Volatile Fatty Acids (VFA) Production and Recovery from Chicken Manure Using a High-Solid Anaerobic Membrane Bioreactor (AnMBR)
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate, Inoculum and Thermal Shock Pretreatment
2.2. Experimental Set Up and Operational Parameters
2.3. Hydrolysis, Acidogenesis, Acetogenesis, Methanogenesis and Hydrogenesis Kinetics under Different pH
2.4. Analytical Methods
3. Results and Discussions
3.1. Effects of pH and OLR on VFA Fermentation
3.2. Effects of Ammonia Nitrogen on VFA Yield from CM
3.3. Kinetic Activities at Different pH
3.4. Membrane Filtration Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Gateway to Poultry Production and Products; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2020; pp. 2–3. [Google Scholar]
- Sakar, S.; Yetilmezsoy, K.; Kocak, E. Anaerobic digestion technology in poultry and livestock waste treatment—A literature review. Waste Manag. Res. 2009, 27, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Qiao, W.; Xiong, L.; Mandy, A.; Wandera, S.M.; Yin, D.; Dong, R.J. Improved high solid anaerobic digestion of chicken manure by moderate in situ ammonia stripping and its relation to metabolic pathway. Renew. Energ. 2020, 146, 2380–2389. [Google Scholar] [CrossRef]
- Yin, D.M.; Westerholm, M.; Qiao, W.; Bi, S.J.; Wandera, S.M.; Fan, R.; Jiang, M.M.; Dong, R.J. An explanation of the methanogenic pathway for methane production in anaerobic digestion of nitrogen-rich materials under mesophilic and thermophilic conditions. Bioresour. Technol. 2018, 264, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Westerholm, M.; Qiao, W.; Xiong, L.; Mahdy, A.; Yin, D.; Song, Y.; Dong, R. Metabolic performance of anaerobic digestion of chicken manure under wet, high solid, and dry conditions. Bioresour. Technol. 2020, 296, 122342. [Google Scholar] [CrossRef]
- Fuchs, W.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China. Renew. Sust. Energ. Rev. 2018, 97, 186–199. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa, A.M.K.; Taherzadeh, M. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Baumann, I.; Westermann, P. Microbial production of short chain fatty acids from lignocellulosic biomass: Current processes and market. Front. Environ. Sci. 2016, 2016, 8469357. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Song, H.S.; Jeon, J.M.; Kim, J.S.; Lee, Y.K.; Yang, Y.H. Poly (3-hydroxybutyrate- co -3-hydroxyhexanoate) production from engineered Ralstonia eutropha using synthetic and anaerobically digested food waste derived volatile fatty acids. Int. J. Biol. M. 2019, 133, 1–10. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, C.; Ai, S.; Wang, H.; Gao, Y.; Yan, L.; Wang, W. Biological pretreatment enhances the activity of functional microorganisms and the ability of methanogenesis during anaerobic digestion. Bioresour. Technol. 2019, 290, 121660. [Google Scholar] [CrossRef]
- Hussain, A.; Filiatrault, M.; Guiot, S.R. Acidogenic digestion of food waste in a thermophilic leach bed reactor: Effect of pH and leachate recirculation rate on hydrolysis and volatile fatty acid production. Bioresour Technol. 2017, 245 Pt A, 1–9. [Google Scholar] [CrossRef]
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour Technol. 2020, 302, 122800. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Liu, J.; Zhang, C.; Zou, L.; Li, Y.Y.; Xu, Z.P. Pretreating anaerobic fermentation liquid with calcium addition to improve short chain fatty acids extraction via in situ synthesis of layered double hydroxides. Bioresour Technol. 2019, 271, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Tampio, E.A.; Lucia, B.; Vainio, M.M.; Kahala, M.M.; Rasi, S.E. Volatile fatty acids (VFAs) and methane from food waste and cow slurry: Comparison of biogas and VFA fermentation processes. GCB Bioenerg. 2019, 11, 72–84. [Google Scholar] [CrossRef]
- Jomnonkhaow, U.; Uwineza, C.; Mahboubi, A.; Wainaina, S.; Reungsang, A.; Taherzadeh, M.J. Membrane bioreactor-assisted volatile fatty acids production and in situ recovery from cow manure. Bioresour Technol. 2021, 321, 124456. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Ros, C.D.; Pavan, P.; Bolzonella, D.J. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Holtzapple, M.T.; Davison, R.R.; Ross, M.K.; Aldrett-Lee, S.; Nagwani, M.; Lee, C.M.; Lee, C.; Adelson, S.; Kaar, W.; Gaskin, D.; et al. Biomass conversion to mixed alcohol fuels using the MixAlco process. Appl. Biochem. Biotech. 1999, 79, 609–631. [Google Scholar] [CrossRef]
