Development and In Vitro Analysis of Layer-by-Layer Assembled Membranes for Potential Wound Dressing: Electrospun Curcumin/Gelatin as Middle Layer and Gentamicin/Polyvinyl Alcohol as Outer Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Middle Layer, Outer Layer, and Multilayer Membrane
2.2.1. Middle Layer Preparation
2.2.2. Outer Layer Preparation
2.2.3. Sandwich Structure Preparation
2.3. Physicochemical Analysis of Multilayer Membranes
2.3.1. Microstructural Observation by Optical Microscopy (OM)
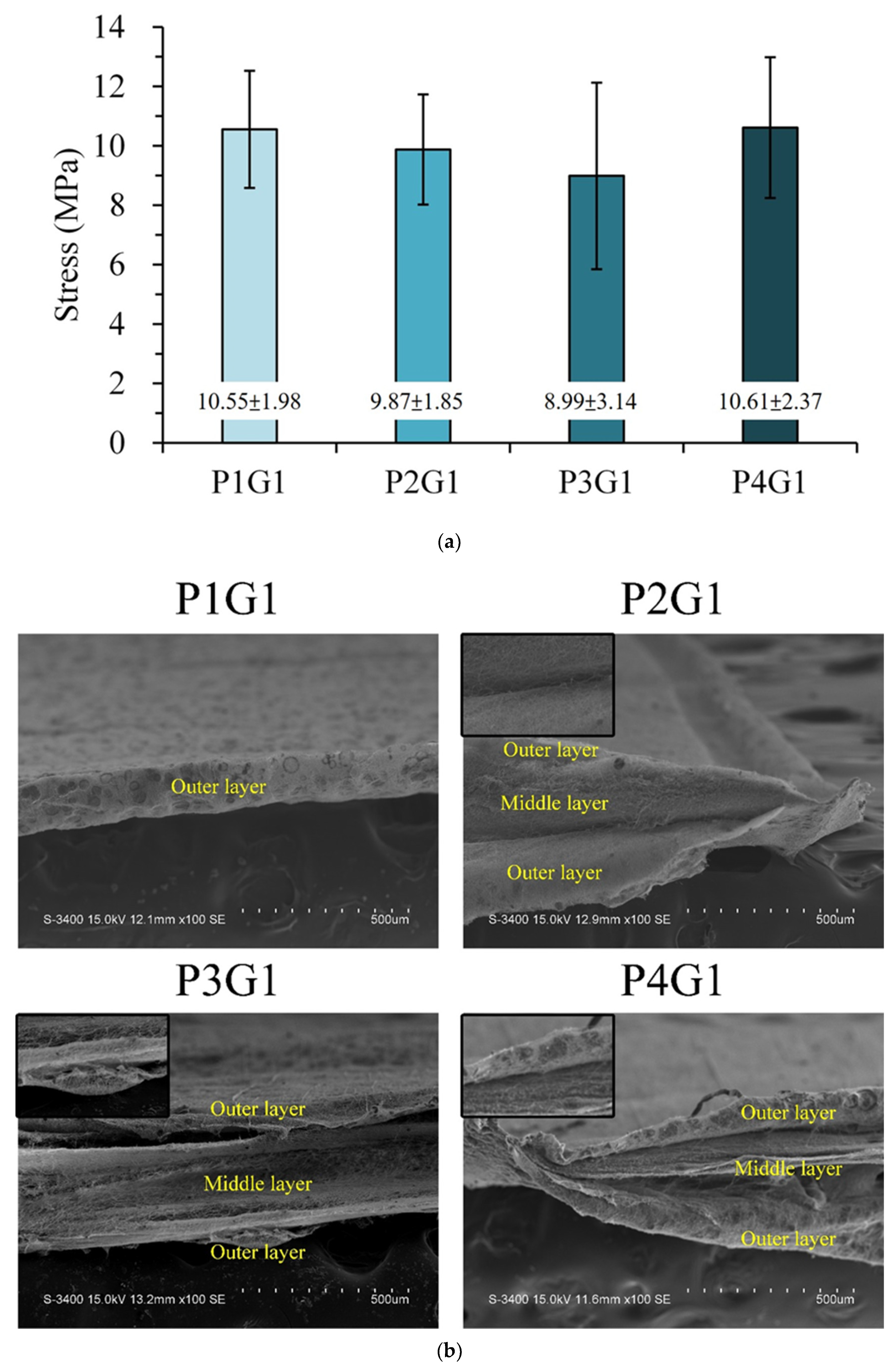
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Fourier Transform Infrared Spectroscopy–Attenuated Total Reflectance (FTIR–ATR)
2.3.4. Tensile Strength Testing
2.3.5. Water Absorption
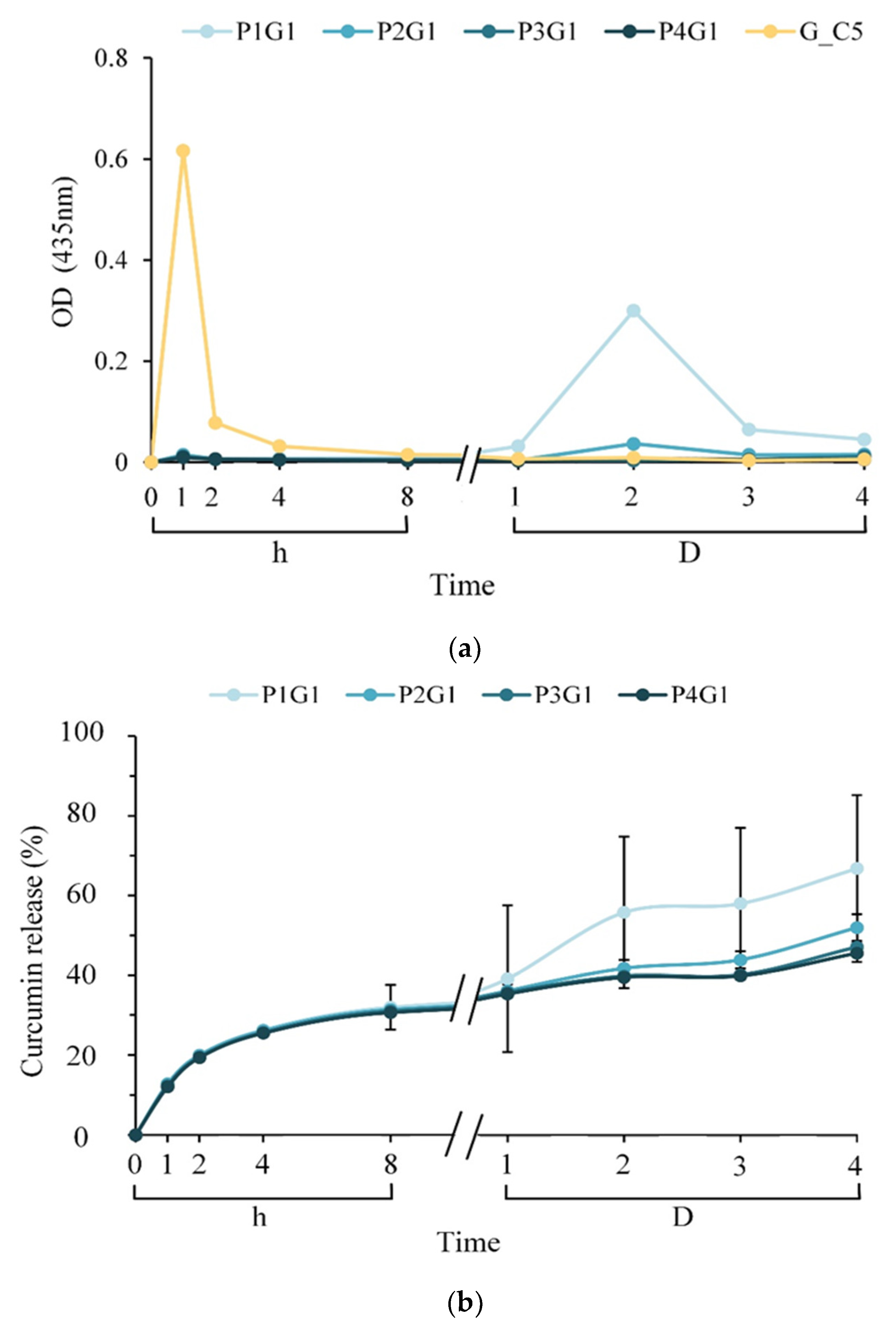
2.3.6. Drug Release In Vitro
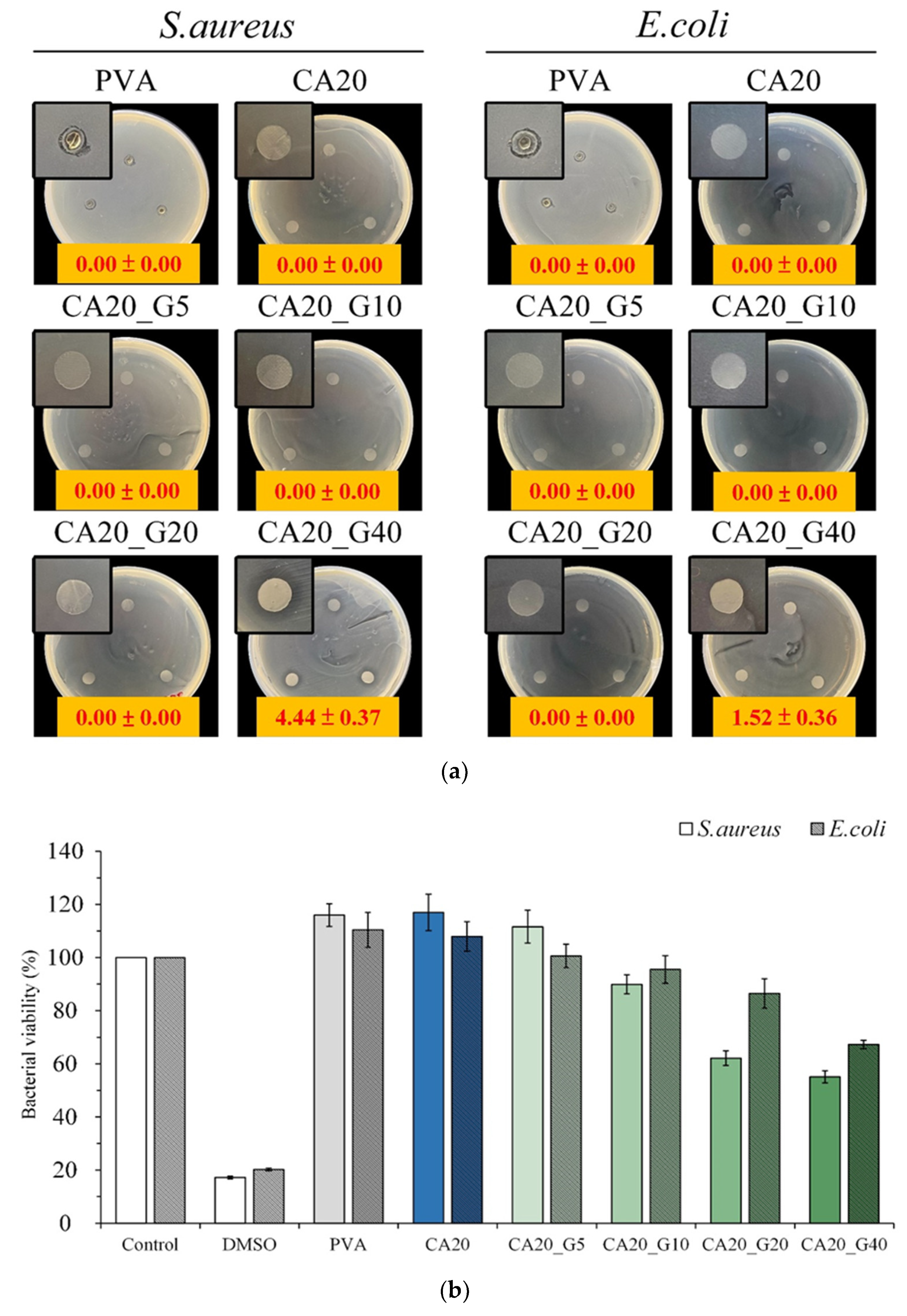
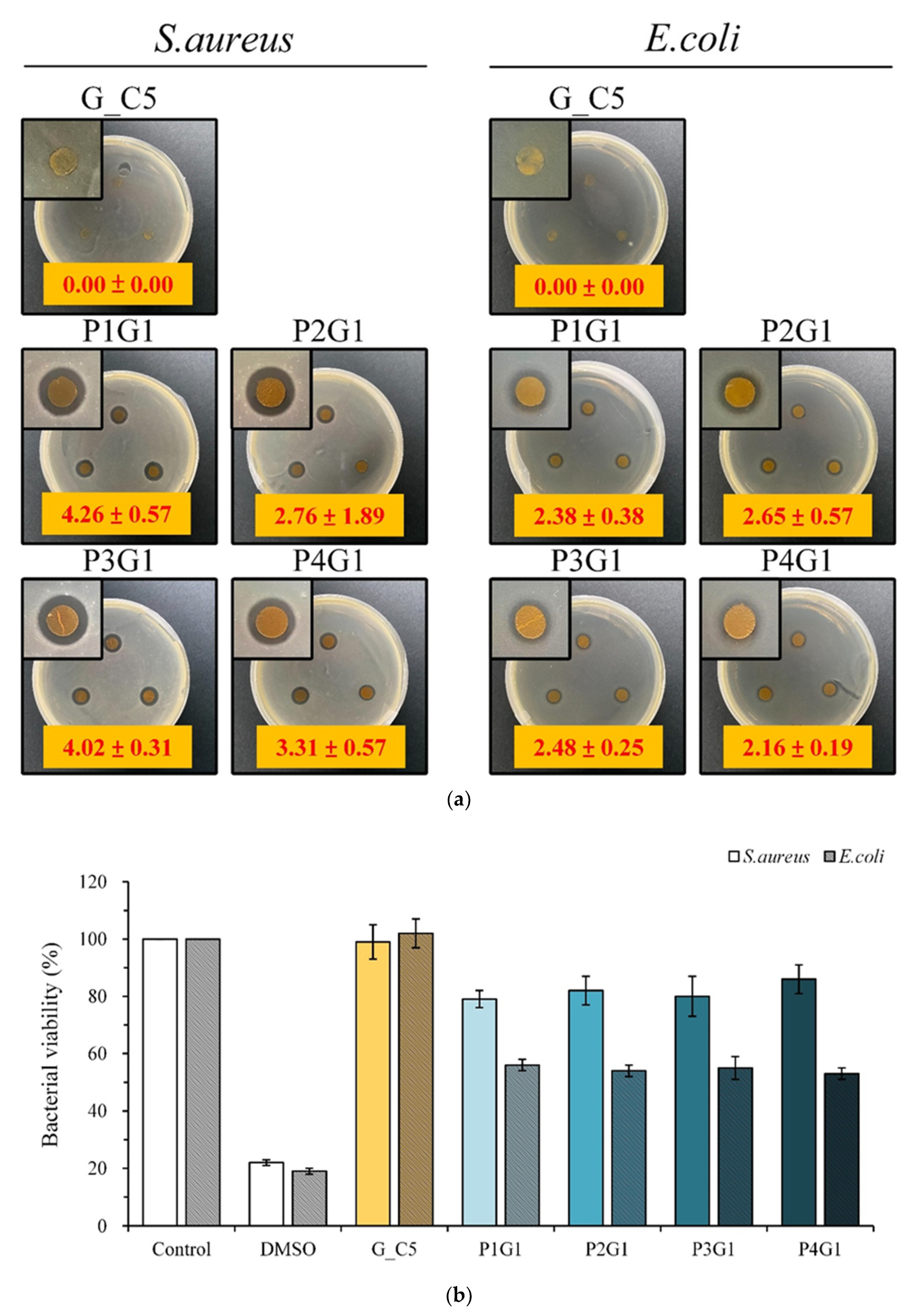
2.4. Antibacterial Activity
2.5. Biocompatibility In Vitro
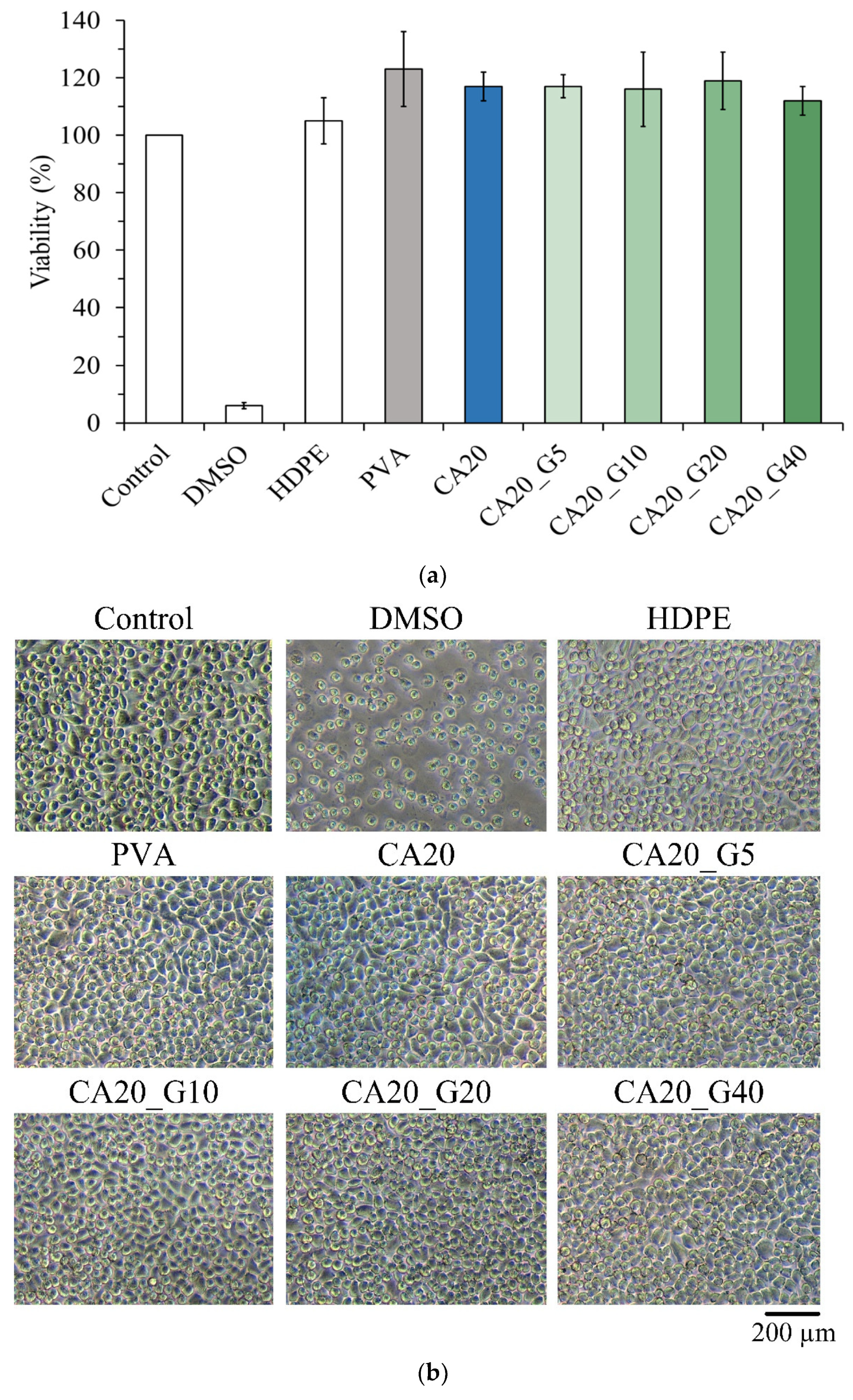
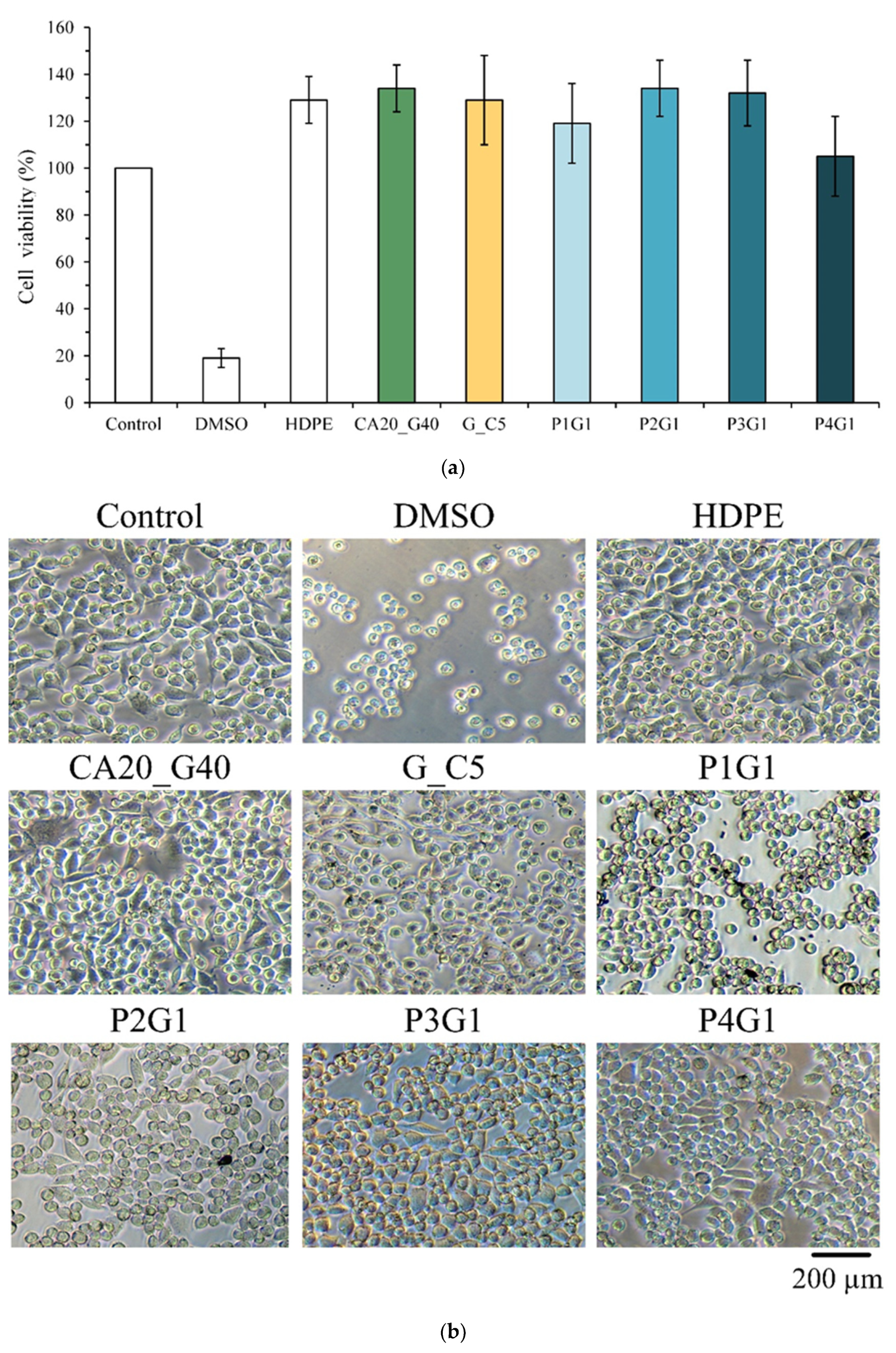
2.5.1. Cytotoxicity
2.5.2. Fibroblast L929 Cell Proliferation and Attachment
2.6. Statistical Analysis
3. Results
3.1. Middle Layer Selection
3.2. Outer Layer Selection
3.3. Sandwich PVA/Gelatin/PVA Membrane Characterizations
3.4. Antibacterial Activity of Sandwich PVA/Gelatin/PVA Membrane
