Evaluation of FAU-type Zeolite Membrane Stability in Transesterification Reaction Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Thermal Analysis of Zeolite Powder after Immersion Treatment with Alcohols and Esters
2.4. Pervaporation Experiment
2.5. Vapor Exposure Test
3. Results
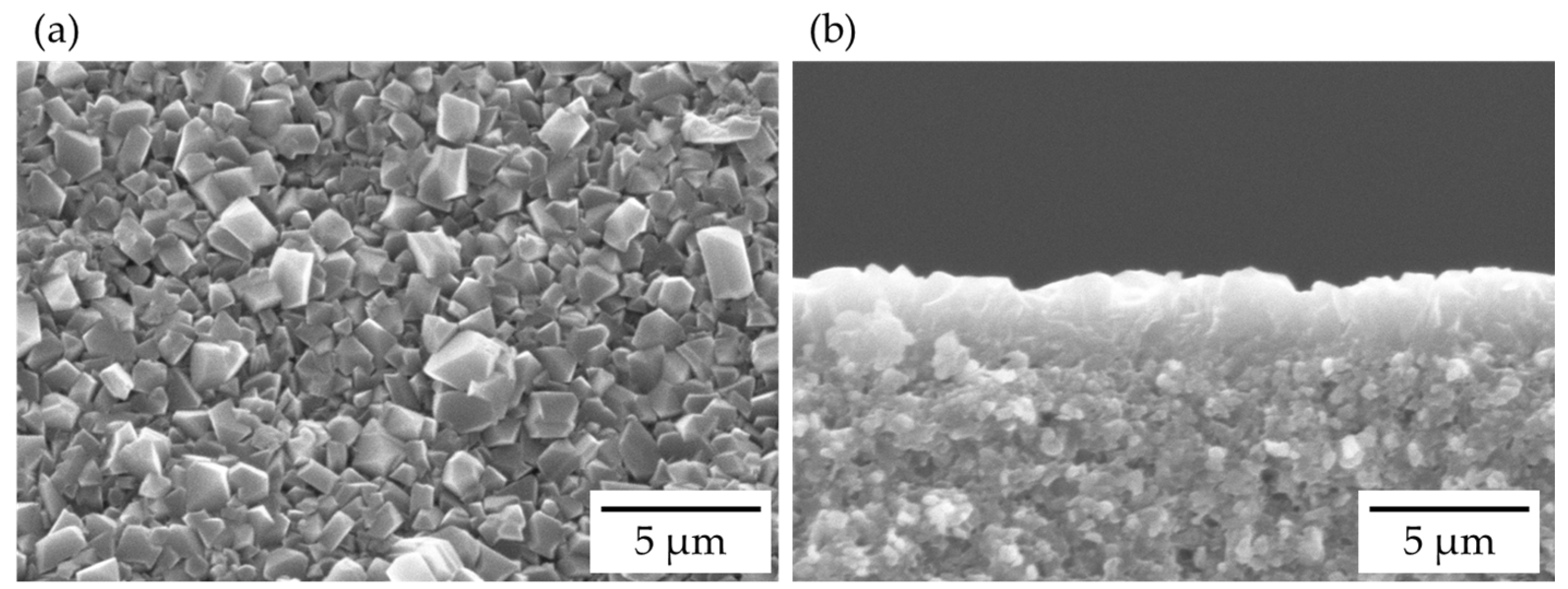
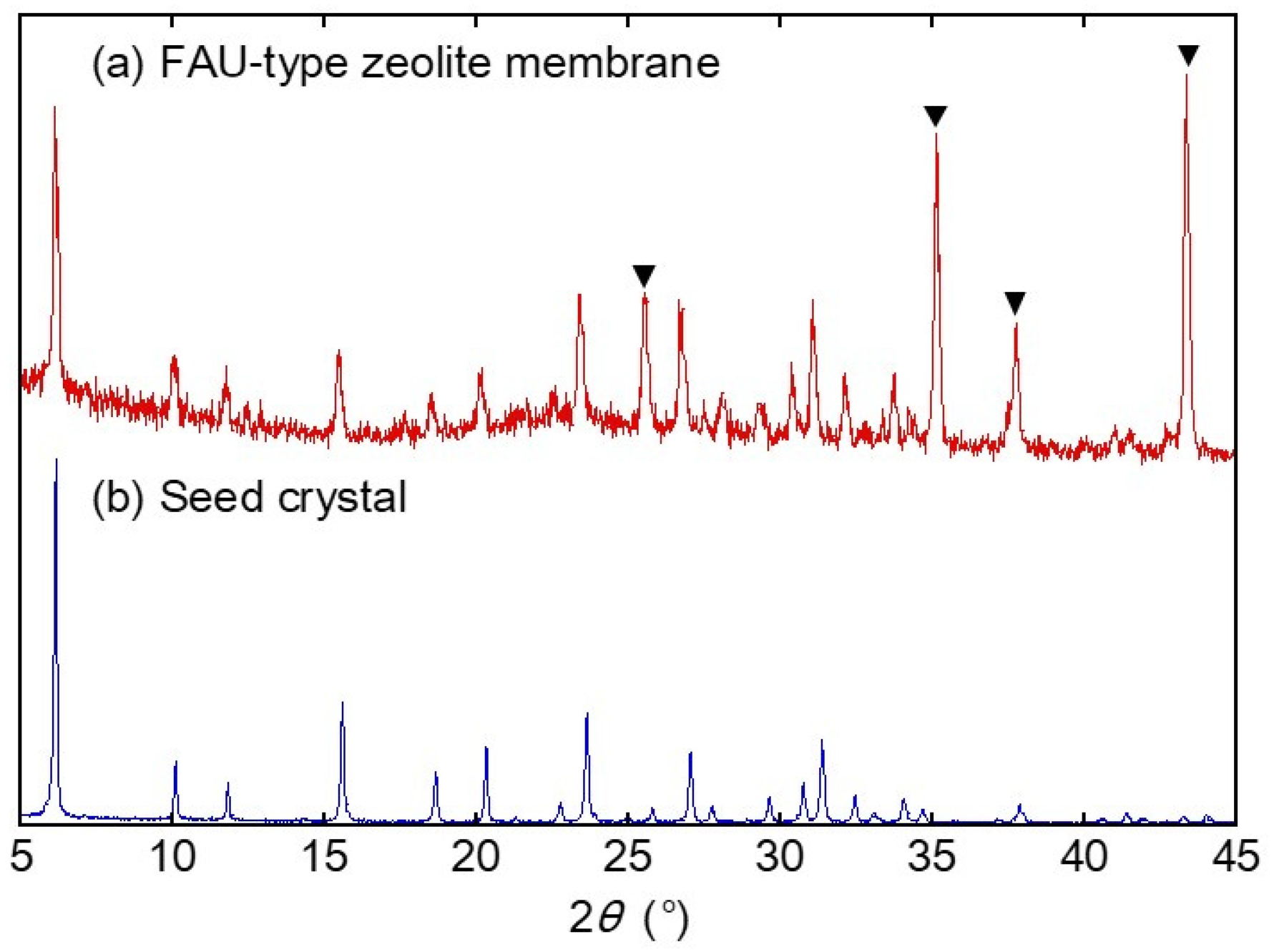
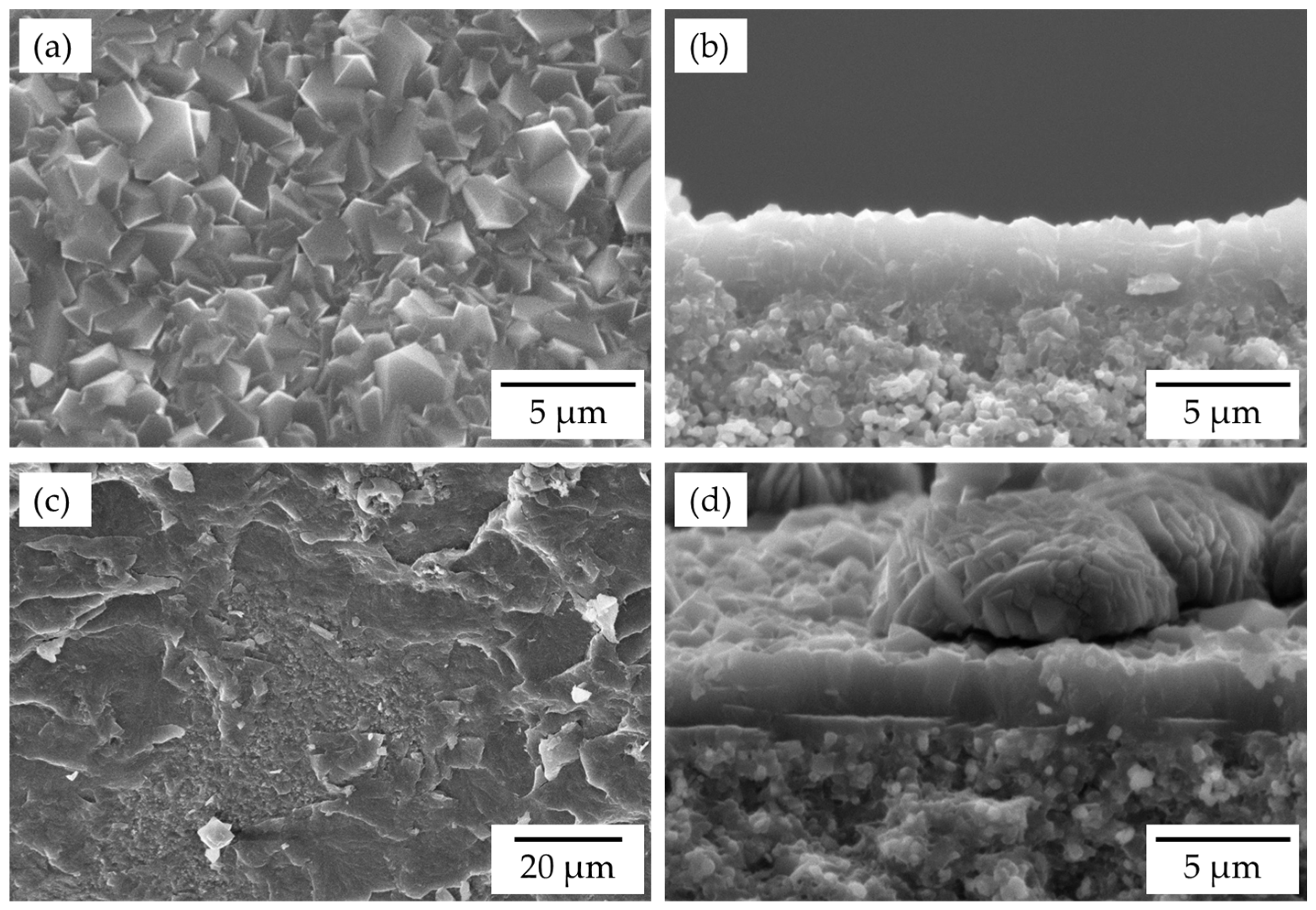
3.1. Characterization
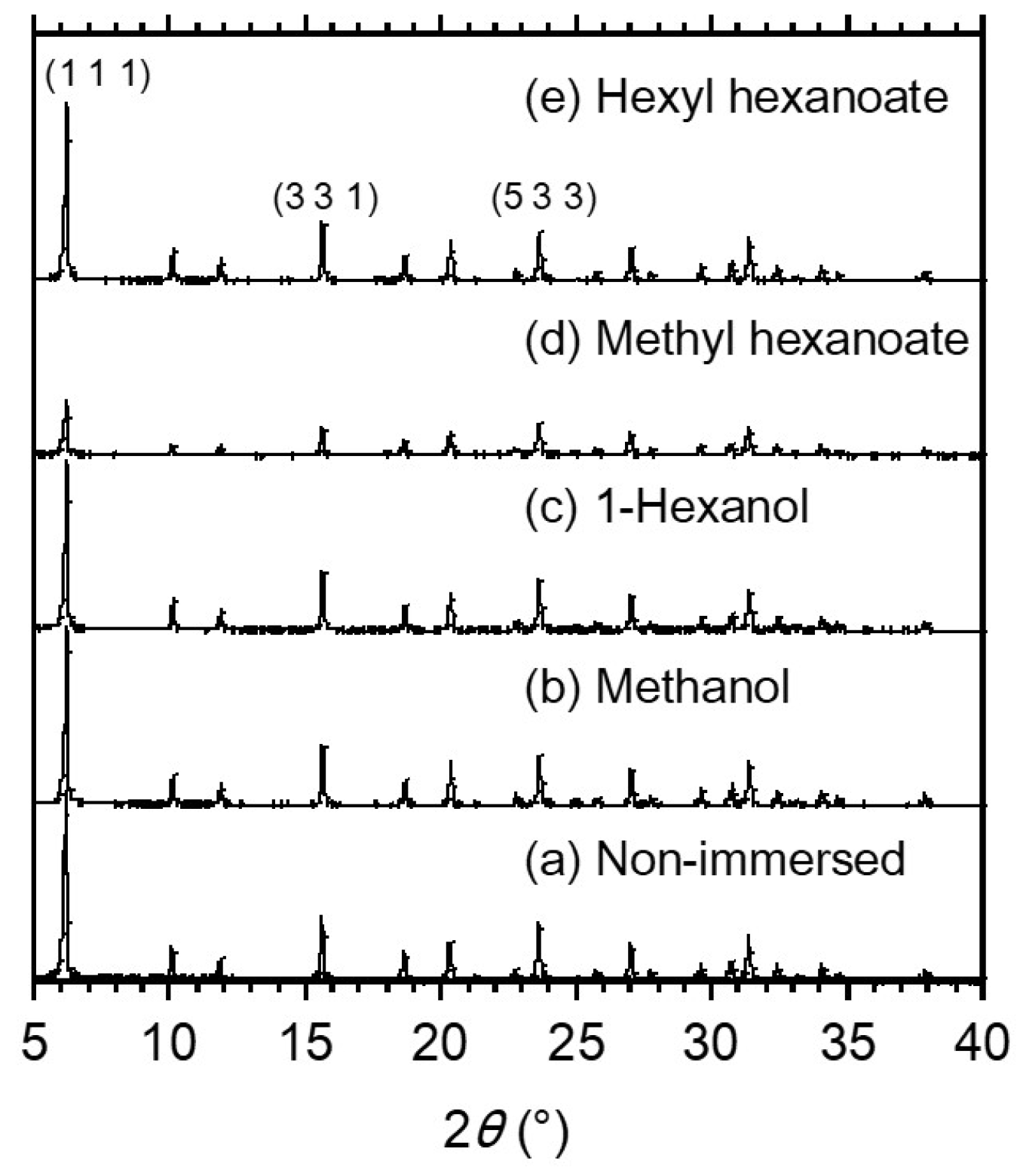
3.2. TG-DTA Measurement of Zeolite Powder Immersed Alcohols and Esters
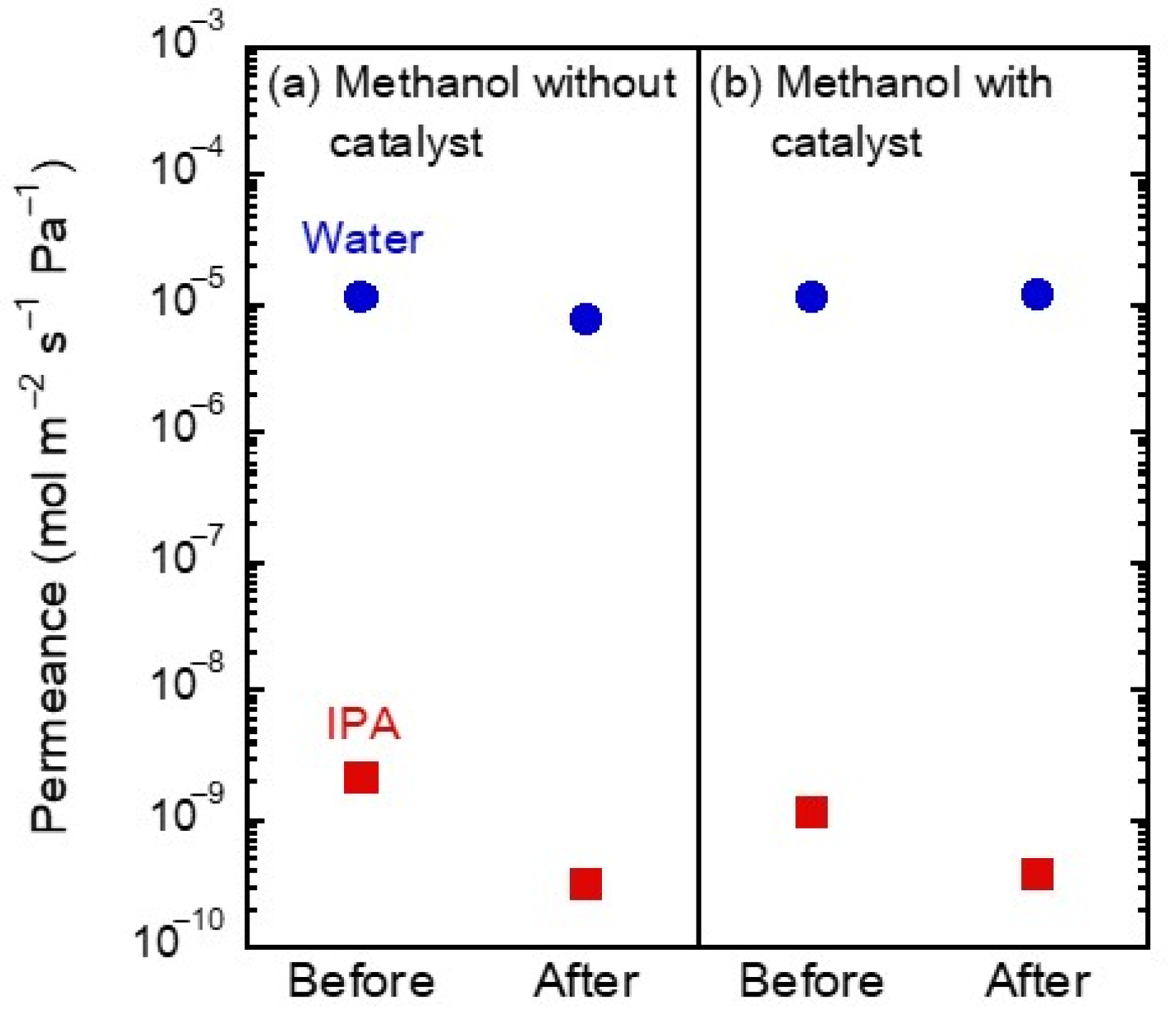
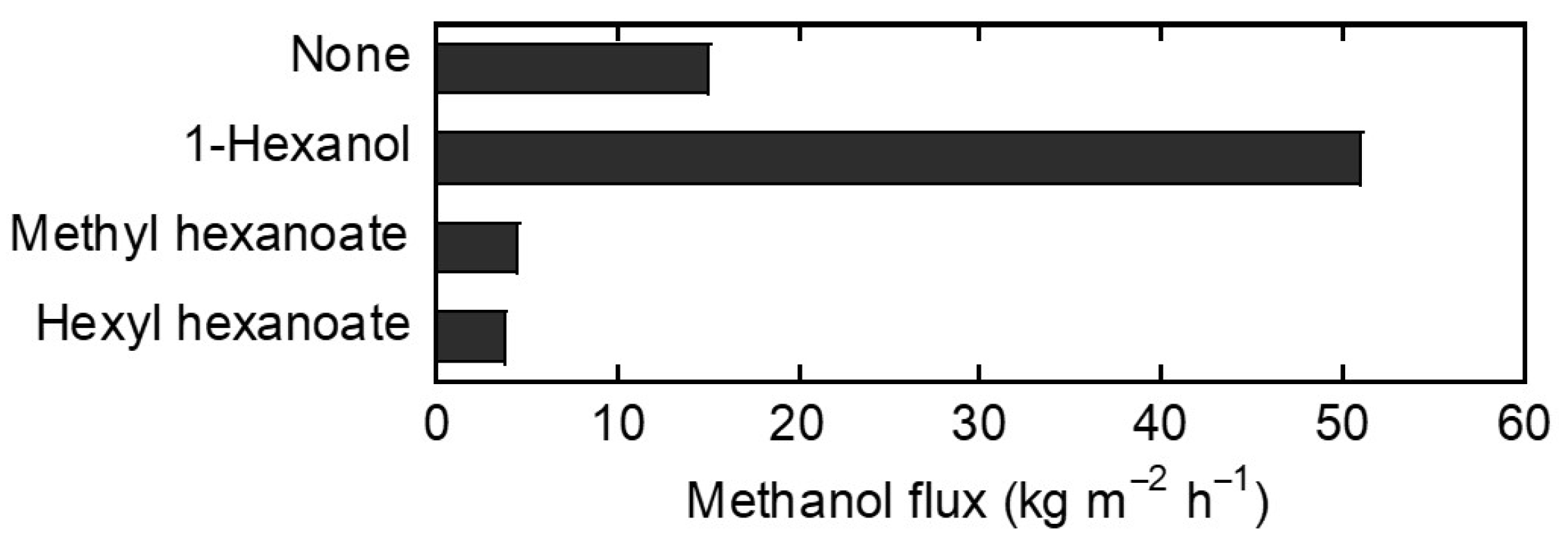
3.3. Vapor Exposure Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horsley, L.H. Table of azeotropes and nonazeotropes. Anal. Chem. 1947, 19, 508–600. [Google Scholar] [CrossRef]
