Gradual Drug Release Membranes and Films Used for the Treatment of Periodontal Disease
Abstract
:1. Introduction
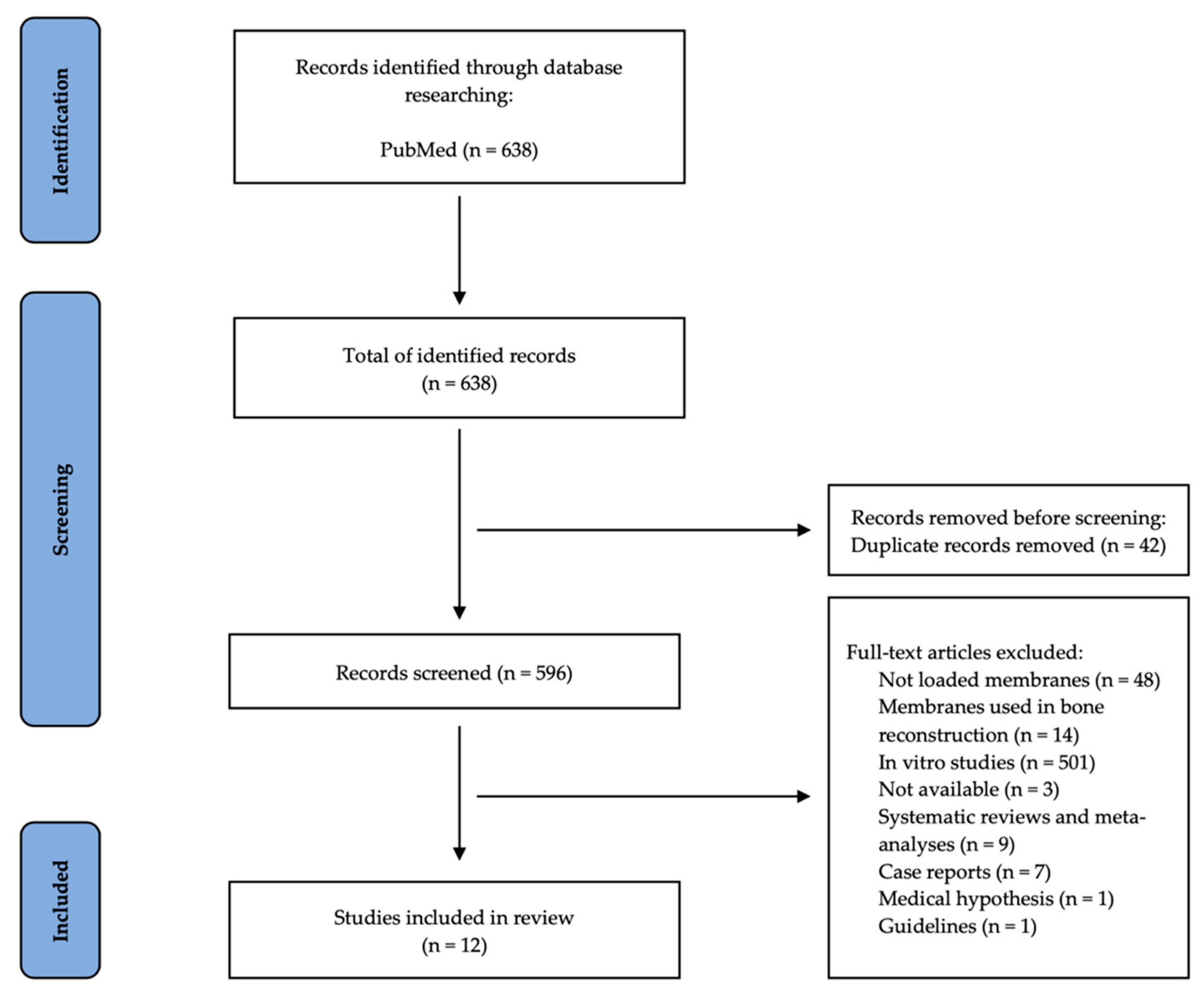
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Collection
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontol. 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontol. 2000 2006, 42, 47–79. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C.; Kornman, K.S. The pathogenesis of human periodontitis: An introduction. Periodontol. 2000 1997, 14, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Attström, R. European Workshop in Periodontology group B. Advances in the pathogenesis of periodontitis. Group B consensus report of the fifth European Workshop in Periodontology. J. Clin. Periodontol. 2005, 32 (Suppl. 6), 130–131. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontol. 2000 2012, 60, 15–39. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Botelho, J.; Proença, L.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. Economic Burden of Periodontal Disease in Europe and the United States of America—An updated forecast. medRxiv 2021, 93, 373–379. [Google Scholar] [CrossRef]
- [GBD] Global Burden of Diseases. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study. Lancet 2015, 388, 1545–1602. [Google Scholar]
- Listl, S.; Galloway, J.; Mossey, P.A.; Marcenes, W. Global Economic Impact of Dental Diseases. J. Dent. Res. 2015, 94, 1355–1361. [Google Scholar] [CrossRef]
- Monsarrat, P.; Blaizot, A.; Kémoun, P.; Ravaud, P.; Nabet, C.; Sixou, M.; Vergnes, J.N. Clinical research activity in periodontal medicine: A systematic mapping of trial registers. J. Clin. Periodontol. 2016, 43, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.M.; Egeberg, A.; Holmstrup, P.; Hansen, P.R. Relation of Periodontitis to Risk of Cardiovascular and All-Cause Mortality (from a Danish Nationwide Cohort Study). Am. J. Cardiol. 2016, 118, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Trombelli, L.; Heitz, F.; Needleman, I.; Moles, D. A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29 (Suppl. 3), 92–102. [Google Scholar] [CrossRef] [PubMed]
- Nowzari, H.; Matian, F.; Slots, J. Periodontal pathogens on polytetrafluoroethylene membrane for guided tissue regeneration inhibit healing. J. Clin. Periodontol. 1995, 22, 469–474. [Google Scholar] [CrossRef]
- Gümüşderelioğlu, M.; Sunal, E.; Tolga Demirtaş, T.; Kiremitçi, A.S. Chitosan-based double-faced barrier membrane coated with functional nanostructures and loaded with BMP-6. J. Mater. Sci. Mater. Med. 2019, 31, 4. [Google Scholar] [CrossRef]
- Sariibrahimoglu, K.; Yang, W.; Leeuwenburgh, S.C.; Yang, F.; Wolke, J.G.; Zuo, Y.; Li, Y.; Jansen, J.A. Development of porous polyurethane/strontium-substituted hydroxyapatite composites for bone regeneration. J. Biomed. Mater. Res. A 2015, 103, 1930–1939. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Janowski, G.M. A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater. 2011, 7, 216–224. [Google Scholar] [CrossRef]