3.5. In Vitro Interaction of L929 Cells with Sandwich PVA/Gelatin/PVA Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993–1003. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun medicated nanofibers for wound healing. Membranes 2021, 11, 7707. [Google Scholar] [CrossRef]
- Leung, V.; Ko, F. Biomedical applications of nanofibers. Polym. Adv. Technol. 2011, 22, 350–3657. [Google Scholar] [CrossRef]
- Razavizadeh, B.M.; Niazmand, R. Characterization of polyamide-6/ propolis blended electrospun fibers. Heliyon 2020, 6, e04784. [Google Scholar] [CrossRef]
- He, P.; Zhong, Q.; Ge, Y.; Guo, Z.; Tian, J.; Zhou, Y.; Ding, S.; Li, H.; Zhou, C. Dual drug loaded coaxial electrospun PLGA/PVP fiber for guided tissue regeneration under control of infection. Mater. Sci. Eng. C 2018, 90, 549–556. [Google Scholar] [CrossRef]
- Zhan, F.; Yan, X.; Li, J.; Sheng, F.; Li, B. Encapsulation of tangeretin in PVA/PAA crosslinking electrospun fibers by emulsion-electrospinning: Morphology characterization, slow-release, and antioxidant activity assessment. Food Chem. 2021, 337, 127763. [Google Scholar] [CrossRef]
- Nada, A.A.; Ali, E.A.; Soliman, A.A.F.; Shen, J.; Abou-Zeid, N.Y.; Hudson, S.M. Multi-layer dressing made of laminated electrospun nanowebs and cellulose-based adhesive for comprehensive wound care. Int. J. Biol. Macromol. 2020, 162, 629–644. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Applications of Poly (Vinyl Alcohol) Hydrogels Produced by Conventional Crosslinking or by Freezing/Thawing Methods. In Biopolymers PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2000; pp. 37–65. [Google Scholar] [CrossRef]