- Luis, P.; Degrève, J.; Bruggen, B.V. Separation of methanol–n-butyl acetate mixtures by pervaporation: Potential of 10 commercial membranes. J. Membr. Sci. 2013, 429, 1–12. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Q.; Shao, J.; Wang, Z.; Chen, X.; Kita, H. Optimization of NaY zeolite membrane preparation for the separation of methanol/methyl methacrylate mixtures. Desalination 2012, 291, 41–47. [Google Scholar] [CrossRef]
- Anggarini, U.; Yu, L.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Microporous nickel-coordinated aminosilica membranes for improved pervaporation performance of methanol/toluene separation. ACS Appl. Mater. Interfaces 2021, 13, 23247–23259. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Nagasawa, H.; Yu, L.; Wang, Q.; Yamamoto, K.; Ohshita, J.; Kanezashi, M.; Tsuru, T. Pervaporation removal of methanol from methanol/organic azeotropes using organosilica membranes: Experimental and modeling. J. Membr. Sci. 2020, 610, 118284. [Google Scholar] [CrossRef]
- Kita, H.; Inoue, T.; Asamura, H.; Tanaka, K.; Okamoto, K. NaY zeolite membrane for the pervaporation separation of methanol–methyl tert-butyl ether mixtures. Chem. Commun. 1997, 1, 45–46. [Google Scholar] [CrossRef]
- Ikeda, A.; Abe, C.; Matsuura, W.; Hasegawa, Y. Development of methanol permselective FAU-type zeolite membranes and their permeation and separation performances. Membranes 2021, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Hasegawa, Y. Efficient transesterification of methyl acetate with 2-propanol by the selective removal of methanol using zeolite membranes. Chem. Lett. 2021, 50, 113–115. [Google Scholar] [CrossRef]