- Korkusuz, F.; Korkusuz, P.; Ekşioĝlu, F.; Gürsel, I.; Hasirci, V. In vivo response to biodegradable controlled antibiotic release systems. Biomed. Mater. Res. 2001, 55, 217–228. [Google Scholar]
- Taubes, G. The bacteria fight back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef]
- Rams, T.E.; Slots, J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol. 2000 1996, 10, 139–159. [Google Scholar] [CrossRef]
- De Oliveira, L.F.; Jorge, A.O.; Dos Santos, S.S. In vitro minocycline activity on superinfecting microorganisms isolated from chronic periodontitis patients. Braz. Oral Res. 2006, 20, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, A.; Spivakovsky, S. Antibiotics in aggressive periodontitis, is there a clinical benefit? Evid.-Based Dent. 2016, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Lecio, G.; Ribeiro, F.V.; Pimentel, S.P.; Reis, A.A.; da Silva, R.; Nociti, F., Jr.; Moura, L.; Duek, E.; Casati, M.; Casarin, R. Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: Randomized clinical, immune and microbiological trial. Clin. Oral Investig. 2020, 24, 1269–1279. [Google Scholar] [CrossRef]
- Chang, H.I.; Lau, Y.C.; Yan, C.; Coombes, A.G. Controlled release of an antibiotic, gentamicin sulphate, from gravity spun polycaprolactone fibers. J. Biomed. Mater. Res. A 2008, 84, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Matsushita, M.; Morisaki, K.; Hayashi, S.; Mayumi, T. Local drug delivery systems for the treatment of periodontal disease. J. Pharmacobiodyn. 1991, 14, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Friedman, M. Sustained-release delivery of antimicrobial drugs for the treatment of periodontal diseases: Fantasy or already reality? Periodontol. 2000 2020, 84, 176–187. [Google Scholar] [CrossRef]
- Steinberg, D.; Friedman, M.; Soskolne, A.; Sela, M.N. A new degradable controlled release device for treatment of periodontal disease: In vitro release study. J. Periodontol. 1990, 61, 393–398. [Google Scholar] [CrossRef]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Wang, S.; Zhang, X.; Yu, J.; Wang, C. Mussel-inspired polydopamine-assisted bromelain immobilization onto electrospun fibrous membrane for potential application as wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110624. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Annamalai, S.K.; Arunachalam, K.D.; Ramakrishna, S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials 2013, 34, 724–734. [Google Scholar] [CrossRef]
- Lan, X.; Wang, H.; Bai, J.; Miao, X.; Lin, Q.; Zheng, J.; Ding, S.; Li, X.; Tang, Y. Multidrug-loaded electrospun micro/nanofibrous membranes: Fabrication strategies, release behaviors and applications in regenerative medicine. J. Control. Release 2021, 330, 1264–1287. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Ga, S.W.; Zello, G.A.; Petersen, J.A. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp (accessed on 20 July 2022).
