Abstract
CO2/H2 separation using membrane technology is an important research area in order to obtain high purity hydrogen as one source of clean energy. Finding a suitable inorganic membrane is one of the critical issues, which needs to be explored for CO2/H2 separation. In the present study, Ba-SAPO-34 zeolite membrane was synthesized and followed by a modification process. CO2/H2 separation of the membrane was investigated by varying the independent process variables (CO2 % in the feed, pressure difference across the membrane and temperature). Modeling and optimization for the responses (CO2/H2 separation selectivity and CO2 permeance) was performed by applying response surface methodology and central composite design, which is available in Design Expert software. The accuracy of the models in predicting the response was tested by comparing with the experimental value of response and the two values were in good agreement. The optimization of the models gave CO2 permeance of 19.23 × 10−7 mol/m2 s Pa and CO2/H2 separation selectivity of 11.6 at 5% CO2 in the feed, a pressure difference of 100 kPa, and temperature of 30 °C for Ba-SAPO-34 zeolite membrane.
1. Introduction
In recent years, gas separation has received enormous attention among researchers due to the issues of energy security and global climate change. Numbers of articles for gas separation processes have been published [1,2,3,4,5]. Hydrogen (H2) separation technologies have gained increasing importance nowadays since hydrogen is one of the important chemical sources for industries. It is also one the main energy sources for transportation fuel and electrical power generation [6]. Separation and purification are important technologies in processes for H2 production, such as thermochemical processes. In order to obtain high purity H2, separation of H2 from carbon dioxide (CO2) is one such important area [5].
Common separation techniques for H2 separation from CO2 are physical absorption with solvents, pressure swing adsorption and cryogenic distillation [5,7]. However, these processes have a number of drawbacks such as complexity of the system, high energy consumption for solvent regeneration, equipment corrosion and flow problems caused by viscosity of solvent [8,9]. Membrane-based technology appears to be a potential alternative for H2 separation in view of its advantages such as sustainable operation and relatively low energy consumption [7]. Palladium membranes have been extensively studied for H2 separation due to its high hydrogen selectivity [10,11,12]. However, the usage of palladium and its alloys have a number of disadvantages, including a high sensitivity to chemicals (i.e., sulphur, chlorine and carbon monoxide in most applications) and their extremely high cost [13]. Polymeric membrane are other candidates for separation of H2 in view of their low cost and low energy requirement [14,15]. However, the application of polymeric membrane in H2 separation is limited by the disadvantages such as the low mechanical stability of rubbery polymers [16].
Zeolite membranes are microporous inorganic membranes that are gaining increasing interests for CO2/H2 separation. Zeolite membranes possess advantages such as uniform pore structure and high chemical stability [17,18]. Different types of zeolite membranes have been studied for gas permeation and separation. These include CHA [17], FAU-type [19,20], PWN-type [21], A-type [22,23,24,25], MFI-type [26,27,28,29,30,31], DDR [32,33], T-type [34,35] and silicoaluminophosphate (SAPO) membranes [18,36,37]. There have been a number of studies on SAPO-34 zeolite membrane for gas permeation and separation due to its small pore structure [18,36,37,38,39,40,41,42,43,44,45,46]. Owing to the pore size of SAPO-34 which is close to the kinetic diameter of the CO2 molecule, the SAPO-34 zeolite membrane has a high potential for separation of H2 from CO2. Hong et al. [39] reported that the SAPO-34 membrane selectively separated CO2 from the CO2/H2 binary gas mixture at low temperature and the membrane became H2 selective at a high temperature of 200 °C.
Design of Experiments is commonly used to perform optimization for the process parameters [47]. Response surface methodology (RSM), available in Design of Experiments, is a statistical tool that could allow reduction in the required numbers of experiments and could be used to investigate the effect of the significant process variable and the effect of the variables’ interaction on the process [48]. There have been numerous studies that have applied RSM and hence showed RSM as an effective tool for optimization of the process [49,50,51,52].
Our previous work [53] has shown that CO2/CH4 separation was selectivity improved from 30 for the H-SAPO-34 zeolite membrane to 103 for the Ba-SAPO-34 zeolite membrane. In the present work, Ba-SAPO-34 zeolite membrane was formed by modifying the pre-synthesized H+-form of SAPO-34 (H-SAPO-34) zeolite membrane. The Ba-SAPO-34 zeolite membrane was subjected to the CO2/H2 separation process by varying three process variables, which are CO2 % (concentration) in the feed, pressure difference and temperature. The objective of the current study was to perform optimization on the operating process conditions of the membrane separation for the CO2/H2 separation selectivity and CO2 permeance. In current work, CO2 permeance was reported instead of H2 permeance because the Ba-SAPO-34 membrane was found to be CO2-selective over the ranges of the process variables studied.
2. Materials and Methods
2.1. Preparation of Zeolite Membrane
H-SAPO-34 membrane was deposited on -alumina disc and then followed by a modification to the Ba-SAPO-34 membrane by following the procedures described in our previous work [43,53]. The synthesis precursor with the molar composition of Al2O3:P2O5:1.2TEAOH:0.3SiO2:80H2O was prepared by mixing deionized water, aluminium isopropoxide (Al(i-C3H7O)3, 98%, Merck, Darmstadt, Germany), tetraethylammonium hydroxide (TEAOH, 35 wt%, Sigma–Aldrich, St. Louis, MI, USA), phosphoric acid (H3PO4, 85%, Sigma–Aldrich) and Ludox AS-40 colloidal silica sol (40 wt%). The synthesis precursor was poured into a Teflon-lined vessel with α-Alumina disc placed in the vessel. The filled Teflon-lined vessel was heated at 200 °C for 2 h in a microwave oven (MARS 5, CEM Corporation, Matthews, NC, Canada). When the microwave heating was done, rinsing and drying were performed on the membrane. The procedures for heating, rinsing and drying were repeated three times. Calcination was performed on the H-SAPO-34 membrane at 400 °C for 15 h in a furnace. In order to modify the H-SAPO-34 membrane to the Ba-SAPO-34 membrane, ion-exchange was performed on the H-SAPO-34 membrane at 70 °C for 5 h by using ion-exchange solution containing Ba2+. Rinsing the membrane with ethanol and followed by drying the membrane at 100 °C overnight were then carried out. The characterization works of the Ba-SAPO-34 membrane were described in our previous work [43,53].