- Yang, J.; Webb, A.R.; Ameer, G.A. Novel citric acid-based biodegradable elastomers for tissue engineering. Adv. Mater. 2004, 16, 511–516. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (PVA)/nano silver hydrogels. Colloids Surf. B 2020, 188, 110757. [Google Scholar] [CrossRef]
- Al Thaher, Y. Tailored gentamicin release from silica nanocarriers coated with polyelectrolyte multilayers. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126210. [Google Scholar] [CrossRef]
- Ward, A. The chemical structure and physical properties of gelatin. J. Photogr. Sci. 1955, 3, 60–67. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chang, P.-J.; Chen, J.-C.; Huang, S.-M.; Liu, S.-M.; Shih, C.-J.; Chen, W.-C. In Vitro Characterizations of Post-Crosslinked Gelatin-Based Microspheres Modified by Phosphatidylcholine or Diammonium Phosphate as Antibiotic Delivery Vehicles. Polymers 2023, 15, 1504. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian Solid Gold. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; pp. 1–75. [Google Scholar] [CrossRef]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef]
- Huang, S.-M.; Liu, S.-M.; Tseng, H.-Y.; Chen, W.-C. Effect of Citric Acid on Swelling Resistance and Physicochemical Properties of Post-Crosslinked Electrospun Polyvinyl Alcohol Fibrous Membrane. Polymers 2023, 15, 1738. [Google Scholar] [CrossRef]
- Zolfagharzadeh, V.; Ai, J.; Soltani, H.; Hassanzadeh, S.; Khanmohammadi, M. Sustain release of loaded insulin within biomimetic hydrogel microsphere for sciatic tissue engineering in vivo. Int. J. Biol. Macromol. 2023, 225, 687–700. [Google Scholar] [CrossRef]
- Ahmad Hariza, A.M.a.; Mohd Yunus, M.H.; Fauzi, M.B.; Murthy, J.K.; Tabata, Y.; Hiraoka, Y. The Fabrication of Gelatin–Elastin–Nanocellulose Composite Bioscaffold as a Potential Acellular Skin Substitute. Polymers 2023, 15, 779. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2020, 308, 125599. [Google Scholar] [CrossRef]
- Briceño, S.; Chavez-Chico, E.A.; González, G. Diatoms decorated with gold nanoparticles by In-situ and Ex-situ methods for in vitro gentamicin release. Mater. Sci. Eng. C 2021, 123, 112018. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A survey of structure− property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhao, J.; Wang, S. Multifunctional hydrogels based on chitosan, hyaluronic acid and other biological macromolecules for the treatment of inflammatory bowel disease: A review. Int. J. Biol. Macromol. 2023, 227, 505–523. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B 2023, 222, 113096. [Google Scholar] [CrossRef] [PubMed]
