An Ionic Supported Liquid Membrane for the Recovery of Bisphenol A from Aqueous Solution
Abstract
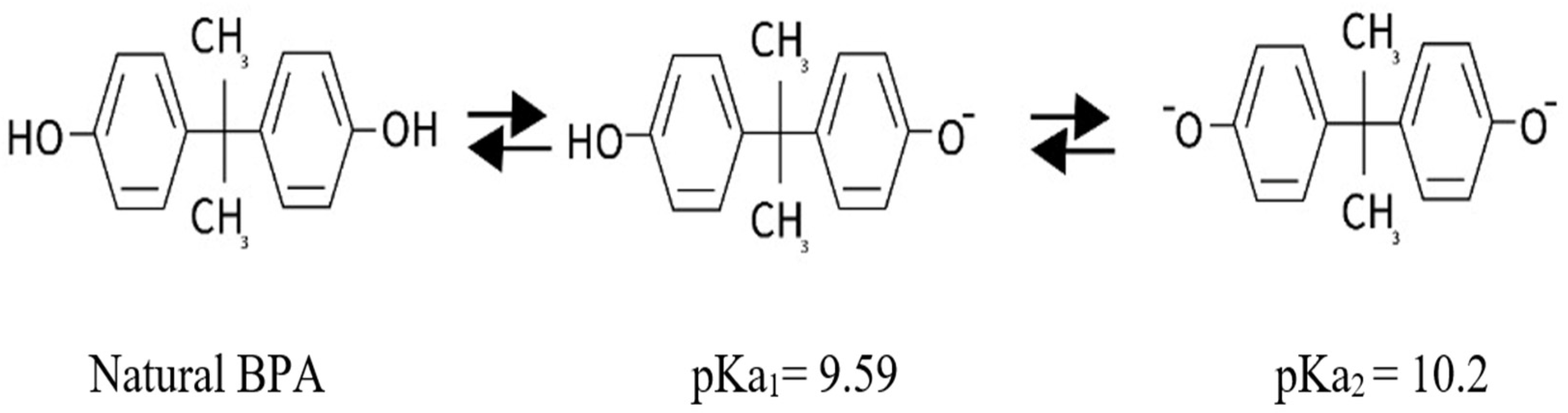
:1. Introduction
2. Experimental
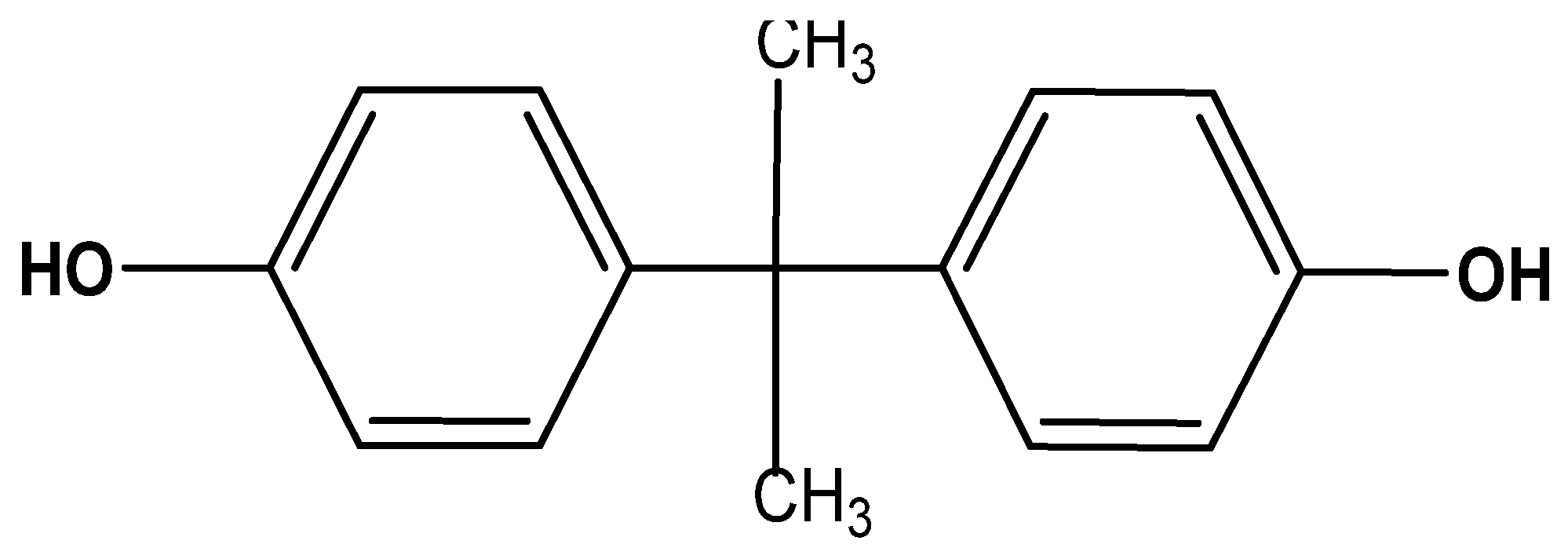
2.1. Materials
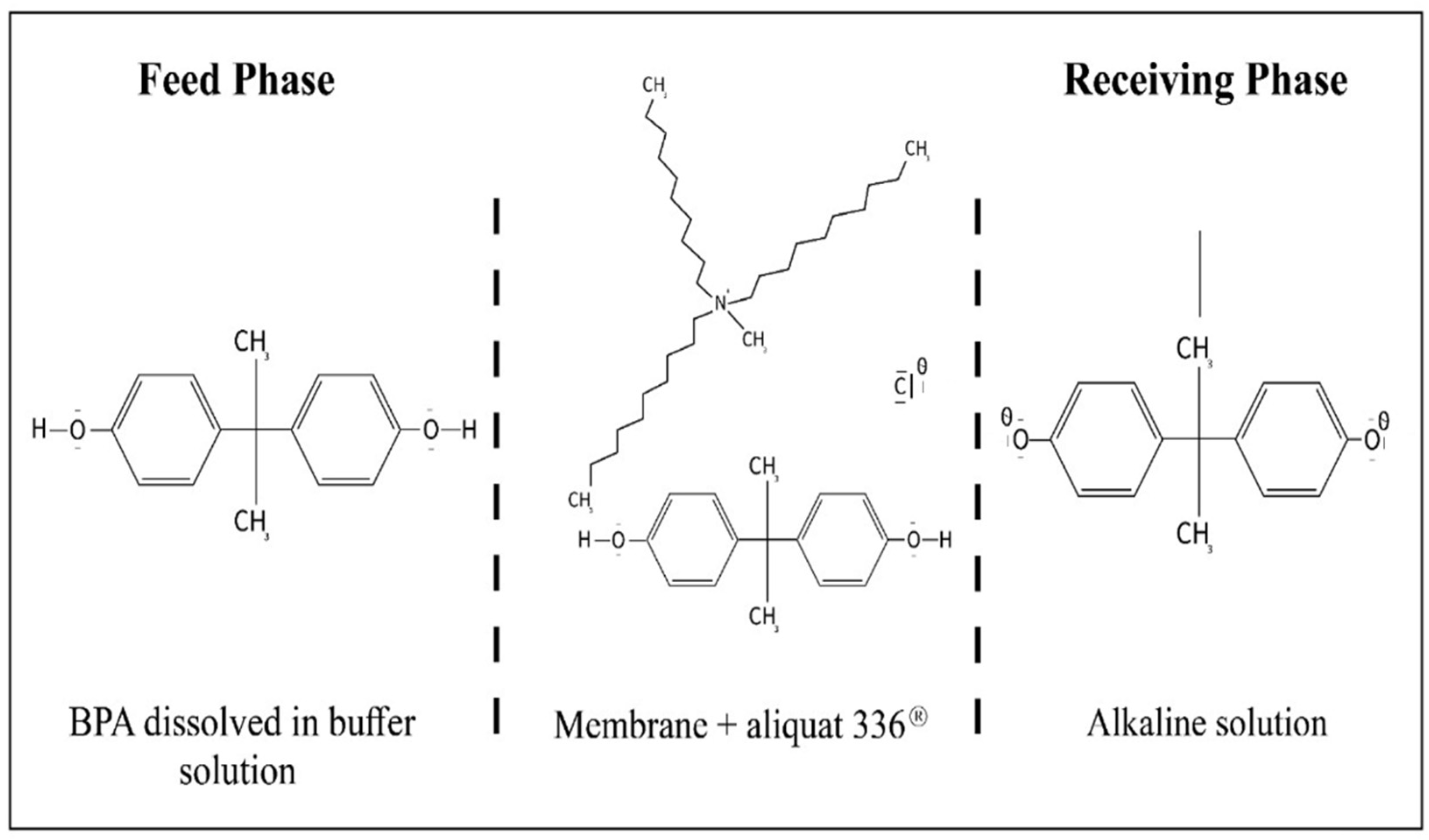
2.2. Membrane Preparation
2.3. Buffer Solutions Preparation
3. Results and Discussion
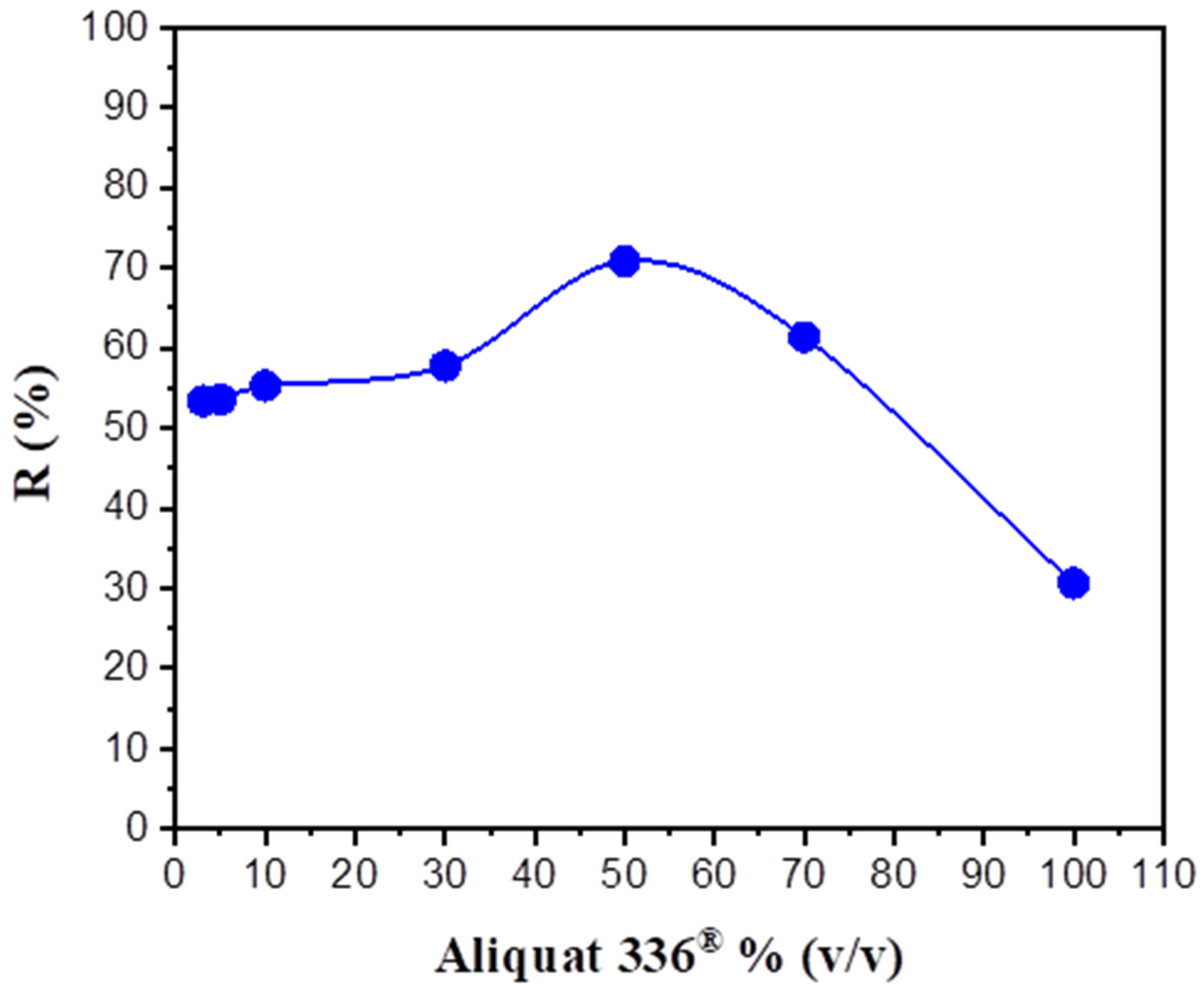
3.1. Effect of Aliquat 336® Percentage
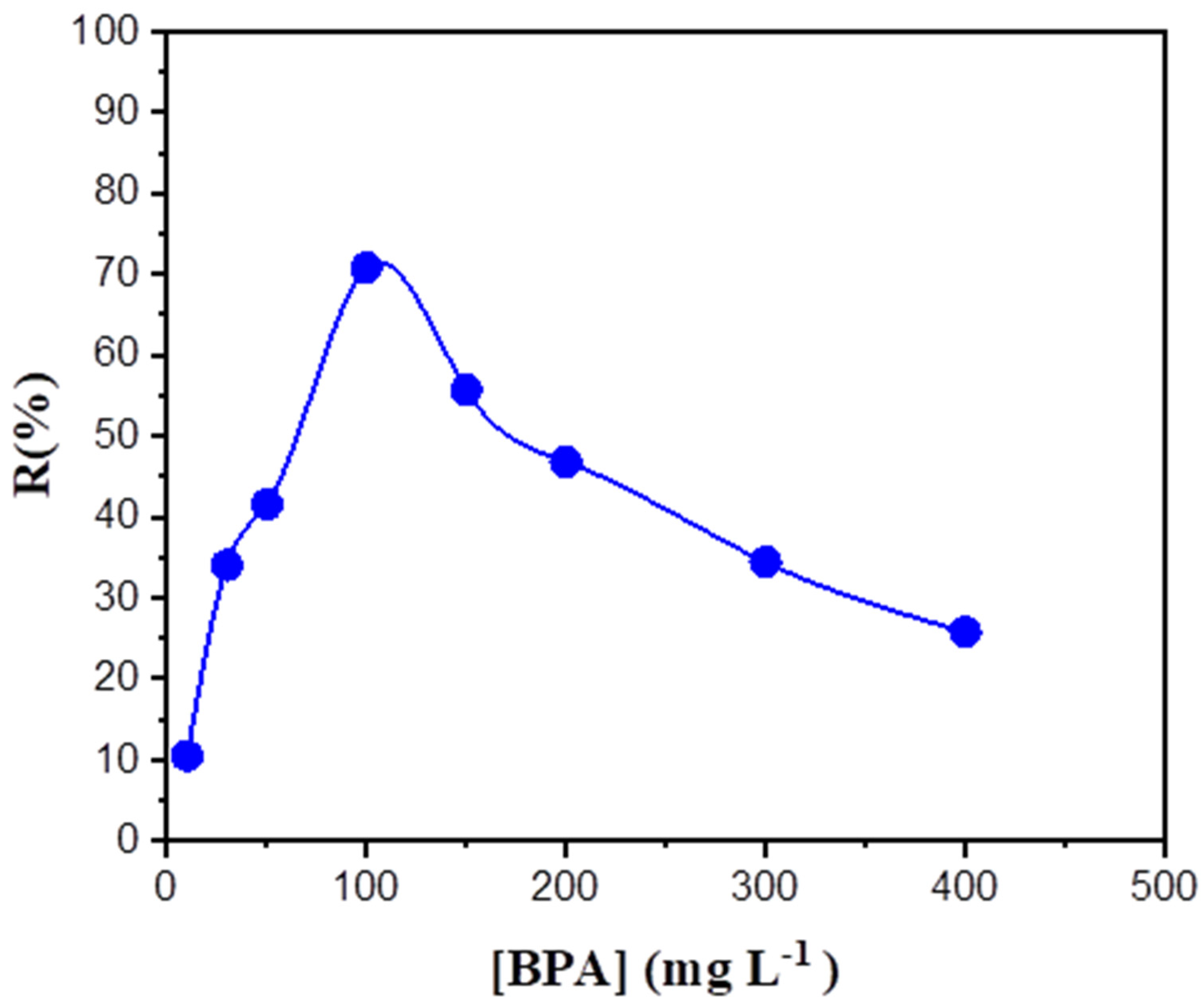
3.2. Effect of BPA Concentration
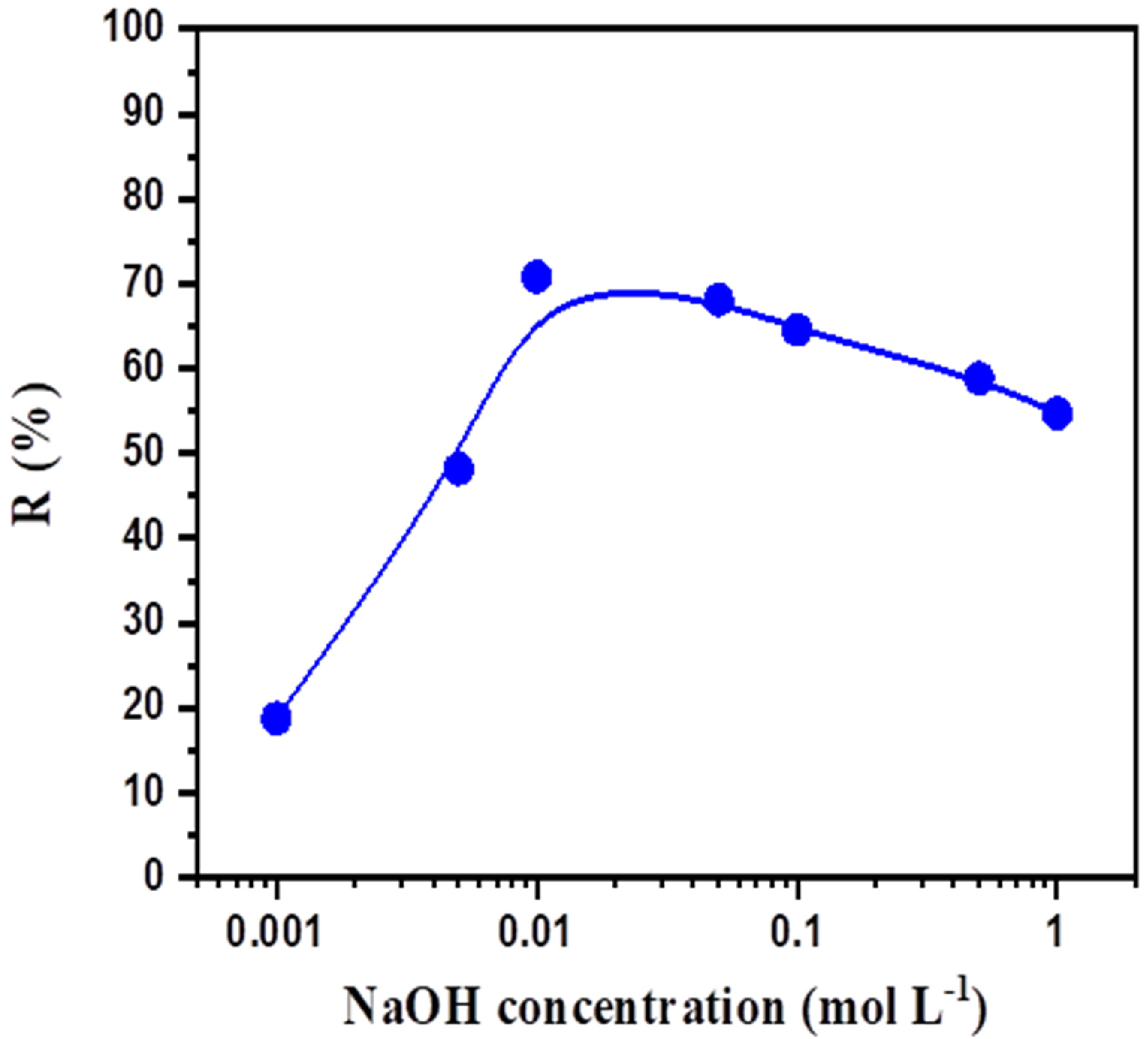
3.3. Effect of NaOH Concentration
3.4. Effect of the Feed Phase pH
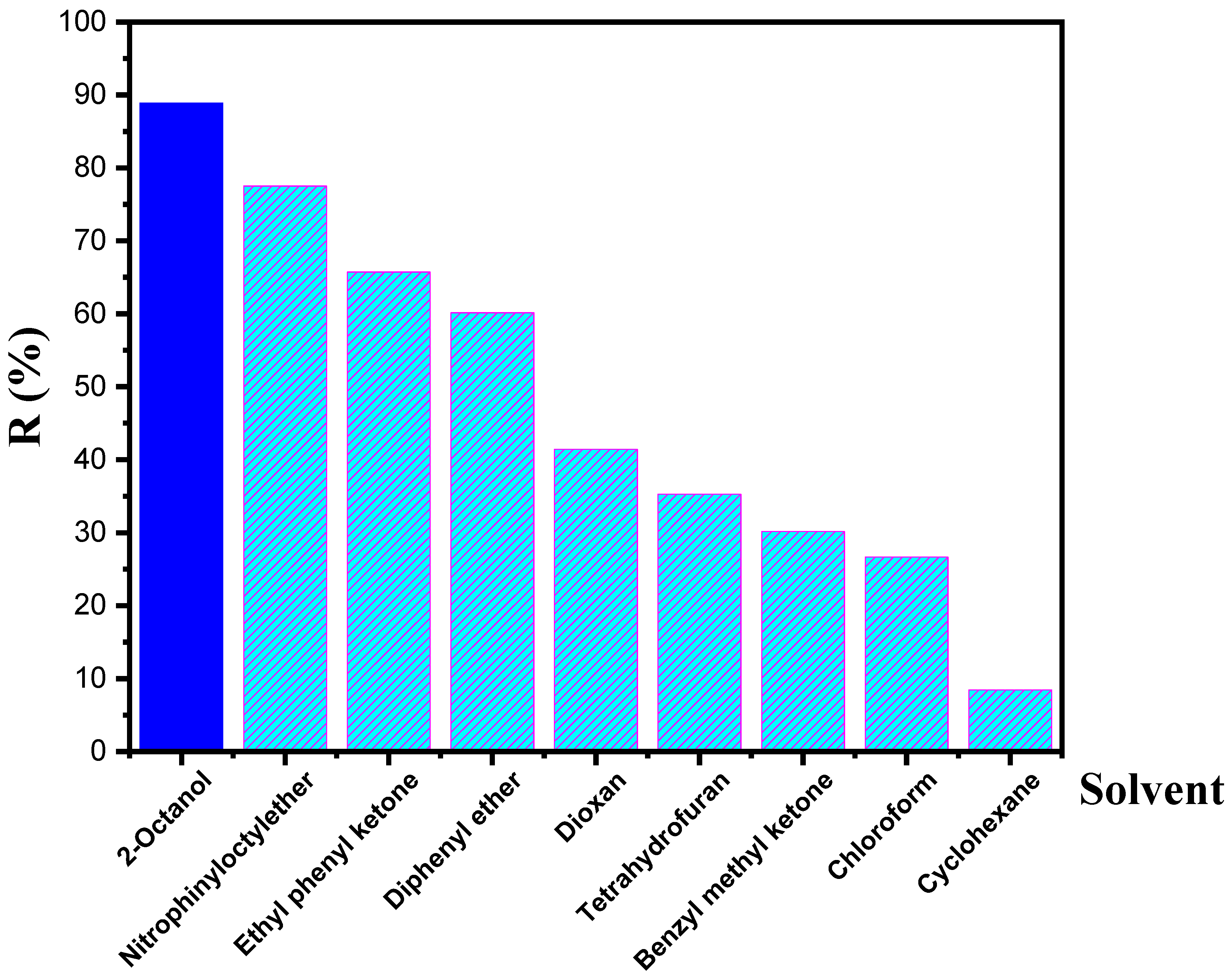
3.5. Effect of the Diluent
3.6. Nature of the Stripping Agent
3.7. Effect of the Polymeric Support Type
3.8. Membrane Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zielinska, M.; Wojnowska-Baryla, I.; Cydzik-Kwiatkowska, A. Sources and Properties of BPA. In Bisphenol A Remov. from Water Wastewater; Springer: Cham, Switzerland, 2019; pp. 3–28. [Google Scholar] [CrossRef]
- Mustieles, V.; D’Cruz, S.C.; Couderq, S.; Rodríguez-Carrillo, A.; Fini, J.B.; Hofer, T.; Steffensen, I.L.; Dirven, H.; Barouki, R.; Olea, N.; et al. Bisphenol A and Its Analogues: A Comprehensive Review to Identify and Prioritize Effect Biomarkers for Human Biomonitoring. Environ. Int. 2020, 144, 105811. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive Removal of Bisphenol A (BPA) from Aqueous Solution: A Review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Bisphenol A—ECHA. Available online: https://echa.europa.eu/hot-topics/bisphenol-a (accessed on 14 June 2021).
- Scientific Opinion on the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs. EFSA J. 2015, 13, 3978. [CrossRef]
- Watanabe, N.; Horikoshi, S.; Kawabe, H.; Sugie, Y.; Zhao, J.; Hidaka, H. Photodegradation Mechanism for Bisphenol A at the TiO2/H2O Interfaces. Chemosphere 2003, 52, 851–859. [Google Scholar] [CrossRef]
- Zhou, D.; Wu, F.; Deng, N.; Xiang, W. Photooxidation of Bisphenol a (BPA) in Water in the Presence of Ferric and Carboxylate Salts. Water Res. 2004, 38, 4107–4116. [Google Scholar] [CrossRef]
