The Human PDZome 2.0: Characterization of a New Resource to Test for PDZ Interactions by Yeast Two-Hybrid
Abstract
1. Introduction
2. Materials and Methods
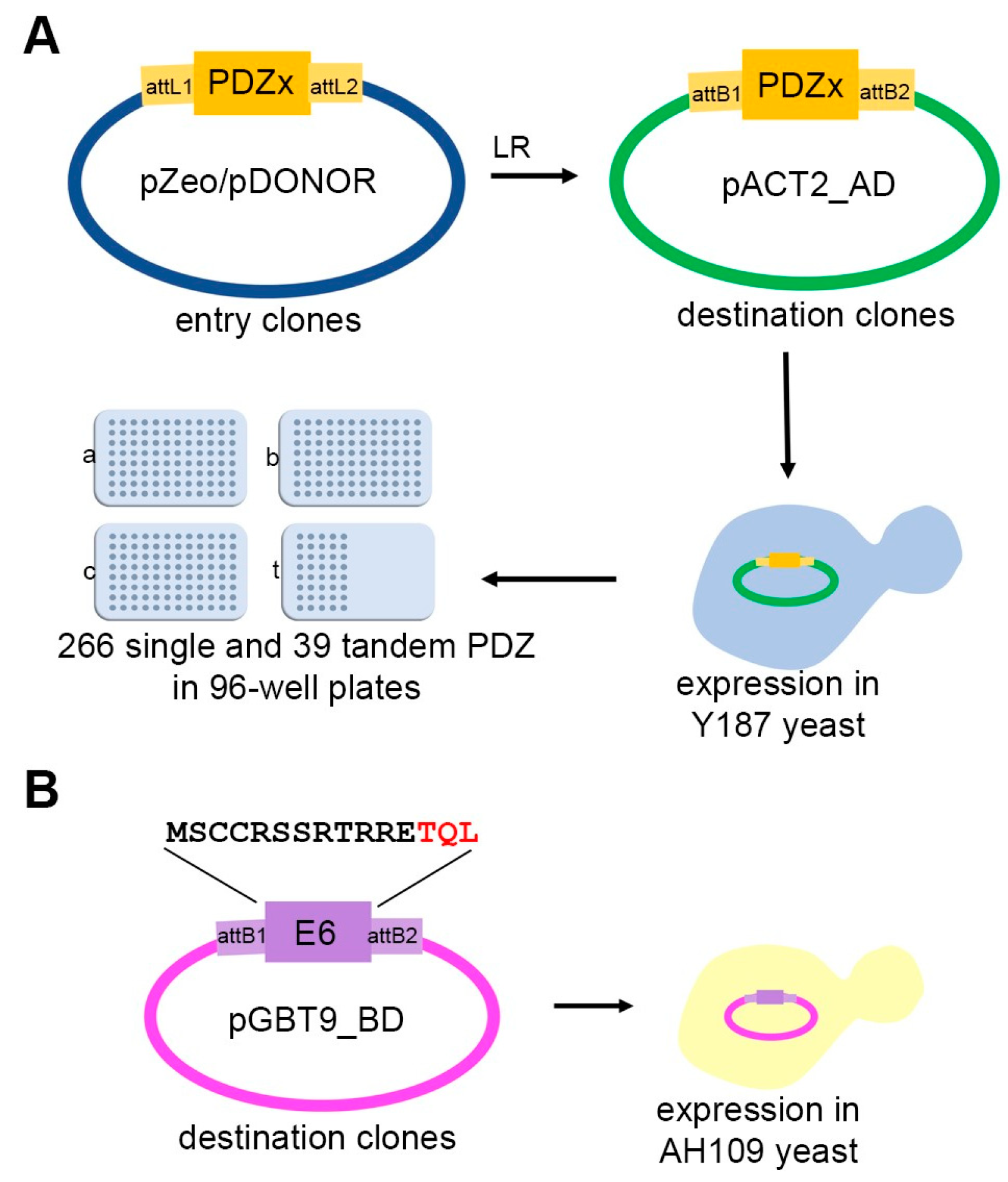
2.1. Sub-Cloning of Prey and Baits
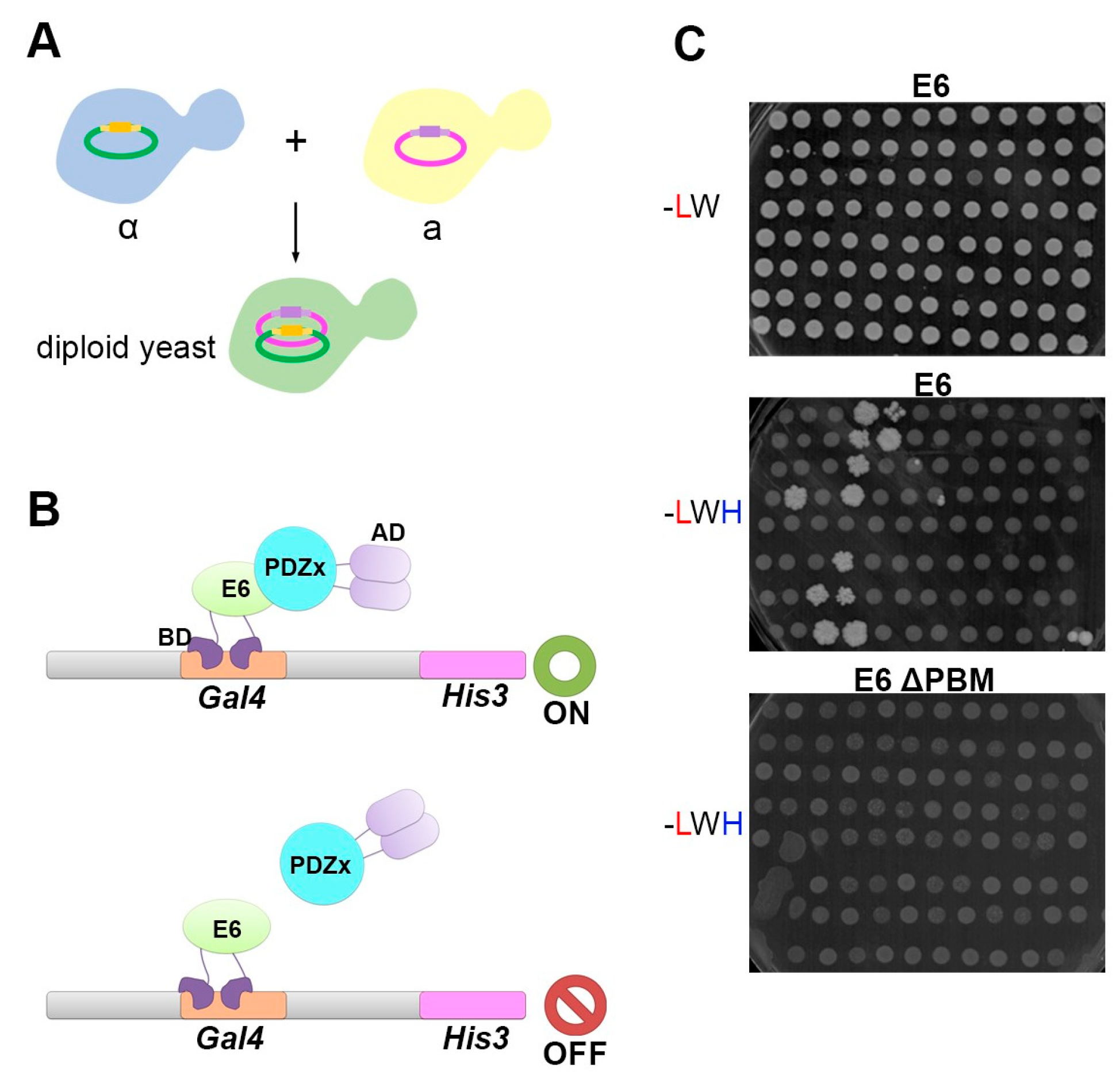
2.2. Yeast Two-Hybrid Assays
3. Results
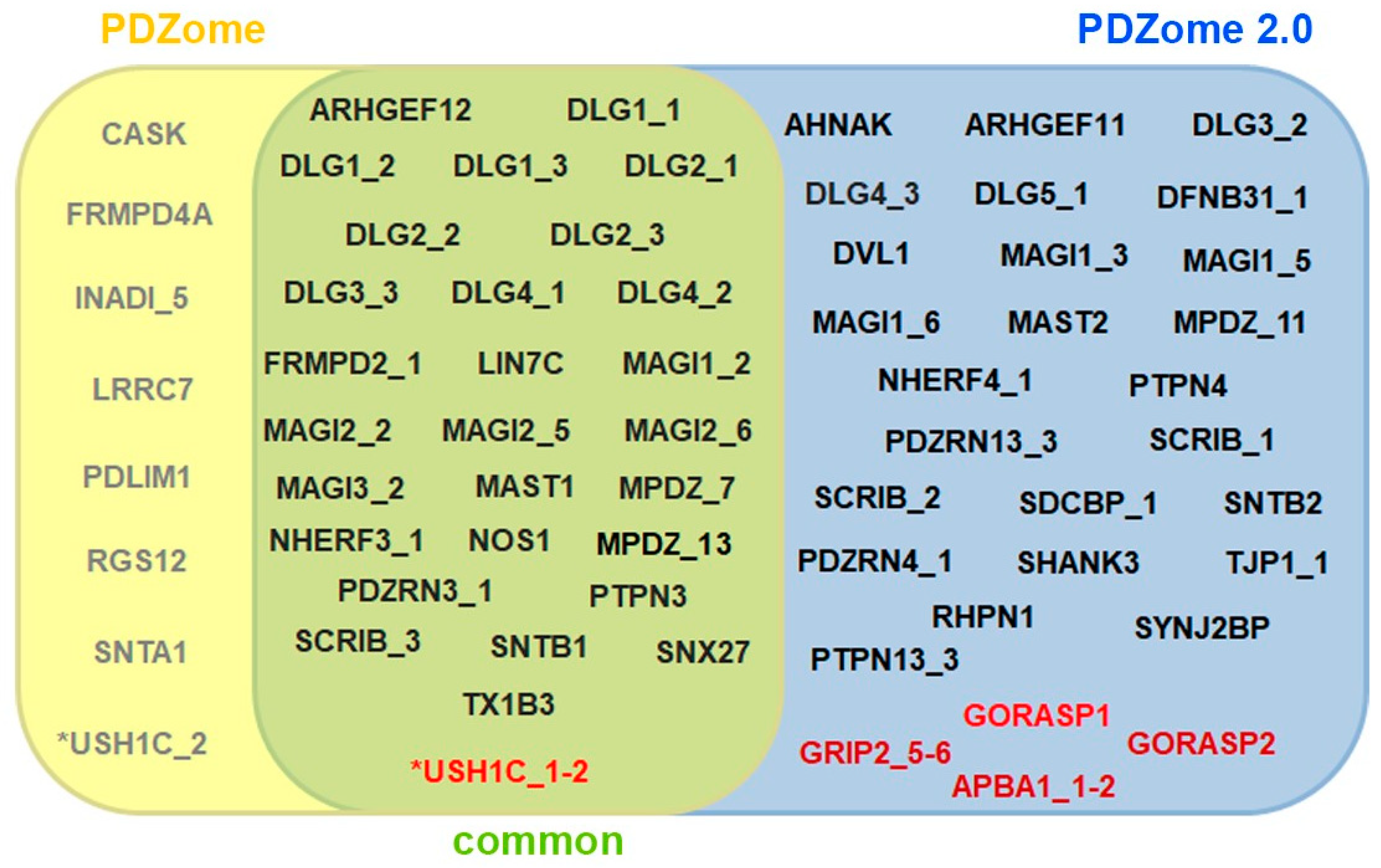
3.1. Construction of the Human PDZ Resource for Yeast Two-Hybrid Assays
3.2. The PDZome 2.0 for Y2H Screenings Is Validated Using the HPV16 E6 Oncoprotein
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harris, B.Z.; Lim, W.A. Mechanism and role of PDZ domains in signaling complex assembly. J. Cell Sci. 2001, 114 Pt 18, 3219–3231. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, G.P.; Ramanujam, P.L.; Galande, S. Structure function relations in PDZ-domain-containing proteins: Implications for protein networks in cellular signalling. J. Biosci. 2018, 43, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Nourry, C.; Grant, S.G.N.; Borg, J.-P. PDZ domain proteins: Plug and play! Sci. STKE 2003, 2003, RE7. [Google Scholar] [CrossRef]
- Cho, K.O.; Hunt, C.A.; Kennedy, M.B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 1992, 9, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Nagafuchi, A.; Yonemura, S.; Kitani-Yasuda, T.; Tsukita, S.; Tsukita, S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J. Cell Biol. 1993, 121, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.B. Origin of PDZ (DHR, GLGF) domains. Trends Biochem. Sci. 1995, 20, 350. [Google Scholar] [CrossRef]
- Woods, D.F.; Bryant, P.J. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 1991, 66, 451–464. [Google Scholar] [CrossRef]
- Woods, D.F.; Bryant, P.J. ZO-1, DlgA and PSD-95/SAP90: Homologous proteins in tight, septate and synaptic cell junctions. Mech. Dev. 1993, 44, 85–89. [Google Scholar] [CrossRef]
- Songyang, Z.; Cantley, L.C. Recognition and specificity in protein tyrosine kinase-mediated signalling. Trends Biochem. Sci. 1995, 20, 470–475. [Google Scholar] [CrossRef]
- Brenman, J.E.; Chao, D.S.; Gee, S.H.; McGee, A.W.; Craven, S.E.; Santillano, D.R.; Wu, Z.; Huang, F.; Xia, H.; Peters, M.F.; et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996, 84, 757–767. [Google Scholar] [CrossRef]
- Harris, B.Z.; Hillier, B.J.; Lim, W.A. Energetic determinants of internal motif recognition by PDZ domains. Biochemistry 2001, 40, 5921–5930. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Meerschaert, K.; Reekmans, G.; Leenaerts, I.; Small, J.V.; Vandekerckhove, J.; David, G.; Gettemans, J. PIP(2)-PDZ domain binding controls the association of syntenin with the plasma membrane. Mol. Cell 2002, 9, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wen, W.; Wei, Z.; Yu, J.; Ye, F.; Liu, C.H.; Hardie, R.C.; Zhang, M. The INAD scaffold is a dynamic, redox-regulated modulator of signaling in the Drosophila eye. Cell 2011, 145, 1088–1101. [Google Scholar] [CrossRef]
- LaLonde, D.P.; Bretscher, A. The scaffold protein PDZK1 undergoes a head-to-tail intramolecular association that negatively regulates its interaction with EBP50. Biochemistry 2009, 48, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- van den Berk, L.C.; Landi, E.; Walma, T.; Vuister, G.W.; Dente, L.; Hendriks, W.J. An allosteric intramolecular PDZ-PDZ interaction modulates PTP-BL PDZ2 binding specificity. Biochemistry 2007, 46, 13629–13637. [Google Scholar] [CrossRef]
- Raghuram, V.; Hormuth, H.; Foskett, J.K. A kinase-regulated mechanism controls CFTR channel gating by disrupting bivalent PDZ domain interactions. Proc. Natl. Acad. Sci. USA 2003, 100, 9620–9625. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, Y. Plasticity of PDZ domains in ligand recognition and signaling. FEBS Lett. 2012, 586, 2638–2647. [Google Scholar] [CrossRef]
- Lee, H.J.; Zheng, J.J. PDZ domains and their binding partners: Structure, specificity, and modification. Cell Commun. Signal. 2010, 8, 8. [Google Scholar] [CrossRef]
- Liu, X.; Fuentes, E.J. Emerging Themes in PDZ Domain Signaling: Structure, Function, and Inhibition. Int. Rev. Cell Mol. Biol. 2019, 343, 129–218. [Google Scholar] [PubMed]
- Doyle, D.A.; Lee, A.; Lewis, J.; Kim, E.; Sheng, M.; MacKinnon, R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell 1996, 85, 1067–1076. [Google Scholar] [CrossRef]
- Tonikian, R.; Zhang, Y.; Sazinsky, S.L.; Currell, B.; Yeh, J.-H.; Reva, B.; Held, H.A.; Appleton, B.A.; Evangelista, M.; Wu, Y.; et al. A specificity map for the PDZ domain family. PLoS Biol. 2008, 6, 2043–2059. [Google Scholar] [CrossRef]
- Garrido-Urbani, S.; Garg, P.; Ghossoub, R.; Arnold, R.; Lembo, F.; Sundell, G.N.; Kim, P.M.; Lopez, M.; Zimmermann, P.; Sidhu, S.S.; et al. Proteomic peptide phage display uncovers novel interactions of the PDZ1-2 supramodule of syntenin. FEBS Lett. 2016, 590, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, Y.; Arnold, R.; McLaughlin, M.; Nim, S.; Joshi, R.; Ray, D.; Liu, B.; Teyra, J.; Pawson, T.; Moffat, J.; et al. Large-scale interaction profiling of PDZ domains through proteomic peptide-phage display using human and viral phage peptidomes. Proc. Natl. Acad. Sci. USA 2014, 111, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P. Evidence for PDZ domains in bacteria, yeast, and plants. Protein Sci. 1997, 6, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Sakarya, O.; Conaco, C.; Egecioglu, O.; Solla, S.A.; Oakley, T.H.; Kosik, K.S. Evolutionary expansion and specialization of the PDZ domains. Mol. Biol. Evol. 2010, 27, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Egea-Jimenez, A.L.; Gallardo, R.; Garcia-Pino, A.; Ivarsson, Y.; Wawrzyniak, A.M.; Kashyap, R.; Loris, R.; Schymkowitz, J.; Rousseau, F.; Zimmermann, P. Frizzled 7 and PIP2 binding by syntenin PDZ2 domain supports Frizzled 7 trafficking and signalling. Nat. Commun. 2016, 7, 12101. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, Y.; Wawrzyniak, A.M.; Kashyap, R.; Polanowska, J.; Betzi, S.; Lembo, F.; Vermeiren, E.; Chiheb, D.; Lenfant, N.; Morelli, X.; et al. Prevalence, specificity and determinants of lipid-interacting PDZ domains from an in-cell screen and in vitro binding experiments. PLoS ONE 2013, 8, e54581. [Google Scholar] [CrossRef]
- Wawrzyniak, A.M.; Vermeiren, E.; Zimmermann, P.; Ivarsson, Y. Extensions of PSD-95/discs large/ZO-1 (PDZ) domains influence lipid binding and membrane targeting of syntenin-1. FEBS Lett. 2012, 586, 1445–1451. [Google Scholar] [CrossRef]
- Zimmermann, P.; Zhang, Z.; Degeest, G.; Mortier, E.; Leenaerts, I.; Coomans, C.; Schulz, J.; N’Kuli, F.; Courtoy, P.J.; David, G. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev. Cell 2005, 9, 377–388. [Google Scholar] [CrossRef]
- Belotti, E.; Polanowska, J.; Daulat, A.M.; Audebert, S.; Thome, V.; Lissitzky, J.C.; Lembo, F.; Blibek, K.; Omi, S.; Lenfant, N.; et al. The human PDZome: A gateway to PSD95-Disc large-zonula occludens (PDZ)-mediated functions. Mol. Cell Proteom. 2013, 12, 2587–2603. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef] [PubMed]
- Spaller, M.R. Act globally, think locally: Systems biology addresses the PDZ domain. ACS Chem. Biol. 2006, 1, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Luck, K.; Charbonnier, S.; Trave, G. The emerging contribution of sequence context to the specificity of protein interactions mediated by PDZ domains. FEBS Lett. 2012, 586, 2648–2661. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Pan, L.; Chen, J.; Zhang, M. Extensions of PDZ domains as important structural and functional elements. Protein Cell 2010, 1, 737–751. [Google Scholar] [CrossRef]
- Zhang, J.; Petit, C.M.; King, D.S.; Lee, A.L. Phosphorylation of a PDZ domain extension modulates binding affinity and interdomain interactions in postsynaptic density-95 (PSD-95) protein, a membrane-associated guanylate kinase (MAGUK). J. Biol. Chem. 2011, 286, 41776–41785. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat. Rev. Neurosci. 2009, 10, 87–99. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, M. Structures and target recognition modes of PDZ domains: Recurring themes and emerging pictures. Biochem. J. 2013, 455, 1–14. [Google Scholar] [CrossRef]
- Grootjans, J.J.; Reekmans, G.; Ceulemans, H.; David, G. Syntenin-syndecan binding requires syndecan-synteny and the co-operation of both PDZ domains of syntenin. J. Biol. Chem. 2000, 275, 19933–19941. [Google Scholar] [CrossRef]
- Duhoo, Y.; Girault, V.; Turchetto, J.; Ramond, L.; Durbesson, F.; Fourquet, P.; Nomine, Y.; Cardoso, V.; Sequeira, A.F.; Bras, J.L.A.; et al. High-Throughput Production of a New Library of Human Single and Tandem PDZ Domains Allows Quantitative PDZ-Peptide Interaction Screening Through High-Throughput Holdup Assay. Methods Mol. Biol. 2019, 2025, 439–476. [Google Scholar]
- Fields, S.; Song, O. A novel genetic system to detect protein-protein interactions. Nature 1989, 340, 245–246. [Google Scholar] [CrossRef]
- Ganti, K.; Broniarczyk, J.; Manoubi, W.; Massimi, P.; Mittal, S.; Pim, D.; Szalmas, A.; Thatte, J.; Thomas, M.; Tomaic, V.; et al. The Human Papillomavirus E6 PDZ Binding Motif: From Life Cycle to Malignancy. Viruses 2015, 7, 3530–3551. [Google Scholar] [CrossRef]
- Thomas, M.; Narayan, N.; Pim, D.; Tomaic, V.; Massimi, P.; Nagasaka, K.; Kranjec, C.; Gammoh, N.; Banks, L. Human papillomaviruses, cervical cancer and cell polarity. Oncogene 2008, 27, 7018–7030. [Google Scholar] [CrossRef] [PubMed]
- Vincentelli, R.; Luck, K.; Poirson, J.; Polanowska, J.; Abdat, J.; Blemont, M.; Turchetto, J.; Iv, F.; Ricquier, K.; Straub, M.L.; et al. Quantifying domain-ligand affinities and specificities by high-throughput holdup assay. Nat. Methods 2015, 12, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Gogl, G.; Zambo, B.; Kostmann, C.; Cousido-Siah, A.; Morlet, B.; Durbesson, F.; Negroni, L.; Eberling, P.; Jane, P.; Nomine, Y.; et al. Quantitative fragmentomics allow affinity mapping of interactomes. Nat. Commun. 2022, 13, 5472. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, A.; Polge, C.; Lentze, N.; Auerbach, D.; Schlattner, U. Yeast two-hybrid, a powerful tool for systems biology. Int. J. Mol. Sci. 2009, 10, 2763–2788. [Google Scholar] [CrossRef]
- Javier, R.T.; Rice, A.P. Emerging theme: Cellular PDZ proteins as common targets of pathogenic viruses. J. Virol. 2011, 85, 11544–11556. [Google Scholar] [CrossRef]
- Neveu, G.; Cassonnet, P.; Vidalain, P.O.; Rolloy, C.; Mendoza, J.; Jones, L.; Tangy, F.; Muller, M.; Demeret, C.; Tafforeau, L.; et al. Comparative analysis of virus-host interactomes with a mammalian high-throughput protein complementation assay based on Gaussia princeps luciferase. Methods 2012, 58, 349–359. [Google Scholar] [CrossRef]
- Pim, D.; Bergant, M.; Boon, S.S.; Ganti, K.; Kranjec, C.; Massimi, P.; Subbaiah, V.K.; Thomas, M.; Tomaic, V.; Banks, L. Human papillomaviruses and the specificity of PDZ domain targeting. FEBS J. 2012, 279, 3530–3537. [Google Scholar] [CrossRef]
- Poirson, J.; Biquand, E.; Straub, M.L.; Cassonnet, P.; Nomine, Y.; Jones, L.; van der Werf, S.; Trave, G.; Zanier, K.; Jacob, Y.; et al. Mapping the interactome of HPV E6 and E7 oncoproteins with the ubiquitin-proteasome system. FEBS J. 2017, 284, 3171–3201. [Google Scholar] [CrossRef]
- Luchow, S.; Sundell, G.N.; Ivarsson, Y. Identification of PDZ Interactions by Proteomic Peptide Phage Display. Methods Mol. Biol. 2021, 2256, 41–60. [Google Scholar]
- Daulat, A.M.; Audebert, S.; Wagner, M.; Camoin, L.; Borg, J.P. Identification of PDZ Interactions by Affinity Purification and Mass Spectrometry Analysis. Methods Mol. Biol. 2021, 2256, 17–40. [Google Scholar] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Imjeti, N.S.; Menck, K.; Egea-Jimenez, A.L.; Lecointre, C.; Lembo, F.; Bouguenina, H.; Badache, A.; Ghossoub, R.; David, G.; Roche, S.; et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl. Acad. Sci. USA 2017, 114, 12495–12500. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Balzano, M.; Lechat, B.; Lambaerts, K.; Egea-Jimenez, A.L.; Lembo, F.; Fares, J.; Meeussen, S.; Kugler, S.; Roebroek, A.; et al. Syntenin-knock out reduces exosome turnover and viral transduction. Sci. Rep. 2021, 11, 4083. [Google Scholar] [CrossRef]
- Lambaerts, K.; Van Dyck, S.; Mortier, E.; Ivarsson, Y.; Degeest, G.; Luyten, A.; Vermeiren, E.; Peers, B.; David, G.; Zimmermann, P. Syntenin, a syndecan adaptor and an Arf6 phosphatidylinositol 4,5-bisphosphate effector, is essential for epiboly and gastrulation cell movements in zebrafish. J. Cell Sci. 2012, 125 Pt 5, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Luyten, A.; Mortier, E.; Van Campenhout, C.; Taelman, V.; Degeest, G.; Wuytens, G.; Lambaerts, K.; David, G.; Bellefroid, E.J.; Zimmermann, P. The postsynaptic density 95/disc-large/zona occludens protein syntenin directly interacts with frizzled 7 and supports noncanonical Wnt signaling. Mol. Biol. Cell 2008, 19, 1594–1604. [Google Scholar] [CrossRef]
- Mortier, E.; Wuytens, G.; Leenaerts, I.; Hannes, F.; Heung, M.Y.; Degeest, G.; David, G.; Zimmermann, P. Nuclear speckles and nucleoli targeting by PIP2-PDZ domain interactions. EMBO J. 2005, 24, 2556–2565. [Google Scholar] [CrossRef]
- Zimmermann, P.; Egea-Jimenez, A.L. Study of PDZ-Peptide and PDZ-Lipid Interactions by Surface Plasmon Resonance/BIAcore. Methods Mol. Biol. 2021, 2256, 75–87. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Cruz, M.; Lembo, F.; Borg, J.-P.; Travé, G.; Vincentelli, R.; Zimmermann, P. The Human PDZome 2.0: Characterization of a New Resource to Test for PDZ Interactions by Yeast Two-Hybrid. Membranes 2023, 13, 737. https://doi.org/10.3390/membranes13080737
Castro-Cruz M, Lembo F, Borg J-P, Travé G, Vincentelli R, Zimmermann P. The Human PDZome 2.0: Characterization of a New Resource to Test for PDZ Interactions by Yeast Two-Hybrid. Membranes. 2023; 13(8):737. https://doi.org/10.3390/membranes13080737
Chicago/Turabian StyleCastro-Cruz, Monica, Frédérique Lembo, Jean-Paul Borg, Gilles Travé, Renaud Vincentelli, and Pascale Zimmermann. 2023. "The Human PDZome 2.0: Characterization of a New Resource to Test for PDZ Interactions by Yeast Two-Hybrid" Membranes 13, no. 8: 737. https://doi.org/10.3390/membranes13080737
APA StyleCastro-Cruz, M., Lembo, F., Borg, J.-P., Travé, G., Vincentelli, R., & Zimmermann, P. (2023). The Human PDZome 2.0: Characterization of a New Resource to Test for PDZ Interactions by Yeast Two-Hybrid. Membranes, 13(8), 737. https://doi.org/10.3390/membranes13080737