2.2. Design of Experiments
By using Design Expert software version 6.0.6 (STAT-EASE Inc., Minneapolis, MN, USA), Design of Experiments was applied for investigating the CO2/H2 separation. The modeling and analysis of problems, which include the generation of model equations by using experimental data, determination of the effect of variables and variables’ interaction on the responses and optimization studies on the responses, were performed by using RSM coupled with central composite design (CCD) [54,55].
Three independent variables were studied for CO2/H2 separation in the current study, which include CO2 % in the feed, pressure difference and temperature, as shown in Table 1. The factor code for CO2 % in the feed, pressure difference and temperature is C, B and A, respectively. As shown in Table 1, the low level and high level are represented by −1 and +1, respectively. CO2 separation selectivity and CO2 permeance are the responses that were investigated in the current study.

Table 1.
Independent variables with ranges for CO2/H2 separation studies in the current study.
Equations (1) and (2) show the polynomial that can be investigated by using Design Expert software for the approximation for the relationship between response (y) and the set of independent variables [50,54,56]:
First order model:
Second order model:
where y is the response, and are the independent variables, is the first order interaction between and , , , and is the regression coefficient for intercept, linear, quadratic and interaction terms, respectively, is the number of independent variables and is the error.
2.3. CO2/H2 Gas Separation Studies
The Ba-SAPO-34 membrane was sealed in a stainless steel module using silicone gaskets and was subjected to CO2/H2 separation studies. Mass flow controllers were used to feed CO2 and H2 gases to the membrane module The CO2 concentration in the feed was varied. The permeate pressure was kept at atmospheric pressure. Back pressure regulator was used to adjust the feed pressure so that the pressure difference across Ba-SAPO-34 membrane can be varied. The temperature for gas separation was varied by changing the temperature of an electronic-controlled oven where the membrane module was located. Online gas chromatography (PERKIN ELMER, CLARUS 500) equipped with CARBOXEN-1010 column and thermal conductivity detector, was used to analyze the composition of the permeate and retentate exit streams.
Permeance, (mol/m2 s Pa) of the gas was determined by using Equation (3).
where is the partial pressure difference of gas i across the membrane (Pa), is the flux of gas i (mol/m2 s), the gas i may corresponds to CO2 or H2.
The CO2/H2 separation selectivity, was determined by using Equation (4).
3. Results
3.1. Characterization Results of Ba-SAPO-34
The Ba-SAPO-34 membrane was characterized by using Scanning Electron Microscopy (SEM) and High-Resolution Transmission Electron Microscopy (HRTEM) in our previous work [43]. The top view SEM image and cross-sectional view SEM image of Ba-SAPO-34 membrane can be found in our previously published works [43]. It was observed from the top view SEM image of the Ba-SAPO-34 membrane that the membrane consists of orthorhombic zeolite crystals with a size of approximately 1 μm [43]. Meanwhile, the cross-sectional view SEM image of the Ba-SAPO-34 membrane showed that Ba-SAPO-34 membrane layer thickness is 4 μm approximately [43]. On the other hand, the HRTEM image of Ba-SAPO-34 can also be found in our previously published works [43] and the HRTEM showed Ba-SAPO-34 with pore channel diameter of less than 0.5 nm.
3.2. Experiment Design Matrix
In the current study, a total of 20 experiment runs for sets of independent variables (C: CO2 % in the feed, B: pressure difference and A: temperature) was suggested by CCD for the CO2/H2 gas separation studies as shown in Table 2. The values of CO2/H2 separation selectivity and CO2 permeance, which were obtained from experimental work, are shown in Table 2 as well. The CO2 permeance was in the range of 1.96 to 19.23 × 10−7 mol/m2 s Pa and the CO2/H2 separation selectivity was in the range of 2.3 to 12.2. The experimental runs at the temperature of 105 °C, pressure difference of 300 kPa and 27.5% CO2 in the feed were repeated another five times (run 15–20 as shown in Table 2) in order to check for the reproducibility of the data. The low values of standard deviations (0.01 for CO2 permeance and 0.05 for CO2/H2 separation selectivity) for the repeated runs indicates good reproducibility of the responses.

Table 2.
Independent variables and responses for the CO2/H2 separation studies.
3.3. Response Surface Modeling
“Inverse” transformation was used to analyze the responses of CO2 permeance and CO2/H2 separation selectivity as defined in Equation (5).
where y is the value of the response and y’ is the transformed value. “Inverse” transformation was applied because this function was able to model and predict the experimental data very well. The CO2 permeance and CO2/H2 separation selectivity was modeled, analyzed in the form of 1/(CO2 permeance) and 1/(CO/H2 separation selectivity) respectively.
3.3.1. Response Surface Modeling of CO2 Permeance
The Analysis of Variance (ANOVA) of the CO2 permeance is shown in Table 3. Equation (6) shows the chosen quadratic model to reflect the relationship between the independent variables and the response.
where C, B and A correspond to the coded value of CO2 % in the feed, pressure difference and temperature, respectively.

Table 3.
ANOVA of the CO2 permeance.
The model F-value of 29,876.61 implies the model was significant. Temperature (A) gave the highest F value of 26,945.27, indicating that it had the most significant effect on CO2 permeance compared to pressure difference (B) and CO2 % in the feed (C).
