Scalant Removal at Acidic pH for Maximum Ammonium Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterisation
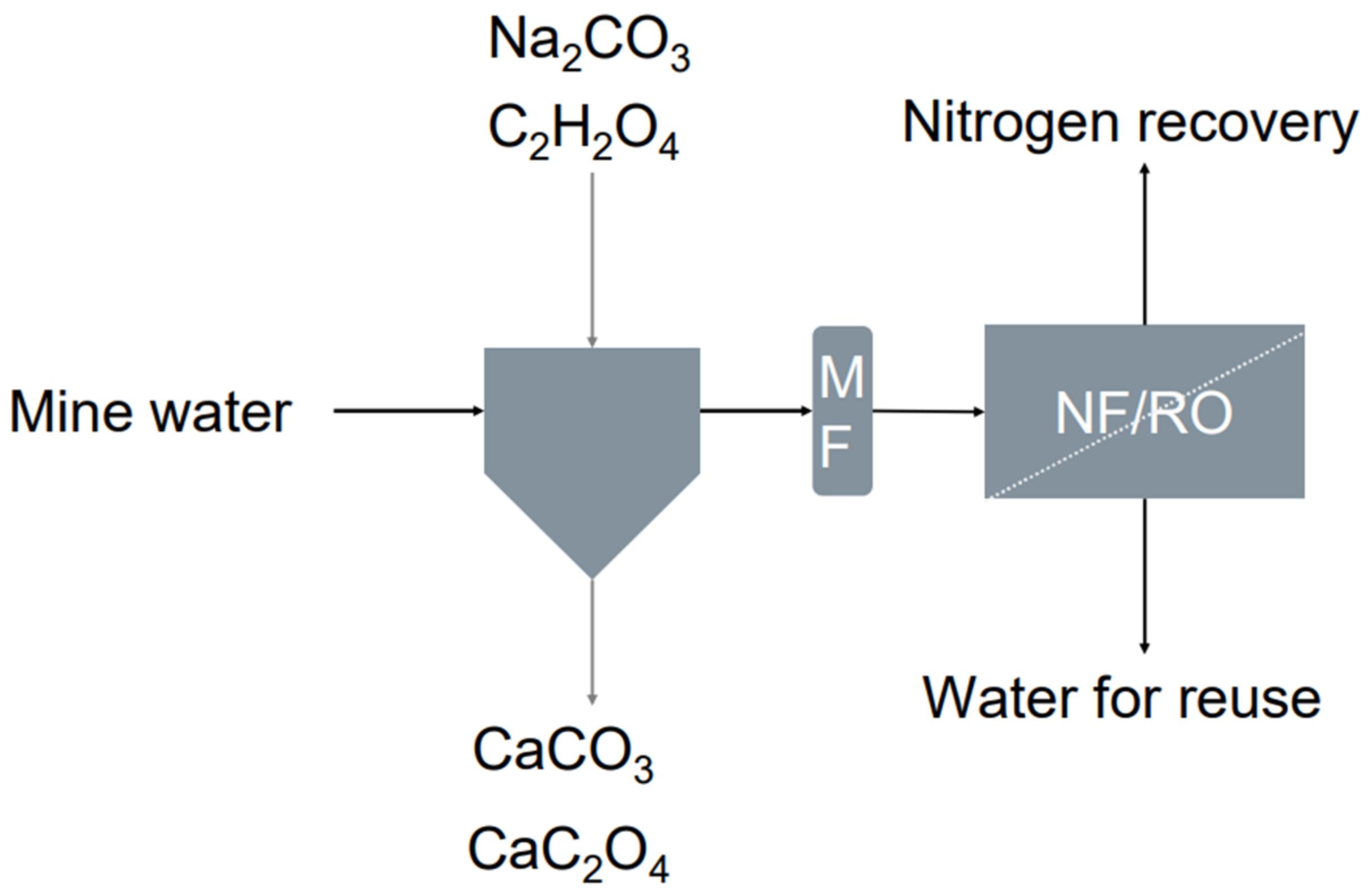
2.2. Precipitation
2.3. Membrane Filtration
3. Results
3.1. Liquids Characterisation
3.2. Precipitation
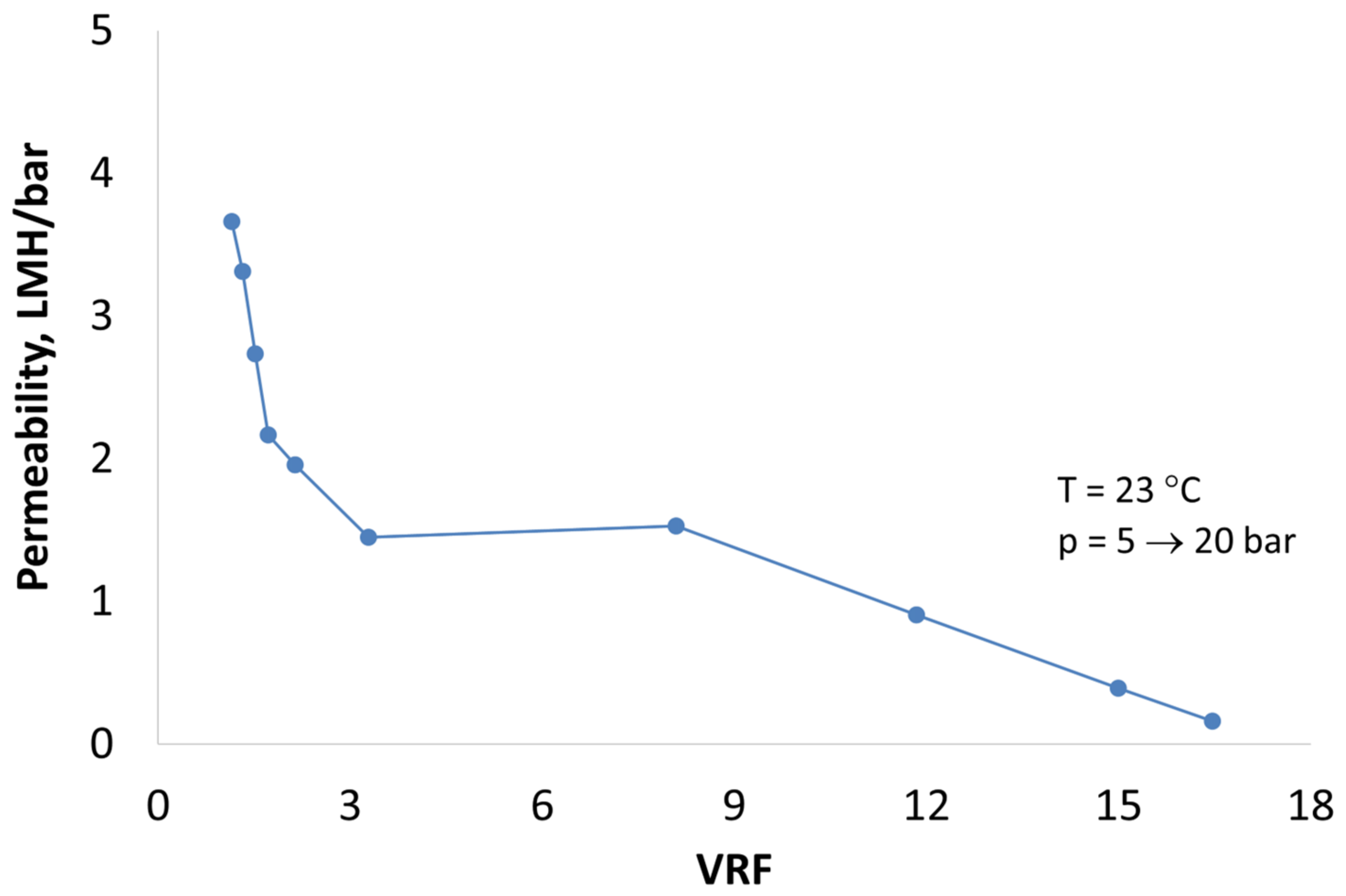
3.3. Membrane Filtration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Z.; Van Linden, N.; Guillen, E.; Spanjers, H.; Van Lier, J.B. Recovery and applications of ammoniacal nitrogen from nitrogen-loaded residual streams: A review. J. Environ. Manag. 2021, 295, 113096. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoek, J.P.; Duijff, R.; Reinstra, O. Nitrogen Recovery from Wastewater: Possibilities, Competition with Other Resources, and Adaptation Pathways. Sustainability 2018, 10, 4605. [Google Scholar] [CrossRef] [Green Version]
- Iddya, A.; Hou, D.; Khor, C.M.; Ren, Z.; Tester, J.; Posmanik, R.; Gross, A.; Jassby, D. Efficient ammonia recovery from wastewater using electrically conducting gas stripping membranes. Environ. Sci. 2020, 7, 1759. [Google Scholar] [CrossRef]
- Kinnunen, P.; Kyllönen, H.; Kaartinen, T.; Mäkinen, J.; Heikkinen, J.; Miettinen, V. Sulphate removal from mine water with chemical, biological and membrane technologies. Water Sci. Technol. 2017, 1, 194–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jermakka, J.; Wendling, L.; Sohlberg, E.; Heinonen, H.; Merta, E.; Laine-Ylijoki, J.; Kaartinen, T.; Mroueh, U.-M. Nitrogen compounds at mines and quarries, Sources, behaviour and removal from mine and quarry waters—Literature study. VTT Technol. 2015, 226, 1–144. [Google Scholar]
- Häyrynen, K.; Pongrácza, E.; Väisänen, V.; Pap, N.; Mänttäri, M.; Langwaldt, J.; Keiski, R.L. Concentration of ammonium and nitrate from mine water by reverse osmosis and nanofiltration. Desalination 2009, 240, 280–289. [Google Scholar] [CrossRef]
