Preparation of Alumina-Sphere-Supported Potassium Chabazite Zeolite Membrane with Excellent Potassium Extraction Performance at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of KCHA Zeolite Membrane
2.3. Modification of KCHA Zeolite Membrane
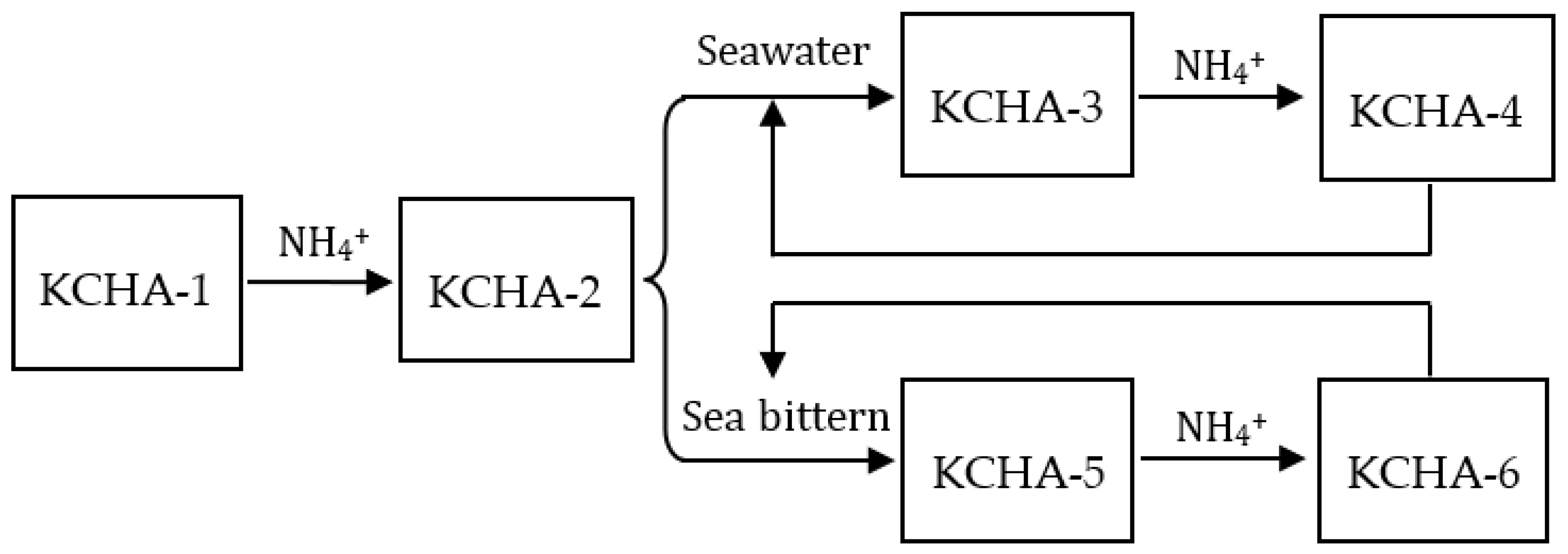
2.4. Potassium Extraction and Removal Process of KCHA Zeolite Membrane
2.5. Characterization
3. Results and Discussion
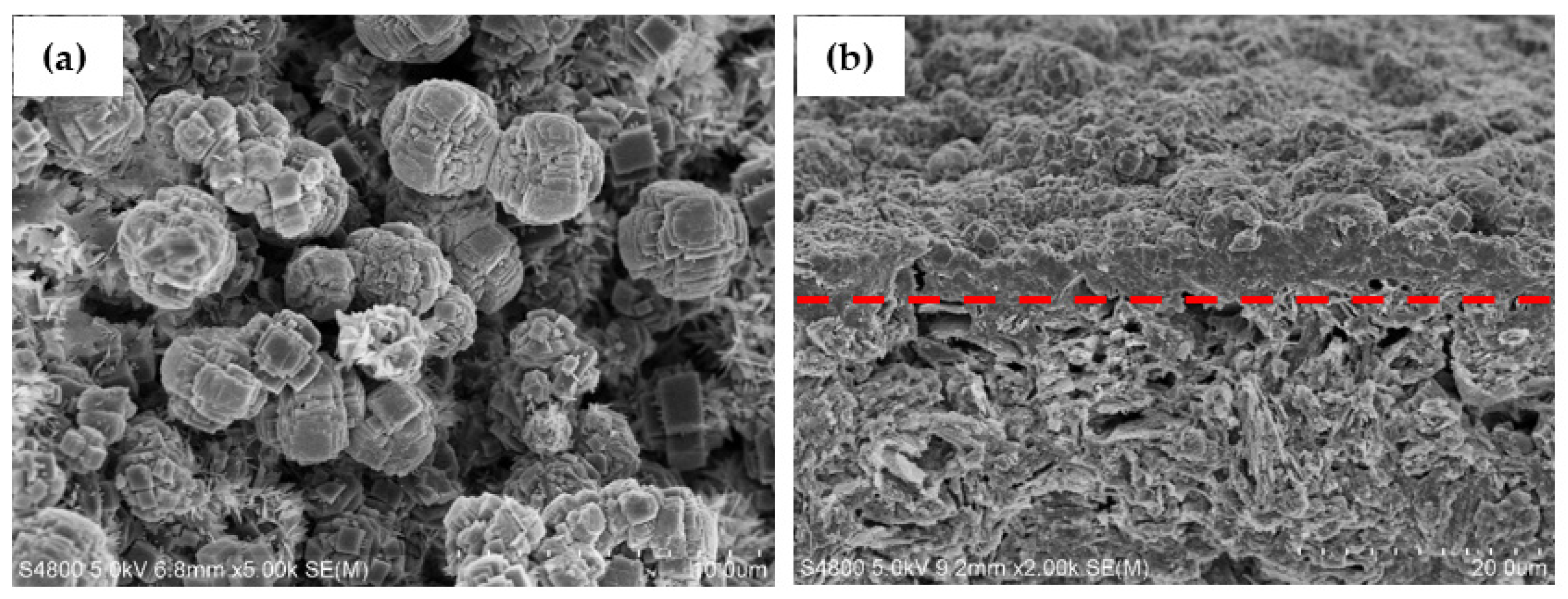
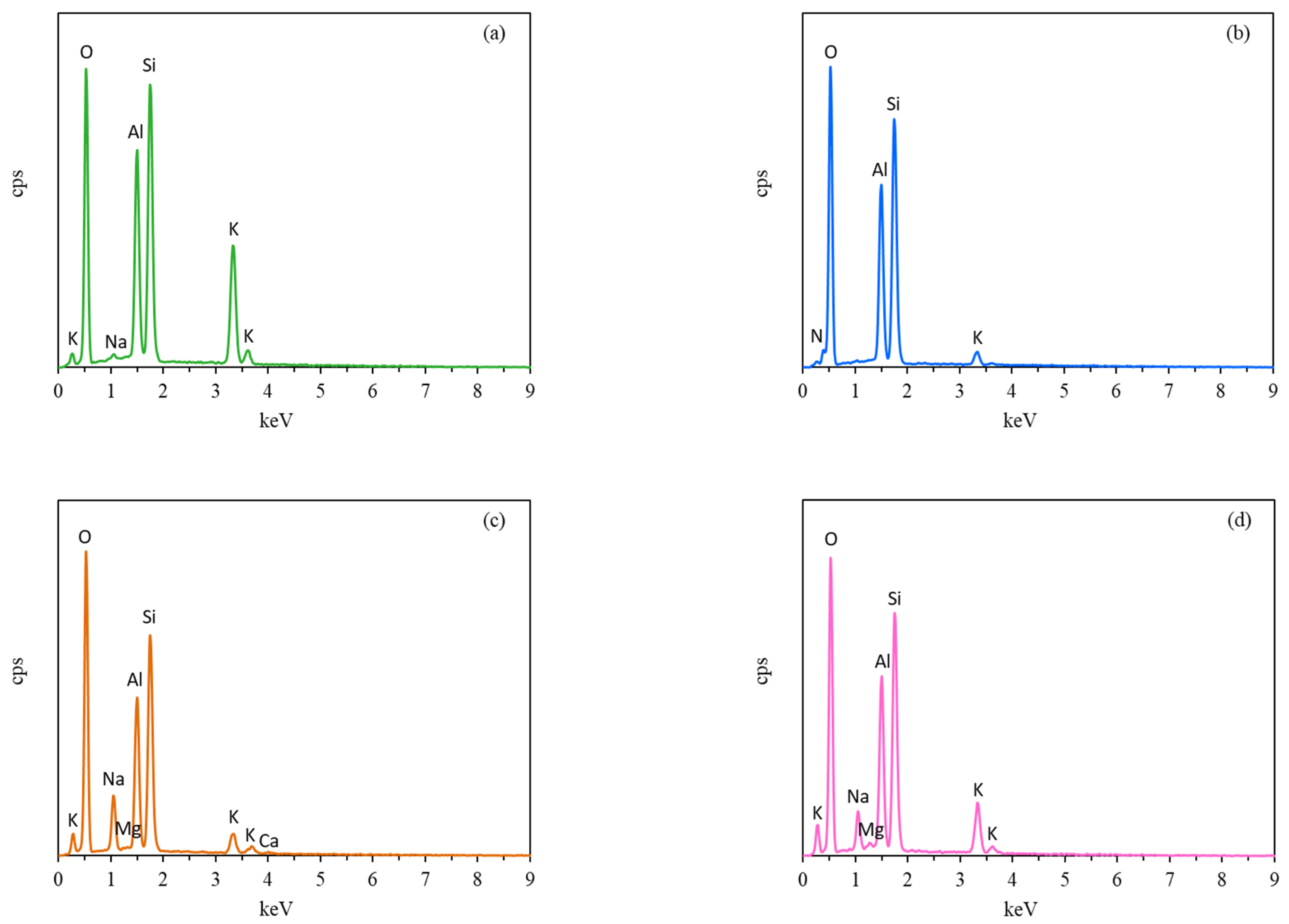
3.1. Characterization of KCHA Zeolite Membrane
3.2. Potassium Extraction Performance of KCHA Zeolite Membrane
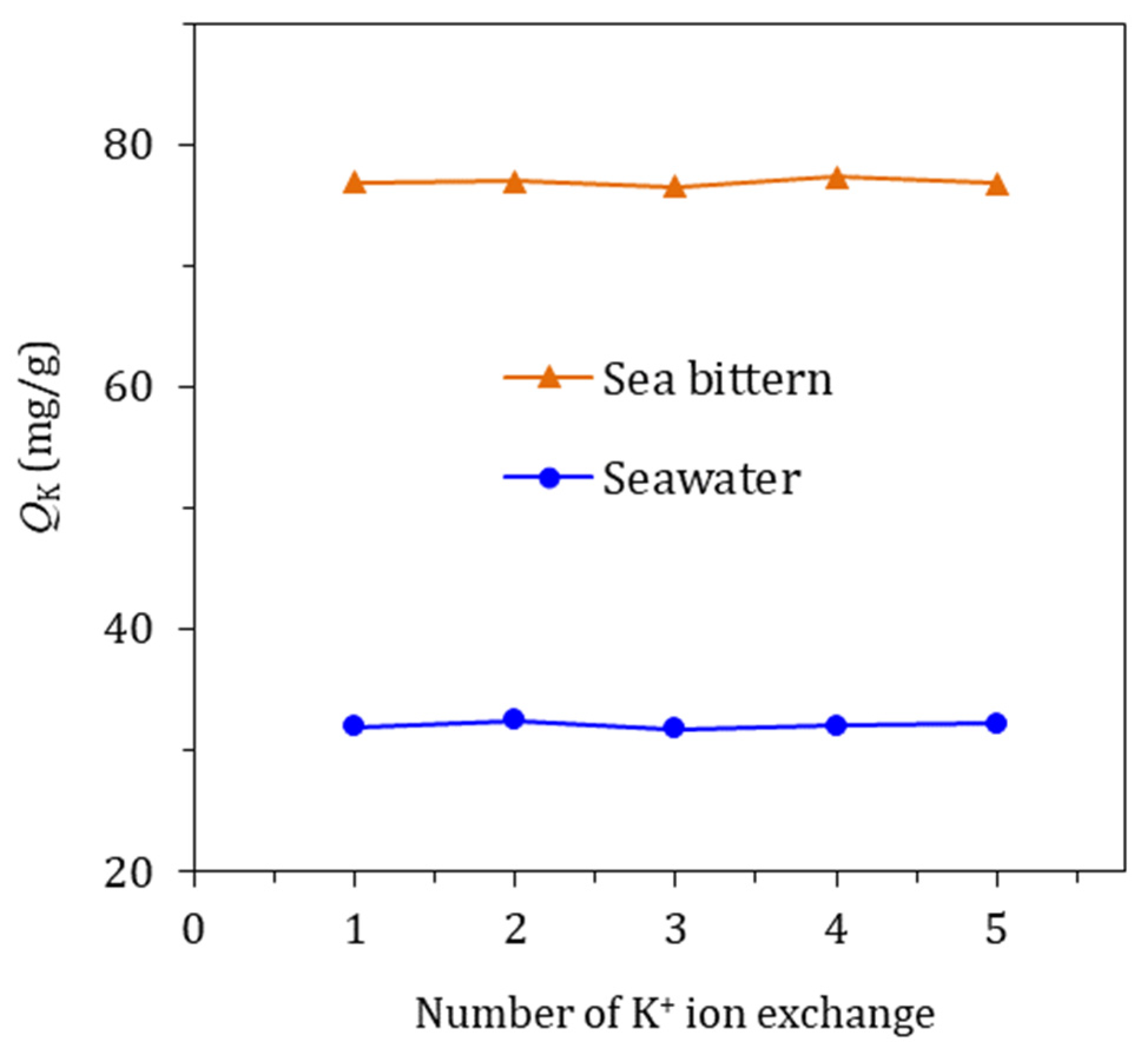
3.3. Reusability of KCHA Zeolite Membrane
3.4. Selective Ion-Exchange Mechanism of KCHA Zeolite Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.H.; Xia, G.M.; Wu, Q.; Chen, W.; Lin, W.H.; Zhang, Z.X.; Chen, Y.L.; Chen, T.T.; Siddique, K.H.M.; Chi, D.C. Zeolite increases grain yield and potassium balance in paddy fields. Geoderma 2022, 405, 115397. [Google Scholar] [CrossRef]
- Rawashdeh, R.A.; Maxwell, P. Analysing the world potash industry. Resour. Policy 2014, 41, 143–151. [Google Scholar] [CrossRef]
- Li, Y.H.; Zheng, J.L.; Wu, Q.; Gong, X.M.; Zhang, Z.X.; Chen, Y.L.; Chen, T.T.; Siddique, K.H.M.; Chi, D.C. Zeolite increases paddy soil potassium fixation, partial factor productivity, and potassium balance under alternate wetting and drying irrigation. Agric. Water Manag. 2022, 260, 107294. [Google Scholar] [CrossRef]
