Combined Separator Based on a Porous Ion-Exchange Membrane for Zinc–Halide Batteries
Abstract
1. Introduction
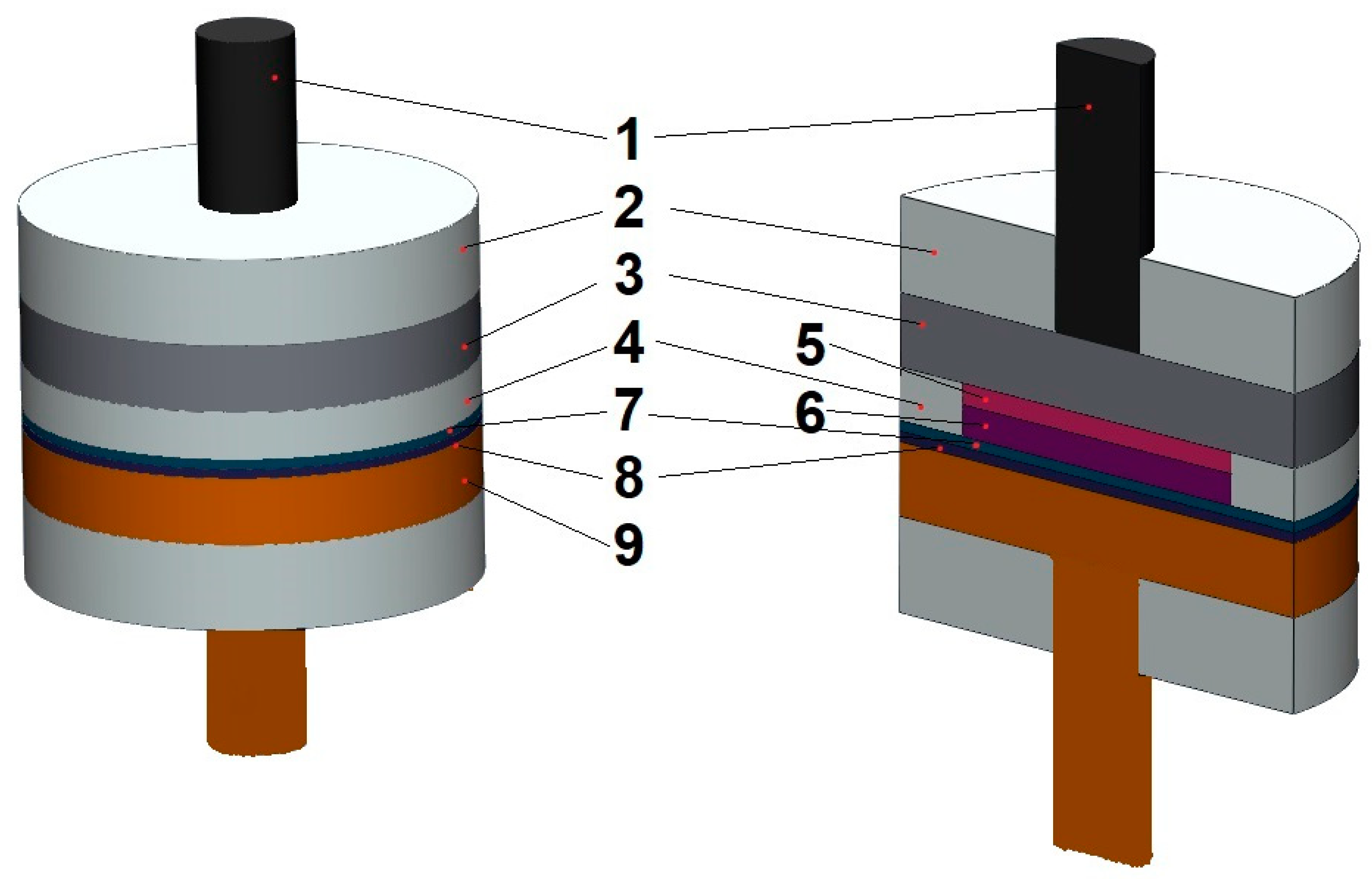
2. Materials and Method of Approach
2.1. Separator Selection and Pretreatment
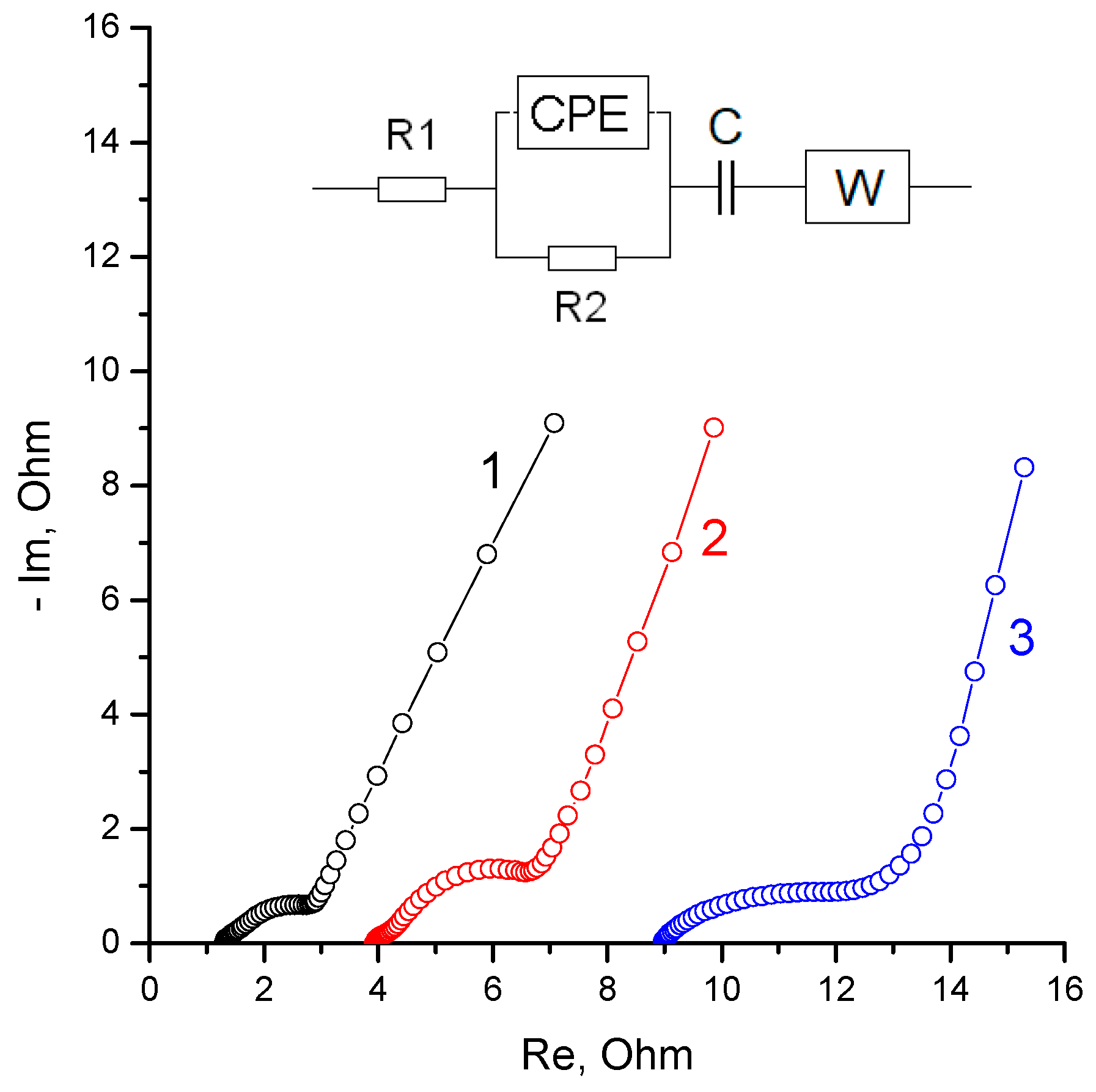
