Platelet-Membrane-Encapsulated Carvedilol with Improved Targeting Ability for Relieving Myocardial Ischemia–Reperfusion Injury
Abstract
1. Introduction
2. Materials and Methods
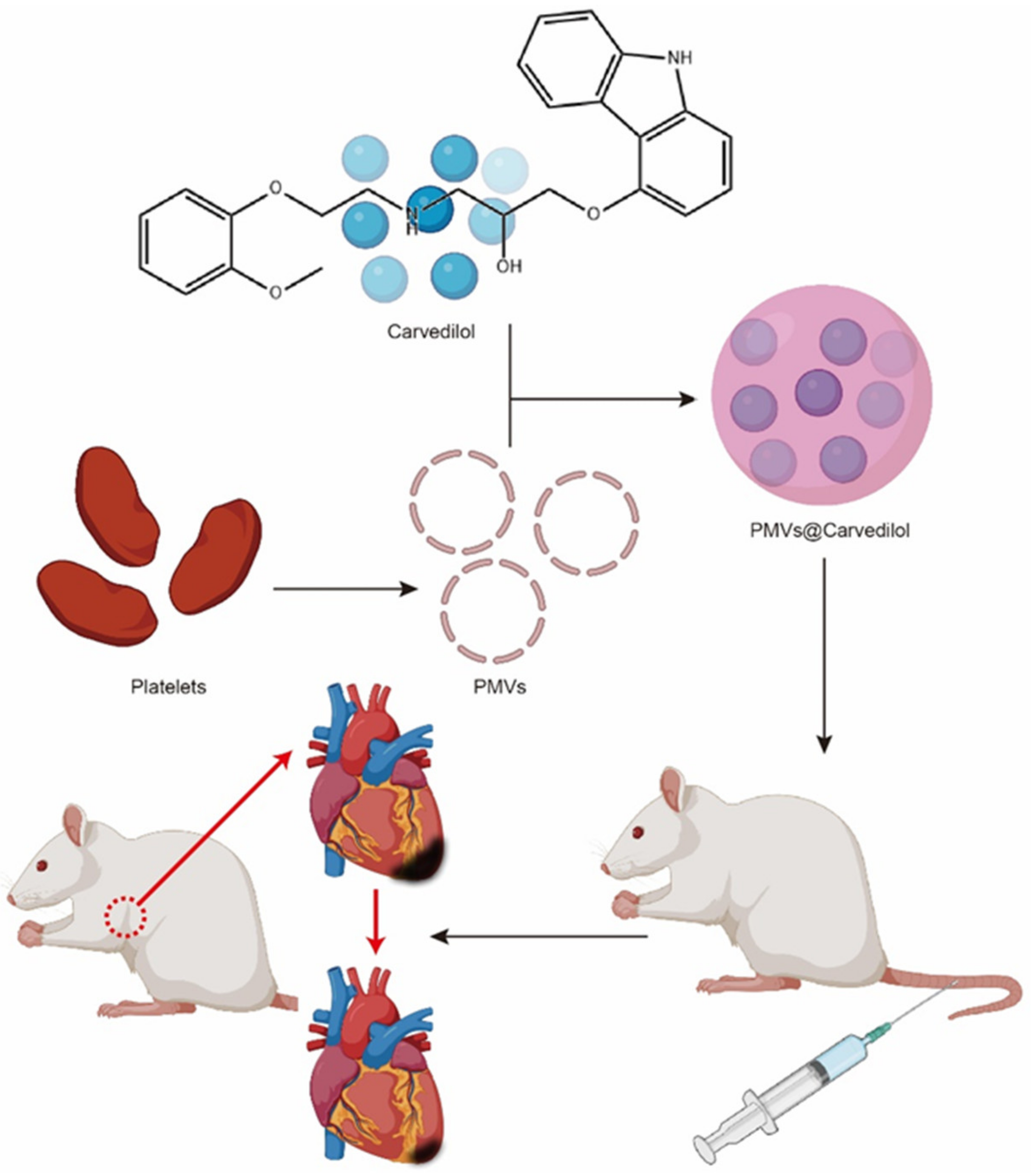
2.1. Separation of Platelets and Generation of PMVs
2.2. Preparation and Toxicity Verification of PMVs@Carvedilol
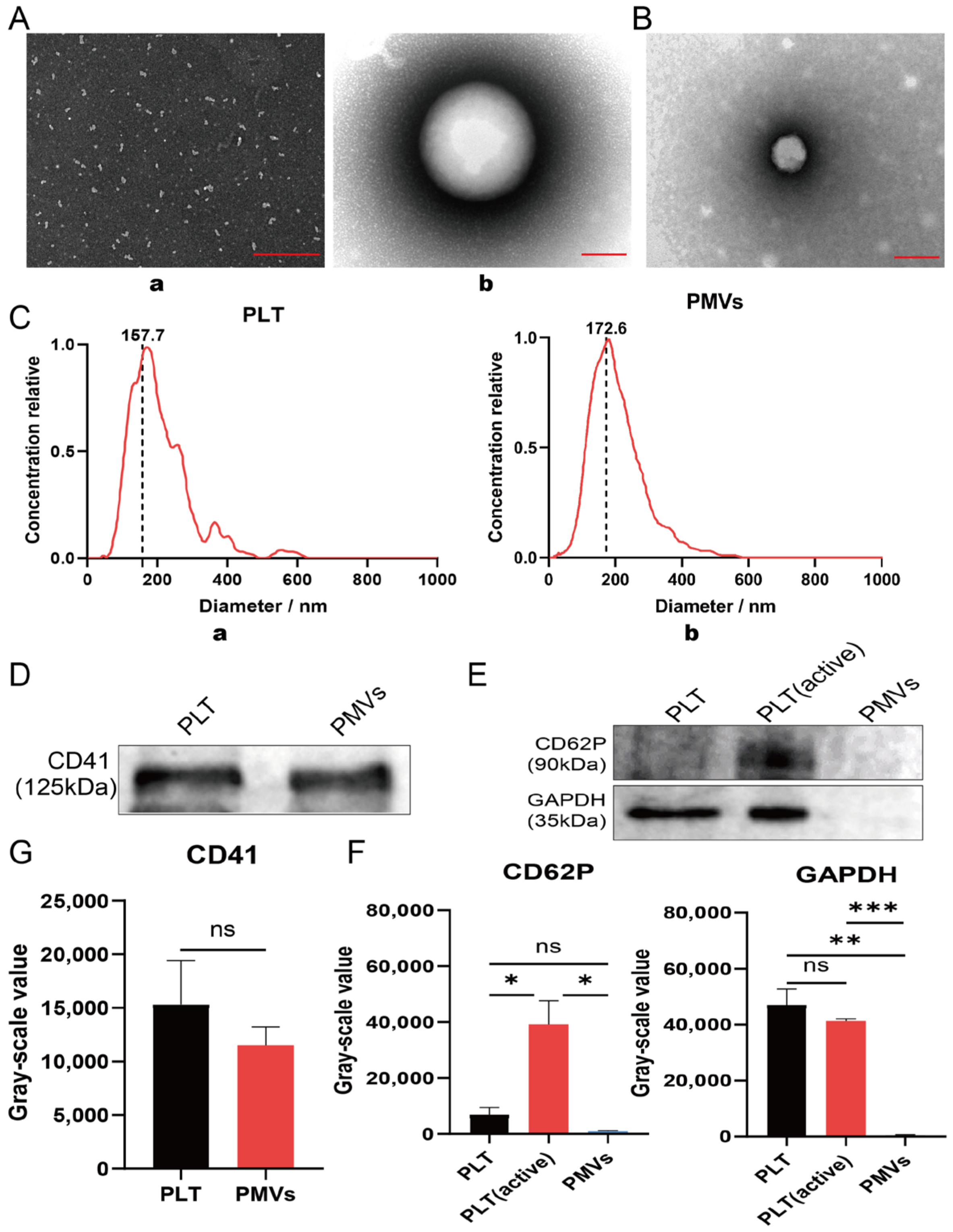
2.3. Detection of Platelet Membrane Vesicle-Specific Surface Markers
2.4. PMV Target Validation
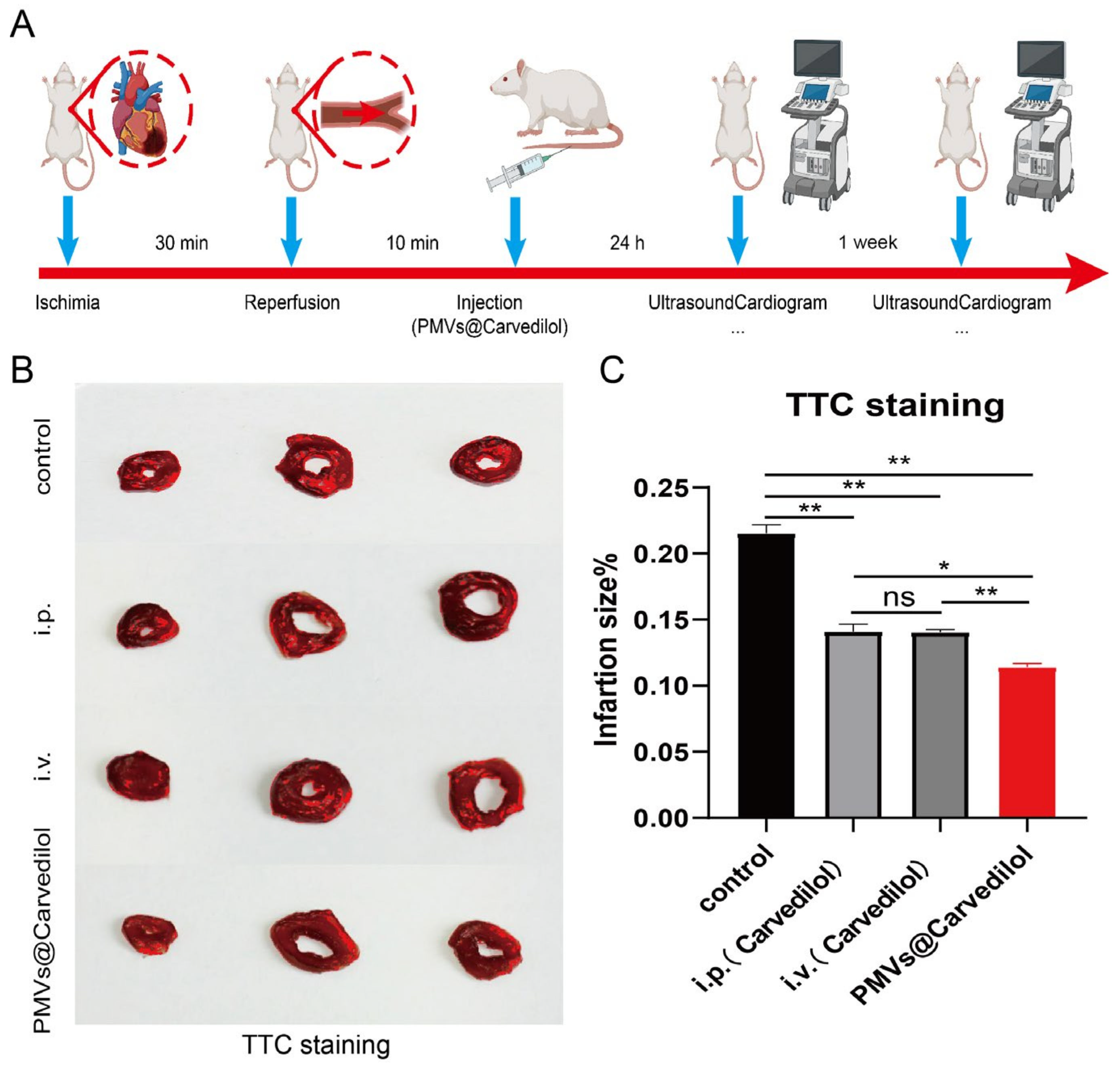
2.5. In Vivo Ischemia/Reperfusion Rat Model
2.6. Fluorescence Imaging Analysis
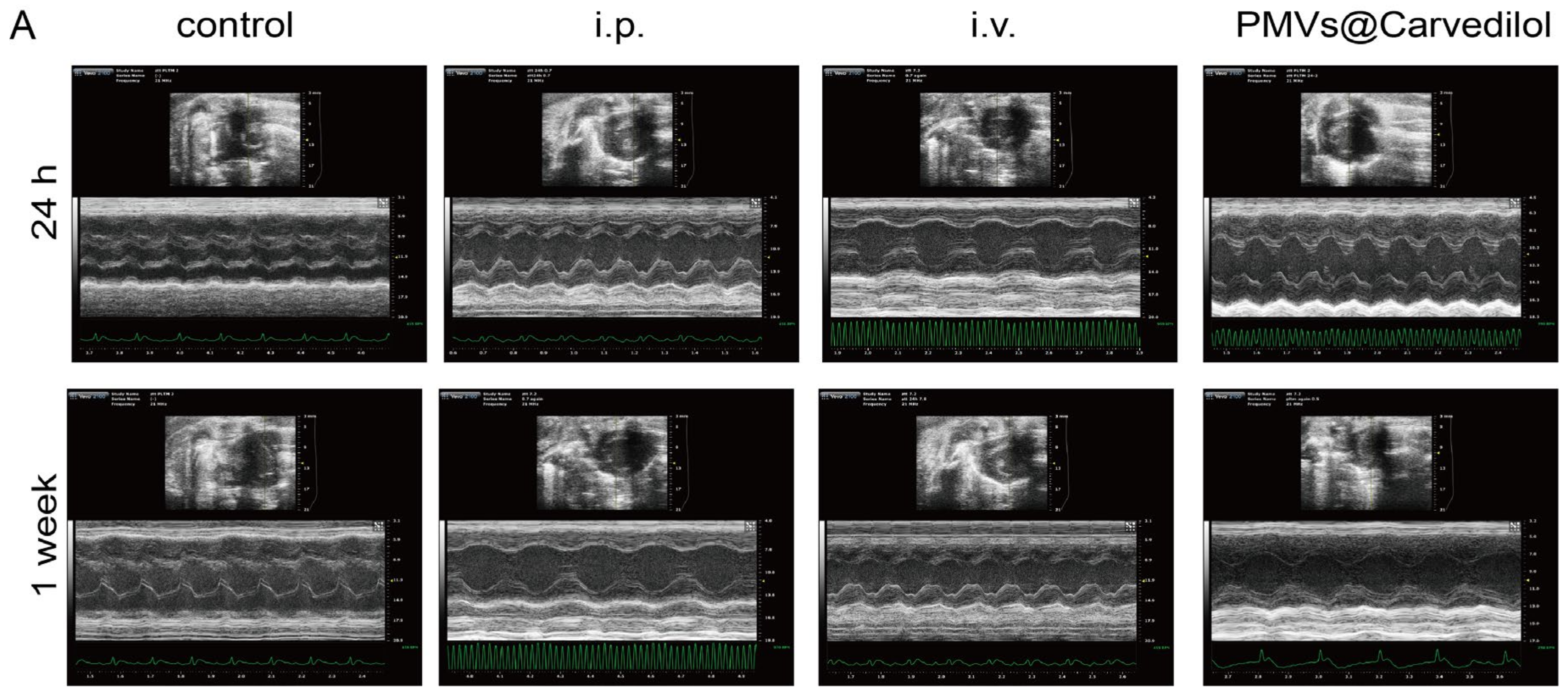
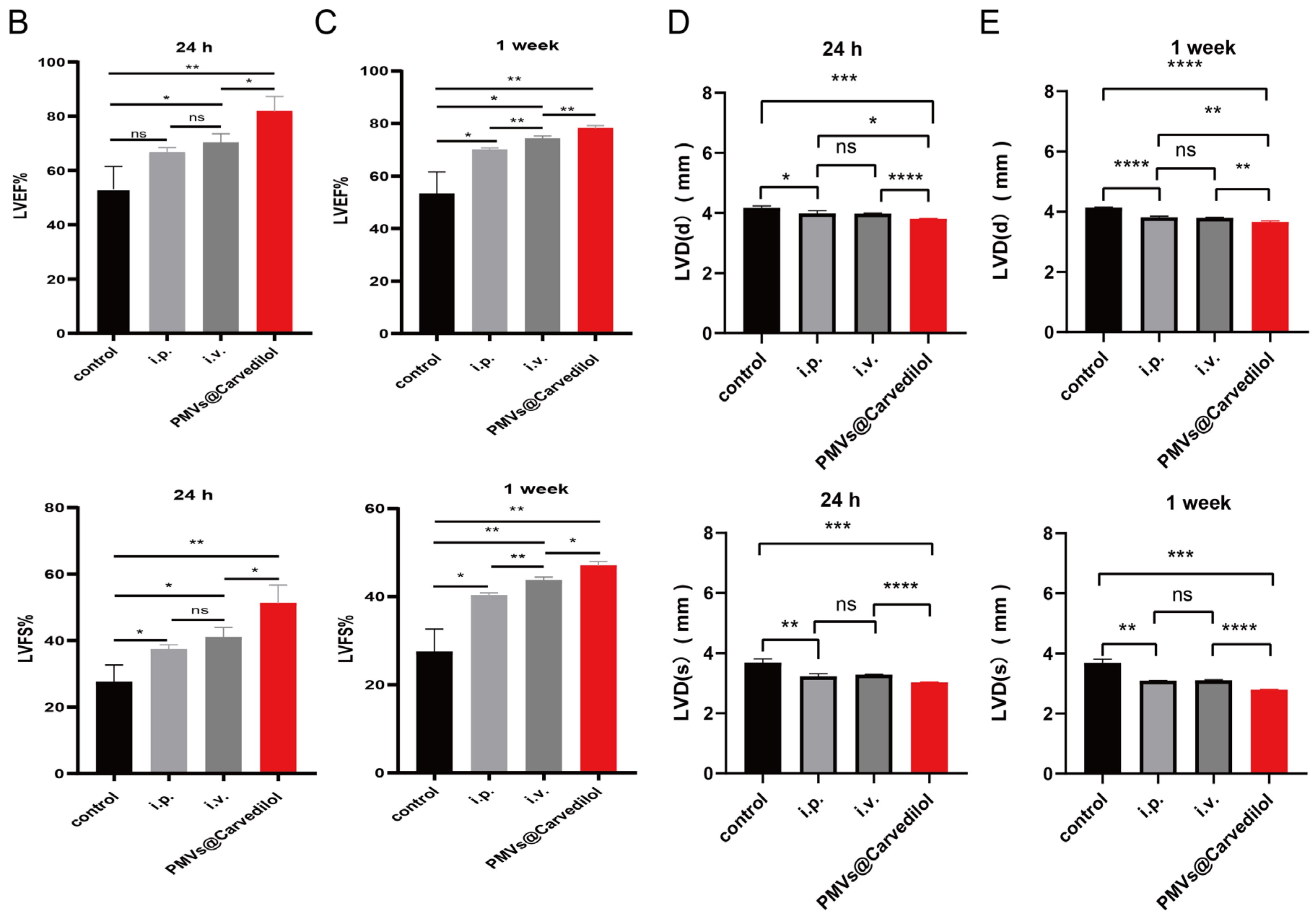
2.7. Cardiac Function Assessment
2.8. TTC Staining
2.9. Statistical Analysis
2.10. Animal Randomization Method
3. Results
3.1. Characterization of Platelet and PMVs In Vitro
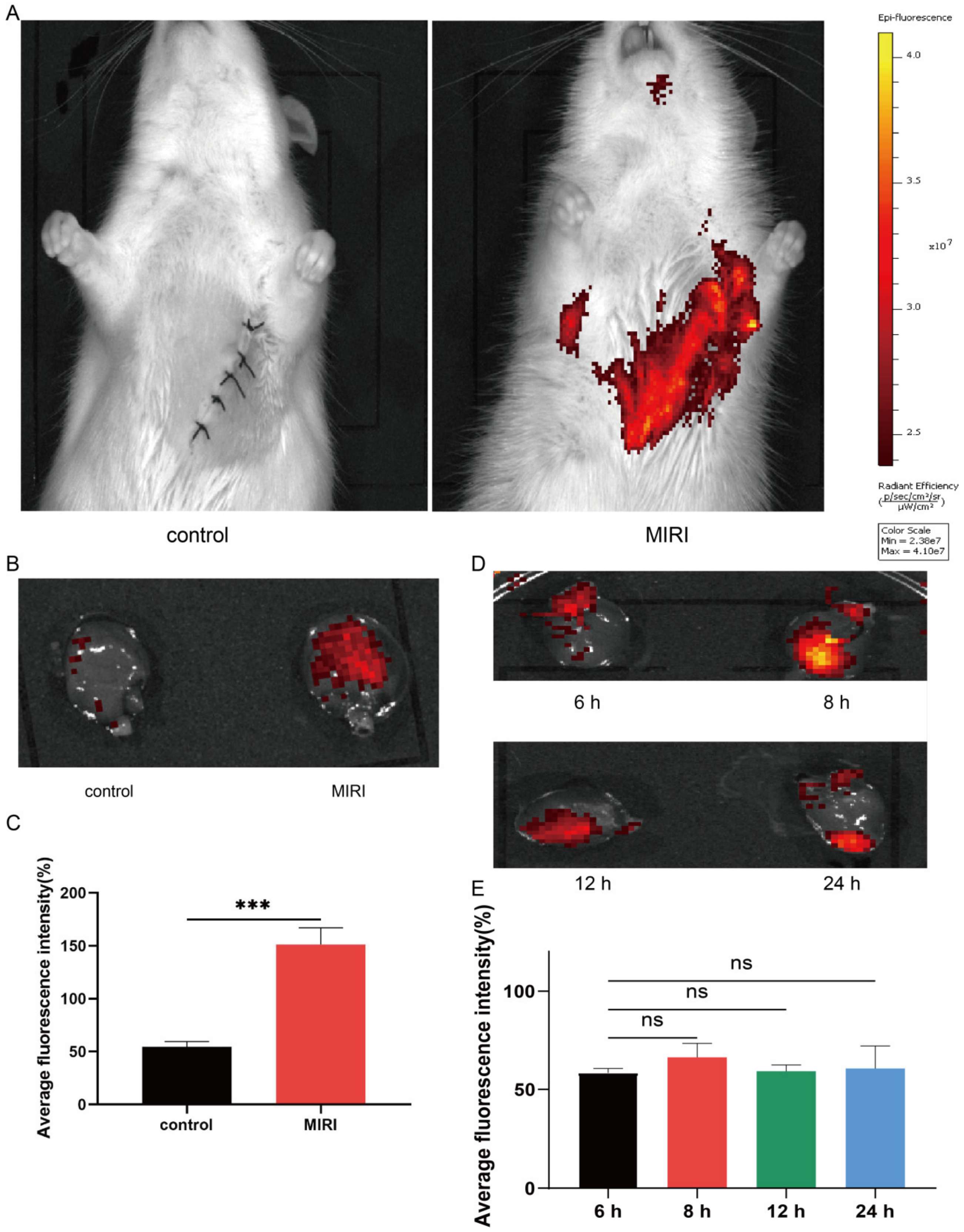
3.2. Intravenously Injected Platelets Targeting MIRI
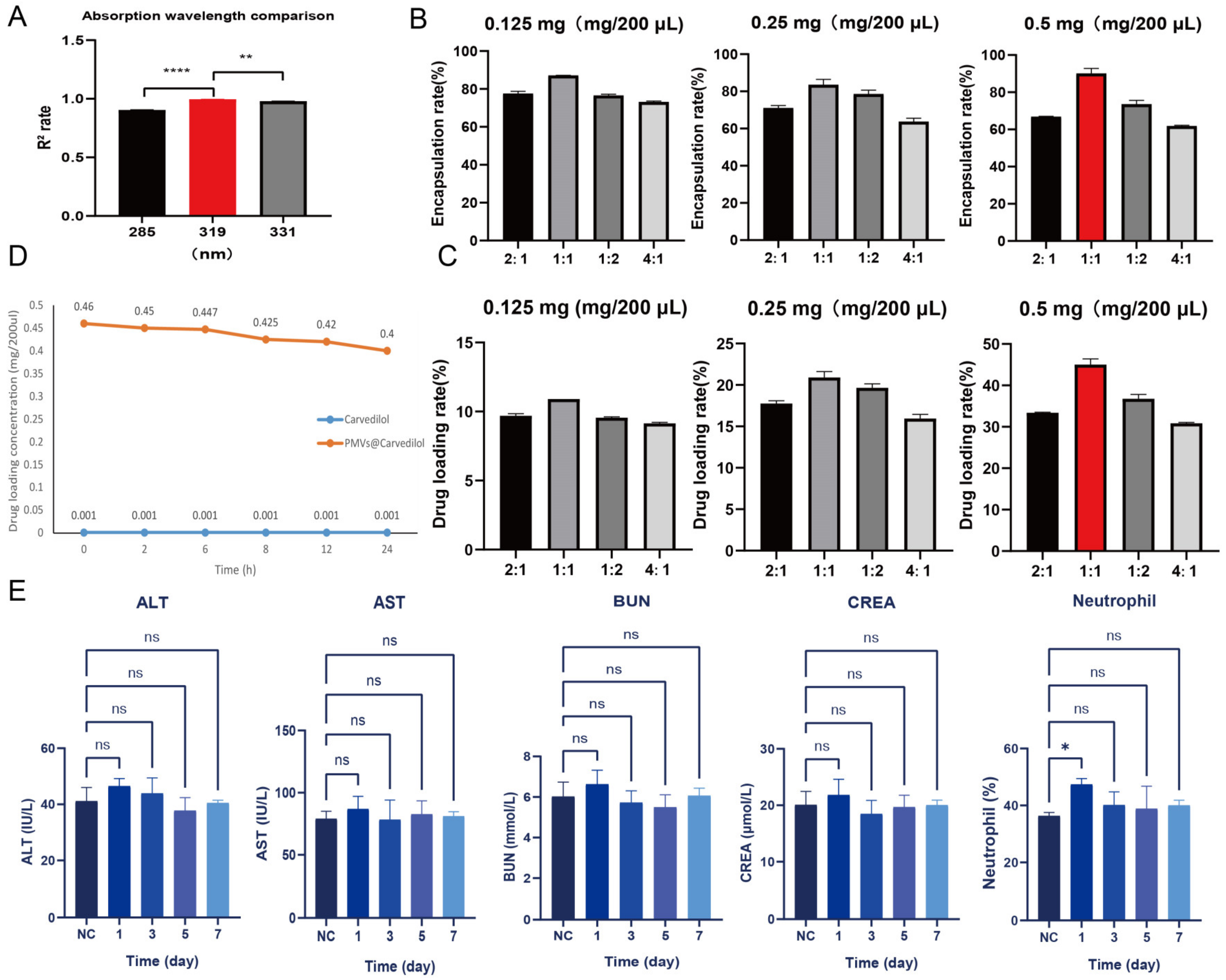
3.3. Determination of Optimal Drug Encapsulation and Loading Rates
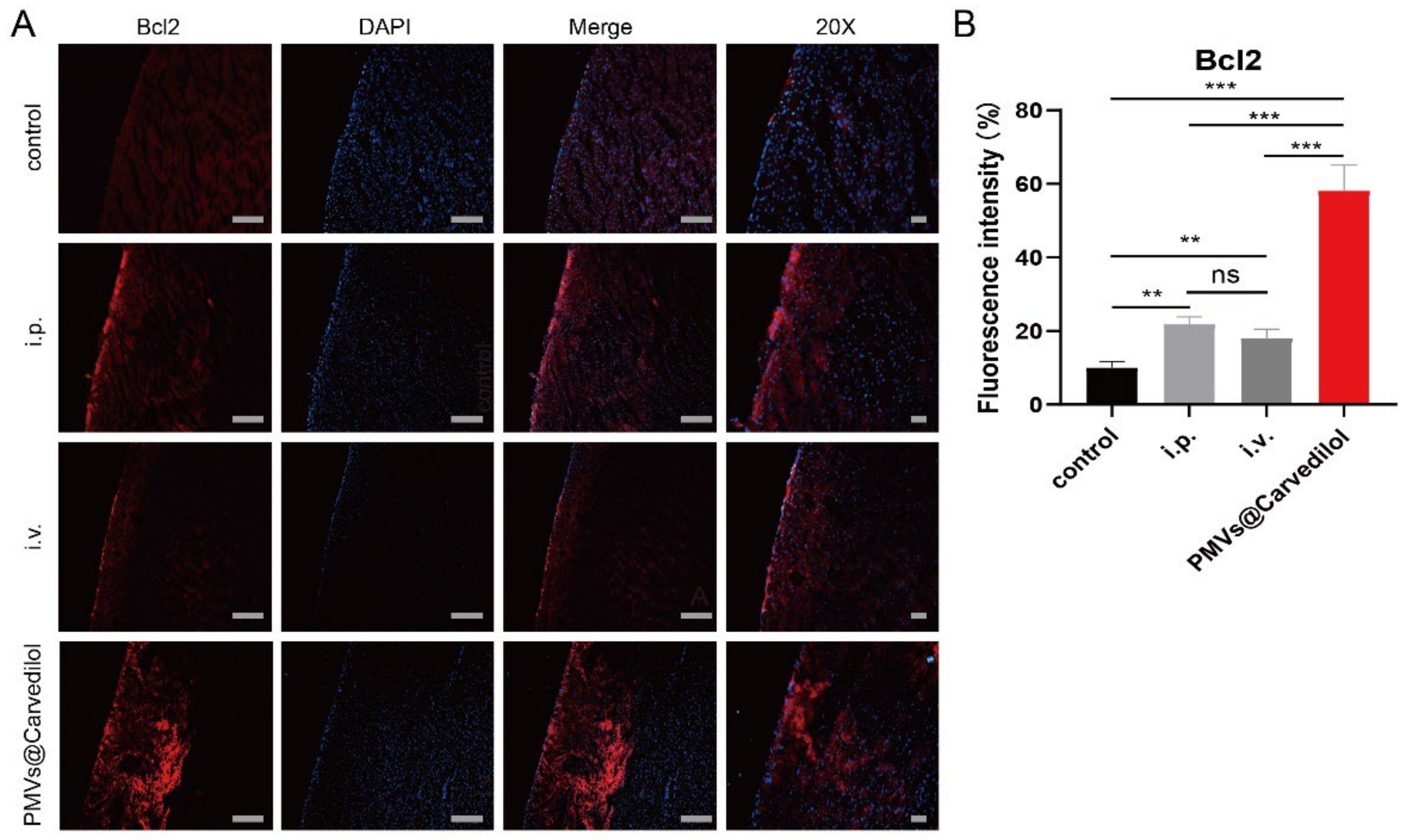
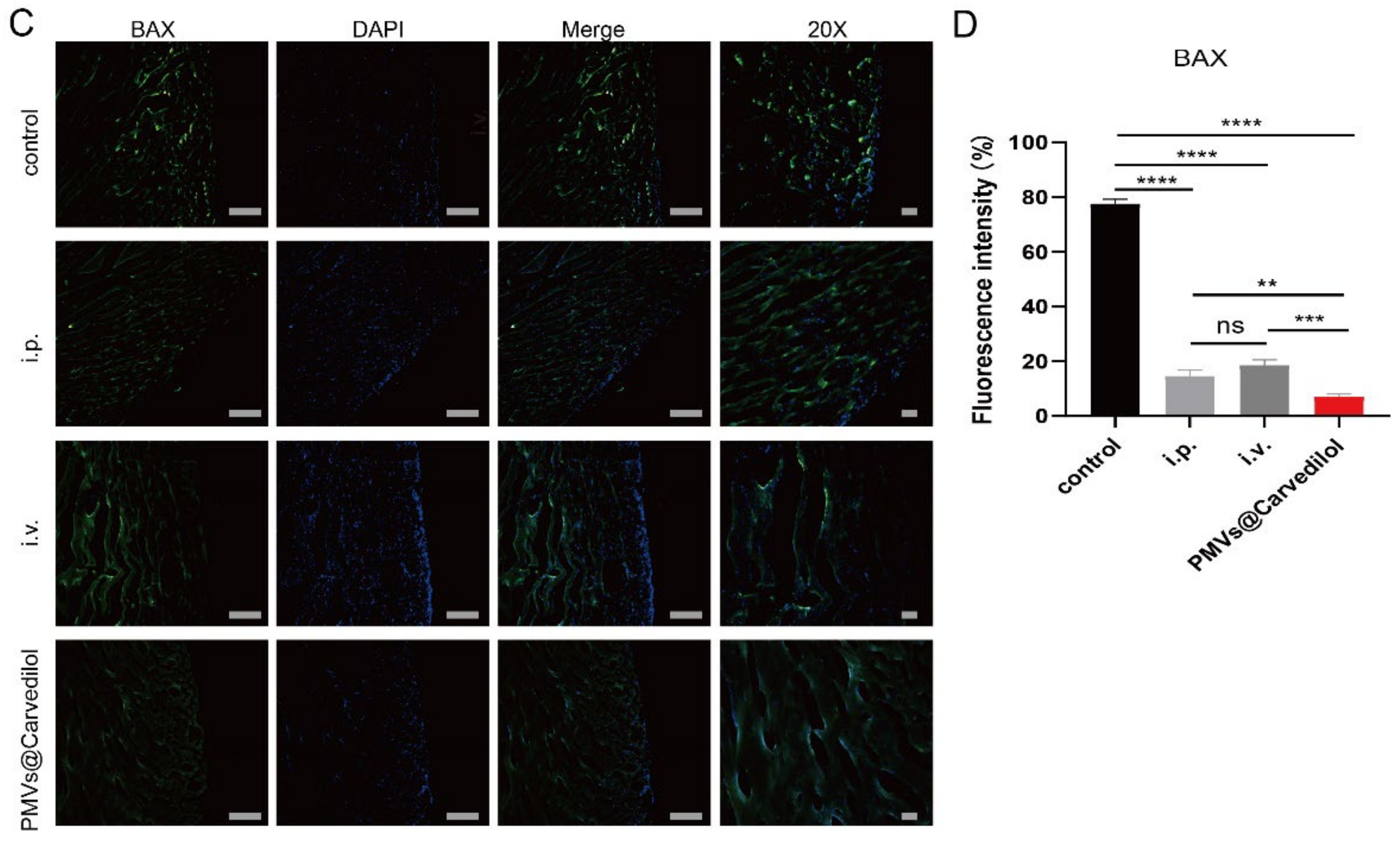
