Ethanol Separation from an Ethanol–Water Solution Using Vacuum Membrane Distillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Feed Solution
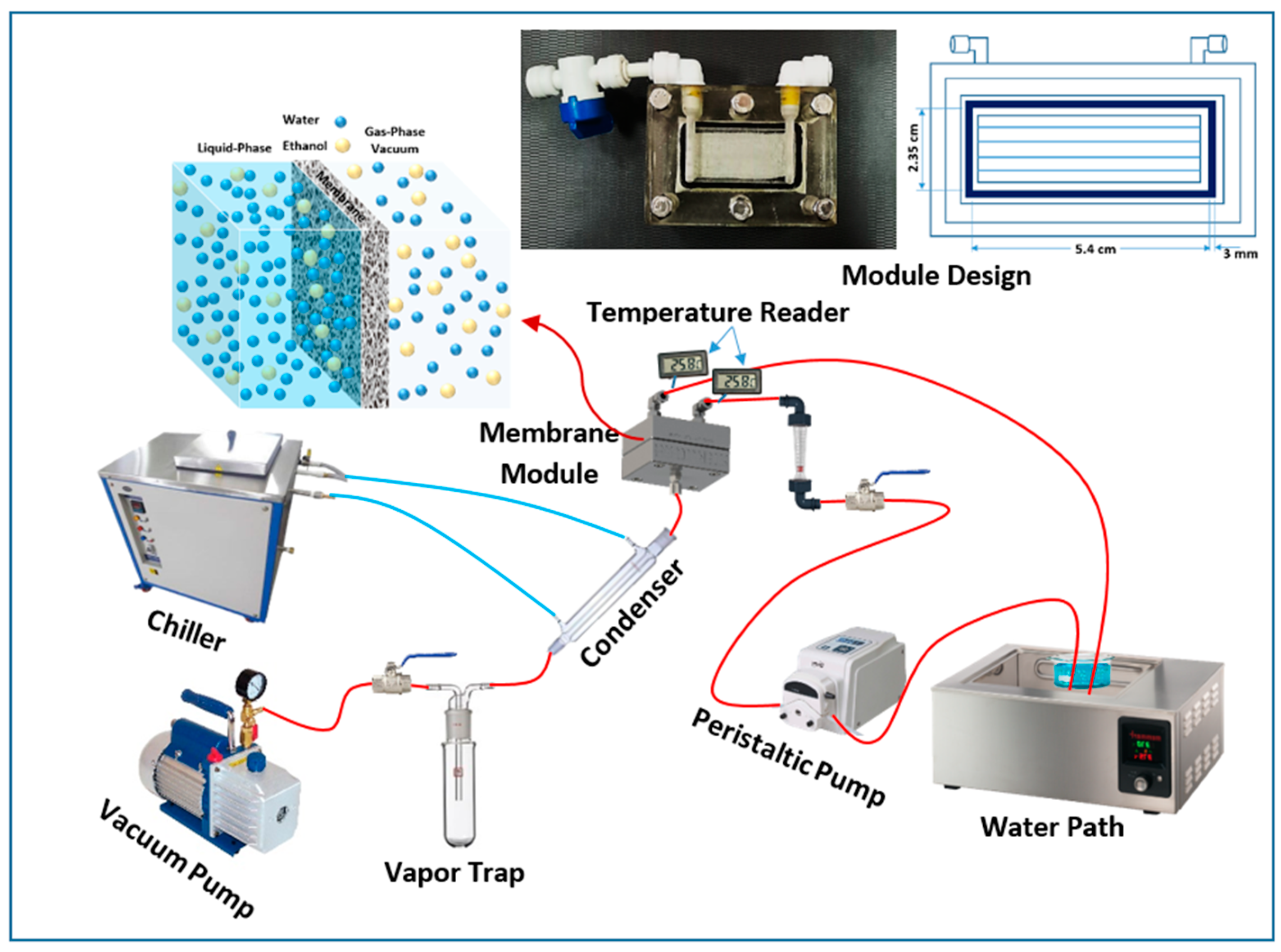
2.3. Experimental Setup
2.4. Characterization Methods
2.5. Experimental Design Using the Aguchi Method
3. Results and Discussion
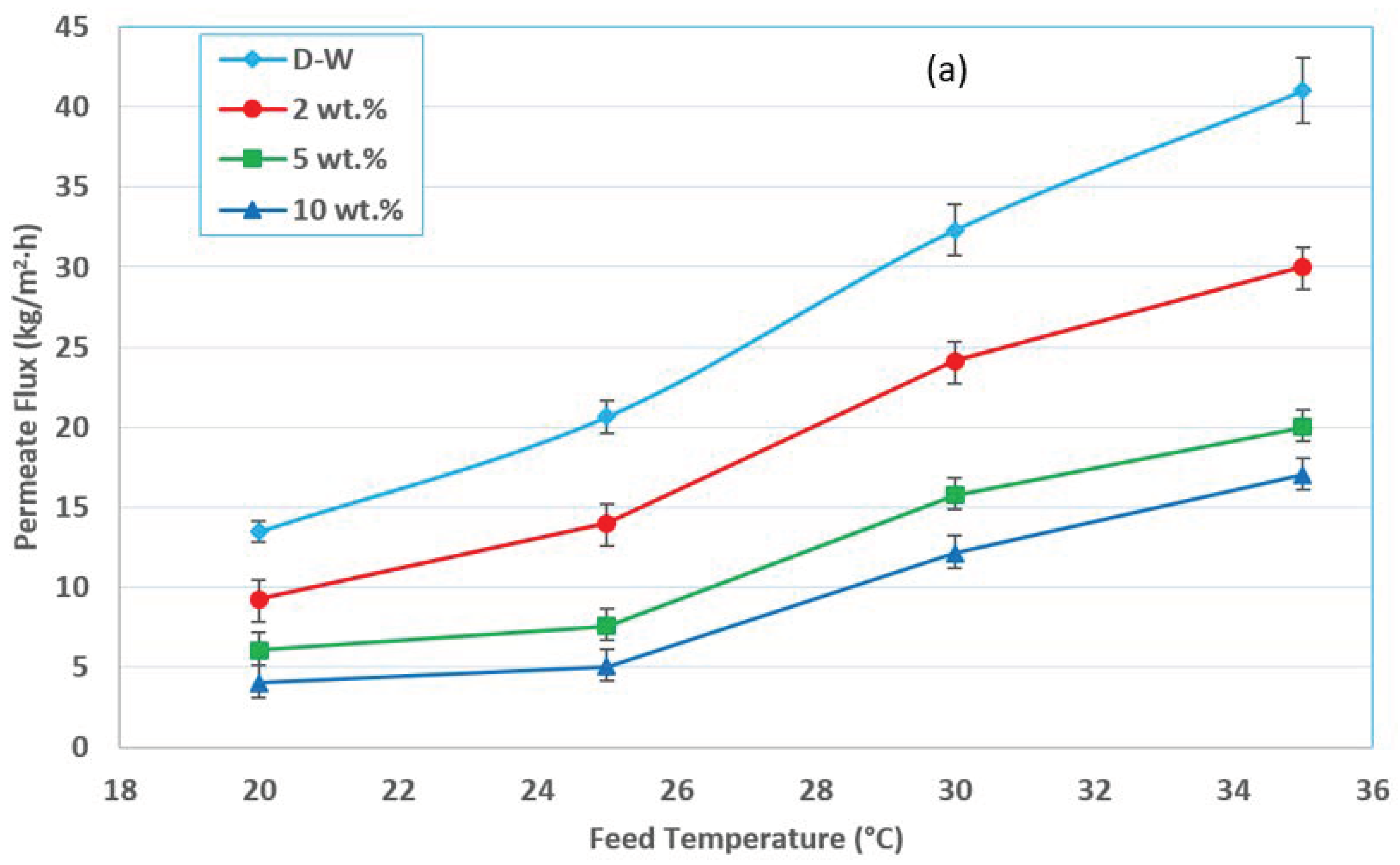
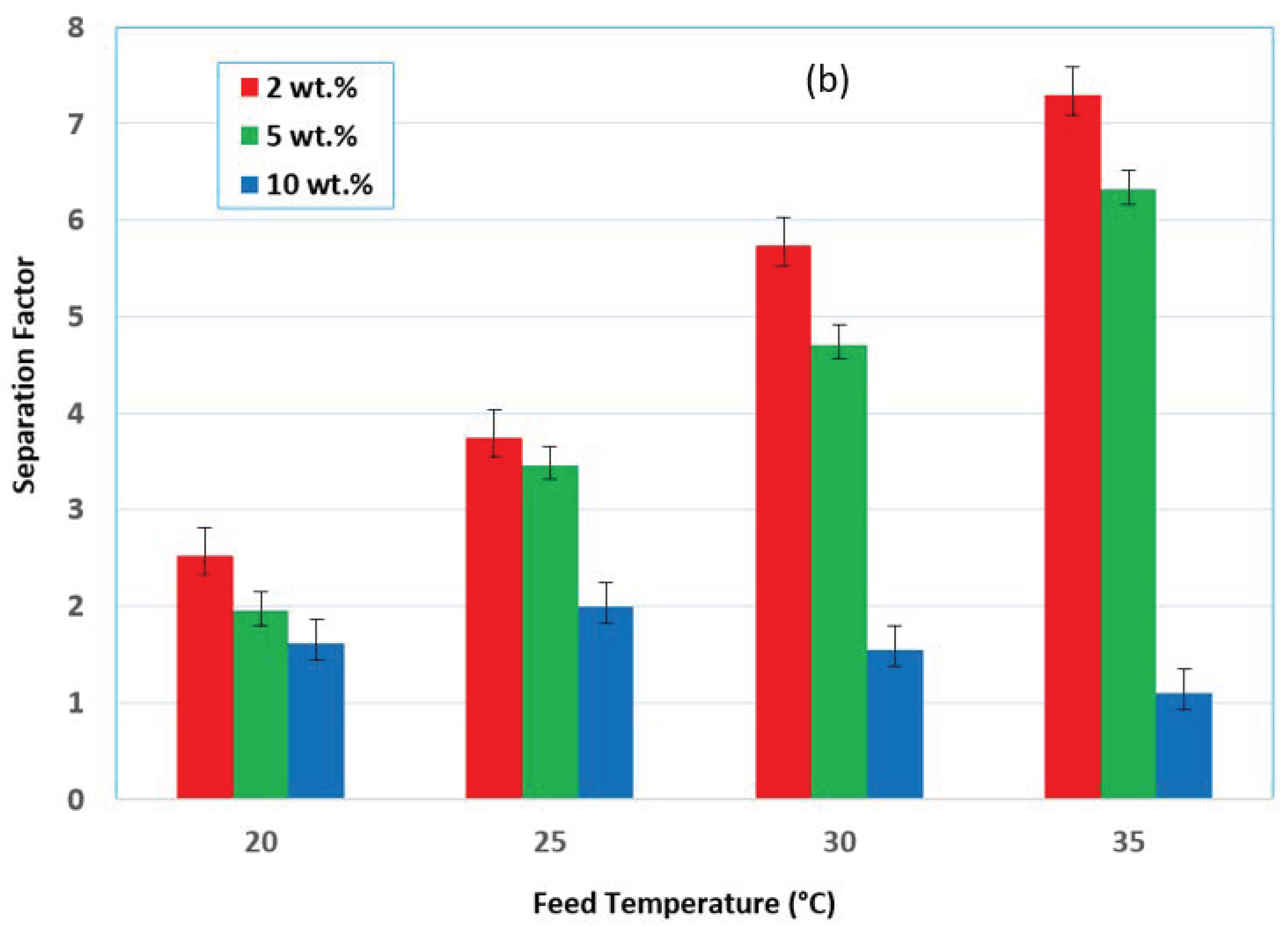
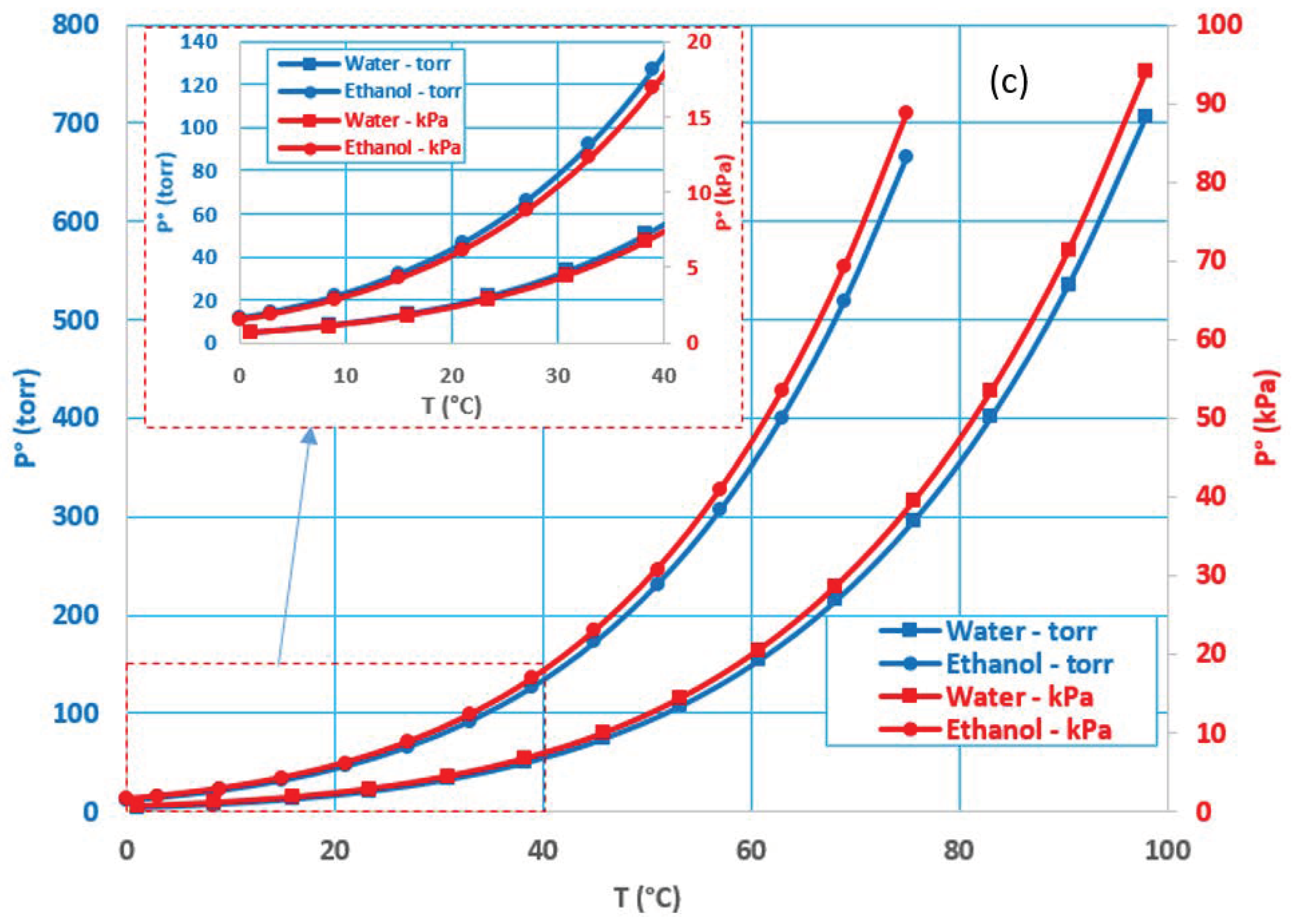
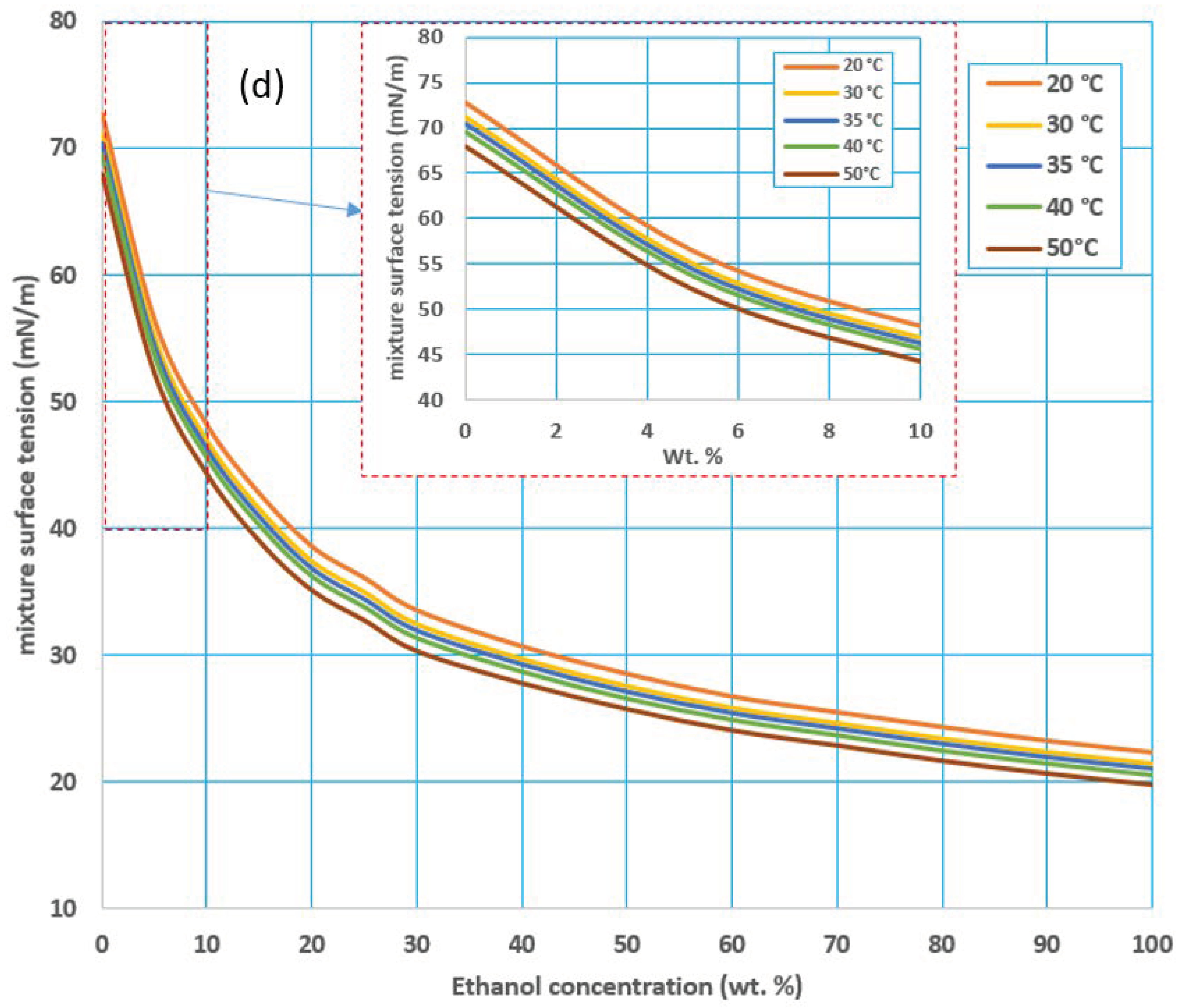
3.1. Effect of Feed Temperature
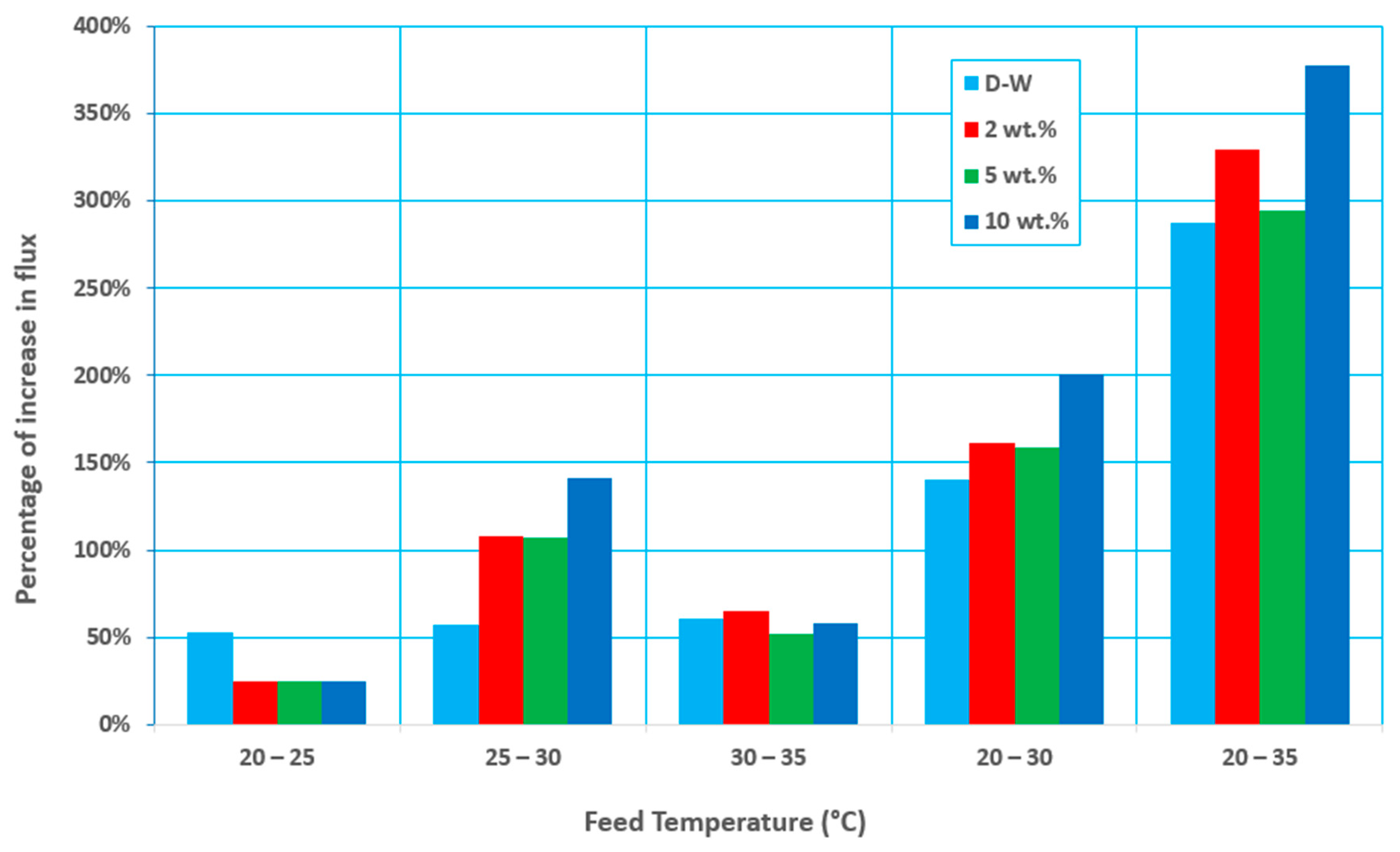
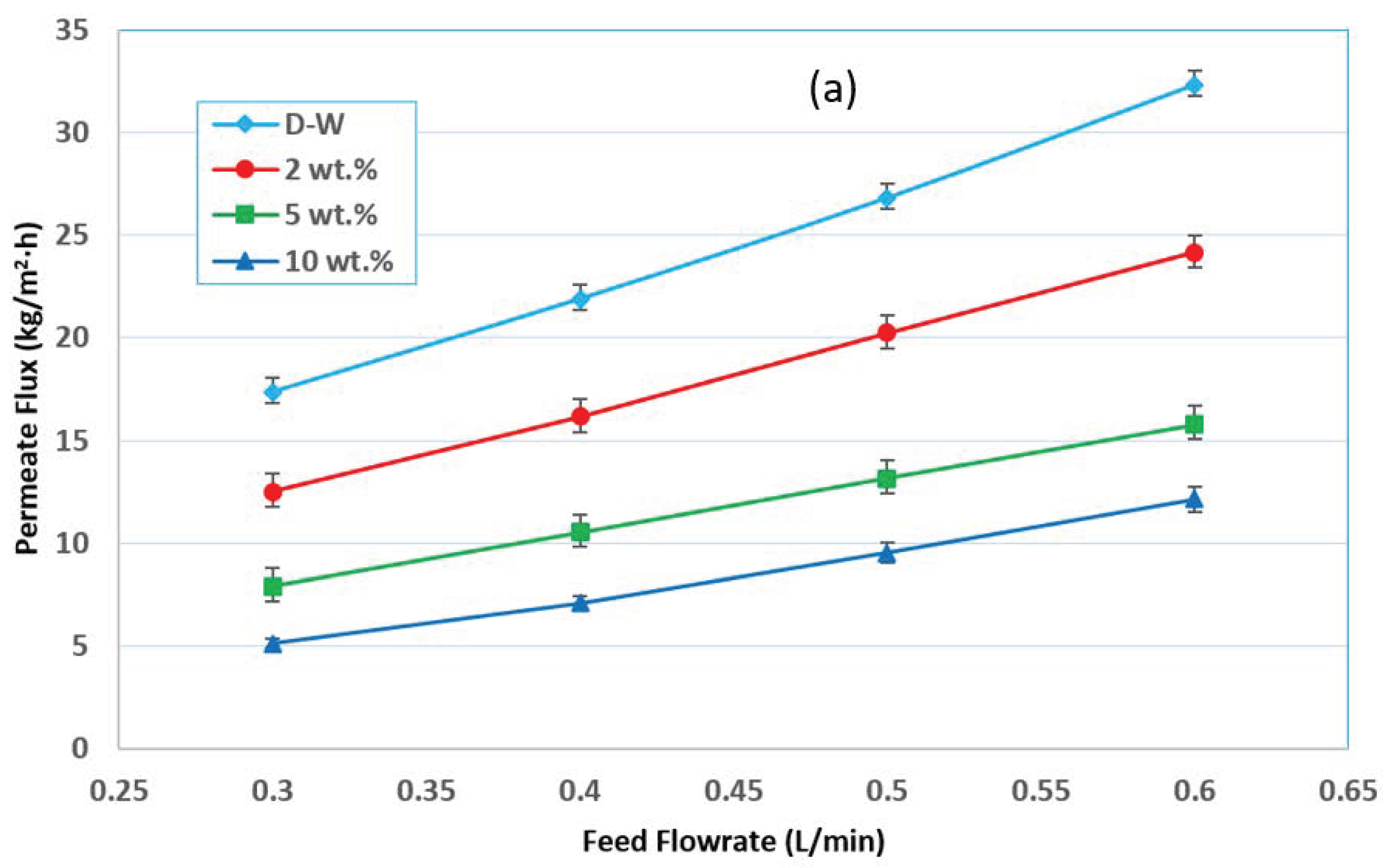
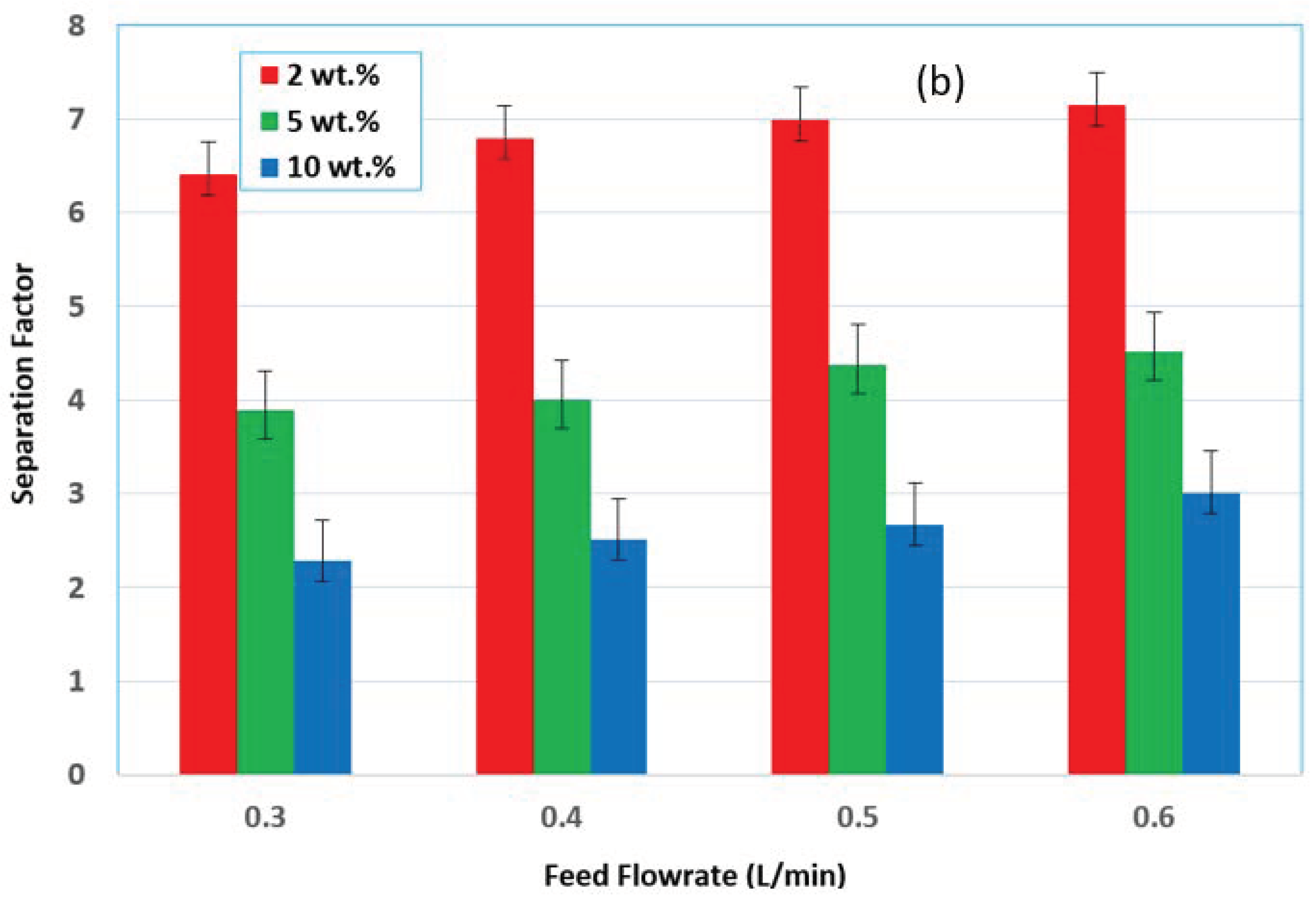
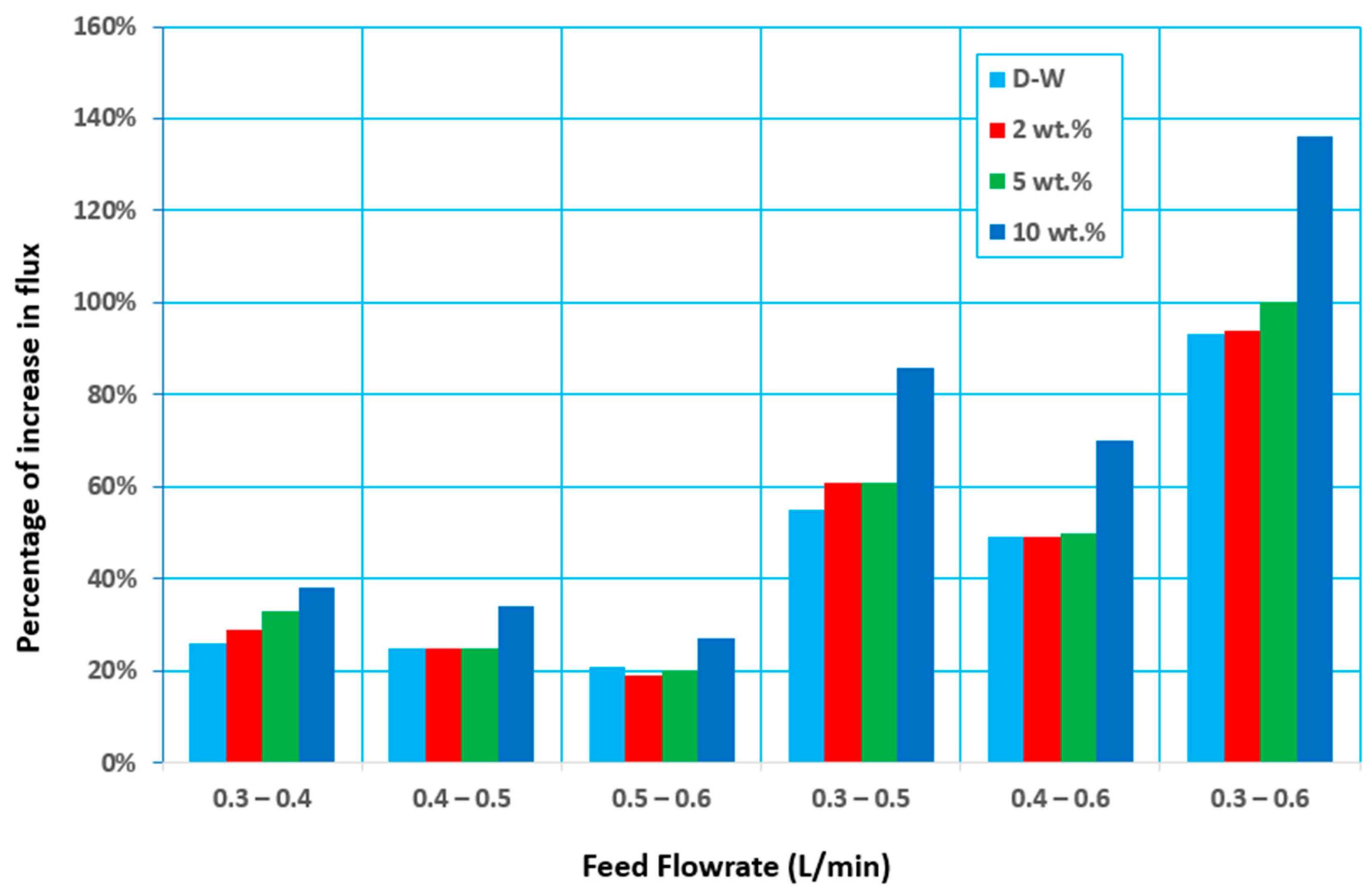
3.2. Effect of Feed Flow Rate
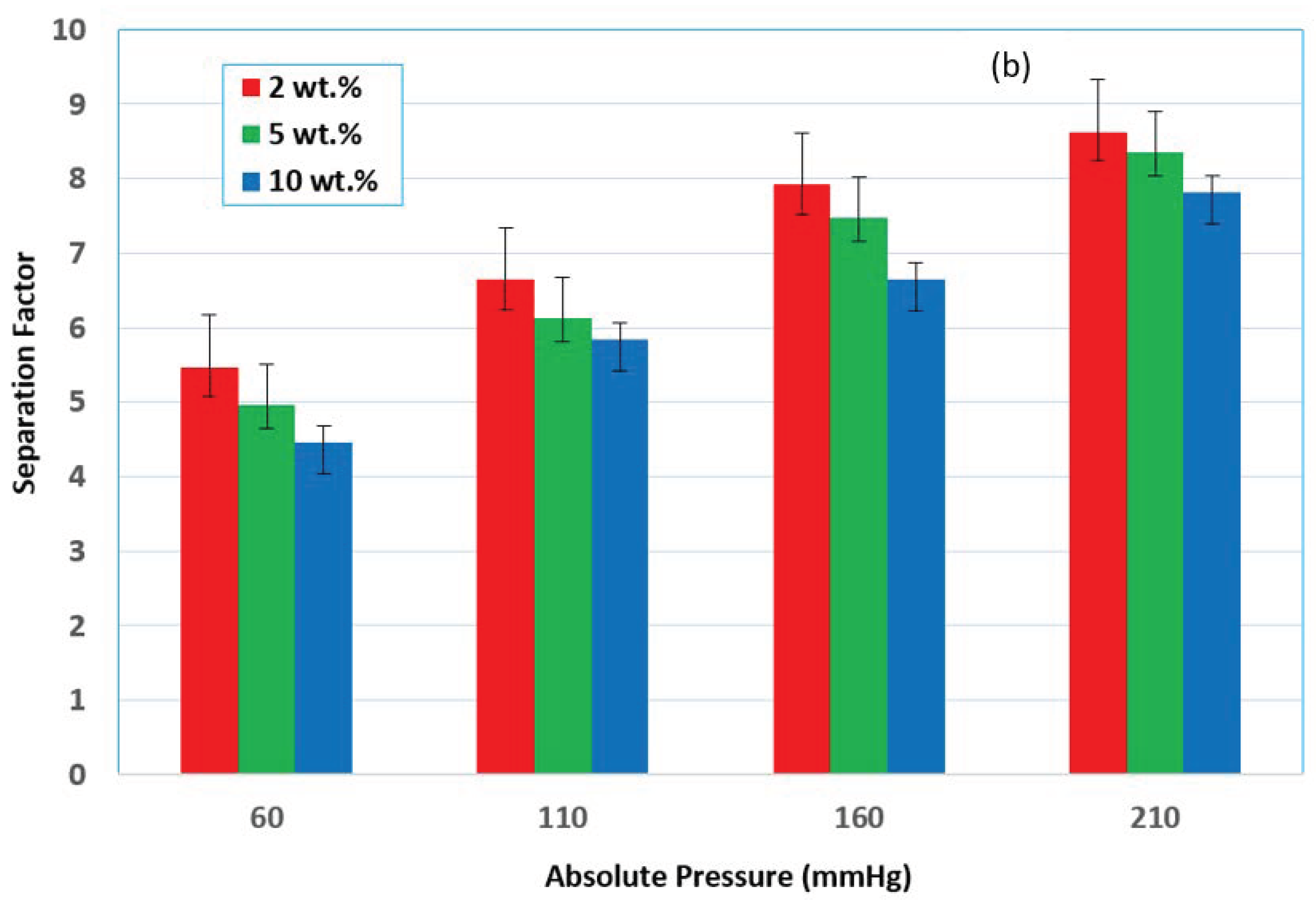
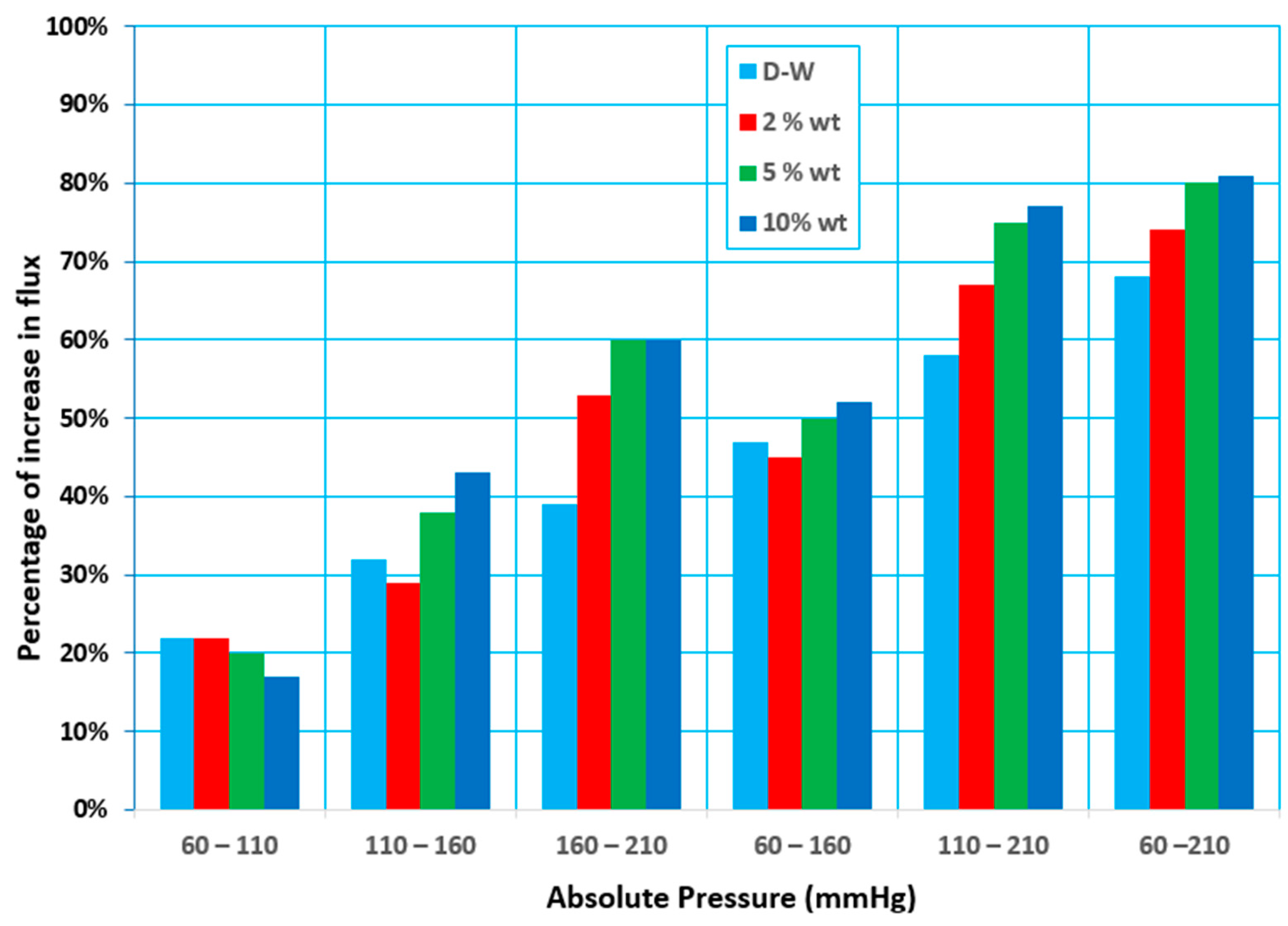
3.3. Effect of Vacuum Pressure
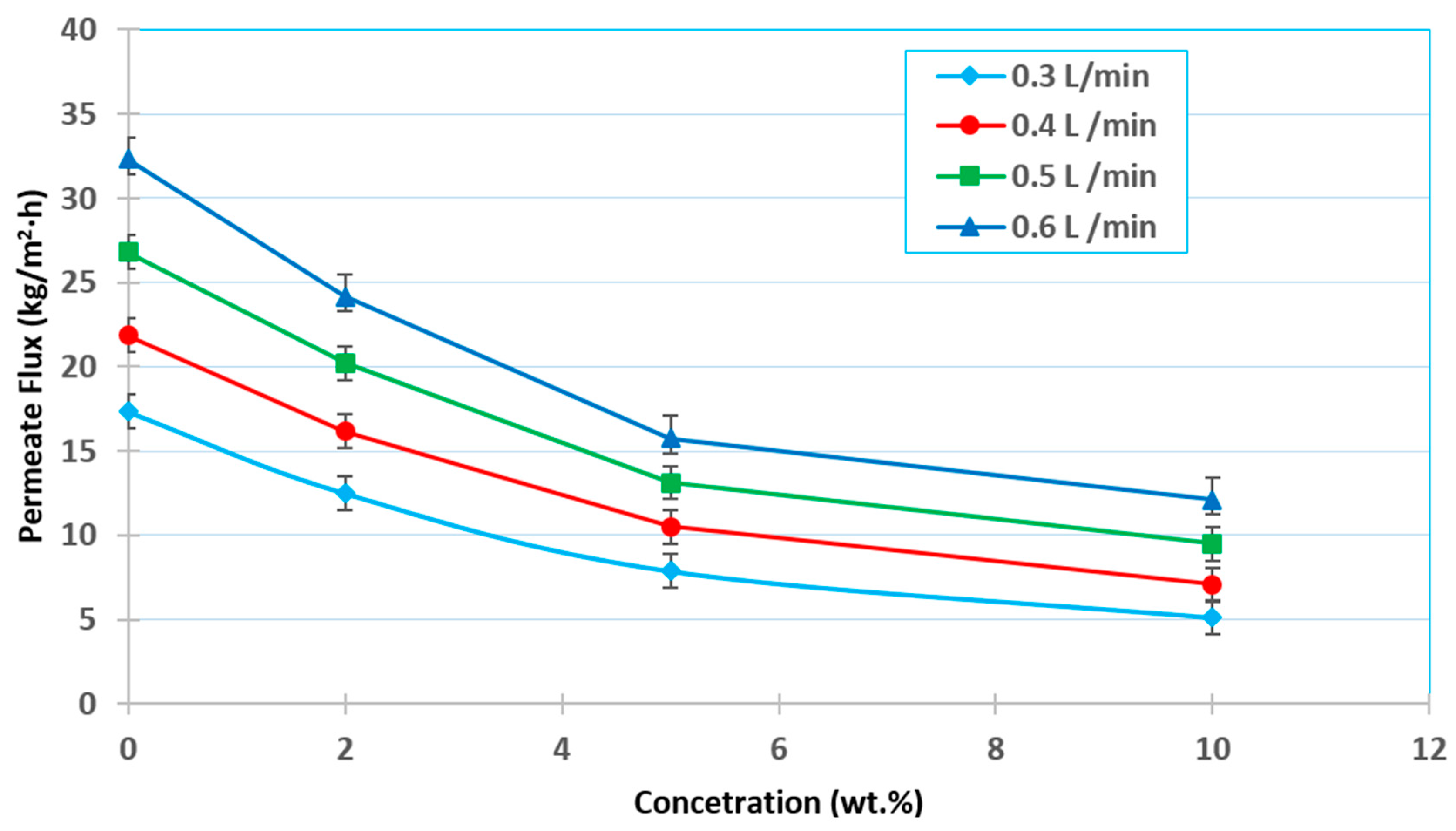
3.4. Effect of the Initial Feed Concentration