- Yeo, M.K.; Kang, M. Photodecomposition of Bisphenol A on Nanometer-Sized TiO2 Thin Film and the Associated Biological Toxicity to Zebrafish (Danio Rerio) during and after Photocatalysis. Water Res. 2006, 40, 1906–1914. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F. Bisphenol A Degradation by Bacteria Isolated from River Water. Arch. Environ. Contam. Toxicol. 2002, 43, 265–269. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Yuan, L.; Yu, J.; Li, J.; Huang, G.; Xi, B.; Liu, H. Aerobic Degradation of Bisphenol A by Achromobacter Xylosoxidans Strain B-16 Isolated from Compost Leachate of Municipal Solid Waste. Chemosphere 2007, 68, 181–190. [Google Scholar] [CrossRef]
- Fan, J.; Fan, Y.; Pei, Y.; Wu, K.; Wang, J.; Fan, M. Solvent Extraction of Selected Endocrine-Disrupting Phenols Using Ionic Liquids. Sep. Purif. Technol. 2008, 61, 324–331. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Feng, H.; Zhou, Y.; Zeng, G.; Lu, Y.; Yu, J.; Ren, X.; Peng, B.; Liu, X. Carbon Felt Cathodes for Electro-Fenton Process to Remove Tetracycline via Synergistic Adsorption and Degradation. Sci. Total Environ. 2019, 670, 921–931. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Guo, J.; Manladan, S.M.; Luo, Z.; Li, Y. Effects of Local Stiffness on the Spot Joints Mechanical Properties: Comparative Study between Resistance Spot Welding and Resistance Spot Clinching Joints. J. Manuf. Process. 2019, 39, 93–101. [Google Scholar] [CrossRef]
- Hama Aziz, K.H. Application of Different Advanced Oxidation Processes for the Removal of Chloroacetic Acids Using a Planar Falling Film Reactor. Chemosphere 2019, 228, 377–383. [Google Scholar] [CrossRef] [PubMed]
- de Cavalcanti, V.O.M.; Santana, R.M.R.; Neves, N.S.d.C.S.; de Lucena, A.L.A.; de Oliveira, M.A.S.; do Nascimento, G.E.; Napoleão, D.C. Treatment of the Drugs Atenolol and Propranolol by Advanced Oxidation Processes, a Kinetic Approach, Toxicity Effects on Seeds, and Chromatographic Analysis. Chem. Pap. 2021, 75, 4391–4403. [Google Scholar] [CrossRef]
