Protons in Gating the Kv1.2 Channel: A Calculated Set of Protonation States in Response to Polarization/Depolarization of the Channel, with the Complete Proposed Proton Path from Voltage Sensing Domain to Gate
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
- (1)
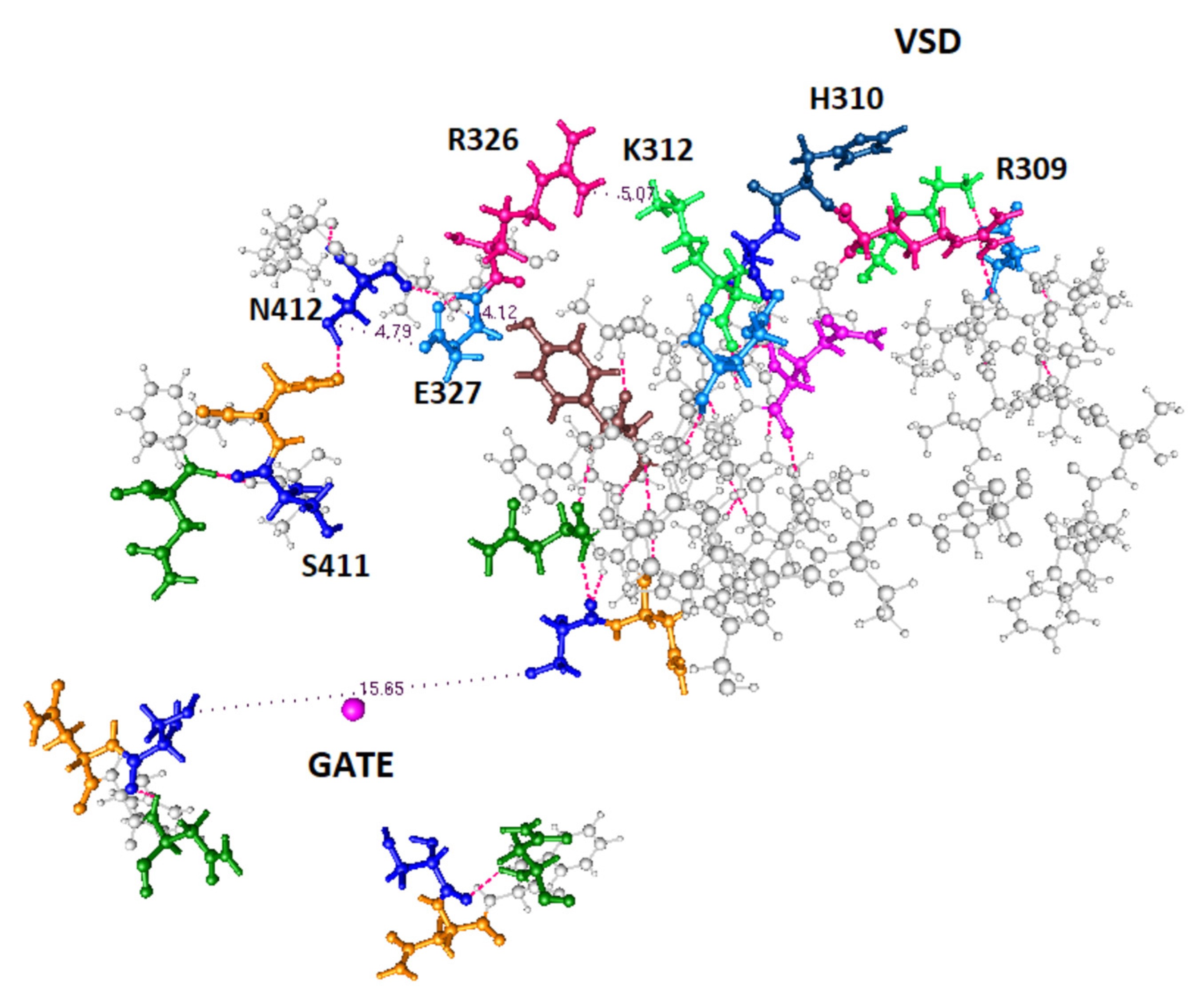
- Two proton paths between the VSD and the gate can be clearly defined. A crossover path between them also exists.