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages a nd limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar] [CrossRef]
- Mahajania, M.; Laddha, R.; Shelke, A.; Gadhiya, N.; Narkhede, S.; Shetty, G.P. Effect of Subgingival Doxycycline Placement on Clinical and Microbiological Parameters in Inflammatory Periodontal Disease: Both in Vivo and in Vitro Studies. J. Contemp. Dent. Pract. 2018, 19, 1228–1234. [Google Scholar]
- Gamal, A.Y.; Kumper, R.M. A novel approach to the use of doxycycline-loaded biodegradable membrane and EDTA root surface etching in chronic periodontitis: A randomized clinical trial. J. Periodontol. 2012, 83, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.; Yadav, S.K.; Patel, R.R.; Nath, G.; Bansal, M.; Mishra, B. Development and Evaluation of Biodegradable Chitosan Films of Metronidazole and Levofloxacin for the Management of Periodontitis. AAPS PharmSciTech 2015, 17, 1312–1325. [Google Scholar] [CrossRef]
- Kassem, A.A.; Ismail, F.A.; Naggar, V.F.; Aboulmagd, E. Preparation and evaluation of periodontal films based on polyelectrolyte complex formation. Pharm. Dev. Technol. 2015, 20, 297–305. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Wang, Y.; Chen, Y.; Xie, J.; Su, J.; Huang, C. One-step treatment of periodontitis based on a core-shell micelle-in-nanofiber membrane with time-programmed drug release. J. Control. Release 2020, 320, 201–213. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Patil, O.N.; Karasik, D.; Razdan, R. Development and evaluation of novel biodegradable chitosan based metformin intrapocket dental film for the management of periodontitis and alveolar bone loss in a rat model. Arch. Oral Biol. 2018, 85, 120–129. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Zahra, S.F.; Razdan, R. Effect of locally administered novel biodegradable chitosan based risedronate/zinc-hydroxyapatite intra-pocket dental film on alveolar bone density in rat model of periodontitis. J. Biomater. Sci. Polym. 2017, 29, 74–91. [Google Scholar] [CrossRef]
- Ma, Y.; Song, J.; Almassri, H.N.S.; Zhang, D.; Zhang, T.; Cheng, Y.; Wu, X. Minocycline-loaded PLGA electrospun membrane prevents alveolar bone loss in experimental peridontitis. Drug Deliv. 2020, 27, 151–160. [Google Scholar] [CrossRef]
- Ho, M.H.; Claudia, J.C.; Tai, W.C.; Huang, K.Y.; Lai, C.H.; Chang, C.H.; Chang, Y.C.; Wu, Y.C.; Kuo, M.Y.; Chang, P.C. The treatment response of barrier membrane with amoxicillin-loaded nanofibers in experimental periodontitis. J. Periodontol. 2021, 92, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Huang, L.; Sun, J.; Sun, J.; Yan, Q.; Duan, B.; Zhang, L.; Shi, B. Multifunctional chitin-based barrier membrane with antibacterial and osteogenic activities for the treatment of periodontal disease. Carbohydr. Polym. 2021, 269, 118276. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, L.; Jin, H.; Wu, Y.; Liu, Y.; Huang, W.; Wei, L.; Zhou, Q.; Chen, F.; Gao, Y.; et al. An enzyme-responsive membrane for antibiotic drug release and local periodontal treatment. Colloids Surf. B Biointerfaces 2019, 183, 110454. [Google Scholar] [CrossRef] [PubMed]