In order to have the terms of the model to be significant at the 95% confidence level, the values of probability should be less than 0.0500 (“Prob > F” less than 0.0500). In this case, all the terms (A, B, C, A2, B2, C2, AB, AC and BC) were found to be significant for the model of 1/(CO2 permeance). The “Lack of Fit F-value” of 1.16 implied that the Lack of Fit was not significant relative to the pure error. It is good to have non-significant Lack of Fit.
Figure 1 presents the comparison between predicted 1/(CO2 permeance) attained by using Equation (6) with the experimental 1/(CO2 permeance). Good agreement between predicted 1/(CO2 permeance) and experimental 1/(CO2 permeance) is indicated by the correlation coefficient value (R2) of 1.000. Hence, this reflects the high accuracy of the generated model Equation (6) to predict the 1/(CO2 permeance) in current work.
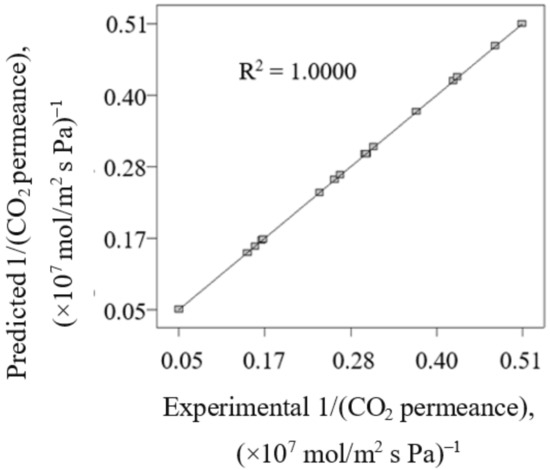
Figure 1.
The comparison between predicted 1/(CO2 permeance) attained by using Equation (6) with the experimental 1/(CO2 permeance).
Figure 2, Figure 3 and Figure 4 present the plots showing the effect of interaction between different independent variables on the 1/(CO2 permeance). It can be observed from Figure 2 that the 1/(CO2 permeance) increased with temperature for all three levels of pressure difference. A similar trend was reported by Li et al. [42]. When the separation temperature increased from 30 to 180 °C, the surface coverage declined and the CO2 diffusivity increased. The decline in surface coverage prevailed the increment in diffusivity when the temperature increased. Subsequently, this resulted in decline in CO2 permeance, and hence increment in the 1/(CO2 permeance) when the temperature increased. The 1/(CO2 permeance also increased with pressure difference as shown in Figure 2. In the current study, gas permeance was calculated by dividing the gas flux with partial pressure difference across the membrane. The increase in CO2 partial pressure gradient was more than the increase in CO2 surface coverage gradient, hence leading to a decrease in CO2 permeance with an increase in pressure difference of 100–500 kPa across the membrane in the current study. Figure 3 and Figure 4 show that the 1/(CO2 permeance) increased with an increase in CO2 % in the feed from 5 to 50%. When the CO2 % in the feed increased, the CO2 permeance decreased. The increase in the CO2 loading approached saturation in the membrane and thus resulted in drop in CO2 permeance as observed and reported by Hong et al. [39].
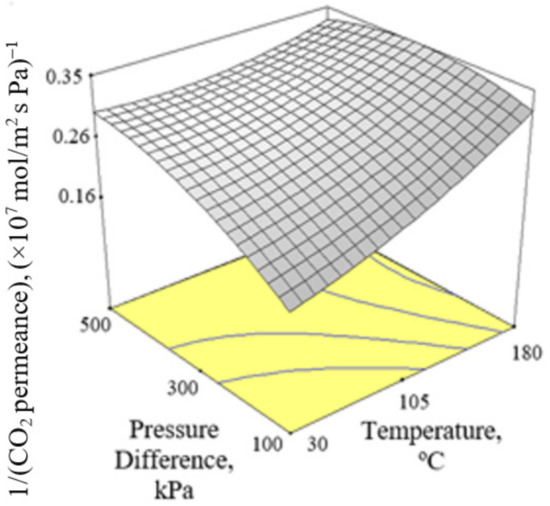
Figure 2.
Effect of interaction between temperature and pressure difference on the 1/(CO2 permeance) at 27.5% CO2 in the feed.
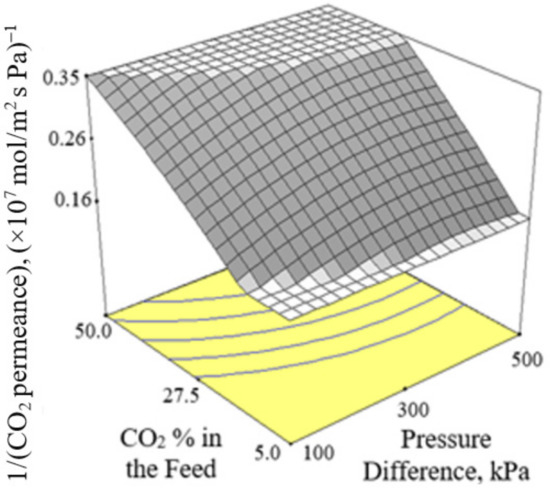
Figure 3.
Effect of interaction between CO2 % in the feed and pressure difference on the 1/(CO2 permeance) at 105 °C.
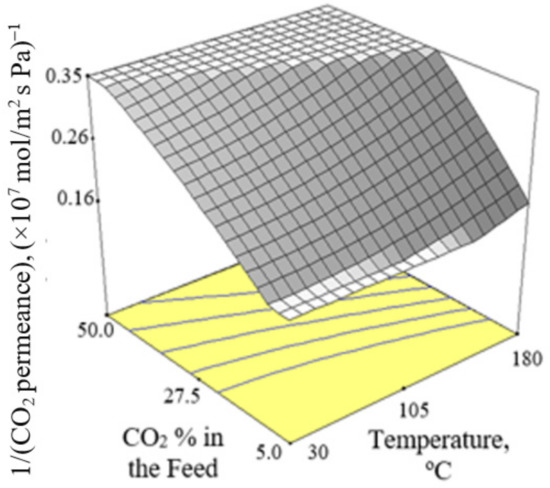
Figure 4.