- da Silva, S.W.; Welter, J.B.; Albornoz, L.L.; Heberle, A.N.A.; Ferreira, J.Z.; Bernardes, A.M. Advanced Electrochemical Oxidation Processes in the Treatment of Pharmaceutical Containing Water and Wastewater: A Review. Curr. Pollut. Rep. 2021, 7, 146–159. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, H.; Zhao, Y.; Deng, X.; Ke, S.; Li, Y.; Tian, M. Implementation of Fluidized-Bed Fenton as Tertiary Treatment of Nitro-Aromatic Industrial Wastewater. Process Saf. Environ. Prot. 2021, 146, 490–498. [Google Scholar] [CrossRef]
- Cañizares, P.; Paz, R.; Sáez, C.; Rodrigo, M.A. Costs of the Electrochemical Oxidation of Wastewaters: A Comparison with Ozonation and Fenton Oxidation Processes. J. Environ. Manag. 2009, 90, 410–420. [Google Scholar] [CrossRef]
- Van, H.T.; Nguyen, L.H.; Hoang, T.K.; Nguyen, T.T.; Tran, T.N.H.; Nguyen, T.B.H.; Vu, X.H.; Pham, M.T.; Tran, T.P.; Pham, T.T.; et al. Heterogeneous Fenton Oxidation of Paracetamol in Aqueous Solution Using Iron Slag as a Catalyst: Degradation Mechanisms and Kinetics. Environ. Technol. Innov. 2020, 18, 100670. [Google Scholar] [CrossRef]
- da Silva, M.P.; de Souza, A.C.A.; de Lima Ferreira, L.E.; Neto, L.M.P.; Nascimento, B.F.; de Araújo, C.M.B.; Fraga, T.J.M.; da Motta Sobrinho, M.A.; Ghislandi, M.G. Photodegradation of Reactive Black 5 and Raw Textile Wastewater by Heterogeneous Photo-Fenton Reaction Using Amino-Fe3O4-Functionalized Graphene Oxide as Nanocatalyst. Environ. Adv. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Panigrahi, A.; Pilli, S.R.; Mohanty, K. Selective Separation of Bisphenol A from Aqueous Solution Using Supported Ionic Liquid Membrane. Sep. Purif. Technol. 2013, 107, 70–78. [Google Scholar] [CrossRef]
- Gupta, S.; Chakraborty, M.; Murthy, Z.V.P. Performance Study of Hollow Fiber Supported Liquid Membrane System for the Separation of Bisphenol A from Aqueous Solutions. J. Ind. Eng. Chem. 2014, 20, 2138–2145. [Google Scholar] [CrossRef]
- Dzygiel, P.; Wieczorek, P.P. Supported Liquid Membranes and Their Modifications: Definition, Classification, Theory, Stability, Application and Perspectives. In Liquid Membranes; Elsevier: Amsterdam, The Netherlands, 2010; pp. 73–140. [Google Scholar] [CrossRef]
- Sastre, A.M.; Kumar, A.; Shukla, J.P.; Singh, R.K. Improved Techniques in Liquid Membrane Separations: An Overview. Sep. Purif. Methods 2008, 27, 213–298. [Google Scholar] [CrossRef]
