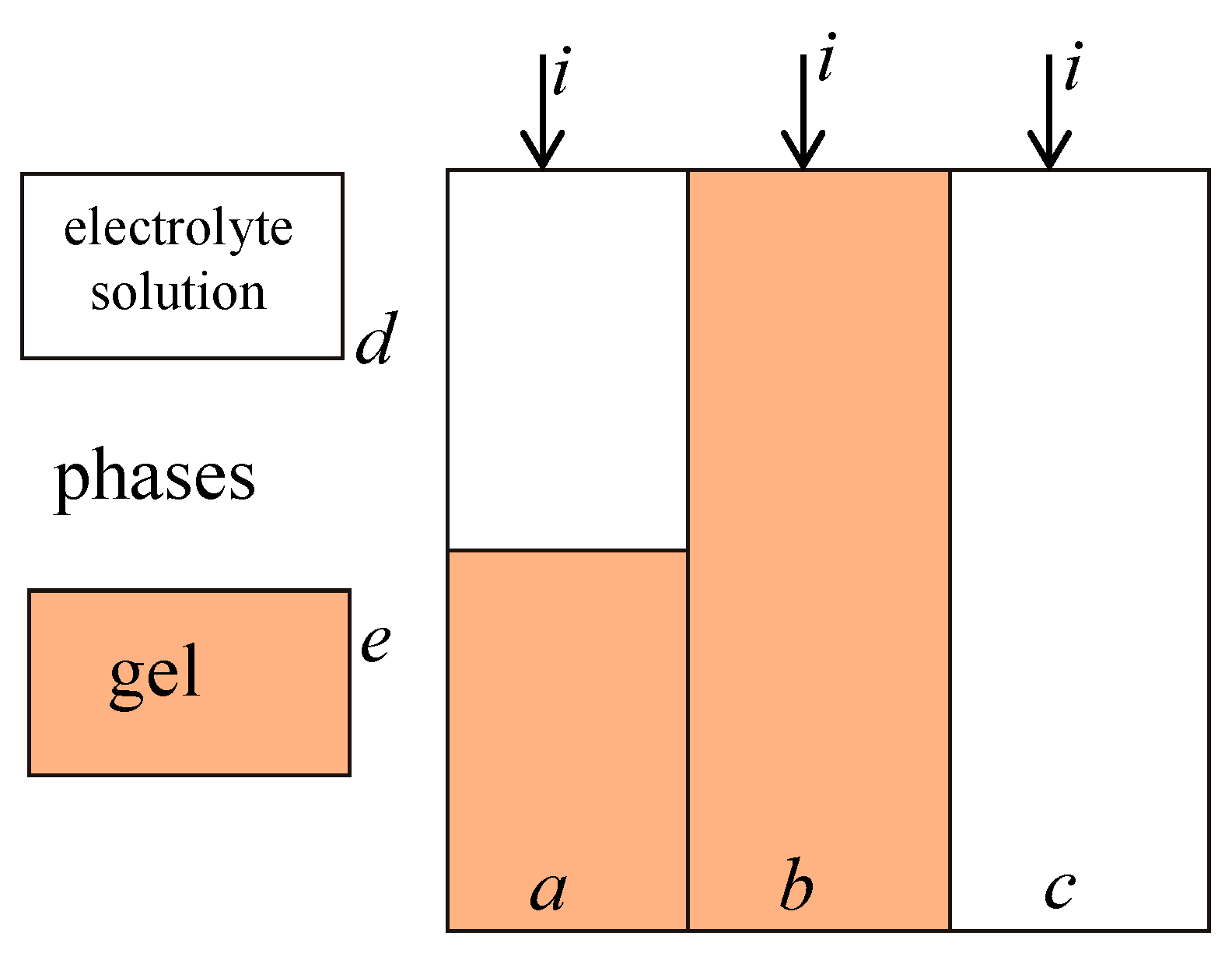
Appendix A.2. The Procedure of t* Calculation
References
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, T.; Siqueira Rodrigues, M.A.; Bernardes, A.M.; Zoppas-Ferreira, J. Closing the Loop in the Electroplating Industry by Electrodialysis. J. Clean. Prod. 2017, 155, 130–138. [Google Scholar] [CrossRef]
- Arana Juve, J.M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for Metal Removal and Recovery: A Review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Cardoso, D.; Stéphano, L.; Antônio, M.; Rodrigues, S.; Moura, A.; Alberto, J.; Tenório, S. Water Recovery from Acid Mine Drainage by Electrodialysis. Miner. Eng. 2013, 40, 82–89. [Google Scholar] [CrossRef]
- Mohammadi, R.; Tang, W.; Sillanpää, M. A Systematic Review and Statistical Analysis of Nutrient Recovery from Municipal Wastewater by Electrodialysis. Desalination 2021, 498, 114626. [Google Scholar] [CrossRef]
- Galama, A.H.; Saakes, M.; Bruning, H.; Rijnaarts, H.H.M.; Post, J.W. Seawater Predesalination with Electrodialysis. Desalination 2014, 342, 61–69. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis Desalination for Water and Wastewater: A Review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Melnikov, S.; Sheldeshov, N.; Zabolotsky, V.; Loza, S.; Achoh, A. Pilot Scale Complex Electrodialysis Technology for Processing a Solution of Lithium Chloride Containing Organic Solvents. Sep. Purif. Technol. 2017, 189, 74–81. [Google Scholar] [CrossRef]
- Melnikov, S.S.; Nosova, E.N.; Melnikova, E.D.; Zabolotsky, V.I. Reactive Separation of Inorganic and Organic Ions in Electrodialysis with Bilayer Membranes. Sep. Purif. Technol. 2021, 268, 118561. [Google Scholar] [CrossRef]
- Melnikov, S.; Loza, S.; Sharafan, M.; Zabolotskiy, V. Electrodialysis Treatment of Secondary Steam Condensate Obtained during Production of Ammonium Nitrate. Technical and Economic Analysis. Sep. Purif. Technol. 2016, 157, 179–191. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Demin, A.V.; Demina, O.A. Ion and Water Transport during Lithium Chloride Concentration from Aqueous Organic Solutions by Electrodialysis. Russ. J. Electrochem. 2011, 47, 327–335. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion Exchange Membranes: New Developments and Applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, H.; Wang, H.; Ladewig, B.P.; Yao, Z. A Comprehensive Review on the Synthesis and Applications of Ion Exchange Membranes. Chemosphere 2021, 282, 130817. [Google Scholar] [CrossRef] [PubMed]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of Ion-Exchange Membrane Materials: Properties vs Structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A. Current Progress in Membranes for Fuel Cells and Reverse Electrodialysis. Mendeleev Commun. 2021, 31, 423–432. [Google Scholar] [CrossRef]
- Thakur, A.K.; Malmali, M. Advances in Polymeric Cation Exchange Membranes for Electrodialysis: An Overview. J. Environ. Chem. Eng. 2022, 10, 108295. [Google Scholar] [CrossRef]
- Roghmans, F.; Evdochenko, E.; Martí-Calatayud, M.C.; Garthe, M.; Tiwari, R.; Walther, A.; Wessling, M. On the Permselectivity of Cation-Exchange Membranes Bearing an Ion Selective Coating. J. Membr. Sci. 2020, 600, 117854. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Sun, W.; Hu, Y.; Tang, H. Membrane Technologies for Li+/Mg2+ Separation from Salt-Lake Brines and Seawater: A Comprehensive Review. J. Ind. Eng. Chem. 2020, 81, 7–23. [Google Scholar] [CrossRef]