- Kim, N.J.; Lim, S.J.; Chang, H.N. Volatile fatty acid platform: Concept and application. In Emerging Areas in Bioengineering; Chang, H.N., Ed.; Wiley-VCH: Weinheim, Germany, 2018; Chapter 10; pp. 255–256. [Google Scholar]
- Yin, D.M.; Mahboubi, A.; Wainaina, S.; Qiao, W.; Taherzadeh, M.J. The effect of mono- and multiple fermentation parameters on volatile fatty acids (VFAs) production from chicken manure via anaerobic digestion. Bioresour. Technol. 2021, 330, 124992. [Google Scholar] [CrossRef]
- Rebecchi, S.; Pinelli, D.; Bertin, L.; Zama, F.; Fava, F.; Frascari, D. Volatile fatty acids recovery from the effluent of an acidogenic digestion process fed with grape pomace by adsorption on ion exchange resins. Chem. Eng. J. 2016, 306, 629–639. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Liu, Y.; Deng, L.; Cheng, C. Selective production of volatile fatty acids at different pH in an anaerobic membrane bioreactor. Bioresour Technol. 2019, 283, 120–128. [Google Scholar] [CrossRef]
- Mazzei, R.; Piacentini, E.; Yihdegom, G.A.; Giorno, L. Membrane bioreactors in food, pharmaceutical and biofuel applications: State of the art, progresses and perspectives. Curr. Org. Chem. 2017, 21, 1671–1701. [Google Scholar] [CrossRef]
- Trad, Z.; Akimbomi, J.; Vial, C.; Larroche, C.; Taherzadeh, M.J.; Fontaine, J.P. Development of a submerged anaerobic membrane bioreactor for concurrent extraction of volatile fatty acids and biohydrogen production. Bioresour. Technol. 2015, 196, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Parchami, M.; Wainaina, S.; Soufiani, A.M.; I’Ons, D.; Taherzadeh, M. MBR-Assisted VFAs production from excess sewage sludge and food waste slurry for sustainable wastewater treatment. Appl. Sci. 2020, 10, 2921. [Google Scholar] [CrossRef]
- Mahboubi, A.; Uwineza, C.; Doyen, W.; Wever, H.D.; Taherzadeh, M.J. Intensification of lignocellulosic bioethanol production process using continuous double-staged immersed membrane bioreactors. Bioresour Technol. 2020, 296, 122314. [Google Scholar] [CrossRef]
