Abstract
To secure existing water resources is one of the imposing challenges to attain sustainability and ecofriendly world. Subsequently, several advanced technologies have been developed for water treatment. The most successful methodology considered so far is the development of water filtration membranes for desalination, ion permeation, and microbes handling. Various types of membranes have been industrialized including nanofiltration, microfiltration, reverse osmosis, and ultrafiltration membranes. Among polymeric nanocomposites, nanocarbon (fullerene, graphene, and carbon nanotubes)-reinforced nanomaterials have gained research attention owing to notable properties/applications. Here, fullerene has gained important stance amid carbonaceous nanofillers due to zero dimensionality, high surface areas, and exceptional physical properties such as optical, electrical, thermal, mechanical, and other characteristics. Accordingly, a very important application of polymer/fullerene C60 nanocomposites has been observed in the membrane sector. This review is basically focused on talented applications of polymer/fullerene nanocomposite membranes in water treatment. The polymer/fullerene nanostructures bring about numerous revolutions in the field of high-performance membranes because of better permeation, water flux, selectivity, and separation performance. The purpose of this pioneering review is to highlight and summarize current advances in the field of water purification/treatment using polymer and fullerene-based nanocomposite membranes. Particular emphasis is placed on the development of fullerene embedded into a variety of polymer membranes (Nafion, polysulfone, polyamide, polystyrene, etc.) and effects on the enhanced properties and performance of the resulting water treatment membranes. Polymer/fullerene nanocomposite membranes have been developed using solution casting, phase inversion, electrospinning, solid phase synthesis, and other facile methods. The structural diversity of polymer/fullerene nanocomposites facilitates membrane separation processes, especially for valuable or toxic metal ions, salts, and microorganisms. Current challenges and opportunities for future research have also been discussed. Future research on these innovative membrane materials may overwhelm design and performance-related challenging factors.
1. Introduction
Clean water is vivacious for human beings and the ecological world environment [1,2]. To achieve unsoiled water, implementation of water treatment or purification technologies have been focused on. As the most successful method, the concentrated fabrication and examination of high-performance membrane technologies has been foreseen. Miscellaneous membrane-dependent technologies, therefore, have been applied for water decontamination [3,4]. Usually, membrane technologies have been claimed for having low cost, fine workability, efficiency, low energy ingesting, and scale-up viabilities [5]. Pressure-driven membranes processes have been applied in conventional membrane filtration [6]. Polymeric membranes have been identified for the high permeability, the stability, the selectivity, the salt rejection, and the low working pressure. Polymeric membranes have been developed using various polymers as polystyrene, polyethylene, polyamide, polyacrylonitrile, poly(vinylidene fluoride), poly(vinyl alcohol), and polyaniline [7,8,9]. Common processes used for the formation of filtration membranes include phase inversion, interfacial polymerization, solution casting, sol-gel processes, and electrospinning [10]. Polymeric membranes have been characterized for the morphology, crystallinity, hydrophilicity, surface roughness, permeation, flux, salt rejection, and antifouling performances. However, the foremost downsides of neat polymeric membranes include the irregularly porous structure, hydrophobicity, membranes fouling, and pore clogging due to effluents [11]. Therefore, the membrane technology has shifted towards the use of nanocomposite membranes instead of neat polymeric membranes [12,13]. For the formation of polymeric nanocomposite membranes, inorganic and organic nanoparticles have been considered [14]. Inorganic nanoparticles such as metal or metal oxides such as Au, Ag, Fe, SiO2, Al2O3, zeolite, and polyhedral oligomeric silsesquioxanes have been filled to form innovative polymeric nanocomposite membranes [15]. Nanocarbon nanomaterials such as fullerene, carbon nanotubes, graphene, nanodiamonds, and carbon nanofiber have also been used to form nanocomposite membranes [16,17,18]. Among various types of nanofiller, carbonaceous nanofillers have revealed low noxiousness, facile preparation, and eco-friendliness to be employed in polymeric membranes [19,20]. Consequently, polymer nanocomposite membranes have the potential for waste water purification towards chemicals, heavy metals, salts, microorganisms, oils, etc. In this regard, polymer/fullerene based microfiltration, nanofiltration, ultrafiltration, desalination, and reverse osmosis membranes have been developed [21,22]. The implication of polymer/fullerene nanocomposites in water treatment membranes has led to genuine innovations in the field of water purification technologies.
This review is revolutionary to portray the scientific development and advancement in the field of polymer/fullerene C60 nanocomposite-based water treatment membranes. Fullerene-based polymer nanocomposite membranes have recently attracted significant attention for waste water treatment and purification, mostly for the removal of toxic metals, microorganisms, chemical compounds, salts, etc. Various literature reports have been found on the design and performance of polymer/fullerene-derived ultrafiltration, nanofiltration, pervaporation, desalination, and reverse osmosis membranes. However, to the best of knowledge, such a specific review on polymer and fullerene has not seen in the literature before. The actual motive behind this review is to develop a pioneering article to portray the developments in the field of polymer/fullerene-based nanocomposite membranes. Significant literature reports on polymer/fullerene nanocomposite membranes were found between 2010 and 2022. Fewer reports developed before 2010 were mentioned in this review, so the main progress in this field during the last two decades was depicted. Thus, this state-of-the-art review highlights some auspicious zones of polymer/fullerene C60 membranes for water treatment. The resourcefulness of polymer/fullerene nanocomposites has accelerated membrane processes for water purification or effluents remediation. Moreover, polymer/fullerene nanocomposites have brought about numerous novelties in the field of high-performance water treatment membranes. Polymer/fullerene nanocomposite membranes have been identified as low-cost, efficient, and easily scalable materials. These membranes have been found highly permeable to water and have stable structures, selectivity, solute rejection, and low fouling. For the formation of polymer/fullerene membranes, polymers such as Nafion, polysulfone, polyamide, and polystyrene have been preferred. Some preparation methods for forming polymeric membranes include solution casting, phase inversion, electrospinning, and solid phase synthesis routes. The ensuing novel membrane materials may have capabilities to overwhelm the challenging aqueous environment for the filtration of undesirable contaminants. In other words, this article offers a groundbreaking and original review on polymer and fullerene-based membrane materials. Despite remarkable properties and the vast potential, devoted future research efforts are desirable to form high-performance nanocomposite membranes for water purification applications and to overcome the associated challenges. Future developments in the field of novel functional polymer/fullerene C60 nanocomposite membranes are not possible for researchers before getting prior knowledge of the reported literature in this field.
2. Fullerene
Fullerene is a symmetrical zero-dimensional nano-allotrope of carbon [23]. It is a hollow ball-like nanostructure having sp2 hybridized carbon atoms in its architecture [24]. The carbon atoms in fullerene form a π conjugation system [25,26]. It is made up of unique polygons, i.e., pentagons and hexagons. The cage-like fullerene nanostructure is ~1 nm [27]. Fullerene was primarily discovered in 1985 [28]. Depending upon the number of carbon atoms in the structure, the fullerene molecules can be of several types such as C24, C28, C60, C70, and C120 (Figure 1) [29]. Fullerene C60 is a commonly known fullerene molecule also referred to as buckminsterfullerene. Then, the next widely researched form of fullerene is C70. Fullerene molecules have revealed sole optical, electrical, magnetic, thermal, mechanical, and biomedical possessions [30,31,32]. Fullerene molecules have been primed using numerous procedures such as the microwave synthesis, arc discharge, plasma techniques, chemical vapor deposition, and numerous chemical approaches [31,32,33]. Nanocarbons such as graphene, carbon nanotube, and nanodiamond have been applied in essential technical applications including nanocomposite formation [34,35,36,37]. Nanocomposites have been formed using the in situ method, solution mixing, melt processing, spinning, printing, and other practices [38,39,40]. Fullerene molecules own exceptional physical features for nanocomposite applications [41,42,43,44]. With polymers, fullerene molecules may form an electron donor-acceptor relationship [45]. The solubility properties of fullerene in number of solvents have been explored for the nanocomposite formation [46,47,48]. The polymers such as poly(vinylpyrrolidone) have also been employed to solubilize fullerene molecules [49,50,51]. Applications areas of fullerene, functional fullerene, and nanocomposites have been observed in optoelectronics [52,53,54], solar cells [55], sensors [56], drug delivery [57], and other practical fields. In the membrane application, fullerene has been reinforced in polymeric matrices to influence final properties [58]. As compared to other nanocarbons, fullerene has a higher surface area to interact with polymers and own a lower aggregation tendency and better dispersion properties [59]. For water treatment purposes, fullerene addition may enhance homogeneity in polymeric membranes; however, appropriate surfactants and stabilizing agents need to be included to form high-performance materials [60,61]. Mostly, fullerene C60 molecules are reinforced in polymeric matrices to form water filtration membranes. Other fullerene forms have been rarely studied in polymeric membranes.
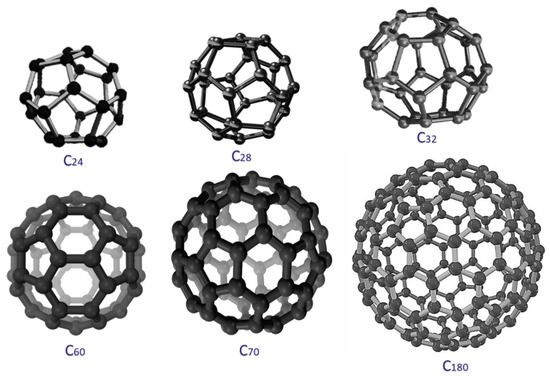
Figure 1.
Fullerene nanostructures.
3. Polymeric Nanocomposite Membranes
Membrane technologies has expanded research interest in methodological applications and technical industries [62,63,64]. Membrane technologies has been used for the remediation of environmental pollutants including noxious gases and water contaminants [65,66,67]. Relative to neat polymeric membranes, nanocomposite membranes have been beneficially applied for water treatment [68]. Membrane technologies have especially targeted domestic water management, industrial waste water treatment, and seawater desalination [69,70,71]. In the membrane filtration process, the trade-off association between the membrane permeability and the membrane selectivity is considered important [72,73,74]. Compared with neat polymeric membranes, nanocomposite membranes exhibit the advantages of superior physicochemical characteristics due to the combination of polymers and nanoparticles [75,76,77,78,79]. Moreover, the enhanced fouling resistance, optimum porosity, hydrophilicity, mechanical robustness, and heat stability have been observed [80,81,82,83,84]. Figure 2 portrays the construction of nanocomposite membranes using a widely applied phase inversion technique [85,86,87,88,89]. This scheme usually encompasses nanoparticle dispersion in a solvent and then amalgamation with a polymer solution [90,91,92]. The phase inversion technique has been used to develop microfiltration, nanofiltration, or ultrafiltration membranes [93,94,95]. Using this approach, nanocarbons such as graphene, graphene oxide, and carbon nanotubes have been reinforced in nylon [96,97,98,99,100], polysulfone [101,102,103,104,105], poly(vinyl alcohol) [106,107,108,109,110], poly(vinyl acetate) [111,112,113,114,115], etc. to develop high-performance membranes. Ammar et. al. [116] fabricated polysulfone and graphene-based membranes with the phase inversion method. The membranes were explored for the morphology and structural crystallinity [117,118,119,120,121]. Owing to electrostatic or van der Waals interactions between the matrix and the nanofiller, the water flux of the nanocomposite membranes was enhanced [122,123]. Ganesh and co-workers [124] reported polysulfone and graphene oxide-derived mixed matrix membranes. The phase inversion method was adopted as a successful method to form membranes [125,126,127,128,129].