- Wang, W.; Hong, G.; Zhang, Y.; Yang, X.; Hu, N.; Zhang, J.; Sorokin, P.; Shao, L. Designing an Energy-Efficient Multi-Stage Selective Electrodialysis Process Based on High-Performance Materials for Lithium Extraction. J. Membr. Sci. 2023, 675, 121534. [Google Scholar] [CrossRef]
- Jeremias, J.S.D.; Lin, J.-Y.; Dalida, M.L.P.; Lu, M.-C. Abatement Technologies for Copper Containing Industrial Wastewater Effluents—A Review. J. Environ. Chem. Eng. 2023, 11, 109336. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Wang, X.; Chen, B.; Wang, Y.; Jiang, C.; Xu, T. Physical and Chemical Synergistic Strategy: A Facile Approach to Fabricate Monovalent Ion Permselective Membranes. Chem. Eng. Sci. 2021, 245, 116873. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Yu, D.; Mondal, A.N.; Hou, L.; Afsar, N.U.; Li, Q.; Xu, T.; Miao, J.; Xu, T. Monovalent Cation Perm-Selective Membranes (MCPMs): New Developments and Perspectives. Chin. J. Chem. Eng. 2017, 25, 1606–1615. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Tao, Y.; Xu, Y.; Pan, J.; Shen, J.; Gao, C. Enhanced Monovalent Selectivity of Cation Exchange Membranes via Adjustable Charge Density on Functional Layers. J. Membr. Sci. 2020, 595, 117544. [Google Scholar] [CrossRef]
- Sata, T.; Ishii, Y.; Kawamura, K.; Matsusaki, K. Composite Membranes Prepared from Cation Exchange Membranes and Polyaniline and Their Transport Properties in Electrodialysis. J. Electrochem. Soc. 1999, 146, 585–591. [Google Scholar] [CrossRef]
- Tan, S.; Viau, V.; Cugnod, D.; Bélanger, D. Chemical Modification of a Sulfonated Membrane with a Cationic Polyaniline Layer to Improve Its Permselectivity. Electrochem. Solid State Lett. 2002, 5, 3–7. [Google Scholar] [CrossRef]
- Sata, T.; Sata, T.; Yang, W. Studies on Cation-Exchange Membranes Having Permselectivity between Cations in Electrodialysis. J. Membr. Sci. 2002, 206, 31–60. [Google Scholar] [CrossRef]
- Tan, S.; Bélanger, D. Characterization and Transport Properties of Nafion/Polyaniline Composite Membranes. J. Phys. Chem. B 2005, 109, 23480–23490. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, P.; Chavan, J.G.; Thakur, A.P.; Hande, V.R.; Samui, A.B. Electrochemical Modification of Cation Exchange Membrane with Polyaniline for Improvement in Permselectivity. Electrochim. Acta 2007, 52, 5046–5052. [Google Scholar] [CrossRef]
- Tan, S.; Laforgue, A.; Bélanger, D. Characterization of a Cation-Exchange/Polyaniline Composite Membrane. Langmuir 2003, 19, 744–751. [Google Scholar] [CrossRef]
- Tan, S.; Tieu, J.H.; Bélanger, D. Chemical Polymerization of Aniline on a Poly(Styrene Sulfonic Acid) Membrane: Controlling the Polymerization Site Using Different Oxidants. J. Phys. Chem. B 2005, 109, 14085–14092. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K.; Trivedi, G.S.; Rangarajan, R. Preparation and Electrochemical Characterization of Cation- and Anion-Exchange/Polyaniline Composite Membranes. J. Colloid Interface Sci. 2004, 277, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Khan, M.A.; AlOthman, Z.A.; Siddiqui, M.R. Polyaniline Modified Organic-Inorganic Hybrid Cation-Exchange Membranes for the Separation of Monovalent and Multivalent Ions. Desalination 2013, 325, 95–103. [Google Scholar] [CrossRef]