- (2)
- We have ruled out a conformation in which K312 and R326 are simultaneously protonated, while the configurations with one or zero protons on these two residues have roughly equal lower energy.
- (3)
- There are appropriate, proton transmitting, residues at each end of the computed path; it is possible to see how the proton connects both to the VSD at one end, and to the gate at the other.
- (4)
- The paths appear to include local energy minima for protons, at which the protons are temporarily immobile. For example, groups of three amino acids which have fairly close oxygens would very likely form such a minimum; calculations on the details are ongoing. In a proton cascade, a proton coming from a higher energy location would displace the proton in such a local minimum, pushing it forward along the proton path.
- (5)
- The charges and the bonding have been determined for all key residues with three proton conformations, two of which are considered to actually be part of the paths.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Doyle, D.A.; Morais Cabral, J.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The Structure of the Potassium Channel: Molecular Basis of K + Conduction and Selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M.; Bezanilla, F. Charge Movement Associated with the Opening and Closing of the Activation Gates of the Na Channels. J. Gen. Physiol. 1974, 63, 533–552. [Google Scholar] [CrossRef]
- Keynes, R.D.; Rojas, E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J. Physiol. 1974, 239, 393–434. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.; Yarov-Yarovoy, V.; Khalili-Araghi, F.; Catterall, W.A.; Klein, M.L.; Tarek, M.; Lindahl, E.; Schulten, K.; Perozo, E.; Bezanilla, F.; et al. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J. Gen. Physiol. 2012, 140, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kariev, A.M.; Green, M.E. Caution Is Required in Interpretation of Mutations in the Voltage Sensing Domain of Voltage Gated Channels as Evidence for Gating Mechanisms. Int. J. Mol. Sci. 2015, 16, 1627–1643. [Google Scholar] [CrossRef] [PubMed]
- Kariev, A.M.; Green, M.E. The Role of Proton Transport in Gating Current in a Voltage Gated Ion Channel, as Shown by Quantum Calculations. Sensors 2018, 18, 3143. [Google Scholar] [CrossRef] [PubMed]