- Jeckson, T.A.; Neo, Y.P.; Sisinthy, S.P.; Gorain, B. Delivery of Therapeutics from Layer-by-Layer Electrospun Nanofiber Matrix for Wound Healing: An Update. J. Pharm. Sci. 2021, 110, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Xu, G.-R.; Liu, X.-Y.; Xu, J.-M.; Li, L.; Su, H.-C.; Zhao, H.-L.; Feng, H.-J. High flux nanofiltration membranes based on layer-by-layer assembly modified electrospun nanofibrous substrate. Appl. Surf. Sci. 2018, 434, 573–581. [Google Scholar] [CrossRef]
- Gul, A.; Gallus, I.; Tegginamath, A.; Maryska, J.; Yalcinkaya, F. Electrospun antibacterial nanomaterials for wound dressings applications. Membranes 2021, 11, 908. [Google Scholar] [CrossRef]
- Huang, R.; Li, Y.; Zhou, X.; Zhang, Q.; Jin, H.; Zhao, J.; Pan, S.; Deng, H. LBL fabricated biopolymer-layered silicate based nanofibrous mats and their cell compatibility studies. Carbohydr. Polym. 2012, 90, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Huang, X.; Shi, X.; Zhan, Y.; Fan, G.; Pan, S.; Tian, J.; Deng, H.; Du, Y. Antimicrobial application of nanofibrous mats self-assembled with quaternized chitosan and soy protein isolate. Carbohydr. Polym. 2015, 133, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, M.; Kikionis, S.; Siamidi, A.; Tragou, K.; Ioannou, E.; Roussis, V.; Tsotinis, A. Modified In Vitro Release of Melatonin Loaded in Nanofibrous Electrospun Mats Incorporated Into Monolayered and Three-Layered Tablets. J. Pharm. Sci. 2019, 108, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.