- Shi, W.; Nie, P.F.; Shang, X.H.; Yang, J.M.; Xie, Z.Z.; Xu, R.; Liu, J.Y. Berlin green-based battery deionization-highly selective potassium recovery in seawater. Electrochim. Acta 2019, 310, 104–112. [Google Scholar] [CrossRef]
- Ji, G.Z.; Wang, W.J.; Chen, H.H.; Yang, S.Y.; Sun, J.; Fu, W.; Yang, B.; Huang, Z.Q. Sustainable potassium chloride production from concentrated KCl brine via a membrane-promoted crystallization process. Desalination 2022, 521, 115389. [Google Scholar] [CrossRef]
- Guo, X.F.; Li, D.; Liu, J.L.; Wang, Z.R.; Wang, J.; Zhao, Y.Y.; Yuan, J.S. Separation of sodium and potassium using adsorption–elution/crystallization scheme from bittern. Chem. Eng. Res. Des. 2020, 16, 72–81. [Google Scholar] [CrossRef]
- An, S.S.; Liu, J.; Wang, J.H.; Wang, M.C.; Ji, Z.Y.; Qi, S.S.; Yuan, J.S. Synthesis and characterization of a plat sheet potassium ion sieve membrane and its performances for separation potassium. Sep. Purif. Technol. 2019, 212, 834–842. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, A.B.; Sun, J.; Ye, Y.; Chen, X.G.; Xia, M.S. Application of ocean manganese nodules for the adsorption of potassium ions from seawater. Miner. Eng. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Ling, R.J.; Chen, W.; Hou, J. Preparation of modified MFI (ZSM-5 and silicalite-1) zeolites for potassium extraction from seawater. Particuology 2018, 36, 190–192. [Google Scholar] [CrossRef]