- Sapronova, A.; Bystrov, V.; Green, M.E. Ion channel gating and proton transport. J. Mol. Struct. 2003, 630, 297–307. [Google Scholar] [CrossRef]
- Green, M.; Sapronova, A.; Bystrov, V.S. Water proton transfer and hydrogen bonding in ion channel gating. Front. Biosci. 2003, 8, s1356–s1370. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.; Zhu, L.; Kallio, K.; Remington, S.J.; Fang, C. Photoinduced proton transfer inside an engineered green fluorescent protein: A stepwise–concerted-hybrid reaction. Phys. Chem. Chem. Phys. 2018, 20, 12517–12526. [Google Scholar] [CrossRef]
- Grigorenko, B.L.; Knyazeva, M.; Nemukhin, A.V. Analysis of proton wires in the enzyme active site suggests a mechanism of c-di-GMP hydrolysis by the EAL domain phosphodiesterases. Proteins Struct. Funct. Bioinform. 2016, 84, 1670–1680. [Google Scholar] [CrossRef]
- Kariev, A.M.; Green, M.E. Quantum Calculation of Proton and Other Charge Transfer Steps in Voltage Sensing in the Kv1.2 Channel. J. Phys. Chem. B 2019, 123, 7984–7998. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Mokrab, Y.; Carvacho, I.; Sands, Z.; Sansom, M.; Clapham, D. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 2010, 17, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Moran, M.M.; Chong, J.A.; Clapham, D.E. A voltage-gated proton-selective channel lacking the pore domain. Nature 2006, 440, 1213–1216. [Google Scholar] [CrossRef]
- Okamura, Y. Biodiversity of voltage sensor domain proteins. Pflug. Arch. Eur. J. Physiol. 2007, 454, 361–371. [Google Scholar] [CrossRef]
- Okamura, Y.; Okochi, Y. Molecular mechanisms of coupling to voltage sensors in voltage-evoked cellular signals. Proc. Jpn. Acad. Ser. B 2019, 95, 111–135. [Google Scholar] [CrossRef] [PubMed]
- Phatak, P.; Frähmcke, J.S.; Wanko, M.; Hoffmann, M.; Strodel, P.; Smith, J.C.; Suhai, S.; Bondar, A.-N.; Elstner, M. Long-Distance Proton Transfer with a Break in the Bacteriorhodopsin Active Site. J. Am. Chem. Soc. 2009, 131, 7064–7078. [Google Scholar] [CrossRef] [PubMed]
- DeCoursey, T. The Voltage-Gated Proton Channel: A Riddle, Wrapped in a Mystery, inside an Enigma. Biochemistry 2015, 54, 3250–3268. [Google Scholar] [CrossRef]