Effect of interaction between CO2 % in the feed and temperature on the 1/(CO2 permeance) at 300 kPa pressure difference.
3.3.2. Response Surface Modeling of CO2/H2 Separation Selectivity
The ANOVA of the CO2/H2 separation selectivity is shown in Table 4. Equation (7) shows the chosen quadratic model to reflect the relationship between the independent variables and the response.
where C, B and A correspond to the coded value of CO2 % in the feed, pressure difference and temperature, respectively.

Table 4.
ANOVA of CO2/H2 separation selectivity.
The model was significant in view of its F-value of 391.84. The significance of variable’s effect on CO2/H2 separation selectivity decreased in the order of temperature (A) > CO2 % in the feed (C) > pressure difference (B) with the F-value in the order of 2209.94 > 586.84 > 5.03, respectively. It is shown in Table 4 that A, B, C, A2, C2, AB, AC, BC were significant terms for the model of 1/(CO2/H2 separation selectivity). However, the term of B2 was included in Equation (7) to obtain a hierarchy model. The “Lack of Fit F-value” of 4.39 implied that the Lack of Fit was not significant relative to the pure error due to noise.
Figure 5 presents the comparison of the predicted 1/(CO2/H2 separation selectivity) attained by using Equation (7) with the experimental 1/(CO2/H2 separation selectivity). Good agreement between predicted 1/(CO2/H2 separation selectivity) and experimental 1/(CO2/H2 separation selectivity) is indicated by the correlation coefficient value (R2) of 0.9972. Hence, this reflects the high accuracy of the generated model Equation (7) to predict the 1/(CO2/H2 separation selectivity) in the current work.
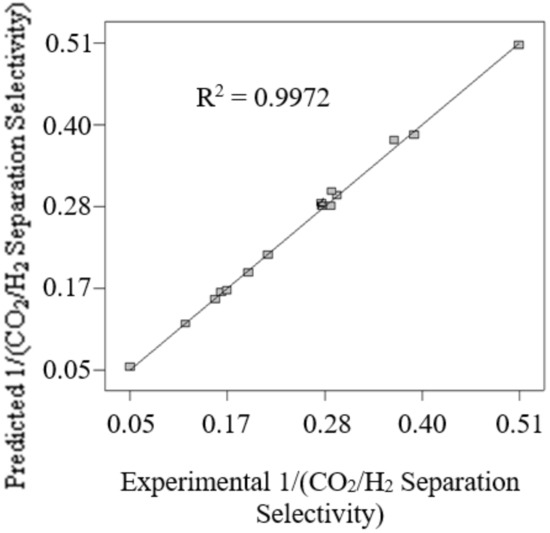
Figure 5.
The comparison of the predicted 1/(CO2/H2 separation selectivity) attained by using Equation (7) with the experimental 1/(CO2/H2 separation selectivity).
Figure 6, Figure 7 and Figure 8 present the plots showing the effect of interaction between different independent variables on the 1/(CO2/H2 separation selectivity). It is shown in Figure 6 that the 1/(CO2/H2 separation selectivity) increased when the temperature increased from 30 to 180 °C. CO2 adsorbed more strongly on Ba-SAPO-34 membrane than H2, and hence permeated faster through the membrane pore despite its larger molecule kinetic diameter than H2 [57]. Therefore, the CO2/H2 separation selectivities obtained in the current study were more than 1. The values became less than 1 when the CO2/H2 separation selectivities were inversed. At temperature as low as 30 °C, strong CO2 adsorption on the membrane inhibited the adsorption and permeance of H2. The degree of CO2 inhibition toward H2 adsorption reduced due to lower CO2 surface coverage when the temperature increased [39]. The CO2 permeance decreased but the H2 permeance increased, led to a drop in CO2/H2 separation selectivity or in other words, an increase in 1/(CO2/H2 separation selectivity) when the temperature increased. When the CO2 % in the feed increased, the 1/(CO2/H2 separation selectivity) increased, as can be seen in Figure 7 and Figure 8.
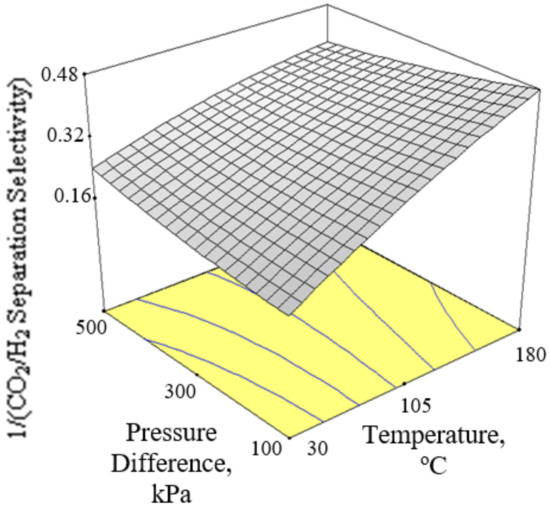
Figure 6.
Effect of interaction between temperature and pressure difference on the 1/(CO2/H2 separation selectivity) at 27.5% CO2 in the feed.
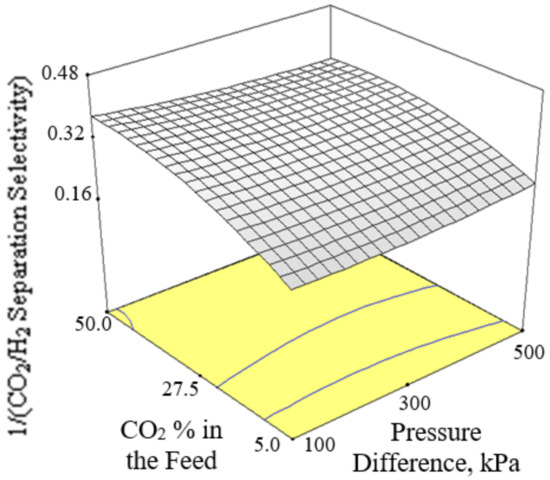
Figure 7.