- Stolwijk, T.B.; Sudholter, E.J.R.; Reinhoudt, D.N. Crown Ether Mediated Transport: A Kinetic Study of Potassium Perchlorate Transport through a Supported Liquid Membrane Containing Dibenzo-18-Crown-6. J. Am. Chem. Soc. 1987, 109, 7042–7047. [Google Scholar] [CrossRef]
- Kemperman, A.J.B.; Bargeman, D.; Van Den Boomgaard, T.; Strathmann, H. Stability of Supported Liquid Membranes: State of the Art. Sep. Sci. Technol. 2006, 31, 2733–2762. [Google Scholar] [CrossRef]
- Teramoto, M.; Sakaida, Y.; Fu, S.S.; Ohnishi, N.; Matsuyama, H.; Maki, T.; Fukui, T.; Arai, K. An Attempt for the Stabilization of Supported Liquid Membrane. Sep. Purif. Technol. 2000, 21, 137–144. [Google Scholar] [CrossRef]
- Goh, S.S.; Rafatullah, M.; Ismail, N.; Alam, M.; Siddiqui, M.R.; Seow, E.-K. Separation of Chromium (VI), Copper and Zinc: Chemistry of Transport of Metal Ions across Supported Liquid Membrane. Membranes 2022, 12, 685. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Ionic Liquids. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 218–225. [Google Scholar] [CrossRef]
- Blaga, A.C.; Tucaliuc, A.; Kloetzer, L. Applications of Ionic Liquids in Carboxylic Acids Separation. Membranes 2022, 12, 771. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; de los Ríos, A.P.; Rubio, M.; Tomás-Alonso, F.; Gómez, D.; Víllora, G. A Novel Application of Supported Liquid Membranes Based on Ionic Liquids to the Selective Simultaneous Separation of the Substrates and Products of a Transesterification Reaction. J. Memb. Sci. 2007, 293, 73–80. [Google Scholar] [CrossRef]
- De los Ríos, A.P.; Hernández-Fernández, F.J.; Tomás-Alonso, F.; Palacios, J.M.; Gómez, D.; Rubio, M.; Víllora, G. A SEM–EDX Study of Highly Stable Supported Liquid Membranes Based on Ionic Liquids. J. Memb. Sci. 2007, 300, 88–94. [Google Scholar] [CrossRef]
- Fortunato, R.; Afonso, C.A.M.; Reis, M.A.M.; Crespo, J.G. Supported Liquid Membranes Using Ionic Liquids: Study of Stability and Transport Mechanisms. J. Memb. Sci. 2004, 242, 197–209. [Google Scholar] [CrossRef]
- Branco, L.C.; Crespo, J.G.; Afonso, C.A.M. Studies on the Selective Transport of Organic Compounds by Using Ionic Liquids as Novel Supported Liquid Membranes. Chem. A Eur. J. 2002, 8, 3865–3871. [Google Scholar] [CrossRef]