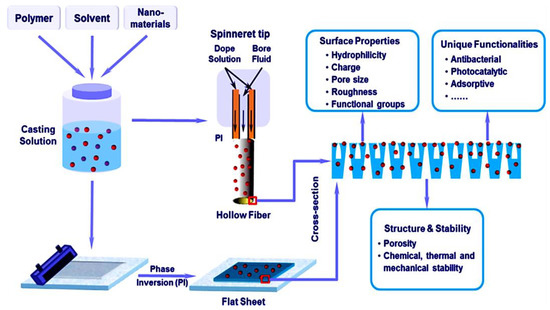
Figure 2.
Fabrication of conventional nanocomposite membranes [130]. Reproduced with permission from Elsevier.
4. Polymer/Fullerene Nanocomposite
Polymer/fullerene nanocomposites have been considered for superior optical, electrical, thermal, mechanical, and processability features [131,132,133]. However, during the polymer/fullerene nanocomposite formation, agglomeration can cause major hinderance to the production of high-performance nanomaterials. The aggregation of fullerene molecules may cause poor solubility in organic solvents [134]. Consequently, the large-scale processing of polymer/fullerene nanocomposites has been studied [135]. To resolve this issue, functional fullerene molecules have been reinforced in polymeric matrices [136,137]. A range of thermoplastics, thermosets, and conjugated polymers have been used to form nanocomposites with fullerene [138,139]. Polymer/fullerene nanocomposites have been applied in wide-ranging methodological fields such as electronics [140], supercapacitors [141], and solar cells [142]. Fullerene C60 has been physically or covalently linked with host polymers to enhance final nanomaterial characteristics [143,144]. The systematic studies on the polymer/fullerene nanocomposites have escorted towards polymeric membranes [145]. In this regard, numerous filtration membranes have been developed using varying polymers (polyamide, polystyrene, polysulfone, etc.) and the fullerene C60 nanofiller [146,147,148]. Nanocomposite membranes have been focused on due to its chemical stability, mechanical resilience, rigidity, porosity, and inexpensiveness [149,150,151]. The inclusion of fullerene contents have significantly improved technological membrane parameters towards the remediation of environmental effluents [152]. Kitjanon et al. [153] developed and studied the cis-1,4-polyisoprene and C60-based nanocomposite membranes via coarse-grained molecular dynamics simulation. Nanoparticles with 0–32 phr fullerene were loaded, and then, simulations were performed over 200 microseconds. The photographs of the cis-1,4-polyisoprene and C60-based nanocomposite are given in Figure 3. In addition, the experimental results for the glass transition temperature, mechanical, and thermodynamical properties were investigated for the high-performance nanocomposites.
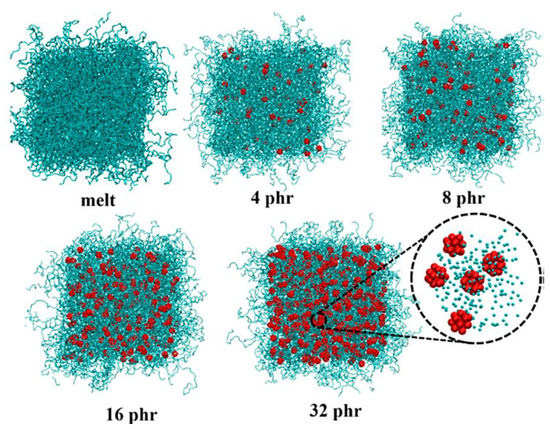
Figure 3.
Snapshots of cis-1,4-polyisoprene and C60-based nanocomposite membranes with varying nanofiller contents from 0 (melt) to 32 phr [153]. Reproduced with permission Creative Commons Attribution License.
5. Implication of Polymer/Fullerene Nanocomposite Membranes in Water Treatment
Polymer/fullerene nanocomposite membranes have been investigated for effective salt removal from water, toxic ion separation, ion pair separation, recovery of expensive metals, and pathogenic microorganisms separation or deterioration [154]. In the sorption method, a huge range of metal ions can be recovered such as nickel, zinc, copper, cobalt, mercury, lead, arsenic, and cadmium [155,156,157]. The design of polymer/fullerene nanocomposite membranes and surface defects may influence their sorption capacities towards metals [158]. Moreover, nanocomposite membranes show better retention time [159]. Perera et al. [160] formed thin-film reverse osmosis membranes having functional hydroxy fullerene C60. The membranes revealed a high water flux of 26.1 L/(m2∙h) and the optimum salt rejection. Moreover, the hydroxy functional fullerene C60-derived membranes presented a high separation efficiency towards Mg2+/Li+ (separation factor: 13.1). The nanocomposite membranes were found effective for the recovery of Li+ ions from seawater [161]. Liu et al. [162] reported epoxy-based membranes with fullerene C60-grafted graphene oxide. Water desalination and ion permeation were studied through the membranes. Figure 4 displays the fabrication procedure, water desalination system, and representation of anions/cations blockage on the membrane surface. The membranes had a high water flux of up to 10.85 L/(m2∙h∙bar) and 0.1883 mol/(m2∙h∙bar) for water desalination and ion permeation, respectively. The inclusion of fullerene C60 revealed fine water adsorption for large water quantities. Table 1 displays the filtration performances of a few systems showing polymeric membranes with fullerene nanoparticles.
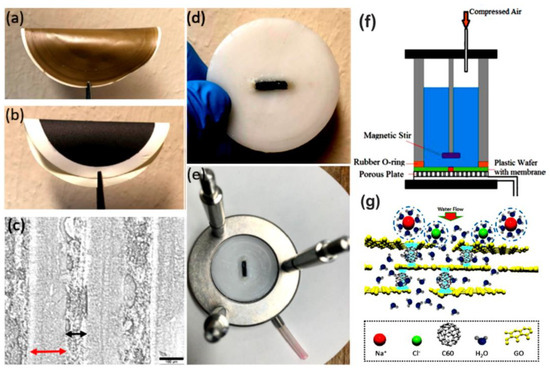
Figure 4.
Fabrication process and water desalination setup using C60-grafted graphene oxide membranes. (a) Graphene oxide membrane without C60; (b) C60-grafted graphene oxide membrane; (c) optical micrograph of a cross-sectional area with a scale bar of 100 µm. The micrograph shows 148 µm thick graphene oxide laminates embedded in 81 µm thick epoxy; (d) graphene oxide-C60 membrane encapsulated with epoxy in a plastic disk of 47 mm; (e) graphene oxide-C60 membrane inside a water desalination setup; (f,g) schematic setup of a flat membrane made of graphene oxide and a C60 hybrid for water desalination [162]. Reproduced with permission from American Chemical Society.

Table 1.
Specifications of a few polymeric membranes with fullerene C60 nanofillers for water purification.
The biocompatibility of fullerene is an ultimate requirement to ensure the safety of drinking water [168]. Fullerene nanofillers embedded into various polymeric membranes influence the resulting membranes performance. The size, shape, and surface properties of fullerenes have been found important to enhance the solute selectivity, water permeability, and stability of nanocomposite membranes. Fullerene nanofillers have been used to achieve desirable pore sizes, larger surface areas, and unique surface functionalities, improving the overall membrane performance. For the fabrication of polymer/fullerene nanocomposite membranes, the surface chemistry of fullerene has been considered important to accomplish better membrane stability [169]. The presence of physical or covalent bonding of fullerene with polymers in nanocomposite membranes have been found to improve hydrophilicity and anti-bacterial, anti-fouling, and water flux performance. Moreover, interactions between fullerene and polymers also aid in the improved membrane thermomechanical stability and long-term durability during filtration and hydraulic cleaning processes. In addition, polymer/fullerene membranes possess low toxicity, long-term organic solvent stability, and enhanced membrane operating times even at higher temperatures. Molecular dynamics (MD) simulations were used to gain insights on the interactions of polymer with fullerene in membranes [170]. The interactions between a single fullerene C60 molecule and a membrane were evaluated by computing the free energy as a function of the distance between the C60 molecule and the polymer layer. The fullerene molecule was found to be absorbed into the polymer hydrophobic part with a marked increase in the stability of the membrane (~30 kcal/mol) and cause no disruption of the hydrophobic environment. According to MD studies, initial fullerene clusters need to be disaggregated to release from the membranes. Furthermore, to make the fullerene molecule soluble from the polymeric membranes, both the energetic and kinetic barriers need to be overcome. Thus, the studies revealed the kinetic stability of polymer/fullerene nanocomposite membranes, and these membranes were not found easily degradable in aqueous environments.
5.1. Nafion/Fullerene Nanocomposite Membranes for Water Treatment
Nafion is a sulfonated tetrafluoroethylene-based fluoropolymer-copolymer [171,172]. It is a widely used synthetic polymer. It has ionic properties such as ionomers. Nafion has been commonly used to form commercial membranes for energy, electronics, and environmental applications [173,174]. Nafion and fullerene-based nanocomposite membranes have been devised [175]. The use of hydroxy-modified fullerene in Nafion results in fine photoconductivity and antimicrobial characteristics. Tasaki et al. [176] fabricated a Nafion/fullerene C60 nanocomposite with the solution casting technique. The neat fullerene C60 and the polyhydroxy fullerene were used as nanofillers. Figure 5 demonstrates the optical micrographs of the solution-cast nanocomposite membranes. Two types of membranes were prepared, i.e., through the doping process and by solution casting. In the doping process, toluene was used as a solvent. On the other hand, dimethyl acetamide was used as a solvent in the solution route. In the doping route, functional fullerene nanoparticles formed large aggregates. In the case of the solution route, finely dispersed fullerene and functional fullerene nanoparticles can be observed. Thus, the solution route was found ideal due to better miscibility properties between the Nafion matrix and the nanofiller. Figure 6 expresses the molecular dynamic simulations of the Nafion/fullerene nanocomposite membranes. The fullerene molecule can be seen totally wrapped by the Nafion oligomer, screening better mutual interactions. The water uptake of the membranes was measured through soaking in water (wet condition) and under a 25% RH (dry condition). The 1 wt % Nafion/C60 and 3 wt % Nafion/C60 membranes were tested. Both the Nafion/C60 nanocomposite membranes were found to hold more water than the neat Nafion, under a 25% RH. Consequently, the higher wet and dry water uptakes of nanocomposite membranes were observed, compared with those of the neat Nafion membrane. The water molecules were suggested to be trapped in the interfaces between the C60 aggregates and Nafion domains, depending upon the morphological form of the nafion/C60 nanocomposite. In the Nafion/fullerene nanofiltration membranes, the internal porosity and permeability were found to be affected by the packing of the polymer chains and dispersion of the fullerene nanoparticles [175]. The presence of fullerene was supposed to interrupt the packing manner of the polymer chains and interfacial morphology to generate nanopores in the Nafion/fullerene nanofiltration membranes for ion separation purposes. Nevertheless, few Nafion/fullerene systems have been studied up till now for water treatment, so further research efforts are found desirable in this category.