- Choung, H.W.; Lee, D.S.; Park, Y.H.; Lee, Y.S.; Bai, S.; Yoo, S.H.; Lee, J.H.; You, H.K.; Park, J.C. The effect of CPNE7 on periodontal regeneration. Connect. Tissue Res. 2019, 60, 419–430. [Google Scholar] [CrossRef]
- Ionel, A.; Lucaciu, O.; Bondor, C.; Moga, M.; Ilea, A.; Feurdean, C.; Buhăţel, D.; Hurubeanu, L.; Câmpian, R.S. Assessment of the relationship between periodontal disease and cardiovascular disorders: A questionnaire-based study. Clujul Med. 2016, 89, 534–541. [Google Scholar] [CrossRef]
- Ionel, A.; Lucaciu, O.; Moga, M.; Buhatel, D.; Ilea, A.; Tabaran, F.; Catoi, C.; Berce, C.; Toader, S.; Campian, R.S. Periodontal disease induced in Wistar rats—Experimental study. HVM Bioflux 2015, 7, 90–95. [Google Scholar]
- Satheesh, K. Successful Strategies for Periodontal Debridement, Dimensions of Dental Hygiene. Dimens. Dent. Hyg. 2017, 15, 39–44. [Google Scholar]
- Guzeldemir-Akcakanat, E. Systemic antibiotics in the treatment of periodontitis. Dent. Med. Res. 2019, 7, 33–34. [Google Scholar] [CrossRef]
- Bogdanovska, L.; Kukeska, S.; Popovska, M.; Petkovska, R.; Goracinova, K. Therapeutic strategies in the treatment of periodontitis. Macedonian pharmaceutical bulletin. Maced. Pharm. Bull. 2012, 58, 3–14. [Google Scholar] [CrossRef]
- Bailey, S.R. Local drug delivery: Current applications. Prog. Cardiovasc. Dis. 1997, 40, 183–204. [Google Scholar] [CrossRef]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef]
- Osorio, R.; Alfonso-Rodríguez, C.A.; Medina-Castillo, A.L.; Alaminos, M.; Toledano, M. Bioactive Polymeric Nanoparticles for Periodontal Therapy. PLoS ONE 2016, 11, e0166217. [Google Scholar] [CrossRef] [PubMed]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Tsalikis, L.; Sakellari, D.; Dagalis, P.; Boura, P.; Konstantinidis, A. Effects of doxycycline on clinical, microbiological and immunological parameters in well-controlled diabetes type-2 patients with periodontal disease: A randomized, controlled clinical trial. J. Clin. Periodontol. 2014, 41, 972–980. [Google Scholar] [CrossRef]
- Stabholz, A.; Sela, M.N.; Friedman, M.; Golomb, G.; Soskolne, A. Clinical and microbiological effects of sustained release chlorhexidine in periodontal pockets. J. Clin. Periodontol. 1986, 13, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, S.; Jalaluddin, M.D.; Khalid, I.; Moon, N.; Shafi, T.K.; Ali, F.M. The Use of Controlled Release Locally Delivered 10% Doxycycline Hyclate Gel as an adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis: Clinical and Microbiological Results. J. Contemp. Dent. Pract. 2013, 14, 1080–1086. [Google Scholar]