3.4. Compared with Other Administration Methods, PMVs@Carvedilol Can Better Reduce Infarct Size, Reduce Myocardial Cell Apoptosis, and Improve Cardiac Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, T.P., Jr.; Covell, J.W.; Sonnenblick, E.H.; Ross, J., Jr.; Braunwald, E. Control of myocardial oxygen consumption: Relative influence of contractile state and tension development. J. Clin. Investig. 1968, 47, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Treatment of myocardial ischemia/reperfusion injury by ischemic and pharmacological postconditioning. Compr. Physiol. 2015, 5, 1123–1145. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Botker, H.E.; Engstrom, T.; Erlinge, D.; Heusch, G.; Ibanez, B.; Kloner, R.A.; Ovize, M.; Yellon, D.M.; Garcia-Dorado, D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: Trials and tribulations. Eur. Heart J. 2017, 38, 935–941. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Boag, S.E.; Andreano, E.; Spyridopoulos, I. Lymphocyte communication in myocardial ischemia/reperfusion injury. Antioxid. Redox Signal. 2017, 26, 660–675. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; He, S.; Sun, G.; Sun, X. Targeting calcium homeostasis in myocardial ischemia/reperfusion injury: An overview of regulatory mechanisms and therapeutic reagents. Front. Pharmacol. 