- Noroozi, R.; Al-Musawi, T.J.; Kazemian, H.; Kalhori, E.M.; Zarrabi, M. Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. J. Water Process Eng. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- Araki, S.; Li, T.; Li, K.; Yamamoto, H. Preparation of zeolite hollow fibers for high-efficiency cadmium removal from waste water. Sep. Purif. Technol. 2019, 221, 393–398. [Google Scholar] [CrossRef]
- Meng, T.; Ren, N.; Ma, Z. Silicalite-1@Cu-ZSM-5 core-shell catalyst for N2O decomposition. J. Mol. Catal. A-Chem. 2015, 404–405, 233–239. [Google Scholar] [CrossRef]
- Flores, C.G.; Schneider, H.; Dornelles, J.S.; Gomes, L.B.; Marcilio, N.R.; Melo, P.J. Synthesis of potassium zeolite from rice husk ash as a silicon source. Clean. Eng. Technol. 2021, 4, 100201. [Google Scholar] [CrossRef]
- Tong, C.H.; Hou, J.; Yang, C.P. Preparation of NH4+-loaded merlinoite for extracting potassium continuously at room temperature. J. Ind. Eng. Chem. 2019, 80, 11–16. [Google Scholar] [CrossRef]
- Hou, J.; Yuan, J.S.; Xu, J.; Sun, L.H. Synthesis and characterization of K-phillipsite (K-PHI) membrane for potassium extraction from seawater. Micropor. Mesopor. Mat. 2013, 172, 217–221. [Google Scholar] [CrossRef]