2.2. Electrochemical Measurements
2.3. Standard Contact Porosimetry
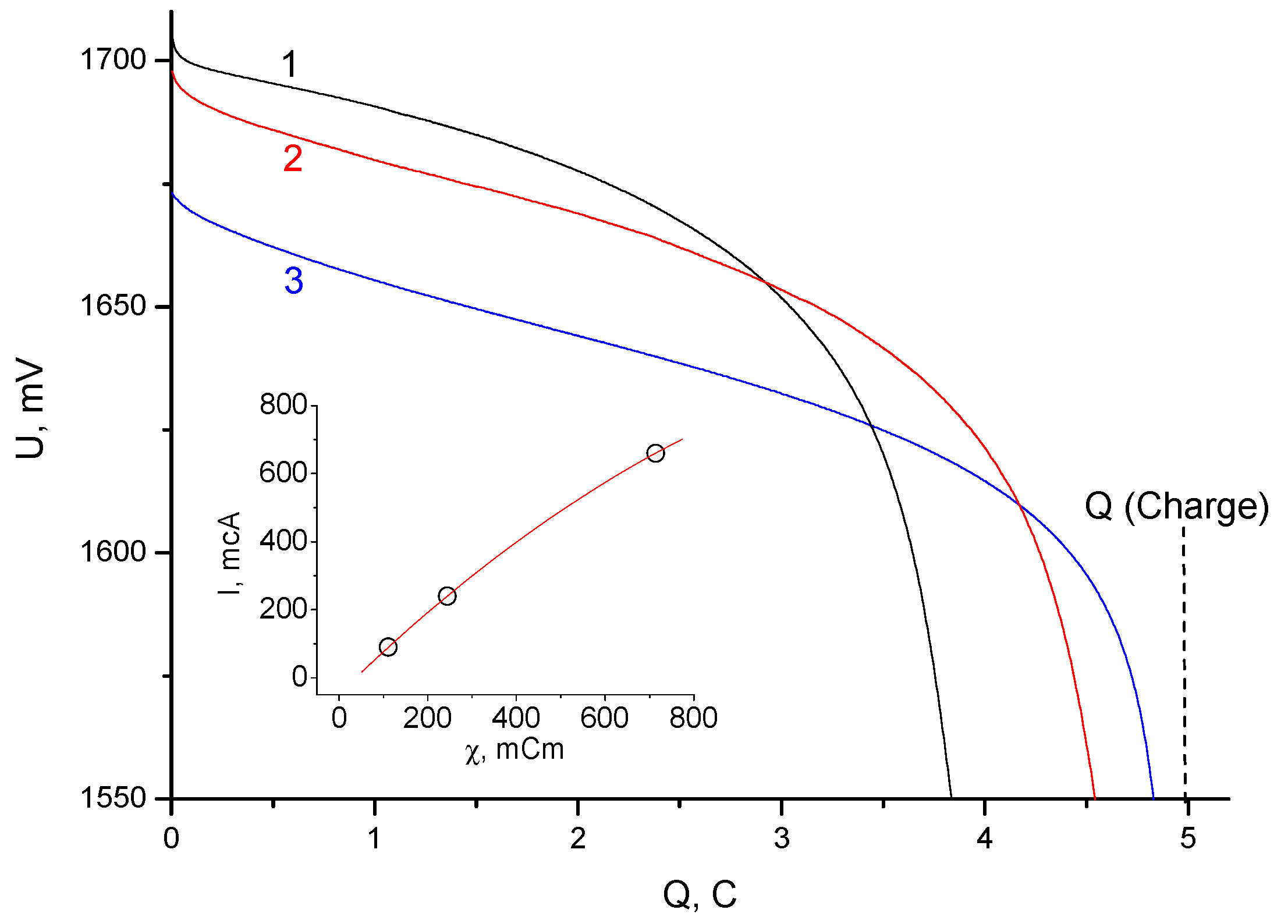
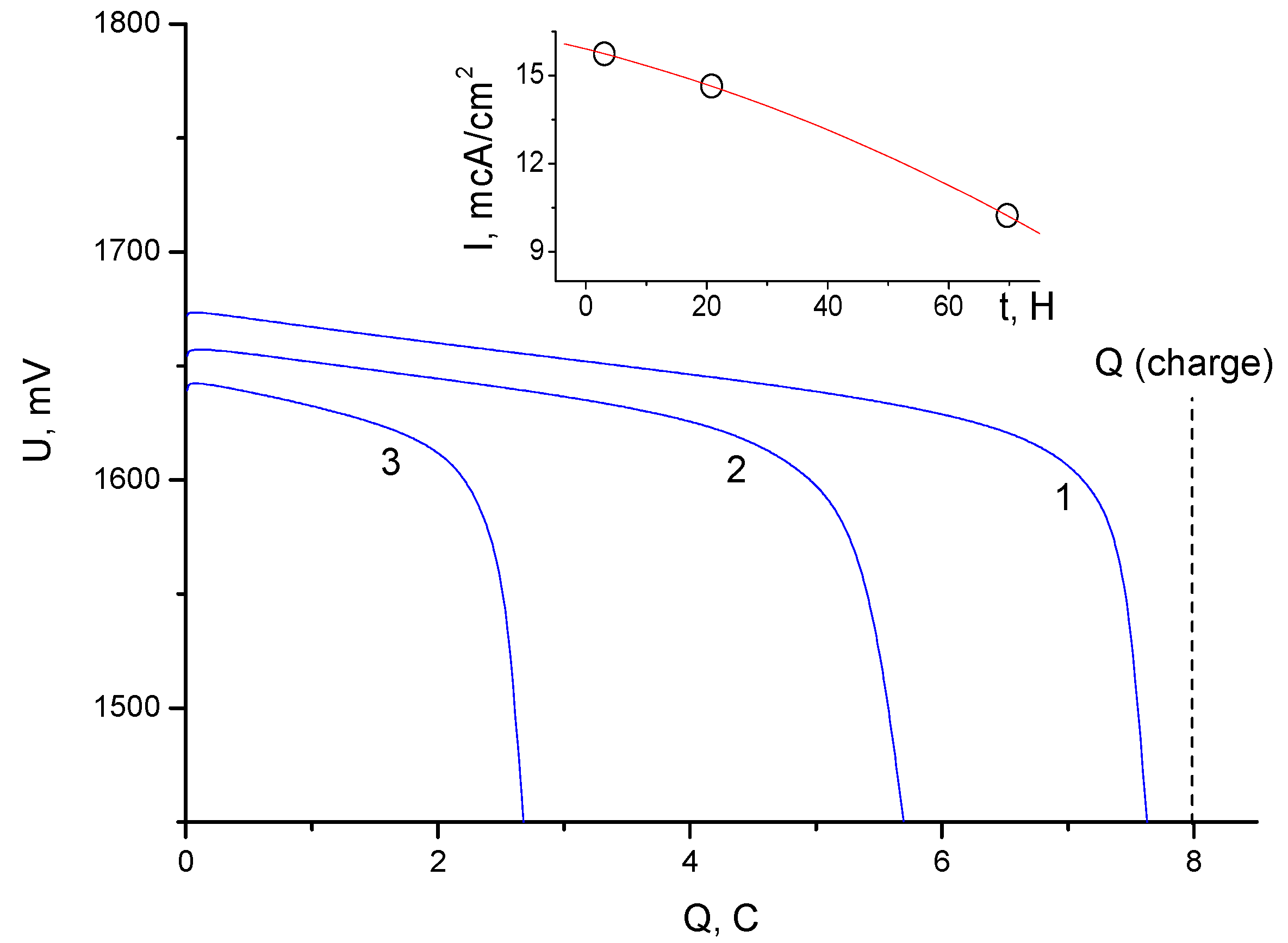
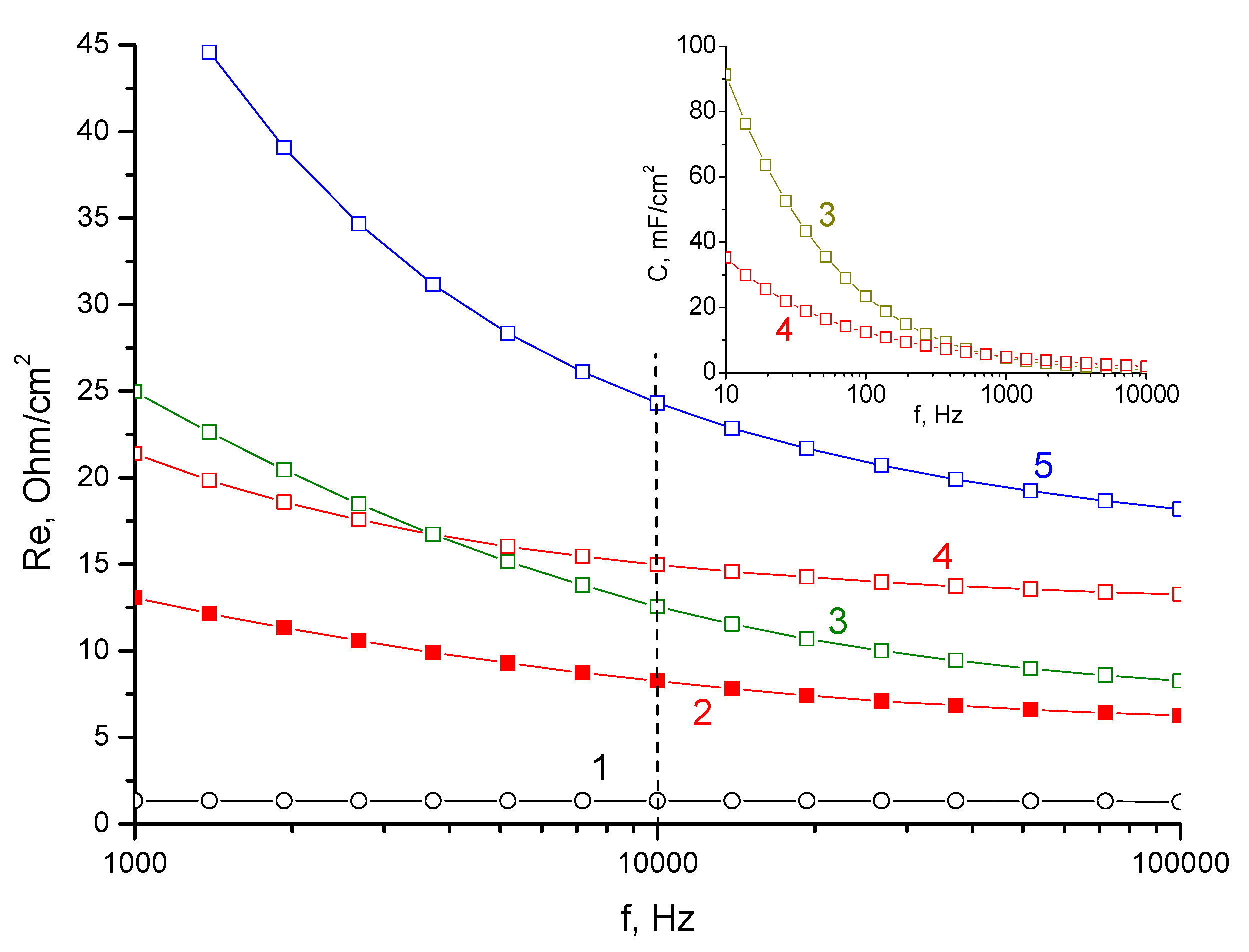
3. Results and Discussion
3.1. Standard Contact Porosimetry