- Pérezpérez-Silva, I.; Galángalán-Vidal, C.A.; Teresa Ramírez-Silva, M.; Rodríguez, J.J.; Arturo, G.; Lvarez-Romero, A.; Paéz-Hernándezhernández, M.E. Phenol Removal Process Development from Synthetic Wastewater Solutions Using a Polymer Inclusion Membrane. Ind. Eng. Chem. Res. 2013, 52, 4919–4923. [Google Scholar] [CrossRef]
- Onac, C.; Kaya, A.; Ataman, D.; Gunduz, N.A.; Alpoguz, H.K. The Removal of Cr(VI) through Polymeric Supported Liquid Membrane by Using Calix[4]Arene as a Carrier. Chin. J. Chem. Eng. 2019, 27, 85–91. [Google Scholar] [CrossRef]
- Xu, D.; Shah, Z.; Sun, G.; Peng, X.; Cui, Y. Recovery of Rare Earths from Phosphate Ores through Supported Liquid Membrane Using N,N,N′,N′-Tetraoctyl Diglycol Amide. Miner. Eng. 2019, 139, 105861. [Google Scholar] [CrossRef]
- Jos’, F.J.; Alguacil, J.; On Caravaca, C.; Martín, M.I. Transport of Chromium(VI) through a Cyanex 921-Supported Liquid Membrane from HCl Solutions. J. Chem. Technol. Biotechnol. 2003, 78, 1048–1053. [Google Scholar] [CrossRef]
- Chaouchi, S.; Hamdaoui, O. Acetaminophen Extraction by Emulsion Liquid Membrane Using Aliquat 336 as Extractant. Sep. Purif. Technol. 2014, 129, 32–40. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the Removal of Phenol from Fluid Streams: A Short Review of Recent Developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Li, J.; Jing, C.; Fang, C.; Liu, Y.; Wang, Y.; Liu, J.; Bi, S.; Chen, Y.; Xiao, Y.; et al. Separation and Recovery of Phenols from an Aqueous Solution by a Green Membrane System. J. Clean. Prod. 2020, 251, 119675. [Google Scholar] [CrossRef]
- Nosrati, S.; Jayakumar, N.S.; Hashim, M.A. Performance Evaluation of Supported Ionic Liquid Membrane for Removal of Phenol. J. Hazard. Mater. 2011, 192, 1283–1290. [Google Scholar] [CrossRef]
- Meng, X.; Gao, G.; Wang, L.; Wang, X.; Tang, W.; Chen, N. Transport of Phenol through Polymer Inclusion Membrane with N,N-Di (1-Methylheptyl) Acetamide as Carriers from Aqueous Solution. J. Membr. Sci. 2015, 493, 615–621. [Google Scholar] [CrossRef]