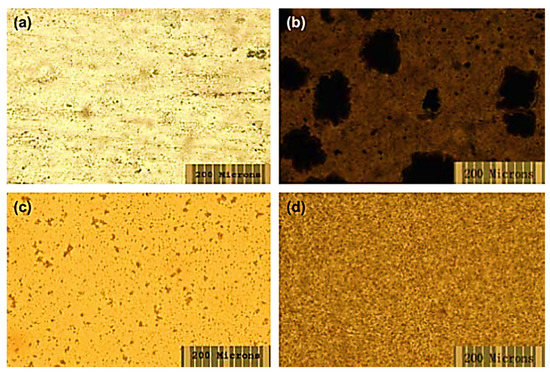
Figure 5.
Optical micrographs of Nafion/C60 fullerene nanocomposites (a) and Nafion/polyhydroxy fullerene nanocomposites (b) by doping and Nafion/C60 fullerene nanocomposites (c) and Nafion/polyhydroxy fullerene nanocomposites (d) by solution casting [176]. Reproduced with permission from Elsevier.
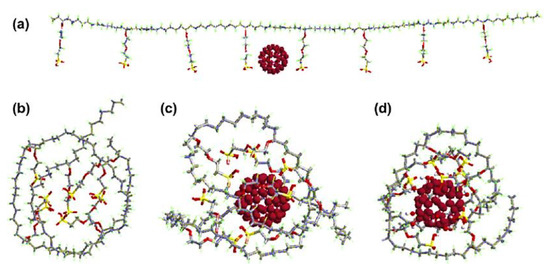
Figure 6.
(a) Initial structure of C60 and Nafion oligomers; (b) snapshots of Nafion oligomers; (c) C60 and Nafion oligomers; and (d) polyhydroxy and Nafion oligomers (taken after 1 ns molecular dynamic simulations) [176]. Reproduced with permission from Elsevier.
5.2. Polysulfone/Fullerene Nanocomposite Membranes in Water Treatment
Polysulfone is a commercial thermoplastic polymer [177]. Polysulfone own fine properties of chemical, thermal, and mechanical stability. Owing to unique processability and physical features, polysulfone has found wide-ranging applications in membranes, coating, nanocomposites, and other technical fields [178]. Penkova et al. [179] fabricated mixed-matrix membranes of polysulfone and fullerene C60 (contents up to 5 wt %). The nanocomposite membrane with a 5 wt % fullerene content revealed fine transport properties towards the pervaporation of an ethyl acetate-water mixture. The sorption and contact angle measurement data for the membranes are given in Table 2. The inclusion of nanofillers enhanced the sorption characteristics. Moreover, the hydrophilic features of the membrane surface were improved with the fullerene addition. The pervaporation mechanism can be explained through the solution-diffusion process [180]. Especially, the permeability is directly related to the solubility and diffusivity of the solute molecules. In this way, the mass transfer of analyte and water through the membranes can be analyzed. However, limited polysulfone/fullerene systems have been studied so far, and thorough research efforts needed to form high-performance membranes in this category.

Table 2.
Sorption characteristics and contact angles of polysulfone and polysulfone/fullerene C60 dense membranes [179]. Reproduced with permission from Springer.
5.3. Polyamide/Fullerene Nanocomposite Membranes towards Water Treatment
Polyamide is a thermoplastic polymer having repeating amide bonds in the backbone. Polyamides occur naturally and can be synthesized using diamine and dicarboxylic acid monomers [181]. Polyamide has been effectively used in membrane applications [182]. Plisko et al. [167] designed a polyamide and hydroxy functional fullerene C60-based thin-film nanocomposite membrane for water treatment. It has been observed that the inclusion of a 5 wt % nanofiller promoted antifouling features and organic matter elimination properties of the membranes. Dmitrenko et al. [166] adopted a polyamide, i.e., polyphenylene isophthalamide filled with 5 wt % fullerene derivatives (carboxyfullerene, polyhydroxylated fullerene and fullerene derivative with L-arginine). In this regard, various pervaporation membranes have been designed as polyphenylene isophthalamide/fullerene (PA/F), polyphenylene isophthalamide/carboxyfullerene (PA/CF), polyphenylene isophthalamide/fullerene derivative with L-arginine (PA/AF), and polyphenylene isophthalamide/polyhydroxylated fullerene (PA/HF). The solid phase synthesis route was used to fabricate mixed matrix pervaporation membranes (Figure 7). The transport properties were studied for the azeotropic methanol-toluene (72/28 wt %) mixture. Figure 8 depicts the permeation flux for the mixed matrix pervaporation, which was increased with the increasing methanol content in feed. Moreover, the inclusion of fullerene derivative to the polymeric membranes enhanced the permeation flux in order of PA < PA/F < PA/CF < PA/AF < PA/HF. Relative to the neat pervaporation membrane, the permeation flux of the nanocomposite membrane was increased by 1.6 times, due to the effect of the inclusion of fullerene nanoparticles. Furthermore, the nanocomposite membranes have enhanced the surface roughness and the surface hydrophilicity properties, relative to the neat polyphenylene isophthalamide membrane. The polyhydroxylated fullerene-based membrane revealed superior performance compared with the other nanocomposite membranes. Consequently, the PA/HF membrane with a 5 wt % nanofiller content increased the permeation flux to 0.084–0.214 kg/(m2∙h) and resulted in the selectivity of 95.9 wt % for methanol. The results were attributed to the affinity of the polyhydroxy functionality of the fullerene molecules permeating methanol. The results were also suggestive of the nanocomposite membrane pore size modification with the addition of altered fullerene derivatives [183]. Comprehensive efforts are still desirable to explore the interaction of the modified fullerene nanoparticles with the polyamide chains actually operating to promote the membrane permeation and selectivity.
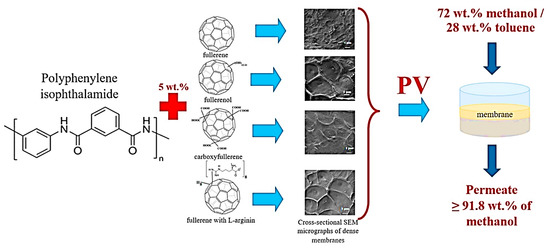
Figure 7.
Graphical development of novel polyphenylene isophthalamide pervaporation (PV) membranes modified with various types of fullerene C60 derivatives [166]. Reproduced with permission from Elsevier.
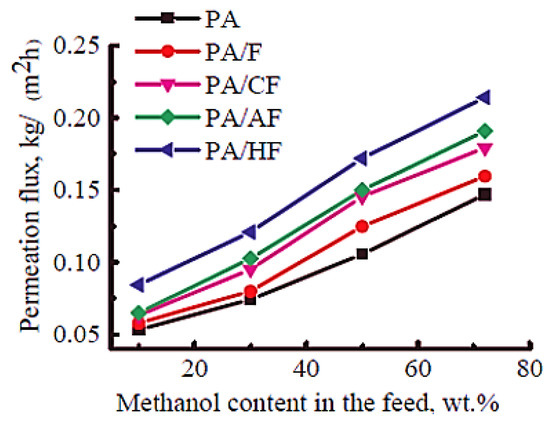
Figure 8.
Dependence of the permeation flux on the methanol content in feed for membranes based on neat PA and nanocomposites with fullerene and fullerene derivatives during pervaporation of methanol-toluene mixtures with 10–72 wt % methanol (22 °C) [166]. PA, polyphenylene isophthalamide; PA/F, polyphenylene isophthalamide/fullerene; PA/CF, polyphenylene isophthalamide/carboxyfullerene; PA/AF, polyphenylene isophthalamide/fullerene derivative with L-arginine; PA/HF, polyphenylene isophthalamide/polyhydroxylated fullerene. Reproduced with permission from Elsevier.
5.4. Polystyrene/Fullerene Nanocomposite Membranes for Water Treatment
Polystyrene is a widely used commercial thermoplastic polymer [184,185]. Polystyrene is usually made from styrene monomer. It is an inexpensive, light-weight, clear, hard, and brittle polymer. Polystyrene has been frequently used to form nanocomposites. Alekseeva et al. [163] formed fullerene C60-filled polystyrene nanocomposite. The ultrafiltration membranes were designed using the phase inversion technique. The static protein sorption tests were carried out for the polystyrene/C60 nanocomposite membranes. With the fullerene nanoparticle loading, protein sorption was reduced along with the flux reduced recovery rates. The result was observed due to better fullerene dispersion and barrier effects in the membranes. Moreover, the Langmuir model was used to study the Cu2+ ions removal efficiency. It was found that the fullerene addition enhanced the membrane affinity towards Cu2+ ions due to better interactions. The fullerene C60 has also been incorporated in polystyrene blend matrices to form water treatment membranes [186,187]. von Reitzenstein et al. [188] performed a comparative study on neat polystyrene, polystyrene/fullerene C60, polystyrene/graphene oxide, and polystyrene/multi-walled carbon nanotubes. The electrospinning technique was used to form nanocomposite nanofibers. Electrospun nanofibers were then used to form nanofiltration membranes. The inclusion of nanofillers in the electrospun polystyrene nanofibers slightly enhanced the diameter, reduced the bead formation and caused the homogeneous surface pore size distribution. Figure 9 displays the surface pore volume distribution of the nanofiber. The mean pore diameters of the neat polymer and polystyrene/graphene oxide was found similar, which were 70–90 nm.
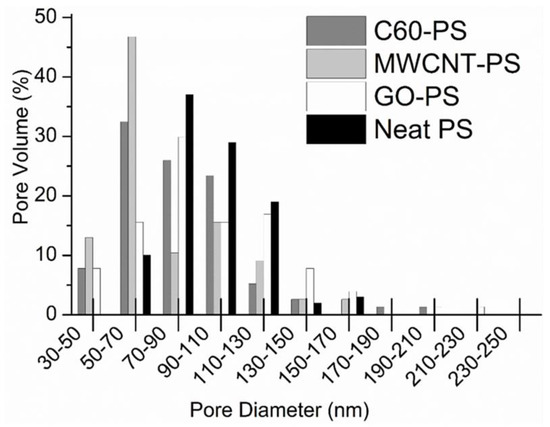
Figure 9.
Distribution of the pore volume measured from scanning electron microscopy of nanofibers [188]. PS, polystyrene; C60-PS, fullerene-polystyrene; MWCNT-PS, multi-walled carbon nanotube-polystyrene; GO-PS, graphene oxide-polystyrene. Reproduced with permission from Elsevier.