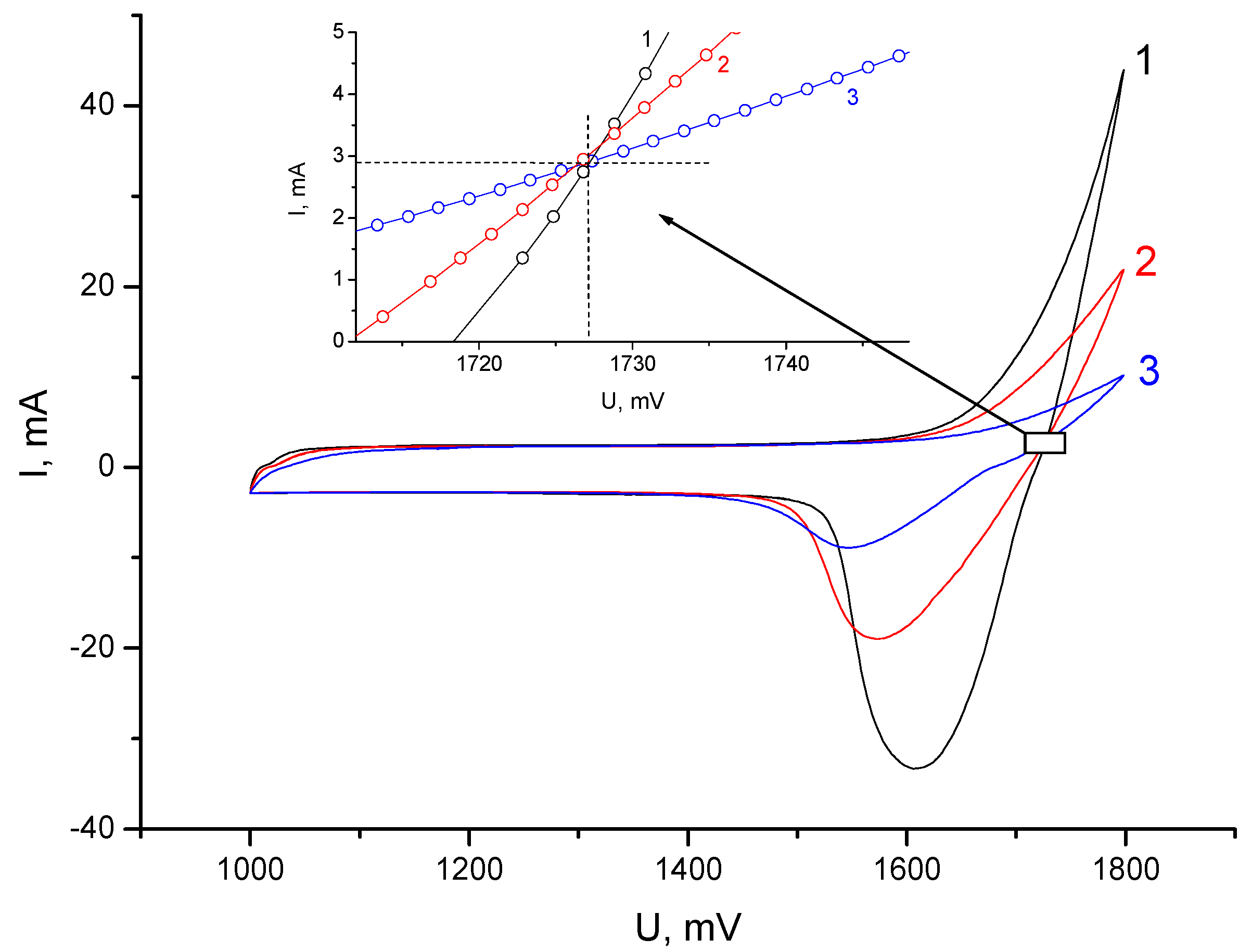
3.2. Electrochemical Measurements
4. Conclusions
- -
- It was shown that the cation exchange membrane made of a highly dispersed resin (KU-2-8, similar to S-100) with a binder of 5% polytetrafluoroethylene significantly slowed down the transfer of bromine to zinc (reduced the bromine leakage current) compared to a traditional mesoporous separator. Significantly lower bromine leakage currents were observed in the cells with a membrane (KU-F5-240), despite the higher porosity in the flooded state.
- -
- It was shown that the electrical conductivity of the water-flooded membrane (KU-F5-240) in the H form strongly depended on the impedance measurement frequency and exceeds the membrane in the Zn form only for the high-frequency region.
