Small Intestinal Submucosa Biomimetic Periosteum Promotes Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Electrospinning of c-PCL/s-SIS Membranes
2.2. Material Characterization
2.3. In Vitro Performance
2.3.1. Cell Culture
2.3.2. EdU Assay
2.3.3. Wound Scratch Assay
2.3.4. Tube Formation Assay
2.3.5. Immunofluorescence
2.3.6. Alizarin Red (AR) Staining
2.3.7. Real-Time Quantitative PCR Analysis
2.4. In Vivo Bone Regeneration Evaluation
2.4.1. Surgical Procedure
2.4.2. Histological Assessment
2.5. Statistical Analysis
3. Results
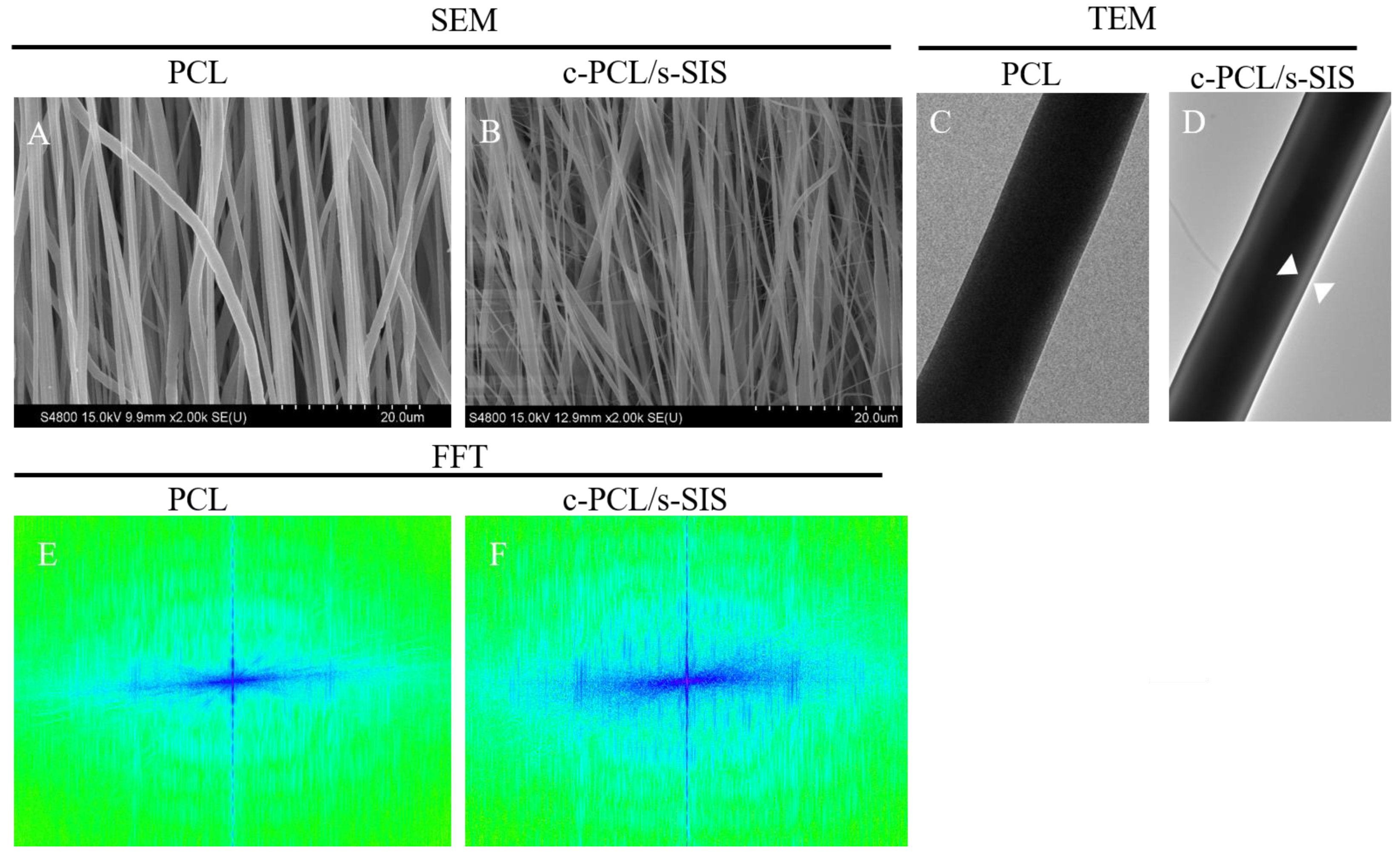
3.1. Construction and Characterization of the Biomimetic Periosteum
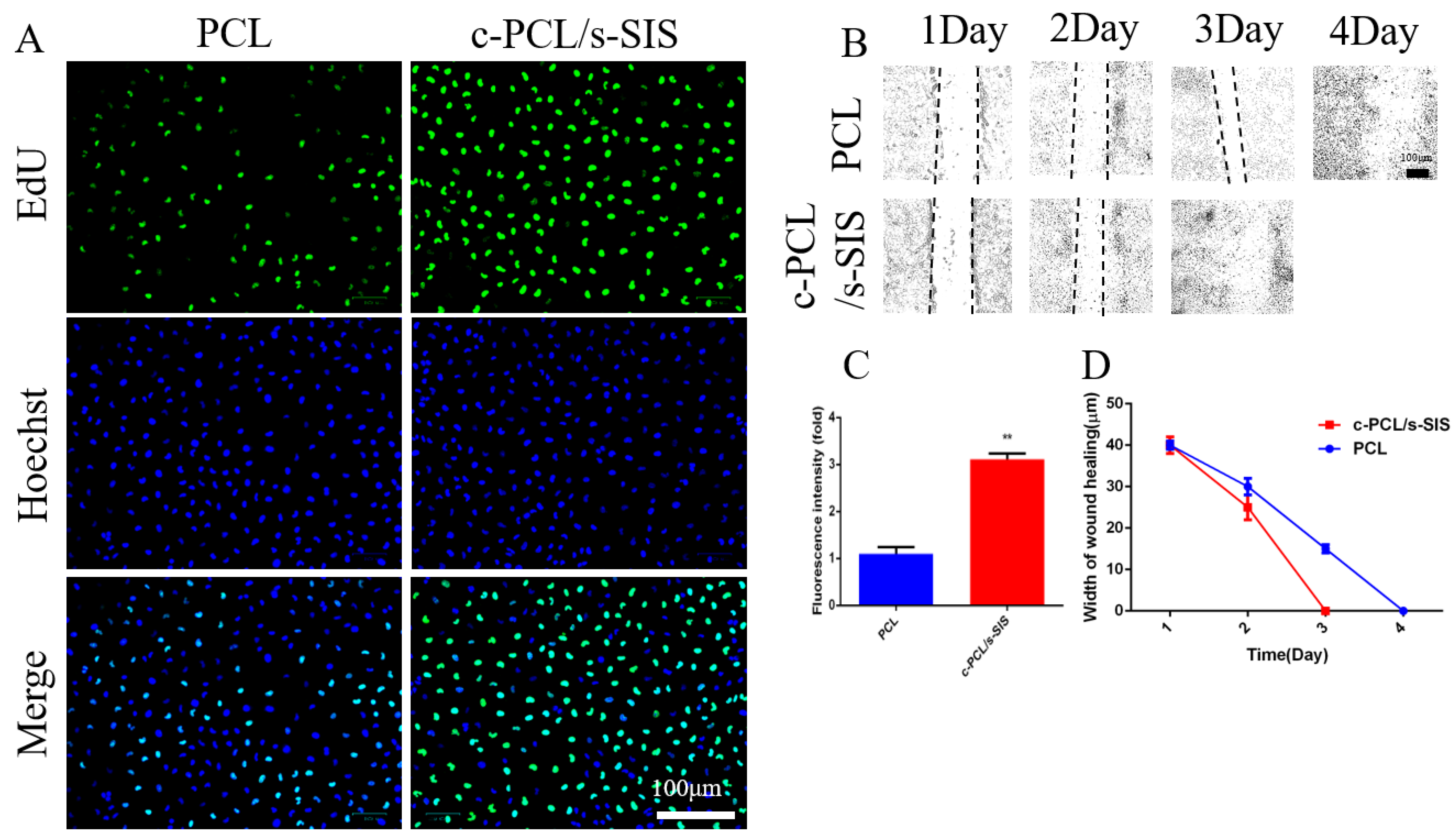
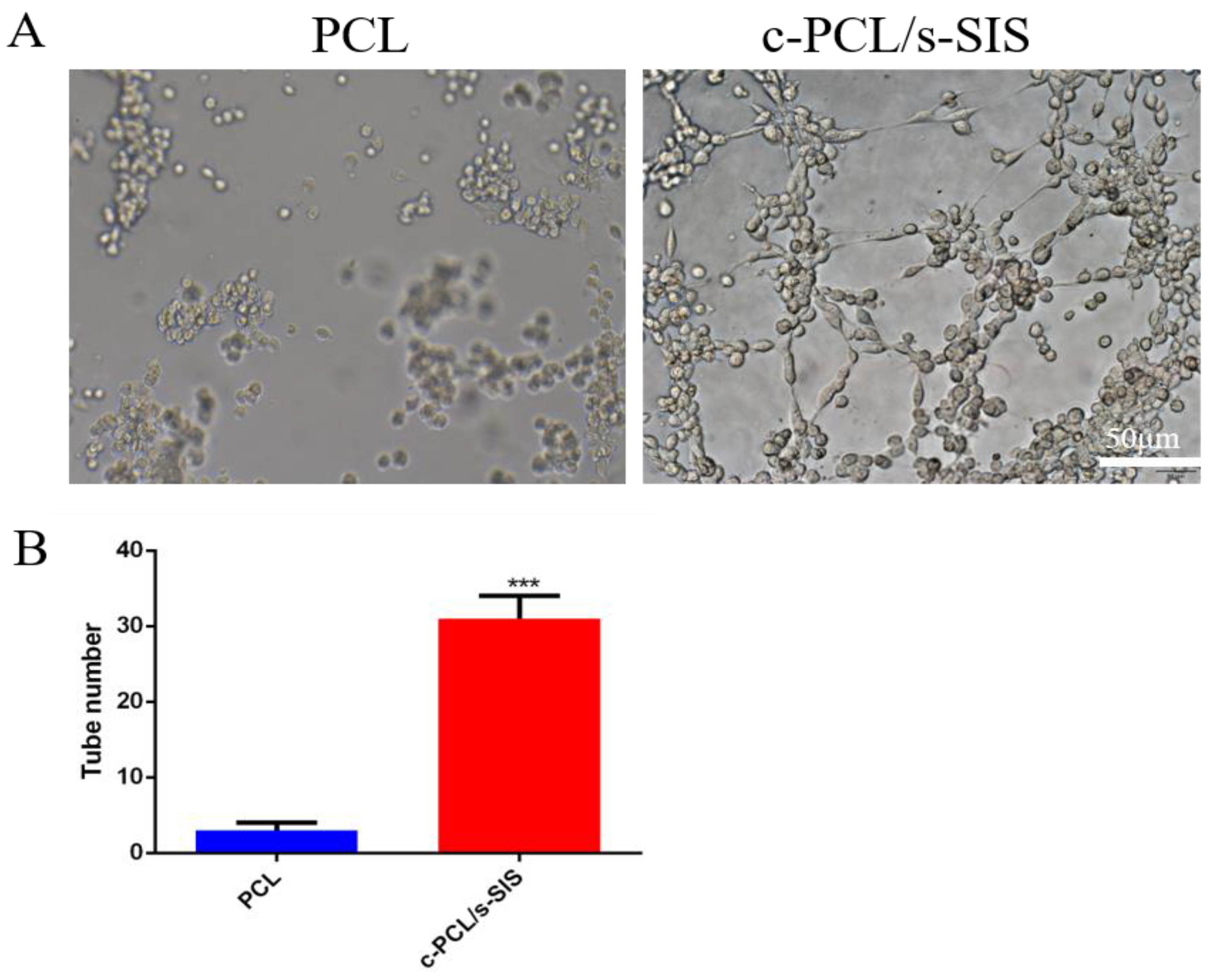
3.2. Biomimetic Periosteum Promotes Vascular Regeneration
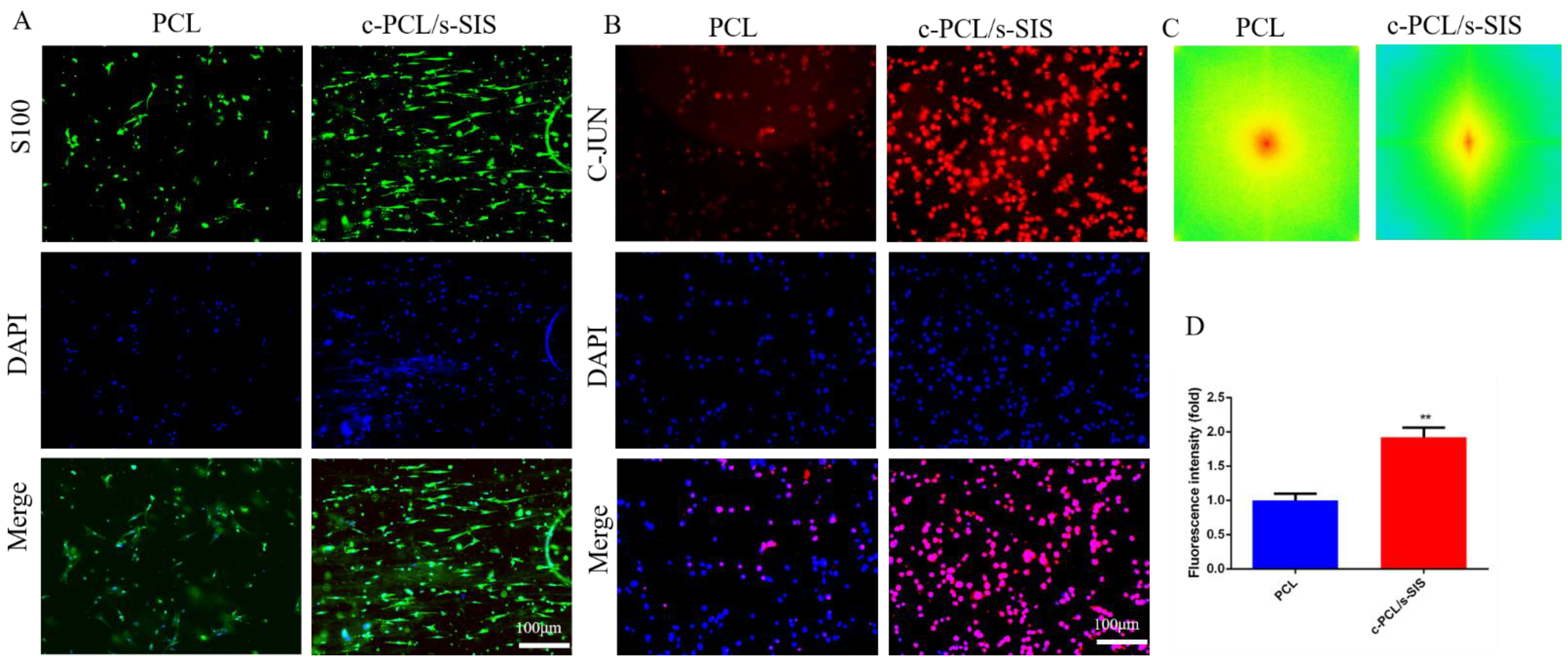
3.3. Effect of the Biomimetic Periosteum on Schwann Cells
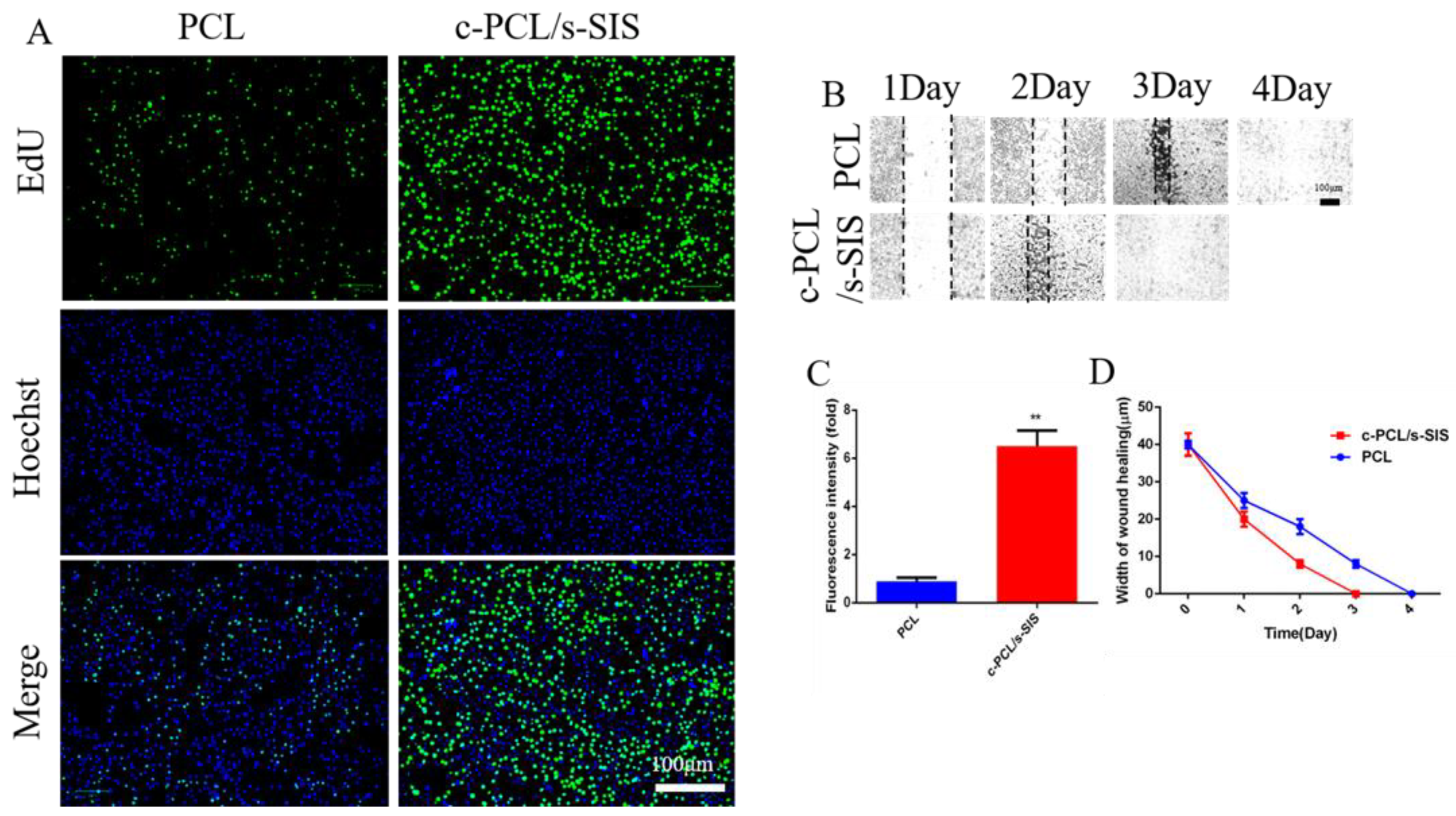
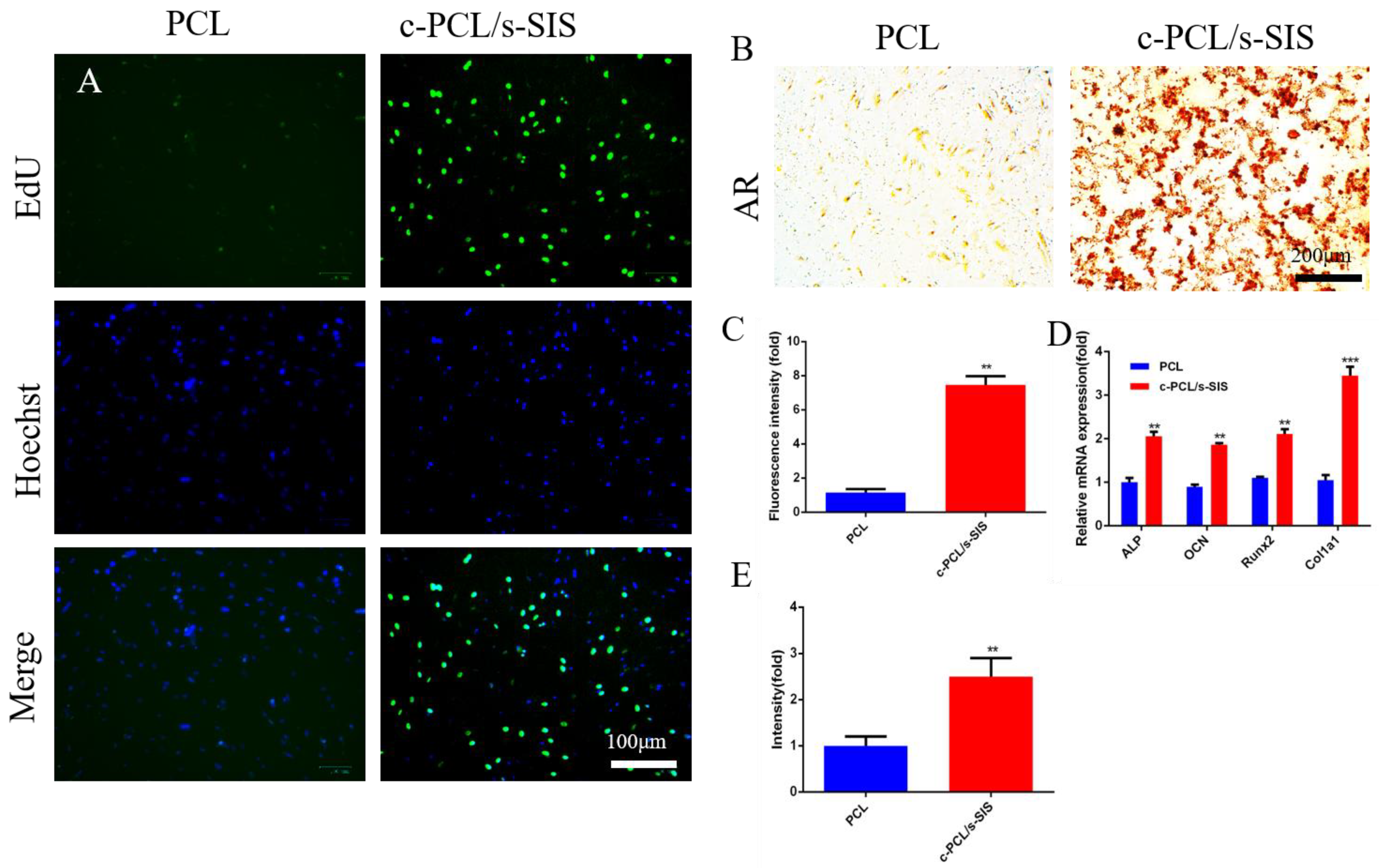
3.4. Effect of the Biomimetic Periosteum on Bone Marrow Mesenchymal Stem Cells
3.5. The Biomimetic Periosteum Promotes Bone Regeneration In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Zhang, M.; Matinlinna, J.P.; Tsoi, J.K.H.; Liu, W.; Cui, X.; Lu, W.W.; Pan, H. Recent developments in biomaterials for long-bone segmental defect reconstruction: A narrative overview. J. Orthop. Transl. 2022, 22, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.R.; Bastos, A.R.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Recent approaches towards bone tissue engineering. Bone 2022, 154, 116256. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Schott, N.G.; Friend, N.E.; Stegemann, J.P. Coupling Osteogenesis and Vasculogenesis in Engineered Orthopedic Tissues. Tissue Eng. Rev. 2021, 27, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, H.; Pan, J.; Li, J.; Zhang, K.; Duan, W.; Liang, H.; Chen, K.; Geng, D.; Shi, Q.; et al. Nanoscaled Bionic Periosteum Orchestrating the Osteogenic Microenvironment for Sequential Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36823–36836. [Google Scholar] [CrossRef]
- Dai, K.; Deng, S.; Yu, Y.; Zhu, F.; Wang, J.; Liu, C. Construction of developmentally inspired periosteum-like tissue for bone regeneration. Bone Res. 2022, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Wang, H.; Ye, G.; Li, Y.; Liu, C.; Yu, M.; Ying, B. Periosteal Tissue Engineering: Current Developments and Perspectives. Adv. Healthc. Mater. 2021, 10, e2100215. [Google Scholar] [CrossRef]
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.F.; Chang, H.; Tate, M.L.K. Elucidating multiscale periosteal mechanobiology: A key to unlocking the smart properties and regenerative capacity of the periosteum? Tissue Eng. Rev. 2013, 19, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Filipowska, J.; Tomaszewski, K.A.; Niedzwiedzki, L.; Walocha, J.A.; Niedzwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pu, P.; Su, Z.; Zhang, X.; Nie, L.; Chang, Y. Schwann Cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem. Biophys. Res. Commun. 2020, 531, 559–565. [Google Scholar] [CrossRef]
- Wang, D.; Lyu, Y.; Yang, Y.; Zhang, S.; Chen, G.; Pan, J.; Tian, W. Schwann cell-derived EVs facilitate dental pulp regeneration through endogenous stem cell recruitment via SDF-1/CXCR4 axis. Acta Biomater. 