- Nazif, A.; Karkhanechi, H.; Saljoughi, E.; Mousavi, S.M.; Matsuyama, H. Recent Progress in Membrane Development, Affecting Parameters, and Applications of Reverse Electrodialysis: A Review. J. Water Process Eng. 2022, 47, 102706. [Google Scholar] [CrossRef]
- Titova, T.S.; Yurova, P.A.; Kuleshova, V.A.; Parshina, A.V.; Stenina, I.A.; Bobreshova, O.V.; Yaroslavtsev, A.B. MF-4SC Membranes Modified by Polyaniline for Potentiometric Determination of Saccharin and Sodium Ions in Aqueous Solutions. Membr. Membr. Technol. 2021, 3, 411–418. [Google Scholar] [CrossRef]
- Parshina, A.; Yelnikova, A.; Kolganova, T.; Titova, T.; Yurova, P.; Stenina, I.; Bobreshova, O.; Yaroslavtsev, A. Perfluorosulfonic Acid Membranes Modified with Polyaniline and Hydrothermally Treated for Potentiometric Sensor Arrays for the Analysis of Combination Drugs. Membranes 2023, 13, 311. [Google Scholar] [CrossRef]
- Yuan, Y.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. Nafion/Polyaniline/Zeolitic Imidazolate Framework-8 Nanocomposite Sensor for the Electrochemical Determination of Dopamine. J. Electroanal. Chem. 2018, 824, 147–152. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, X.; Zuo, Y.; Ju, P.; Tang, Y. Electrodeposited Pd-Ni-Mo Film as a Cathode Material for Hydrogen Evolution Reaction. Electrochim. Acta 2015, 174, 1041–1049. [Google Scholar] [CrossRef]
- Masoumi, H.; Aslani, A.; Ghaemi, A.; Farrokhzad, H. Engineering and Chemistry Aspects of the Well-Known Conductive Polymers as Sensors: Characterization, Mechanism, Synthesis, Scale-up: A Review. Sens. Int. 2023, 4, 100240. [Google Scholar] [CrossRef]
- Do, J.S.; Chang, Y.H. Optimizing the Sensing Performance of Amperometric Creatinine Detection Based on Creatinine Deiminase/Nafion®-Nanostructured Polyaniline Composite Film by Mixture Design Method. Sens. Actuators Rep. 2023, 5, 100135. [Google Scholar] [CrossRef]
- Kumar, A.; Ibraheem, S.; Ali, S.; Maiyalagan, T.; Javed, M.S.; Gupta, R.K.; Saad, A.; Yasin, G. Polypyrrole and Polyaniline-Based Membranes for Fuel Cell Devices: A Review. Surf. Interfaces 2022, 29, 101738. [Google Scholar] [CrossRef]
- Huang, Q.M.; Zhang, Q.L.; Huang, H.L.; Li, W.S.; Huang, Y.J.; Luo, J.L. Methanol Permeability and Proton Conductivity of Nafion Membranes Modified Electrochemically with Polyaniline. J. Power Sources 2008, 184, 338–343. [Google Scholar] [CrossRef]
- Zhiani, M.; Gharibi, H.; Kakaei, K. Optimization of Nafion Content in Nafion-Polyaniline Nano-Composite Modified Cathodes for PEMFC Application. Int. J. Hydrogen Energy 2010, 35, 9261–9268. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Sulfonated Poly(Ether Ether Ketone)/Polyaniline Composite Proton-Exchange Membrane. J. Membr. Sci. 2006, 280, 389–396. [Google Scholar] [CrossRef]
- Munar, A.; Suarez, K.; Solorza, O.; Berezina, N.P.; Compañ, V. Performance of Hydrogen Fuel Cell MEAs Based on Perfluorinated Nanocomposite Membranes Modified by Polyaniline. J. Electrochem. Soc. 2010, 157, B1186. [Google Scholar] [CrossRef]
- Mehboob, S.; Lee, J.Y.; Hun Ahn, J.; Abbas, S.; Do, X.H.; Kim, J.; Shin, H.J.; Henkensmeier, D.; Ha, H.Y. Perfect Capacity Retention of All-Vanadium Redox Flow Battery Using Nafion/Polyaniline Composite Membranes. J. Ind. Eng. Chem. 2023, 121, 348–357. [Google Scholar] [CrossRef]