- Huang, W.W.; Huang, W.L.; Yuan, T.; Zhao, Z.; Cai, W.; Zhang, Z.; Lei, Z.; Feng, C. Volatile fatty acids (VFAs) production from swine manure through short-term dry anaerobic digestion and its separation from nitrogen and phosphorus resources in the digestate. Water Res. 2016, 90, 344–353. [Google Scholar] [CrossRef]
- Kullavanijaya, P.; Chavalparit, O. The production of volatile fatty acids from Napier grass via an anaerobic leach bed process: The influence of leachate dilution, inoculum, recirculation, and buffering agent addition. J. Environ. Chem. Eng. 2019, 7, 103458. [Google Scholar] [CrossRef]
- Wainaina, S.; Kisworini, A.D.; Fanani, M.; Wikandari, R.; Taherzadeh, M.J. Utilization of food waste-derived volatile fatty acids for production of edible Rhizopus oligosporus fungal biomass. Bioresour Technol. 2020, 310, 123444. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, J.; Zhang, Y.; Li, K. Effect of initial total solids concentration on volatile fatty acid production from food waste during anaerobic acidification. Environ. Technol. 2015, 36, 1884–1891. [Google Scholar] [CrossRef]
- Béligon, V.; Noblecourt, A.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P.J.B. Proof of concept for biorefinery approach aiming at two bioenergy production compartments, hydrogen and biodiesel, coupled by an external membrane. Biofuels 2018, 9, 163–174. [Google Scholar] [CrossRef]
- Yin, D.M.; Taherzadeh, M.J.; Lin, M.; Jiang, M.M.; Qiao, W.; Dong, R.J. Upgrading the anaerobic membrane bioreactor treatment of chicken manure by introducing in-situ ammonia stripping and hyper-thermophilic pretreatment. Bioresour. Technol. 2020, 310, 123470. [Google Scholar] [CrossRef]
- Yin, D.M.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R.J. Enhancing hyper-thermophilic hydrolysis pre-treatment of chicken manure for biogas production by in-situ gas phase ammonia stripping. Bioresour. Technol. 2019, 287, 121470. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF): Washington, DC, USA, 2012. [Google Scholar]
- Wainaina, S.; Awasthi, M.K.; Horvath, I.S.; Taherzadeh, M.J. Anaerobic digestion of food waste to volatile fatty acids and hydrogencat high organic loading rates in immersed membrane bioreactors. Renew. Energ. 2020, 152, 1140–1148. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Ahn, J.; Kim, J.; Lee, S.; Lee, I.; Kim, S.; Chang, S.; Chung, W.J.S. Volatile fatty acid production from food waste leachate using enriched bacterial culture and soil bacteria as co- digester. Sustainability 2021, 13, 9606. [Google Scholar] [CrossRef]