- Ogunbiyi, O.; Saththasivam, J.; Al-Masri, D.; Manawi, Y. Sustainable brine management from the perspectives of water, energy and mineral recovery: A comprehensive review. Desalination 2021, 513, 115055. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, J.; Gao, J.; Ji, Z.; Xiaofu, G.X.; Liu, J.; Yuan, J. Trash to treasure: Seawater pretreatment by CO2 mineral carbonation using brine pretreatment waste of soda ash plant as alkali source. Desalination 2017, 407, 85–92. [Google Scholar] [CrossRef]
- Shenvi, S.; Isloor, A.; Ismail, A. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Kyllönen, H.; Grönroos, A.; Järvelä, E.; Heikkinen, J.; Tang, C. Experimental Aspects of Scaling Control in Membrane Filtration of Mine Water. Mine Water Environ. 2016, 36, 193–198. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, H.; Xie, Y.; Yuan, J.; Ji, Z.; Yan, Z. Mechanism studies of a CO2 participant softening pretreatment process for seawater desalination. Desalination 2016, 393, 166–173. [Google Scholar] [CrossRef]
- Ubbink, B.J. Controlled Precipitation of Calcium Carbonate in Spatial Dimension with Multiple Calcium Chloride and Sodium Carbonate Pulses. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2013. [Google Scholar]
- Aghajanian, S.; Nieminen, H.; Koiranen, T. Precipitation of calcium carbonate in highly alkaline solution through carbonated water Precipitation of calcium carbonate in highly alkaline solution through carbonated water. In Proceedings of the 2nd International Process Intensification Conference, Leuven, Belgium, 27 May 2019. [Google Scholar]