On the other hand, the polystyrene/fullerene C60 and the polystyrene/multi-walled carbon nanotube had a reduced mean pore diameter of 50–70 nm. The effect was observed probably due to more stability and less pore formation tendency of fullerene and nanotube-filled nanocomposites. The transmission electron microscopy (TEM) was used to inspect the morphologies of the neat polystyrene nanofiber and the nanocarbon-dispersed nanocomposite nanofiber (Figure 10). The polystyrene/graphene oxide-based nanofiber showed a flaky appearance due to the presence of nanosheets. The nanotube can be observed as tangled threads in the polystyrene/multi-walled carbon nanotube nanocomposites. The polystyrene/fullerene nanofibers were opaque and dense, although few fullerene aggregates can be identified as flaky edges on the fiber surface. The polymer/nanocarbon nanofibers for the filtration membranes must be further investigated for the mechanism of pore creation, pore formation frequency control, and precise nanofiber dimensions [189].
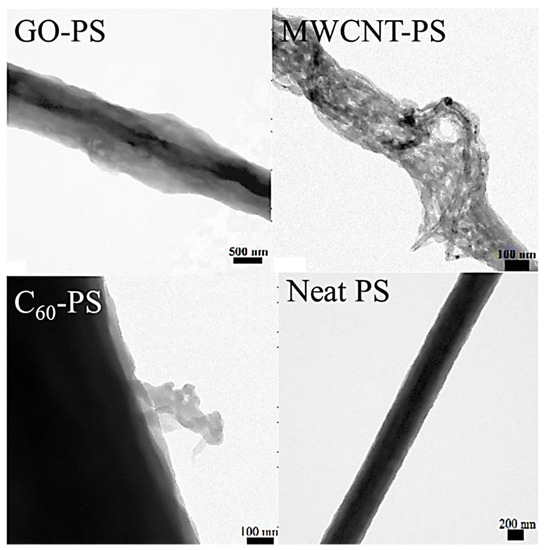
Figure 10.
Transmission electron microscopic images of the neat PS and the nanocomposite nanofiber [188]. PS, polystyrene; C60-PS, fullerene-polystyrene; MWCNT-PS, multi-walled carbon nanotube-polystyrene; GO-PS, graphene oxide-polystyrene. Reproduced with permission from Elsevier.
6. Challenges, Future, and Summary
There is a mammoth necessity of better-quality approaches to the use of fullerenes and derived nanomaterials in high tech industries [190]. Most prominently, fullerene-derived nanomaterials have been absorbed for membranes or coatings [191], electronics [192], energy devices [193], biomedical areas [194], and other fields. The future implication of fullerene-based nanomaterials has been suggested for the aerospace or automotive, civil engineering, and other high-performance applications. In membrane applications, polymer/fullerene nanocomposites have been applied due to remarkable morphological, mechanical, barrier, electrical, and other physical properties [195]. In polymer/fullerene nanocomposite membranes, nanofiller dispersal and matrix-nanofiller interactions have been found desirable to develop high-performance nanostructures. Accordingly, the fullerene dispersion has been identified as an important challenge of these membranes. In this regard, modified fullerene derivatives need to be employed. Moreover, precise controls over the pore size, pore structure, microstructure, membrane surface roughness, membrane wettability, etc. (defining the membrane performance parameters) remain as major encounters [196,197]. Furthermore, comprehensive studies are still desired to explore the mechanism of membrane separation processes. Solution-diffusion processes leading to water filtration need to be explored experimentally and theoretically, to profoundly identify the separation mechanism. Another important challenge is the discovery of additional all-inclusive categories of polymer/fullerene membranes beyond Nafion/fullerene, polyamide/fullerene, polysulfone/fullerene, and polystyrene/fullerene nanocomposite membranes known so far towards water treatment. The above-mentioned challenges can be overcome by using modified fullerene nanoparticles to attain promising forthcoming membrane nanomaterials. For future membrane utility, the self-assembly of fullerene molecules and network formation with polymeric matrices must be considered [198,199,200].
Consequently, polymer/fullerene nanocomposites capture a special position as separation membranes in the water treatment industry. These membranes have attained increasing research attention due to performance advantages, low operating expenses, high selectivity, and easy scale-up. In fact, the primary function of fullerene has been found as physical/chemical binders within the polymeric chains of nanocomposite membranes for optimizing essential mechanical properties, structural stability, thermal stability, and membrane properties. Certainly, inclusion of fullerene in the matrix creates van der Waals forces and/or covalent interactions with the polymer chains, leading to reinforcement and superior physical features. Moreover, the variations in fullerene loading levels cause dominant effects on the reinforcement and membrane properties. Subsequently, polymer/fullerene nanocomposites have been found as fast emerging separation membranes for clean water resources. Among all available polymer/nanocarbon membranes, polymer/fullerene nanocomposites have been considered as the energy-efficient and green technology for separation of impurities from aqueous solutions due to optimum nanoporous membrane features. Recently, polymer/fullerene nanocomposite membranes have been considered significant for resolving technical and commercial challenges towards separation and purification technologies. The development of distinct polymer/fullerene nanostructured membranes with exclusive properties not only solves the trade-off issue related to water treatment technologies, but also opens new pathways towards real-time applications. The newly fabricated polymer/fullerene membranes have been found superior in terms of selectivity, permeability, and long-term stability. Thus, the research on these novel nanocomposite membranes may continue to develop better cost-effective water treatment systems to decrease the overall capital investment.
In short, this cutting-edge overview grants an analysis on the use of polymer/fullerene C60 nanocomposite membranes for water remediation. Fullerene and modified fullerene nanofillers have been incorporated in polymers to develop nanocomposite membranes. The enhanced polymer/fullerene nanocomposite properties lead to several prospects to revolutionize the related potential for waste water purification. Plentiful research efforts have been fixated on refining the polymer/fullerene nanocomposite membrane properties such as water permeability, salt rejection, ion separation, overall separation efficiency, antifouling performance, and other membrane topographies. Moreover, fullerene-based nanofillers improve the membrane surface properties, have low cost, and enhance the long-term stability of the membranes. To widen the use of polymer/fullerene in membranes, devotion must be taken to augment polymer-nanocarbon interactions, nanofiller dispersibility, membrane stability, membrane permeability, separation competence, and practicable membrane parameters to solve current glitches in this field.
Author Contributions
Conceptualization, A.K.; data curation, A.K.; writing of the original draft preparation, A.K.; review and editing, A.K., I.A., M.M. and M.H.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dmitrenko, M.; Chepeleva, A.; Liamin, V.; Kuzminova, A.; Mazur, A.; Semenov, K.; Penkova, A. Novel PDMS-b-PPO Membranes Modified with Graphene Oxide for Efficient Pervaporation Ethanol Dehydration. Membranes 2022, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Gas separation and filtration membrane applications of polymer/graphene nanocomposites. In Graphene to Polymer/Graphene Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 197–222. [Google Scholar] [CrossRef]
- Yue, C.; Chen, Y.; Zhang, W.; Zheng, Y.; Hu, X.; Shang, B. Direct Purification of Digestate Using Polymeric Ultrafiltration Membranes: Influence of Materials on Filtration Behavior and Fouling Characteristics. Membranes 2022, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.L.; Chen, Y.-S.; Juang, R.-S. Fouling Analysis in One-Stage Ultrafiltration of Precipitation-Treated Bacillus subtilis Fermentation Liquors for Biosurfactant Recovery. Membranes 2022, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Aumesquet-Carreto, M.; Ortega-Delgado, B.; García-Rodríguez, L. Opportunities of Reducing the Energy Consumption of Seawater Reverse Osmosis Desalination by Exploiting Salinity Gradients. Membranes 2022, 12, 1045. [Google Scholar] [CrossRef]
- Yang, D.; Cho, Y.; Kang, H. Effects of the Structure of Benzenesulfonate-Based Draw Solutes on the Forward Osmosis Process. Membranes 2022, 12, 1067. [Google Scholar] [CrossRef]
- Naseer, M.N.; Dutta, K.; Zaidi, A.A.; Asif, M.; Alqahtany, A.; Aldossary, N.A.; Jamil, R.; Alyami, S.H.; Jaafar, J. Research Trends in the Use of Polyaniline Membrane for Water Treatment Applications: A Scientometric Analysis. Membranes 2022, 12, 777. [Google Scholar] [CrossRef]
- Bardhan, A.; Subbiah, S.; Mohanty, K.; Ibrar, I.; Altaee, A. Feasibility of Poly (Vinyl Alcohol)/Poly (Diallyldimethylammonium Chloride) Polymeric Network Hydrogel as Draw Solute for Forward Osmosis Process. Membranes 2022, 12, 1097. [Google Scholar] [CrossRef]
- Hallinan, D.T., Jr.; Minelli, M.; Oparaji, O.; Sardano, A.; Iyiola, O.; Garcia, A.R.; Burnett, D.J. Effect of Polystyrene Synthesis Method on Water Sorption and Glass Transition. Membranes 2022, 12, 1059. [Google Scholar] [CrossRef]
- Lu, X.; Elimelech, M. Fabrication of desalination membranes by interfacial polymerization: History, current efforts, and future directions. Chem. Soc. Rev. 2021, 50, 6290–6307. [Google Scholar] [CrossRef]
- Ng, Z.; Lau, W.; Matsuura, T.; Ismail, A. Thin film nanocomposite RO membranes: Review on fabrication techniques and impacts of nanofiller characteristics on membrane properties. Chem. Eng. Res. Des. 2020, 165, 81–105. [Google Scholar] [CrossRef]
- Subaer, S.; Fansuri, H.; Haris, A.; Misdayanti, M.; Ramadhan, I.; Wibawa, T.; Putri, Y.; Ismayanti, H.; Setiawan, A. Pervaporation Membranes for Seawater Desalination Based on Geo–rGO–TiO2 Nanocomposites: Part 2—Membranes Performances. Membranes 2022, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Q.; Akbarzadeh, R.; Gharbia, S.S.; Ndungu, P.G. Design of Multi-Layer Graphene Membrane with Descending Pore Size for 100% Water Desalination by Simulation Using ReaxFF. Membranes 2022, 12, 1038. [Google Scholar] [CrossRef]
