The Role of Inorganic Phosphate Transporters in Highly Proliferative Cells: From Protozoan Parasites to Cancer Cells
Abstract
1. Introduction
2. Na+-Dependent Pi Transport
2.1. Cancer Cells
2.2. Apicomplexa
2.3. Trypanosomatids
2.3.1. Genus Trypanosoma
2.3.2. Genus Leishmania
2.3.3. Genus Phytomonas
3. H+-Dependent Pi Transport
3.1. Cancer Cells
3.2. Apicomplexa
3.3. Trypanosomatids
3.4. Other Unicellular Protozoa
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auesukaree, C.; Homma, T.; Tochio, H.; Shirakawa, M.; Kaneko, Y.; Harashima, S. Intracellular Phosphate Serves as a Signal for the Regulation of the PHO Pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 17289–17294. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Taketani, Y.; Morita, K.; Tatsumi, S.; Katai, K.; Nii, T.; Yamamoto, H.; Miyamoto, K.I. Molecular mechanisms of mammalian inorganic phosphate homeostasis. Adv. Enzym. Regul. 2000, 40, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Dick, C.F.; Dos-Santos, A.L.; Meyer-Fernandes, J.R. Inorganic phosphate uptake in unicellular eukaryotes. Biochim. Biophys. Acta 2014, 1840, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med. 2013, 34, 386–395. [Google Scholar] [CrossRef]
- Dick, C.F.; Dos-Santos, A.L.; Majerowicz, D.; Gondim, K.C.; Caruso-Neves, C.; Silva, I.V.; Vieyra, A.; Meyer-Fernandes, J.R. Na+-dependent and Na+-independent mechanisms for inorganic phosphate uptake in Trypanosoma rangeli. Biochim. Biophys. Acta 2012, 1820, 1001–1008. [Google Scholar] [CrossRef]
- Dick, C.F.; Dos-Santos, A.L.; Majerowicz, D.; Paes, L.S.; Giarola, N.L.; Gondim, K.C.; Vieyra, A.; Meyer-Fernandes, J.R. Inorganic phosphate uptake in Trypanosoma cruzi is coupled to K+ cycling and to active Na+ extrusion. Biochim. Biophys. Acta 2013, 1830, 4265–4273. [Google Scholar] [CrossRef]
- Dick, C.F.; Dos-Santos, A.L.; Fonseca-de-Souza, A.L.; Rocha-Ferreira, J.; Meyer-Fernandes, J.R. Trypanosoma rangeli: Differential expression of ecto-phosphatase activities in response to inorganic phosphate starvation. Exp. Parasitol. 2010, 124, 386–393. [Google Scholar] [CrossRef]
- Elser, J.J.; Kyle, M.M.; Smith, M.S.; Nagy, J.D. Biological stoichiometry in human cancer. PLoS ONE 2007, 10, e1028. [Google Scholar] [CrossRef]
- Papaloucas, C.D.; Papaloucas, M.D.; Kouloulias, V.; Neanidis, K.; Pistevou-Gompaki, K.; Kouvaris, J.; Zygogianni, A.; Mystakidou, K.; Papaloucas, A.C. Measurement of blood phosphorus: A quick and inexpensive method for detection of the existence of cancer in the body. Too good to be true, or forgotten knowledge of the past? Med. Hypotheses 2014, 82, 24–25. [Google Scholar] [CrossRef]
- Jin, H.; Xu, C.X.; Lim, H.T.; Park, S.J.; Shin, J.Y.; Chung, Y.S.; Park, S.C.; Chang, S.H.; Youn, H.J.; Lee, K.H.; et al. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68. [Google Scholar] [CrossRef]
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R., Jr. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.; Caramujo, M.J. Tumour metastasis as an adaptation of tumour cells to fulfil their phosphorus requirements. Med. Hypotheses 2012, 78, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Michigami, T.; Kawai, M.; Yamazaki, M.; Ozono, K. Phosphate as a Signaling Molecule and Its Sensing Mechanism. Physiol. Rev. 2018, 98, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Saliba, K.J.; Martin, R.E.; Bröer, A.; Henry, R.I.; McCarthy, C.S.; Downie, M.J.; Allen, R.J.; Mullin, K.A.; McFadden, G.I.; Bröer, S.; et al. Sodium-dependent uptake of inorganic phosphate by the intracellular malaria parasite. Nature 2006, 443, 582–585. [Google Scholar] [CrossRef]
