The Optimized Conformation Dynamics of the KcsA Filter as a Probe for Lateral Membrane Effects: A First Principle Based Femto-Sec Resolution MD Study
Abstract
1. Introduction
2. Materials and Methods
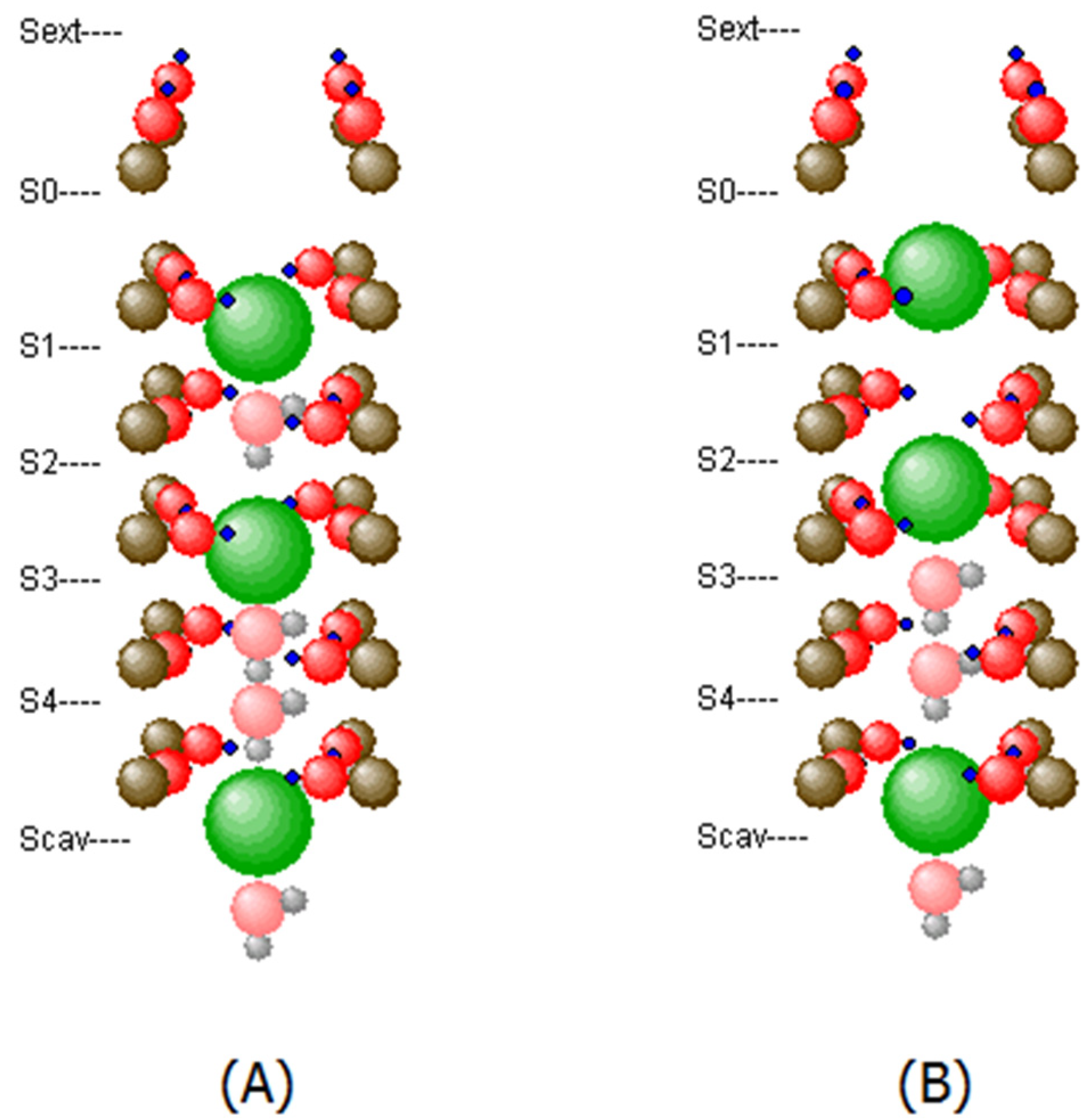
2.1. The Selectivity Filter of the KcsA Potassium Channel
2.2. Modeling the Selectivity Filter
2.3. Motion of K+ and H2O through the SF
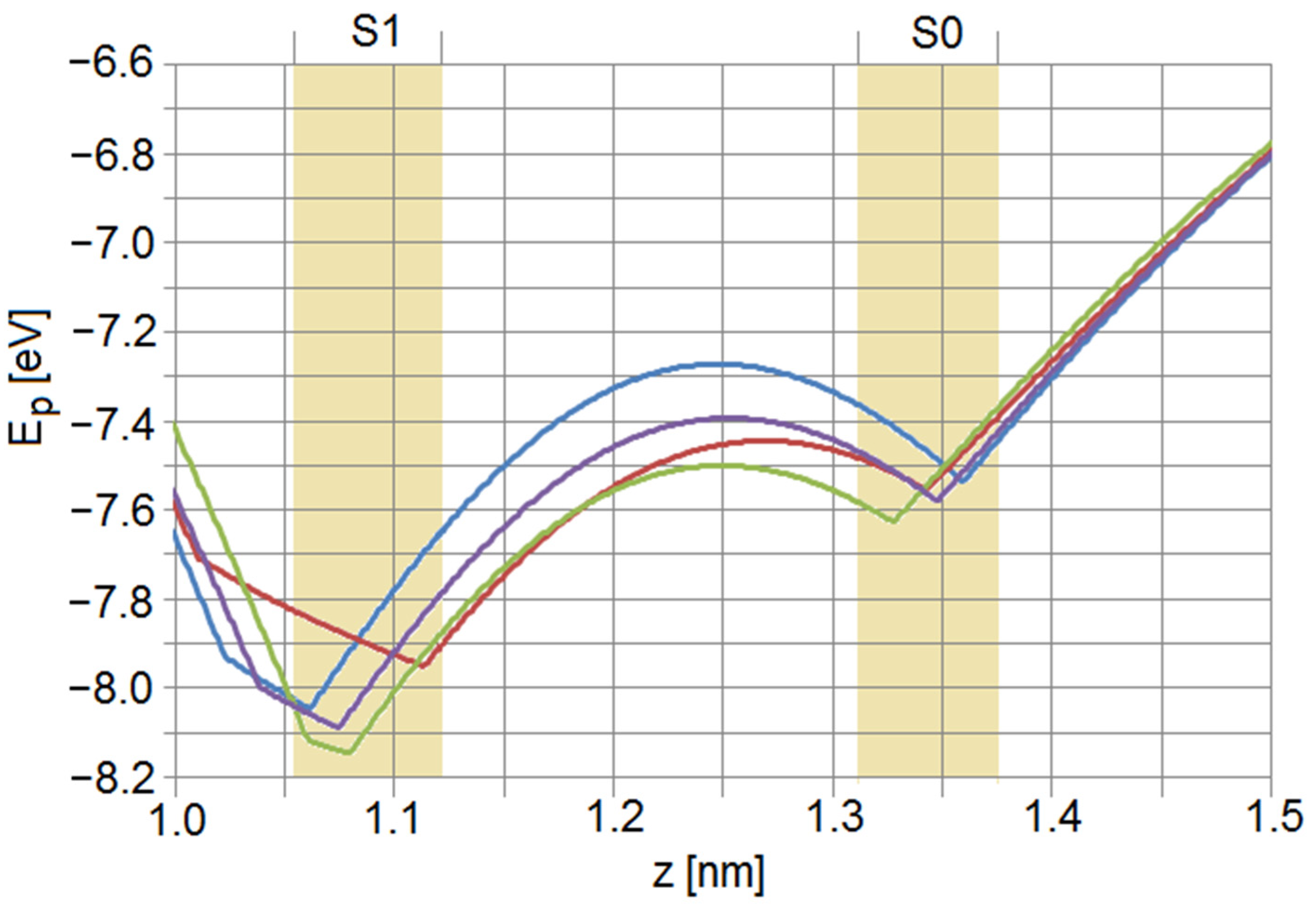
2.4. Lateral Membrane Effects
3. Results
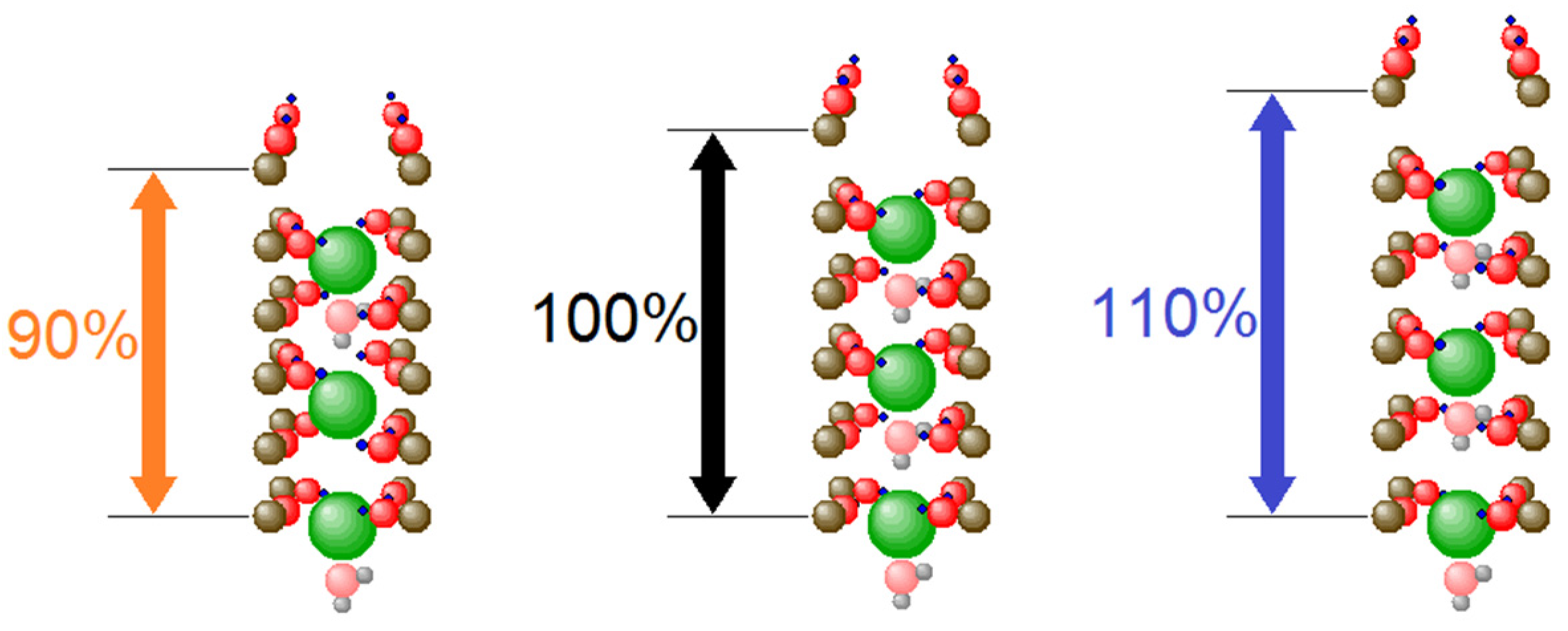
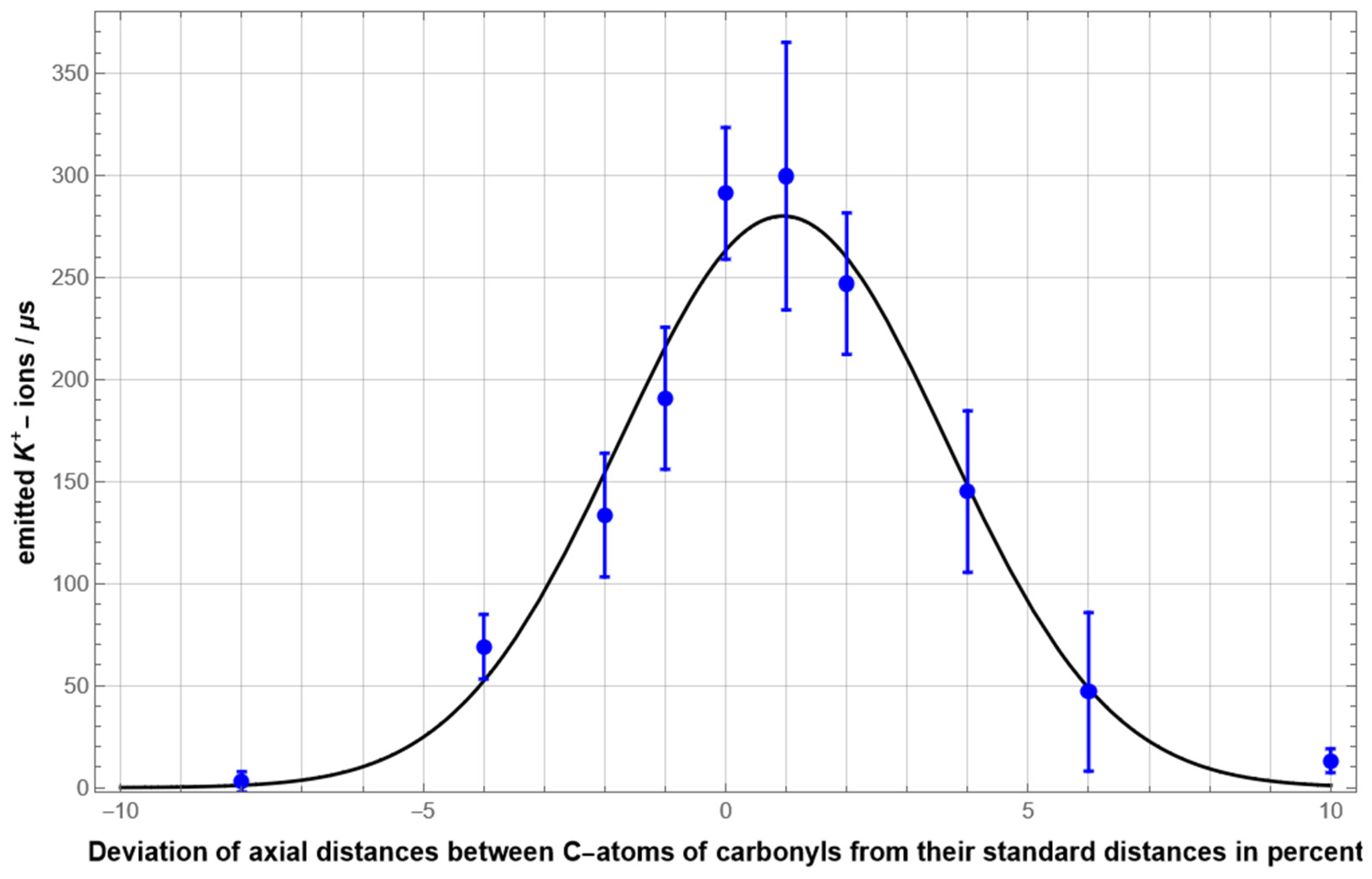
3.1. Optimized Conduction Rates at Standard Configuration
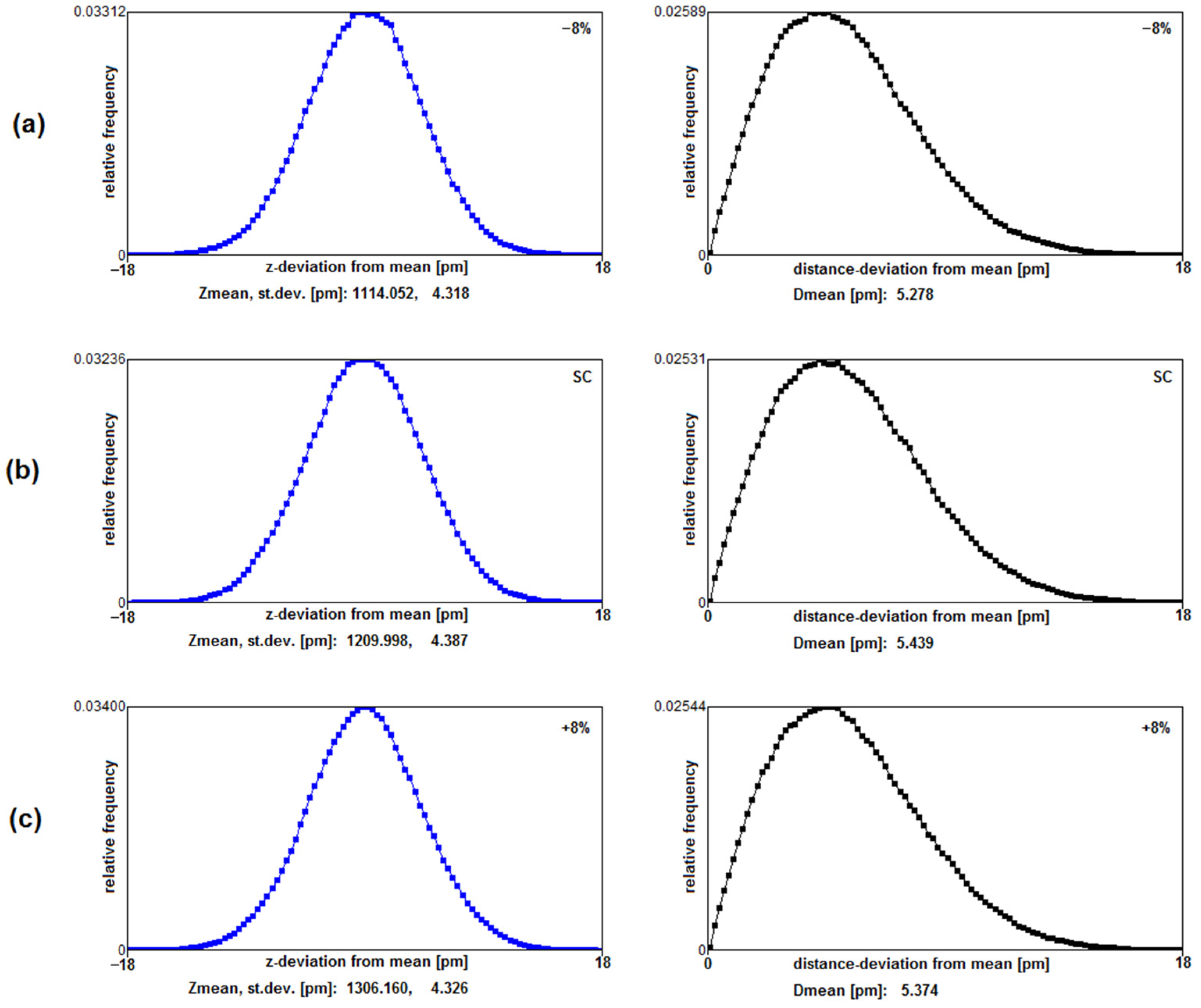
3.2. Coordination Mobility
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Forces between Particles
Examples of Forces between the Particles
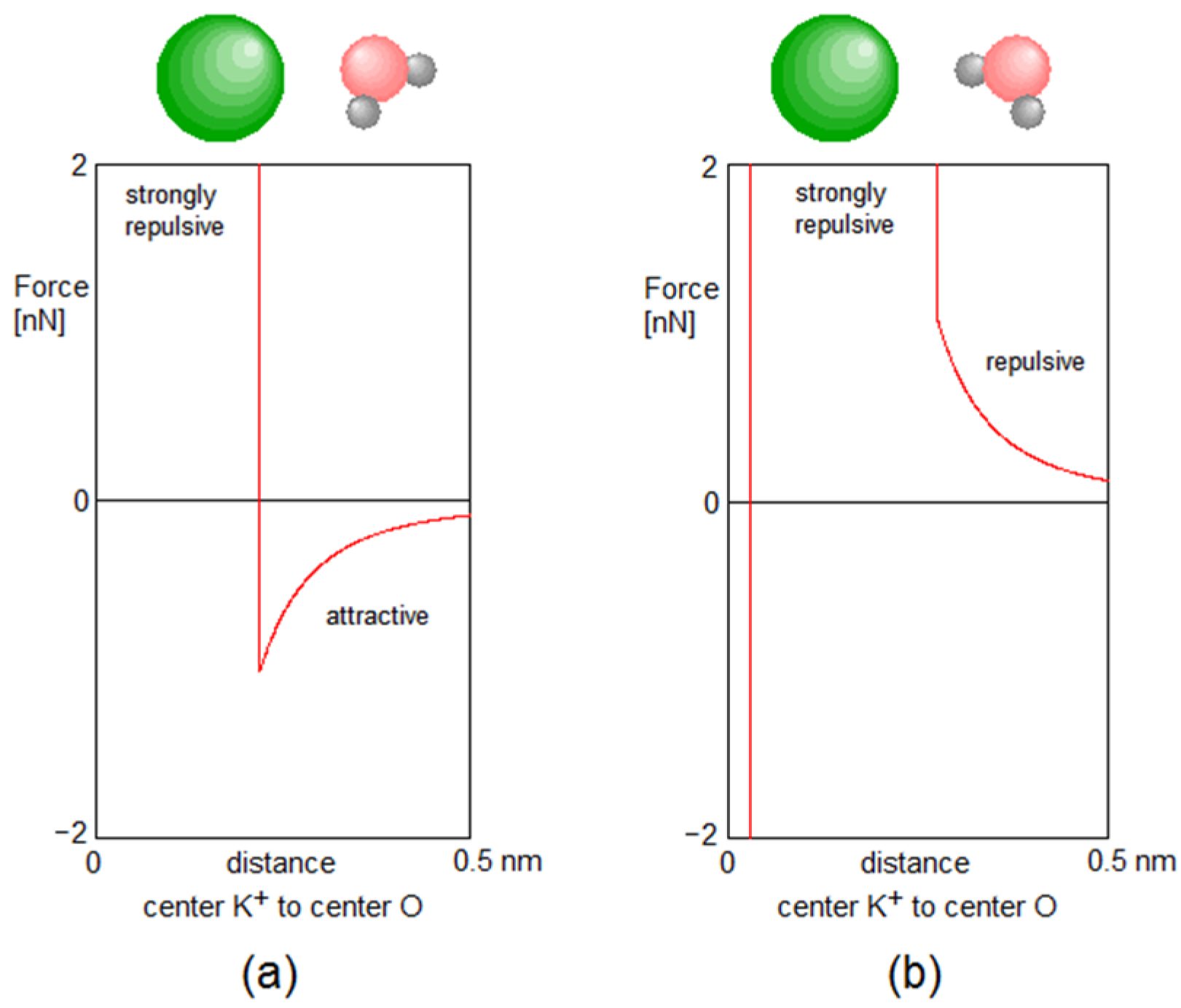

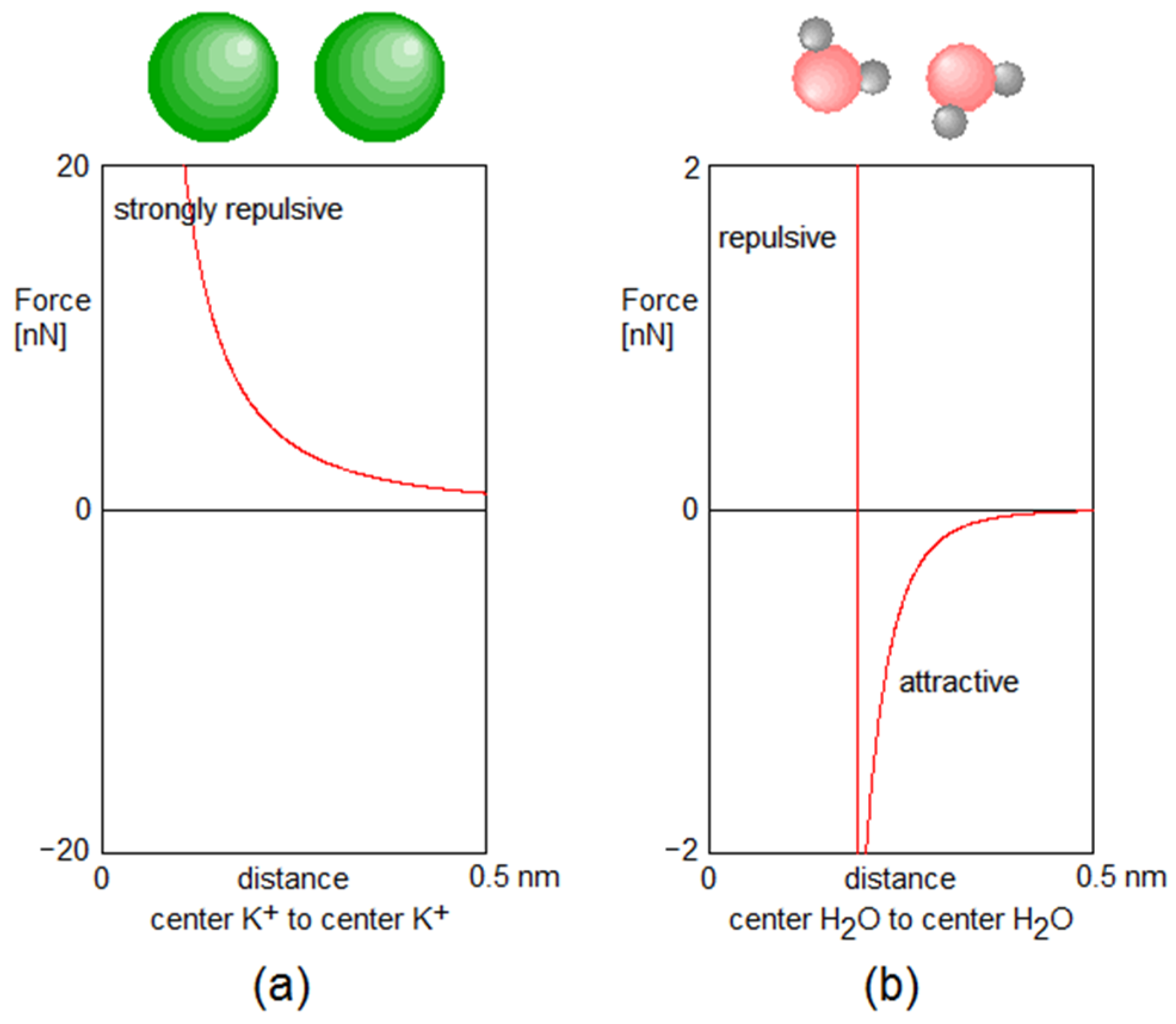
Appendix B
| [cm−1] | [Hz] | Oszillations-Period [fs] | Bending Constant [N/rad] | Energies for | |
|---|---|---|---|---|---|
| [J] | [meV] | ||||
| 300 | 9 × 1012 | 111.1 | 1.0443 × 10−8 | 4.2450 × 10−21 | 26.50 |