- Helms, V.; Gu, W. Proton Travel in Green Fluorescent Protein; Fluorescent Proteins I; Springer: Berlin/Heidelberg, Germany, 2012; Volume 11, pp. 171–182. [Google Scholar]
- Chowdhury, S.; Haehnel, B.M.; Chanda, B. Interfacial gating triad is crucial for electromechanical transduction in voltage-activated potassium channels. J. Gen. Physiol. 2014, 144, 457–467. [Google Scholar] [CrossRef]
- Roepke, M.; Saura, P.; Riepl, D.; Pöverlein, M.C.; Kaila, V.R. Functional water wires catalyze long-range proton pumping in the mammalian respiratory complex I. J. Am. Chem. Soc. 2020, 142, 21758–21766. [Google Scholar] [CrossRef]
- Salna, B.; Benabbas, A.; Sage, J.T.; van Thor, J.; Champion, P.M. Wide-dynamic-range kinetic investigations of deep proton tunnelling in proteins. Nat. Chem. 2016, 8, 874–880. [Google Scholar] [CrossRef]
- Paulino, J.; Yi, M.; Hung, I.; Gan, Z.; Wang, X.; Chekmenev, E.Y.; Zhou, H.-X.; Cross, T.A. Functional stability of water wire–carbonyl interactions in an ion channel. Proc. Natl. Acad. Sci. USA 2020, 117, 11908–11915. [Google Scholar] [CrossRef]
- Zhao, L.; Qi, Z.; Zhang, X.; Bi, L.; Jin, G. Regulatory role of the extreme C-terminal end of the S6 inner helix in C-terminal-truncated Kv1.2 channel activation. Cell Biol. Int. 2010, 34, 433–439. [Google Scholar] [CrossRef]
- Chamberlin, A.; Qiu, F.; Wang, Y.; Noskov, S.Y.; Larsson, H.P. Mapping the Gating and Permeation Pathways in the Voltage-Gated Proton Channel Hv1. J. Mol. Biol. 2015, 427, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Intharathep, P.; Hannongbua, S. Evaluating how rimantadines control the proton gating of the influenza A M2-proton port via allosteric binding outside of the M2-channel: MD simulations. J. Enzym. Inhib. Med. Chem. 2011, 26, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, L.; Pouyssegur, J. Regulation of the Na+/H+ exchanger isoform NHE1: Role of phosphorylation. Kidney Int. 1996, 49, 1038–1041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babich, V.; Vadnagara, K.; Di, S.F. The biophysical and molecular basis of intracellular pH sensing by Na+/H+ exchanger-3. FASEB J. 2013, 27, 4646–4658. [Google Scholar] [CrossRef]
