Ultra-Selective CMSMs Derived from Resorcinol-Formaldehyde Resin for CO2 Separation
Abstract
:1. Introduction
2. Materials and Methods
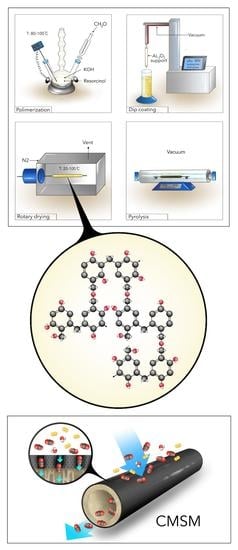
2.1. CMSMs Fabrication
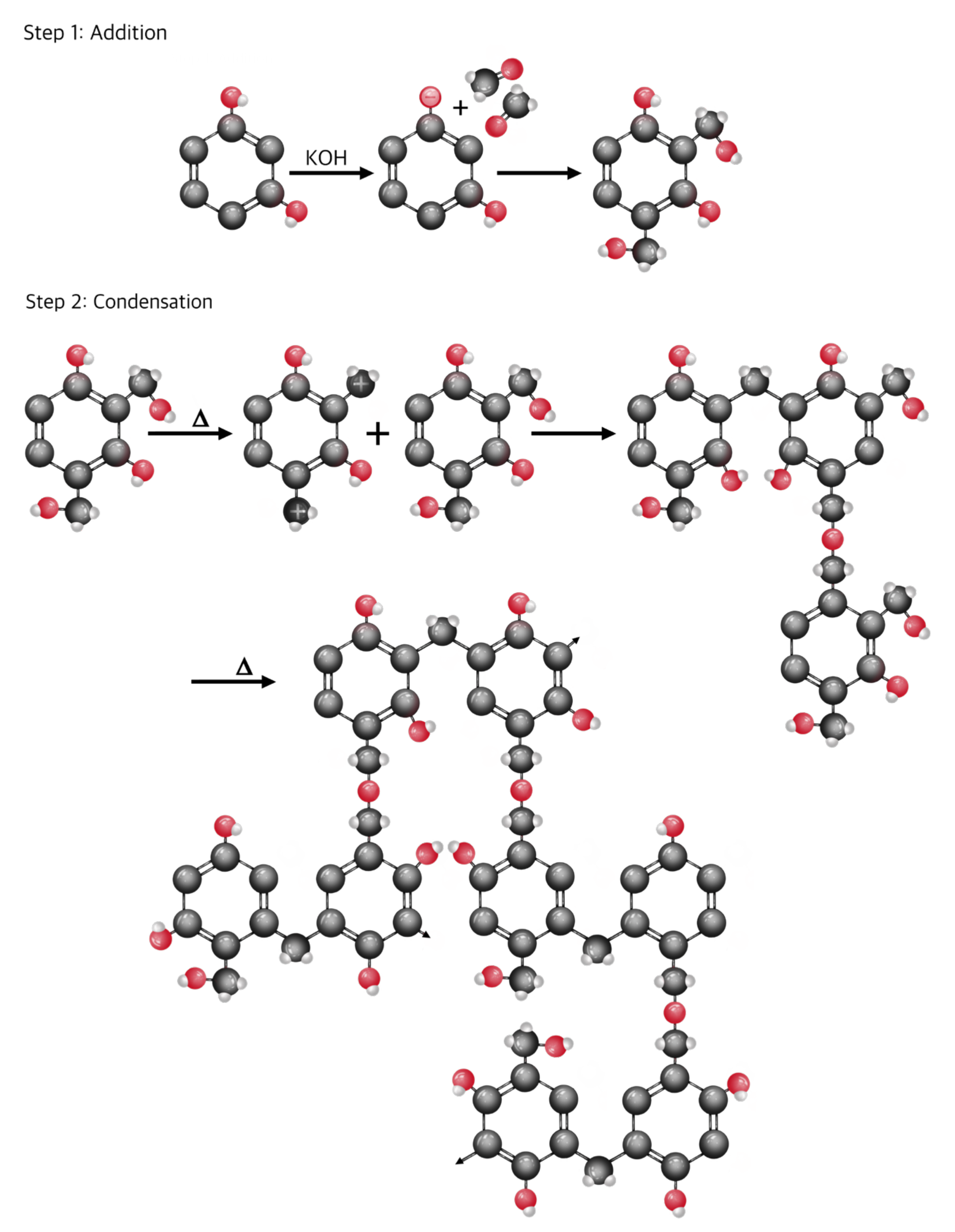
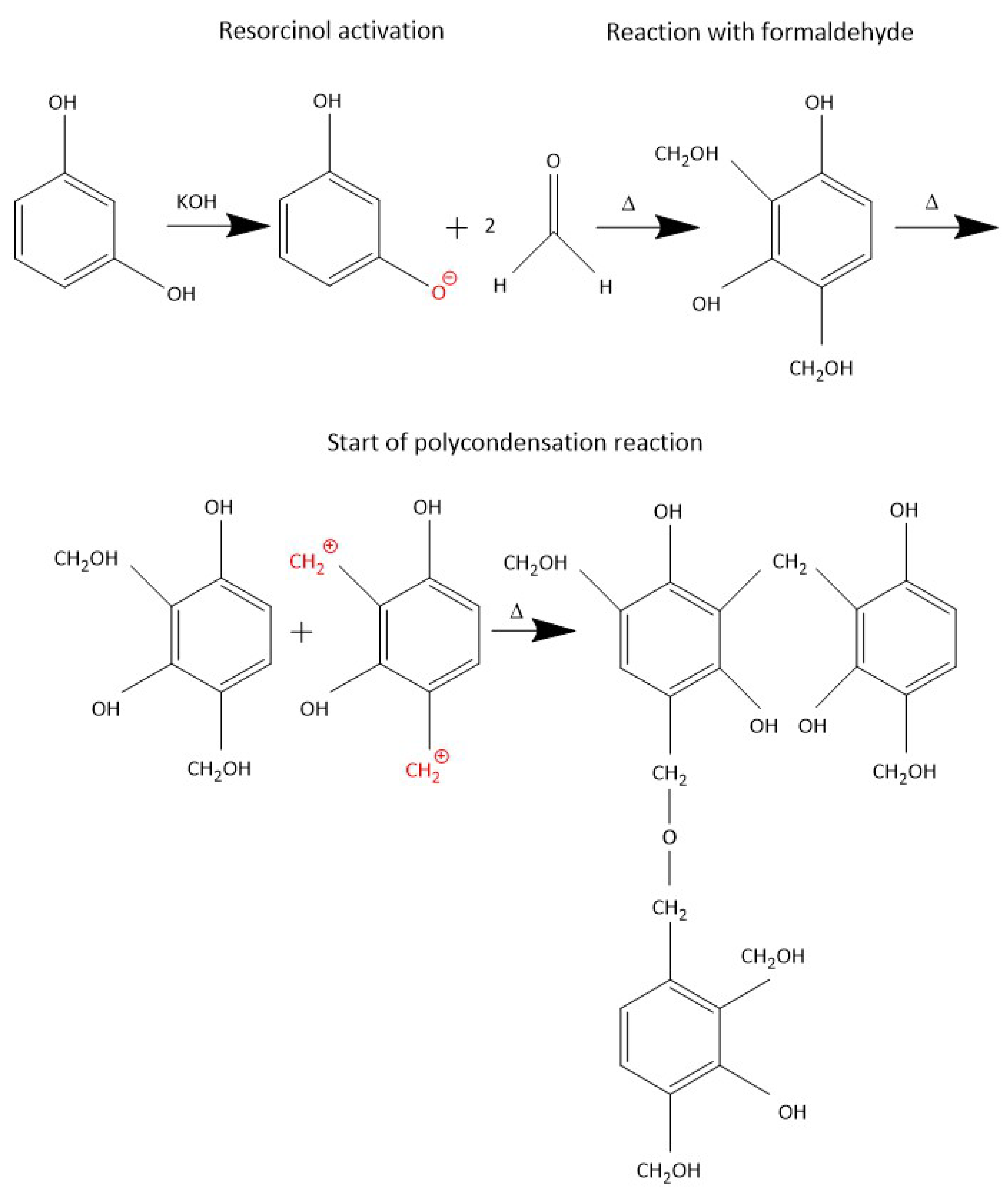
2.1.1. Preparation of the Resorcinol-Formaldehyde Precursor
2.1.2. Dipping Solution Preparation
2.1.3. Dip Coating and Polymerization
2.1.4. Carbonization
2.1.5. Post Treatment
2.2. CMSMs Characterization
2.2.1. Gel Permeation Chromatography (GPC)
2.2.2. CHO Analysis
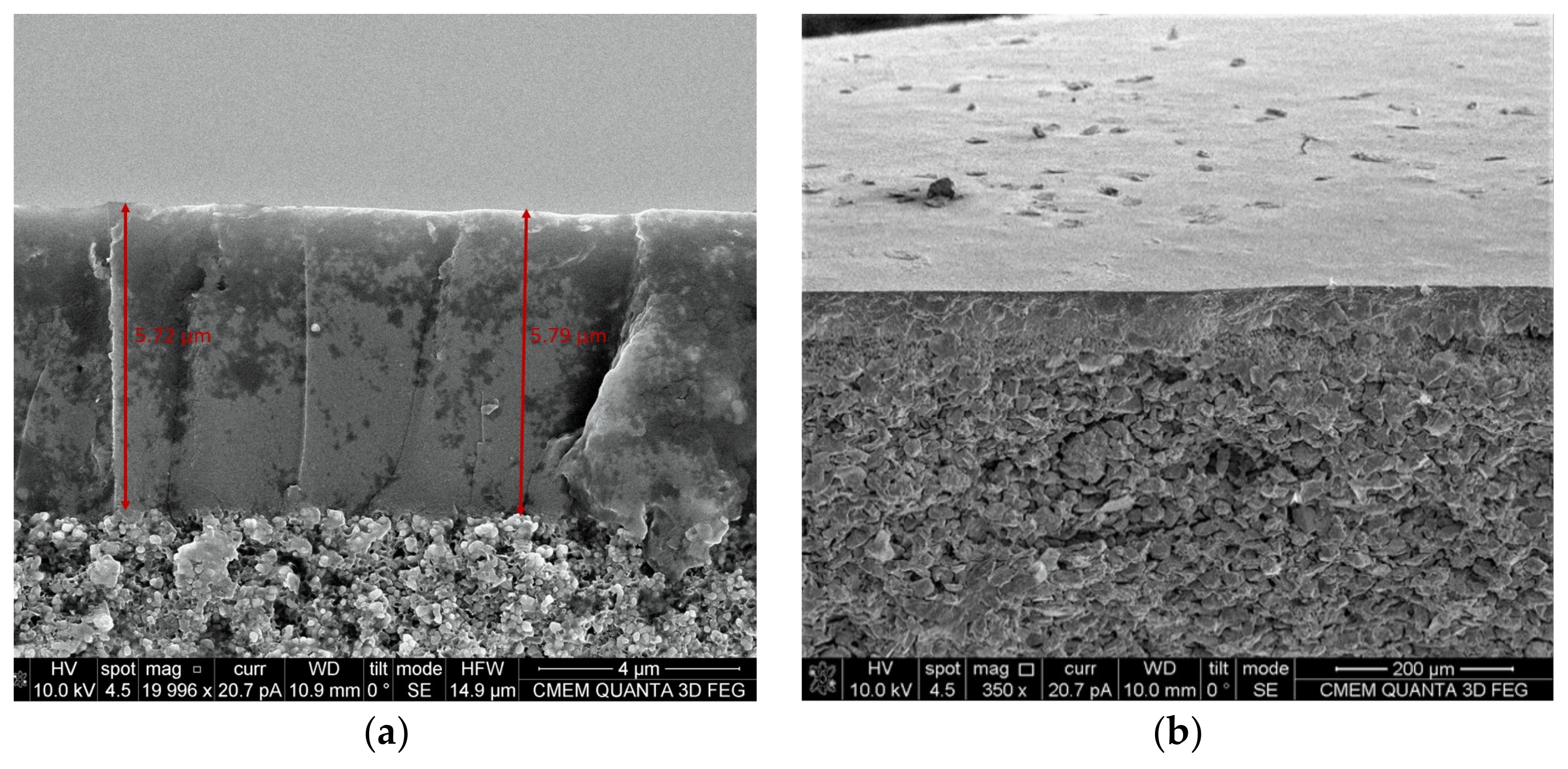
2.2.3. Scanning Electron Microscopy (SEM) Energy Dispersive X-ray Analyzer (EDX)
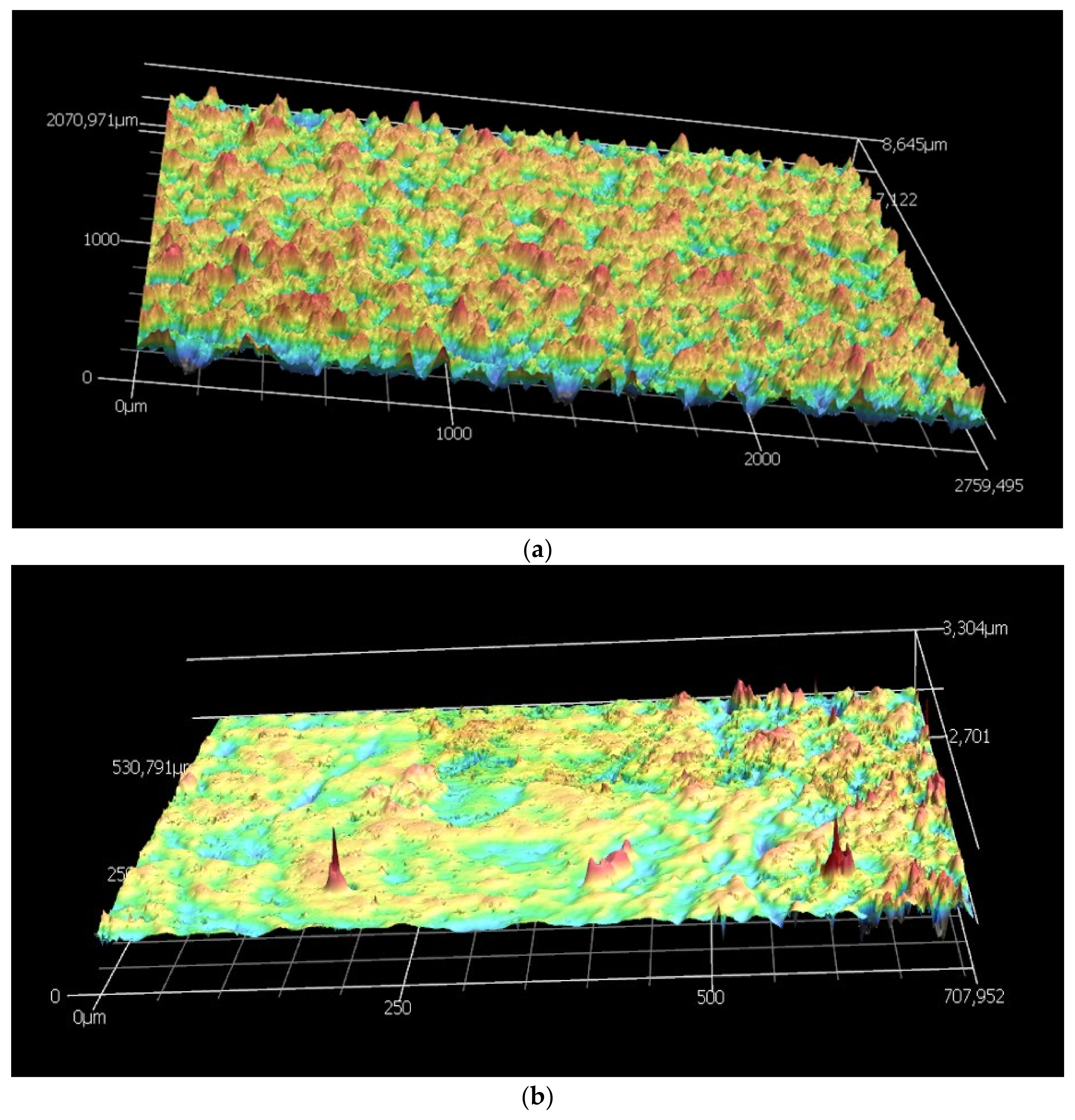
2.2.4. D Laser Confocal Microscopy
2.2.5. Perm-Porometry Tests
2.3. CMSMs Performance Tests
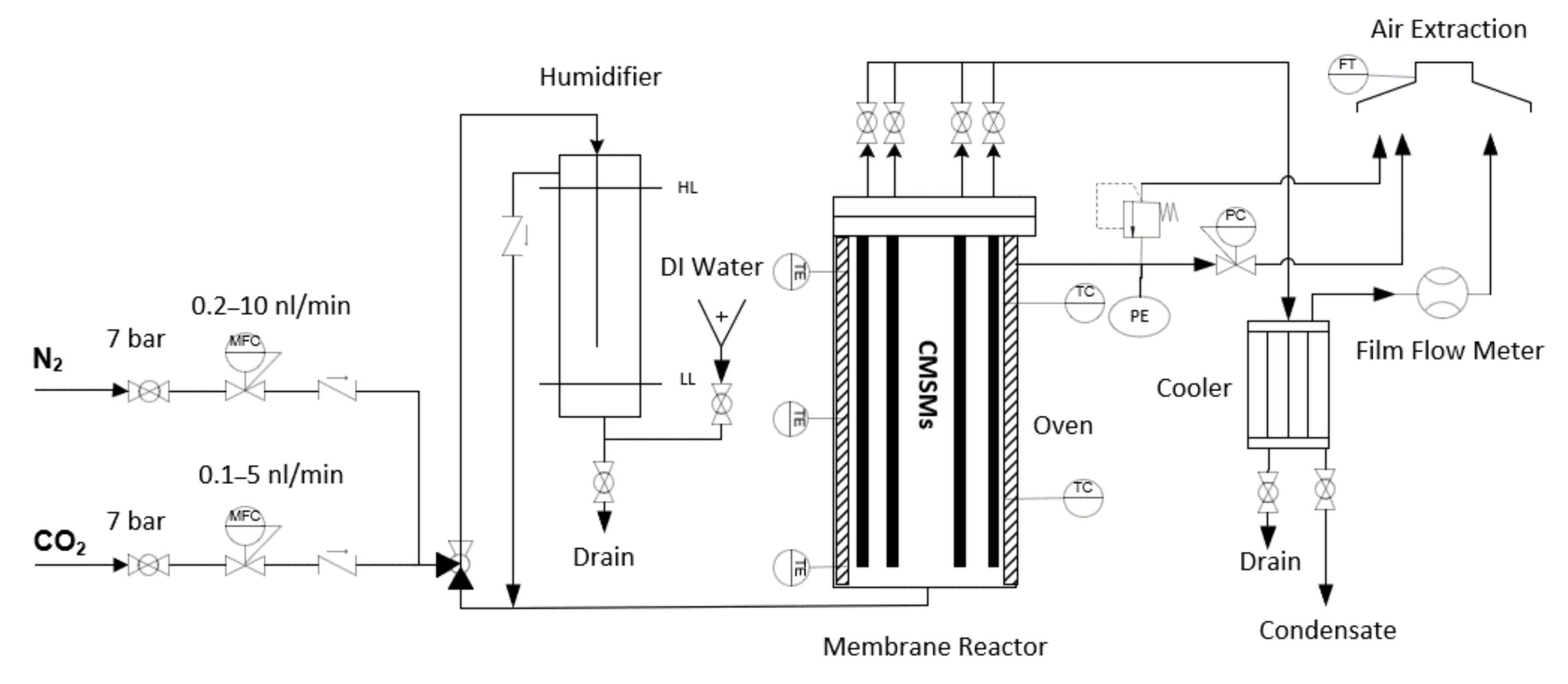
2.3.1. Gas Permeation Setup
2.3.2. Gas Permeation Tests
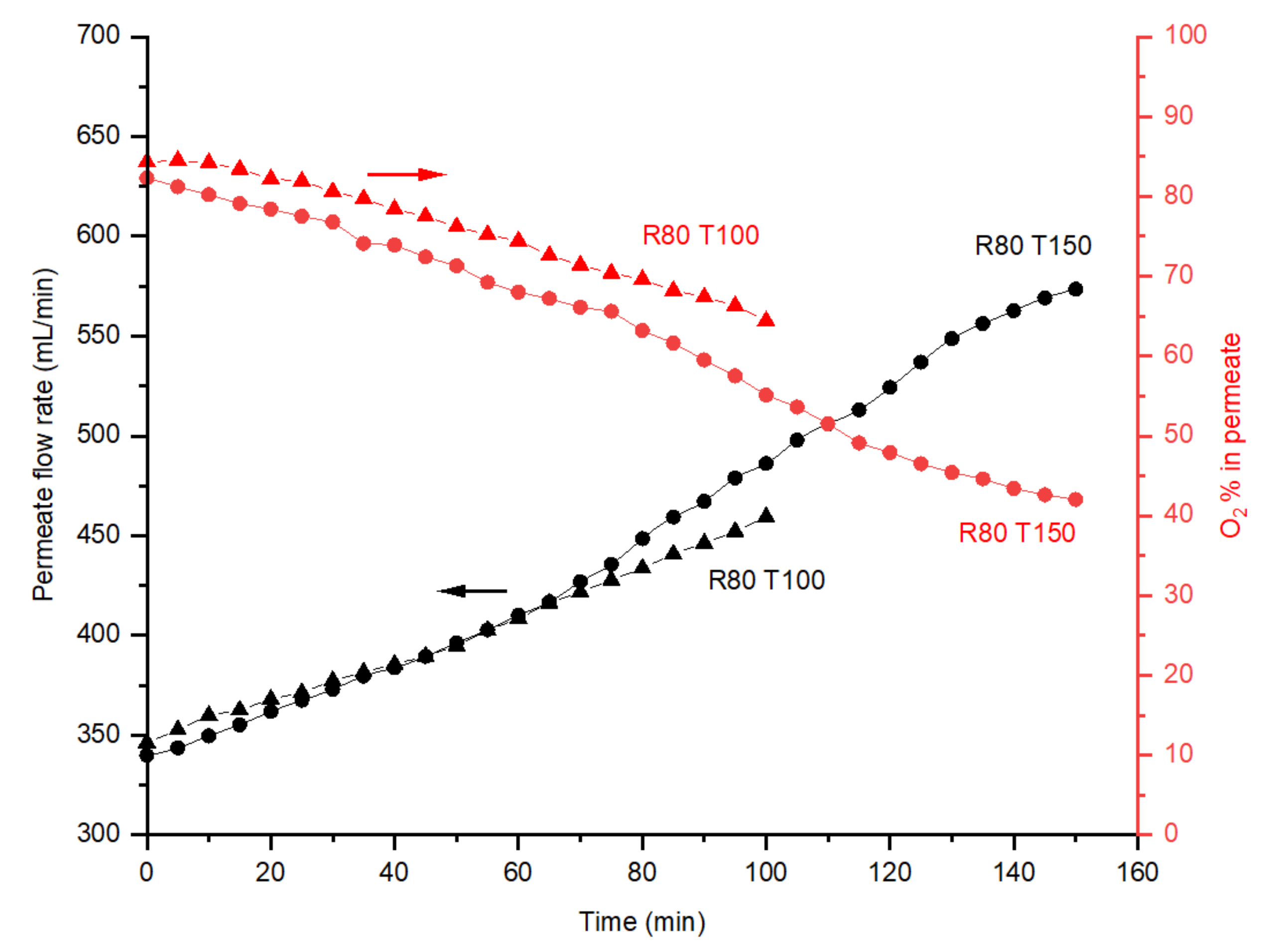
2.3.3. Post Treatment with Oxygen
3. Results
4. Discussion
4.1. CMSMs Permeation Tests
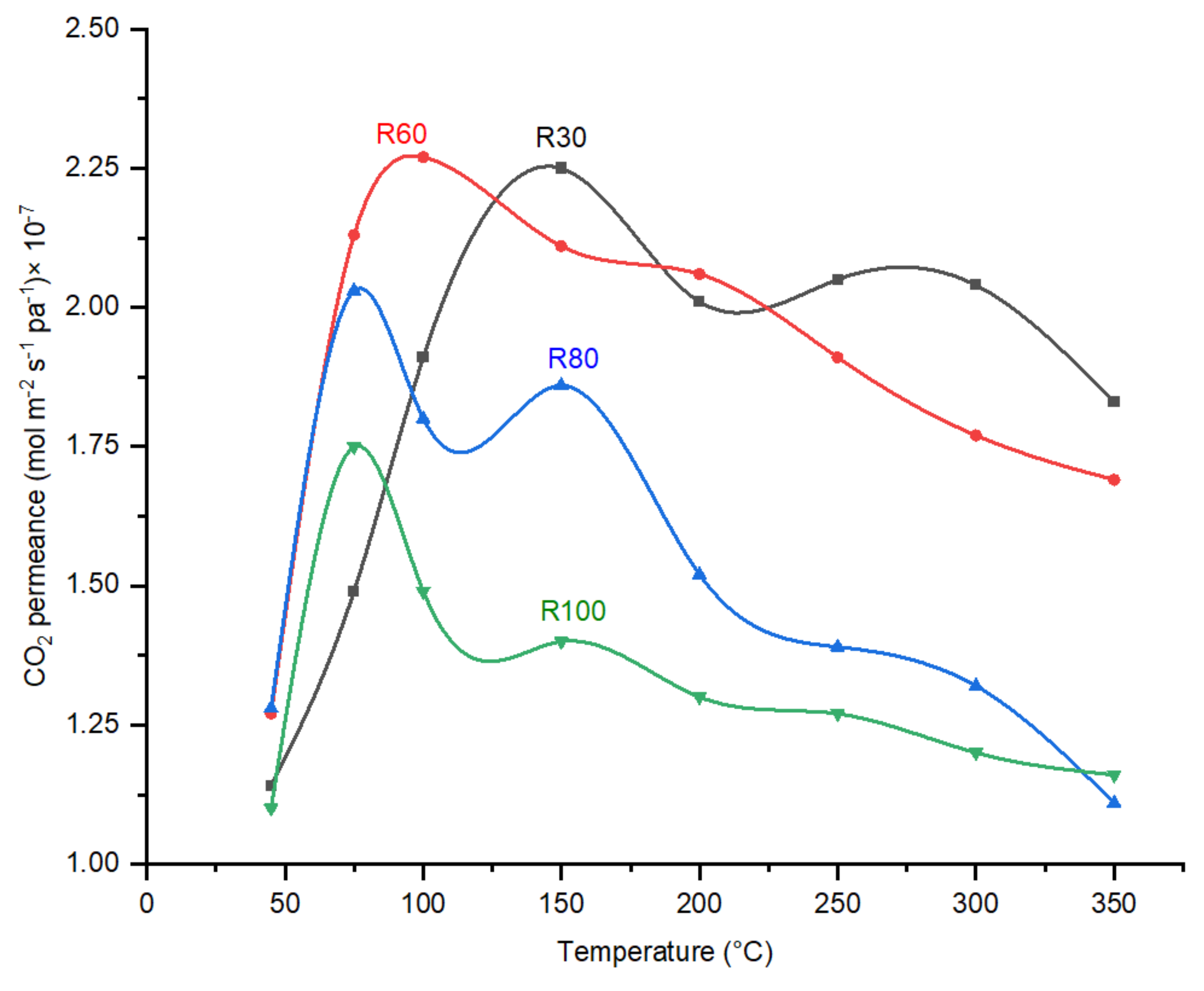
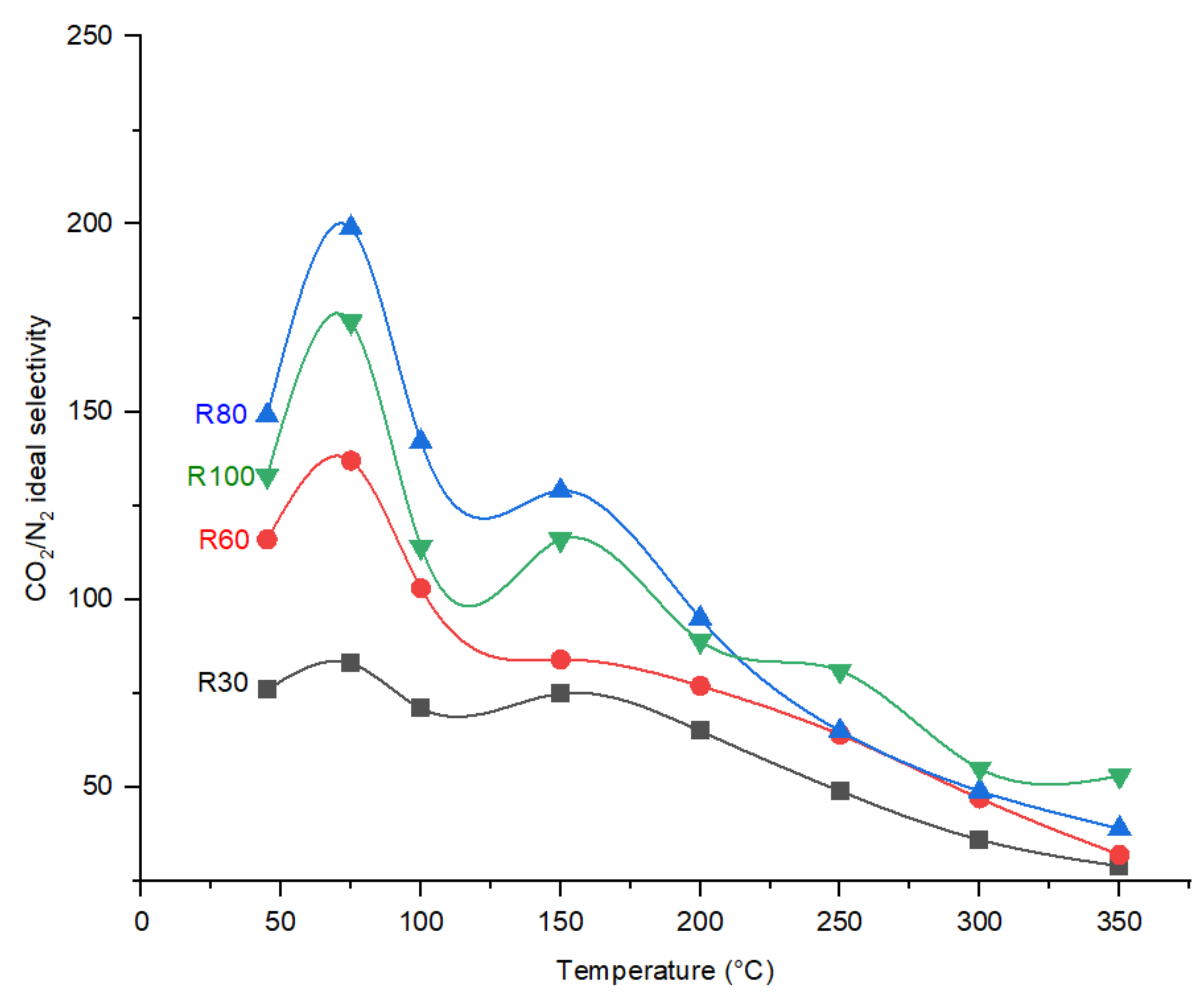
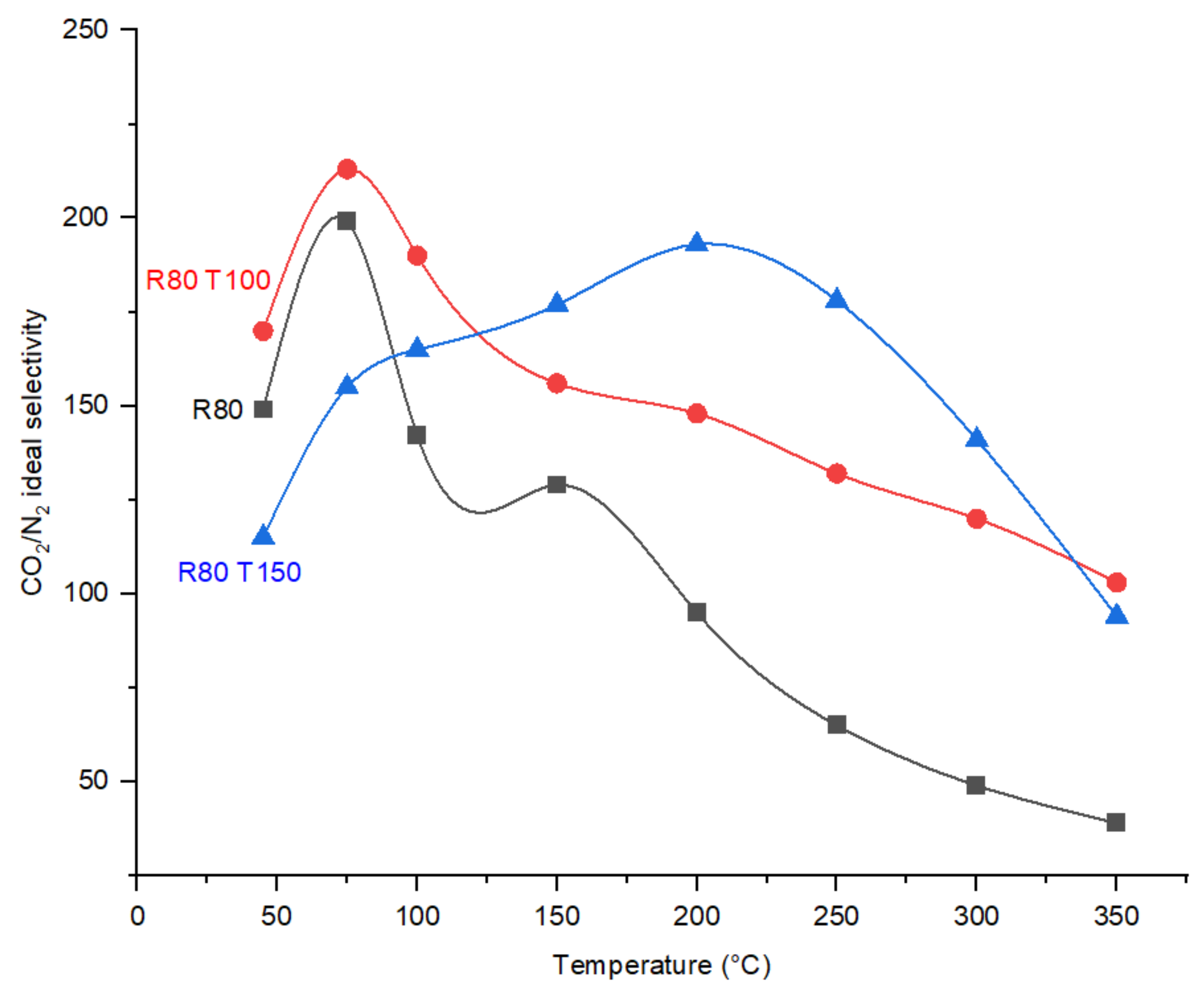
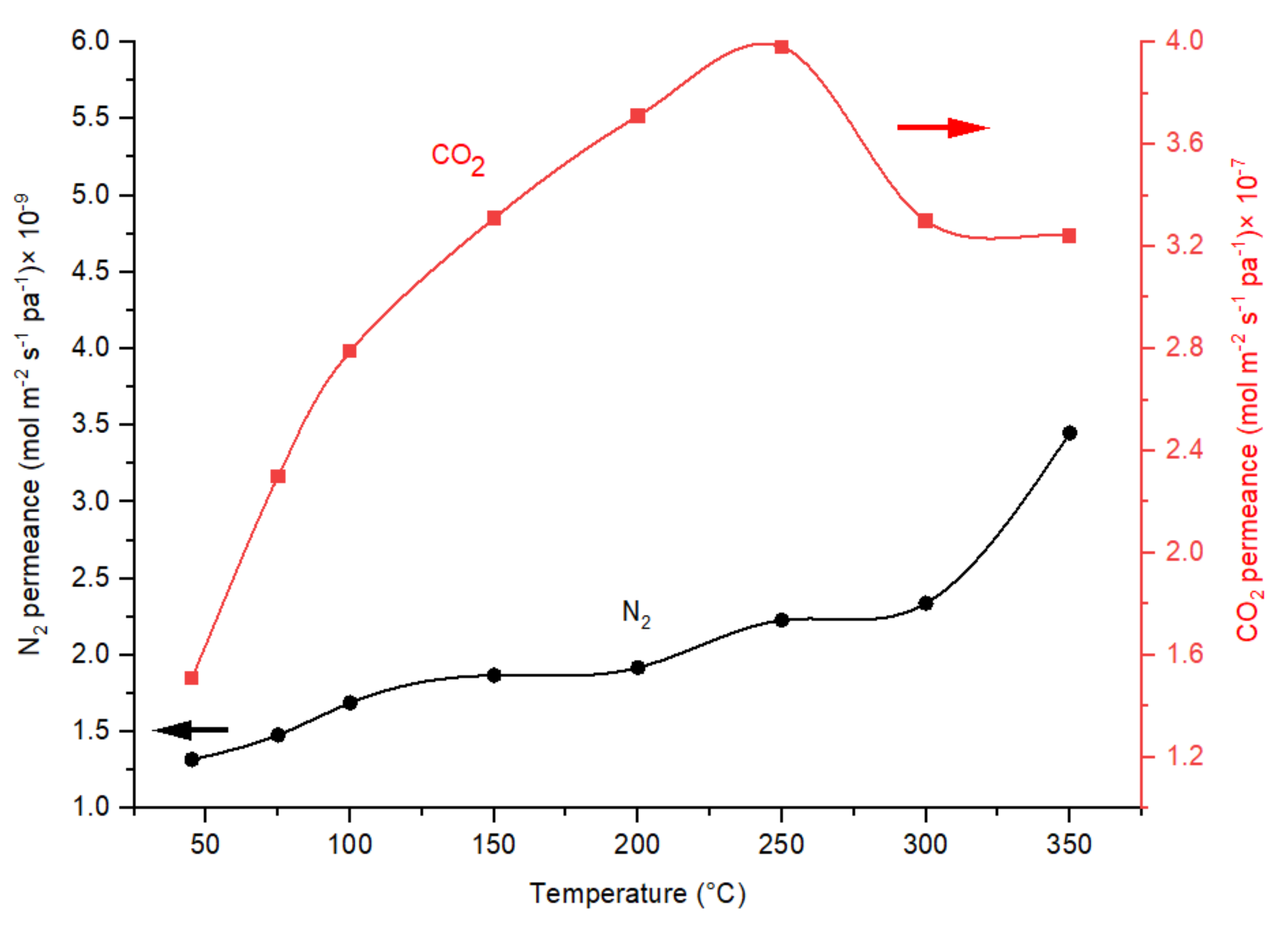
Effect of Temperature on CO2/N2 Ideal Selectivity and CO2 Permeance
4.2. Effect of DP
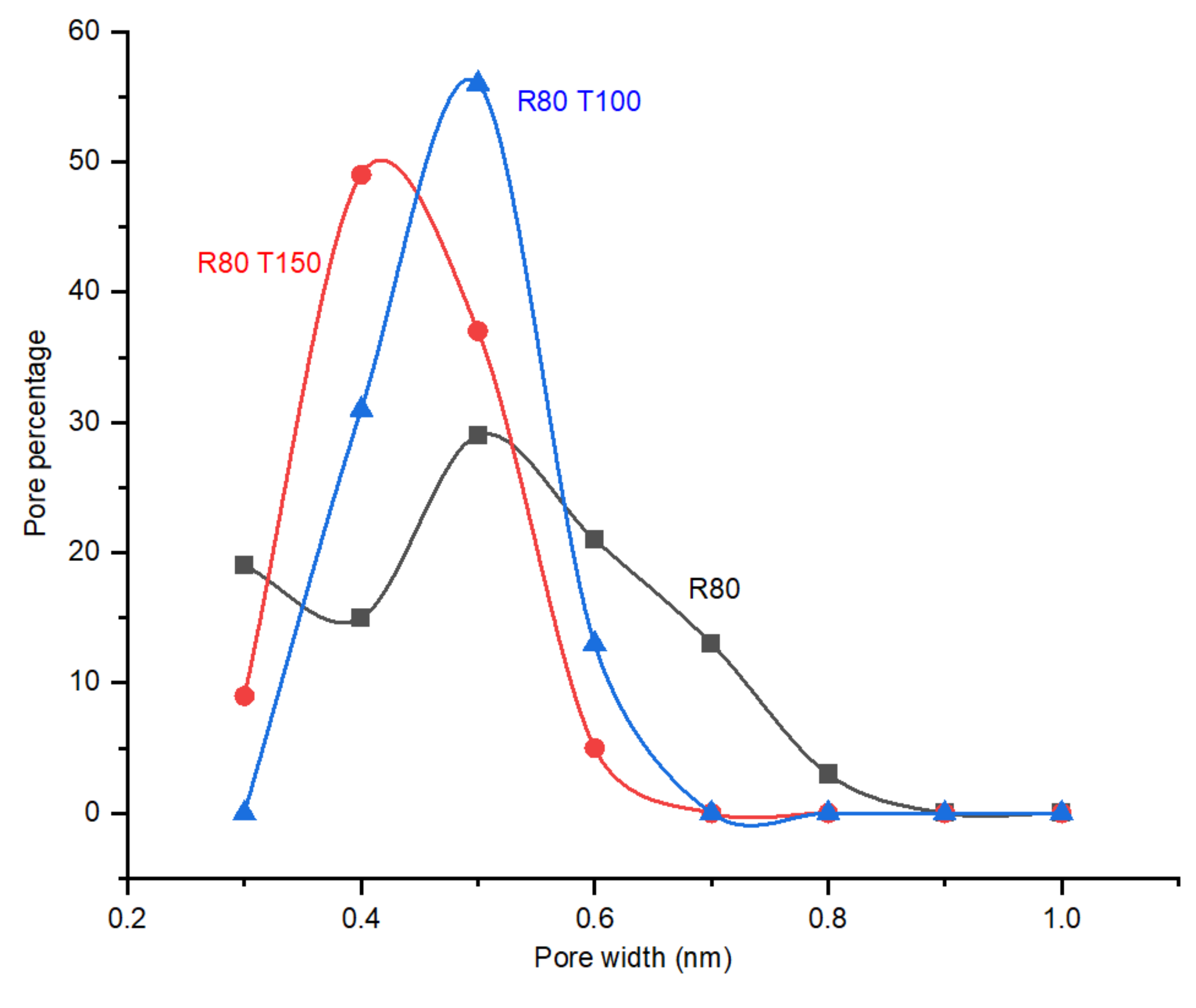
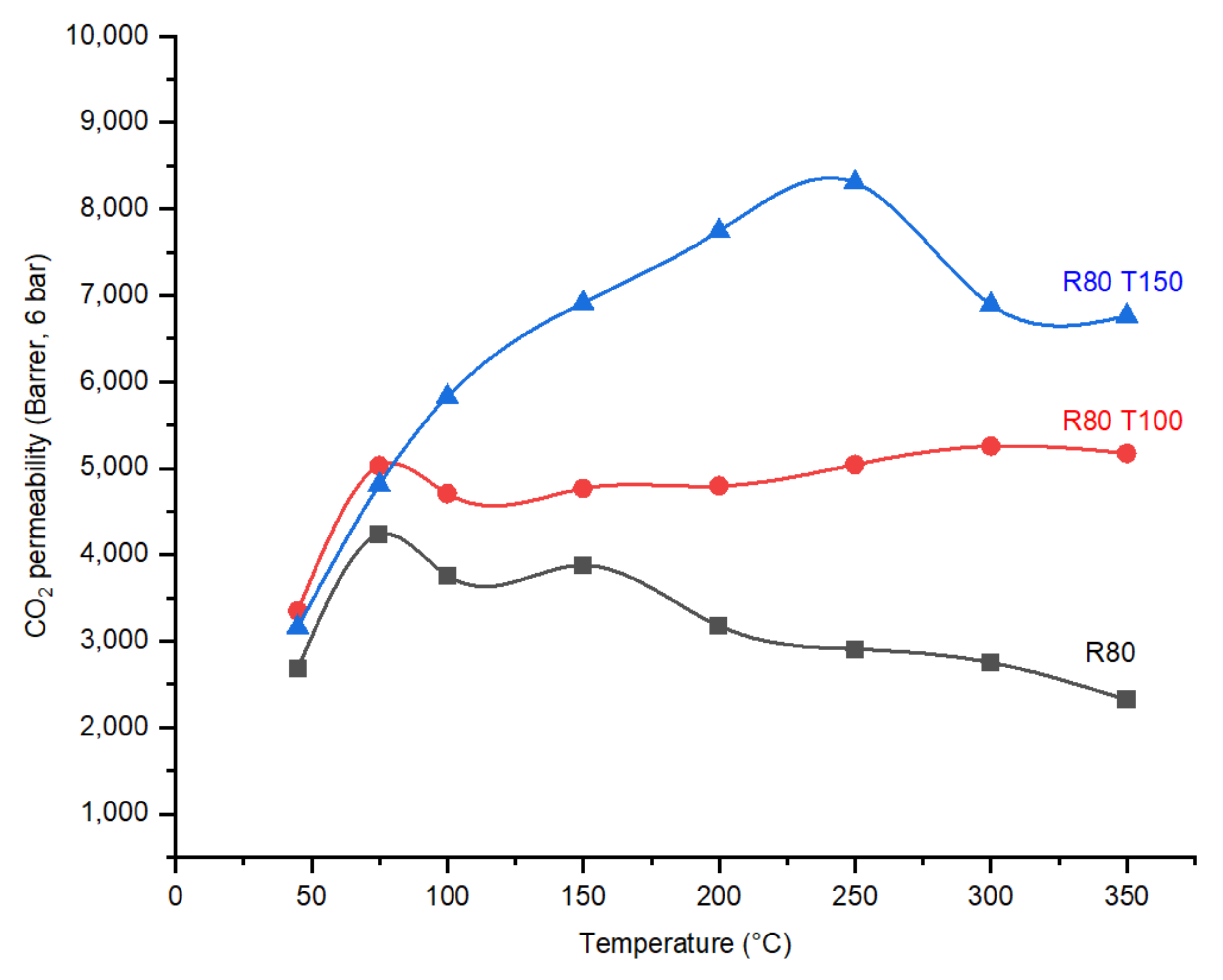
4.3. Effect of Post Treatment
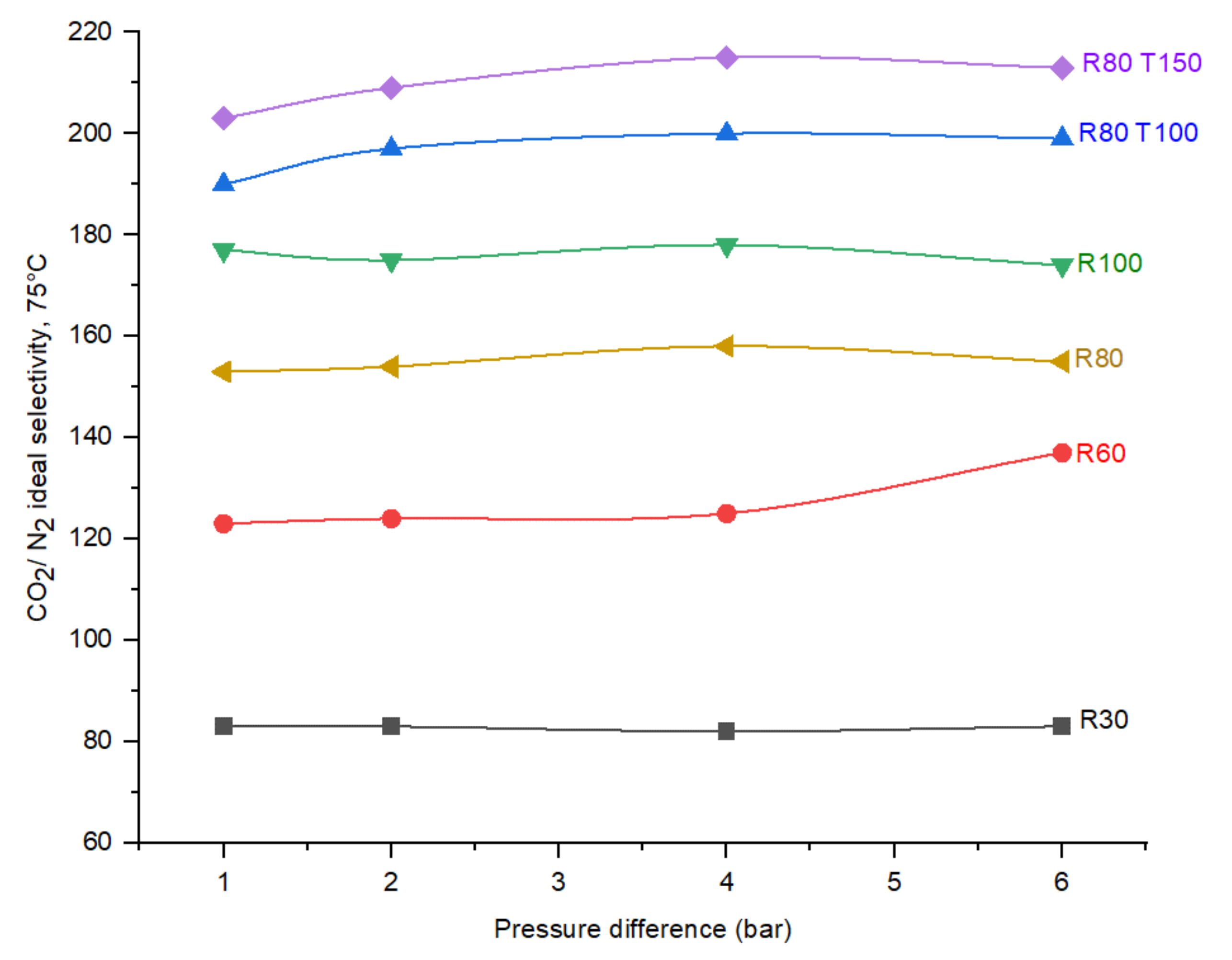
4.4. Effect of Pressure
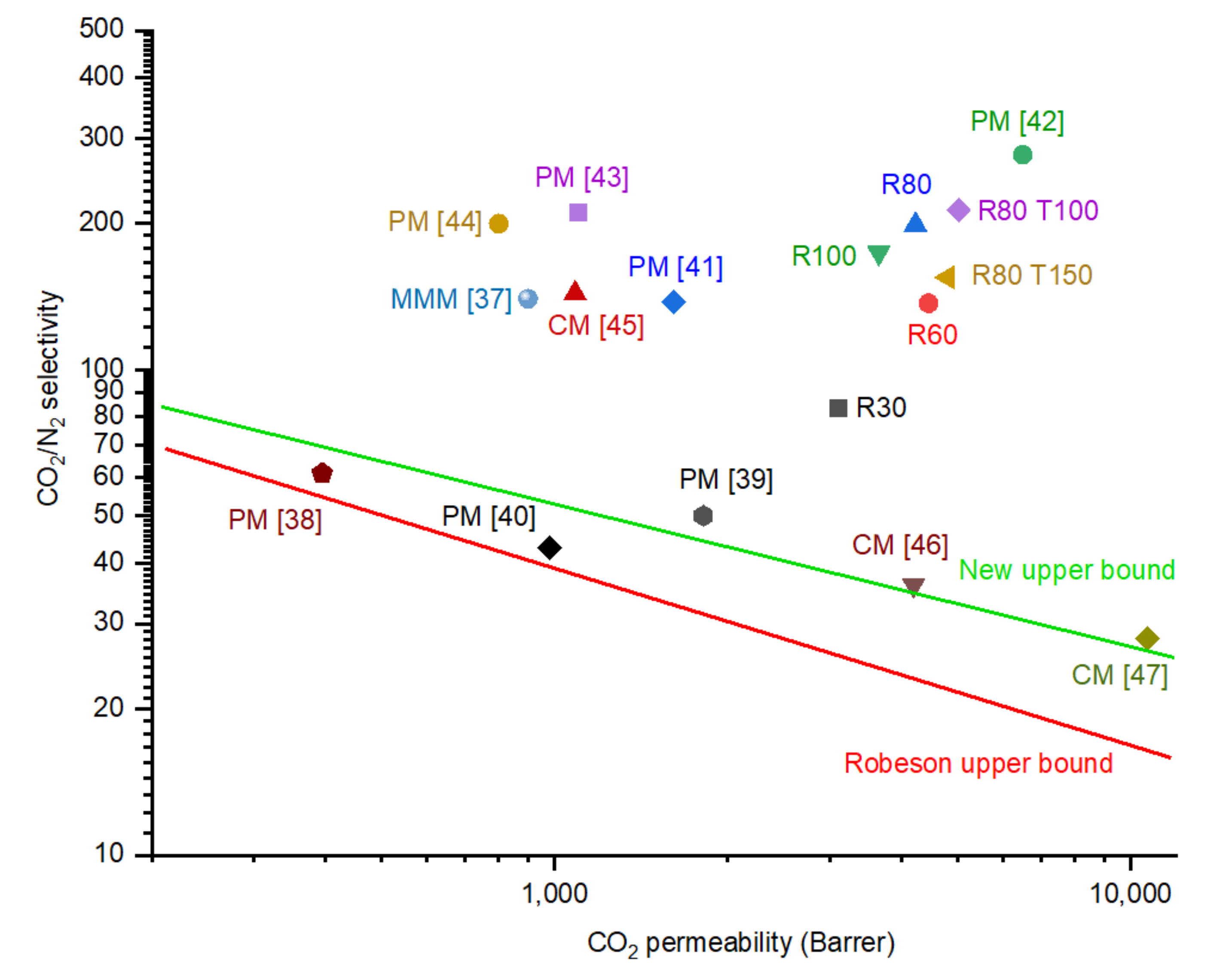
4.5. Comparison of Membranes’ Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Olivier, J. Trends in Global CO2 and Total Greenhouse Gas Emissions; 2020 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherland, 2020; p. 4331. [Google Scholar]
- Deutz, S.; Bardow, A. Life-Cycle Assessment of an Industrial Direct Air Capture Process Based on Temperature–Vacuum Swing Adsorption. Nat. Energy 2021, 6, 203–213. [Google Scholar] [CrossRef]