-T.; Drăgan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An overview of biopolymeric electrospun nanofibers based on polysaccharides for wound healing management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent trends on burn wound care: Hydrogel dressings and scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef]
- Sharma, D.; Srivastava, S.; Kumar, S.; Sharma, P.K.; Hassani, R.; Dailah, H.G.; Khalid, A.; Mohan, S. Biodegradable Electrospun Scaffolds as an Emerging Tool for Skin Wound Regeneration: A Comprehensive Review. Pharmaceuticals 2023, 16, 325. [Google Scholar] [CrossRef]
- Ahmad, N. In Vitro and In Vivo Characterization Methods for Evaluation of Modern Wound Dressings. Pharmaceutics 2022, 15, 42. [Google Scholar] [CrossRef]
- Kataria, K.; Gupta, A.; Rath, G.; Mathur, R.B.; Dhakate, S.R. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int. J. Pharm. 2014, 469, 102–110. [Google Scholar] [CrossRef]
- Kozlik, P.; Bosakova, Z.; Marekova, D.; Holan, V.; Sykova, E. Controlled gentamicin release from multi-layered electrospun nanofibrous structures of various thicknesses. Int. J. Nanomed. 2012, 7, 5315–5325. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef]
- Kharat, M.; Zhang, G.; McClements, D.J. Stability of curcumin in oil-in-water emulsions: Impact of emulsifier type and concentration on chemical degradation. Food Res. Int. 2018, 111, 178–186. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Pei, Y.; Xiong, W.; Zhang, C.; Xu, W.; Liu, S.; Li, B. Curcumin encapsulated in the complex of lysozyme/carboxymethylcellulose and implications for the antioxidant activity of curcumin. Food Res. Int. 2015, 75, 98–105. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Wang, X.; Li, X.; Xiao, B. Effect of particle size on the cellular uptake and anti-inflammatory activity of oral nanotherapeutics. Colloids Surf. B 2020, 187, 110880. [Google Scholar] [CrossRef]
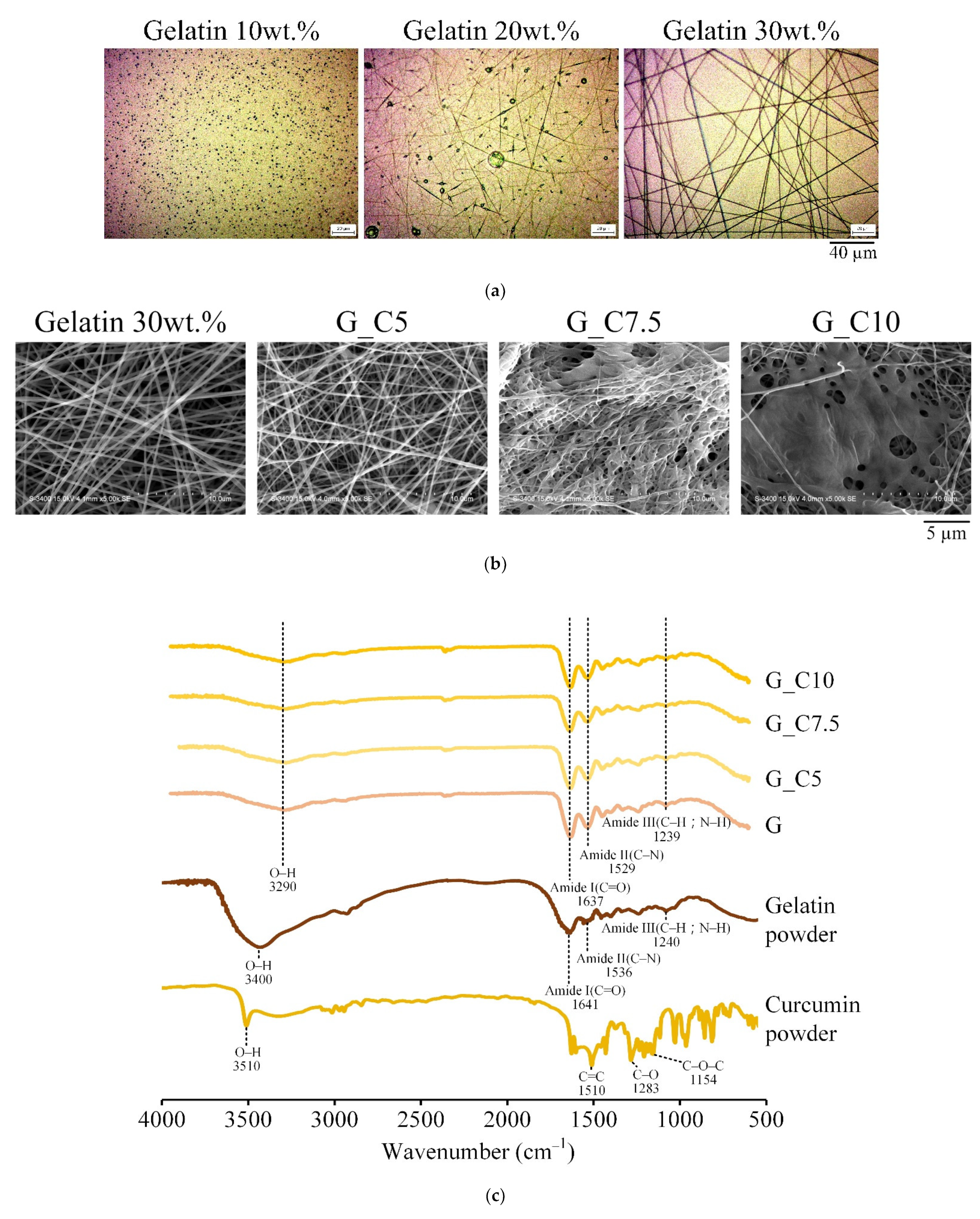
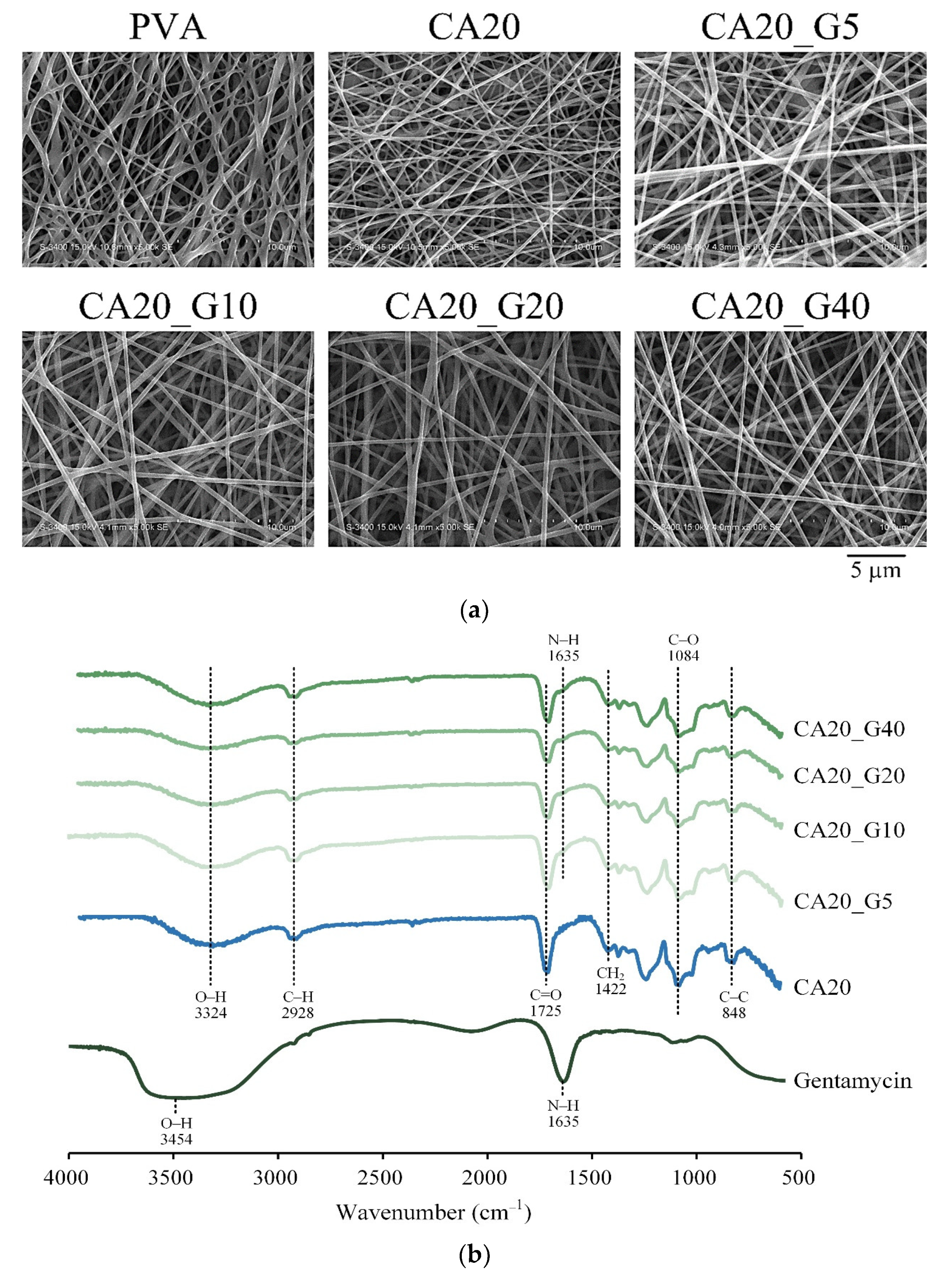


| Designated Groups | Gelatin a (wt.%) | Curcumin (% by Weight of Gelatin) |
|---|---|---|
| G | 30 | 0 |
| G_C5 | 30 | 5.0 |
| G_C7.5 | 30 | 7.5 |
| G_C10 | 30 | 10.0 |
| Designated Groups | PVA b (wt.%) | Citric Acid (% by Weight of PVA) | Gentamycin (mg/10 mL Spinning Solution) |
|---|---|---|---|
| CA20_G5 | 8 | 20 | 5 |
| CA20_G10 | 8 | 20 | 10 |
| CA20_G20 | 8 | 20 | 20 |
| CA20_G40 | 8 | 20 | 40 |
| Designated Membrane with a Sandwich Structure of PVA-Gelatin-PVA | Gelatin Double-Sided Outer Layer PVA Spinning Time (h) | Middle Layer Gelatin Spinning Time (h) |
|---|---|---|
| P1G1 | 1 | 1 |
| P2G1 | 2 | 1 |