- Cao, J.L.; Liu, X.W.; Fu, R.; Tan, Z.Y. Magnetic P zeolites: Synthesis, characterization and the behavior in potassium extraction from seawater. Sep. Purif. Technol. 2008, 63, 92–100. [Google Scholar] [CrossRef]
- Dong, D.Q.; Zhou, Z.Y.; Zhong, J.; Liu, Y.F. K-type mordenite synthesized by acid advancing and its remembering exchange to K+. Chin. J. Inorg. Chem. 2000, 16, 580–584. [Google Scholar]
- Ivanov, V.A.; Timofeevskaja, V.D.; Gavlina, O.T.; Gorshkov, V.I. Dual-temperature reagent-less ion-exchange separations of alkali metal salts on zeolites. Micropor. Mesopor. Mat. 2003, 65, 257–265. [Google Scholar] [CrossRef]
- Torkia, Y.B.; Sghaier, W.; Bouaziz, N.; Lamine, A.B. Xenon adsorption isotherms on chabazite. Statistical physics modeling investigation: Adsorption energy and pore size distributions computation. J. Environ. Chem. Eng. 2021, 9, 104733. [Google Scholar] [CrossRef]
- Alver, B.E.; Sakızcı, M. Hydrogen (H2) adsorption on natural and cation-exchanged clinoptilolite, mordenite and chabazite. Int. J. Hydrog. Energy 2019, 44, 6748–6755. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Chen, X.; Zhu, M.; Zhang, F.; Gui, T.; Hu, N.; Chen, X.; Kita, H. Preparation of chabazite zeolite membranes by a two-stage varying-temperature hydrothermal synthesis for water-ethanol separation. Sep. Purif. Technol. 2020, 234, 116055. [Google Scholar] [CrossRef]