- Goglio, P.; Williams, A.G.; Balta-Ozkan, N.; Harris, N.R.P.; Williamson, P.; Huisingh, D.; Zhang, Z.; Tavoni, M. Advances and Challenges of Life Cycle Assessment (LCA) of Greenhouse Gas Removal Technologies to Fight Climate Changes. J. Clean. Prod. 2020, 244, 118896. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in Carbon Capture Technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Chawla, M.; Saulat, H.; Khan, M.M.; Khan, M.M.; Rafiq, S.; Cheng, L.; Iqbal, T.; Rasheed, M.I.; Farooq, M.Z.; Saeed, M.; et al. Membranes for CO2/CH4 and CO2/N2 Gas Separation. Chem. Eng. Technol. 2020, 43, 184–199. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Qiao, G.G.; Webley, P.A. Recent Progress on Fabrication Methods of Polymeric Thin Film Gas Separation Membranes for CO2 Capture. J. Membr. Sci. 2019, 572, 38–60. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Zhou, A.; Dong, S.; Shi, K.; Li, B.; Han, J.; O’Hare, D. High-Efficiency CO2 Separation Using Hybrid LDH-Polymer Membranes. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Fang, M.; He, Z.; Merkel, T.C.; Okamoto, Y. High-Performance Perfluorodioxolane Copolymer Membranes for Gas Separation with Tailored Selectivity Enhancement. J. Mater. Chem. A 2018, 6, 652–658. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.; Zhang, Z.; Xu, S.; Ma, C.; Li, N. Enhanced Molecular Selectivity and Plasticization Resistance in Ring-Opened Tröger’s Base Polymer Membranes. J. Membr. Sci. 2021, 634, 119399. [Google Scholar] [CrossRef]
- Nemestóthy, N.; Bakonyi, P.; Lajtai-Szabó, P.; Bélafi-Bakó, K. The Impact of Various Natural Gas Contaminant Exposures on CO2/CH4 Separation by a Polyimide Membrane. Membranes 2020, 10, 324. [Google Scholar] [CrossRef]