- Ryu, M.Y.; You, K.S.; Ahn, J.W.; Kim, H. Effect of the pH and Basic Additives on the Precipitation of Calcium Carbonate during Carbonation Reaction. Resour. Process. 2007, 54, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Kontrec, J.; Tomaši, N.; Mlinari, N.M.; Kralj, D.; Džakula, B.N. Effect of pH and Type of Stirring on the Spontaneous Precipitation of CaCO3 at Identical Initial Supersaturation, Ionic Strength and (Ca2+)/(CO32−) Ratio. Crystals 2021, 11, 1075. [Google Scholar] [CrossRef]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Nilvebrand, N.-O.; Reimann, A.; De Sousa, F.; Cassland, P.; Larsson, S.; Hong, F.; Jönsson, L.J. Enzymatic degradation of oxalic acid for prevention of scaling. In Biotechnology in the Pulp and Paper Industry 8th ICBPPI; Viikari, L., Lantto, R., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2002; Volume 21, pp. 231–238. [Google Scholar]
- Damtie, M.; Volpin, F.; Yao, M.; Tijing, L.D.; Hailemariam, R.H.; Bao, T.; Park, K.-D.; Shon, H.K.; Choi, J.-S. Ammonia recovery from human urine as liquid fertilizers in hollow fiber membrane contactor: Effects of permeate chemistry. Environ. Eng. Res. 2021, 26, 190523. [Google Scholar] [CrossRef]
- Masse, L.; Masse, D.I.; Pellerin, Y. Review Paper: SE—Structures and Environment The use of membranes for the treatment of manure: A critical literature review. Biosyst. Eng. 2007, 98, 371–380. [Google Scholar] [CrossRef]
- Vecino, X.; Reiga, M.; Bhushana, B.; Giberta, O.; Valderramaa, C.; Cortinaa, J.L. Liquid fertilizer production by ammonia recovery from treated ammonia rich regenerated streams using liquid-liquid membrane contactors. Chem. Eng. J. 2019, 360, 890–899. [Google Scholar] [CrossRef]
- Arogo, J.; Westerman, P.W.; Heber, A.J.; Robarge, W.P.; Classen, J.J. Ammonia Emissions from Animal Feeding Operations. White Paper Prepared for: National Center for Manure and Animal Waste Management North Carolina State University 2014, Raleigh, N.C. Available online: https://www.researchgate.net/publication/238176842_Ammonia_Emissions_from_Animal_Feeding_Operations (accessed on 1 August 2022).