Effect of interaction between CO2 % in the feed and pressure difference on the 1/(CO2/H2 separation selectivity) at 105 °C.
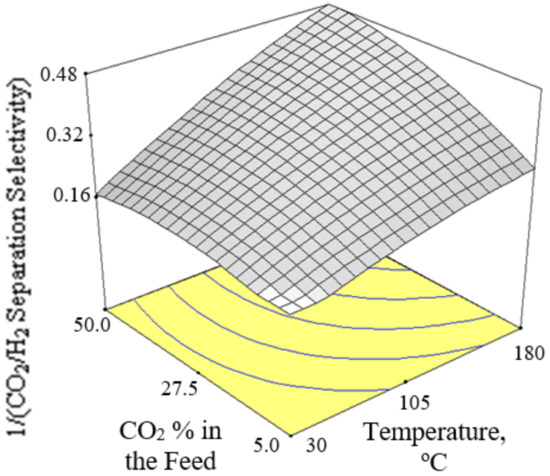
Figure 8.
Effect of interaction between CO2 % in the feed and temperature on the 1/(CO2/H2 separation selectivity) at 300 kPa pressure difference.
3.4. Optimization Studies
The goal set for the responses and variables, that need to be satisfied simultaneously for optimizing the responses by using Design Expert, is presented in Table 5. It was the goal for the responses to minimize the 1/(CO2/H2 separation selectivity) and the 1(CO2 permeance), or in other words, to maximize the CO2/H2 separation selectivity and the CO2 permeance.

Table 5.
Goal set for optimization of the studies of CO2/H2 separation.
Design Expert generated solutions (optimum conditions) with different total desirability as shown in Table 6. The desirability function approach was applied in the RSM to optimize the operating conditions in the present work.

Table 6.
Optimum conditions for the 1/(CO2/H2 separation selectivity) and the 1/(CO2 permeance) generated by Design Expert.
This approach was applied to determine the operating condition that results in response to the highest desirability. Each estimated response variable was transformed into an individual desirability value, di, using the desirability function [58]. The value of the desirability varies over the range
where (di = 1) reflects a completely ideal response value and (di = 0) reflects a completely undesirable response value. Then, the individual desirability values were combined in order to determine the value of the total desirability, D, as shown in Equation (9).
where m is the number of responses. In view of the highest total desirability of 0.996 displayed by Solution 1 as shown in Table 6. Solution 1 was selected as the optimum condition. With the optimum condition of Solution 1, 1/(CO2/H2 separation selectivity) value of 0.086 and the 1/(CO2 permeance) value of 0.052 (×10−7 mol/m2 s Pa)−1, were attained, which are equivalent to CO2/H2 separation selectivity of 11.6 and CO2 permeance of 19.23 × 10−7 mol/m2 s Pa.
An additional five experiments were conducted at the optimum operating condition (temperature of 30 °C, pressure difference of 100 kPa, 5% CO2 in the feed) generated by Design of Experiments in order to check the accuracy of the Design of Experiments. The separation result is presented in Table 7. The experimental values of CO2/H2 separation selectivity and CO2 permeance were compared with the values predicted by using the models. The attainment of mean error of 3.64% for CO2/H2 separation selectivity and the mean error of 1.46% for CO2 permeance reflects good agreement between the predicted values and the experimental values. This implies that Design of Experiments with RSM is an accurate tool used to model and predict CO2/H2 separation performance of the membrane in the current work.

Table 7.
The results of the additional CO2/H2 separation experiments conducted at optimum operating condition generated by Design of Experiments.
3.5. Comparison of CO2/H2 Separation Performance with the Other Zeolite Membranes Reported in the Literature
The Ba-SAPO-34 zeolite membrane in the present study was also compared for its CO2/H2 gas separation performance with the other zeolite membranes reported in the literature, and the comparison is shown in Table 8. Yin et al. [59] prepared a stainless-steel-net-supported P/NaX composite, which displayed CO2/H2 selectivity of ~4.1–6.4 and CO2 permeance of ~0.18–1.68 × 10−7 mol/m2 s Pa. Mirfendereski et al. [60] reported H2/CO2 selectivity of 0.11–0.22, which is equivalent to CO2/H2 selectivity of ~9.1–4.5 for ZSM-5 zeolite membranes. In the other studies reported by Aydani et al. [61], the SSZ-13 membrane was synthesized by dynamic rub coating. CO2 permeance of 5.8 × 10−7 mol/m2 s Pa and CO2/H2 selectivity of 17 were obtained for the synthesized SSZ-13 membrane [61]. On the other hand, CO2/H2 selectivity of 17 was reported for the DDR membrane by Zito et al. [62].

Table 8.
Comparison of CO2/H2 separation performance with the other zeolite membranes reported in the literature.
Xu et al. [63] prepared Na-LTA and Cs-LTA membranes. Xu et al. [63] reported an H2/CO2 separation factor of 5.9 and 8 for Na-LTA and Cs-LTA membranes, respectively. Moreover, Li et al. [64] reported the synthesis of the AlPO4-LTA membrane and obtained an H2/CO2 separation factor of 7.3 for the AlPO4-LTA membrane. The reported H2/CO2 separation factor values of greater than one for these membranes indicates that these membranes are H2-selective.