2020, 11, 872. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: Implications for pharmacological cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1341–H1352. [Google Scholar] [CrossRef]
- Lippi, G.; Franchini, M.; Cervellin, G. Diagnosis and management of ischemic heart disease. Semin. Thromb. Hemost. 2013, 39, 202–213. [Google Scholar] [CrossRef]
- Rubak, P.; Nissen, P.H.; Kristensen, S.D.; Hvas, A.M. Investigation of platelet function and platelet disorders using flow cytometry. Platelets 2016, 27, 66–74. [Google Scholar] [CrossRef]
- Xu, Y.; Huo, Y.; Toufektsian, M.C.; Ramos, S.I.; Ma, Y.; Tejani, A.D.; French, B.A.; Yang, Z. Activated platelets contribute importantly to myocardial reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H692–H699. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Chen, J.; Liu, Y.; Cheng, X.; Yang, F.; Gu, N. Platelet membrane biomimetic magnetic nanocarriers for targeted delivery and in situ generation of nitric oxide in early ischemic stroke. ACS Nano 2020, 14, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Hu, M.; Wang, Z.; Zhang, W.; Li, J.; Yang, H.; Lin, J.; Xing, B. Recent advances of membrane-cloaked nanoplatforms for biomedical applications. Bioconjug. Chem. 2018, 29, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, R.; Yodsanit, N.; Ye, M.; Wang, Y.; Wang, B.; Guo, L.-W.; Kent, K.C.; Gong, S. Hydrogen peroxide-responsive platelet membrane-coated nanoparticles for thrombus therapy. Biomater. Sci. 2021, 9, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zuo, H.; Chen, B.; Wang, R.; Ahmed, A.; Hu, Y.; Ouyang, J. Erratum: Doxorubicin-loaded platelets as a smart drug delivery system: An improved therapy for lymphoma. Sci. Rep. 2017, 7, 44974. [Google Scholar] [CrossRef]
- Li, Q.-R.; Xu, H.-Z.; Xiao, R.-C.; Liu, Y.; Tang, J.-M.; Li, J.; Yu, T.-T.; Liu, B.; Li, L.-G.; Wang, M.-F.; et al. Platelets are highly efficient and efficacious carriers for tumor-targeted nano-drug delivery. Drug Deliv. 2022, 29, 937–949. [Google Scholar] [CrossRef]
- Kunde, S.S.; Wairkar, S. Platelet membrane camouflaged nanoparticles: Biomimetic architecture for targeted therapy. Int. J. Pharm. 2021, 598, 120395. [Google Scholar] [CrossRef]