- Zakil, F.A.; Kamarudin, S.K.; Basri, S. Modified Nafion Membranes for Direct Alcohol Fuel Cells: An Overview. Renew. Sustain. Energy Rev. 2016, 65, 841–852. [Google Scholar] [CrossRef]
- Schwenzer, B.; Kim, S.; Vijayakumar, M.; Yang, Z.; Liu, J. Correlation of Structural Differences between Nafion/Polyaniline and Nafion/Polypyrrole Composite Membranes and Observed Transport Properties. J. Membr. Sci. 2011, 372, 11–19. [Google Scholar] [CrossRef]
- Yurova, P.A.; Karavanova, Y.A.; Yaroslavtsev, A.B. Diffusion Characteristics of Surface-Modified Ion-Exchange Membranes Based on MK-40, MF-4SK, and Polyaniline. Pet. Chem. 2012, 52, 593–597. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, L.; Chen, Q.; Lu, H.; Wang, J. Modification of Cation Exchange Membranes with Conductive Polyaniline for Electrodialysis Applications. J. Membr. Sci. 2019, 582, 211–223. [Google Scholar] [CrossRef]
- Farrokhzad, H.; Darvishmanesh, S.; Genduso, G.; Van Gerven, T.; Van Der Bruggen, B. Development of Bivalent Cation Selective Ion Exchange Membranes by Varying Molecular Weight of Polyaniline. Electrochim. Acta 2015, 158, 64–72. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Kompan, M.E.; Malyshkin, V.V.; Rosanov, V.V.; Stejskal, J. Properties of Proton-Conducting Nafion-Type Membranes with Nanometer-Thick Polyaniline Surface Layers. Russ. J. Electrochem. 2009, 45, 697–706. [Google Scholar] [CrossRef]
- Yurova, P.A.; Karavanova, Y.A.; Gorbunova, Y.G.; Yaroslavtsev, A.B. Transport Properties of Asymmetric Ion-Exchange Membranes Based on MC-40, MF-4SC, and Polyaniline. Pet. Chem. 2014, 54, 551–555. [Google Scholar] [CrossRef]
- Parshina, A.; Yelnikova, A.; Titova, T.; Kolganova, T.; Yurova, P.; Stenina, I.; Bobreshova, O.; Yaroslavtsev, A. Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Polyaniline and PEDOT for Multicomponent Analysis of Sulfacetamide Pharmaceuticals. Polymers 2022, 14, 2545. [Google Scholar] [CrossRef] [PubMed]
- Sata, T.; Ogura, S.; Kishimoto, F. Properties of Composite Membranes from Ion Exchange Membranes and Conducting Polymers. III. Changes in Acid Transport. J. Membr. Sci. 1993, 84, 259–269. [Google Scholar] [CrossRef]
- Protasov, K.V.; Shkirskaya, S.A.; Berezina, N.P.; Zabolotskii, V.I. Composite Sulfonated Cation-Exchange Membranes Modified with Polyaniline and Applied to Salt Solution Concentration by Electrodialysis. Russ. J. Electrochem. 2010, 46, 1131–1140. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Loza, N.V.; Shkirskaya, S.A.; Falina, I.V.; Khanukaeva, D.Y. Influence of Conditions of Polyaniline Synthesis in Perfluorinated Membrane on Electrotransport Properties and Surface Morphology of Composites. J. Solid State Electrochem. 2015, 19, 2623–2631. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Loza, N.V.; Andreeva, M.A.; Shkirskaya, S.A.; Dammak, L. Influence of Electric Field during the Chemical Synthesis of Polyaniline on the Surface of Heterogeneous Sulfonated Cation-Exchange Membranes on the Their Structure and Properties. Membr. Membr. Technol. 2019, 1, 229–237. [Google Scholar] [CrossRef]
- Loza, N.V.; Loza, S.A.; Kononenko, N.A.; Magalyanov, A.V. Ion Transport in Sulfuric Acid Solution through Anisotropic Composites Based on Heterogeneous Membranes and Polyaniline. Pet. Chem. 2015, 55, 724–729. [Google Scholar] [CrossRef]