- -
- A method for modifying a porous cation exchange membrane (KU-F5-240) by impregnation in a dilute solution of TBA bromide was proposed and tested, due to which the leakage current for bromine was further reduced to values of 10–20 μA/cm2. Such leakage currents made it possible to achieve a high Coulomb efficiency (more than 90%) under the conditions of sufficiently long cycles. An analysis of the electrochemical behavior of the cells suggested that the modification of the porous cation exchange membrane led to the formation of a combined (matrix) cation–anion exchange membrane. The impregnation technique, in this case, made it possible to regulate the most important ion exchange characteristics of the membrane. Despite the fact that the mechanism of charge transfer on the combined membrane was not studied in this work, the asymmetric nature of the charge and discharge modes was noted.
- -
- With a relatively high internal resistance of the battery cell, the maximum discharge current significantly exceeded the charge current.
- -
- Based on the results of the work, a conclusion was made about the prospects of using modified ion exchange membranes in zinc–halide batteries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, B.A.; Holmes, E.L. Adsorptive powers of synthetic zeolites. J. Soc. Chem. Ind. London 1935, 54, 1–6. [Google Scholar]
- Connoly, D.J.; Gresham, W.F. Fluorocarbon Vinyl Esters Polymers. U.S Patent 3,282,875, 1 November 1966. [Google Scholar]
- Glüsen, A.; Stolten, D. Membranes for polymer electrolyte fuel cells. Chem. Ingeneur. Technik. 2003, 75, 1591–1597. [Google Scholar] [CrossRef]
- Sata, T. Ion Exchange Membranes: Preparation, Characterization, Modification and Application; Royal Society of Chemistry: London, UK, 2004; p. 314. [Google Scholar]
- Wood, J.; Gifford, J. Improvements in continuous electrodeionization for power plant applications. In Proceedings of the Industrial Water Conference, Orlando, FL, USA, 11–12 December 2002. [Google Scholar]
- Yaroslavtsev, A.B.; Nikonenko, V.V. Ion-exchange membrane materials: Properties, modification, and practical application. Nanotechnol. Russ. 2009, 4, 137–159. [Google Scholar] [CrossRef]
- Ryu, W.-H.; Gittleson, F.S.; Schwab, M.; Goh, T.; Taylor, A.D. A mesoporous catalytic membrane architecture for lithium-oxygen battery systems. Nano Lett. 2015, 15, 434–441. [Google Scholar] [CrossRef]
- Sherazi, T.A.; Ahmad, S.; Kashmiri, M.A.; Kim, D.S.; Guiver, M.D. Radiation induced grafting of styrene onto ultra-high molecular weight polyethylene powder and subsequent film fabrication for application as polymer electrolyte membrane. J. Membr. Sci. 2009, 333, 59–67. [Google Scholar] [CrossRef][Green Version]
- Yao, S.; He, Y.; Wang, Y.; Bi, M.; Liang, Y.; Majeed, A.; Yang, Z.; Shen, X. CoFe2O4 nanoparticles loaded N-doped carbon nanofibers networks as electrocatalyst for enhancing redox kinetics in Li-S batteries. Appl. Surf. Sci. 2021, 560, 1449908. [Google Scholar]
- Yao, S.; Bi, M.; Yu, H.; Zhang, C.; Zhang, X.; Liu, H.; Zhang, T.; Xiang, J.; Shen, X. Spinel manganese-cobalt oxide nanospheres anchored on nitrogen-containing carbon nanofibers as a highly efficient redox electrocatalyst in lithium/polysulfides batteries. Appl. Surf. Sci. 2022, 598, 153787. [Google Scholar] [CrossRef]
- ZBM2 Zinc-Bromine Flow Battery. Available online: https://www.redflow.com/products/redflow-zbm2/ (accessed on 25 May 2021).
- The Future of Storage Is Long. Available online: https://www.primuspower.com/en/ (accessed on 25 May 2021).