- Kononova, S.V.; Gubanova, G.N.; Korytkova, E.N.; Sapegin, D.A.; Setnickova, K.; Petrychkovych, R.; Uchytil, P. Polymer nanocomposite membranes. Appl. Sci. 2018, 8, 1181. [Google Scholar] [CrossRef]
- Dmitrieva, E.; Grushevenko, E.; Razlataya, D.; Golubev, G.; Rokhmanka, T.; Anokhina, T.; Bazhenov, S. Alginate Ag for Composite Hollow Fiber Membrane: Formation and Ethylene/Ethane Gas Mixture Separation. Membranes 2022, 12, 1090. [Google Scholar] [CrossRef]
- Eremin, Y.; Grekhov, A.; Belogorlov, A. Percolation Effects in Mixed Matrix Membranes with Embedded Carbon Nanotubes. Membranes 2022, 12, 1100. [Google Scholar] [CrossRef]
- Kausar, A.; Bocchetta, P. Polymer/Graphene Nanocomposite Membranes: Status and Emerging Prospects. J. Compos. Sci. 2022, 6, 76. [Google Scholar] [CrossRef]
- Kausar, A. Polymeric Nanofibers as Electrodes for Fuel Cells. In Organic Electrodes; Springer: Berlin/Heidelberg, Germany, 2022; pp. 155–169. [Google Scholar]
- Teow, Y.H.; Ooi, B.S.; Ahmad, A.L.; Lim, J.K. Investigation of Anti-fouling and UV-Cleaning Properties of PVDF/TiO2 Mixed-Matrix Membrane for Humic Acid Removal. Membranes 2020, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Samree, K.; Srithai, P.-U.; Kotchaplai, P.; Thuptimdang, P.; Painmanakul, P.; Hunsom, M.; Sairiam, S. Enhancing the Antibacterial Properties of PVDF Membrane by Hydrophilic Surface Modification Using Titanium Dioxide and Silver Nanoparticles. Membranes 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-E.; Zhu, W.-W.; Xu, S.-J.; Xu, Z.-L.; Shen, Q.; Sun, W.-G.; Wu, Q.; Zheng, X.-P. A PVDF/PVB composite UF membrane improved by F-127-wrapped fullerene for protein waste-water separation. RSC Adv. 2016, 6, 83510–83519. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Kuzminova, A.I.; Zolotarev, A.A.; Korniak, A.S.; Ermakov, S.S.; Su, R.; Penkova, A.V. Novel mixed matrix membranes based on polyelectrolyte complex modified with fullerene derivatives for enhanced pervaporation and nanofiltration. Sep. Purif. Technol. 2022, 298, 121649. [Google Scholar] [CrossRef]
- Jehoulet, C.; Obeng, Y.S.; Kim, Y.T.; Zhou, F.; Bard, A.J. Electrochemistry and Langmuir trough studies of fullerene C60 and C70 films. J. Am. Chem. Soc. 1992, 114, 4237–4247. [Google Scholar] [CrossRef]
- Chang, C.-W. Electrical and Thermal Transport Measurements on Nano-Structured Materials. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2006. [Google Scholar]
- Radford, C.L.; Mudiyanselage, P.D.; Stevens, A.L.; Kelly, T.L. Heteroatoms as Rotational Blocking Groups for Non-Fullerene Acceptors in Indoor Organic Solar Cells. ACS Energy Lett. 2022, 7, 1635–1641. [Google Scholar] [CrossRef]
- Tian, C.; Sun, C.; Chen, J.; Song, P.; Hou, E.; Xu, P.; Liang, Y.; Yang, P.; Luo, J.; Xie, L.; et al. Fullerene Derivative with Flexible Alkyl Chain for Efficient Tin-Based Perovskite Solar Cells. Nanomaterials 2022, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Epitome of Fullerene in Conducting Polymeric Nanocomposite—Fundamentals and Beyond. Polym.-Plast. Technol. Mater. 2022, 61, 1–14. [Google Scholar] [CrossRef]
- Lopez, A.M.; Mateo-Alonso, A.; Prato, M. Materials chemistry of fullerene C 60 derivatives. J. Mater. Chem. 2011, 21, 1305–1318. [Google Scholar] [CrossRef]
- Blanter, M.S.; Borisova, P.A.; Brazhkin, V.V.; Lyapin, S.G.; Sviridova, T.A.; Filonenko, V.P.; Kondratev, O.A. The influence of metals on the phase transformations of fullerenes at high pressure and high temperatures. Mater. Lett. 2022, 318, 132199. [Google Scholar] [CrossRef]
- Giacalone, F.; Martin, N. Fullerene polymers: Synthesis and properties. Chem. Rev. 2006, 106, 5136–5190. [Google Scholar] [CrossRef]
- Akasaka, T.; Wakahara, T.; Nagase, S.; Kobayashi, K.; Waelchli, M.; Yamamoto, K.; Kondo, M.; Shirakura, S.; Maeda, Y.; Kato, T. Structural determination of the La@ C82 isomer. J. Phys. Chem. B 2001, 105, 2971–2974. [Google Scholar] [CrossRef]
- Withers, J.C.; Loutfy, R.O.; Lowe, T.P. Fullerene commercial vision. Fuller. Nanotub. Carbon Nanostruct. 1997, 5, 1–31. [Google Scholar] [CrossRef]
- Mojica, M.; Alonso, J.A.; Méndez, F. Synthesis of fullerenes. J. Phys. Org. Chem. 2013, 26, 526–539. [Google Scholar] [CrossRef]
- Das, D.; Rahaman, H. Carbon Nanotube and Graphene Nanoribbon Interconnects; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Biswas, C.; Lee, Y.H. Graphene Versus Carbon Nanotubes in Electronic Devices. Adv. Funct. Mater. 2011, 21, 3806–3826. [Google Scholar] [CrossRef]
- Yamashita, S. A Tutorial on Nonlinear Photonic Applications of Carbon Nanotube and Graphene. J. Light. Technol. 2011, 30, 427–447. [Google Scholar] [CrossRef]
- Song, N.; Jiao, D.; Cui, S.; Hou, X.; Ding, P.; Shi, L. Highly Anisotropic Thermal Conductivity of Layer-by-Layer Assembled Nanofibrillated Cellulose/Graphene Nanosheets Hybrid Films for Thermal Management. ACS Appl. Mater. Interfaces 2017, 9, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Boysen, R.I.; Schwarz, L.J.; Nicolau, D.V.; Hearn, M.T.W. Molecularly imprinted polymer membranes and thin films for the separation and sensing of biomacromolecules. J. Sep. Sci. 2016, 40, 314–335. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Aghamohammadi, M.; Himmelberger, S.; Sonar, P.; Garriga, M.; Salleo, A.; Campoy-Quiles, M. One-Step Macroscopic Alignment of Conjugated Polymer Systems by Epitaxial Crystallization during Spin-Coating. Adv. Funct. Mater. 2013, 23, 2368–2377. [Google Scholar] [CrossRef]
- Abdelghani, R.; Hassan, H.S.; Morsi, I.; Kashyout, A. Nano-architecture of highly sensitive SnO2–based gas sensors for acetone and ammonia using molecular imprinting technique. Sens. Actuators B Chem. 2019, 297, 126668. [Google Scholar] [CrossRef]
- Shen, Y.; Nakanishi, T. Fullerene assemblies toward photo-energy conversions. Phys. Chem. Chem. Phys. 2014, 16, 7199–7204. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Möhwald, H.; Nakanishi, T. Recent progress in morphology control of supramolecular fullerene assemblies and its applications. Chem. Soc. Rev. 2010, 39, 4021–4035. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef]
- Yavarinasab, A.; Janfaza, S.; Tasnim, N.; Tahmooressi, H.; Dalili, A.; Hoorfar, M. Graphene/poly (methyl methacrylate) electrochemical impedance-transduced chemiresistor for detection of volatile organic compounds in aqueous medium. Anal. Chim. Acta 2020, 1109, 27–36. [Google Scholar] [CrossRef]
- Kausar, A. Fullerene nanofiller reinforced epoxy nanocomposites—Developments, progress and challenges. Mater. Res. Innov. 2021, 25, 175–185. [Google Scholar] [CrossRef]
- Nierengarten, J.-F. Chemical modification of C 60 for materials science applications. New J. Chem. 2004, 28, 1177–1191. [Google Scholar] [CrossRef]
- Dmitruk, N.; Borkovskaya, O.Y.; Havrylenko, T.; Naumenko, D.; Petrik, P.; Meza-Laguna, V.; Basiuk, E. Effect of chemical modification of thin C60 fullerene films on the fundamental absorption edge. Semicond. Phys. Quantum Electron. Optoelectron. 2010, 13, 180–185. [Google Scholar] [CrossRef]
- Wang, W.; Hanindita, F.; Hamamoto, Y.; Li, Y.; Ito, S. Fully conjugated azacorannulene dimer as large diaza [80] fullerene fragment. Nat. Commun. 2022, 13, 1498. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Strongly optical absorptive nanofluids and rheology in bonded fullerene C60 via poly(vinyl pyrrolidone) molecules in water. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 143–150. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Variation of optical properties, rheology, and microstructure in fullerene/poly (vinyl pyrrolidone) nanofluids with fullerene content in n-butanol. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 154–161. [Google Scholar] [CrossRef]
- Baskar, A.V.; Benzigar, M.R.; Talapaneni, S.N.; Singh, G.; Karakoti, A.S.; Yi, J.; Al-Muhtaseb, A.a.H.; Ariga, K.; Ajayan, P.M.; Vinu, A. Self-Assembled Fullerene Nanostructures: Synthesis and Applications. Adv. Funct. Mater. 2022, 32, 2106924. [Google Scholar] [CrossRef]
- Qi, F.; Jones, L.O.; Jiang, K.; Jang, S.-H.; Kaminsky, W.; Oh, J.; Zhang, H.; Cai, Z.; Yang, C.; Kohlstedt, K.L.; et al. Regiospecific N-alkyl substitution tunes the molecular packing of high-performance non-fullerene acceptors. Mater. Horiz. 2021, 9, 403–410. [Google Scholar] [CrossRef]
- Abbas, F.; Ali, U.; Ahmad, H.M.R.; Tallat, A.; Shehzad, A.; Zeb, Z.; Hussain, I.; Saeed, A.; Tariq, M. Role of Iodo-Substituted Subphthalocyanine (Subpcs) π-conjugated aromatic N-fused di-Iminoisonidole units on the performance of non-fullerene small organic solar cells. Comput. Theor. Chem. 2021, 1207, 113508. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Durantini, J.E.; Durantini, E.N. Fullerene C60 derivatives as antimicrobial photodynamic agents. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100471. [Google Scholar] [CrossRef]
- Chae, S.-R.; Therezien, M.; Budarz, J.F.; Wessel, L.; Lin, S.; Xiao, Y.; Wiesner, M.R. Comparison of the photosensitivity and bacterial toxicity of spherical and tubular fullerenes of variable aggregate size. J. Nanopart. Res. 2011, 13, 5121–5127. [Google Scholar] [CrossRef]
- Modi, A.; Koratkar, N.; Lass, E.; Wei, B.; Ajayan, P.M. Miniaturized gas ionization sensors using carbon nanotubes. Nature 2003, 424, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Favila, A.; Glossman-Mitnik, D. DFT studies of functionalized carbon nanotubes and fullerenes as nanovectors for drug delivery of antitubercular compounds. Chem. Phys. Lett. 2007, 447, 105–109. [Google Scholar] [CrossRef]