- Liu, X.; Bian, Y.; Zhao, J.; Wang, Y.; Zhao, L. Menthol-Based Deep Eutectic Solvent in Dispersive Liquid-Liquid Microextraction Followed by Solidification of Floating Organic Droplet for the Determination of Three Bisphenols with UPLC-MS/MS. Microchem. J. 2020, 159, 105438. [Google Scholar] [CrossRef]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A Review of the Environmental Fate, Effects, and Exposures of Bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Degradation of Bisphenol A by Ozonation: Rate Constants, Influence of Inorganic Anions, and by-Products. Maejo Int. J. Sci. Technol. 2012, 6, 77–94. [Google Scholar]
- Venkateswaran, P.; Palanivelu, K. Recovery of Phenol from Aqueous Solution by Supported Liquid Membrane Using Vegetable Oils as Liquid Membrane. J. Hazard. Mater. 2006, 131, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Chimuka, L.; Msagati, T.A.M.; Cukrowska, E.; Tutu, H. Critical Parameters in a Supported Liquid Membrane Extraction Technique for Ionizable Organic Compounds with a Stagnant Acceptor Phase. J. Chromatogr. A. 2010, 1217, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, V.; Surucu, A.; Kunduracioglu, A. Synergistic Extraction of Cr(VI) from Ni(II) and Co(II) by Flat Sheet Supported Liquid Membranes Using TIOA and TBP as Carriers. Polish J. Chem. Technol. 2015, 17, 34–42. [Google Scholar] [CrossRef]
- Pilli, S.R.; Banerjee, T.; Mohanty, K. 1-Butyl-2,3-Dimethylimidazolium Hexafluorophosphate as a Green Solvent for the Extraction of Endosulfan from Aqueous Solution Using Supported Liquid Membrane. Chem. Eng. J. 2014, 257, 56–65. [Google Scholar] [CrossRef]
- Rosly, M.B.; Jusoh, N.; Othman, N.; Rahman, H.A.; Noah, N.F.M.; Sulaiman, R.N.R. Effect and Optimization Parameters of Phenol Removal in Emulsion Liquid Membrane Process via Fractional-Factorial Design. Chem. Eng. Res. Des. 2019, 145, 268–278. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ohtani, T.; Kondo, K. Comparison of Solvent Extraction and Supported Liquid Membrane Permeation Using an Ionic Liquid for Concentrating Penicillin G. J. Memb. Sci. 2007, 289, 92–96. [Google Scholar] [CrossRef]