- Falina, I.; Loza, N.; Loza, S.; Titskaya, E.; Romanyuk, N. Permselectivity of Cation Exchange Membranes Modified by Polyaniline. Membranes 2021, 11, 227. [Google Scholar] [CrossRef]
- Loza, S.A.; Romanyuk, N.A.; Falina, I.V.; Loza, N.V. Electrodialysis Separation and Selective Concentration of Sulfuric Acid and Nickel Sulfate Using Membranes Modified with Polyaniline. Membr. Membr. Technol. 2023, 5, 236–256. [Google Scholar] [CrossRef]
- Kononenko, N.; Nikonenko, V.; Grande, D.; Larchet, C.; Dammak, L.; Fomenko, M.; Volfkovich, Y. Porous Structure of Ion Exchange Membranes Investigated by Various Techniques. Adv. Colloid Interface Sci. 2017, 246, 196–216. [Google Scholar] [CrossRef]
- Gebel, G. Structural Evolution of Water Swollen Perfluorosulfonated Ionomers from Dry Membrane to Solution. Polymer 2000, 41, 5829–5838. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B. Perfluorinated Ion-Exchange Membranes. Polym. Sci. Ser. A 2013, 55, 674–698. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Kranina, N.A.; Malykhin, M.D.; Akberova, E.M.; Zhiltsova, A.V. The Surface Inhomogeneity of Ion-Exchange Membranes by SEM and AFM Data. J. Surf. Investig. 2013, 7, 144–153. [Google Scholar] [CrossRef]
- Vasil’eva, V.; Goleva, E.; Pismenskaya, N.; Kozmai, A.; Nikonenko, V. Effect of Surface Profiling of a Cation-Exchange Membrane on the Phenylalanine and NaCl Separation Performances in Diffusion Dialysis. Sep. Purif. Technol. 2019, 210, 48–59. [Google Scholar] [CrossRef]
- Volodina, E.; Pismenskaya, N.; Nikonenko, V.; Larchet, C.; Pourcelly, G. Ion Transfer across Ion-Exchange Membranes with Homogeneous and Heterogeneous Surfaces. J. Colloid Interface Sci. 2005, 285, 247–258. [Google Scholar] [CrossRef]
- Berezina, N.P.; Volfkovich, Y.M.; Kononenko, N.A.; Blinov, I.A. The Study of the Distribution of Water in the Heterogeneous Ion-Exchanging Membranes Using Reference Porometry. Sov. Electrochem. 1987, 23, 912–916. [Google Scholar]
- Brovkina, M.A.; Kutenko, N.A.; Loza, N.V. Electrochemical Behavior of Polyaniline-Modified Cation-Exchange Heterogeneous Membranes in Solutions Containing Mono- and Bivalent Cations. Membr. Membr. Technol. 2023, 5, 178–192. [Google Scholar] [CrossRef]
- Titorova, V.D.; Moroz, I.A.; Mareev, S.A.; Pismenskaya, N.D.; Sabbatovskii, K.G.; Wang, Y.; Xu, T.; Nikonenko, V.V. How Bulk and Surface Properties of Sulfonated Cation-Exchange Membranes Response to Their Exposure to Electric Current during Electrodialysis of a Ca2+ Containing Solution. J. Membr. Sci. 2022, 644, 120149. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Jia, Y.X.; Wang, X.L. The Improvement of Comprehensive Transport Properties to Heterogeneous Cation Exchange Membrane by the Covalent Immobilization of Polyethyleneimine. Sep. Purif. Technol. 2015, 140, 69–76. [Google Scholar] [CrossRef]
- Titorova, V.; Sabbatovskiy, K.; Sarapulova, V.; Kirichenko, E.; Sobolev, V.; Kirichenko, K. Characterization of MK-40 Membrane Modified by Layers of Cation Exchange and Anion Exchange Polyelectrolytes. Membranes 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Kozaderova, O.A.; Kim, K.B.; Gadzhiyevа, C.S.; Niftaliev, S.I. Electrochemical Characteristics of Thin Heterogeneous Ion Exchange Membranes. J. Membr. Sci. 2020, 604, 118081. [Google Scholar] [CrossRef]