- Kida, D.; Karolewicz, B.; Junka, A.; Sender-Janeczek, A.; Duś, I.; Marciniak, D.; Szulc, M. Metronidazole-Loaded Porous Matrices for Local Periodontitis Treatment: In Vitro Evaluation and In Vivo Pilot. Study Appl. Sci. 2019, 9, 4545. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural Polymers for the Maintenance of Oral Health: Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 10337. [Google Scholar] [CrossRef]
- Wirth, R.; Pap, B.; Maróti, G.; Vályi, P.; Komlósi, L.; Barta, N.; Strang, O.; Minárovits, J.; Kovács, K.L. Toward Personalized Oral Diagnosis: Distinct Microbiome Clusters in Periodontitis Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 747814. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Egle, K.; Salma, I.; Dubnika, A. From Blood to Regenerative Tissue: How Autologous Platelet-Rich Fibrin Can Be Combined with Other Materials to Ensure Controlled Drug and Growth Factor Release. Int. J. Mol. Sci. 2021, 22, 11553. [Google Scholar] [CrossRef] [PubMed]
- Melo-Ferraz, A.; Coelho, C.; Miller, P.; Criado, M.B.; Monteiro, M.C. Platelet activation and antimicrobial activity of L-PRF: A preliminary study. Mol. Biol. Rep. 2021, 48, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Perez, K.; Dym, H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent. Clin. N. Am. 2020, 64, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Rockwell, H. The Benefits of Platelet-Rich Fibrin. Facial Plast. Surg. Clin. N. Am. 2019, 27, 331–340. [Google Scholar] [CrossRef]
- Egle, K.; Skadins, I.; Grava, A.; Micko, L.; Dubniks, V.; Salma, I.; Dubnika, A. Injectable Platelet-Rich Fibrin as a Drug Carrier Increases the Antibacterial Susceptibility of Antibiotic-Clindamycin Phosphate. Int. J. Mol. Sci. 2022, 23, 7407. [Google Scholar] [CrossRef] [PubMed]
- Straub, A.; Vollmer, A.; Lâm, T.T.; Brands, R.C.; Stapf, M.; Scherf-Clavel, O.; Bittrich, M.; Fuchs, A.; Kübler, A.C.; Hartmann, S. Evaluation of advanced platelet-rich fibrin (PRF) as a bio-carrier for ampicillin/sulbactam. Clin. Oral. Investig. 2022; advance online publication. [Google Scholar] [CrossRef]
- Dubnika, A.; Egle, K.; Skrinda-Melne, M.; Skadins, I.; Rajadas, J.; Salma, I. Development of Vancomycin Delivery Systems Based on Autologous 3D Platelet-Rich Fibrin Matrices for Bone Tissue Engineering. Biomedicines 2021, 9, 814. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y. Autologous liquid platelet rich fibrin: A novel drug delivery system. Acta Biomater. 2018, 75, 35–51. [Google Scholar] [CrossRef]
- Di Liddo, R.; Bertalot, T.; Borean, A.; Pirola, I.; Argentoni, A.; Schrenk, S.; Cenzi, C.; Capelli, S.; Conconi, M.T.; Parnigotto, P.P. Leucocyte and Platelet-rich Fibrin: A carrier of autologous multipotent cells for regenerative medicine. J. Cell. Mol. Med. 2018, 22, 1840–1854. [Google Scholar] [CrossRef]
- Rafiee, A.; Memarpour, M.; Taghvamanesh, S.; Karami, F.; Karami, S.; Morowvat, M.H. Drug Delivery Assessment of a Novel Triple Antibiotic-Eluting Injectable Platelet-Rich Fibrin Scaffold: An In Vitro Study. Curr. Pharm. Biotechnol. 2021, 22, 380–388. [Google Scholar] [CrossRef]