- An, H.; Cho, K.Y.; Yu, S.; Kim, K.C.; Shin, J.H.; Nam, K.J.; Park, J.H.; Lee, J.S. Triple-Ligand Zeolitic Imidazolate Frameworks for Highly CO2 Selective Mixed Matrix Membranes. Chem. Eng. J. 2022, 433, 133606. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Martin-Gil, V.; Supinkova, T.; Lambert, P.; Castro-Muñoz, R.; Hrabanek, P.; Kocirik, M.; Fila, V. Novel MMM Using CO2 Selective SSZ-16 and High-Performance 6FDA-Polyimide for CO2/CH4 Separation. Sep. Purif. Technol. 2021, 254, 117582. [Google Scholar] [CrossRef]
- Norahim, N.; Yaisanga, P.; Faungnawakij, K.; Charinpanitkul, T.; Klaysom, C. Recent Membrane Developments for CO2 Separation and Capture. Chem. Eng. Technol. 2018, 41, 211–223. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, S.; Lee, M.; Hong, S.; Jang, M.G.; Choi, N.; Hwang, K.S.; Baik, H.; Kim, J.K.; Yip, A.C.K.; et al. A Hybrid Zeolite Membrane-Based Breakthrough for Simultaneous CO2 Capture and CH4 Upgrading from Biogas. ACS Appl. Mater. Interfaces 2022, 14, 2893–2907. [Google Scholar] [CrossRef] [PubMed]
- Takayama, D.; Ishii, K.; Nomura, M.; Onoki, T.; Okuno, T.; Tawarayama, H.; Ishikawa, S. Development of Pure Silica Cha Membranes for CO2 Separation. Membranes 2021, 11, 926. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, F.; Sun, K.; Jin, X.; Zhang, Y.; Liu, B.; Zhou, R. Green Synthesis of Highly CO2-Selective CHA Zeolite Membranes in All-Silica and Fluoride-Free Solution for CO2/CH4 Separations. Energy Fuels 2020, 34, 11307–11314. [Google Scholar] [CrossRef]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Role of Amine Type in CO2 Separation Performance within Amine Functionalized Silica/Organosilica Membranes: A Review. Appl. Sci. 2018, 8, 1032. [Google Scholar] [CrossRef]
- Kusuki, Y.; Shimazaki, H.; Tanihara, N.; Nakanishi, S.; Yoshinaga, T. Gas Permeation Properties and Characterization of Asymmetric Carbon Membranes Prepared by Pyrolyzing Asymmetric Polyimide Hollow Fiber Membrane. J. Membr. Sci. 1997, 134, 245–253. [Google Scholar] [CrossRef]