- Capitanio, N.; Palese, L.L.; Capitanio, G.; Martino, P.L.; Richter, O.-M.H.; Ludwig, B.; Papa, S. Allosteric interactions and proton conducting pathways in proton pumping aa3 oxidases: Heme a as a key coupling element. Biochim. Biophys. Acta 2012, 1817, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Rahman, M.; Zhu, D. Protons inhibit Cl-conductance by direct or allosteric interaction with the GABA-binding site in the rat recombinant α1β2γ2L and α1β2 GABAA receptor. Eur. J. Pharmacol. 2005, 528, 1–6. [Google Scholar] [CrossRef]
- Bassetto, C.A.; Carvalho-De-Souza, J.L.; Bezanilla, F. Molecular basis for functional connectivity between the voltage sensor and the selectivity filter gate in Shaker K+ channels. eLife 2021, 10, e63077. [Google Scholar] [CrossRef]
- Zhao, L.L.; Zhao, L.L.; Wu, A.; Bi, L.J.; Liu, P.; Zhang, X.E.; Jiang, T.; Jin, G.; Qi, Z. Length-dependent regulation of teh Kv1.2 channel activation. Mol. Membr. Biol. 2009, 26, 186–193. [Google Scholar] [CrossRef]
- Garczarek, F.; Brown, L.S.; Lanyi, J.K.; Gerwert, K. Proton binding within a membrane protein by a protonated water cluster. Proc. Natl. Acad. Sci. USA 2005, 102, 3633–3638. [Google Scholar] [CrossRef] [PubMed]
- Garczarek, F.; Gerwert, K. Functional waters in intraprotein proton transfer monitored by FTIR difference spectroscopy. Nature 2006, 439, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Woelke, A.L.; Wagner, A.; Galstyan, G.; Meyer, T.; Knapp, E.-W. Proton Transfer in the K-Channel Analog of B-Type Cytochrome c Oxidase from Thermus thermophilus. Biophys. J. 2014, 107, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Freier, E.; Potschies, M.; Hofmann, E.; Gerwert, K. Directional Proton Transfer in Membrane Proteins Achieved through Protonated Protein-Bound Water Molecules: A Proton Diode. Angew. Chem. Int. Ed. 2010, 49, 6889–6893. [Google Scholar] [CrossRef] [PubMed]
- Sheets, F.M.; Fozzard, H.A.; Hanck, D.A. Important role of asparagine in coupling the poer and voltage sensing domain in voltage=gated sodium channels. Biophys. J. 2015, 109, 2277–2286. [Google Scholar] [CrossRef][Green Version]
- Duzhyy, E.D.; Sakai, Y.; Sokolowski, B.H.A. Cloning and developmental expression of Shaker potassium channels in the cochlea of chicken. Mol. Brain Res. 2004, 121, 70–85. [Google Scholar] [CrossRef]
- Dudev, T.; Musset, B.; Morgan, D.; Cherny, V.V.; Smith, S.M.E.; Mazmanian, K.; DeCoursey, T.; Lim, C. Selectivity Mechanism of the Voltage-gated Proton Channel, HV1. Sci. Rep. 2015, 5, 10320. [Google Scholar] [CrossRef]