- Kouki, N.; Tayeb, R.; Dhahbi, M. A Flat-Sheet Supported Liquid Membrane Based on Aliquat® 336 as Carrier for the Removal of Salicylic Acid from Aqueous Solution. New Pub. Balaban 2014, 52, 4745–4754. [Google Scholar] [CrossRef]
- Kouki, N.; Tayeb, R.; Dhahbi, M. Recovery of Acetaminophen from Aqueous Solutions Using a Supported Liquid Membrane Based on a Quaternary Ammonium Salt as Ionophore. Chem. Pap. 2014, 68, 457–464. [Google Scholar] [CrossRef]
- Zaghbani, A.; Tayeb, R.; Dhahbi, M.; Hidalgo, M.; Vocanson, F.; Bonnamour, I.; Seta, P.; Fontàs, C. Selective Thiacalix[4]Arene Bearing Three Amide Groups as Ionophore of Binary Pd(II) and Au(III) Extraction by a Supported Liquid Membrane System. Sep. Purif. Technol. 2007, 57, 374–379. [Google Scholar] [CrossRef]
- Zidi, C.; Tayeb, R.; Ben, M.; Ali, S.; Dhahbi, M. Liquid–liquid Extraction and Transport across Supported Liquid Membrane of Phenol Using Tributyl Phosphate. J. Memb. Sci. 2010, 360, 334–340. [Google Scholar] [CrossRef]
- Fontàs, C.; Hidalgo, M.; Salvadó, V.; Anticó, E. Selective Recovery and Preconcentration of Mercury with a Benzoylthiourea-Solid Supported Liquid Membrane System. Anal. Chim. Acta 2005, 547, 255–261. [Google Scholar] [CrossRef]






| The pH Buffer | Method of Preparation |
|---|---|
| pH 12 | Glycine–NaOH buffer Stock solution: A: 0.2 M solution of glycine (1.5 g in 100 mL) B: 0.2 M NaOH 50 mL of A + 46.5 mL of B, diluted to a total of 200 mL |
| pH 10.6 | Glycine–NaOH buffer Stock solution: A: 0.2 M solution of glycine (1.5 g in 100 mL) B: 0.2 M NaOH 50 mL of A + 45.5 mL of B, diluted to a total of 200 mL |
| pH 8.6 | Glycine–NaOH buffer Stock solution: A: 0.2 M solution of glycine (1.5 g in 100 mL) B: 0.2 M NaOH 50 mL of A + 4 mL of B, diluted to a total of 200 mL |
| pH 6 | Succinic buffer Stock solution: A: 0.2 M solution of succinic acid (5.9 g in 250 mL) B: 0.2 M NaOH 25 mL of A + 43.5 mL of B, diluted to a total of 100 mL |
| pH 4 | Succinic buffer Stock solution: A: 0.2 M solution of succinic acid (5.9 g in 250 mL) B: 0.2 M NaOH. 25 mL of A + 10 mL of B, diluted to a total of 100 mL |
| pH 2 | Hydrochloric acid–Potassium chloride buffer Stock solution: A: 0.2 M solution of KCl (7.46 g in 50 mL) B: 0.2 M HCl. 50 mL of A + 10.6 mL of B, diluted to a total of 200 mL |