- Sharafan, M.; Zabolotsky, V. Study of Electric Mass Transfer Peculiarities in Electromembrane Systems by the Rotating Membrane Disk Method. Desalination 2014, 343, 194–197. [Google Scholar] [CrossRef]
- Stockmeier, F.; Schatz, M.; Habermann, M.; Linkhorst, J.; Mani, A.; Wessling, M. Direct 3D Observation and Unraveling of Electroconvection Phenomena during Concentration Polarization at Ion-Exchange Membranes. J. Membr. Sci. 2021, 640, 119846. [Google Scholar] [CrossRef]
- Belloň, T.; Polezhaev, P.; Vobecká, L.; Svoboda, M.; Slouka, Z. Experimental Observation of Phenomena Developing on Ion-Exchange Systems during Current-Voltage Curve Measurement. J. Membr. Sci. 2019, 572, 607–618. [Google Scholar] [CrossRef]
- Luo, T.; Roghmans, F.; Wessling, M. Ion Mobility and Partition Determine the Counter-Ion Selectivity of Ion Exchange Membranes. J. Membr. Sci. 2020, 597, 117645. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Sharafan, M.V.; Shel’Deshov, N.V. Influence of the Nature of Membrane Ionogenic Groups on Water Dissociation and Electrolyte Ion Transport: A Rotating Membrane Disk Study. Russ. J. Electrochem. 2008, 44, 1127–1134. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V.; Pismenskaya, N.D.; Laktionov, E.V.; Urtenov, M.K.; Strathmann, H.; Wessling, M.; Koops, G.H. Coupled Transport Phenomena in Overlimiting Current Electrodialysis. Sep. Purif. Technol. 1998, 14, 255–267. [Google Scholar] [CrossRef]
- Nikonenko, V.; Urtenov, M.; Mareev, S.; Pourcelly, G. Mathematical Modeling of the Effect of Water Splitting on Ion Transfer in the Depleted Diffusion Layer near an Ion-exchange Membrane. Membranes 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Nikonenko, V.V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of Electroconvection and Its Use in Intensifying the Mass Transfer in Electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Korzhova, E.; Pismenskaya, N.; Lopatin, D.; Baranov, O.; Dammak, L.; Nikonenko, V. Effect of Surface Hydrophobization on Chronopotentiometric Behavior of an AMX Anion-Exchange Membrane at Overlimiting Currents. J. Membr. Sci. 2016, 500, 161–170. [Google Scholar] [CrossRef]
- Chamoulaud, G.; Bélanger, D. Modification of Ion-Exchange Membrane Used for Separation of Protons and Metallic Cations and Characterization of the Membrane by Current-Voltage Curves. J. Colloid Interface Sci. 2005, 281, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Porozhnyy, M.V.; Shkirskaya, S.A.; Butylskii, D.Y.; Dotsenko, V.V.; Safronova, E.Y.; Yaroslavtsev, A.B.; Deabate, S.; Huguet, P.; Nikonenko, V.V. Physicochemical and Electrochemical Characterization of Nafion-Type Membranes with Embedded Silica Nanoparticles: Effect of Functionalization. Electrochim. Acta 2021, 370, 137689. [Google Scholar] [CrossRef]
- Ibanez, R.; Stamatialis, D.F.; Wessling, M. Role of Membrane Surface in Concentration Polarization at Cation Exchange Membranes. J. Membr. Sci. 2004, 239, 119–128. [Google Scholar] [CrossRef]
- Rubinstein, I.; Staude, E.; Kedem, O. Role of the Membrane Surface in Concentration Polarization at Ion-Exchange Membrane. Desalination 1988, 69, 101–114. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.H.; Moon, S.H. Heterogeneity of Ion-Exchange Membranes: The Effects of Membrane Heterogeneity on Transport Properties. J. Colloid Interface Sci. 2001, 241, 120–126. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Akberova, E.M.; Zabolotsky, V.I.; Novak, L.; Kostylev, D.V. Effect of Dispersity of a Sulfonated Cation-Exchanger on the Current–Voltage Characteristics of Heterogeneous Membranes Ralex CM Pes. Pet. Chem. 2018, 58, 1133–1143. [Google Scholar] [CrossRef]