- Temudo, M.F.; Kleerebezem, R.; Loosdrecht, M.V. Influence of the pH on (open) mixed culture fermentation of glucose: A chemostat study. Biotechnol. Bioeng. 2007, 98, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Coats, E.R.; Gregg, M.; Crawford, R.L. Effect of organic loading and retention time on dairy manure fermentation. Bioresour. Technol. 2011, 102, 2572–2577. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yu, X.; Wang, K.; Shen, D. Acidogenic fermentation of the main substrates of food waste to produce volatile fatty acids. Int. J.Hydrogen. 2016, 41, 21713–21720. [Google Scholar] [CrossRef]
- Nie, H.; Jacobi, F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Mono-fermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Luongo, V.; Frunzo, L.; Pirozzi, F.; Lens, P.; Esposito, G. Continuous biohydrogen production by thermophilic dark fermentation of cheese whey: Use of buffalo manure as buffering agent. Int. J. Hydrog. Energ. 2016, 42, 4861–4869. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Mirzaman, Z.; Wayne, J.P. Characterization of hydrolysis kinetics in staged anaerobic digestion of wastewater treatment sludge. Water Environ. Res. 2018, 8, 5–12. [Google Scholar]
- Gustin, S.; Marinsek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process. Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Jiang, M.; Qiao, W.; Jiang, P.; Wu, Z.; Lin, M.; Sun, Y.; Dong, R. Mitigating membrane fouling in a high solid food waste thermophilic anaerobic membrane bioreactor by incorporating fixed bed bio-carriers. Chemosphere 2022, 292, 133488. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Qiao, W.; Liu, Y.; Yao, J.; Gu, C.; Zheng, X.; Dong, R. Contribution of chemical precipitation to the membrane fouling in a high-solids type anaerobic membrane bioreactor treating OFMSW leachate. J. Membrane. Sci. 2022, 647, 120298. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Rania, H.; Joo, H.T. Membrane bioreactor (MBR) technology for wastewater treatment and reclamation: Membrane fouling. Membrane 2016, 6, 33. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Hu, Y.; Guo, G.; Li, Y.Y. Bioenergy recovery from methanogenic co-digestion of food waste and sewage sludge by a high-solid anaerobic membrane bioreactor (AnMBR): Mass balance and energy potential. Bioresour. Technol. 2021, 326, 124754. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Calli, B. Long-term inuence of trace element deciency on anaerobic mono-digestion of chicken manure. J. Environ. Manag. 2018, 223, 743–748. [Google Scholar] [CrossRef]
- Sambusiti, C.; Myriam, S.; Gauchou, V.; Segues, B.; Leca, M.A.; Baldoni-Andrey, P.; Jacob, M. Influence of HRT reduction on pilot scale flat sheet submerged membrane bioreactor (sMBR) performances for Oil & Gas wastewater treatment. J. Membr. Sci. 2019, 594, 117459. [Google Scholar]
- Buzatu, P.; Qiblawey, H.; Odai, A.; Jamaleddin, J.; Nasser, M.; Judd, S.J. Clogging vs. fouling in immersed membrane bioreactors. Water Res. 2018, 144, 46–54. [Google Scholar] [CrossRef]
- Kim, Y.; Li, S.; Chekli, L.; Woo, Y.C.; Wei, C.H.; Phuntsho, S.; Ghaffour, N.; Leiknes, T.; Shon, H.K. Assessing the removal of organic micro-pollutants from anaerobic membrane bioreactor effluent by fertilizer-drawn forward osmosis. J. Membr. Sci. 2017, 533, 84–95. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Y.; Yao, J.; Zheng, X.; Wandera, S.M.; Dong, R.; Li, Y.Y.; Qiao, W. The materials flow and membrane filtration performance in treating the organic fraction of municipal solid waste leachate by a high solid type of submerged anaerobic membrane bioreactor. Bioresour. Technol. 2021, 329, 124927. [Google Scholar] [CrossRef]
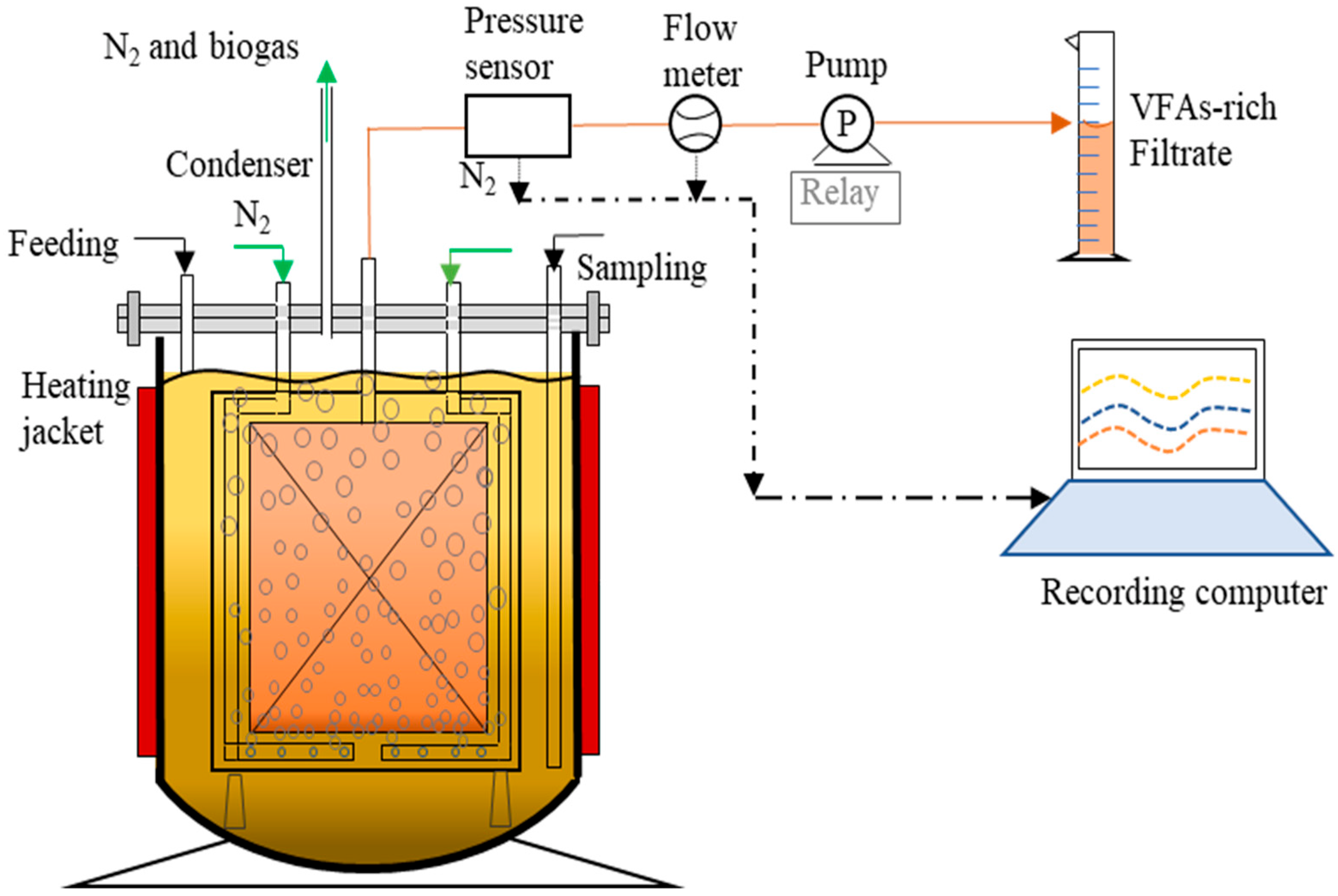

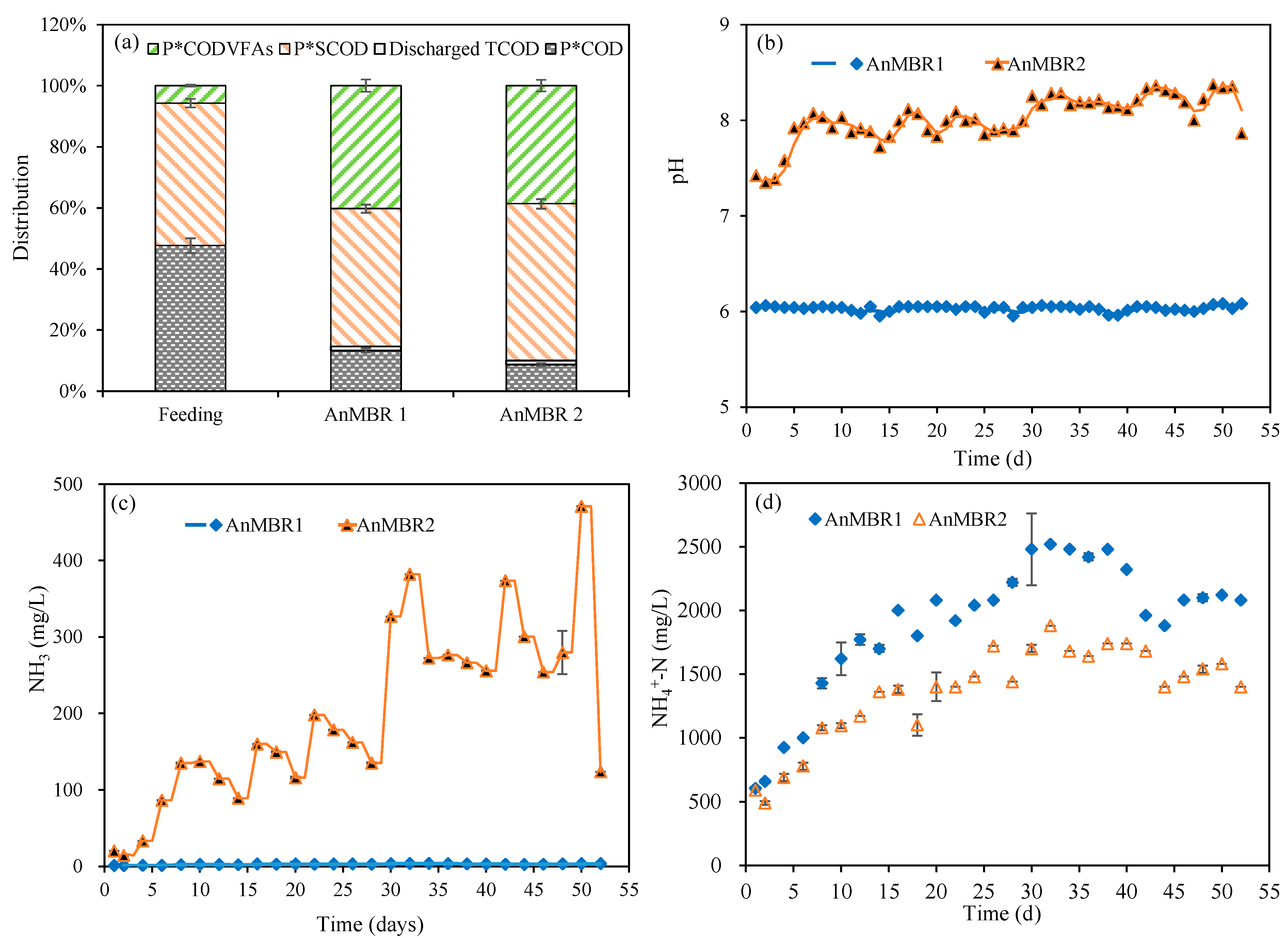

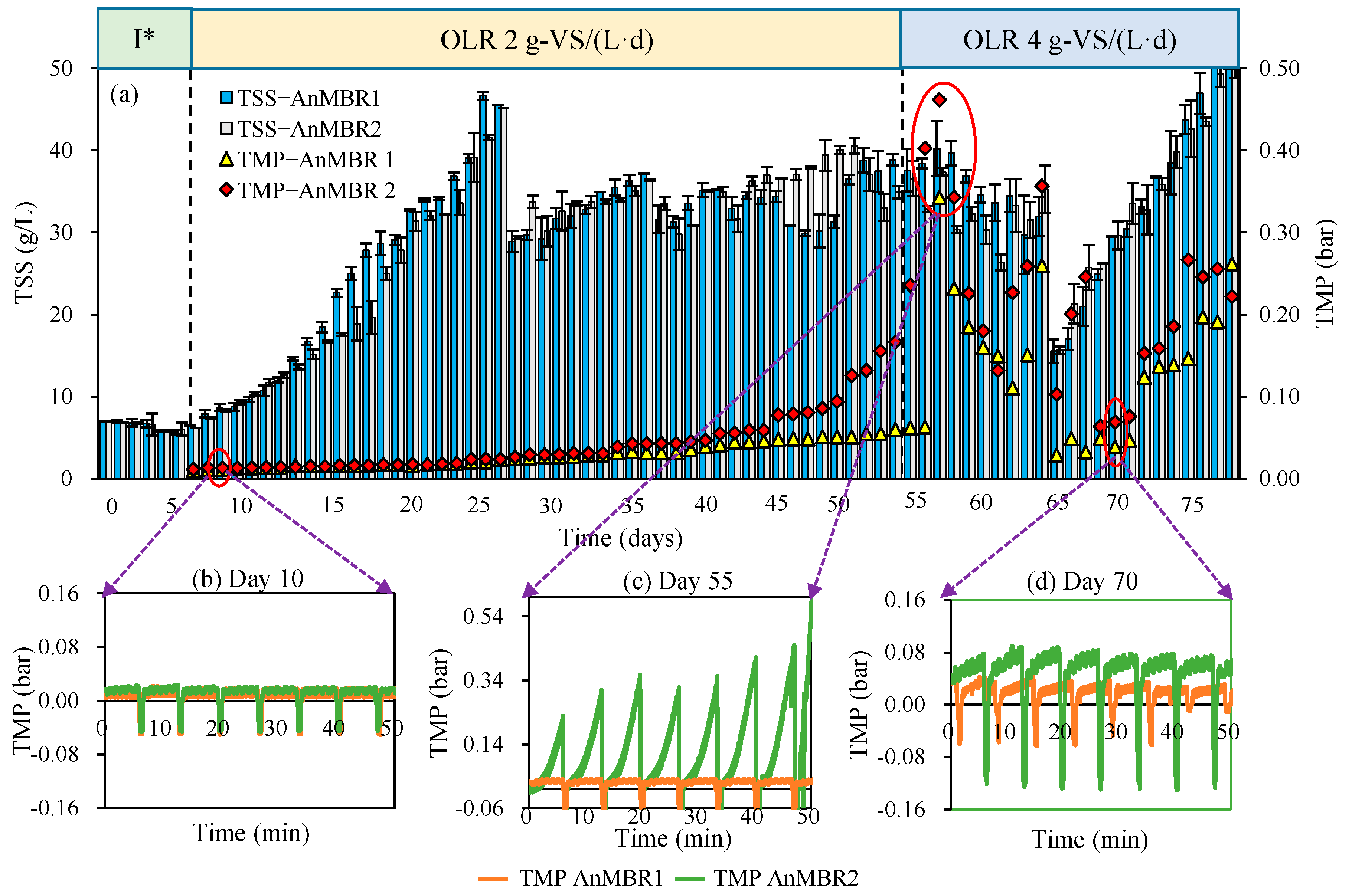
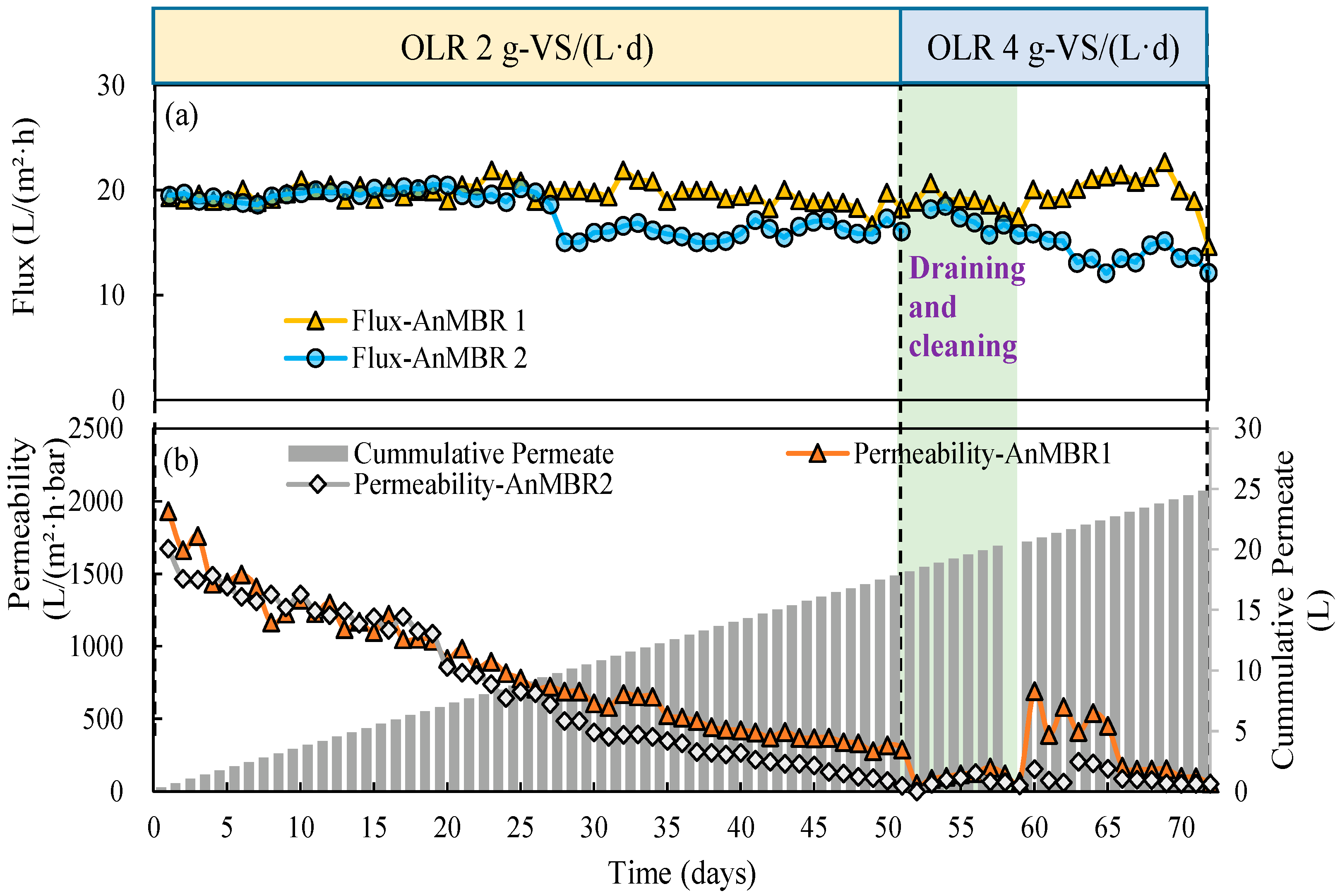
| Units | Raw CM | Inoculum CM (80 °C, 15 min) | Sieved Thermally Shocked CM (80 °C, 90 min) | |
|---|---|---|---|---|
| pH | - | - | 7.1 | 7.8 |
| TS | g/L | 327 ± 2 | 14.6 ± 0.7 | 63.9 ± 4.9 |
| VS | g/L | 220 ± 1 | 10.1 ± 0.7 | 45.1 ± 0.2 |
| VS/TS | %TS | 67 ± 1 | 68.3 ± 2.4 | 70.5 ± 4.7 |
| TSS | g/L | 282 ± 5.0 | 7.1 ± 0.1 | 37.8 ± 0.9 |
| VSS | g/L | 183 ± 2 | 3.9 ± 0.1 | 25.4 ± 0.8 |