- Beck, L.; Leroy, C.; Salaün, C.; Margall-Ducos, G.; Desdouets, C.; Friedlander, G. Identification of a novel function of PiT1 critical for cell proliferation and independent of its phosphate transport activity. J. Biol. Chem. 2009, 284, 31363–31374. [Google Scholar] [CrossRef]
- Russo-Abrahão, T.; Alves-Bezerra, M.; Majerowicz, D.; Freitas-Mesquita, A.L.; Dick, C.F.; Gondim, K.C.; Meyer-Fernandes, J.R. Transport of inorganic phosphate in Leishmania infantum and compensatory regulation at low inorganic phosphate concentration. Biochim. Biophys. Acta 2013, 1830, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Russo-Abrahão, T.; Koeller, C.M.; Steinmann, M.E.; Silva-Rito, S.; Marins-Lucena, T.; Alves-Bezerra, M.; Lima-Giarola, N.L.; de-Paula, I.F.; Gonzalez-Salgado, A.; Sigel, E.; et al. H+-dependent inorganic phosphate uptake in Trypanosoma brucei is influenced by myo-inositol transporter. J. Bioenerg. Biomembr. 2017, 49, 183–194. [Google Scholar] [CrossRef]
- Vieira-Bernardo, R.; Gomes-Vieira, A.L.; Carvalho-Kelly, L.F.; Russo-Abrahão, T.; Meyer-Fernandes, J.R. The biochemical characterization of two phosphate transport systems in Phytomonas serpens. Exp. Parasitol. 2017, 173, 1–8. [Google Scholar] [CrossRef]
- Russo-Abrahão, T.; Lacerda-Abreu, M.A.; Gomes, T.; Cosentino-Gomes, D.; Carvalho-de-Araújo, A.D.; Rodrigues, M.F.; Oliveira, A.C.L.; Rumjanek, F.D.; Monteiro, R.Q.; Meyer-Fernandes, J.R. Characterization of inorganic phosphate transport in the triple-negative breast cancer cell line, MDA-MB-231. PLoS ONE 2018, 13, e0191270. [Google Scholar] [CrossRef]
- Sindhu, K.J.; Kureel, A.K.; Saini, S.; Kumari, S.; Verma, P.; Rai, A.K. Characterization of phosphate transporter(s) and understanding their role in Leishmania donovani parasite. Acta Parasitol. 2018, 63, 75–88. [Google Scholar] [CrossRef]
- Carvalho-Kelly, L.F.; Gomes-Vieira, A.L.; Paes-Vieira, L.; da Silva, A.D.Z.; Meyer-Fernandes, J.R. Leishmania amazonensis inorganic phosphate transporter system is increased in the proliferative forms. Mol. Biochem. Parasitol. 2019, 233, 111212. [Google Scholar] [CrossRef] [PubMed]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Cosentino-Gomes, D.; Nascimento, M.T.C.; Carvalho-Kelly, L.F.; Gomes, T.; Rodrigues, M.F.; König, S.; Rumjanek, F.D.; Monteiro, R.Q.; et al. H+-dependent inorganic phosphate transporter in breast cancer cells: Possible functions in the tumor microenvironment. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Asady, B.; Dick, C.F.; Ehrenman, K.; Sahu, T.; Romano, J.D.; Coppens, I. A single Na+-Pi cotransporter in Toxoplasma plays key roles in phosphate import and control of parasite osmoregulation. PLoS Pathog. 2020, 16, e1009067. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Kelly, L.F.; Pralon, C.F.; Rocco-Machado, N.; Nascimento, M.T.; Carvalho-de-Araújo, A.D.; Meyer-Fernandes, J.R. Acanthamoeba castellanii phosphate transporter (AcPHS) is important to maintain inorganic phosphate influx and is related to trophozoite metabolic processes. J. Bioenerg. Biomembr. 2020, 52, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Rocco-Machado, N.; Cosentino-Gomes, D.; Dick, C.F.; Carvalho-Kelly, L.F.; Cunha Nascimento, M.T.; Rocha-Vieira, T.C.; Meyer-Fernandes, J.R. Hydrogen Peroxide Generation as an Underlying Response to High Extracellular Inorganic Phosphate (Pi) in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 10096. [Google Scholar] [CrossRef]
- Carvalho-Kelly, L.F.; Dick, C.F.; Rocco-Machado, N.; Gomes-Vieira, A.L.; Paes-Vieira, L.; Meyer-Fernandes, J.R. Anaerobic ATP synthesis pathways and inorganic phosphate transport and their possible roles in encystment in Acanthamoeba castellanii. Cell Biol. Int. 2022, 46, 1288–1298. [Google Scholar] [CrossRef]