| 400 | 1.2 × 1013 | 83.3 | 1.8565 × 10−8 | 7.5467 × 10−21 | 47.11 |
| 500 | 1.5 × 1013 | 66.7 | 2.9007 × 10−8 | 1.1791 × 10−20 | 73.60 |
| 600 | 1.8 × 1013 | 55.6 | 4.1771 × 10−8 | 1.6980 × 10−20 | 105.99 |
| 700 | 2.1 × 1013 | 47.6 | 5.6855 × 10−8 | 2.3112 × 10−20 | 144.27 |
References
- Elinder, F.; Liin, S.I. Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front. Physiol. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Roux, B.; Schulten, K. Computational Studies of Membrane Channels. Structure 2004, 12, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Miloshevsky, G.V.; Jordan, P.C. Permeation in ion channels: The interplay of structure and theory. Trends Neurosci. 2004, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Morales, J.F.; Vasquez, V. How lipids contribute to ion channel function, a fat perspective on direct and indirect interactions. Curr. Opin. Struct. Biol. 2018, 51, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Bagatolli, L.A.; Mouritsen, O.G. Is the fluid mosaic (and the accompanying raft hypothesis) a suitable model to describe fundamental features of biological membranes? What may be missing? Front. Plant Sci. 2013, 4, 457. [Google Scholar] [CrossRef]
- Czysz, A.H.; Rasenick, M.M. G-Protein Signaling, Lipid Rafts and the Possible Sites of Action for the Antidepressant Effects of n-3 Polyunsaturated Fatter Acids. CNS Neurol. Disord. Drug Targets 2013, 12, 466–473. [Google Scholar] [CrossRef]
- Yazdi, S.; Stein, M.; Elinder, F.; Andersson, M.; Lindahl, E. The Molecular Basis of Polyunsaturated Fatty Acid Interactions with the Shaker Voltage-Gated Potassium Channel. PLOS Comput. Biol. 2016, 12, e1004704. [Google Scholar] [CrossRef]
- Börjesson, S. Polyunsaturated Fatty Acids Modifying Ion Channel Voltage Gating. Ph.D. Thesis, Linköping University, Faculty of Health Sciences, Linköping, Sweden, 2012. [Google Scholar]
- Doyle, D.A.; Morais Cabral, J.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef]
- Fowler, P.W.; Abad, E.; Beckstein, O.; Sansom, M.S. Energetics of Multi-ion Conduction Pathways in Potassium Ion Channels. JCTC 2013, 9, 5176–5189. [Google Scholar] [CrossRef]