| P3G1 | 3 | 1 |
| P4G1 | 4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-M.; Liu, S.-M.; Tseng, H.-Y.; Chen, W.-C. Development and In Vitro Analysis of Layer-by-Layer Assembled Membranes for Potential Wound Dressing: Electrospun Curcumin/Gelatin as Middle Layer and Gentamicin/Polyvinyl Alcohol as Outer Layers. Membranes 2023, 13, 564. https://doi.org/10.3390/membranes13060564
Huang S-M, Liu S-M, Tseng H-Y, Chen W-C. Development and In Vitro Analysis of Layer-by-Layer Assembled Membranes for Potential Wound Dressing: Electrospun Curcumin/Gelatin as Middle Layer and Gentamicin/Polyvinyl Alcohol as Outer Layers. Membranes. 2023; 13(6):564. https://doi.org/10.3390/membranes13060564
Chicago/Turabian StyleHuang, Ssu-Meng, Shih-Ming Liu, Hua-Yi Tseng, and Wen-Cheng Chen. 2023. "Development and In Vitro Analysis of Layer-by-Layer Assembled Membranes for Potential Wound Dressing: Electrospun Curcumin/Gelatin as Middle Layer and Gentamicin/Polyvinyl Alcohol as Outer Layers" Membranes 13, no. 6: 564. https://doi.org/10.3390/membranes13060564
APA StyleHuang, S.-M., Liu, S.-M., Tseng, H.-Y., & Chen, W.-C. (2023). Development and In Vitro Analysis of Layer-by-Layer Assembled Membranes for Potential Wound Dressing: Electrospun Curcumin/Gelatin as Middle Layer and Gentamicin/Polyvinyl Alcohol as Outer Layers. Membranes, 13(6), 564. https://doi.org/10.3390/membranes13060564