- Carvalho-de-Araújo, A.D.; Carvalho-Kelly, L.F.; Dick, C.F.; Meyer-Fernandes, J.R. Inorganic phosphate transporter in Giardia duodenalis and its possible role in ATP synthesis. Mol. Biochem. Parasitol. 2022, 251, 111504. [Google Scholar] [CrossRef]
- Cui, J.; Yang, X.; Yang, J.; Jia, R.; Feng, Y.; Shen, B. A Coccidia-Specific Phosphate Transporter Is Essential for the Growth of Toxoplasma gondii Parasites. Microbiol. Spectr. 2022, 12, e0218622. [Google Scholar] [CrossRef]
- Werner, A.; Kinne, R.K. Evolution of the Na-P(i) cotransport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R301–R312. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Monteiro, R.Q.; Rumjanek, F.D. Meyer-Fernandes JR. Inorganic phosphate transporters in cancer: Functions, molecular mechanisms and possible clinical applications. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 291–298. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Meyer-Fernandes, J.R. The Roles of Sodium-Independent Inorganic Phosphate Transporters in Inorganic Phosphate Homeostasis and in Cancer and Other Diseases. Int. J. Mol. Sci. 2020, 21, 9298. [Google Scholar] [CrossRef] [PubMed]
- Lacerda-Abreu, M.A.; Meyer-Fernandes, J.R. Extracellular Inorganic Phosphate-Induced Release of Reactive Oxygen Species: Roles in Physiological Processes and Disease Development. Int. J. Mol. Sci. 2021, 22, 7768. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Meyer-Fernandes, J.R. Inorganic phosphate (Pi) in the breast cancer microenvironment: Production, transport and signal transduction as potential targets for anticancer strategies. Curr. Cancer Drug Targets 2022. [Google Scholar] [CrossRef]
- Samyn, D.R.; Van der Veken, J.; Van Zeebroeck, G.; Persson, B.L.; Karlsson, B.C. Key Residues and Phosphate Release Routes in the Saccharomyces cerevisiae Pho84 Transceptor: The role of tyr179 in functional regulation. J. Biol. Chem. 2016, 291, 26388–26398. [Google Scholar] [CrossRef]
- Salcedo-Sora, J.E.; Caamano-Gutierrez, E.; Ward, S.A.; Biagini, G.A. The proliferating cell hypothesis: A metabolic framework for Plasmodium growth and development. Trends Parasitol. 2014, 30, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.A.; Rathmell, J.C. Metabolic pathways in T cell fate and function. Trends Immunol. 2012, 33, 168–173. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Oca, P.; Zaka, R.; Dion, A.S.; Freeman, T.A.; Williams, C.J. Phosphate and calcium are required for TGFbeta-mediated stimulation of ANK expression and function during chondrogenesis. J. Cell. Physiol. 2010, 224, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Binet, M.R.; Lee, L.J.; Ma, R.; Graham, A.I.; McLeod, C.W.; Poole, R.K. Expression of the PitA phosphate/metal transporter of Escherichia coli is responsive to zinc and inorganic phosphate levels. FEMS Microbiol. Lett. 2008, 289, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Biber, J.; Hernando, N.; Forster, I. Phosphate transporters and their function. Annu. Rev. Physiol. 2013, 75, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Phosphate transport kinetics and structure-function relationships of SLC34 and SLC20 proteins. Curr. Top. Membr. 2012, 70, 313–356. [Google Scholar] [CrossRef] [PubMed]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Proximal tubular handling of phosphate: A molecular perspective. Kidney Int. 2006, 70, 1548–1559. [Google Scholar] [CrossRef]