- Haider, S.; Lindbråthen, A.; Lie, J.A.; Andersen, I.C.T.; Hägg, M.B. CO2 Separation with Carbon Membranes in High Pressure and Elevated Temperature Applications. Sep. Purif. Technol. 2018, 190, 177–189. [Google Scholar] [CrossRef]
- Swaidan, R.; Ma, X.; Litwiller, E.; Pinnau, I. High Pressure Pure- and Mixed-Gas Separation of CO2/CH4 by Thermally-Rearranged and Carbon Molecular Sieve Membranes Derived from a Polyimide of Intrinsic Microporosity. J. Membr. Sci. 2013, 447, 387–394. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Ismail, A.F.; Ismail, N.H.; Kadirgama, K. A Brief Review on Carbon Selective Membranes from Polymer Blends for Gas Separation Performance. Rev. Chem. Eng. 2021, 37, 339–362. [Google Scholar] [CrossRef]
- Hägg, M.B.; Lie, J.A.; Lindbråthen, A. Carbon Molecular Sieve Membranes. Ann. N. Y. Acad. Sci. 2003, 984, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; David, L.I.B. A Review on the Latest Development of Carbon Membranes for Gas Separation. J. Membr. Sci. 2001, 193, 1–18. [Google Scholar] [CrossRef]
- Torres, D.; Pérez-Rodríguez, S.; Cesari, L.; Castel, C.; Favre, E.; Fierro, V.; Celzard, A. Review on the Preparation of Carbon Membranes Derived from Phenolic Resins for Gas Separation: From Petrochemical Precursors to Bioresources. Carbon 2021, 183, 12–33. [Google Scholar] [CrossRef]
- Lei, L.; He, X. Carbon Membrane Preparation. In Carbon Membrane Technology; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–20. ISBN 9780429445989. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, W.; Mahurin, S.M.; Wang, S.; Chen, H.; Cheng, L.; Jie, K.; Meyer, H.M.; Jiang, D.; Liu, G.; et al. Surpassing Robeson Upper Limit for CO2/N2 Separation with Fluorinated Carbon Molecular Sieve Membranes. Chem 2020, 6, 631–645. [Google Scholar] [CrossRef]