- Ikeda, A.; Matsuura, W.; Abe, C.; Hasegawa, Y. Effect of reaction substrates on membrane-assisted transesterification reactions. Chem. Eng. Process. 2021, 165, 108443. [Google Scholar] [CrossRef]
- Ikeda, A.; Abe, C.; Matsuura, W.; Hasegawa, Y. Kinetic study of the zeolite membrane-assisted transesterification reaction with methanol removal. Chem. Eng. Process. 2022, 172, 108778. [Google Scholar] [CrossRef]
- Zhang, F.; Rezac, M.E.; Majumdar, S.; Kosaraju, P.; Nemser, S. Improving chemical production processes by selective by-product removal in a pervaporation membrane reactor. Sep. Sci. Technol. 2014, 49, 1289–1297. [Google Scholar] [CrossRef][Green Version]
- Sato, T.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Enhanced production of butyl acetate via methanol-extracting transesterification membrane reactors using organosilica membrane: Experiment and modeling. Chem. Eng. J. 2022, 429, 13218. [Google Scholar] [CrossRef]
- Kumakiri, I.; Hashimoto, K.; Nakagawa, Y.; Inoue, Y.; Kanehiro, Y.; Tanaka, K.; Kita, H. Application of FAU zeolite membranes to alcohol/acrylate mixture systems. Catal. Today 2014, 236, 86–91. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kimura, K.; Nemoto, Y.; Nagase, T.; Kiyozumi, Y.; Nishide, T.; Mizukami, F. Real-time monitoring of permeation properties through polycrystalline MFI-type zeolite membranes during pervaporation using mass- spectrometry. Sep. Purif. Technol. 2008, 58, 386–392. [Google Scholar] [CrossRef]
- Ohe, S. Data Book of Vapor-Liquid Equilibrium Computation Program by Excel; Shuzo Ohe: Tokyo, Japan, 2002. [Google Scholar]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Patterns for Zeolites, 5th ed.; The Structure Commission of the International Zeolite Association; Elsevier: Amsterdam, The Netherlands, 2008; pp. 166–167. [Google Scholar]
- Iglesia, O.; Mallada, R.; Coronas, M.M.J. Continuous zeolite membrane reactor for esterification of ethanol and acetic acid. Chem. Eng. J. 2007, 131, 35–39. [Google Scholar] [CrossRef]
- Tanemura, K.; Rohand, T. Activated charcoal as an effective additive for alkaline and acidic hydrolysis of esters in water. Tetrahedron Lett. 2020, 61, 152467. [Google Scholar] [CrossRef]
- Wu, H.; Gong, Q.; Olson, D.H.; Li, J. Commensurate adsorption of hydrocarbons and alcohols in microporous metal organic frameworks. Chem. Rev. 2012, 112, 836–868. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 20 December 2022).