- Petrov, M.M.; Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Loktionov, P.A.; Pichugov, R.D.; Kartashova, N.V.; Glazkov, A.T.; Abunaeva, L.Z.; Andreev, V.N.; et al. Redox flow batteries: Role in modern electric power industry and comparative characteristics of the main types. Russ. Chem. Rev. 2021, 90, 677–702. [Google Scholar] [CrossRef]
- Fomichev, A.M. Bromium-Zinc Storage Battery with Landlocked Electrolyte. Patent RU 2400871C1, 10 December 2009. [Google Scholar]
- Kim, R.; Yuk, S.; Lee, J.H.; Choi, C.; Kim, S.; Heo, J. Scaling the water cluster size of Nafion membranes for a high performance Zn/Br redox flow battery. Membr. Sci. 2018, 564, 852–858. [Google Scholar] [CrossRef]
- Han, D.; Gikunoo, E.K.; Shanmugam, S. A zwitterionic composite membrane for a high performance zinc/bromine flowless battery. J. Mater. Chem. A 2022, 10, 18598–18601. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Huang, J.; Chi, X.; Yang, J. A high-energy efficiency static membrane-free zinc–bromine battery enabled by a high concentration hybrid electrolyte. Sustain. Energy Fuels 2022, 6, 1148–1155. [Google Scholar] [CrossRef]
- Bae, S.; Lee, J.; Kim, D.S. The effect of Cr3+-Functionalized additive in zinc-bromine flow battery. J. Power Sources 2019, 413, 167–173. [Google Scholar] [CrossRef]
- Wu, M.C.; Zhao, T.S.; Wei, L.; Jiang, H.R.; Zhang, R.H. Improved electrolyte for zinc-bromine flow batteries. J. Power Sources 2018, 384, 232–239. [Google Scholar] [CrossRef]
- Karpenko, L.V.; Demina, O.A.; Dvorkina, G.A. Comparative Study of Methods Used for the Determination of Electroconductivity of Ion-Exchange Membranes. Russ. J. Electrochem. 2001, 37, 287–293. [Google Scholar] [CrossRef]
- Stenina, I.A.; Sistat, P.; Rebrov, A.I.; Pourcelly, G.; Yarolavtsev, A.B. Ion mobility in Nafion-117 membranes. Desalination 2004, 170, 49–57. [Google Scholar] [CrossRef]
- Volfkovich, Y.; Mikhalin, A.; Rychagov, A.; Sosenkin, V.; Bograchev, D. Activated Carbons as Nanoporous Electron-Ion-Exchangers. Russ. J. Electrochem. 2020, 5, 869–882. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Bagotzky, V.S.; Sosenkin, V.E.; Blinov, I.A. The standard contact porosimetry. Colloid Surf. A 2001, 187, 349–365. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Filippov, A.N.; Bagotsky, V.S. Structural Properties of Porous Materials and Powders Used in Different Fields of Science and Technology; Springer: London, UK, 2014. [Google Scholar]
- Volfkovich, Y.M.; Bagotzky, V.S. The method of standard porosimetry. Investigation of the formation of porous structures. J. Power Sources 1994, 48, 339. [Google Scholar] [CrossRef]