- Miller, D.G.; Edwards, R.H.; Miller, A.D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc. Natl. Acad. Sci. USA 1994, 91, 78–82. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, B.; Johann, S.V.; Klinger, H.P.; Blair, D.G.; Rubinson, H.; Dunn, K.J.; Sass, P.; Vitek, S.M.; Robins, T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990, 1, 119–127. [Google Scholar]
- Kavanaugh, M.P.; Kabat, D. Identification and characterization of a widely expressed phosphate transporter/retrovirus receptor family. Kidney Int. 1996, 49, 959–963. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Bogaert, Y.E.; Levi, M.; Sorribas, V. Characterization of phosphate transport in rat vascular smooth muscle cells: Implications for vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1030–1036. [Google Scholar] [CrossRef]
- Virkki, L.V.; Biber, J.; Murer, H.; Forster, I.C. Phosphate transporters: A tale of two solute carrier families. Am. J. Physiol. Ren. Physiol. 2007, 293, F643–F654. [Google Scholar] [CrossRef]
- Forster, I.; Hernando, N.; Sorribas, V.; Werner, A. Phosphate transporters in renal, gastrointestinal, and other tissues. Adv. Chronic Kidney Dis. 2011, 18, 63–76. [Google Scholar] [CrossRef]
- Persson, B.L.; Lagerstedt, J.O.; Pratt, J.R.; Pattison-Granberg, J.; Lundh, K.; Shokrollahzadeh, S.; Lundh, F. Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr. Genet. 2003, 43, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Bun-Ya, M.; Nishimura, M.; Harashima, S.; Oshima, Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol. Cell. Biol. 1991, 11, 3229–3238. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.; Persson, B.L. Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol. Gen. Genet. 1998, 258, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Zuckerkandl, E.; Pauling, L. Evolutionary divergence and convergence in proteins. In Evolving Genes and Proteins; Bryson, V., Vogel, H.J., Eds.; Academic Press: New York, NY, USA, 1965; pp. 97–166. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Mann, B.J.; Bowman, B.J.; Grotelueschen, J.; Metzenberg, R.L. Nucleotide sequence of pho-4+, encoding a phosphate-repressible phosphate permease of Neurospora crassa. Gene 1989, 83, 281–289. [Google Scholar] [CrossRef]
- Petit, V.; Massonnet, G.; Maciorowski, Z.; Touhami, J.; Thuleau, A.; Némati, F.; Laval, J.; Château-Joubert, S.; Servely, J.L.; Vallerand, D.; et al. Optimization of tumor xenograft dissociation for the profiling of cell surface markers and nutrient transporters. Lab. Investig. 2013, 93, 611–621. [Google Scholar] [CrossRef]
- Jiang, L.; Dai, Y.; Liu, X.; Wang, C.; Wang, A.; Chen, Z.; Heidbreder, C.E.; Kolokythas, A.; Zhou, X. Identification and experimental validation of G protein alpha inhibiting activity polypeptide 2 (GNAI2) as a microRNA-138 target in tongue squamous cell carcinoma. Hum. Genet. 2011, 129, 189–197. [Google Scholar] [CrossRef]
- Puech, C.; Prevot, N.; Perek, N. Gefitinib inhibits sodium phosphate co-transporter III isoform 1 in a model of human malignant glioma. Anticancer Res. 2014, 34, 6527–6535. [Google Scholar]
- Andersen, J.N.; Sathyanarayanan, S.; Di Bacco, A.; Chi, A.; Zhang, T.; Chen, A.H.; Dolinski, B.; Kraus, M.; Roberts, B.; Arthur, W.; et al. Pathway-based identification of biomarkers for targeted therapeutics: Personalized oncology with PI3K pathway inhibitors. Sci. Transl. Med. 2010, 2, 43ra55. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Bakiri, L.; Mairhorfer, A.; Schweifer, N.; Haslinger, C.; Kenner, L.; Komnenovic, V.; Scheuch, H.; Beug, H.; Wagner, E.F. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat. Genet. 