- Gil, V.V.; Andreeva, M.A.; Jansezian, L.; Han, J.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Dammak, L. Impact of Heterogeneous Cation-Exchange Membrane Surface Modification on Chronopotentiometric and Current–Voltage Characteristics in NaCl, CaCl2 and MgCl2 Solutions. Electrochim. Acta 2018, 281, 472–485. [Google Scholar] [CrossRef]
- Akberova, E.M.; Vasil’eva, V.I. Effect of the Resin Content in Cation-Exchange Membranes on Development of Electroconvection. Electrochem. Commun. 2020, 111, 106659. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Akberova, E.M.; Zabolotskii, V.I. Electroconvection in Systems with Heterogeneous Ion-Exchange Membranes after Thermal Modification. Russ. J. Electrochem. 2017, 53, 398–410. [Google Scholar] [CrossRef]
- Nebavskaya, K.A.; Sarapulova, V.V.; Sabbatovskiy, K.G.; Sobolev, V.D.; Pismenskaya, N.D.; Sistat, P.; Cretin, M.; Nikonenko, V.V. Impact of Ion Exchange Membrane Surface Charge and Hydrophobicity on Electroconvection at Underlimiting and Overlimiting Currents. J. Membr. Sci. 2017, 523, 36–44. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Mareev, S.A.; Pokhidnya, E.V.; Larchet, C.; Dammak, L.; Nikonenko, V.V. Effect of Surface Modification of Heterogeneous Anion-Exchange Membranes on the Intensity of Electroconvection at Their Surfaces. Russ. J. Electrochem. 2019, 55, 1203–1220. [Google Scholar] [CrossRef]
- Akberova, E.M.; Vasil’eva, V.I.; Zabolotsky, V.I.; Novak, L. Effect of the Sulfocation-Exchanger Dispersity on the Surface Morphology, Microrelief of Heterogeneous Membranes and Development of Electroconvection in Intense Current Modes. J. Membr. Sci. 2018, 566, 317–328. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Dolgopolov, S.V.; Loza, N.V.; Shel’deshov, N.V. Effects of PH Variation in Solutions under the Polarization Conditions of the MF-4SK Membrane with Surface Modified by Polyaniline. Russ. J. Electrochem. 2015, 51, 19–24. [Google Scholar] [CrossRef]
- Loza, N.V.; Dolgopolov, S.V.; Kononenko, N.A.; Andreeva, M.A.; Korshikova, Y.S. Effect of Surface Modification of Perfluorinated Membranes with Polyaniline on Their Polarization Behavior. Russ. J. Electrochem. 2015, 51, 538–545. [Google Scholar] [CrossRef]
- Loza, N.V.; Kutenko, N.A.; Kononenko, N.A.; Volfkovich, Y.M.; Sosenkin, V.E. Transport Properties and Structure of Anisotropic Composites Based on Cation-Exchange Membranes and Polyaniline. Membr. Membr. Technol. 2023, 5, 193–208. [Google Scholar] [CrossRef]
- Loza, S.; Loza, N.; Kutenko, N.; Smyshlyaev, N. Profiled Ion-Exchange Membranes for Reverse and Conventional Electrodialysis. Membr. Technol. 2022, 12, 985. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Filippov, A.N.; Bagotsky, V.S. Structural Properties of Porous Materials and Powders Used in Different Fields of Science and Technology; Springer: London, UK, 2014; ISBN 978-1-4471-6377-0. [Google Scholar]