- Köpfer, D.A.; Song, C.; Gruene, T.; Sheldrick, G.M.; Zachariae, U.; de Groot, B.L. Ion permeation in K+ channels occurs by direct Coulomb knock-on. Science 2014, 346, 352–355. [Google Scholar] [CrossRef]
- Roux, B. Theoretical and computational models of ion channels. Curr. Opin. Struct. Biol. 2002, 12, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Garofoli, S.; Jordan, P.C. Modeling permeation energetics in the KcsA potassium channel. Biophys. J. 2003, 84, 2814–2830. [Google Scholar] [CrossRef]
- Summhammer, J.; Sulyok, G.; Bernroider, G. Quantum Mechanical Coherence of K+ Ion Wave Packets Increases Conduction in the KcsA Ion Channel. Appl. Sci. 2020, 10, 4250. [Google Scholar] [CrossRef]
- Sumino, A.; Sumikama, T.; Iwamoto, M.; Dewa, T.; Oiki, S. The Open Gate Structure of the Membrane-Embedded KcsA Potassium Channel viewed from the Cytoplasmic Side. Sci. Rep. 2013, 3, 1063. [Google Scholar] [CrossRef] [PubMed]
- Sansom, M.S.; Shrivastava, I.H.; Bright, J.N.; Tate, J.; Capener, C.E.; Biggin, P.C. Potassium channels: Structures, models, simulations. Biochim. Biophys. Acta (BBA)—Biomembr. 2002, 156, 294–307. [Google Scholar] [CrossRef]
- Zhou, Y.; Morais-Cabral, J.H.; Kaufman, A.; MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0 Å resolution. Nature 2001, 414, 43–48. [Google Scholar] [CrossRef]
- Cantor, R.S. Lateral pressures in cell membranes: A mechanism for modulation of protein function. J. Phys. Chem. B 1997, 101, 1723–1725. [Google Scholar] [CrossRef]
- Guidoni, L.; Carloni, P. Potassium permeation through the KcsA channel: A density functional study. Biochim. Biophys. Acta (BBA)—Biomembr. 2002, 1563, 1–6. [Google Scholar] [CrossRef]
- Chung, S.H.; Corry, B. Conduction properties of KcsA measured using Brownian Dynamics with flexible carbonyl groups in the selectivity filter. Biophys. J. 2007, 93, 44–53. [Google Scholar] [CrossRef]
- Berneche, S.; Roux, B. A gate in the selectivity filter of potassium channels. Structure 2005, 13, 591–600. [Google Scholar] [CrossRef]