- Medrano, J.A.; Llosa-Tanco, M.A.; Tanaka, D.A.P.; Gallucci, F. Membranes Utilization for Biogas Upgrading to Synthetic Natural Gas. In Substitute Natural Gas from Waste; Elsevier: Amsterdam, The Netherlands, 2019; pp. 245–274. ISBN 9780128155547. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Whitley, R.; Mendes, A. Preparation and Characterization of Carbon Molecular Sieve Membranes Based on Resorcinol–Formaldehyde Resin. J. Membr. Sci. 2014, 459, 207–216. [Google Scholar] [CrossRef]
- Yoshimune, M.; Yamamoto, T.; Nakaiwa, M.; Haraya, K. Preparation of Highly Mesoporous Carbon Membranes via a Sol–Gel Process Using Resorcinol and Formaldehyde. Carbon 2008, 46, 1031–1036. [Google Scholar] [CrossRef]
- Dong, Y.R.; Nakao, M.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Gas Permeation and Pervaporation of Water/Alcohols through the Microporous Carbon Membranes Prepared from Resorcinol/Formaldehyde/Quaternary Ammonium Compounds. Sep. Purif. Technol. 2010, 73, 2–7. [Google Scholar] [CrossRef]
- Tanaka, D.A.P.; Tanco, M.A.L.; Okazaki, J.; Wakui, Y.; Mizukami, F.; Suzuki, T.M. Preparation of “Pore-Fill” Type Pd–YSZ–γ-Al2O3 Composite Membrane Supported on α-Al2O3 Tube for Hydrogen Separation. J. Membr. Sci. 2008, 320, 436–441. [Google Scholar] [CrossRef]
- Tanco, M.A.L.; Tanaka, D.A.P.; Rodrigues, S.C.; Texeira, M.; Mendes, A. Composite-Alumina-Carbon Molecular Sieve Membranes Prepared from Novolac Resin and Boehmite. Part I: Preparation, Characterization and Gas Permeation Studies. Int. J. Hydrog. Energy 2015, 40, 5653–5663. [Google Scholar] [CrossRef]
- Rahimalimamaghani, A.; Tanaka, D.A.P.; Tanco, M.A.L.; D’Angelo, F.N.; Gallucci, F. Effect of Aluminium Acetyl Acetonate on the Hydrogen and Nitrogen Permeation of Carbon Molecular Sieves Membranes. Int. J. Hydrog. Energy 2022, 47, 14570–14579. [Google Scholar] [CrossRef]