| DS | g/L | 45 ± 13.0 | 3.1 ± 0.7 | 26.1 ± 5.8 |
| VSD | g/L | 37 ± 1.0 | 3.1 ± 0.7 | 19.7 ± 0.6 |
| TCOD | g/L | 287 ± 4.0 | 12.8 ± 0.4 | 95.5 ± 0.7 |
| SCOD | g/L | 36 ± 2.0 | 5.5 ± 0.1 | 44.5 ± 0.7 |
| Ace | g/L | 3.2 ± 0.2 | 1.9 ± 0.3 | 3.8 ± 0.3 |
| Pro. | g/L | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.5 ± 0.2 |
| But. | g/L | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| total VFA | g/L | 3.8 ± 0.2 | 3.1 ± 0.1 | 4.7 ± 0.1 |
| NH4+-N | mg/L | ND | 495 ± 7 | 4550 ± 71 |
| Bicarbonate alkalinity | mg-Ca(HCO3)2/L | ND | 1063 ± 18 | 11,875 ± 355 |
| Total alkalinity | mg-CaCO3/L | ND | 1438 ± 35 | 12,500 ± 575 |
| TKN | % | 26.6 ± 0.3 | ND | 2.5 ± 0.1 |
| VFA (g/L) | Evaporation Coefficient (g/(L·h)) | t1 (h) | t2 (h) | Volatile Concentration (g/(L·d)) | ||||
|---|---|---|---|---|---|---|---|---|
| k1 | R2 | k2 | R2 | |||||
| AnMBR1 (pH 6.0) | 10 | −0.10 | 0.98 | −0.13 | 0.99 | 22.5 | 1.5 | 2.5 |
| 20 | −0.14 | 0.99 | −0.16 | 0.98 | 22.5 | 1.5 | 3.4 | |
| AnMBR2 (pH~8) | 10 | −0.07 | 0.89 | −0.06 | 0.95 | 22.5 | 1.5 | 1.7 |
| 20 | −0.07 | 0.97 | −0.12 | 0.96 | 21.5 | 2.5 | 1.8 | |
| Units | OLR 2 g-VS/(L·d) | OLR 4 g-VS/(L·d) | |||
|---|---|---|---|---|---|
| AnMBR1 (pH 6.0) | AnMBR2 (pH Uncontrolled) | AnMBR1 (pH 6.0) | AnMBR2 (pH Uncontrolled) | ||
| pH | \ | 6.0 ± 0.1 | 8.2 ± 0.2 | 6.0 ± 0.0 | 8.2 ± 0.3 |
| TS | g/L | 52.6 ± 6.9 | 43.1 ± 5.7 | 53.3 ± 16.2 | 46.5 ± 10.1 |
| VS | g/L | 28.4 ± 2.6 | 29.6 ± 2.6 | 33.7 ± 9.1 | 32.2 ± 7.1 |
| VS/TS | %TS | 54.7 ± 6.6 | 69.0 ± 2.9 | 63.9 ± 3.2 | 69.5 ± 4.9 |
| TSS | g/L | 33.0 ± 2.3 | 34.0 ± 2.5 | 34.8 ± 10.3 | 33.5 ± 8.5 |
| VSS | g/L | 25.1 ± 2.8 | 24.8 ± 1.4 | 26.2 ± 6.0 | 25.0 ± 5.0 |
| TCOD | g/L | 22.0 ± 5.6 | 21.7 ± 4.7 | 62.1 ± 6.1 | 68.7 ± 6.5 |
| SCOD | g/L | 13.4 ± 1.4 | 17.0 ± 1.5 | 21.6 ± 4.7 | 21.7 ± 4.7 |
| TOC | g/L | 6.0 ± 1.4 | 4.8 ± 0.6 | 6.0 ± 1.4 | 4.8 ± 0.6 |
| Ace. | g/L | 8.5 ± 0.7 | 11.9 ± 0.8 | 5.8 ± 1.3 | 7.0 ± 2.8 |
| Pro. | g/L | 2.3 ± 0.3 | 3.1 ± 0.2 | 1.8 ± 0.2 | 2.0 ± 0.4 |
| But. | g/L | 2.2 ± 0.2 | 1.4 ± 0.2 | 2.4 ± 0.3 | 1.3 ± 0.3 |
| VFA * | g/L | 14.5 ± 1.2 | 18.0 ± 0.7 | 13.3 ± 1.3 | 12.9 ± 0.7 |
| NH4+-N | mg/L | 2262 ± 236 | 1645 ± 146 | 2042 ± 518 | 1381 ± 307 |