- Gwan, J.-F.; Baumgaertner, A. Cooperative transport in a potassium ion channel. J. Chem. Phys. 2007, 127, 045103. [Google Scholar] [CrossRef]
- Ader, C.; Pongs, O.; Becker, S.; Baldus, M. Protein dynamics detected in a membrane-embedded potassium channel using two-dimensional solid-state NMR spectroscopy. Biochim. Biophys. Acta (BBA)—Biomembr. 2010, 1798, 286–290. [Google Scholar] [CrossRef]
- Summhammer, J.; Sulyok, G.; Bernroider, G. Quantum dynamics and non-local effects behind ion transition states during permeation in membrane channel proteins. Entropy 2018, 20, 558. [Google Scholar] [CrossRef] [PubMed]
- Bernroider, G.; Summhammer, J. Can quantum entanglement between ion-transition states effect action potential initiation? Cogn. Comput. 2012, 4, 29–37. [Google Scholar] [CrossRef]
- Tonello, L.; Cocchi, M.; Gabrielli, F.; Minuto, C. Human and Animal Brain Phospholipids Fatty Acids, Evolution and Mood Disorders. J. Phylogenetics Evol. Biol. 2014, 2, 1000128. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicholson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Cocchi, M.; Minuto, C.; Tonello, L.; Gabrielli, F.; Bernroider, G.; Tuszynski, J.A.; Cappello, F.; Rasenick, M. Linolic acid: Is this the key that unlocks the quantum brain? Insights linking broken symmetries in molecular biology, mood disorders and personalistic emergentism. BMC Neurosci. 2017, 18, 38. [Google Scholar] [CrossRef]
- Cournia, Z.; Allen, T.W.; Andricioaei, I.; Antonny, B.; Baum, D.; Brannigan, G.; Buchete, N.-V.; Deckman, J.T.; Delemotte, L.; Del Val, C.; et al. Membrane Protein Structure, Function and Dynamics: A Perspective from Experiments and Theory. J. Membr. Biol. 2015, 248, 611–640. [Google Scholar] [CrossRef]
- Van den Brink-van der Laan, E.; Killian, J.A.; de Kruijff, B. Noonbilayer lipids affect peripheral and integralmembrane proteins via changes in the lateral pressure profile. Biochim. Biophys. Acta (BBA)—Biomembr. 2004, 1666, 275–288. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summhammer, J.; Sulyok, G.; Bernroider, G.; Cocchi, M. The Optimized Conformation Dynamics of the KcsA Filter as a Probe for Lateral Membrane Effects: A First Principle Based Femto-Sec Resolution MD Study. Membranes 2022, 12, 1183. https://doi.org/10.3390/membranes12121183
Summhammer J, Sulyok G, Bernroider G, Cocchi M. The Optimized Conformation Dynamics of the KcsA Filter as a Probe for Lateral Membrane Effects: A First Principle Based Femto-Sec Resolution MD Study. Membranes. 2022; 12(12):1183. https://doi.org/10.3390/membranes12121183
Chicago/Turabian StyleSummhammer, Johann, Georg Sulyok, Gustav Bernroider, and Massimo Cocchi. 2022. "The Optimized Conformation Dynamics of the KcsA Filter as a Probe for Lateral Membrane Effects: A First Principle Based Femto-Sec Resolution MD Study" Membranes 12, no. 12: 1183. https://doi.org/10.3390/membranes12121183
APA StyleSummhammer, J., Sulyok, G., Bernroider, G., & Cocchi, M. (2022). The Optimized Conformation Dynamics of the KcsA Filter as a Probe for Lateral Membrane Effects: A First Principle Based Femto-Sec Resolution MD Study. Membranes, 12(12), 1183. https://doi.org/10.3390/membranes12121183