- Dudev, T.; Grauffel, C.; Lim, C. Influence of the Selectivity Filter Properties on Proton Selectivity in the Influenza A M2 Channel. J. Am. Chem. Soc. 2016, 138, 13038–13047. [Google Scholar] [CrossRef]
- Kalstrup, T.; Blunck, R. S4-S5 linker movement during activation and inactivation in voltage-gated K(+) channels. Proc. Natl. Acad. Sci. USA 2018, 115, E6751–E6759. [Google Scholar] [CrossRef]
- Kalstrup, T.; Blunck, R. Dynamics of internal pore opening in KV channels probed by a fluorescent unnatural amino acid. Proc. Natl. Acad. Sci. USA 2013, 110, S8272/1–S8272/2. [Google Scholar] [CrossRef]
- Cowgill, J.; Chanda, B. The contribution of voltage clamp fluorometry to the understanding of channel and transporter mechanisms. J. Gen. Physiol. 2019, 151, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Cowgill, J.; Chanda, B. Mapping Electromechanical Coupling Pathways in Voltage-Gated Ion Channels: Challenges and the Way Forward. J. Mol. Biol. 2021, 433, 167104. [Google Scholar] [CrossRef]
- Asamoah, O.K.; Wuskell, J.P.; Loew, L.; Bezanilla, F. A Fluorometric Approach to Local Electric Field Measurements in a Voltage-Gated Ion Channel. Neuron 2003, 37, 85–98. [Google Scholar] [CrossRef]
- Ishida, I.G.; Rangel-Yescas, G.E.; Carrasco-Zanini, J.; Islas, L.D. Voltage-dependent gating and gating charge measurements in the Kv1.2 potassium channel. J. Gen. Physiol. 2015, 145, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Islas, L.D. Functional diversity of potassium channel voltage-sensing domains. Channels 2016, 10, 202–213. [Google Scholar] [CrossRef]
- Yi, B.A.; Minor, D.L.; Lin, Y.-F.; Jan, Y.N.; Jan, L.Y. Controlling potassium channel activities: Interplay between the membrane and intracellular factors. Proc. Natl. Acad. Sci. USA 2001, 98, 11016–11023. [Google Scholar] [CrossRef]
- Minor, D.L.; Lin, Y.-F.; Mobley, B.C.; Avelar, A.; Jan, Y.N.; Jan, L.; Berger, J.M. The Polar T1 Interface Is Linked to Conformational Changes that Open the Voltage-Gated Potassium Channel. Cell 2000, 102, 657–670. [Google Scholar] [CrossRef]