- Li, Z.; Hu, S.; Cheng, K. Platelets and their biomimetics for regenerative medicine and cancer therapies. J. Mater. Chem. B 2018, 6, 7354–7365. [Google Scholar] [CrossRef]
- Banskota, S.; Yousefpour, P.; Chilkoti, A. Cell-based biohybrid drug delivery systems: The best of the synthetic and natural worlds. Macromol. Biosci. 2017, 17, 1600361. [Google Scholar] [CrossRef]
- Shi, Q.; Montgomery, R.R. Platelets as delivery systems for disease treatments. Adv. Drug Deliv. Rev. 2010, 62, 1196–1203. [Google Scholar] [CrossRef]
- Wei, X.; Ying, M.; Dehaini, D.; Su, Y.; Kroll, A.V.; Zhou, J.; Gao, W.; Fang, R.H.; Chien, S.; Zhang, L. Nanoparticle functionalization with platelet membrane enables multifactored biological targeting and detection of atherosclerosis. ACS Nano 2018, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Xu, J.; Liu, G.; Di, C.; Zhao, X.; Li, X.; Li, Y.; Pang, N.; Yang, C.; et al. Engineered nanoplatelets for targeted delivery of plasminogen activators to reverse thrombus in multiple mouse thrombosis models. Adv. Mater. 2020, 32, e1905145. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Gong, L.; Zou, Y.; Yang, D.; Liu, H.; Liang, Y.; Sun, N.; Zhao, L.; Zhang, Q.; Lin, Z. Platelet membrane-cloaked paclitaxel-nanocrystals augment postoperative chemotherapeutical efficacy. J. Control. Release 2020, 324, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Park, W.; Park, S.-B.; Rhim, W.-K.; Han, D.K. Recent trends in cell membrane-cloaked nanoparticles for therapeutic applications. Methods 2020, 177, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Peer, D. Platelet mimicry: The emperor’s new clothes? Nanomedicine 2016, 12, 245–248. [Google Scholar] [CrossRef]
- Bahmani, B.; Gong, H.; Luk, B.T.; Haushalter, K.J.; De Teresa, E.; Previti, M.; Zhou, J.; Gao, W.; Bui, J.D.; Zhang, L.; et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat. Commun. 2021, 12, 1999. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Williams, G.R.; Fan, Q.; Niu, S.; Wu, J.; Xie, X.; Zhu, L.-M. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J. Nanobiotechnol. 2019, 17, 60. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Blatter, L.A. Effect of carvedilol on atrial excitation-contraction coupling, Ca release, and arrhythmogenicity. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1245–H1255. [Google Scholar] [CrossRef]