- Rouquerol, J.; Baron, G.; Denoyel, R.; Giesche, H.; Groen, J.; Klobes, P.; Levitz, P.; Neimark, A.V.; Rigby, S.; Skudas, R.; et al. Liquid intrusion and alternative methods for the characterization of macroporous materials. Pure Appl. Chem. 2012, 84, 107–136. [Google Scholar] [CrossRef]
- Gierke, T.D.; Munn, G.E.; Wilson, F.C. The morphology in nafion perfluorinated membrane products, as determined by wide- and small-angle x-ray studies. J. Polym. Sci. Polym. Phys. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Gierke, T.D. Ion transport and clustering in nafion perfluorinated membranes. J. Membr. Sci. 1983, 13, 307–326. [Google Scholar] [CrossRef]
- Kuo, A.-T.; Okazaki, S.; Shinoda, W. Transferable coarse-grained model for perfluorosulfonic acid polymer membranes. J. Chem. Phys. 2017, 147, 094904. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Gnusin, N.P.; Berezina, N.P.; Parshikov, S.B. Modeling transport asymmetry in bilayered ion-exchange membranes interacting with surface-active organic substances. Russ. J. Electrochem. 2002, 38, 828–833. [Google Scholar] [CrossRef]

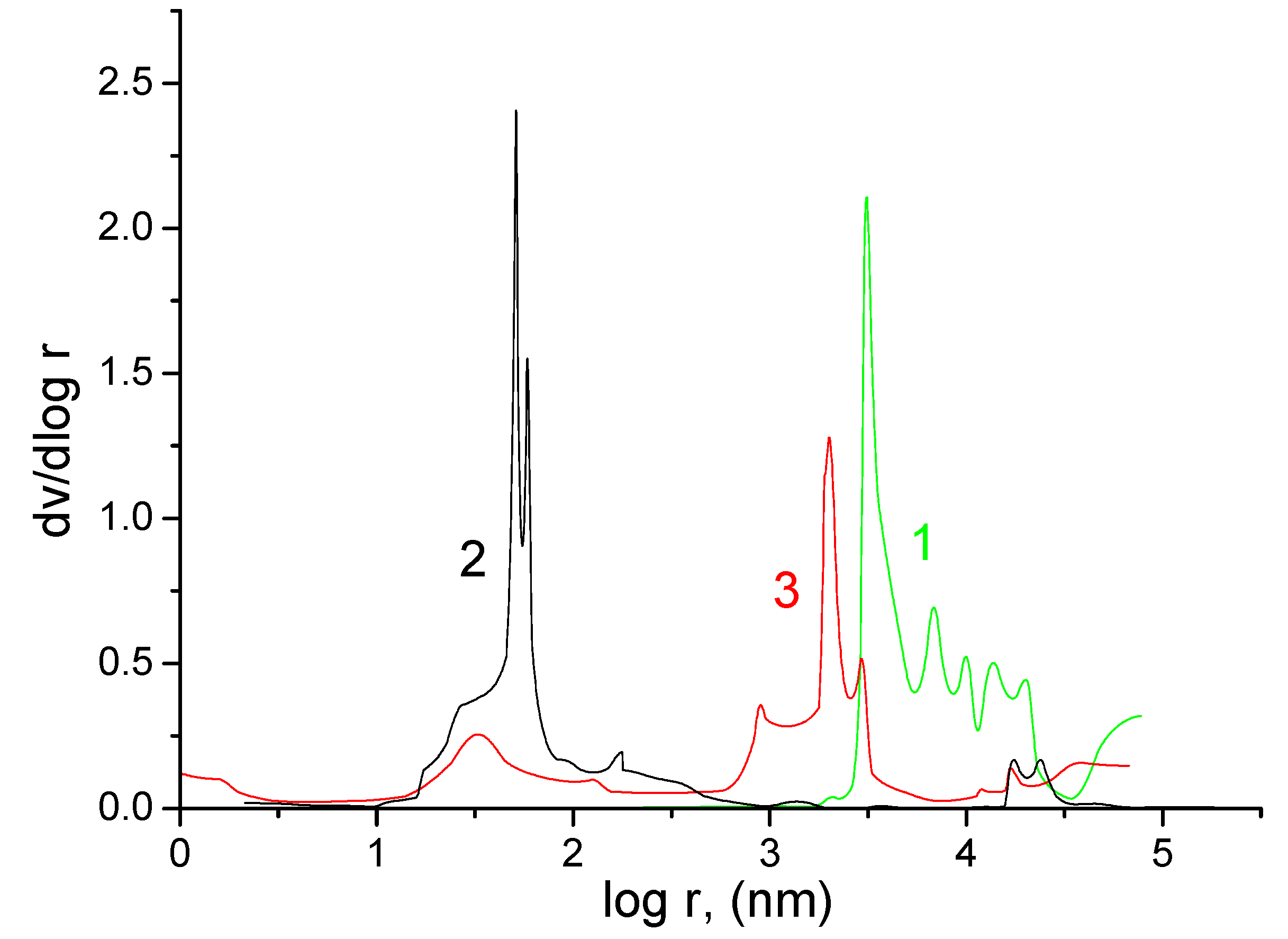

| Sample | Porosity, cm3/cm3 | Specific Surface Area of Mesopores, m2/g | Total Specific Surface Area, m2/g | Average Pore Radius, nm |
|---|---|---|---|---|
| FS 2226 | 0.655 | 0 | 0.8 | 13,550 |
| KU-F5-240 | 0.765 | 158 | 753 | 5031 |
| Grace | 0.515 | 40.2 | 40.5 | 2320 |
| No | Type | Ionic Conductivity, mS/cm |
|---|---|---|
| 1 | Grace separator, in electrolyte | 3.40 |
| 2 | FS 2226 separator, in electrolyte | 3.15 |
| 3 | KU-F5-240 membrane, in electrolyte | 3.55 |
| 4 | Modified TBA membrane, in electrolyte | 2.84 |
| 5 | KU-F5-240 membrane, in water (N-form) | 1.82 |
| 6 | Modified TBA membrane, in water (N-form) | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rychagov, A.Y.; Volfkovich, Y.M.; Sosenkin, V.E.; Seliverstov, A.F.; Izmailova, M.Y. Combined Separator Based on a Porous Ion-Exchange Membrane for Zinc–Halide Batteries. Membranes 2023, 13, 67. https://doi.org/10.3390/membranes13010067
Rychagov AY, Volfkovich YM, Sosenkin VE, Seliverstov AF, Izmailova MY. Combined Separator Based on a Porous Ion-Exchange Membrane for Zinc–Halide Batteries. Membranes. 2023; 13(1):67. https://doi.org/10.3390/membranes13010067
Chicago/Turabian StyleRychagov, Alexey Y., Yury M. Volfkovich, Valentin E. Sosenkin, Alexsandr F. Seliverstov, and Marianna Y. Izmailova. 2023. "Combined Separator Based on a Porous Ion-Exchange Membrane for Zinc–Halide Batteries" Membranes 13, no. 1: 67. https://doi.org/10.3390/membranes13010067
APA StyleRychagov, A. Y., Volfkovich, Y. M., Sosenkin, V. E., Seliverstov, A. F., & Izmailova, M. Y. (2023). Combined Separator Based on a Porous Ion-Exchange Membrane for Zinc–Halide Batteries. Membranes, 13(1), 67. https://doi.org/10.3390/membranes13010067