- Kuehnel, R.U.; Schroeter, F.; Mueller, T.; Ostovar, R.; Albes, J.M. Platelet-Rich Fibrin in Combination with Local Antibiotics Optimizes Wound Healing After Deep Sternal Wound Problems and Prevents Reinfection. Surg. Technol. Int. 2021, 39, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Kornsuthisopon, C.; Pirarat, N.; Osathanon, T.; Kalpravidh, C. Autologous platelet-rich fibrin stimulates canine periodontal regeneration. Sci. Rep. 2020, 10, 1850. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, H.S.; Gummaluri, S.S.; Astekar, M.; Gummaluri, R.K. Novel method of determining the periodontal regenerative capacity of T-PRF and L-PRF: An immunohistochemical study. Dent. Med. Probl. 2020, 57, 137–144. [Google Scholar] [CrossRef] [PubMed]
| Study | Selection | Comparability | Outcome | NOS Score |
|---|---|---|---|---|
| Human Studies | ||||
| Mahajania et al., 2018 [34] | *** | ** | *** | 8 |
| Gamal and Kumper, 2012 [35] | ** | ** | *** | 7 |
| Khan et al., 2015 [36] | *** | ** | *** | 8 |
| Kassem et al., 2015 [37] | ** | ** | *** | 7 |
| Animal Studies | ||||
| Liu et al., 2020 [38] | ** | * | *** | 6 |
| Khajuria et al., 2018 [39] | *** | ** | *** | 8 |
| Khajuria et al., 2017 [40] | *** | ** | *** | 8 |
| Ma et al., 2020 [41] | *** | ** | *** | 8 |
| Ho et al., 2021 [42] | *** | * | *** | 7 |
| Wu et al., 2021 [43] | * | ** | *** | 7 |
| Li et al., 2019 [44] | *** | ** | *** | 8 |
| Choung et al., 2019 [45] | ** | ** | *** | 7 |
| References | Number of Patients | Type of Membrane | Loaded Drug | Results |
|---|---|---|---|---|
| Mahajania et al., 2018 [34] | n = 19 | Hydroxy-propyl methylcellulose films. | Doxycicline (DOX) | Local release of doxycicline is an effective antibiotic of choice in periodontitis. |
| Gamal and Kumper, 2012 [35] | n = 30 | Biodegradable collagen membrane (COL) | Doxycycline (DOX) | The results of the study suggest that the DOX–COL + EDTA-guided tissue antibacterial regimen is a convenient method for obtaining a prolonged drug release without compromising the space that should be occupied by regenerating tissues or by inducing smearing of the root surface by DOX gel. |
| Khan et al., 2015 [36] | n = 10 | Biodegradable films of chitosan (CS) | Metronidazole (MZ) and levofloxacin (LF) | The films of MZ and LF were successful for the management of periodontitis. |
| Kassem et al., 2015 [37] | n = 5 | Chitosan-alginate and chitosan-pectin polyelectrolyte complex (PEC) films | Tetracycline hydrochloride (Tc) | PEC films could be exploited as a prolonged drug release devices for treatment of periodontal pockets. |
| References | Type of Subjects (Number) | Type of Membrane | Type of Load | Results |
|---|---|---|---|---|
| Liu et al., 2020 [38] | Dogs (n = 9) | Core-shell nanofiber membrane. | SP600125 and BMP-2 | Membranes with sequential release of SP600125 and BMP-2 represent a good therapy for periodontitis. |