- Liu, B.; Kita, H.; Yogo, K. Preparation of Si-rich LTA zeolite membrane using organic template-free solution for methanol dehydration. Sep. Purif. Technol. 2020, 239, 116533. [Google Scholar] [CrossRef]
- Leeuwen, M.E. Derivation of Stockmayer potential parameters for polar fluids. Fluid Phase Equilibria 1994, 99, 1–18. [Google Scholar] [CrossRef]


| Component | Antoine Constant | Wilson Parameter | |||
|---|---|---|---|---|---|
| A | B | C | ΛAW | ΛWA | |
| Water | 8.02754 | 1705.616 | 231.405 | - | - |
| Methanol | 8.07919 | 1581.34 | 239.65 | 0.55148 | 0.89781 |
| 2-Propanol | 6.6604 | 813.055 | 132.93 | 0.04857 | 0.77714 |
| Immersion Solution | Intensity Ratio (-) | |
|---|---|---|
| I(1 1 1)/I(5 3 3) | I(3 3 1)/I(5 3 3) | |
| None | 3.3 | 1.1 |
| Methanol | 3.5 | 1.2 |
| 1-Hexanol | 3.1 | 1.1 |
| Methyl hexanoate | 1.6 | 0.8 |
| Hexyl hexanoate | 3.4 | 1.1 |
| Exposed Vapor | Intensity Ratio (-) | ||
|---|---|---|---|
| I(1 1 1)/Iα-alumina | I(3 3 1)/Iα-alumina | I(5 3 3)/Iα-alumina | |
| None | 0.27 | 0.10 | 0.14 |
| Hexyl methyl ether | 0.13 | 0.07 | 0.09 |
| Hexyl ether | 0.22 | 0.09 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, A.; Matsuura, W.; Abe, C.; Lundin, S.-T.B.; Hasegawa, Y. Evaluation of FAU-type Zeolite Membrane Stability in Transesterification Reaction Conditions. Membranes 2023, 13, 68. https://doi.org/10.3390/membranes13010068
Ikeda A, Matsuura W, Abe C, Lundin S-TB, Hasegawa Y. Evaluation of FAU-type Zeolite Membrane Stability in Transesterification Reaction Conditions. Membranes. 2023; 13(1):68. https://doi.org/10.3390/membranes13010068
Chicago/Turabian StyleIkeda, Ayumi, Wakako Matsuura, Chie Abe, Sean-Thomas Bourne Lundin, and Yasuhisa Hasegawa. 2023. "Evaluation of FAU-type Zeolite Membrane Stability in Transesterification Reaction Conditions" Membranes 13, no. 1: 68. https://doi.org/10.3390/membranes13010068
APA StyleIkeda, A., Matsuura, W., Abe, C., Lundin, S.-T. B., & Hasegawa, Y. (2023). Evaluation of FAU-type Zeolite Membrane Stability in Transesterification Reaction Conditions. Membranes, 13(1), 68. https://doi.org/10.3390/membranes13010068