2007, 39, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, S.; Pirmettis, I.; Chatzipanagiotou, T.; Ptohis, N.; Papantoniou, V. Pentavalent technetium-99m dimercaptosuccinic acid [99m Tc-(V)DMSA] brain scintitomography--a plausible non-invasive depicter of glioblastoma proliferation and therapy response. J. Neurooncol. 2007, 85, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Perek, N.; Le Jeune, N.; Frere, D.; Dubois, F. Evidence that 99mTc-(V)-DMSA uptake is mediated by NaPi cotransporter type III in tumour cell lines. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 77–84. [Google Scholar] [CrossRef]
- Denoyer, D.; Perek, N.; Le Jeune, N.; Cornillon, J.; Dubois, F. Correlation between 99mTc-(V)-DMSA uptake and constitutive level of phosphorylated focal adhesion kinase in an in vitro model of cancer cell lines. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 820–827. [Google Scholar] [CrossRef]
- Rangel, L.B.; Sherman-Baust, C.A.; Wernyj, R.P.; Schwartz, D.R.; Cho, K.R.; Morin, P.J. Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene 2003, 22, 7225–7232. [Google Scholar] [CrossRef]
- Jarzab, B.; Wiench, M.; Fujarewicz, K.; Simek, K.; Jarzab, M.; Oczko-Wojciechowska, M.; Wloch, J.; Czarniecka, A.; Chmielik, E.; Lange, D.; et al. Gene expression profile of papillary thyroid cancer: Sources of variability and diagnostic implications. Cancer Res. 2005, 65, 1587–1597. [Google Scholar] [CrossRef]
- Chen, D.R.; Chien, S.Y.; Kuo, S.J.; Teng, Y.H.; Tsai, H.T.; Kuo, J.H.; Chung, J.G. SLC34A2 as a novel marker for diagnosis and targeted therapy of breast cancer. Anticancer Res. 2010, 30, 4135–4140. [Google Scholar]
- Jiang, Z.; Hao, Y.; Ding, X.; Zhang, Z.; Liu, P.; Wei, X.; Xi, J. The effects and mechanisms of SLC34A2 on tumorigenicity in human non-small cell lung cancer stem cells. Tumour Biol. 2016, 37, 10383–10392. [Google Scholar] [CrossRef]
- Soares, I.C.; Simões, K.; de Souza, J.E.; Okamoto, O.K.; Wakamatsu, A.; Tuma, M.; Ritter, G.; Alves, V.A. In silico analysis and immunohistochemical characterization of NaPi2b protein expression in ovarian carcinoma with monoclonal antibody Mx35. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 165–172. [Google Scholar] [CrossRef]
- Leslie, B.R.; Gerwin, L.E.; Taylor, S.I. Sodium-Glucose Cotransporter-2 Inhibitors: Lack of a Complete History Delays Diagnosis. Ann. Intern. Med. 2019, 171, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.G.; Matuschewski, K.; Zhang, M.; Rug, M. Plasmodium falciparum. Trends Parasitol. 2019, 35, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K. Ion Regulation in the Malaria Parasite. Annu. Rev. Microbiol. 2015, 69, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Staines, H.M.; Ellory, J.C.; Kirk, K. Perturbation of the pump-leak balance for Na+ and K+ in malaria-infected erythrocytes. Am. J. Physiol. Cell Physiol. 2001, 280, C1576–C1587. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, M.P.; Miller, D.G.; Zhang, W.; Law, W.; Kozak, S.L.; Kabat, D.; Miller, A.D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. USA 1994, 91, 7071–7075. [Google Scholar] [CrossRef]
- Bai, L.; Collins, J.F.; Ghishan, F.K. Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am. J. Physiol. Cell Physiol. 2000, 279, C1135–C1143. [Google Scholar] [CrossRef]
- Luft, B.J.; Remington, J.S. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 1992, 15, 211–222. [Google Scholar] [CrossRef]
- Schwab, J.C.; Beckers, C.J.; Joiner, K.A. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. USA 1994, 91, 509–513. [Google Scholar] [CrossRef]