- Freney, J.R.; Simpson, J.R.; Denmead, O.T. Volatilization of Ammonia, Gaseous Loss of Nitrogen from Plant-Soil Systems; Part of the Developments in Plant and Soil Sciences Book Series; Springer Science + Business Media: Dordrecht, The Netherlands, 1983; Volume 9, pp. 1–32. [Google Scholar] [CrossRef]
- Kyllönen, H.; Heikkinen, J.; Järvelä, E.; Sorsamäki, L.; Siipola, V.; Grönroos, A. Wastewater Purification with Nutrient and Carbon Recovery in a Mobile Resource Container. Membranes 2021, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Hevia, G.; Serralta, J.; Seco, A.; Ferrer, J. Economic analysis of the scale-up and implantation of a hollow fibre membrane contactor plant for nitrogen recovery in a full-scale wastewater treatment plant. Sep. Purif. Technol. 2021, 275, 119128. [Google Scholar] [CrossRef]
- LCK Cuvette Test System|HACH. Available online: https://uk.hach.com/lck (accessed on 16 November 2022).



| Parameter | Unit | Process Water |
|---|---|---|
| pH | 7.1 | |
| Conductivity | mS/cm | 6.1 |
| Osmotic pressure | bar | 1.6 |
| Total-N | mg/L | 174 |
| NH4-N | mg/L | 170 |
| NO3-N | mg/L | 13 |
| Al | mg/L | <0.03 |
| Ca | mg/L | 390 |
| Cu | mg/L | <0.003 |
| Mg | mg/L | 55 |
| Mn | mg/L | 0.12 |
| Fe | mg/L | <0.05 |
| Ni | mg/L | 0.051 |
| Cr | mg/L | <0.003 |
| P | mg/L | <0.01 |
| Ba | mg/L | 0.007 |
| S | mg/L | 920 |
| SO4 | mg/L | 2600 |
| Na | mg/L | 680 |
| K | mg/L | 36 |
| Si | mg/L | 8.2 |
| Zn | mg/L | <0.005 |
| Chemical | Dose g/L | pH | NH4-N mg/L | Ca mg/L | Mg mg/L |
|---|---|---|---|---|---|
| - | - | 8.1 | 170 | 390 | 77 |
| Na2CO3 | 0.8 | 8.3 | 140 | 170 | 59 |
| Na2CO3 | 1.7 | 9.5 | 150 | 140 | 23 |
| Na2CO3 | 4.0 | 10.1 | 150 | 7 | 34 |
| C2H2O4 | 0.13 | 6.0 | 140 | 310 | 77 |
| C2H2O4 | 0.77 | 2.2 | 170 | 81 | 56 |
| Chemical | Dose g/L | pH | π Bar | NH4-N mg/L | NO3-N mg/L | Ca mg/L | Mg mg/L | SO4 mg/L | K mg/L | COD mg/L |
|---|---|---|---|---|---|---|---|---|---|---|
| - | - | 8.1 | 1.6 | 170 | 13 | 390 | 77 | 2600 | 69 | 160 |
| Na2CO3 | 4.0 | 10.1 | 3.5 | 150 | 12 | 7 | 34 | 2300 | 45 | na |
| C2H2O4 | 0.77 | 2.2 | 2.0 | 170 | 12 | 81 | 56 | 2200 | 41 | 240 |
| Membrane/pH | VRF | Sample | NH4-N mg/L | NO3-N mg/L | Ca mg/L | Mg mg/L | SO4 mg/L | K mg/L |
|---|---|---|---|---|---|---|---|---|
| NF270/2.2 | Feed | 170 | 12 | 81 | 56 | 2200 | 41 | |
| 25 | Concentrate | 710 | 16 | 1000 | 1300 | 13,900 | 260 | |
| Permeate | 130 | 10 | 6 | 14 | 550 | 24 | ||
| NF90/2.2 | Feed | 170 | 12 | 81 | 56 | 2200 | 41 | |