4. Conclusions
In this study, the CO2/H2 separation process over Ba-SAPO-34 zeolite membrane was investigated. Modeling and optimization for the responses (CO2/H2 separation selectivity and CO2 permeance) as a function of the independent process variables (CO2 % in the feed, pressure difference and temperature) was performed by applying response surface methodology and central composite design, which is available in Design Expert software. The obtained model equations were able to predict the responses over the ranges of CO2 % in the feed, pressure difference and temperature studied. In addition, optimum CO2 permeance of 19.23 × 10−7 mol/m2 s Pa and CO2/H2 separation selectivity of 11.6 were obtained at 5% CO2 in the feed, pressure difference of 100 kPa and temperature of 30 °C for the Ba-SAPO-34 zeolite membrane.
Author Contributions
Conceptualization, T.L.C.; methodology, T.L.C.; validation, T.L.C.; formal analysis, T.L.C.; investigation, T.L.C.; writing—original draft preparation, T.L.C.; writing—review and editing, T.Y.S.N., V.V. and Y.F.Y.; visualization, C.-D.H. and Z.A.J.; supervision, A.L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by YUTP-Fundamental Research Grant (Cost center: 015LC0-258), Research University Grant (by Universiti Sains Malaysia) (811043) and Ministry of Higher Education Malaysia’s (MOHE) Fundamental Research Grant Scheme (FRGS) Ref: FRGS/1/2020/TK0/UTP/02/28 (Cost center: 015MA0-123). The APC was funded by YUTP-Fundamental Research Grant (Cost center: 015LC0-258).
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to acknowledge the financial support from YUTP-Fundamental Research Grant (Cost center: 015LC0-258) and Ministry of Higher Education Malaysia’s (MOHE) Fundamental Research Grant Scheme (FRGS) Ref: FRGS/1/2020/TK0/UTP/02/28 (Cost center: 015MA0-123). This research work was also supported by Universiti Teknologi PETRONAS, Institute of Contaminant Management UTP, CO2 Research Centre (CO2RES), UTP. The authors would also like to acknowledge the financial support given by Universiti Sains Malaysia (USM, Malaysia) in the form of Research University Grant (811043).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ismail, A.F.; David, L.I.B. A review on the latest development of carbon membranes for gas separatio. J. Membr. Sci. 2001, 193, 1–18. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S. Hydrogen Membrane Separation Techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Lu, G.Q.; Diniz da Costa, J.C.; Duke, M.; Giessler, S.; Socolow, R.; Williams, R.H.; Kreutz, T. Inorganic membranes for hydrogen production and purification: A critical review and perspective. J. Colloid Interface Sci. 2007, 314, 589–603. [Google Scholar] [CrossRef]
- Moon, J.-H.; Bae, J.-H.; Han, Y.-J.; Lee, C.-H. Adsorbent/membrane hybrid (AMH) system for hydrogen separation: Synergy effect between zeolite 5A and silica membrane. J. Membr. Sci. 2010, 356, 58–69. [Google Scholar] [CrossRef]
- Bai, X.; Shi, Z.; Xia, H.; Li, S.; Liu, Z.; Liang, H.; Liu, Z.; Wang, B.; Qiao, Z. Machine-Learning-Assisted High-Throughput computational screening of Metal—Organic framework membranes for hydrogen separation. Chem. Eng. J. 2022, 446, 136783. [Google Scholar] [CrossRef]
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Pennline, H.; Baltrus, J.P.; Stevens, R.W., Jr.; Khatri, R.; Chuang, S.S.C.; Filburn, T. Improved immobilized carbon dioxide capture sorbents. Fuel Proc. Technol. 2005, 86, 1449–1455. [Google Scholar] [CrossRef]
- Zheng, F.; Tran, D.N.; Busche, B.J.; Fryxell, G.E.; Addleman, R.S.; Zemanian, T.S.; Aardahl, C.L. Ethylenediamine-modified SBA-15 as regenerable CO2 sorbent. Ind. Eng. Chem. Res. 2005, 44, 3099–3105. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Devlopment of palladium/ceramic membranes for hydrogen separation. Int. J. Hydrogen Energy 2011, 36, 4498–4506. [Google Scholar] [CrossRef]
- Shi, L.; Goldbach, A.; Xu, H. High-flux H2 separation membranes from (Pd/Au)n nanolayers. Int. J. Hydrogen Energy 2011, 36, 2281–2284. [Google Scholar] [CrossRef]
- Nagata, K.; Miyamoto, M.; Watabe, T.; Fujioka, Y.; Yogo, K. Preparation of pore-fill-type palladium-porous alumina composite membrane for hydrogen separation. Chem. Lett. 2011, 40, 19–21. [Google Scholar] [CrossRef]
- Li, X.; Zhou, C.; Lin, Z.; Rocha, J.; Lito, P.F.; Santiago, A.S.; Silva, C.M. Titanosilicate AM-3 membrane: A new potential candidate for H2 separation. Microporous Mesoporous Mater. 2011, 137, 43–48. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas permeation and diffusion in cross-linked poly(ethylene glycol diacrylate). Macromolecules 2006, 39, 3568–3580. [Google Scholar] [CrossRef]
- Lin, H.; Van Wagner, E.; Freeman, B.D.; Toy, L.G.; Gupta, R.P. Plasticization-enhanced hydrogen purification using polymeric membranes. Science 2006, 311, 639–642. [Google Scholar] [CrossRef]