- Rodrigues, C.O.; Ruiz, F.A.; Rohloff, P.; Scott, D.A.; Moreno, S.N. Characterization of isolated acidocalcisomes from Toxoplasma gondii tachyzoites reveals a novel pool of hydrolyzable polyphosphate. J. Biol. Chem. 2002, 277, 48650–48656. [Google Scholar] [CrossRef]
- Lahti, R. Microbial inorganic pyrophosphatases. Microbiol. Rev. 1983, 47, 169–178. [Google Scholar] [CrossRef]
- Moreno, B.; Bailey, B.N.; Luo, S.; Martin, M.B.; Kuhlenschmidt, M.; Moreno, S.N.; Docampo, R.; Oldfield, E. (31)P NMR of apicomplexans and the effects of risedronate on Cryptosporidium parvum growth. Biochem. Biophys. Res. Commun. 2001, 284, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.A.; Gigley, J.P.; Bzik, D.J. Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int. J. Parasitol. 2004, 34, 323–331. [Google Scholar] [CrossRef]
- Figueiredo, M.B.; Genta, F.A.; Garcia, E.S.; Azambuja, P. Lipid mediators and vector infection: Trypanosoma rangeli inhibits Rhodnius prolixus hemocyte phagocytosis by modulation of phospholipase A2 and PAF-acetylhydrolase activities. J. Insect Physiol. 2008, 54, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Watanabe Costa, R.; Batista, M.F.; Meneghelli, I.; Vidal, R.O.; Nájera, C.A.; Mendes, A.C.; Andrade-Lima, I.A.; da Silveira, J.F.; Lopes, L.R.; Ferreira, L.R.P.; et al. Comparative Analysis of the Secretome and Interactome of Trypanosoma cruzi and Trypanosoma rangeli Reveals Species Specific Immune Response Modulating Proteins. Front. Immunol. 2020, 11, 1774. [Google Scholar] [CrossRef]
- Fonseca-de-Souza, A.L.; Dick, C.F.; dos Santos, A.L.; Fonseca, F.V.; Meyer-Fernandes, J.R. Trypanosoma rangeli: A possible role for ecto-phosphatase activity on cell proliferation. Exp. Parasitol. 2009, 122, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ulrich, P.N.; Storey, M.; Johnson, D.; Tischer, J.; Tovar, J.A.; Moreno, S.N.; Orlando, R.; Docampo, R. Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells. PLoS Pathog. 2014, 10, e1004555. [Google Scholar] [CrossRef]
- Herwaldt, B.L. Leishmaniasis. Lancet 1999, 354, 1191–1199. [Google Scholar] [CrossRef]
- Rodrigues, V.; Cordeiro-da-Silva, A.; Laforge, M.; Silvestre, R.; Estaquier, J. Regulation of immunity during visceral Leishmania infection. Parasit. Vectors 2016, 9, 118. [Google Scholar] [CrossRef]
- Meyer-Fernandes, J.R.; Saad-Nehme, J.; Peres-Sampaio, C.E.; Belmont-Firpo, R.; Bisaggio, D.F.; Do Couto, L.C.; Fonseca-de-Souza, A.L.; Lopes, A.H.; Souto-Padrón, T. A Mg-dependent ecto-ATPase is increased in the infective stages of Trypanosoma cruzi. Parasitol. Res. 2004, 93, 41–50. [Google Scholar] [CrossRef]
- De Almeida-Amaral, E.E.; Belmont-Firpo, R.; Vannier-Santos, M.A.; Meyer-Fernandes, J.R. Leishmania amazonensis: Characterization of an ecto-phosphatase activity. Exp. Parasitol. 2006, 114, 334–340. [Google Scholar] [CrossRef]
- Peres-Sampaio, C.E.; de Almeida-Amaral, E.E.; Giarola, N.L.; Meyer-Fernandes, J.R. Leishmania amazonensis: Effects of heat shock on ecto-ATPase activity. Exp. Parasitol. 2008, 119, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Giarola, N.L.; Silveira, T.S.; Inacio, J.D.; Vieira, L.P.; Almeida-Amaral, E.E.; Meyer-Fernandes, J.R. Leishmania amazonensis: Increase in ectoATPase activity and parasite burden of vinblastine-resistant protozoa. Exp. Parasitol. 2014, 146, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Verma, P.; Kureel, A.K.; Saini, S.; Rai, A.K. Pi inhibits intracellular accumulation of methylglyoxal in promastigote form of L. donovani. Mol. Biochem. Parasitol. 2016, 207, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Coombs, G.H.; Craft, J.A.; Hart, D.T. A comparative study of Leishmania mexicana amastigotes and promastigotes. Enzyme activities and subcellular locations. Mol. Biochem. Parasitol. 1982, 5, 199–211. [Google Scholar] [CrossRef]