- Krishna, S.H.; Jones, C.B.; Gounder, R. Temperature dependence of Cu(I) oxidation and Cu(II) reduction kinetics in the selective catalytic reduction of NOx with NH3 on Cu-chabazite zeolites. J. Catal. 2021, 404, 873–882. [Google Scholar] [CrossRef]
- Leyva-Ramo, R.; Monsivais-Rocha, J.E.; Aragon-Piña, A.; Berber-Mendoza, M.S.; Guerrero-Coronado, R.M.; Alonso-Davila, P.; Mendoza-Barron, J. Removal of ammonium from aqueous solution by ion exchange on natural and modified chabazite. J. Environ. Manag. 2010, 91, 2662–2668. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.; Hu, N.; Gui, T.; Li, Y.; Zhang, F.; Zhou, R.; Chen, X.; Kita, H. Synthesis of low-silica CHA zeolite chabazite in fluoride media without organic structural directing agents and zeolites. Micropor. Mesopor. Mat. 2014, 196, 270–276. [Google Scholar] [CrossRef]
- Volkov, A.G.; Paula, S.; Deamer, D.W. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem. Bioenerg. 1997, 42, 153–160. [Google Scholar] [CrossRef]
- Kazemimoghadam, M.; Mohammadi, T. Synthesis of MFI zeolite membranes for water desalination. Desalination 2007, 206, 547–553. [Google Scholar] [CrossRef]


| Cation | Seawater | Sea Bittern |
|---|---|---|
| K+ (mg∙mL−1) | 0.38 | 10.67 |
| Na+ (mg∙mL−1) | 10.62 | 39.71 |
| Mg2+ (mg∙mL−1) | 1.28 | 57.59 |
| Ca2+ (mg∙mL−1) | 0.40 | -- |
| Sample | KCHA-1 | KCHA-2 | KCHA-3 | KCHA-4 | KCHA-5 | KCHA-6 | |