- Iqbal, Z.; Shamair, Z.; Usman, M.; Gilani, M.A.; Yasin, M.; Saqib, S.; Khan, A.L. One pot synthesis of UiO-66@IL composite for fabrication of CO2 selective mixed matrix membranes. Chemosphere 2022, 303, 135122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, S.; Liu, B.; Zhou, R.; Xing, W. Scalable fabrication of highly selective SSZ-13 membranes on 19-channel monolithic supports for efficient CO2 capture. Sep. Purif. Technol. 2022, 293, 121122. [Google Scholar] [CrossRef]
- Wang, B.; Huang, W.; Zhu, Y.; Zhou, R.; Xing, W. Ultra-permeable high-selective SAPO-34 membranes for efficient CO2 capture. J. Membr. Sci. 2022, 650, 120420. [Google Scholar] [CrossRef]
- Gu, X.; Dong, J.; Nenoff, T.M. Synthesis of defect-free FAU-type zeolite membranes and separation for dry and moist CO2/N2 mixtures. Ind. Eng. Chem. Res. 2005, 44, 937–944. [Google Scholar] [CrossRef]
- Sato, K.; Sugimoto, K.; Sekine, Y.; Takada, M.; Matsukata, M.; Nakane, T. Application of FAU-type zeolite membranes to vapor/gas separation under high pressure and high temperature up to 5 MPa and 180 °C. Microporous Mesoporous Mater. 2007, 101, 312–318. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Pakdel, S.; Azamat, J.; Erfan-Niya, H.; Khataee, A. Theoretical Study of CO2/N2 Gas Mixture Separation through a High-Silica PWN-type Zeolite Membrane. Ind. Eng. Chem. Res. 2022, 61, 5593–5599. [Google Scholar] [CrossRef]
- Nishiyama, N.; Yamaguchi, M.; Katayama, T.; Hirota, Y.; Miyamoto, M.; Egashira, Y.; Ueyama, K.; Nakanishi, K.; Ohta, T.; Mizusawa, A.; et al. Hydrogen-permeable membranes composed of zeolite nano-blocks. J. Membr. Sci. 2007, 306, 349–354. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liu, J.; Yang, W. Microwave synthesis of LTA zeolite membranes without seeding. J. Membr. Sci. 2006, 277, 230–239. [Google Scholar] [CrossRef]
- Das, N.; Kundu, D.; Chatterjee, M. The effect of intermediate layer on synthesis and gas permeation properties of NaA zeolite membrane. J. Coat. Technol. Res. 2010, 7, 383–390. [Google Scholar] [CrossRef]
- Xu, X.; Yang, W.; Liu, J.; Lin, L.; Stroh, N.; Brunner, H. Synthesis of NaA zeolite membrane on a ceramic hollow fiber. J. Membr. Sci. 2004, 229, 81–85. [Google Scholar] [CrossRef]
- Sebastián, V.; Kumakiri, I.; Bredesen, R.; Menéndez, M. Zeolite membrane for CO2 removal: Operating at high pressure. J. Membr. Sci. 2007, 292, 92–97. [Google Scholar] [CrossRef]
- Mabande, G.T.P.; Noack, M.; Avhale, A.; Kölsch, P.; Georgi, G.; Schwieger, W.; Caro, J. Permeation properties of bi-layered Al-ZSM-5/Silicalite-1 membranes. Microporous Mesoporous Mater. 2007, 98, 55–61. [Google Scholar] [CrossRef]
- Bernal, M.P.; Coronas, J.; Menéndez, M.; Santamaría, J. Separation of CO2/N2 mixtures using MFI-type zeolite membranes. AIChE J. 2004, 50, 127–135. [Google Scholar] [CrossRef]
- Piera, E.; Brenninkmeijer, C.A.M.; Santamaría, J.; Coronas, J. Separation of traces of CO from air using MFI-type zeolite membranes. J. Membr. Sci. 2002, 201, 229–232. [Google Scholar] [CrossRef]
- Poshusta, J.C.; Noble, R.D.; Falconer, J.L. Temperature and pressure effects on CO2 and CH4 permeation through MFI zeolite membranes. J. Membr. Sci. 1999, 160, 115–125. [Google Scholar] [CrossRef]
- Shin, D.W.; Hyun, S.H.; Cho, C.H.; Han, M.H. Synthesis and CO2/N2 gas permeation characteristics of ZSM-5 zeolite membranes. Microporous Mesoporous Mater. 2005, 85, 313–323. [Google Scholar] [CrossRef]
- Himeno, S.; Tomita, T.; Suzuki, K.; Nakayama, K.; Yajima, K.; Yoshida, S. Synthesis and permeation properties of a DDR-type zeolite membrane for separation of CO2/CH4 gaseous mixtures. Ind. Eng. Chem. Res. 2007, 46, 6989–6997. [Google Scholar] [CrossRef]
- Tomita, T.; Nakayama, K.; Sakai, H. Gas separation characteristics of DDR type zeolite membrane. Microporous Mesoporous Mater. 2004, 68, 71–75. [Google Scholar] [CrossRef]
- Mirfendereski, S.M.; Mazaheri, T.; Sadrzadeh, M.; Mohammadi, T. CO2 and CH4 permeation through T-type zeolite membranes: Effect of synthesis parameters and feed pressure. Sep. Purif. Technol. 2008, 61, 317–323. [Google Scholar] [CrossRef]
- Cui, Y.; Kita, H.; Okamoto, K.-i. Preparation and gas separation performance of zeolite T membrane. J. Mater. Chem. 2004, 14, 924–932. [Google Scholar] [CrossRef]
- Li, S.; Carreon, M.A.; Zhang, Y.; Funke, H.H.; Noble, R.D.; Falconer, J.L. Scale-up of SAPO-34 membranes for CO2/CH4 separation. J. Membr. Sci. 2010, 352, 7–13. [Google Scholar] [CrossRef]
- Li, S.; Fan, C.Q. High-flux SAPO-34 membrane for CO2/N2 separation. Ind. Eng. Chem. Res. 2010, 49, 4399–4404. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Funke, H.F.; Falconer, J.L.; Noble, R.D. Ion-exchanged SAPO-34 membranes for light gas separations. Microporous Mesoporous Mater. 2007, 106, 140–146. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Falconer, J.L.; Noble, R.D. Hydrogen purification using a SAPO-34 membrane. J. Membr. Sci. 2008, 307, 277–283. [Google Scholar] [CrossRef]
- Li, S.; Alvarado, G.; Noble, R.D.; Falconer, J.L. Effects of impurities on CO2/CH4 separations through SAPO-34 membranes. J. Membr. Sci. 2005, 251, 59–66. [Google Scholar] [CrossRef]