- Verner, Z.; Cermáková, P.; Skodová, I.; Kováčová, B.; Lukeš, J.; Horváth, A. Comparative analysis of respiratory chain and oxidative phosphorylation in Leishmania tarentolae, Crithidia fasciculata, Phytomonas serpens and procyclic stage of Trypanosoma brucei. Mol. Biochem. Parasitol. 2014, 193, 55–65. [Google Scholar] [CrossRef]
- Pedersen, B.P.; Kumar, H.; Waight, A.B.; Risenmay, A.J.; Roe-Zurz, Z.; Chau, B.H.; Schlessinger, A.; Bonomi, M.; Harries, W.; Sali, A.; et al. Crystal structure of a eukaryotic phosphate transporter. Nature 2013, 496, 533–536. [Google Scholar] [CrossRef]
- Ito, M.; Matsuka, N.; Izuka, M.; Haito, S.; Sakai, Y.; Nakamura, R.; Segawa, R.; Kuwahata, M.; Yamamoto, H.; Pike, W.J.; et al. Characterization of inorganic phosphate transport in osteoclast-like cells. Am. J. Physiol. Cell Physiol. 2004, 288, 921–993. [Google Scholar] [CrossRef][Green Version]
- Wykoff, D.D.; O’Shea, E.K. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 2001, 159, 1491–1499. [Google Scholar] [CrossRef]
- Mouillon, J.M.; Persson, B.L. New aspects on phosphate sensing and signalling in Saccharomyces cerevisiae. FEMS Yeast Res. 2006, 6, 171–176. [Google Scholar] [CrossRef]
- Versaw, W.K.; Metzemberg, R.L. Repressible cation-phosphate symporters in Neurospora crassa. Proc. Natl. Acad. Sci. USA 1995, 92, 3884–3887. [Google Scholar] [CrossRef]
- Wykoff, D.D.; Rizvi, A.H.; Raser, J.M.; Margolin, B.; O’Shea, E.K. Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol. Cell 2007, 27, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Versaw, W.K. A phosphate-repressible, high-affinity phosphate permease is encoded by the pho-5+ gene of Neurospora crassa. Gene 1995, 153, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.W.; Levinson, C. Phosphate concentration and transport in Ehrlich ascites tumor cells: Effect of sodium. J. Cell. Physiol. 1982, 110, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Antoine, J.C.; Prina, E.; Jouanne, C.; Bongrand, P. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect. Immun. 1990, 58, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.P.; Paletta-Silva, R.; Saraiva, E.M.; Lopes, A.H.; Meyer-Fernandes, J.R. Leishmania chagasi: An ecto-3’-nucleotidase activity modulated by inorganic phosphate and its possible involvement in parasite-macrophage interaction. Exp. Parasitol. 2011, 127, 702–707. [Google Scholar] [CrossRef]
- Persson, B.L.; Berhe, A.; Fristedt, U.; Martinez, P.; Pattison, J.; Petersson, J.; Weinander, R. Phosphate permeases of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1998, 1365, 23–30. [Google Scholar] [CrossRef]
- Pinheiro, C.M.; Martins-Duarte, E.S.; Ferraro, R.B.; Fonseca-de-Souza, A.L.; Gomes, M.T.; Lopes, A.H.; Vannier-Santos, M.A.; Santos, A.L.; Meyer-Fernandes, J.R. Leishmania amazonensis: Biological and biochemical characterization of ecto-nucleoside triphosphate diphosphohydrolase activities. Exp. Parasitol. 2006, 114, 16–25. [Google Scholar] [CrossRef]
- Gonzalez-Salgado, A.; Steinmann, M.E.; Greganova, E.; Rauch, M.; Mäser, P.; Sigel, E.; Bütikofer, P. Myo-Inositol uptake is essential for bulk inositol phospholipid but not glycosylphosphatidylinositol synthesis in Trypanosoma brucei. J. Biol. Chem. 2012, 287, 13313–13323. [Google Scholar] [CrossRef]
- González-Salgado, A.; Steinmann, M.; Major, L.L.; Sigel, E.; Reymond, J.L.; Smith, T.K.; Bütikofer, P. Trypanosoma brucei Bloodstream Forms Depend upon Uptake of myo-Inositol for Golgi Complex Phosphatidylinositol Synthesis and Normal Cell Growth. Eukaryot. Cell. 2015, 14, 616–624. [Google Scholar] [CrossRef][Green Version]