- Kausar, A. Advances in Condensation Polymer Containing zero-dimensional Nanocarbon reinforcement—Fullerene, Carbon nanoonion, and Nanodiamond. Polym.-Plast. Technol. Mater. 2021, 60, 695–713. [Google Scholar]
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrdjanovic, J. Review of Synthesis and Antioxidant Potential of Fullerenol Nanoparticles. J. Nanomater. 2015, 16, 280. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef]
- Caetano, A.; Drioli, E.; de Pinho, M.; Muntau, H. Membrane Technology: Applications to Industrial Wastewater Treatment: Applications to Industrial Wastewater Treatment; Springer Science & Business Media: Berlin, Germany, 1995. [Google Scholar]
- Nunes, S.P.; Peinemann, K.-V. Membrane Technology: In the Chemical Industry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Molinari, R.; Palmisano, L.; Drioli, E.; Schiavello, M. Studies on various reactor configurations for coupling photocatalysis and membrane processes in water purification. J. Membr. Sci. 2002, 206, 399–415. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Ho-Bum, P. Separation Membrane Including Graphene. Patent EP2511002B1, 14 March 2015. [Google Scholar]
- Smith, Z.P.; Freeman, B.D. Graphene Oxide: A New Platform for High-Performance Gas- and Liquid-Separation Membranes. Angew. Chem. Int. Ed. 2014, 53, 10286–10288. [Google Scholar] [CrossRef]
- Celebi, K.; Buchheim, J.; Wyss, R.M.; Droudian, A.; Gasser, P.; Shorubalko, I.; Kye, J.-I.; Lee, C.; Park, H.G. Ultimate Permeation Across Atomically Thin Porous Graphene. Science 2014, 344, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Adeola, A.O.; Nomngongo, P.N. Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective. Polymers 2022, 14, 2462. [Google Scholar] [CrossRef] [PubMed]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Trieb, F.; Müller-Steinhagen, H. Concentrating solar power for seawater desalination in the Middle East and North Africa. Desalination 2008, 220, 165–183. [Google Scholar] [CrossRef]
- Linares, R.V.; Li, Z.; Sarp, S.; Bucs, S.; Amy, G.; Vrouwenvelder, J. Forward osmosis niches in seawater desalination and wastewater reuse. Water Res. 2014, 66, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Q.; Deng, F.; Huang, H.; Wan, Q.; Liu, M.; Wei, Y. Mussel-inspired fabrication of functional materials and their environmental applications: Progress and prospects. Appl. Mater. Today 2017, 7, 222–238. [Google Scholar] [CrossRef]
- Zuo, K.; Chang, J.; Liu, F.; Zhang, X.; Liang, P.; Huang, X. Enhanced organics removal and partial desalination of high strength industrial wastewater with a multi-stage microbial desalination cell. Desalination 2017, 423, 104–110. [Google Scholar] [CrossRef]
- Miller, S.; Shemer, H.; Semiat, R. Energy and environmental issues in desalination. Desalination 2015, 366, 2–8. [Google Scholar] [CrossRef]
- Lau, W.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef]
- Saraswathi, M.S.S.A.; Nagendran, A.; Rana, D. Tailored polymer nanocomposite membranes based on carbon, metal oxide and silicon nanomaterials: A review. J. Mater. Chem. A 2019, 7, 8723–8745. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, L.; Uliana, A.; Hou, J.; Wang, J.; Zhang, Y.; Li, X.; Yuan, S.; Li, J.; Tian, M.; et al. Elevated Performance of Thin Film Nanocomposite Membranes Enabled by Modified Hydrophilic MOFs for Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, J.; Wang, Z.; Shi, F.; Liu, Q. Enhanced Separation Performance of PVDF/PVP-g-MMT Nanocomposite Ultrafiltration Membrane Based on the NVP-Grafted Polymerization Modification of Montmorillonite (MMT). Langmuir 2012, 28, 4776–4786. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.; Harami, H.R.; Rezakazemi, M. Accurate prediction of solubility of gases within H 2 -selective nanocomposite membranes using committee machine intelligent system. Int. J. Hydrogen Energy 2018, 43, 6614–6624. [Google Scholar] [CrossRef]
- He, W.; Liu, P.; Jiang, J.; Liu, M.; Li, H.; Zhang, J.; Luo, Y.; Cheung, H.-Y.; Yao, X. Development of multifunctional liquid-infused materials by printing assisted functionalization on porous nanocomposites. J. Mater. Chem. A 2018, 6, 4199–4208. [Google Scholar] [CrossRef]
- Díaz, U.; Corma, A. Ordered covalent organic frameworks, COFs and PAFs. From preparation to application. Coord. Chem. Rev. 2016, 311, 85–124. [Google Scholar] [CrossRef]
- He, Y.; Zhuang, X.; Lei, C.; Lei, L.; Hou, Y.; Mai, Y.; Feng, X. Porous carbon nanosheets: Synthetic strategies and electrochemical energy related applications. Nano Today 2019, 24, 103–119. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Nakhjiri, A.T.; Ghadiri, M.; Marjani, A.; Heydarinasab, A.; Shirazian, S. Computational fluid dynamics simulation of NO2 molecular sequestration from a gaseous stream using NaOH liquid absorbent through porous membrane contactors. J. Mol. Liq. 2020, 313, 113584. [Google Scholar] [CrossRef]
- Ye, X.; Cui, Y.; Ke, L.; Gao, K.; Huang, X.; Shi, B. Fabrication of 3D porous superhydrophobic sponges using plant polyphenol-Fe3+ complexes as adhesive and their applications in oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 9–16. [Google Scholar] [CrossRef]
- Ee, S.L.; Duan, X.; Liew, J.; Nguyen, Q.D. Droplet size and stability of nano-emulsions produced by the temperature phase inversion method. Chem. Eng. J. 2008, 140, 626–631. [Google Scholar] [CrossRef]
- Fernandez, P.; André, V.; Rieger, J.; Kühnle, A. Nano-emulsion formation by emulsion phase inversion. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 53–58. [Google Scholar] [CrossRef]
- Yang, C.; Jin, C.; Chen, F. Micro-tubular solid oxide fuel cells fabricated by phase-inversion method. Electrochem. Commun. 2010, 12, 657–660. [Google Scholar] [CrossRef]
- Jin, C.; Yang, C.; Chen, F. Effects on microstructure of NiO–YSZ anode support fabricated by phase-inversion method. J. Membr. Sci. 2010, 363, 250–255. [Google Scholar] [CrossRef]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, J.C.; Feng, J.; Garcia-Celma, M.J.; Azemar, A.N.; Solans, C. Phase Behavior and Nano-emulsion Formation by the Phase Inversion Temperature Method. Langmuir 2004, 20, 6594–6598. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Shariaty-Niassar, M.; Matsuura, T.; Ismail, A.F. Janus graphene oxide nanosheet: A promising additive for enhancement of polymeric membranes performance prepared via phase inversion. J. Colloid Interface Sci. 2018, 527, 10–24. [Google Scholar] [CrossRef]
- Baldino, L.; Sarno, M.; Cardea, S.; Irusta, S.; Ciambelli, P.; Santamaria, J.; Reverchon, E. Formation of cellulose acetate–graphene oxide nanocomposites by supercritical CO2 assisted phase inversion. Ind. Eng. Chem. Res. 2015, 54, 8147–8156. [Google Scholar] [CrossRef]
- Wang, X.; Feng, M.; Liu, Y.; Deng, H.; Lu, J. Fabrication of graphene oxide blended polyethersulfone membranes via phase inversion assisted by electric field for improved separation and antifouling performance. J. Membr. Sci. 2019, 577, 41–50. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.; Mohamma, A.; Arabi, M.A. A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modelling, and atomic force microscopy. Desalination 2004, 170, 281–308. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef]
- Fan, L.; Harris, J.L.; Roddick, F.A.; A Booker, N. Influence of the characteristics of natural organic matter on the fouling of microfiltration membranes. Water Res. 2001, 35, 4455–4463. [Google Scholar] [CrossRef]
- Holmes, D.R.; Bunn, C.W.; Smith, D.J. The crystal structure of polycaproamide: Nylon 6. J. Polym. Sci. 1955, 17, 159–177. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Cho, J.; Paul, D. Nylon 6 nanocomposites by melt compounding. Polymer 2001, 42, 1083–1094. [Google Scholar] [CrossRef]
- Newman, B.; Chen, P.; Pae, K.; Scheinbeim, J. Piezoelectricity in nylon 11. J. Appl. Phys. 1980, 51, 5161–5164. [Google Scholar] [CrossRef]
- Dobo, E.J.; Kim, D.W.; Mallonee, W.C. Process for Producing a Nylon Non-Woven Fabric. Patent US4187343A, 5 February 1980. [Google Scholar]
- Noshay, A.; Robeson, L. Sulfonated polysulfone. J. Appl. Polym. Sci. 1976, 20, 1885–1903. [Google Scholar] [CrossRef]
- Park, J.Y.; Acar, M.H.; Akthakul, A.; Kuhlman, W.; Mayes, A.M. Polysulfone-graft-poly(ethylene glycol) graft copolymers for surface modification of polysulfone membranes. Biomaterials 2006, 27, 856–865. [Google Scholar] [CrossRef]
- Erb, A.; Paul, D. Gas sorption and transport in polysulfone. J. Membr. Sci. 1981, 8, 11–22. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, Y.; Dong, C.; Sheng, J. Morphology of ultrafine polysulfone fibers prepared by electrospinning. Polym. Int. 2004, 53, 1704–1710. [Google Scholar] [CrossRef]
- Rivaton, A.; Gardette, J.L. Photodegradation of polyethersulfone and polysulfone. Polym. Degrad. Stab. 1999, 66, 385–403. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Sreekumar, T.; Kumar, S.; Moore, V.C.; Hauge, R.H.; Smalley, R.E. Poly (vinyl alcohol)/SWNT composite film. Nano Lett. 2003, 3, 1285–1288. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced mechanical properties of graphene-based poly (vinyl alcohol) composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Strawhecker, K.; Manias, E. Structure and properties of poly (vinyl alcohol)/Na+ montmorillonite nanocomposites. Chem. Mater. 2000, 12, 2943–2949. [Google Scholar] [CrossRef]
- Liu, P.; Gong, K.; Xiao, P.; Xiao, M. Preparation and characterization of poly(vinyl acetate)-intercalated graphite oxide nanocomposite. J. Mater. Chem. 2000, 10, 933–935. [Google Scholar] [CrossRef]