|---|---|---|---|---|---|---|---|
| Element | |||||||
| N (wt.%) | -- | 7.54 | -- | 7.64 | -- | 7.96 | |
| K (wt.%) | 18.33 | 2.75 | 4.25 | 1.06 | 9.46 | 1.77 | |
| Na (wt.%) | 0.31 | -- | 5.57 | 0.24 | 3.46 | 0.16 | |
| Mg (wt.%) | -- | -- | 0.11 | -- | 0.36 | ||
| Ca (wt.%) | -- | -- | 1.63 | -- | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Wei, H.; Hou, J. Preparation of Alumina-Sphere-Supported Potassium Chabazite Zeolite Membrane with Excellent Potassium Extraction Performance at Room Temperature. Membranes 2022, 12, 604. https://doi.org/10.3390/membranes12060604
Ouyang J, Wei H, Hou J. Preparation of Alumina-Sphere-Supported Potassium Chabazite Zeolite Membrane with Excellent Potassium Extraction Performance at Room Temperature. Membranes. 2022; 12(6):604. https://doi.org/10.3390/membranes12060604
Chicago/Turabian StyleOuyang, Jie, Heng Wei, and Jin Hou. 2022. "Preparation of Alumina-Sphere-Supported Potassium Chabazite Zeolite Membrane with Excellent Potassium Extraction Performance at Room Temperature" Membranes 12, no. 6: 604. https://doi.org/10.3390/membranes12060604
APA StyleOuyang, J., Wei, H., & Hou, J. (2022). Preparation of Alumina-Sphere-Supported Potassium Chabazite Zeolite Membrane with Excellent Potassium Extraction Performance at Room Temperature. Membranes, 12(6), 604. https://doi.org/10.3390/membranes12060604