3.5. Taguchi Results
3.5.1. The Statistical Analysis
3.5.2. Predicted Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | membrane effective area (m2) |
| C | concentration (wt.%) |
| DOF | degree of freedom |
| J | permeate flux (kg/m2·h) |
| K | over all mass transfer coefficient |
| mass transfer coefficient of feed layer | |
| mass transfer coefficient of membrane layer | |
| mass transfer coefficient of feed layer | |
| L | effective length of membrane (m) |
| vapor pressure of the solution at the membrane feed side | |
| vapor pressure of the solution at the membrane permeate (vacuum) side | |
| P° | the vapor pressure for pure water |
| P | number of parameters |
| Q | the rate of feed flow in (L/min) |
| R2 | coefficient of determination |
| R | coefficient of correlation |
| S/N | signal to noise ratio |
| feed temperature (°C) | |
| feed temperature at the membrane surface (°C) | |
| t | operational time (hour) |
| V | volume of fresh water (L) |
| W | effective width of membrane (m) |
| the mole fractions of ethanol in the bulk liquid feed | |
| the mole fractions of water in the bulk liquid feed | |
| the mole fractions of ethanol in the permeate | |
| the mole fractions of water in the permeate | |
| Greek Symbols | |
| δ | membrane thickness |
| Ɛ | porosity |
| density (kg/L) | |
| separation factor |
References
- Bastos, V.D. Etanol, alcoolquímica e biorrefinarias. BNDES Set. 2007, 25, 5–38. [Google Scholar]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Prada, O.A.; Guevara-Lastre, C.A.; Barón-Núñez, F.W.; Barajas-Ferreira, C.; Ortiz-Basurto, R.I.; Torrestiana-Sánchez, B.; Muvdi-Nova, C.J. Vacuum membrane distillation: Modeling and analysis for recovery of ethanol from ethanol/water solutions. CTyF-Cienc. Tecnol. Future 2013, 5, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, N.; Ng, D.; Ike, I.A.; Xie, Z.; Gray, S. Environmental Science membrane distillation using a dual-layer. Environ. Sci. Water Res. Technol. 2019, 5, 119–130. [Google Scholar] [CrossRef]
- Lewandowicz, G.; Białas, W.; Marczewski, B.; Szymanowska, D. Application of membrane distillation for ethanol recovery during fuel ethanol production. J. Membr. Sci. 2011, 375, 212–219. [Google Scholar] [CrossRef]
- la Espriella, R.J.C.; Barón-Núñez, F.W.; Muvdi-Nova, C.J. Operating conditions influence on VMD and SGMD for ethanol recovery from aqueous solutions. CTyF-Cienc. Tecnol. Future 2015, 6, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Ameen, N.A.M.; Ibrahim, S.S.; Alsalhy, Q.F.; Figoli, A. Highly salinewater desalination using direct contact membrane distillation (DCMD): Experimental and simulation study. Water 2020, 12, 1575. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Fan, S.; Xiao, Z.; Liu, Y.; Liu, J.; Qiu, B.; Wang, Y. Bioethanol production in vacuum membrane distillation bioreactor by permeate fractional condensation and mechanical vapor compression with polytetrafluoroethylene (PTFE) membrane. Bioresour. Technol. 2018, 268, 708–714. [Google Scholar] [CrossRef]
- Izquierdo-Gil, M.A.; Jonsson, G. Factors affecting flux and ethanol separation performance in vacuum membrane distillation (VMD). J. Membr. Sci. 2003, 214, 113–130. [Google Scholar] [CrossRef]
- Gryta, M.; Morawski, A.W.; Tomaszewska, M. Ethanol production in membrane distillation bioreactor. Catal. Today 2000, 56, 159–165. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Membrane Distillation Principles and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; Available online: http://www.sciencedirect.com/science/article/pii/B9780444531261100168 (accessed on 1 January 2020).