- Li, S.; Martinek, J.G.; Falconer, J.L.; Noble, R.D.; Gardner, T.Q. High-pressure CO2/CH4 separation using SAPO-34 membranes. Ind. Eng. Chem. Res. 2005, 44, 3220–3228. [Google Scholar] [CrossRef]
- Li, S.; Falconer, J.L.; Noble, R.D. SAPO-34 membranes for CO2/CH4 separations: Effect of Si/Al ratio. Microporous Mesoporous Mater. 2008, 110, 310–317. [Google Scholar] [CrossRef]
- Chew, T.L.; Yeong, Y.F.; Ho, C.D.; Ahmad, A.L. Ion-Exchanged Silicoaluminophosphate-34 Membrane for Efficient CO2/N2 Separation with Low CO2 Concentration in the Gas Mixture. Ind. Eng. Chem. Res. 2019, 58, 729–735. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, L.; Wang, Z.; Qiu, S.; Zhu, G. Synthesis of a SAPO-34 membrane on macroporous supports for high permeance separation of a CO2/CH4 mixture. J. Mater. Chem. 2009, 19, 7698–7703. [Google Scholar] [CrossRef]
- Falconer, J.L.; Carreon, M.A.; Li, S.; Noble, R.D. Synthesis of zeolites and zeolite membranes using multiple structure directing agents. U.S. Patent No. 8,302,782, 2012, 18 September 2008. [Google Scholar]
- Carreon, M.A.; Li, S.; Falconer, J.L.; Noble, R.D. SAPO-34 seeds and membranes prepared using multiple structure directing agents. Adv. Mater. 2008, 20, 729–732. [Google Scholar] [CrossRef]
- Tong, L.-I.; Chang, Y.-C.; Lin, S.-H. Determining the optimal re-sampling strategy for a classification model with imbalanced data using design of experiments and response surface methodologies. Expert Syst. Appl. 2011, 38, 4222–4227. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J. Optimisation of integrated biodiesel production. Part I. A study of the biodiesel purity and yield. Biores. Technol. 2007, 98, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Hydrogen production from carbon dioxide reforming of methane over Ni-Co/MgO-ZrO2 catalyst: Process optimization. Int. J. Hydrogen Energy 2011, 36, 4875–4886. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, W.; Zhang, W.; Hu, Q.; Zeng, X. Optimizing the extraction of phenolic antioxidants from kudingcha made frrom Ilex kudingcha C.J. Tseng by using response surface methodology. Sep. Purif. Technol. 2011, 78, 311–320. [Google Scholar] [CrossRef]
- Low, K.L.; Tan, S.H.; Zein, S.H.S.; McPhail, D.S.; Boccaccini, A.R. Optimization of the mechanical properties of calcium phosphate/multi-walled carbon nanotubes/bovine serum albumin composites using response surface methodology. Mater. Des. 2011, 32, 3312–3319. [Google Scholar] [CrossRef]
- Switzar, L.; Giera, M.; Lingeman, H.; Irth, H.; Niessen, W.M.A. Protein digestion optimization for characterization of drug-protein adducts using response surface modeling. J. Chromatogr. A 2011, 1218, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.L.; Ahmad, A.L.; Bhatia, S. Ba-SAPO-34 membrane synthesized from microwave heating and its performance for CO2/CH4 gas separation. Chem. Eng. J. 2011, 171, 1053–1059. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Yeong, Y.F.; Abdullah, A.Z.; Ahmad, A.L.; Bhatia, S. Process optimization studies of p-xylene separation from binary xylene mixture over silicalite-1 membrane using response surface methodology. J. Membr. Sci. 2009, 341, 96–108. [Google Scholar] [CrossRef]
- Wee, S.L.; Tye, C.T.; Bhatia, S. Process optimization studies for the dehydration of alcohol-water system by inorganic membrane based pervaporation separation using design of experiments (DOE). Sep. Purif. Technol. 2010, 71, 192–199. [Google Scholar] [CrossRef]
- Hong, M.; Falconer, J.L.; Noble, R.D. Modification of zeolite membranes for H2 separation by catalytic cracking of methyldiethoxysilane. Ind. Eng. Chem. Res. 2005, 44, 4035–4041. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Yin, X.; Zhu, G.; Wang, Z.; Yue, N.; Qiu, S. Zeolite P/NaX composite membrane for gas separation. Microporous Mesoporous Mater. 2007, 105, 156–162. [Google Scholar] [CrossRef]
- Mirfendereski, S.M.; Mazaheri, T. Preparation of high performance ZSM-5 zeolite membranes for CO2/H2 separation. J. Ind. Eng. Chem. 2021, 94, 240–252. [Google Scholar] [CrossRef]
- Aydani, A.; Brunetti, A.; Maghsoudi, H.; Barbieri, G. CO2 separation from binary mixtures of CH4, N2, and H2 by using SSZ-13 zeolite membrane. Sep. Purif. Technol. 2021, 256, 117796. [Google Scholar] [CrossRef]
- Zito, P.F.; Brunetti, A.; Drioli, E.; Barbieri, G. CO2 Separation via a DDR Membrane: Mutual Influence of Mixed Gas Permeation. Ind. Eng. Chem. Res. 2020, 59, 7054–7060. [Google Scholar] [CrossRef]
- Xu, C.; Wei, W.; He, Y. Enhanced hydrogen separation performance of Linde Type-A zeolite molecular sieving membrane by cesium ion exchange. Mater. Lett. 2022, 324, 132680. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Wang, B.; Zi, W.; Ji, Q.; Li, Y.; Zhang, X.; Wang, Y.; Ding, Y.; Liu, J.; et al. Ultrafast synthesis of discrete submicron AlPO4-LTA molecular sieve crystals and their application in molecular sieve membrane. Microporous Mesoporous Mater. 2022, 334, 111771. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).