- Lewicki, J.P.; Fox, C.A.; Worsley, M.A. On the Synthesis and Structure of Resorcinol-Formaldehyde Polymeric Networks—Precursors to 3D-Carbon Macroassemblies. Polymer 2015, 69, 45–51. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson Upper Bounds for CO2/CH4 and CO2/N2 Separations Using a Series of Ultrapermeable Benzotriptycene-Based Polymers of Intrinsic Microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Huang, G.; Isfahani, A.P.; Muchtar, A.; Sakurai, K.; Shrestha, B.B.; Qin, D.; Yamaguchi, D.; Sivaniah, E.; Ghalei, B. Pebax/Ionic Liquid Modified Graphene Oxide Mixed Matrix Membranes for Enhanced CO2 Capture. J. Membr. Sci. 2018, 565, 370–379. [Google Scholar] [CrossRef]
- Habib, N.; Shamair, Z.; Tara, N.; Nizami, A.S.; Akhtar, F.H.; Ahmad, N.M.; Gilani, M.A.; Bilad, M.R.; Khan, A.L. Development of Highly Permeable and Selective Mixed Matrix Membranes Based on Pebax®1657 and NOTT-300 for CO2 Capture. Sep. Purif. Technol. 2020, 234, 116101. [Google Scholar] [CrossRef]
- Yave, W.; Huth, H.; Car, A.; Schick, C. Peculiarity of a CO2-Philic Block Copolymer Confined in Thin Films with Constrained Thickness: “A Super Membrane for CO2-Capture”. Energy Environ. Sci. 2011, 4, 4656–4661. [Google Scholar] [CrossRef]
- Xia, J.; Liu, S.; Chung, T.S. Effect of End Groups and Grafting on the CO2 Separation Performance of Poly(Ethylene Glycol) Based Membranes. Macromolecules 2011, 44, 7727–7736. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Yu, X.; Wang, J.; Wang, S. High-Performance Membranes with Multi-Permselectivity for CO2 Separation. Adv. Mater. 2012, 24, 3196–3200. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, Z.; Zhang, C.; Yuan, S.; Zhu, Y.; Wang, J.; Wang, S. PVAm–PIP/PS Composite Membrane with High Performance for CO2/N2 Separation. AIChE J. 2013, 59, 215–228. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, W.S.W. High-Molecular-Weight Polyvinylamine/Piperazine Glycinate Membranes for CO2 Capture from Flue Gas. J. Membr. Sci. 2016, 514, 376–384. [Google Scholar] [CrossRef]