- Demina, O.A.; Kononenko, N.A.; Falina, I.V. New Approach to the Characterization of Ion-Exchange Membranes Using a Set of Model Parameters. Pet. Chem. 2014, 54, 515–525. [Google Scholar] [CrossRef]


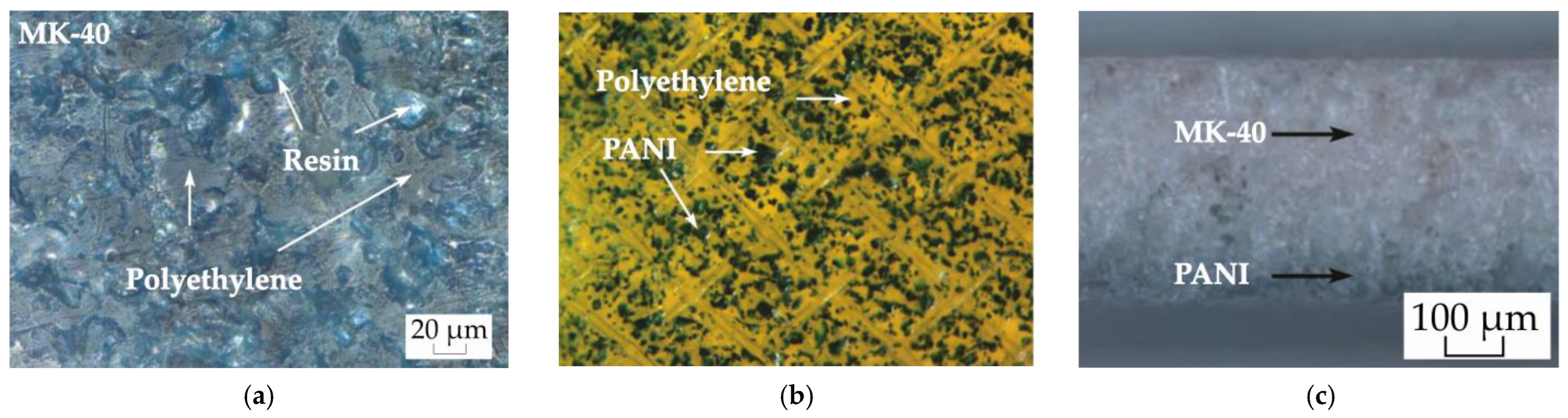
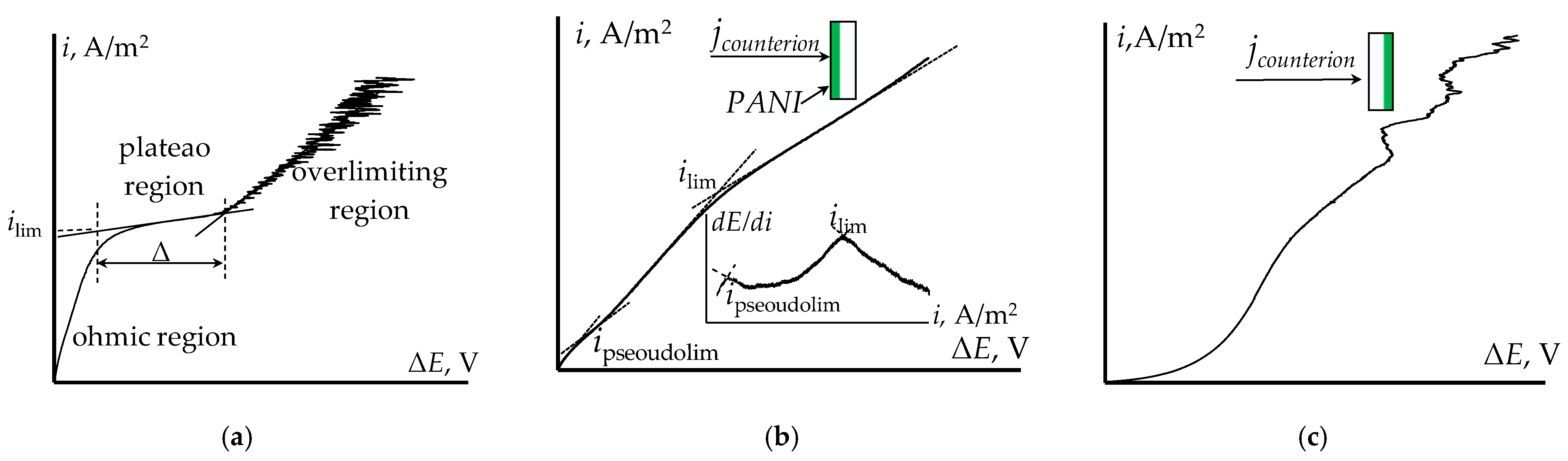

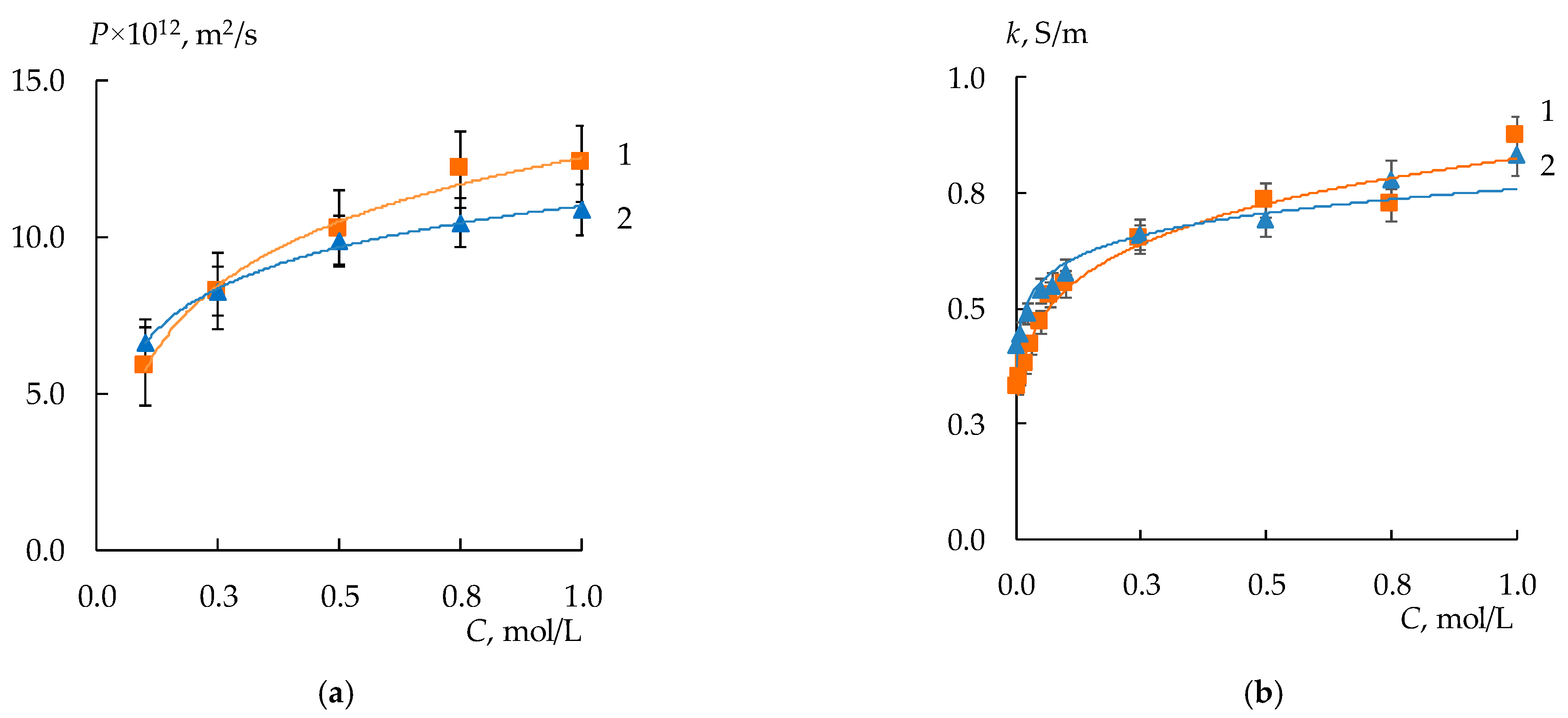

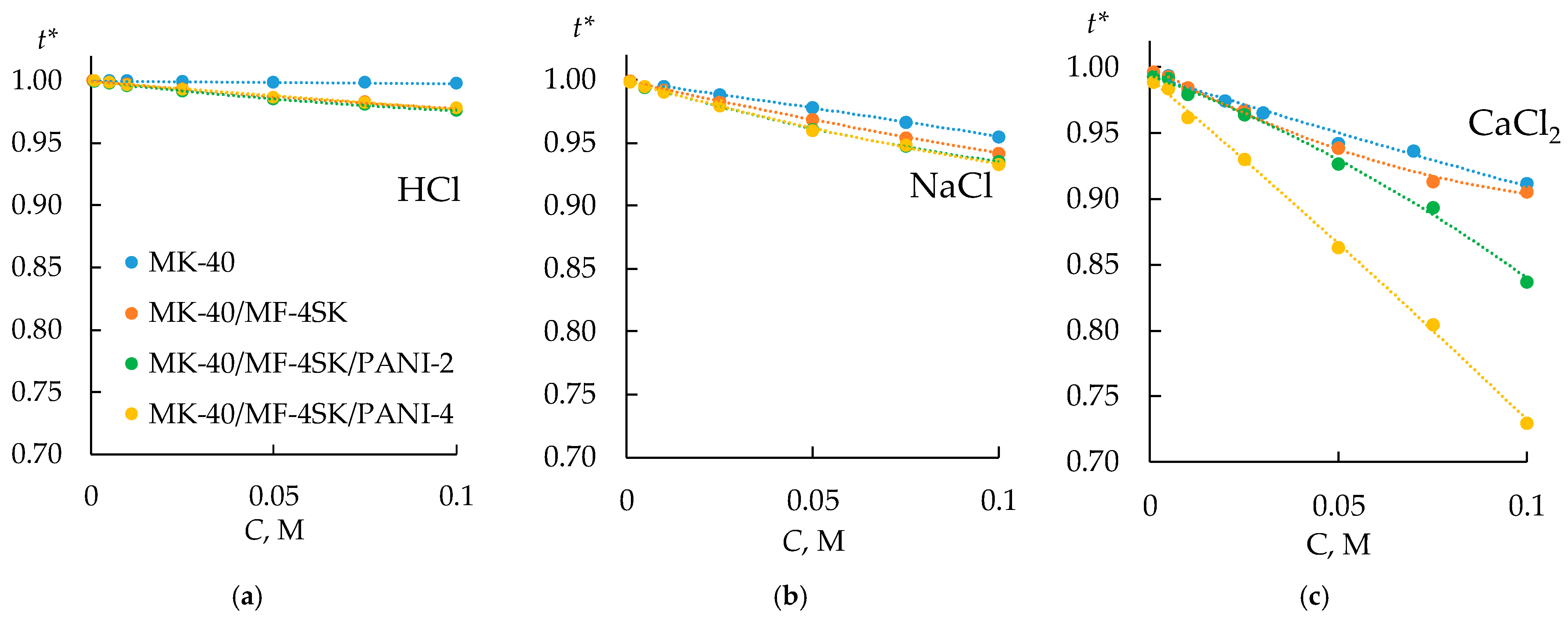


| Membrane | Q, mmol-eq/g | l, μm |
|---|---|---|
| MK-40 | 1.6 ± 0.1 | 560 ± 10 |
| MK-40/MF-4SK | 1.7 ± 0.2 | 550 ± 20 |
| MK-40/MF-4SK/PANI-2 | 1.6 ± 0.1 | 550 ± 10 |
| MK-40/MF-4SK/PANI-4 | 1.6 ± 0.1 | 540 ± 15 |
| Membrane | Counterion | V0, cm3/gdry | S, m2/g | ||
|---|---|---|---|---|---|
| MK-40/MF-4SK | H+ | 0.771 | 0.34 | 0.31 | 400 |
| MK-40/MF-4SK/PANI-2 | 0.608 | 0.43 | 0.24 | 386 | |
| MK-40/MF-4SK/PANI-4 | 0.560 | 0.49 | 0.20 | 375 | |
| MK-40/MF-4SK | Ca2+ | 0.747 | 0.34 | 0.31 | 402 |
| MK-40/MF-4SK/PANI-2 | 0.531 | 0.47 | 0.21 | 420 | |
| MK-40/MF-4SK/PANI-4 | 0.541 | 0.47 | 0.21 | 438 |
| Membrane | ilim, A/m2 | Δ, V | Δiohm/ΔEohm | |||
|---|---|---|---|---|---|---|
| Bilayer Membrane Orientation | ||||||
| I | II | I | II | I | II | |
| NaCl | ||||||
| MK-40 | 37.2 ± 0.4 | 0.97 ± 0.02 | 348 ± 73 | |||
| MK-40/MF-4SK | 34 ± 1 | 32.9 ± 0.2 | 0.85 ± 0.07 | 0.83 ± 0.03 | 227 ± 18 | 229 ± 5 |
| MK-40/MF-4SK/PANI-2 | 40 ± 1 | 36 ± 1 | 1.27 ± 0.07 | 0.98 ± 0.08 | 171 ± 12 | 180 ± 3 |
| MK-40/MF-4SK_PANI-4 | 36 ± 1 | 38 ± 2 | 1.12 ± 0.05 | 0.93 ± 0.09 | 191 ± 6 | 201 ± 7 |
| HCl | ||||||
| MK-40 | 173 ± 11 | 0.96 ± 0.04 | 1545 ± 106 | |||
| MK-40/MF-4SK | 171 ± 18 | 142 ± 29 | 0.98 ± 0.07 | 0.80 ± 0.05 | 1692.7 ± 121.4 | 1221 ± 158 |
| MK-40/MF-4SK/PANI-2 | 165 ± 26 | 160 ± 5 | 3.8 ± 0.3 | 1.8 ± 0.1 | 1079.8 ± 142.6 | 1159 ± 90 |
| MK-40/MF-4SK_PANI-4 | 166 ± 3 | 184 ± 6 | 1.6 ± 0.2 | 1.75 ± 0.3 | 1350.9 ± 250.2 | 1250 ± 232 |
| CaCl2 | ||||||
| MK-40 | 31.7 ± 0.2 | 0.99 ± 0.01 | 240.4 ± 14.3 | |||
| MK-40/MF-4SK | 29.7 ± 0.3 | 29.7 ± 0.4 | 0.83 ± 0.01 | 0.71 ± 0.01 | 159.8 ± 3.2 | 168 ± 3 |
| MK-40/MF-4SK/PANI-2 | 30.2 ± 0.4 | 30.2 ± 0.4 | 1.66 ± 0.01 | 1.4 ± 0.1 | 36.0 ± 0.5 | 57 ± 6 |
| MK-40/MF-4SK_PANI-4 | 30 ± 1 | 32.7 ± 0.7 | 1.9 ± 0.2 | 1.6 ± 0.1 | 66.1 ± 2.8 | 81 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loza, N.; Falina, I.; Kutenko, N.; Shkirskaya, S.; Loza, J.; Kononenko, N. Bilayer Heterogeneous Cation Exchange Membrane with Polyaniline Modified Homogeneous Layer: Preparation and Electrotransport Properties. Membranes 2023, 13, 829. https://doi.org/10.3390/membranes13100829
Loza N, Falina I, Kutenko N, Shkirskaya S, Loza J, Kononenko N. Bilayer Heterogeneous Cation Exchange Membrane with Polyaniline Modified Homogeneous Layer: Preparation and Electrotransport Properties. Membranes. 2023; 13(10):829. https://doi.org/10.3390/membranes13100829
Chicago/Turabian StyleLoza, Natalia, Irina Falina, Natalia Kutenko, Svetlana Shkirskaya, Julia Loza, and Natalia Kononenko. 2023. "Bilayer Heterogeneous Cation Exchange Membrane with Polyaniline Modified Homogeneous Layer: Preparation and Electrotransport Properties" Membranes 13, no. 10: 829. https://doi.org/10.3390/membranes13100829
APA StyleLoza, N., Falina, I., Kutenko, N., Shkirskaya, S., Loza, J., & Kononenko, N. (2023). Bilayer Heterogeneous Cation Exchange Membrane with Polyaniline Modified Homogeneous Layer: Preparation and Electrotransport Properties. Membranes, 13(10), 829. https://doi.org/10.3390/membranes13100829