- Gajria, A.M.; Davé, V.; Gross, R.A.; McCarthy, S.P. Miscibility and biodegradability of blends of poly(lactic acid) and poly(vinyl acetate). Polymer 1996, 37, 437–444. [Google Scholar] [CrossRef]
- Plazek, D.J. The Temperature Dependence of the Viscoelastic Behavior of Poly(vinyl acetate). Polym. J. 1980, 12, 43–53. [Google Scholar] [CrossRef]
- Sato, Y.; Takikawa, T.; Takishima, S.; Masuoka, H. Solubilities and diffusion coefficients of carbon dioxide in poly(vinyl acetate) and polystyrene. J. Supercrit. Fluids 2001, 19, 187–198. [Google Scholar] [CrossRef]
- McKinney, J.E.; Goldstein, M. PVT relationships for liquid and glassy poly(vinyl acetate). J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1974, 78A, 331–353. [Google Scholar] [CrossRef]
- Ammar, A.; Al-Enizi, A.M.; AlMaadeed, M.A.; Karim, A. Influence of graphene oxide on mechanical, morphological, barrier, and electrical properties of polymer membranes. Arab. J. Chem. 2016, 9, 274–286. [Google Scholar] [CrossRef]
- Barick, A.K.; Tripathy, D.K. Effect of organoclay on the morphology, mechanical, thermal, and rheological properties of organophilic montmorillonite nanoclay based thermoplastic polyurethane nanocomposites prepared by melt blending. Polym. Eng. Sci. 2010, 50, 484–498. [Google Scholar] [CrossRef]
- Pírková, M.; Brus, J.; Brožová, L.; Strachota, A.; Baldrian, J.; Urbanová, M.; Kotek, J.; Strachotová, B.; Šlouf, M. A view from inside onto the surface of self-assembled nanocomposite coatings. Prog. Org. Coat. 2008, 61, 145–155. [Google Scholar] [CrossRef]
- McDaniel, P.B.; Deitzel, J.M.; Gillespie, J.W., Jr. Structural hierarchy and surface morphology of highly drawn ultra high molecular weight polyethylene fibers studied by atomic force microscopy and wide angle X-ray diffraction. Polymer 2015, 69, 148–158. [Google Scholar] [CrossRef]
- Karino, T.; Ikeda, Y.; Yasuda, Y.; Kohjiya, S.; Shibayama, M. Nonuniformity in natural rubber as revealed by small-angle neutron scattering, small-angle X-ray scattering, and atomic force microscopy. Biomacromolecules 2007, 8, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Majdan, M.; Maryuk, O.; Pikus, S.; Olszewska, E.; Kwiatkowski, R.; Skrzypek, H. Equilibrium, FTIR, scanning electron microscopy and small wide angle X-ray scattering studies of chromates adsorption on modified bentonite. J. Mol. Struct. 2005, 740, 203–211. [Google Scholar] [CrossRef]
- Ayyaru, S.; Ahn, Y.-H. Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J. Membr. Sci. 2017, 525, 210–219. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Blanco, J.-F.; Sublet, J.; Nguyen, Q.T.; Schaetzel, P. Formation and morphology studies of different polysulfones-based membranes made by wet phase inversion process. J. Membr. Sci. 2006, 283, 27–37. [Google Scholar] [CrossRef]
- Itta, A.K.; Tseng, H.-H.; Wey, M.-Y. Effect of dry/wet-phase inversion method on fabricating polyetherimide-derived CMS membrane for H2/N2 separation. Int. J. Hydrogen Energy 2010, 35, 1650–1658. [Google Scholar] [CrossRef]
- Asghar, M.R.; Zhang, Y.; Wu, A.; Yan, X.; Shen, S.; Ke, C.; Zhang, J. Preparation of microporous Cellulose/Poly(vinylidene fluoride-hexafluoropropylene) membrane for lithium ion batteries by phase inversion method. J. Power Sources 2018, 379, 197–205. [Google Scholar] [CrossRef]
- Sizov, V.E.; Kondratenko, M.S.; Gallyamov, M.O.; Stevenson, K.J. Advanced porous polybenzimidazole membranes for vanadium redox batteries synthesized via a supercritical phase-inversion method. J. Supercrit. Fluids 2018, 137, 111–117. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.; Abdullah, M.; Ng, B. Preparation of polyvinylidene fluoride hollow fiber membranes for CO2 absorption using phase-inversion promoter additives. J. Membr. Sci. 2010, 355, 200–207. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Kausar, A. Advances in Polymer/Fullerene Nanocomposite: A Review on Essential Features and Applications. Polym. Technol. Eng. 2016, 56, 594–605. [Google Scholar] [CrossRef]
- Etxebarria, I.; Ajuria, J.; Pacios, R. Polymer: Fullerene solar cells: Materials, processing issues, and cell layouts to reach power conversion efficiency over 10%, a review. J. Photonics Energy 2015, 5, 057214. [Google Scholar] [CrossRef]
- Harris, P.J. Fullerene polymers: A brief review. C 2020, 6, 71. [Google Scholar] [CrossRef]
- Mackay, M.E.; Tuteja, A.; Duxbury, P.M.; Hawker, C.J.; Van Horn, B.; Guan, Z.; Chen, G.; Krishnan, R.S. General Strategies for Nanoparticle Dispersion. Science 2006, 311, 1740–1743. [Google Scholar] [CrossRef]
- Bartelt, J.A.; Douglas, J.D.; Mateker, W.R.; El Labban, A.; Tassone, C.J.; Toney, M.F.; Fréchet, J.M.J.; Beaujuge, P.M.; McGehee, M.D. Controlling Solution-Phase Polymer Aggregation with Molecular Weight and Solvent Additives to Optimize Polymer-Fullerene Bulk Heterojunction Solar Cells. Adv. Energy Mater. 2014, 4, 1301733. [Google Scholar] [CrossRef]
- Song, S.; Hill, R.; Choi, K.; Wojciechowski, K.; Barlow, S.; Leisen, J.; Snaith, H.J.; Marder, S.R.; Park, T. Surface modified fullerene electron transport layers for stable and reproducible flexible perovskite solar cells. Nano Energy 2018, 49, 324–332. [Google Scholar] [CrossRef]
- Tabata, Y.; Murakami, Y.; Ikada, Y. Antitumor Effect of Poly(Ethylene Glycol)-Modified Fullerene. Fuller. Sci. Technol. 1997, 5, 989–1007. [Google Scholar] [CrossRef]
- Tumbleston, J.R.; Yang, L.; You, W.; Ade, H. Morphology linked to miscibility in highly amorphous semi-conducting polymer/fullerene blends. Polymer 2014, 55, 4884–4889. [Google Scholar] [CrossRef]
- Kausar, A. Cutting-edge Shape Memory Polymer/Fullerene Nanocomposite: Design and Contemporary Status. Polym.-Plast. Technol. Mater. 2022, 61, 1–14. [Google Scholar] [CrossRef]
- McCamey, D.; Seipel, H.A.; Paik, S.-Y.; Walter, M.J.; Borys, N.J.; Lupton, J.M.; Boehme, C. Spin Rabi flopping in the photocurrent of a polymer light-emitting diode. Nat. Mater. 2008, 7, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Anas, M.; Ebrahim, S.; Soliman, M.; Abou-Aly, A. Polyaniline/fullerene derivative nanocomposite for highly efficient supercapacitor electrode. Int. J. Hydrogen Energy 2020, 45, 16254–16265. [Google Scholar] [CrossRef]
- Campoy-Quiles, M.; Ferenczi, T.A.M.; Agostinelli, T.; Etchegoin, P.G.; Kim, Y.; Anthopoulos, T.D.; Stavrinou, P.N.; Bradley, D.D.C.; Nelson, J. Morphology evolution via self-organization and lateral and vertical diffusion in polymer: Fullerene solar cell blends. Nat. Mater. 2008, 7, 158–164. [Google Scholar] [CrossRef]
- Jani, M.; Arcos-Pareja, J.A.; Ni, M. Engineered Zero-Dimensional Fullerene/Carbon Dots-Polymer Based Nanocomposite Membranes for Wastewater Treatment. Molecules 2020, 25, 4934. [Google Scholar] [CrossRef]
- Abbo, H.S.; Gupta, K.C.; Khaligh, N.G.; Titinchi, S.J.J. Carbon Nanomaterials for Wastewater Treatment. ChemBioEng Rev. 2021, 8, 463–489. [Google Scholar] [CrossRef]
- Goh, P.; Kang, H.; Ismail, A.; Khor, W.; Quen, L.; Higgins, D. Nanomaterials for microplastic remediation from aquatic environment: Why nano matters? Chemosphere 2022, 299, 134418. [Google Scholar] [CrossRef]
- Tarabukina, E.; Krasnov, I.; Ratnikova, O.; Melenevskaya, E.; Filippov, A. Effect of Centrifugal Field upon Hydrodynamic Characteristics of Fullerene C60 and Poly(N-vinylpyrrolidone) Complex in Aqueous Solutions. Int. J. Polym. Anal. Charact. 2007, 12, 203–220. [Google Scholar] [CrossRef]
- Krasnou, I.; Tarabukina, E.; Melenevskaya, E.; Filippov, A.; Aseyev, V.; Hietala, S.; Tenhu, H. Rheological behavior of poly (vinylpyrrolidone)/fullerene C60 complexes in aqueous medium. J. Macromol. Sci. Part B Phys. 2008, 47, 500–510. [Google Scholar] [CrossRef]
- Chubarova, E.; Melenevskaya, E.Y.; Sudareva, N.; Andreeva, O.; Malachova, I.; Ratnikova, O. Degradation of macromolecular chains in fullerene C60–polystyrene composites. J. Macromol. Sci. Part B Phys. 2005, 44, 455–469. [Google Scholar] [CrossRef]
- Stylianakis, M.M. Distinguished Contributions in the Fields of Biomedical and Environmental Applications Incorporating Nanostructured Materials and Composites. Molecules 2021, 26, 2112. [Google Scholar] [CrossRef]
- Sudareva, N.; Penkova, A.; Kostereva, T.; Polotskii, A.; Polotskaya, G. Properties of casting solutions and ultrafiltration membranes based on fullerene-polyamide nanocomposites. Express Polym. Lett. 2012, 6, 173–188. [Google Scholar] [CrossRef]
- Semenov, K.N.; Andrusenko, E.V.; Charykov, N.A.; Litasova, E.V.; Panova, G.G.; Penkova, A.V.; Murin, I.V.; Piotrovskiy, L.B. Carboxylated fullerenes: Physico-chemical properties and potential applications. Prog. Solid State Chem. 2017, 47–48, 19–36. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Atta, R.; Zolotarev, A.; Plisko, T.; Mazur, A.; Solovyev, N.; Ermakov, S. The development and study of novel membrane materials based on polyphenylene isophthalamide—Pluronic F127 composite. Mater. Des. 2019, 165, 107596. [Google Scholar] [CrossRef]
- Kitjanon, J.; Khuntawee, W.; Phongphanphanee, S.; Sutthibutpong, T.; Chattham, N.; Karttunen, M.; Wong-Ekkabut, J. Nanocomposite of Fullerenes and Natural Rubbers: MARTINI Force Field Molecular Dynamics Simulations. Polymers 2021, 13, 4044. [Google Scholar] [CrossRef]
- Brunet, L.; Lyon, D.Y.; Hotze, E.M.; Alvarez, P.J.J.; Wiesner, M.R. Comparative Photoactivity and Antibacterial Properties of C60 Fullerenes and Titanium Dioxide Nanoparticles. Environ. Sci. Technol. 2009, 43, 4355–4360. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zheng, X.; Li, H.-F.; Lin, J.-M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta 2013, 784, 1–17. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Samonin, V.V.; Nikonova, V.Y.; Podvyaznikov, M.L. Carbon adsorbents on the basis of the hydrolytic lignin modified with fullerenes in producing. Russ. J. Appl. Chem. 2014, 87, 190–193. [Google Scholar] [CrossRef]