| Compound | Formula | Viscosity η (cP) | Dielectric Constant ɛr |
|---|---|---|---|
| Tetrahydrofuran | C4H8O | 0.55 | 7.58 |
| Chloroform | CHCl3 | 0.57 | 4.81 |
| Cyclohexane | C6H12 | 1 | 18.5 |
| Dioxane | C4H8O | 1.54 | 2.21 |
| Diphenylether | C12H10O | 3.9 | - |
| 2-Octanol | C8H18O | 10.6 | 3.4 |
| Nitrophenyloctylether | C14H21NO3 | 12.8 | 23.1 |
| Benzylmethylketone | C9H10O | - | - |
| Ethylphenylketone | C9H10O | - | - |
| Polymeric Support | Durapore® GVSP Millipore, USA | Celgard 2500 Celgard Inc., USA | Accurel® PP-2E Enka | Accurel® PP 2E-HF Membrana, USA |
|---|---|---|---|---|
| Material | Polyvinylidene Difluoride | Polypropylene | Polypropylene | Polypropylene |
| Thickness d0 (μm) | 120 | 25 | 130–180 | 160 |
| Pore diameter d (μm) | 0.2 | 0.064 | 0.2 | 0.2 |
| Porosity ɛ (%) | 65 | 55 | 70 | 75 |
| Tortuosity (τ = 1 − lnε) | 1.43 | 1.598 | 1.357 | 1.29 |
| ε/d0τ (10−3 µm−1) | 3.788 | 13.76 | 3.328 | 3.633 |
| Jexp (10−6 mol m−2 s−1) | 4.8 | 0.32 | 0.48 | 0.16 |
| JN (10−6 mol m−2 s−1) | 4.6 | 0.084 | 0.524 | 0.16 |
| JN (10−6 mol m−2 s−1) | 8.6 [53] | 6.9 [53] | ||
| JN (10−6 mol m−2 s−1) | 0.43 [54] | 4 [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldwaish, M.; Kouki, N.; Algreiby, A.; Tar, H.; Tayeb, R.; Hafiane, A. An Ionic Supported Liquid Membrane for the Recovery of Bisphenol A from Aqueous Solution. Membranes 2022, 12, 869. https://doi.org/10.3390/membranes12090869
Aldwaish M, Kouki N, Algreiby A, Tar H, Tayeb R, Hafiane A. An Ionic Supported Liquid Membrane for the Recovery of Bisphenol A from Aqueous Solution. Membranes. 2022; 12(9):869. https://doi.org/10.3390/membranes12090869
Chicago/Turabian StyleAldwaish, Manal, Noura Kouki, Azizah Algreiby, Haja Tar, Rafik Tayeb, and Amor Hafiane. 2022. "An Ionic Supported Liquid Membrane for the Recovery of Bisphenol A from Aqueous Solution" Membranes 12, no. 9: 869. https://doi.org/10.3390/membranes12090869
APA StyleAldwaish, M., Kouki, N., Algreiby, A., Tar, H., Tayeb, R., & Hafiane, A. (2022). An Ionic Supported Liquid Membrane for the Recovery of Bisphenol A from Aqueous Solution. Membranes, 12(9), 869. https://doi.org/10.3390/membranes12090869