| 25 | Concentrate | 2950 | 15 | 620 | 1300 | 22,100 | 860 | |
| Permeate | 9 | 11 | 1 | 4 | 120 | 2 | ||
| NF90/8.1 | Feed | 170 | 13 | 370 | 77 | 2600 | 69 | |
| No precipitation | 7 | Concentrate | 770 | 27 | 1400 | 550 | 9500 | 200 |
| Permeate | 18 | 9.8 | 0 | 3 | 33 | 0 | ||
| NF90/10.1 | Feed | 150 | 12 | 7 | 34 | 2300 | 45 | |
| 17 | Concentrate | 370 | 40 | 100 | 630 | 24,000 | 780 | |
| Permeate | 80 | 12 | 0 | 5 | 30 | 1 | ||
| NF90/7.5 | Feed | 130 | 12 | 18 | 36 | 4200 | 38 | |
| 14 | Concentrate | 1490 | 10 | 600 | 180 | 4400 | 510 | |
| Permeate | 25 | 12 | 0 | 0 | 15 | 6 | ||
| LG SW/10.1 | Feed | 120 | 12 | 7 | 34 | 2300 | 45 | |
| 17 | Concentrate | 440 | 250 | 110 | 800 | 36,300 | 910 | |
| Peremate | 70 | 2 | 0 | 2 | 45 | 3 | ||
| LG SW/7.5 | Feed | 130 | 12 | 18 | 36 | 4200 | 38 | |
| 17 | Concentrate | 2010 | 150 | 290 | 830 | 70,600 | 740 | |
| Permeate | 41 | 2 | 0 | 0 | 90 | 1 |
| pH | π mS/cm | NH4-N mg/L | NO3-N mg/L | Ca mg/L | K mg/L | Mg mg/L | Cl mg/L | SO4 mg/L | COD mg/L | |
|---|---|---|---|---|---|---|---|---|---|---|
| Original | 7.1 | 6.1 | 160 | 12.3 | 440 | 58 | 82 | 19 | 1900 | 160 |
| Feed | 2.1 | 8.4 | 160 | 13.8 | 110 | 73 | 110 | 21 | 2990 | 240 |
| Concentrate | 1.7 | 56 | 1910 | 15.7 | 540 | 660 | 980 | 20 | 36,300 | 110 |
| Permeate | 2.4 | 1.8 | 4.8 | 1.9 | 1 | 4 | 2 | 5.4 | 150 | 160 |
| Al mg/L | Ba mg/L | Cr mg/L | Cu mg/L | Fe mg/L | Mn mg/L | Na mg/L | Ni mg/L | P mg/L | Si mg/L | S mg/L | Zn mg/L | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed | <0.03 | 0.007 | <0.003 | <0.003 | <0.05 | 0.12 | 680 | 0.051 | <0.01 | 8.2 | 920 | <0.005 |
| Concentrate | 0.67 | 0.13 | 0.28 | 5.9 | 24 | 5.3 | 8800 | 1.2 | <5 | 76 | 13,000 | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyllönen, H.; Heikkinen, J.; Järvelä, E.; Grönroos, A. Scalant Removal at Acidic pH for Maximum Ammonium Recovery. Membranes 2022, 12, 1233. https://doi.org/10.3390/membranes12121233
Kyllönen H, Heikkinen J, Järvelä E, Grönroos A. Scalant Removal at Acidic pH for Maximum Ammonium Recovery. Membranes. 2022; 12(12):1233. https://doi.org/10.3390/membranes12121233
Chicago/Turabian StyleKyllönen, Hanna, Juha Heikkinen, Eliisa Järvelä, and Antti Grönroos. 2022. "Scalant Removal at Acidic pH for Maximum Ammonium Recovery" Membranes 12, no. 12: 1233. https://doi.org/10.3390/membranes12121233
APA StyleKyllönen, H., Heikkinen, J., Järvelä, E., & Grönroos, A. (2022). Scalant Removal at Acidic pH for Maximum Ammonium Recovery. Membranes, 12(12), 1233. https://doi.org/10.3390/membranes12121233