- Zhao, Z.; Ma, F.; Liu, W.; Liu, D. Concentration of ginseng extracts aqueous solution by vacuum membrane distillation. 1. Effects of operating conditions. Desalination 2008, 234, 152–157. [Google Scholar] [CrossRef]
- Qusay, F.A.; Ibrahim, S.S.; Khaleel, S.R. Performance of vacuum poly(propylene) membrane distillation (VMD) for saline water desalination. Chem. Eng. Process. Process Intensif. 2017, 120, 68–80. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Ameen, N.A.M. Desalination of Highly Saline Water Using Direct Contact Membrane Distillation (DCMD). Al-Khwarizmi Eng. J. 2019, 14, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Safi, N.N.; Ibrahim, S.S.; Zouli, N.; Majdi, H.S.; Alsalhy, Q.F.; Drioli, E.; Figoli, A. A systematic framework for optimizing a sweeping gas membrane distillation (SGMD). Membranes 2020, 10, 254. [Google Scholar] [CrossRef]
- Hussein, S.S.; Ibrahim, S.S.; Toma, M.A.; Alsalhy, Q.F.; Drioli, E. Novel chemical modification of polyvinyl chloride membrane by free radical graft copolymerization for direct contact membrane distillation (DCMD) application. J. Membr. Sci. 2020, 611, 118266. [Google Scholar] [CrossRef]
- Bandini, S.; Saavedra, A.; Sarti, G.C. Vacuum Membrane Distillation: Experiments and Modeling. AIChE J. 1997, 43, 398–408. [Google Scholar] [CrossRef]
- Rao, S.R.; Padmanabhan, G. Application of Taguchi methods and ANOVA in optimization of process parameters for metal removal rate in electrochemical machining of Al/5% SiC composites. Int. J. Eng. Res. 2012, 2, 192–197. Available online: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Application+of+Taguchi+methods+and+ANOVA+in+optimization+of+process+parameters+for+metal+removal+rate+in+electrochemical+machining+of+Al+/+5+%25+SiC+composites#0 (accessed on 15 May 2012).
- Manjaiah, M.; Laubscher, R.F.; Kumar, A.; Basavarajappa, S. Parametric optimization of MRR and surface roughness in wire electro discharge machining (WEDM) of D2 steel using Taguchi-based utility approach. Int. J. Mech. Mater. Eng. 2016, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, M.M.A.; Kargari, A.; Tabatabaei, M. Sweeping Gas Membrane Distillation (SGMD) as an Alternative for Integration of Bioethanol Processing: Study on a Commercial Membrane and Operating Parameters. Chem. Eng. Commun. 2015, 202, 457–466. [Google Scholar] [CrossRef]
- Vazquez, G.; Alvarez, E.; Navaza, J.M. Surface Tension of Alcohol + Water from 20 to 50 °C. J. Chem. Eng. Data 1995, 40, 611–614. [Google Scholar] [CrossRef]
- Khattab, I.S.; Bandarkar, F.; Fakhree, M.A.A.; Jouyban, A. Density, viscosity, and surface tension of water+ethanol mixtures from 293 to 323K. Korean J. Chem. Eng. 2012, 29, 812–817. [Google Scholar] [CrossRef]
- Shi, J.Y.; Zhao, Z.P.; Zhu, C.Y. Studies on simulation and experiments of ethanol-water mixture separation by VMD using a PTFE flat membrane module. Sep. Purif. Technol. 2014, 123, 53–63. [Google Scholar] [CrossRef]
- Rice, O.K. The effect of pressure on surface tension. J. Chem. Phys. 1947, 15, 333–335. [Google Scholar] [CrossRef]
- Pangarkar, B.L.; Sane, M.G.; Parjane, S.B.; Guddad, M. Vacuum Membrane Distillation for Desalination of Ground Water by using Flat Sheet Membrane. Int. J. Chem. Mol. Eng. 2011, 5, 797–802. [Google Scholar]
- Nakao, S.i.; Saitoh, F.; Asakura, T.; Toda, K.; Kimura, S. Continuous ethanol extraction by pervaporation from a membrane bioreactor. J. Membr. Sci. 1987, 30, 273–287. [Google Scholar] [CrossRef]
- Bandim, S.; Gostoh, C.; Sartl, G.C. Separation efficiency in vacuum membrane distillation. J. Membr. Sci. 1992, 73, 217–229. [Google Scholar] [CrossRef]
- Sarti, G.C.; Gostoli, C.; Bandini, S. Extraction of organic components from aqueous streams by vacuum membrane distillation. J. Membr. Sci. 1993, 80, 21–33. [Google Scholar] [CrossRef]
- Diban, N.; Voinea, O.C.; Urtiaga, A.; Ortiz, I. Vacuum membrane distillation of the main pear aroma compound: Experimental study and mass transfer modeling. J. Membr. Sci. 2009, 326, 64–75. [Google Scholar] [CrossRef]
- Rom, A.; Strommer, M.; Friedl, A. Comparison of sweepgas and vacuum membrane distillation as in-situ separation of ethanol from aqueous solutions. Chem. Eng. Trans. 2014, 39, 985–990. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Białończyk, L. Ethanol production from whey in a bioreactor coupled with direct contact membrane distillation. Catal. Today 2016, 268, 156–163. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, C.; Lo, Y.; Nagarajan, D.; Hu, J. Ethanol production by modified polyvinyl alcohol-immobilized Zymomonas mobilis and in situ membrane distillation under very high gravity condition. Appl. Energy 2017, 202, 1–5. [Google Scholar] [CrossRef]
- Blume, I.; Wijmans, J.G.; Baker, R.W. The separation of dissolved organics from water by pervaporation. J. Membr. Sci. 1990, 49, 253–286. [Google Scholar] [CrossRef]
- Craig, J.C. Ethanol production in a continuous fermentation/membrane pervaporation system. Appl. Microbiol. Biotechnol. 1996, 44, 699–704. [Google Scholar]
- Padukone, N. Evaluation of PTMSP membranes in achieving enhanced ethanol removal from fermentations by pervaporation. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 1997, 63–65, 469–482. [Google Scholar] [CrossRef]
- Chen, X.; Ping, Z.; Long, Y. Separation properties of alcohol-water mixture through silicalite-I-filled silicone rubber membranes by pervaporation. J. Appl. Polym. Sci. 1998, 67, 629–636. [Google Scholar] [CrossRef]
- O’Brien, D.J.; Roth, L.H.; McAloon, A.J. Ethanol production by continuous fermentation-pervaporation: A preliminary economic analysis. J. Membr. Sci. 2000, 166, 105–111. [Google Scholar] [CrossRef]
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for bioethanol production by pervaporation. Biotechnol. Biofuels 2021, 14, 10. [Google Scholar] [CrossRef]
- O’Brien, D.J.; Senske, G.E.; Kurantz, M.J.; Craig, C. Ethanol recovery from corn fiber hydrolysate fermentations by pervaporation. Bioresour. Technol. 2004, 92, 15–19. [Google Scholar] [CrossRef]
- Aroujalian, A.; Belkacemi, K.; Davids, S.J.; Turcotte, G.; Pouliot, Y. Effect of residual sugars in fermentation broth on pervaporation flux and selectivity for ethanol. Desalination 2006, 193, 103–108. [Google Scholar] [CrossRef]
- Lewandowska, M.; Kujawski, W. Ethanol production from lactose in a fermentation/pervaporation system. J. Food Eng. 2007, 79, 430–437. [Google Scholar] [CrossRef]
- Ding, W.W.; Wu, Y.T.; Tang, X.Y.; Yuan, L.; Xiao, Z.Y. Continuous ethanol fermentation in a closed-circulating system using an immobilized cell coupled with PDMS membrane pervaporation. J. Chem. Technol. Biotechnol. 2011, 86, 82–87. [Google Scholar] [CrossRef]
- Chovau, S.; Gaykawad, S.; Straathof, A.J.J.; van der Bruggen, B. Bioresource Technology Influence of fermentation by-products on the purification of ethanol from water using pervaporation. Bioresour. Technol. 2011, 102, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Sanz, M.T.; Beltrán, S. Separation by pervaporation of ethanol from aqueous solutions and effect of other components present in fermentation broths. J. Chem. Technol. Biotechnol. 2009, 84, 1873–1882. [Google Scholar] [CrossRef]
- Dobrak, A.; Figoli, A.; Chovau, S.; Galiano, F.; Simone, S.; Vankelecom, I.F.J.; Drioli, E.; Van der Bruggen, B. Performance of PDMS membranes in pervaporation: Effect of silicalite fillers and comparison with SBS membranes. J. Colloid Interface Sci. 2010, 346, 254–264. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, E.J.; Kim, Y.G.; Choi, I.S.; Bae, H.J. Pervaporative separation of bioethanol using a polydimethylsiloxane/polyetherimide composite hollow-fiber membrane. Bioresour. Technol. 2012, 109, 110–115. [Google Scholar] [CrossRef]
- Bello, R.H.; Linzmeyer, P.; Franco, C.M.B.; Souza, O.; Sellin, N.; Medeiros, S.H.W.; Marangoni, C. Pervaporation of ethanol produced from banana waste. Waste Manag. 2014, 34, 1501–1509. [Google Scholar] [CrossRef]
- Naik, P.V.; Kerkhofs, S.; Martens, J.A.; Vankelecom, I.F.J. PDMS mixed matrix membranes containing hollow silicalite sphere for ethanol/water separation by pervaporation. J. Membr. Sci. 2016, 502, 48–56. [Google Scholar] [CrossRef]
- Fan, S.; Xiao, Z.; Li, M. Energy efficient of ethanol recovery in pervaporation membrane bioreactor with mechanical vapor compression eliminating the cold traps. Bioresour. Technol. 2016, 211, 24–30. [Google Scholar] [CrossRef]
- Sun, W.; Jia, W.; Xia, C.; Zhang, W.; Ren, Z. Study of in situ ethanol recovery via vapor permeation from fermentation. J. Membr. Sci. 2017, 530, 192–200. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Podtynnikov, I.A.; Golubev, G.S.; Volkov, V.V.; Borisov, I.L. Polyheptylmethylsiloxane—A Novel Material for Removal of Oxygenates from Water by Pervaporation. Pet. Chem. 2018, 58, 941–948. [Google Scholar] [CrossRef]
- Golubev, G.S.; Borisov, I.L.; Volkov, V.V. Performance of Commercial and Laboratory Membranes for Recovering Bioethanol from Fermentation Broth by Thermopervaporation. Russ. J. Appl. Chem. 2018, 91, 1375–1381. [Google Scholar] [CrossRef]




| Parameter | Present Work | Previous Work [8,15,16] |
|---|---|---|
| Membrane type/Code | PTFE/M1 | PTFE/M2 |
| Average pore size (µm) | 0.2 | 0.22 |
| Thickness (µm) | 35 | 96 |
| Porosity Ɛ% | 71 | 78 |
| Contact angle (°) | 118.02 | 114 |
| Effected membrane dimensions (cm) | 4.1 × 3.1 | 4.1 × 3.1 |
| LEP (bar) | 5.5 | unkown |
| Ref. | System | Mem.-Type | Characteristic of Membrane | Operating Conditions | J (kg/m2·h) | A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ (µm) | A (m2) | ε (%) | P.S. (µm) | Q (L/min) | Concentration | Tf (°C) | Pvac (mbar(abs)) | |||||
| [27] | E-W | PTFE-FS | 80 | 0.02 | 78 | 0.2 | ø | 5–10 wt.% | 30 | 5 | 5.7 | 5.4 |
| [28] | E-W | PTFE-FS | 60 | ø | 60 | 0.2 | 0.627–4.764 | 0–5 wt.% | 35–60 | 33.3–53.3 | 6.9–19.9 | 5.3–8.8 |
| [29] | E-W | PP-HF | 25 | 3 × 10−3 | 35 | 0.4 | ø | 5 wt.% | 35 | 26–60 | 0.72–4.68 | 0.24 |
| [18] | A-E | PTFE-FS | 60 | ø | 60 | 0.2 | 0.05–3 | 2–10 wt.% A-E, IPA-W | 25–35 | 10–80 | 3.6–28.8 | ø |
| IPA-W | 10–70 | 1.5–25.2 | ||||||||||
| [10] | E-W | PVDF-FS | 120 | 3.705 × 10−3 | 75 | 0.2 | 1.9–7.4 | 0.15–0.25 wt.% | 20–45 | 1–49 | 17.1 | 5.2–7.9 |
| [30] | E-W | PP-HF | 1550 | 0.0389 | 75 | 0.2 | 0.06–1.31 | 0.15–3% v/v | 23–32 | 28–40.4 | 0.36–0.5 | 4.4 |
| [4] | E-W | PP-HF PVDF-FS PP-FS | 25–120 | 0.003–9.8 × 10−4 | 35–79 | 0.02 | ø | 0.25–5 wt.% | 25–70 | 20–60 | ø | ø |