- Adam, R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dringen, R. Differential effects of iodoacetamide and iodoacetate on glycolysis and glutathione metabolism of cultured astrocytes. Front. Neuroenerg. 2009, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.; Jarrol, E.L.; Khan, N.A. Carbohydrate analysis of Acanthamoeba castellanii. Exp. Parasitol. 2009, 122, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Moon, E.Y.; Hong, Y.; Chung, D.I.; Kong, H.H. Autophagy protein 12 plays an essential role in Acanthamoeba encystations. Exp. Parasitol. 2015, 159, 46–52. [Google Scholar] [CrossRef]
- Dick, C.F.; Dos-Santos, A.L.A.; Meyer-Fernandes, J.R. Inorganic phosphate as an important regulator of phosphatases. Enzyme Res. 2011, 2011, 103980. [Google Scholar] [CrossRef]
- Moon, E.K.; Hong, Y.; Chung, D.I.; Kong, H.H. Cysteine protease involving in autophagosomal degradation of mitochondria during encystation of Acanthamoeba. Mol. Biochem. Parasitol. 2012, 185, 121–126. [Google Scholar] [CrossRef]
- Aqeel, Y.; Siddiqui, R.; Farooq, M.; Khan, N.A. Anaerobic respiration: In vitro efficacy of nitazoxanide against mitochondriate Acanthamoeba castellanii of the T4 genotype. Exp. Parasitol. 2015, 157, 170–176. [Google Scholar] [CrossRef]
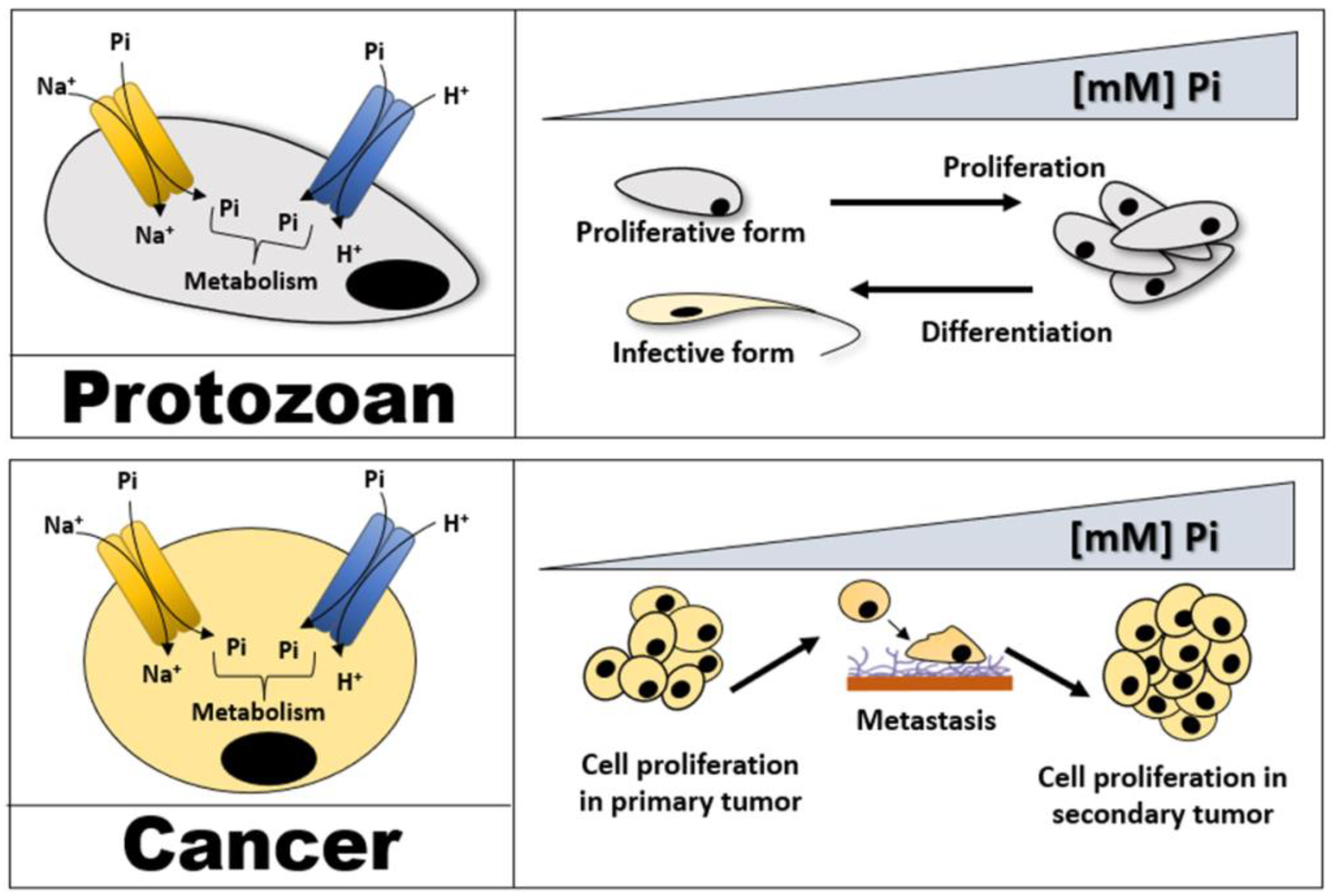
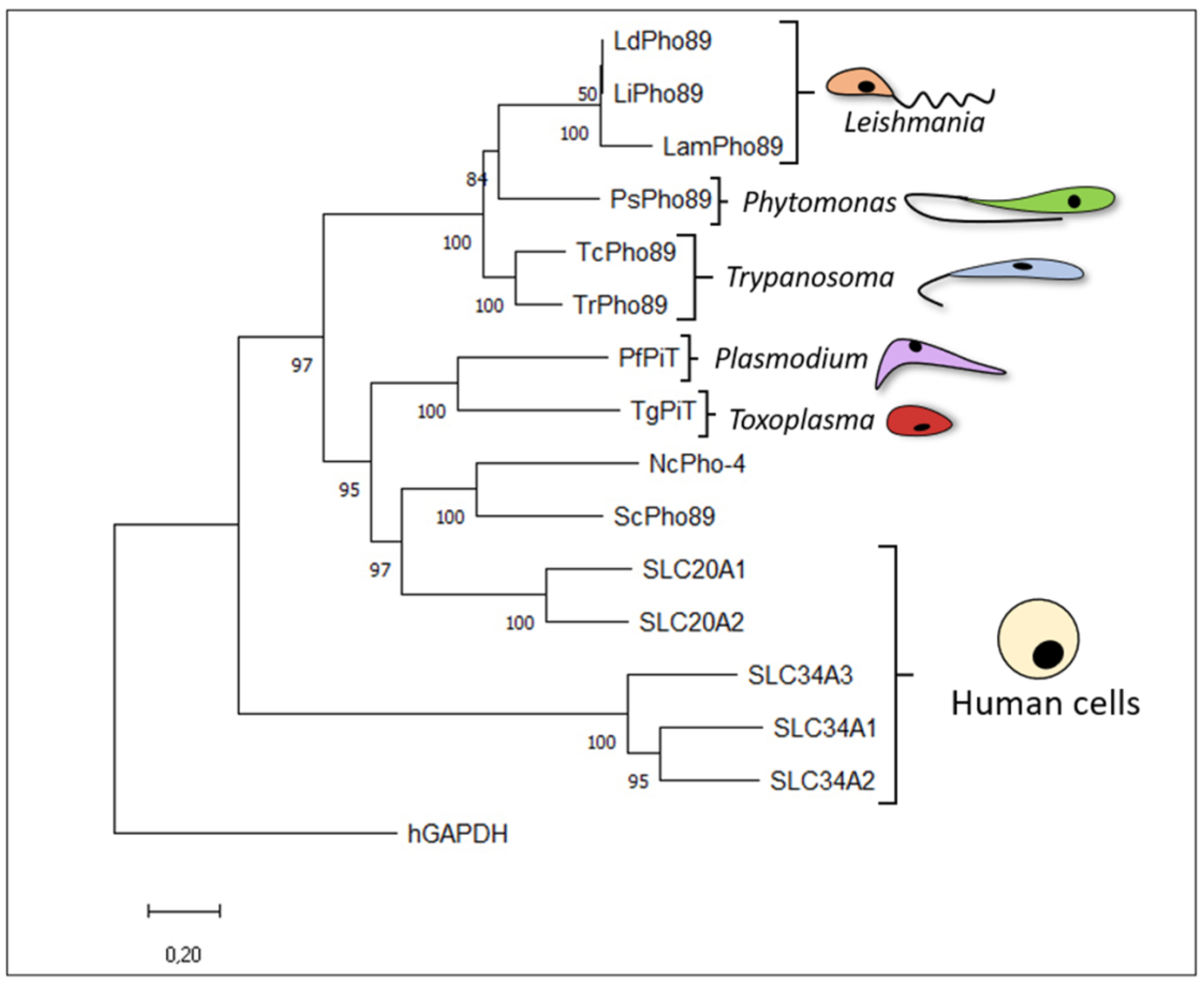


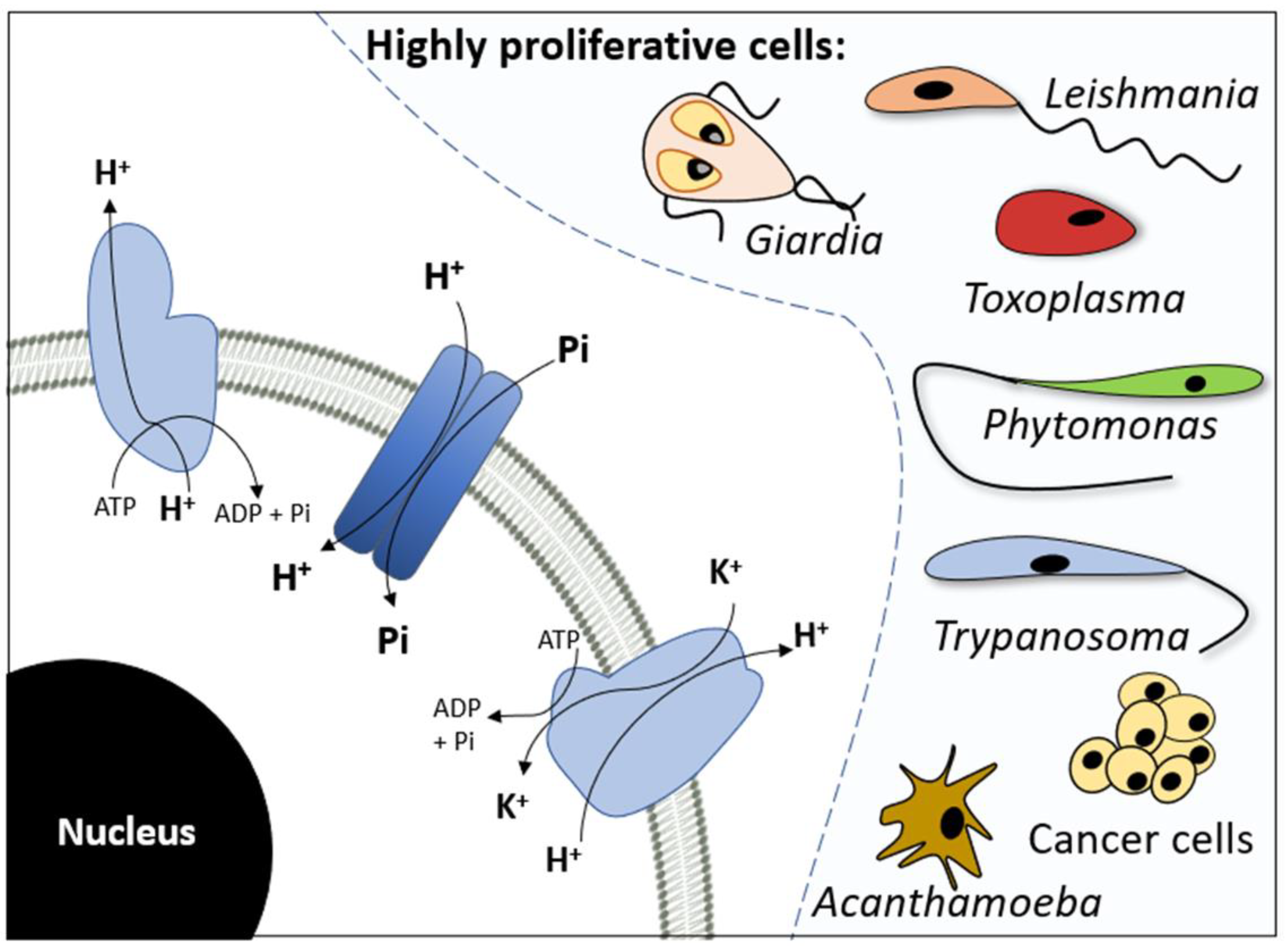
| Cells | Protein | K0.5 Pi | K0.5 Na | Reference |
|---|---|---|---|---|
| MDA-MB-231 (breast cancer) | NaPi-IIb | 84.9 ± 10.4 μM | 14.1 ± 2.8 mM | [19] |
| Plasmodium falciparum | PfPiT | 118.0 ± 15.0 µM | ND 1 | [14] |
| Toxoplasma gondii | TgPiT | 22.9 ± 5.7 μM | 3.7 ± 0.5 mM | [23] |
| Trypanosoma rangeli | TrPho89 | 58 ± 3 μM | 1.2 ± 0.3 mM | [5] |
| Trypanosoma cruzi | TcPho89 | 9.2 ± 2.2 μM | 4.5 ± 0.7 mM | [6] |
| Phytomonas serpens | PsPho89 | 7.1 ± 0.7 μM | 21.3 ± 0.2 mM | [18] |
| Cells | Protein | K0.5 Pi | Reference |
|---|---|---|---|
| MDA-MB-231 (breast cancer) | ND 1 | 1387.0 ± 167.4 μM | [22] |
| Leishmania infantum | LiPho84 | 16.0 ± 2.0 μM | [16] |
| Leishmania amazonensis | LaPho84 | 43.4 ± 4.3 μM | [21] |
| Trypanosoma rangeli | TrPho84 | 45.0 ± 7.0 μM | [5] |
| Trypanosoma brucei | TbHMIT | 93.0 ± 8.0 μM | [17] |
| Phytomonas serpens | PsPho84 | 2.8 ± 0.4 μM | [18] |
| Giardia duodenalis | GdPho84 | 67.7 ± 7.1 μM | [27] |
| Acanthamoeba castellanii | AcPHS1 | 88.8 ± 6.8 μM | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacerda-Abreu, M.A.; Dick, C.F.; Meyer-Fernandes, J.R. The Role of Inorganic Phosphate Transporters in Highly Proliferative Cells: From Protozoan Parasites to Cancer Cells. Membranes 2023, 13, 42. https://doi.org/10.3390/membranes13010042
Lacerda-Abreu MA, Dick CF, Meyer-Fernandes JR. The Role of Inorganic Phosphate Transporters in Highly Proliferative Cells: From Protozoan Parasites to Cancer Cells. Membranes. 2023; 13(1):42. https://doi.org/10.3390/membranes13010042
Chicago/Turabian StyleLacerda-Abreu, Marco Antonio, Claudia Fernanda Dick, and José Roberto Meyer-Fernandes. 2023. "The Role of Inorganic Phosphate Transporters in Highly Proliferative Cells: From Protozoan Parasites to Cancer Cells" Membranes 13, no. 1: 42. https://doi.org/10.3390/membranes13010042
APA StyleLacerda-Abreu, M. A., Dick, C. F., & Meyer-Fernandes, J. R. (2023). The Role of Inorganic Phosphate Transporters in Highly Proliferative Cells: From Protozoan Parasites to Cancer Cells. Membranes, 13(1), 42. https://doi.org/10.3390/membranes13010042