- Güven, B.; Kara, Z.; Onay-Beşikci, A. Metabolic effects of carvedilol through β-arrestin proteins: Investigations in a streptozotocin-induced diabetes rat model and in C2C12 myoblasts. Br. J. Pharmacol. 2020, 177, 5580–5594. [Google Scholar] [CrossRef]
- Chen, S.-J.; Tsui, P.-F.; Chuang, Y.-P.; Chiang, D.M.-L.; Chen, L.W.; Liu, S.-T.; Lin, F.-Y.; Huang, S.-M.; Lin, S.-H.; Wu, W.-L.; et al. Carvedilol ameliorates experimental atherosclerosis by regulating cholesterol efflux and exosome functions. Int. J. Mol. Sci. 2019, 20, 5202. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Duan, J.; Zhu, B.; Yang, L. Carvedilol exerts myocardial protection via regulation of AMPK-mTOR-dependent autophagy? Biomed. Pharmacother. 2019, 118, 109283. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; De Velasco, M.A.; Saitou, Y.; Nose, K.; Nishioka, T.; Ishii, T.; Uemura, H. Carvedilol protects tubular epithelial cells from ischemia-reperfusion injury by inhibiting oxidative stress. Int. J. Urol. 2010, 17, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Teoh, J.P.; Wang, Y.; Broskova, Z.; Bayoumi, A.S.; Tang, Y.; Su, H.; Weintraub, N.L.; Kim, I.M. Carvedilol-responsive microRNAs, miR-199a-3p and -214 protect cardiomyocytes from simulated ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H371–H383. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, S.; Haruyama, A.; Inami, S.; Arikawa, T.; Saito, F.; Watanabe, R.; Sakuma, M.; Abe, S.; Nakajima, T.; Tanaka, A.; et al. Effects of carvedilol vs. bisoprolol on inflammation and oxidative stress in patients with chronic heart failure. J. Cardiol. 2020, 75, 140–147. [Google Scholar] [CrossRef]
- Sessa, M.; Rasmussen, D.B.; Jensen, M.T.; Kragholm, K.; Torp-Pedersen, C.; Andersen, M. Metoprolol versus carvedilol in patients with heart failure, chronic obstructive pulmonary disease, diabetes mellitus, and renal failure. Am. J. Cardiol. 2020, 125, 1069–1076. [Google Scholar] [CrossRef]
- Grandinetti, V.; Carlos, F.P.; Antonio, E.L.; de Oliveira, H.A.; Dos Santos, L.F.N.; Yoshizaki, A.; Mansano, B.S.D.M.; Silva, F.A.; Porte, L.A.; Albuquerque-Pontes, G.M.; et al. Photobiomodulation therapy combined with carvedilol attenuates post-infarction heart failure by suppressing excessive inflammation and oxidative stress in rats. Sci. Rep. 2019, 9, 9425. [Google Scholar] [CrossRef]
- Harima, M.; