Production and Optimization of Biodiesel in a Membrane Reactor, Using a Solid Base Catalyst
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis and Evaluation of Catalysts
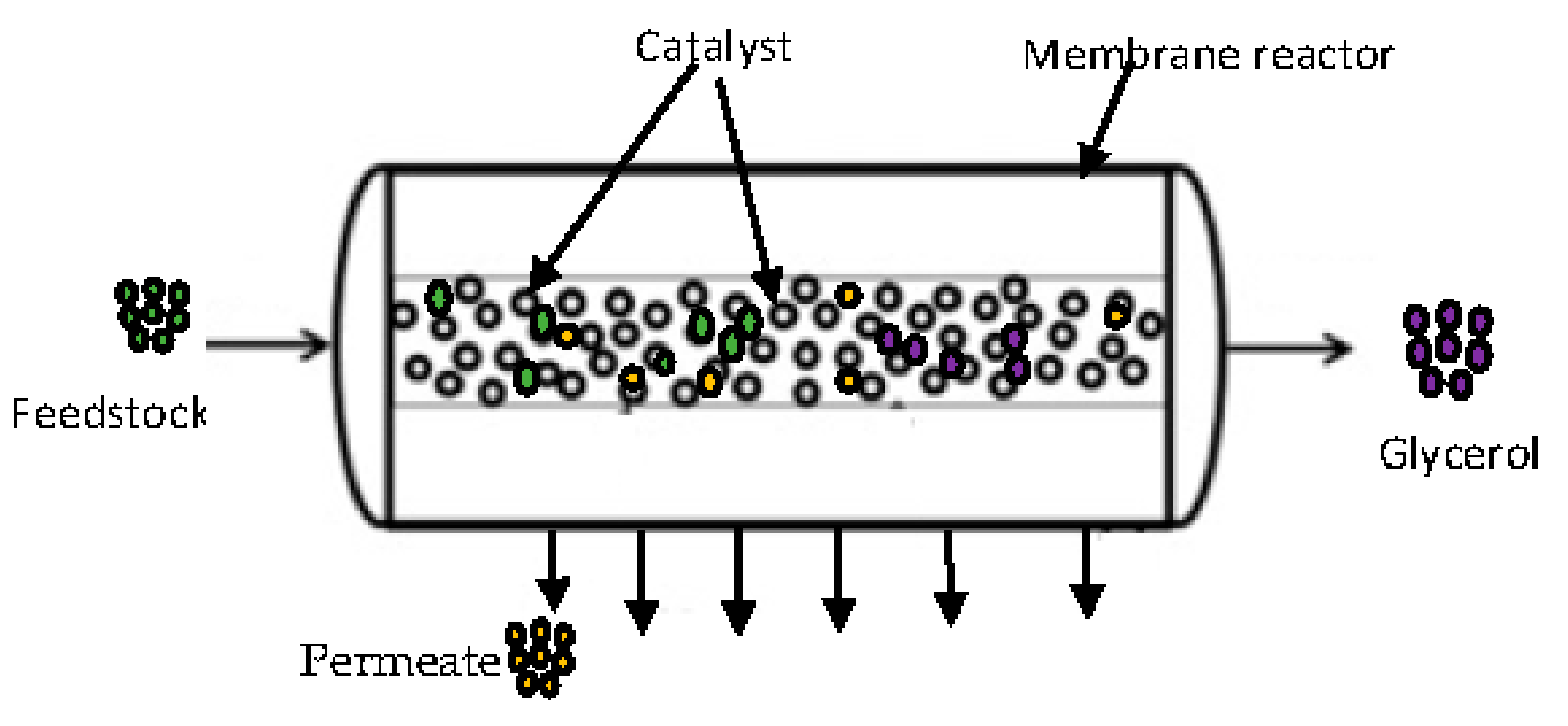
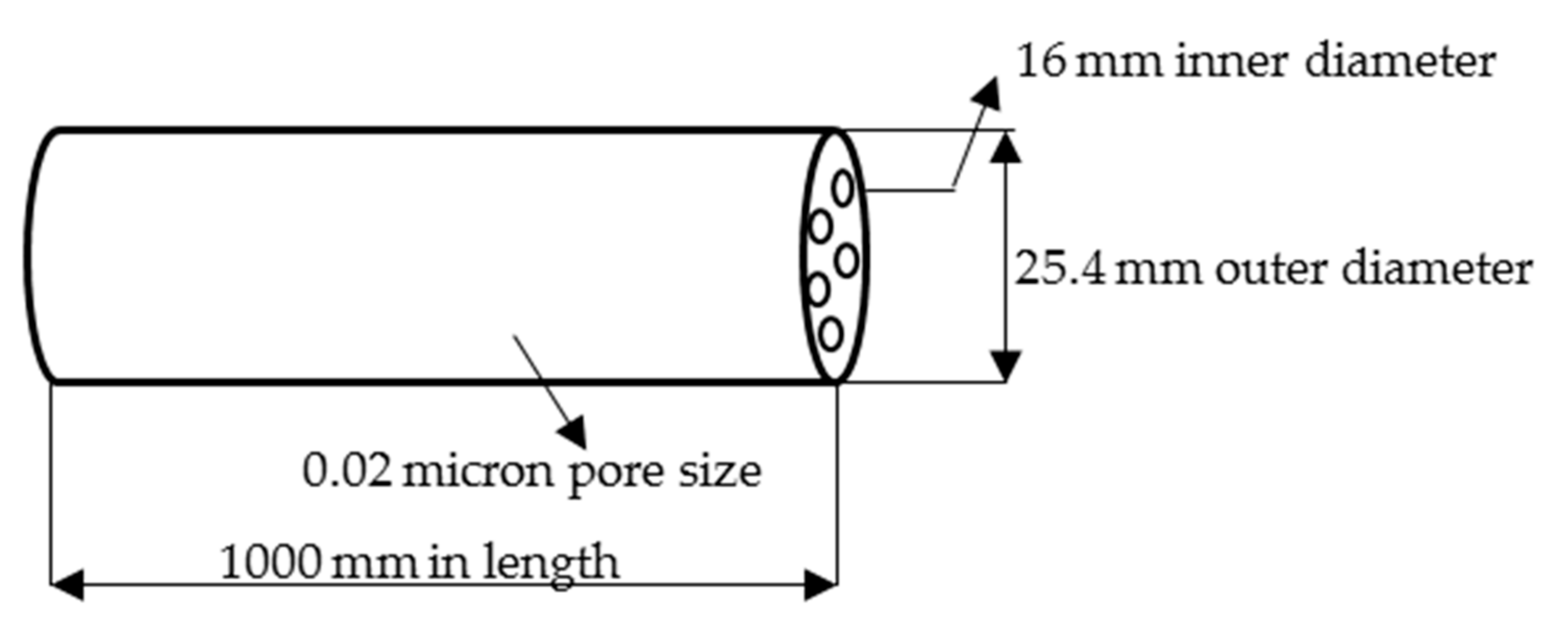
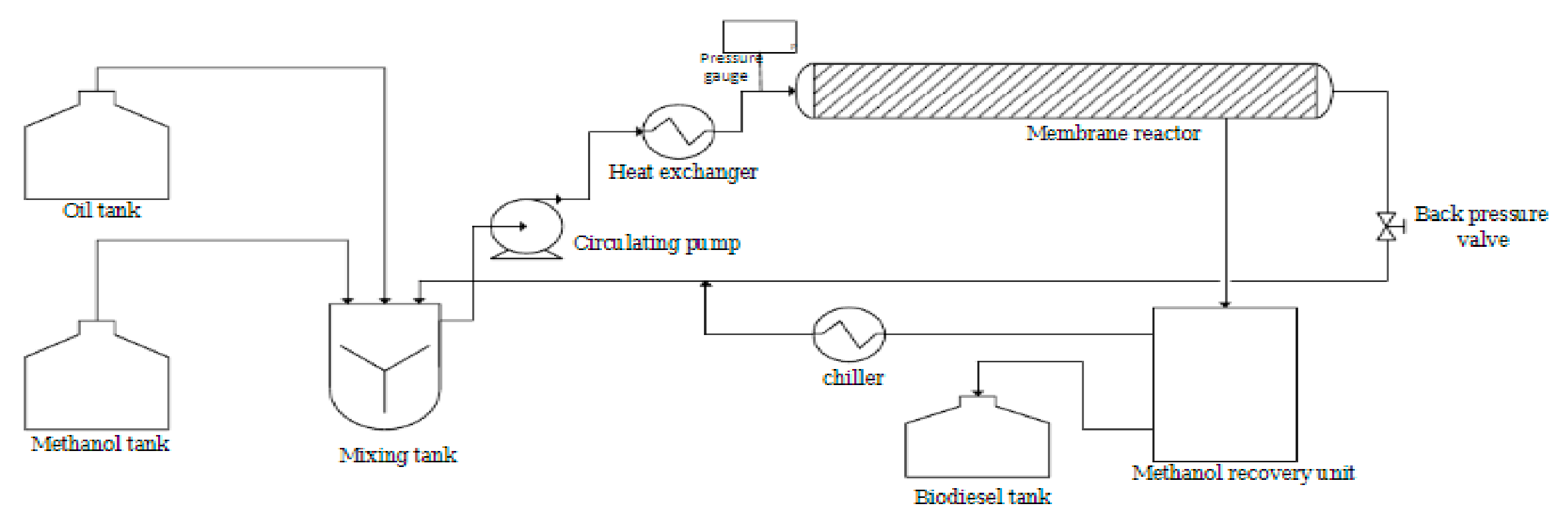
2.3. Transesterification Process in Membrane Reactor
2.4. Design of Experiments
2.5. Analytical Statistics (ANOVA)
3. Results and Discussion
3.1. Characteristics of Catalysts
3.2. Experimental Design Based on Central Composite Design
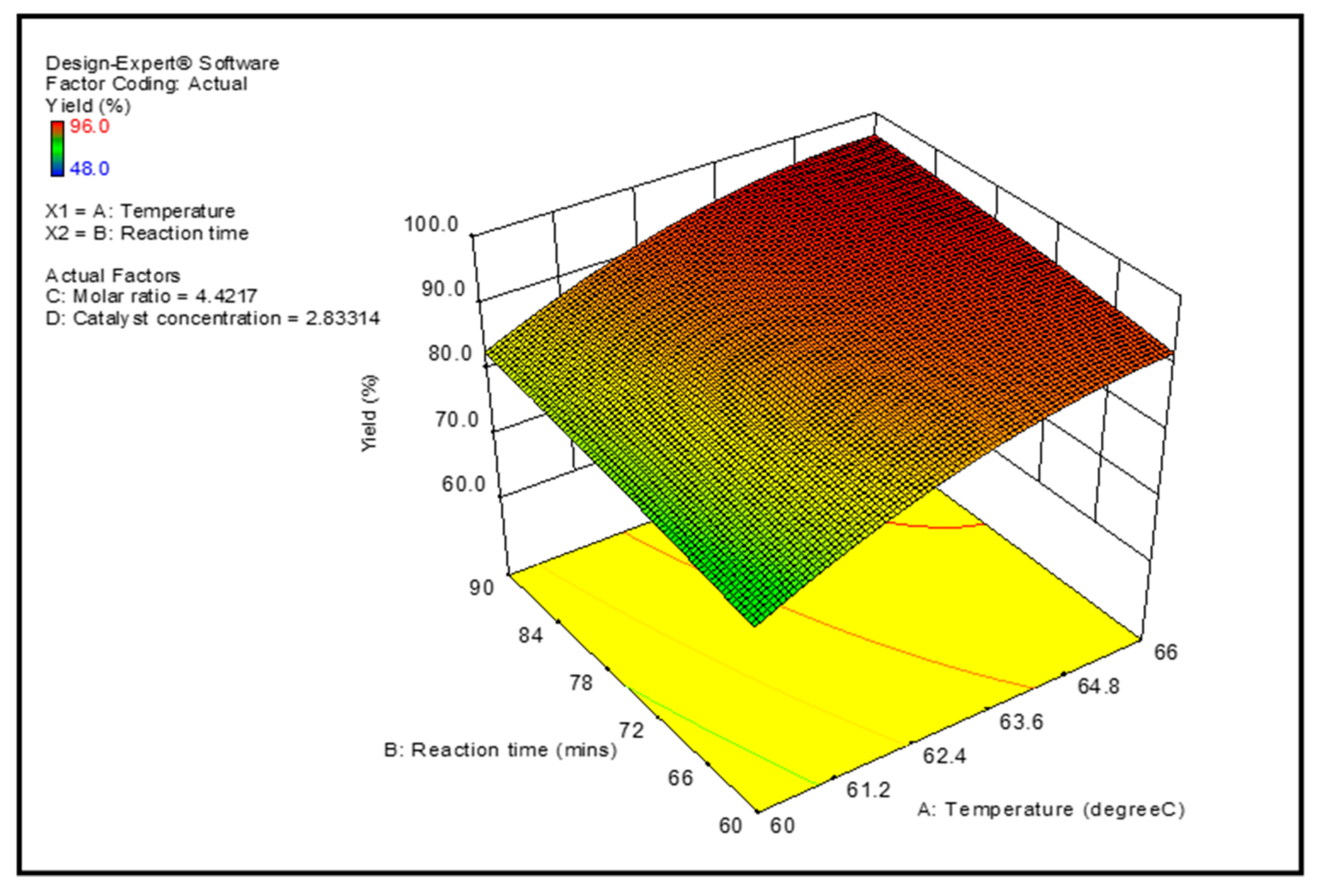
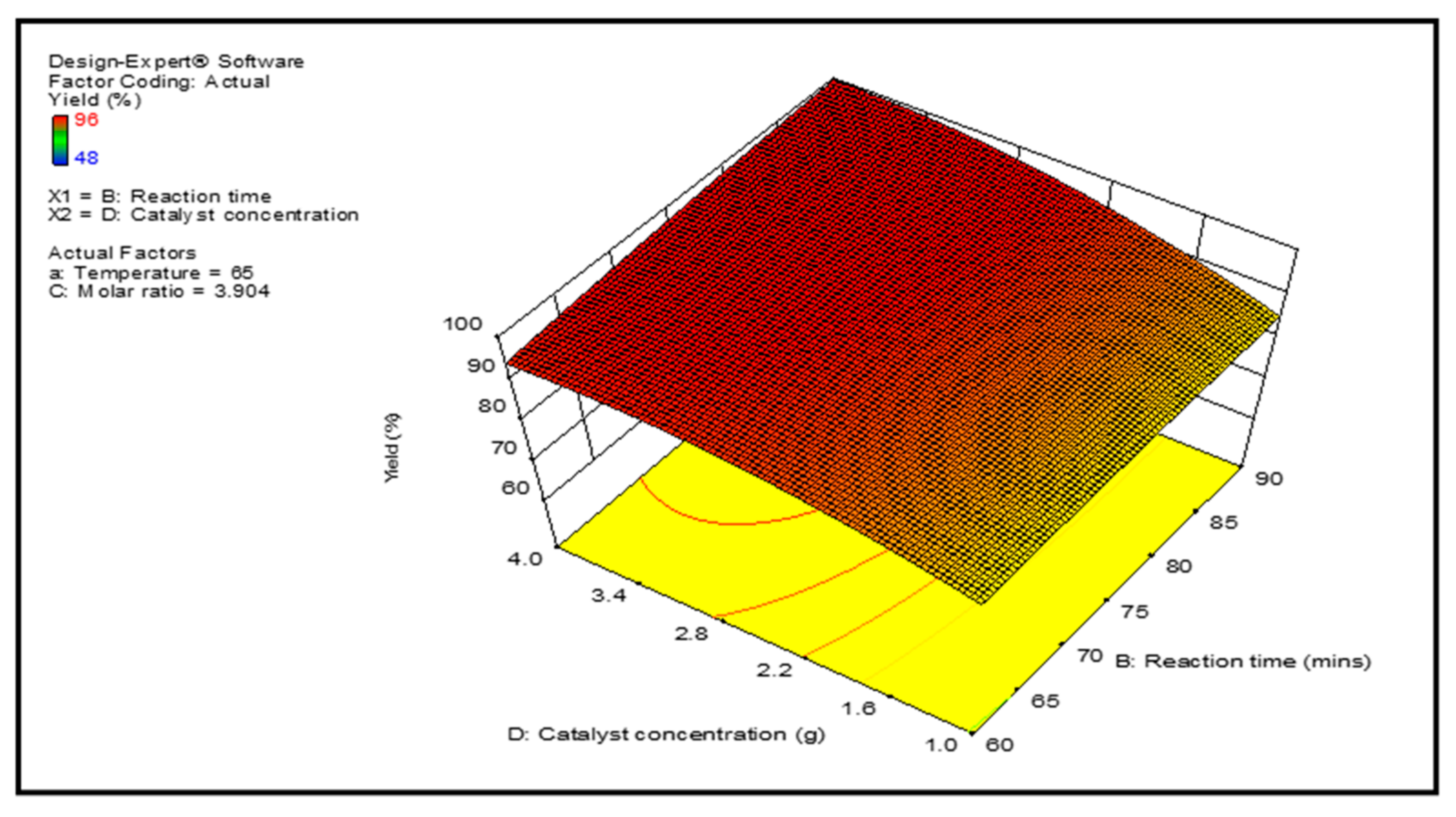
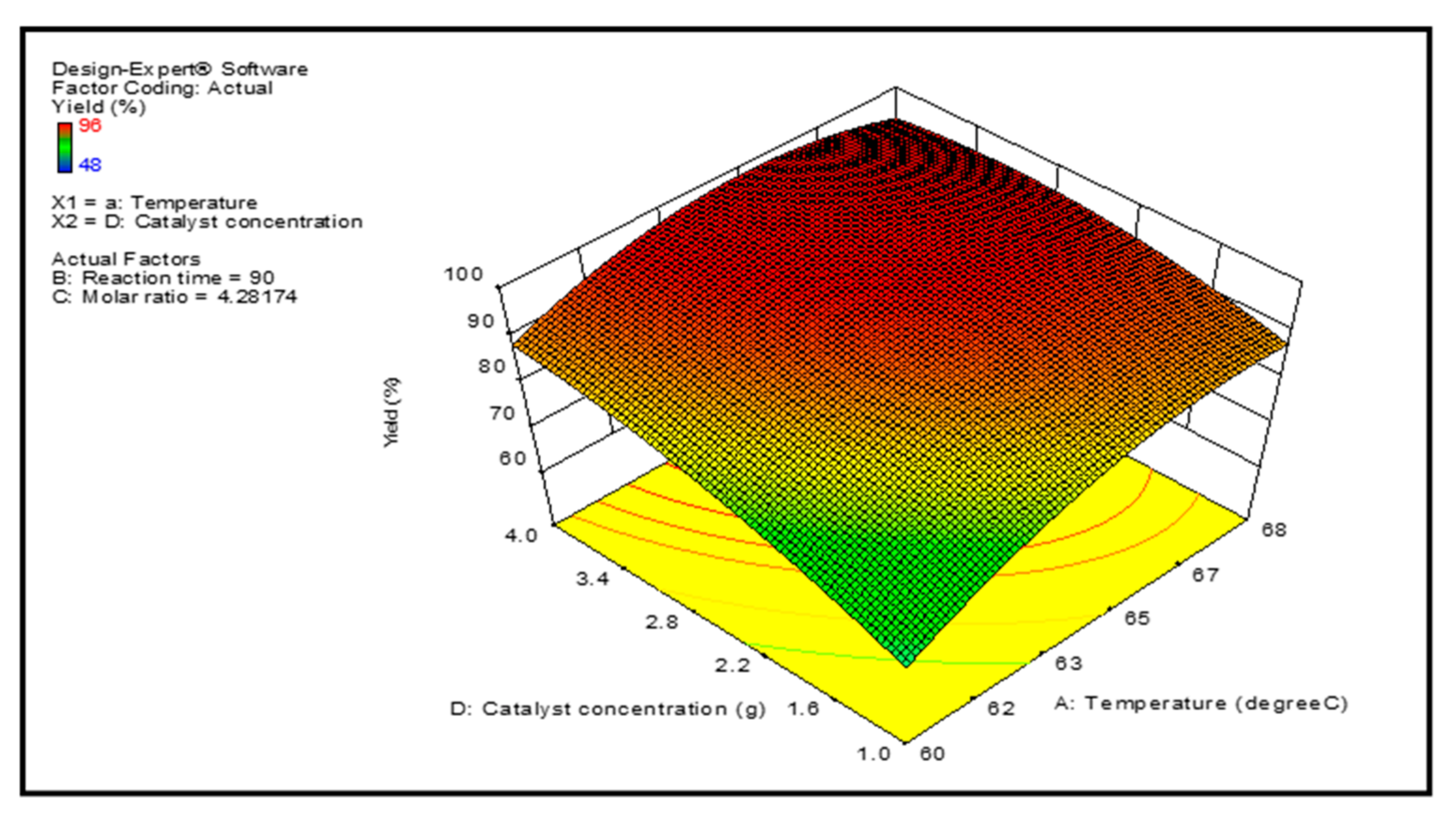
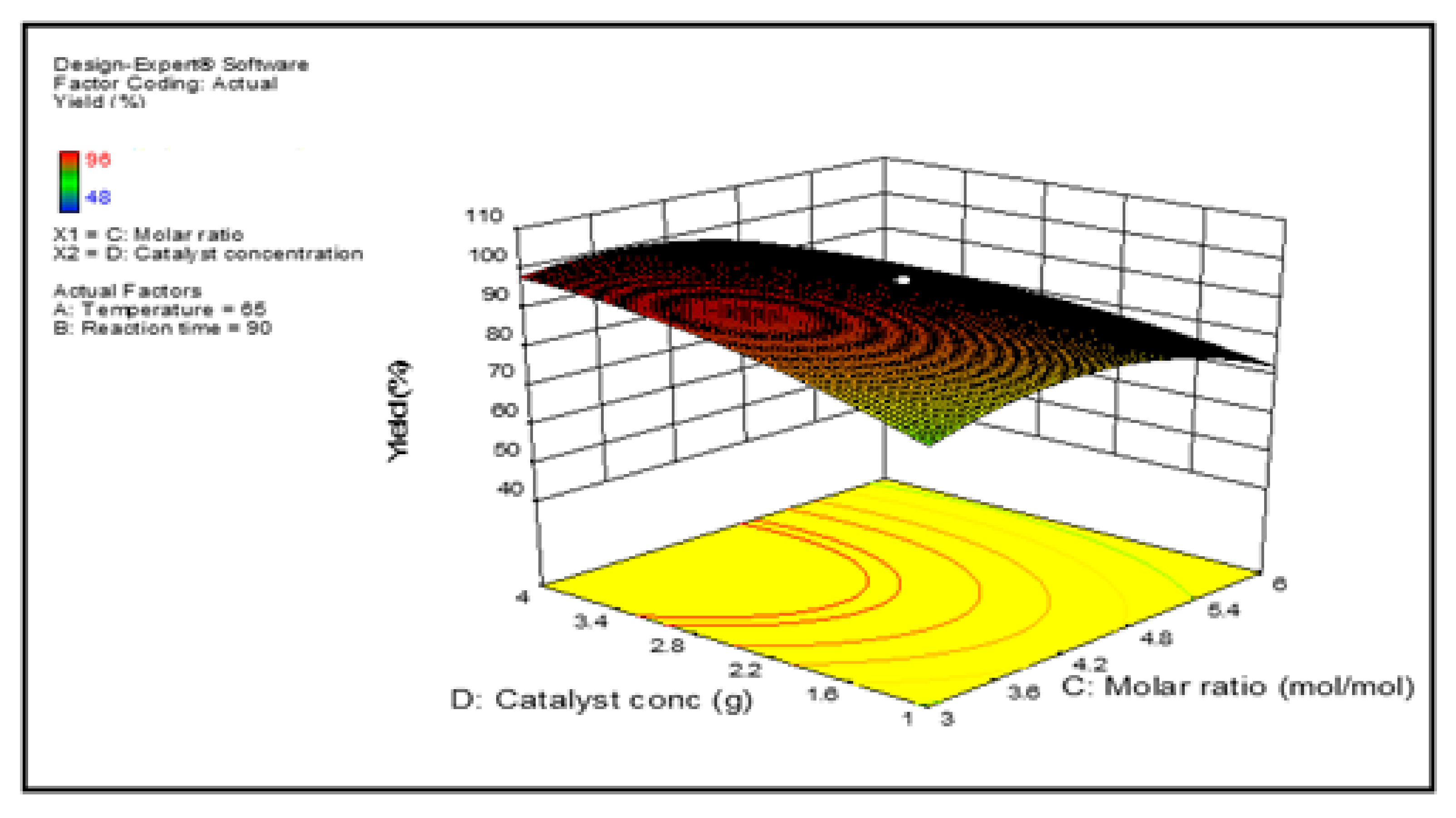
3.3. Optimization Study
3.4. Biodiesel Characterization
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malode, S.; Prabhu, K.; Mascarenhas, R.; Shetti, N.; Aminabhavi, T. Recent advances and viability in biofuel production. Energy Convers. Manag. X 2021, 10, 100070. [Google Scholar] [CrossRef]
- Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Tobar, M.; Núñez, G.A. Supercritical transesterification of microalgae triglycerides for biodiesel production: Effect of alcohol type and co-solvent. J. Supercrit. Fluids 2018, 137, 50–56. [Google Scholar] [CrossRef]
- Litinas, A.; Geivanidis, S.; Faliakis, A.; Courouclis, Y.; Samaras, Z.; Keder, A.; Krasnoholovets, V.; Gandzha, I.; Zabulonov, Y.; Puhach, O.; et al. Biodiesel production from high FFA feedstocks with a novel chemical multifunctional process intensifier. Biofuel Res. J. 2020, 7, 1170. [Google Scholar] [CrossRef]
- Barahira, D.S.; Okudoh, V.I.; Eloka-Eboka, A.C. Suitability of crop residues as feedstock for biofuel production in South Africa. A sustainable win-win scenario. J. Oleo Sci. 2021, 70, 213–226. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S. A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng. 2020, 8, 104523. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Moyo, L.B.; Iyuke, S.E.; Muvhiiwa, R.F.; Simate, G.S.; Hlabangana, N. Application of response surface methodology for optimization of biodiesel production parameters from waste cooking oil using a membrane reactor. S. Afr. J. Chem. Eng. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Baroutian, S.; Aroua, M.K.; Raman, A.A.A.; Sulaiman, N.M.N. A packed bed membrane reactor for production of biodiesel using activated carbon supported catalyst. Bioresour. Technol. 2011, 102, 1095–1102. [Google Scholar] [CrossRef]
- Sharma, A.; Kodgire, P.; Kachhwaha, S.S. Investigation of ultrasound-assisted KOH and CaO catalyzed transesterification for biodiesel production from waste cotton-seed cooking oil: Process optimization and conversion rate evaluation. J. Clean. Prod. 2020, 259, 120982. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Calcium diglyceroxide catalyzed biodiesel production from waste cooking oil in the presence of microwave: Optimization and kinetic studies. Renew. Energy 2018, 121, 757–767. [Google Scholar] [CrossRef]
- Borah, M.J.; Das, A.; Das, V.; Bhuyan, N.; Deka, D. Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst. Fuel 2019, 242, 345–354. [Google Scholar] [CrossRef]
- Sindhu, R.; Raina, D.; Binod, P.; Mathew, G.M.; Saran, S.; Pugazhendi, A.; Pandey, A.; Narisetty, V.; Kumar, V. Recent Advances in Biodiesel Production: Challenges and Solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar]
- Asasucharit, C. Investigation on Properties of Jatropha oil from storage of seed, oil and different storage tanks at different period. Int. J. Sci. Innov. Technol. 2019, 2, 73–80. [Google Scholar]
- Osorio-González, C.S.; Gómez-Falcon, N.; Sandoval-Salas, F.; Saini, R.; Brar, S.K.; Ramírez, A.A. Production of biodiesel from castor oil: A review. Energies 2020, 13, 2467. [Google Scholar] [CrossRef]
- Etim, A.O.; Musonge, P.; Eloka-Eboka, A.C. Transesterification via parametric modelling and optimization of marula (Sclerocarya birrea) seed oil methyl ester synthesis. J. Oleo Sci. 2021, 70, 77–93. [Google Scholar] [CrossRef]
- Olagunju, O.A.; Musonge, P. Production of biodiesel using a membrane reactor to minimize separation cost. Earth Environ. Sci. IOP Conf. Ser. 2017, 78, 012019. [Google Scholar] [CrossRef] [Green Version]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Valorisation of high acid value waste cooking oil into biodiesel using supercritical methanolysis: Experimental assessment and statistical optimisation on typical Egyptian feedstock. Energy 2018, 162, 408–420. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Chen, W.H.; Kusumo, F.; Dharma, S.; Sebayang, A.H. Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Dharma, S.M.H.H.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Silitonga, A.S.; Kusumo, F.; Mahlia, T.M.I. Optimization of biodiesel production process for mixed Jatropha curcas–Ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- Elkelawy, M.; Bastawissi, H.A.E.; Esmaeil, K.K.; Radwan, A.M.; Panchal, H.; Sadasivuni, K.K.; Ponnamma, D.; Walvekar, R. Experimental studies on the biodiesel production parameters optimization of sunflower and soybean oil mixture and DI engine combustion, performance, and emission analysis fueled with diesel/biodiesel blends. Fuel 2019, 255, 115791. [Google Scholar] [CrossRef]
- Li, Z.; Ding, S.; Chen, C.; Qu, S.; Du, L.; Lu, J.; Ding, J. Recyclable Li/NaY zeolite as a heterogeneous alkaline catalyst for biodiesel production: Process optimization and kinetics study. Energy Convers. Manag. 2019, 192, 335–345. [Google Scholar] [CrossRef]



| Parameters | Symbol | −1 | 0 | 1 |
|---|---|---|---|---|
| Temperature (°C) | X1 | 60 | 65 | 70 |
| Reaction time (minutes) | X2 | 60 | 90 | 120 |
| Molar ratio | X3 | 3:1 | 4:1 | 6:1 |
| Catalyst concentration | X4 | 1 | 2.5 | 4 |
| Output | ||||
| Biodiesel yield (%) | Y |
| Analysis | Method | Result |
|---|---|---|
| Pore volume | BET | 0.152 cm3/g |
| Micro pore volume | BET | 0.121 cm3/g |
| Average pore width | BET | 2.87 nm |
| BET surface area | BET | 240.51 m2/g |
| Active concentration sites | TPD-CO2 | 1.436 mmol/g |
| Standard Runs | Randomized Runs | Coded Factors | Response Y | |||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | |||
| 1 | 29 | −1 | −1 | −1 | −1 | 62 |
| 2 | 5 | 1 | −1 | −1 | −1 | 90 |
| 3 | 14 | −1 | 1 | −1 | −1 | 60 |
| 4 | 12 | −1 | 1 | −1 | −1 | 75 |
| 5 | 13 | −1 | −1 | 1 | −1 | 49 |
| 6 | 2 | 1 | −1 | 1 | −1 | 66 |
| 7 | 18 | −1 | 1 | 1 | −1 | 55 |
| 8 | 8 | 1 | 1 | 1 | −1 | 79 |
| 9 | 24 | −1 | −1 | −1 | 1 | 84 |
| 10 | 27 | 1 | −1 | −1 | 1 | 92 |
| 11 | 17 | −1 | 1 | −1 | 1 | 89 |
| 12 | 11 | 1 | 1 | −1 | 1 | 95 |
| 13 | 6 | −1 | −1 | 1 | 1 | 50 |
| 14 | 10 | 1 | −1 | 1 | 1 | 60 |
| 15 | 30 | −1 | 1 | 1 | 1 | 78 |
| 16 | 3 | 1 | 1 | 1 | 1 | 74 |
| 17 | 25 | −2 | 0 | 0 | 0 | 50 |
| 18 | 22 | 2 | 0 | 0 | 0 | 60 |
| 19 | 4 | 0 | −2 | 0 | 0 | 78 |
| 20 | 15 | 0 | 2 | 0 | 0 | 95 |
| 21 | 23 | 0 | 0 | −2 | 0 | 65 |
| 22 | 21 | 0 | 0 | 2 | 0 | 48 |
| 23 | 1 | 0 | 0 | 0 | −2 | 62 |
| 24 | 9 | 0 | 0 | 0 | 2 | 94 |
| 25 | 20 | 0 | 0 | 0 | 0 | 93 |
| 26 | 7 | 0 | 0 | 0 | 0 | 94 |
| 27 | 26 | 0 | 0 | 0 | 0 | 92 |
| 28 | 28 | 0 | 0 | 0 | 0 | 95 |
| 29 | 19 | 0 | 0 | 0 | 0 | 93 |
| 30 | 16 | 0 | 0 | 0 | 0 | 96 |
| Analysis of Variance Table [Partial Sum of Squares-Type III] | ||||||
|---|---|---|---|---|---|---|
| Sum of | Mean | F | p-Value | |||
| Source | Squares | df | Square | Value | Prob > F | |
| Model | 8183.89 | 14 | 584.56 | 24.05 | <0.0001 | significant |
| X1: A-Temperature | 2412.32 | 1 | 2412.32 | 99.26 | <0.0001 | |
| X2: B-Reaction time | 1199.92 | 1 | 1199.92 | 49.37 | <0.0001 | |
| X3: C-Molar ratio | 310.32 | 1 | 310.32 | 12.77 | 0.0028 | |
| X4: D-Catalyst concentration | 933.75 | 1 | 933.75 | 38.42 | <0.0001 | |
| X1X2: AB | 31.08 | 1 | 31.08 | 1.28 | 0.2759 | |
| X1X3: AC | 6.63 | 1 | 6.63 | 0.27 | 0.6091 | |
| X1X4: AD | 253.61 | 1 | 253.61 | 10.44 | 0.0056 | |
| X2X3: BC | 303.63 | 1 | 303.63 | 12.49 | 0.0030 | |
| X2X4: BD | 145.81 | 1 | 145.81 | 6.00 | 0.0271 | |
| X3X4: CD | 222.76 | 1 | 222.76 | 9.17 | 0.0085 | |
| X12: A2 | 2223.26 | 1 | 2223.26 | 91.48 | <0.0001 | |
| X22: B2 | 61.97 | 1 | 61.97 | 2.55 | 0.1311 | |
| X32: C2 | 643.77 | 1 | 643.77 | 26.49 | 0.0001 | |
| X42: D2 | 361.05 | 1 | 361.05 | 14.86 | 0.0016 | |
| Residual | 364.54 | 15 | 24.30 | |||
| Lack of Fit | 360.66 | 10 | 36.07 | 46.54 | 0.1533 | Not significant |
| Pure Error | 3.88 | 5 | 0.78 | |||
| Cor Total | 8548.43 | 29 | ||||
| Parameter | Goal | Experimental Region | Optimum Condition | ||
|---|---|---|---|---|---|
| Lower | Upper | Theoretical Value | Experimental Value | ||
| Temperature (°C) | In range | 60 | 70 | 65 | 65 |
| Reaction time (min) | In range | 60 | 120 | 90 | 90 |
| Catalyst concentration | target | - | 3 | 3 | 3 |
| Molar ratio | In range | 3:1 | 6:1 | 4.2:1 | 4.2:1 |
| Yield (%) | Maximize | 97.7 | 96.9 | ||
| Characteristic | Test | Units | ASTM and SANS 1935 Specification Limit | Result |
|---|---|---|---|---|
| Density @ 15 °C | ASTM D7042 | g/mL | 0.86–0.9 | 0.87 |
| Viscosity @ 40 °C | ASTM D7042 | cSt | 3.5–5 | 3.8 |
| Flash point | ASTM D93 | °C | 120 min | 167 |
| Water content | ASTM D6304 | % | 0.05 max | - |
| Total acid number | - | mgKOH/g | 0.5 max | 0.21 |
| Total Contamination | IP 440 | mg/Kg | 24 max | 2 |
| Sulphur | ASTM D4294 | ppm | 10 max | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olagunju, O.A.; Musonge, P.; Kiambi, S.L. Production and Optimization of Biodiesel in a Membrane Reactor, Using a Solid Base Catalyst. Membranes 2022, 12, 674. https://doi.org/10.3390/membranes12070674
Olagunju OA, Musonge P, Kiambi SL. Production and Optimization of Biodiesel in a Membrane Reactor, Using a Solid Base Catalyst. Membranes. 2022; 12(7):674. https://doi.org/10.3390/membranes12070674
Chicago/Turabian StyleOlagunju, Olusegun Ayodeji, Paul Musonge, and Sammy Lewis Kiambi. 2022. "Production and Optimization of Biodiesel in a Membrane Reactor, Using a Solid Base Catalyst" Membranes 12, no. 7: 674. https://doi.org/10.3390/membranes12070674
APA StyleOlagunju, O. A., Musonge, P., & Kiambi, S. L. (2022). Production and Optimization of Biodiesel in a Membrane Reactor, Using a Solid Base Catalyst. Membranes, 12(7), 674. https://doi.org/10.3390/membranes12070674