- Blinova, N.V.; Svec, F. Functionalized Polyaniline-Based Composite Membranes with Vastly Improved Performance for Separation of Carbon Dioxide from Methane. J. Membr. Sci. 2012, 423–424, 514–521. [Google Scholar] [CrossRef]
- Hu, C.P.; Polintan, C.K.; Tayo, L.L.; Chou, S.C.; Tsai, H.A.; Hung, W.S.; Hu, C.C.; Lee, K.R.; Lai, J.Y. The Gas Separation Performance Adjustment of Carbon Molecular Sieve Membrane Depending on the Chain Rigidity and Free Volume Characteristic of the Polymeric Precursor. Carbon 2019, 143, 343–351. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, H.; Zhang, S.; Zhang, F.; Jin, J. Carbon Molecular Sieve Membranes Derived from Tröger’s Base-Based Microporous Polyimide for Gas Separation. ChemSusChem 2018, 11, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.G.; Fu, S.; Itta, A.K.; Qiu, W.; Liu, G.; Swaidan, R.; Koros, W.J. 6FDA-DETDA: DABE Polyimide-Derived Carbon Molecular Sieve Hollow Fiber Membranes: Circumventing Unusual Aging Phenomena. J. Membr. Sci. 2018, 546, 197–205. [Google Scholar] [CrossRef]


| Sample (Polymer) | C | H | O | MW | DP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal | Obs * | Cal | Cal | Obs * | Cal | Obs * | Cal | Obs ** | ||||
| (%) | # | (%) | # | (%) | # | (g mol−1) | ## | |||||
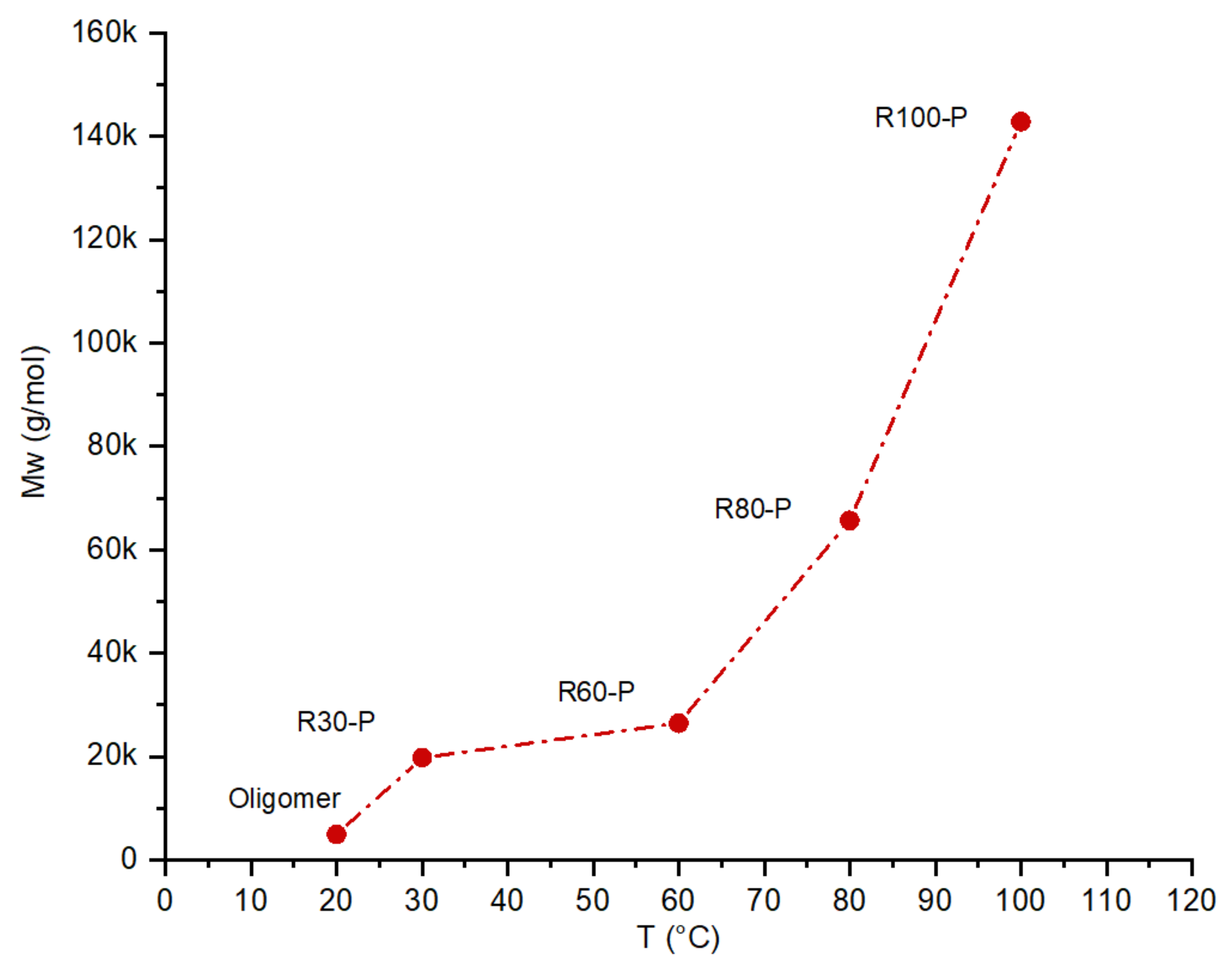
| Oligomer | 61.5 | 61.9 | 250 | 5.7 | 5.5 | 280 | 32.8 | 32.6 | 100 | 4880 | 4939 | 10 |
| R30-P | 61.4 | 62.2 | 1025 | 5.7 | 5.8 | 1148 | 32.9 | 32 | 410 | 20,008 | 19,766 | 41 |
| R60-P | 61.5 | 62.4 | 1350 | 5.7 | 5.6 | 1512 | 32.8 | 32 | 540 | 26,352 | 26,420 | 54 |