- Yashas, S.R.; Shahmoradi, B.; Wantala, K.; Shivaraju, H.P. Potentiality of polymer nanocomposites for sustainable environmental applications: A review of recent advances. Polymer 2021, 233, 124184. [Google Scholar] [CrossRef]
- Ma, J.; Guo, Q.; Gao, H.-L.; Qin, X. Synthesis of C60/graphene composite as electrode in supercapacitors. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 477–482. [Google Scholar] [CrossRef]
- Perera, M.G.N.; Galagedara, Y.R.; Ren, Y.; Jayaweera, M.; Zhao, Y.; Weerasooriya, R. Fabrication of fullerenol-incorporated thin-film nanocomposite forward osmosis membranes for improved desalination performances. J. Polym. Res. 2018, 25, 199. [Google Scholar] [CrossRef]
- Shen, Q.; Xu, S.J.; Xu, Z.L.; Zhang, H.Z.; Dong, Z.Q. Novel thin-film nanocomposite membrane with water-soluble polyhydroxylated fullerene for the separation of Mg2+/Li+ aqueous solution. J. Appl. Polym. Sci. 2019, 136, 48029. [Google Scholar] [CrossRef]
- Liu, Y.; Phillips, B.; Li, W.; Zhang, Z.; Fang, L.; Qiu, J.; Wang, S. Fullerene-Tailored Graphene Oxide Interlayer Spacing for Energy-Efficient Water Desalination. ACS Appl. Nano Mater. 2018, 1, 6168–6175. [Google Scholar] [CrossRef]
- Alekseeva, O.V.; Bagrovskaya, N.A.; Noskov, A.V. Sorption of heavy metal ions by fullerene and polystyrene/fullerene film compositions. Prot. Met. Phys. Chem. Surf. 2016, 52, 443–447. [Google Scholar] [CrossRef]
- Jin, X.; Hu, J.; Tint, M.; Ong, S.; Biryulin, Y.; Polotskaya, G. Estrogenic compounds removal by fullerene-containing membranes. Desalination 2007, 214, 83–90. [Google Scholar] [CrossRef]
- Penkova, A.V.; Polotskaya, G.A.; Toikka, A.M.; Trchová, M.; Šlouf, M.; Urbanová, M.; Brus, J.; Brožová, L.; Pientka, Z. Structure and pervaporation properties of poly (phenylene-iso-phthalamide) membranes modified by fullerene C60. Macromol. Mater. Eng. 2009, 294, 432–440. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Kuzminova, A.; Atta, R.; Zolotarev, A.; Mazur, A.; Vezo, O.; Lahderanta, E.; Markelov, D.; Ermakov, S. Development and investigation of novel polyphenylene isophthalamide pervaporation membranes modified with various fullerene derivatives. Sep. Purif. Technol. 2019, 226, 241–251. [Google Scholar] [CrossRef]
- Plisko, T.V.; Liubimova, A.S.; Bildyukevich, A.V.; Penkova, A.V.; Dmitrenko, M.E.; Mikhailovskii, V.Y.; Melnikova, G.B.; Semenov, K.N.; Doroshkevich, N.V.; Kuzminova, A.I. Fabrication and characterization of polyamide-fullerenol thin film nanocomposite hollow fiber membranes with enhanced antifouling performance. J. Membr. Sci. 2018, 551, 20–36. [Google Scholar] [CrossRef]
- Halenova, T.; Raksha, N.; Savchuk, O.; Ostapchenko, L.; Prylutskyy, Y.; Ritter, U.; Scharff, P. Evaluation of the biocompatibility of water-soluble pristine C60 fullerenes in rabbit. BioNanoScience 2020, 10, 721–730. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, F.; Li, S.; Hu, Z.; Zhao, Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale 2011, 3, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Russ, K.; Elvati, P.; Parsonage, T.; Dews, A.; Jarvis, J.; Ray, M.; Schneider, B.; Smith, P.; Williamson, P.; Violi, A. C 60 fullerene localization and membrane interactions in RAW 264.7 immortalized mouse macrophages. Nanoscale 2016, 8, 4134–4144. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Peron, J.; Mani, A.; Zhao, X.; Edwards, D.; Adachi, M.; Soboleva, T.; Shi, Z.; Xie, Z.; Navessin, T.; Holdcroft, S. Properties of Nafion® NR-211 membranes for PEMFCs. J. Membr. Sci. 2010, 356, 44–51. [Google Scholar] [CrossRef]
- Maiti, T.K.; Singh, J.; Majhi, J.; Ahuja, A.; Maiti, S.; Dixit, P.; Bhushan, S.; Bandyopadhyay, A.; Chattopadhyay, S. Advances in polybenzimidazole based membranes for fuel cell applications that overcome Nafion membranes constraints. Polymer 2022, 255, 125151. [Google Scholar] [CrossRef]
- Wan, Y.H.; Sun, J.; Jian, Q.P.; Fan, X.; Zhao, T.S. A Nafion/polybenzimidazole composite membrane with consecutive proton-conducting pathways for aqueous redox flow batteries. J. Mater. Chem. A 2022, 10, 13021–13030. [Google Scholar] [CrossRef]
- Li, Y.; He, G.; Wang, S.; Yu, S.; Pan, F.; Wu, H.; Jiang, Z. Recent advances in the fabrication of advanced composite membranes. J. Mater. Chem. A 2013, 1, 10058–10077. [Google Scholar] [CrossRef]
- Tasaki, K.; Gasa, J.; Wang, H.; DeSousa, R. Fabrication and characterization of fullerene–Nafion composite membranes. Polymer 2007, 48, 4438–4448. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Sokolova, M.P.; Chen, B.; Plisko, T.V.; Markelov, D.A.; Ermakov, S.S. Impact of fullerene loading on the structure and transport properties of polysulfone mixed-matrix membranes. J. Mater. Sci. 2016, 51, 7652–7659. [Google Scholar] [CrossRef]
- Aryafard, E.; Rahmatmand, B.; Rahimpour, M.R. Application of computational fluid dynamics technique in pervaporation processes. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–268. [Google Scholar]
- Vojdani, M.; Giti, R. Polyamide as a Denture Base Material: A Literature Review. J. Dent. (Shiraz Iran) 2015, 16, 1–9. [Google Scholar]
- Tan, X.F.; Liu, Y.G.; Gu, Y.L.; Xu, Y.; Zeng, G.M.; Hu, X.J.; Liu, S.B.; Wang, X.; Liu, S.M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, M.; Mohebbi, A. Carbon-Based Materials for Desalination. In Sustainable Materials and Systems for Water Desalination; Springer: Berlin/Heidelberg, Germany, 2021; pp. 197–212. [Google Scholar]
- Jin, B.; Du, Z.; Zhang, C.; Yu, Z.; Wang, X.; Hu, J.; Li, Z. Eu-Chelate Polystyrene Microsphere-Based Lateral Flow Immunoassay Platform for hs-CRP Detection. Biosensors 2022, 12, 977. [Google Scholar] [CrossRef]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2017, 38, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hansen, C.J.; Wu, C.-C.; Robinette, E.J.; Peterson, A.M. Effect of surface wettability on the interfacial adhesion of a thermosetting elastomer on glass. RSC Adv. 2021, 11, 31142–31151. [Google Scholar] [CrossRef] [PubMed]
- Niinivaara, E.; Ouzas, A.; Fraschini, C.; Berry, R.M.; Dubé, M.A.; Cranston, E.D. How latex film formation and adhesion at the nanoscale correlate to performance of pressure sensitive adhesives with cellulose nanocrystals. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2021, 379, 20200330. [Google Scholar] [CrossRef] [PubMed]
- von Reitzenstein, N.H.; Baghirzade, B.S.; Pruitt, E.; Hristovski, K.; Westerhoff, P.; Apul, O.G. Comparing the morphologies and adsorption behavior of electrospun polystyrene composite fibers with 0D fullerenes, 1D multiwalled carbon nanotubes and 2D graphene oxides. Chem. Eng. J. Adv. 2022, 9, 100199. [Google Scholar] [CrossRef]
- Cao, X.; Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun Porous Nanofibers: Pore-Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater. Polymers 2022, 14, 3990. [Google Scholar] [CrossRef] [PubMed]
- Chavalarias, D.; Cointet, J.-P. Bottom-up scientific field detection for dynamical and hierarchical science mapping, methodology and case study. Scientometrics 2008, 75, 37–50. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Z.; Gholizadeh, M.; Zhang, S.; Lam, C.H.; Xiong, Z.; Wang, Y. Coke Formation during Thermal Treatment of Bio-oil. Energy Fuels 2020, 34, 7863–7914. [Google Scholar] [CrossRef]
- Gudjonsdottir, S.; Van Der Stam, W.; Koopman, C.; Kwakkenbos, B.; Evers, W.H.; Houtepen, A.J. On the Stability of Permanent Electrochemical Doping of Quantum Dot, Fullerene, and Conductive Polymer Films in Frozen Electrolytes for Use in Semiconductor Devices. ACS Appl. Nano Mater. 2019, 2, 4900–4909. [Google Scholar] [CrossRef]
- Zheng, T.; Fan, L.; Zhou, H.; Zhao, Y.; Jin, B.; Peng, R. Engineering of Electron Extraction and Defect Passivation via Anion-Doped Conductive Fullerene Derivatives as Interlayers for Efficient Invert Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 24747–24755. [Google Scholar] [CrossRef]
- Djordjević, A.; Bogdanović, G.; Dobrić, S. Fullerenes in biomedicine. J. Buon 2006, 11, 391–404. [Google Scholar]
- Amooghin, A.E.; Sanaeepur, H.; Pedram, M.Z.; Omidkhah, M.; Kargari, A. New advances in polymeric membranes for CO2 separation. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Research Center: Badajoz, Spain, 2016; pp. 354–368. [Google Scholar]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Amooghin, A.E.; Bandehali, S.; Moghadassi, A.; Matsuura, T.; Van der Bruggen, B. Polyimides in membrane gas separation: Monomer’s molecular design and structural engineering. Prog. Polym. Sci. 2019, 91, 80–125. [Google Scholar] [CrossRef]
- Barrau, S.; Heiser, T.; Richard, F.; Brochon, C.; Ngov, C.; Van De Wetering, K.; Hadziioannou, G.; Anokhin, D.V.; Ivanov, D.A. Self-Assembling of Novel Fullerene-Grafted Donor–Acceptor Rod-Coil Block Copolymers. Macromolecules 2008, 41, 2701–2710. [Google Scholar] [CrossRef]
- Lin, J.-H.; Chen, C.-S.; Ma, H.-L.; Chang, C.-W.; Hsu, C.-Y.; Chen, H.-W. Self-assembling of multi-walled carbon nanotubes on a porous carbon surface by catalyst-free chemical vapor deposition. Carbon 2008, 46, 1619–1623. [Google Scholar] [CrossRef]
- Rybkin, A.Y.; Belik, A.Y.; Goryachev, N.; Mikhaylov, P.; Kraevaya, O.; Filatova, N.; Parkhomenko, I.; Peregudov, A.; Terent’ev, A.; Larkina, E. Self-assembling nanostructures of water-soluble fullerene [60]–chlorin e6 dyads: Synthesis, photophysical properties, and photodynamic activity. Dye. Pigment. 2020, 180, 108411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).