| Khajuria et al., 2018 [39] | Rats (n = 40) | Bioabsorbable chitosan-metformin based intrapocket dental film (CMIDF) | Metformin hydrochloride | This study indicates potential antibacterial and osteoprotective efficacy of novel CMIDF in experimental periodontitis. |
| Khajuria et al., 2017 [40] | Rats (n = 60) | Bioresorbable chitosan-based risedronate/zinc-hydroxyapatite intrapocket dental film (CRZHDF) | Risedronate/zinc-hydroxyapatite nanoparticles | The study reported here reveals that novel CRZHDF treatment effectively reduced alveolar bone destruction and contributes to periodontal healing in a rat model of experimental periodontitis. |
| Ma et al., 2020 [41] | Rats (n = 25) | Poly (lactic-co-glycolic acid) electrospun membrane (PLGA) | Minocycline | This study states that Minocycline-loaded PLGA could be used to stimulate bone regeneration in periodontal disease. |
| Ho et al., 2021 [42] | Rats (n = 27) | Poly-DL-lactic acid nanofibers (PDLLA) | Amoxicilin (AMX) | PDDLA-AMX decreased inflammation and accelerated periodontal regeneration. |
| Wu et al., 2021 [43] | Rats (-) | chitin hydrogel membrane (ChT-1%ZnO) | zinc oxide nanoparticles | The results suggested that ChT-1%ZnO performs better than pure ChT in periodontal bone defects regeneration, but it could not fully repair the defect to the primary level. |
| Li et al., 2019 [44] | Rats (n = 12) | chitosan membrane | polyphosphoester and minocycline hydrochloride (PPEM) | Incorporation of 0.6% (W/V) MH drug provided the membrane with efficient antibacterial activity and that it could sustain drug release in vitro to achieve longer drug concentration maintenance. |
| Choung et al., 2019 [45] | Dogs (n = 8) | collagen carrier | protein copine7 (Cpne7) | The PDL fibers’ physiological organization and periodontal cells’ attachment to cementum may be supported by Cpne7. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrescu, N.; Crisan, B.; Aghiorghiesei, O.; Sarosi, C.; Mirica, I.C.; Lucaciu, O.; Iușan, S.A.L.; Dirzu, N.; Apostu, D. Gradual Drug Release Membranes and Films Used for the Treatment of Periodontal Disease. Membranes 2022, 12, 895. https://doi.org/10.3390/membranes12090895
Petrescu N, Crisan B, Aghiorghiesei O, Sarosi C, Mirica IC, Lucaciu O, Iușan SAL, Dirzu N, Apostu D. Gradual Drug Release Membranes and Films Used for the Treatment of Periodontal Disease. Membranes. 2022; 12(9):895. https://doi.org/10.3390/membranes12090895
Chicago/Turabian StylePetrescu, Nausica, Bogdan Crisan, Ovidiu Aghiorghiesei, Codruta Sarosi, Ioana Codruta Mirica, Ondine Lucaciu, Simina Angela Lăcrimioara Iușan, Noemi Dirzu, and Dragos Apostu. 2022. "Gradual Drug Release Membranes and Films Used for the Treatment of Periodontal Disease" Membranes 12, no. 9: 895. https://doi.org/10.3390/membranes12090895
APA StylePetrescu, N., Crisan, B., Aghiorghiesei, O., Sarosi, C., Mirica, I. C., Lucaciu, O., Iușan, S. A. L., Dirzu, N., & Apostu, D. (2022). Gradual Drug Release Membranes and Films Used for the Treatment of Periodontal Disease. Membranes, 12(9), 895. https://doi.org/10.3390/membranes12090895