| R80-P | 61.5 | 61.9 | 3375 | 5.7 | 5.6 | 3780 | 32.8 | 32.5 | 1350 | 65,880 | 65,640 | 135 |
| R100-P | 61.5 | 62 | 7325 | 5.7 | 5.8 | 8204 | 32.8 | 32.2 | 2930 | 142,984 | 142,790 | 293 |
| Membrane | SEM-EDX (wt%) | Organic Elemental Analysis (wt%) | |||
|---|---|---|---|---|---|
| C | O | C | O | H | |
| R30 | 90.6 | 6.3 | 92.1 | 4.9 | 3 |
| R60 | 92.7 | 5.7 | 94.6 | 3.1 | 2.3 |
| R80 | 94.2 | 3.8 | 96.1 | 2.6 | 1.3 |
| R100 | 96.5 | 3.2 | 97.2 | 2.1 | 0.7 |
| R80T100 | 91.1 | 5.9 | 95.9 | 2.7 | 1.4 |
| R80T150 | 88.9 | 7.4 | 95.3 | 2.9 | 1.8 |
| Membrane | Before Post Treatment (nm) | After Post Treatment (nm) | ||
|---|---|---|---|---|
| Ra | Rz | Ra | Rz | |
| R80T100 | 1347 | 8843 | 39 | 399 |
| R80T150 | 1266 | 8690 | 164 | 1099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahimalimamaghani, A.; Pacheco Tanaka, D.A.; Llosa Tanco, M.A.; Neira D’Angelo, M.F.; Gallucci, F. Ultra-Selective CMSMs Derived from Resorcinol-Formaldehyde Resin for CO2 Separation. Membranes 2022, 12, 847. https://doi.org/10.3390/membranes12090847
Rahimalimamaghani A, Pacheco Tanaka DA, Llosa Tanco MA, Neira D’Angelo MF, Gallucci F. Ultra-Selective CMSMs Derived from Resorcinol-Formaldehyde Resin for CO2 Separation. Membranes. 2022; 12(9):847. https://doi.org/10.3390/membranes12090847
Chicago/Turabian StyleRahimalimamaghani, Arash, David Alfredo Pacheco Tanaka, Margot A. Llosa Tanco, Maria Fernanda Neira D’Angelo, and Fausto Gallucci. 2022. "Ultra-Selective CMSMs Derived from Resorcinol-Formaldehyde Resin for CO2 Separation" Membranes 12, no. 9: 847. https://doi.org/10.3390/membranes12090847
APA StyleRahimalimamaghani, A., Pacheco Tanaka, D. A., Llosa Tanco, M. A., Neira D’Angelo, M. F., & Gallucci, F. (2022). Ultra-Selective CMSMs Derived from Resorcinol-Formaldehyde Resin for CO2 Separation. Membranes, 12(9), 847. https://doi.org/10.3390/membranes12090847