2022, 140, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Jiang, H.J.; Su, X.Y.; Feng, L.; Zhan, N.Z.; Li, S.S.; Chen, Z.J.; Chang, B.H.; Cheng, P.Z.; Yang, L.; et al. Schwann Cells Accelerate Osteogenesis via the Mif/CD74/FOXO1 Signaling Pathway In Vitro. Stem Cells Int. 2022, 2022, 4363632. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.; Ahmed, M.; Wieringa, P.; Moroni, L. Schwann cells promote endothelial cell migration. Cell Adh. Migr. 2015, 9, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jiang, X.; Jiang, S.; Cai, X.; Yu, S.; Pei, G. Schwann cells promote prevascularization and osteogenesis of tissue-engineered bone via bone marrow mesenchymal stem cell-derived endothelial cells. Stem Cell Res. Ther. 2021, 12, 382. [Google Scholar] [CrossRef]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Fang, Q.; Wang, F.; Ao, Q.; Wang, X.; Tian, X.; Tong, H.; Bai, S.; Fan, J. Surface modification of small intestine submucosa in tissue engineering. Regen. Biomater. 2020, 7, 339–348. [Google Scholar] [CrossRef]
- Cao, G.; Huang, Y.; Li, K.; Fan, Y.; Xie, H.; Li, X. Small intestinal submucosa: Superiority, limitations and solutions, and its potential to address bottlenecks in tissue repair. J. Mater. Chem. 2019, 7, 5038–5055. [Google Scholar] [CrossRef]
- Deng, R.; Luo, Z.; Rao, Z.; Lin, Z.; Chen, S.; Zhou, J.; Zhu, Q.; Liu, X.; Bai, Y.; Quan, D. Decellularized Extracellular Matrix Containing Electrospun Fibers for Nerve Regeneration: A Comparison Between Core–Shell Structured and Preblended Composites. Adv. Fiber Mater. 2022, 4, 503–519. [Google Scholar] [CrossRef]
- Sun, T.; Liu, M.; Yao, S.; Ji, Y.; Shi, L.; Tang, K.; Xiong, Z.; Yang, F.; Chen, K.; Guo, X. Guided osteoporotic bone regeneration with composite scaffolds of mineralized ECM/heparin membrane loaded with BMP2-related peptide. Int. J. Nanomed. 2018, 13, 791–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Liu, M.; Yao, S.; Ji, Y.; Xiong, Z.; Tang, K.; Chen, K.; Yang, H.; Guo, X. Biomimetic Composite Scaffold Containing Small Intestinal Submucosa and Mesoporous Bioactive Glass Exhibits High Osteogenic and Angiogenic Capacity. Tissue Eng. 2018, 24, 1044–1056. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yao, M.; Zhang, Y.; Lin, Z.; Zou, W.; Li, J.; Habibovic, P.; Du, C. Biomimetic three-layered membranes comprising (poly)-epsilon-caprolactone, collagen and mineralized collagen for guided bone regeneration. Regen. Biomater. 2021, 8, rbab065. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, J.; Niu, K.; Li, W.; Jiang, N.; Li, J.; Yu, Q.; Wu, C. Electrospun artificial periosteum loaded with DFO contributes to osteogenesis via the TGF-beta1/Smad2 pathway. Biomater. Sci. 2021, 9, 2090–2102. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Liu, X.; Jiao, Y.; Wang, F.; Jiang, G.; Wang, L. Tricalcium Phosphate Sol-Incorporated Poly(epsilon-caprolactone) Membrane with Improved Mechanical and Osteoinductive Activity as an Artificial Periosteum. ACS Biomater. Sci. Eng. 2020, 6, 4631–4643. [Google Scholar] [CrossRef]
- Elgali, I.; Turri, A.; Xia, W.; Norlindh, B.; Johansson, A.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration using resorbable membrane and different bone substitutes: Early histological and molecular events. Acta Biomater. 2016, 29, 409–423. [Google Scholar] [CrossRef]
- le Visage, C.; Yang, S.-H.; Kadakia, L.; Sieber, A.N.; Kostuik, J.P.; Leong, K.W. Small Intestinal Submucosa as a Potential Bioscaffold for Intervertebral Disc Regeneration. Spine 2006, 31, 2423–2430. [Google Scholar] [CrossRef] [Green Version]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (epsilon-caprolactone)-based electrospun nano-featured substrate for tissue engineering applications: A review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.-M.; Xu, X.; Lim, C.T.; Ramakrishna, S. Preparation of Core−Shell Structured PCL-r-Gelatin Bi-Component Nanofibers by Coaxial Electrospinning. Chem. Mater. 2004, 16, 3406–3409. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Primer | Sequence (5′→3′) |
|---|---|
| β-actin-F | GGAGATTACTGCCCTGGCTCCTA |
| β-actin-R | GACTCATCGTACTCCTGCTTGCTG |
| Pdlim3(Alp)-F | TCATAATTCCAGGCCGAACCA |
| Pdlim3(Alp)-R | GGCCATCTTAGCAGCAACTTTCA |
| Bglap(ocn)-F | CCCTCTCTCTGCTCACTCTGCT |
| Bglap(ocn)-R | CTTACTGCCCTCCTGCTTGG |
| Col1a1(col1)-F | GACATGTTCAGCTTTGTGGACCTC |
| Col1a1(col1)-R | AGGGACCCTTAGGCCATTGTGTA |
| Runx2-F | CATGGCCGGGAATGATGAG |
| Runx2-R | TGTGAAGACCGTTATGGTCAAAGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Ye, B.; Zeng, L.; Xiong, Z.; Sun, T.; Chen, K.; Ding, Q.; Su, W.; Jing, X.; Gao, Q.; et al. Small Intestinal Submucosa Biomimetic Periosteum Promotes Bone Regeneration. Membranes 2022, 12, 719. https://doi.org/10.3390/membranes12070719
Su Y, Ye B, Zeng L, Xiong Z, Sun T, Chen K, Ding Q, Su W, Jing X, Gao Q, et al. Small Intestinal Submucosa Biomimetic Periosteum Promotes Bone Regeneration. Membranes. 2022; 12(7):719. https://doi.org/10.3390/membranes12070719
Chicago/Turabian StyleSu, Yanlin, Bing Ye, Lian Zeng, Zekang Xiong, Tingfang Sun, Kaifang Chen, Qiuyue Ding, Weijie Su, Xirui Jing, Qing Gao, and et al. 2022. "Small Intestinal Submucosa Biomimetic Periosteum Promotes Bone Regeneration" Membranes 12, no. 7: 719. https://doi.org/10.3390/membranes12070719
APA StyleSu, Y., Ye, B., Zeng, L., Xiong, Z., Sun, T., Chen, K., Ding, Q., Su, W., Jing, X., Gao, Q., Huang, G., Wan, Y., Yang, X., & Guo, X. (2022). Small Intestinal Submucosa Biomimetic Periosteum Promotes Bone Regeneration. Membranes, 12(7), 719. https://doi.org/10.3390/membranes12070719