| NH3 | mg/L | 3.2 ± 0.4 | 269 ± 85 | 3.0 ± 0.9 | 245 ± 152 |
| Viscosity | mPa·s | 9.13 ± 1.04 | 3.03 ± 0.64 | 5.73 ± 3.90 | 3.50 ± 1.89 |
| TKN | % | 1.6 ± 0.1 | 0.3 ± 0.0 | 1.7 ± 0.1 | 0.2 ± 0.0 |
| Time (d) | 2nd | 4th | 6th | 8th | 10th | |
|---|---|---|---|---|---|---|
| AnMBR1 | Hydrolysis (%) | 16.8 ± 0.1 | 37.2 ± 0.2 | 65.0 ± 1.8 | 72.8 ± 1.7 | 73.9 ± 1.6 |
| Acidogenesis (%) | 14.9 ± 0.1 | 31.9 ± 0.8 | 47.2 ± 0.8 | 65.9 ± 2.2 | 71.7 ± 2.2 | |
| Methanogenesis (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.0 | 1.4 ± 0.0 | |
| Hydrogenesis (%) | 1.3 ± 0.0 | 3.2 ± 0.1 | 4.1 ± 0.1 | 5.0 ± 0.0 | 5.5 ± 0.1 | |
| AnMBR2 | Hydrolysis (%) | 30.4 ± 0.3 | 62.8 ± 0.4 | 73.0 ± 2.5 | 77.0 ± 2.6 | 80.9 ± 2.3 |
| Acidogenesis (%) | 17.9 ± 0.2 | 40.2 ± 0.3 | 59.5 ± 1.2 | 70.4 ± 2.1 | 76.4 ± 1.5 | |
| Methanogenesis (%) | 0.0 ± 0.0 | 0.1 ± 0.1 | 1.0 ± 0.1 | 2.4 ± 0.1 | 3.2 ± 0.1 | |
| Hydrogenesis (%) | 1.6 ± 0.0 | 4.9 ± 0.1 | 5.8 ± 0.2 | 6.2 ± 0.2 | 6.3 ± 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, D.M.; Uwineza, C.; Sapmaz, T.; Mahboubi, A.; De Wever, H.; Qiao, W.; Taherzadeh, M.J. Volatile Fatty Acids (VFA) Production and Recovery from Chicken Manure Using a High-Solid Anaerobic Membrane Bioreactor (AnMBR). Membranes 2022, 12, 1133. https://doi.org/10.3390/membranes12111133
Yin DM, Uwineza C, Sapmaz T, Mahboubi A, De Wever H, Qiao W, Taherzadeh MJ. Volatile Fatty Acids (VFA) Production and Recovery from Chicken Manure Using a High-Solid Anaerobic Membrane Bioreactor (AnMBR). Membranes. 2022; 12(11):1133. https://doi.org/10.3390/membranes12111133
Chicago/Turabian StyleYin, Dong Min, Clarisse Uwineza, Tugba Sapmaz, Amir Mahboubi, Heleen De Wever, Wei Qiao, and Mohammad J. Taherzadeh. 2022. "Volatile Fatty Acids (VFA) Production and Recovery from Chicken Manure Using a High-Solid Anaerobic Membrane Bioreactor (AnMBR)" Membranes 12, no. 11: 1133. https://doi.org/10.3390/membranes12111133
APA StyleYin, D. M., Uwineza, C., Sapmaz, T., Mahboubi, A., De Wever, H., Qiao, W., & Taherzadeh, M. J. (2022). Volatile Fatty Acids (VFA) Production and Recovery from Chicken Manure Using a High-Solid Anaerobic Membrane Bioreactor (AnMBR). Membranes, 12(11), 1133. https://doi.org/10.3390/membranes12111133