- Lee, J.; Kang, M.; Kim, S.; Chang, I. Structural and molecular insight into the pH-induced low-permeability of the voltage-gated potassium channel Kv1.2 through dewetting of the water cavity. PLoS Comput. Biol. 2020, 16, e1007405. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chen, X.; Wang, Q.; Ni, F.; Ma, J. Structure of the full-length Shaker potassium channel Kv1.2 by normal-mode-based X-ray crystallographic refinement. Proc. Natl. Acad. Sci. USA 2010, 107, 11352–11357. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO Version 3.1. In Gaussian Suite of Programs; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Wolf, S.; Freier, E.; Gerwert, K. A Delocalized Proton-Binding Site within a Membrane Protein. Biophys. J. 2014, 107, 174–184. [Google Scholar] [CrossRef]

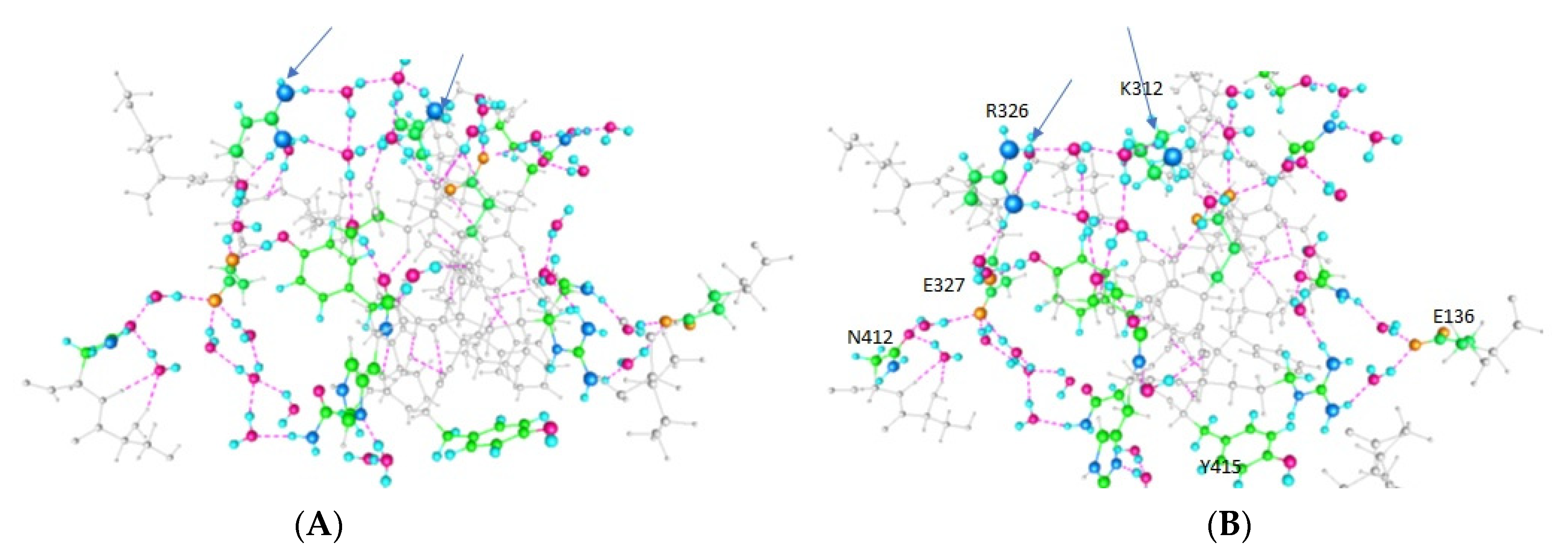
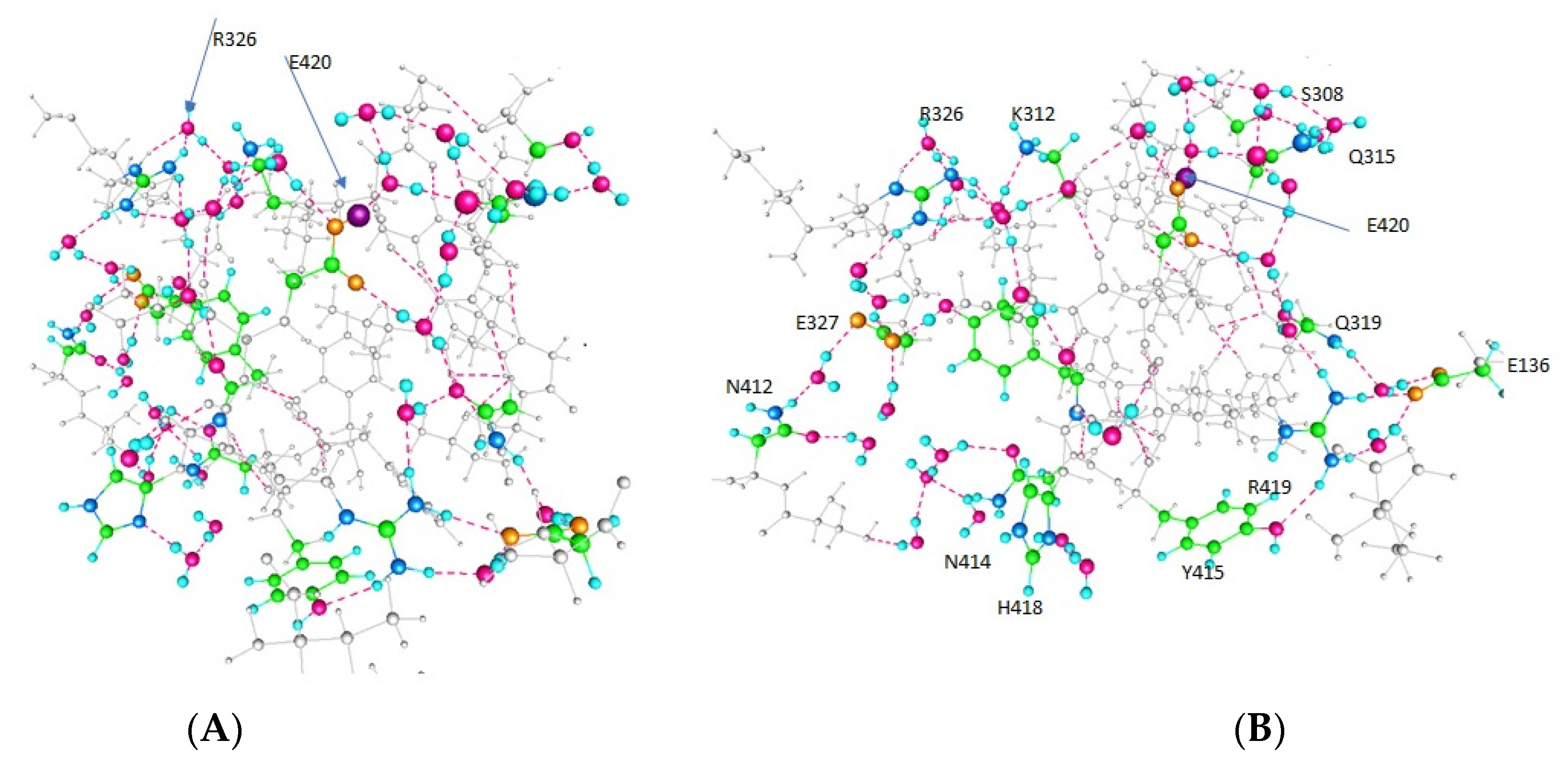
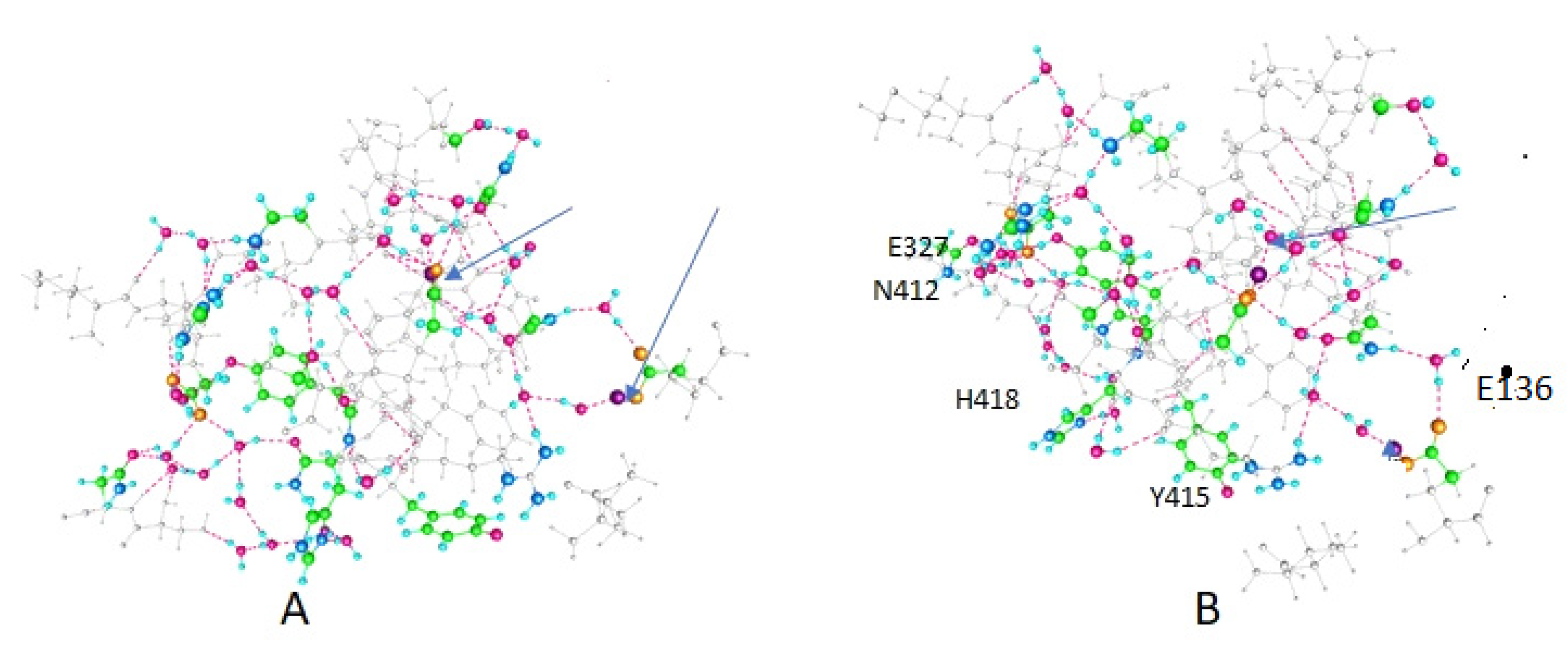
| Amino Acid | Figure 2 | Figure 3 | Figure 4 |
|---|---|---|---|
| E420 | -- | + | + |
| Y415 | + | + | -- |
| K312 | + | -- | -- |
| E136 (In T1) | -- | -- | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariev, A.M.; Green, M.E. Protons in Gating the Kv1.2 Channel: A Calculated Set of Protonation States in Response to Polarization/Depolarization of the Channel, with the Complete Proposed Proton Path from Voltage Sensing Domain to Gate. Membranes 2022, 12, 718. https://doi.org/10.3390/membranes12070718
Kariev AM, Green ME. Protons in Gating the Kv1.2 Channel: A Calculated Set of Protonation States in Response to Polarization/Depolarization of the Channel, with the Complete Proposed Proton Path from Voltage Sensing Domain to Gate. Membranes. 2022; 12(7):718. https://doi.org/10.3390/membranes12070718
Chicago/Turabian StyleKariev, Alisher M., and Michael E. Green. 2022. "Protons in Gating the Kv1.2 Channel: A Calculated Set of Protonation States in Response to Polarization/Depolarization of the Channel, with the Complete Proposed Proton Path from Voltage Sensing Domain to Gate" Membranes 12, no. 7: 718. https://doi.org/10.3390/membranes12070718
APA StyleKariev, A. M., & Green, M. E. (2022). Protons in Gating the Kv1.2 Channel: A Calculated Set of Protonation States in Response to Polarization/Depolarization of the Channel, with the Complete Proposed Proton Path from Voltage Sensing Domain to Gate. Membranes, 12(7), 718. https://doi.org/10.3390/membranes12070718