| [24] | E-W | PTFE-FS | 50 | 0.002 | 60–70 | 0.22 | 0.83 | 0–5 wt.% | 45–60 | 73–123 | 5–24 | 6.5–9 |
| [31] | E-W | PP-Ca | ø | 0.1 | ø | 0.2 | 6–25 | 0.5–5 wt.% | 20–50 | 25–50 | 3.6 | 4.5–5.5 |
| [7] | E-W | PTFE-FS | 220–265 | 9.08 × 10−4 | 72–76 | 0.2–5 | 0.16–2.27 | 0.5–10 wt.% | 30–50 | 111.4–304 | 2.17–44.7 | 1.7–2.3 |
| [32] | E-W | PP-Ca | 50–450 | 0.087 | 73 | 0.22 | ø | 5.2–12.3% v/v | 26.7–47 | 62–114 | 0.6–3.2 | 0.7–7.4 |
| 0.067 | 30.4–40.7 | |||||||||||
| [33] | E-W glucose | PTFE-Ca | 220 | 0.0266 | 80 | 0.2 | ø | 175 g/L glucose 86.8 g/L Ethanol | 23 | ø | 0.51 | 2.9 |
| [9] | E-W glucose | PTFE-FS | 65 | 0.024 | 72 | 0.5 | 0–2.6 | 5–35 g/L Ethanol 0–150 glucose | 20–50 | ø | 1–11.5 | 3.5–6.6 |
| Present work | E-W | PTFE-FS | 21–51 | 1.269 × 10−3 | 71 | 0.2 | 0.3–0.6 | 0–10 wt.% | 20–35 | 79.9–279.9 | 24.145 | 8.63 |
| Ref. | Mem.-Type | A (m2) | Pvac (mbar(abs)) | Tf (°C) | J (kg/m2·h) | A |
|---|---|---|---|---|---|---|
| [27] | PDMS | 0.02 | 5 | 30 | 0.06 | 8.1 |
| [34] | PDMS | 0.17 | 27 | 30 | 0.39 | 6.3 |
| [35] | PDMS | 0.1 | 5 | 35 | 0.37 | 4.1 |
| [36] | PTMSP | 0.0055 | 4 | 25 | 0.15 | 9.9 |
| [37] | PDMS with zeolites | 0.0028 | 0.3 | 50 | 0.13 | 13.4 |
| [38] | PDMS | 0.22 | 5 | 34 | 0.15 | 10.3 |
| [39] | MFI with silicalite | 0.00052 | 3 | 25 | 0.02 | 10 |
| [40] | PDMS | 0.22 | 5 | 35 | 0.09 | 8.2 |
| [41] | PDMS | 0.01 | 13 | 40 | 0.25 | 10 |
| [42] | PDMS | 0.017 | 1 | 65 | 1.6 | 7.8 |
| [43] | PDMS in PA support | 0.08 | 50 | 28 | 0.39 | 6.4 |
| [44] | PDMS | 0.002 | 3 | 30 | 0.55 | 8.4 |
| [45] | POMS | 0.017 | 1 | 43 | 0.52 | 9.1 |
| [46] | PDMS | 0.00088 | 1 | 41 | 0.17 | 5.5 |
| [47] | PDMS | 0.00224 | 10 | 40 | 0.5 | 8.3 |
| [48] | PDMS/PEI | 0.9 | 7 | 40 | 0.23 | 6.8 |
| [49] | PDMS | 0.04 | 7 | 27 | 0.004 | 10.6 |
| [50] | PDMS with silicalite | 0.00159 | 1 | 40 | 0.1 | 14.9 |
| [51] | PDMS | 0.16 | 46 | 35 | 0.4 | 8.5 |
| [52] | PDMS/PEI | 0.00196 | 40 | 35 | 0.21 | 7.2 |
| [53] | PHepMS | 1.385 × 10−3 | ~0.05 | 30 | 0.0028 | 19.9 |
| POMS | 0.0026 | 18.8 | ||||
| PDMS | 0.0055 | 31 | ||||
| [54] | PDMS | 2.55 × 10−3 | ø | 40–80 | 5.4 | 10.2 |
| Run | Operating Parameters | Flux (Exp.) (kg/m2·h) | Flux (Pred.) (kg/m2·h) | S/N | Mean | |||
|---|---|---|---|---|---|---|---|---|
| C (wt.%) | T (°C) | Q (L/min) | (mmHg (abs)) | |||||
| 1 | 2 | 25 | 0.4 | 160 | 6.2802 | 6.1967 | 15.9594 | 6.2802 |
| 2 | 2 | 30 | 0.5 | 110 | 14.1844 | 12.6885 | 23.0362 | 14.1844 |
| 3 | 2 | 35 | 0.6 | 60 | 21.5431 | 21.4596 | 26.6661 | 21.5431 |
| 4 | 5 | 25 | 0.5 | 60 | 9.9593 | 10.8394 | 19.9645 | 9.9593 |
| 5 | 5 | 30 | 0.6 | 160 | 8.5892 | 9.9468 | 18.6790 | 8.5892 |
| 6 | 5 | 35 | 0.4 | 110 | 12.8383 | 13.2610 | 22.1701 | 12.8383 |
| 7 | 10 | 25 | 0.6 | 110 | 7.4951 | 6.6984 | 17.4955 | 7.4951 |
| 8 | 10 | 30 | 0.4 | 60 | 7.5951 | 7.7333 | 17.6106 | 7.5951 |
| 9 | 10 | 35 | 0.5 | 160 | 9.4593 | 9.1200 | 19.5171 | 9.4593 |
| Factor | DOF | SS | Variance | F | P |
|---|---|---|---|---|---|
| Concentration (wt.%) | 2 | 51.593 | 25.796 | 3.229 | 28.667% |
| Temperature (°C) | 2 | 69.97 | 34.986 | 4.379 | 38.879% |
| Flow rate (L/min) | 2 | 20.31 | 10.153 | 1.270 | 11.284% |
| Absolute pressure (mmHg (abs)) | 2 | 38.10 | 19.050 | 2.384 | 21.170% |
| Error | 9 | 71.901 | 7.989 | ||
| Total | 17 | 179.98 | |||
| descriptive statistics | |||||
| Sample | N | Mean | StDev | SE Mean | 95% CI |
| Concentration (wt.%) | 9 | 5.67 | 3.50 | 1.17 | (2.98, 8.36) |
| Temperature (°C) | 9 | 30.00 | 4.33 | 1.44 | (26.67, 33.33) |
| Flow rate (L/min) | 9 | 0.5000 | 0.0866 | 0.0289 | (0.4334, 0.5666) |
| Absolute pressure (mmHg (abs)) | 9 | 110.0 | 43.3 | 14.4 | (76.7, 143.3) |
| Flux (kg/m2·h) | 9 | 10.88 | 4.74 | 1.58 | (7.24, 14.53) |
| Separation factor | 9 | 4.979 | 1.241 | 0.414 | (4.025, 5.933) |
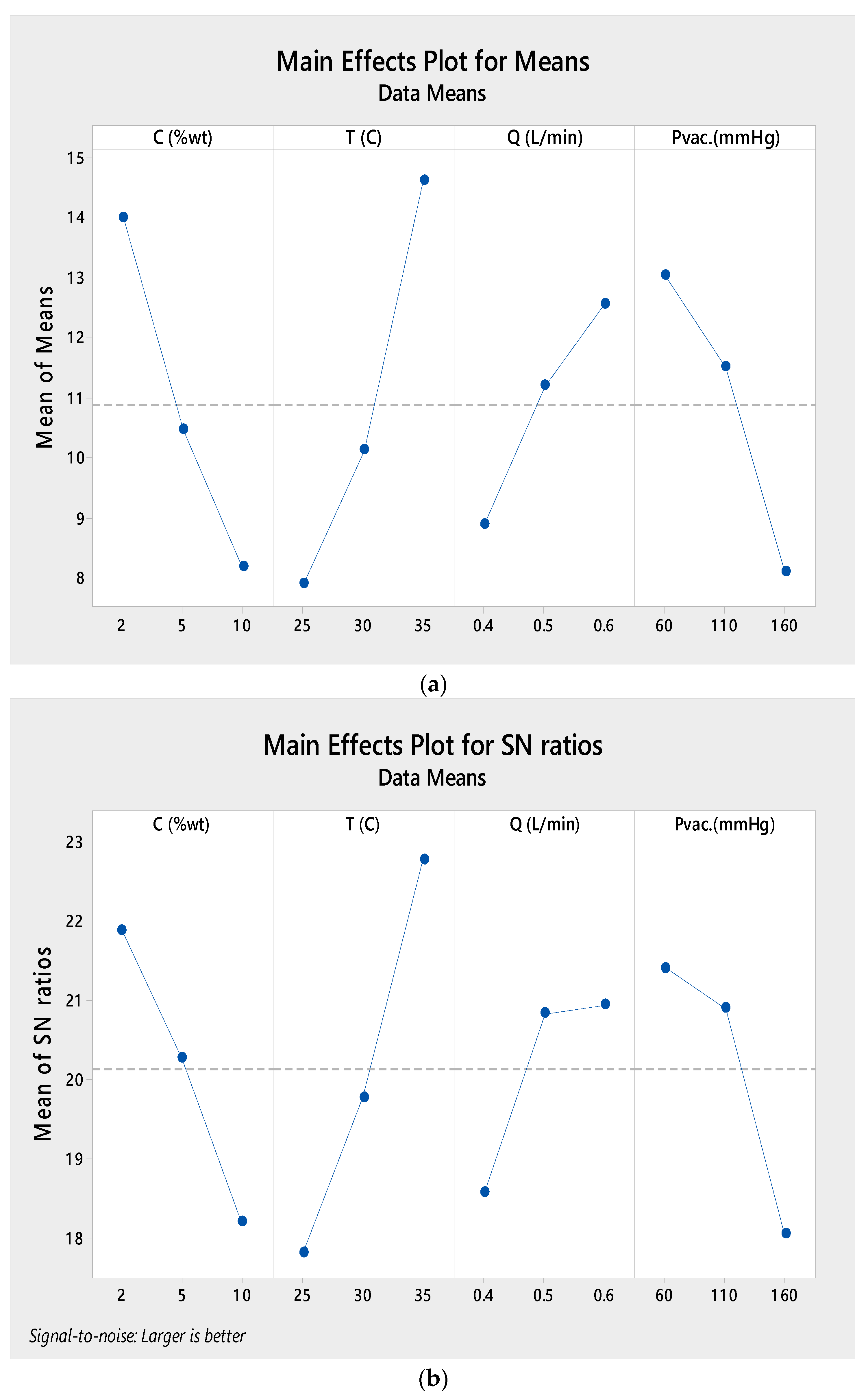
| Parameters | Level | Mean Flux (kg/m2·h) | S/N |
|---|---|---|---|
| Temperature (°C) | 25 | 7.912 | 17.58 |
| 30 | 10.123 | 19.78 | |
| 35 | 14.614 | 22.78 | |
| Absolute pressure (mmHg (abs)) | 60 | 13.033 | 21.41 |
| 110 | 11.506 | 20.90 | |
| 160 | 8.110 | 18.05 | |
| Feed flow rate (L/min) | 0.4 | 8.905 | 18.58 |
| 0.5 | 11.201 | 20.84 | |
| 0.6 | 12.542 | 20.95 | |
| Concentration (wt.%) | 2 | 14.003 | 21.89 |
| 5 | 10.462 | 20.27 | |
| 10 | 8.183 | 18.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassif, A.G.; Ibrahim, S.S.; Majdi, H.S.; Alsalhy, Q.F. Ethanol Separation from an Ethanol–Water Solution Using Vacuum Membrane Distillation. Membranes 2022, 12, 807. https://doi.org/10.3390/membranes12080807
Nassif AG, Ibrahim SS, Majdi HS, Alsalhy QF. Ethanol Separation from an Ethanol–Water Solution Using Vacuum Membrane Distillation. Membranes. 2022; 12(8):807. https://doi.org/10.3390/membranes12080807
Chicago/Turabian StyleNassif, Abeer G., Salah S. Ibrahim, Hasan Sh. Majdi, and Qusay F. Alsalhy. 2022. "Ethanol Separation from an Ethanol–Water Solution Using Vacuum Membrane Distillation" Membranes 12, no. 8: 807. https://doi.org/10.3390/membranes12080807
APA StyleNassif, A. G., Ibrahim, S. S., Majdi, H. S., & Alsalhy, Q. F. (2022). Ethanol Separation from an Ethanol–Water Solution Using Vacuum Membrane Distillation. Membranes, 12(8), 807. https://doi.org/10.3390/membranes12080807








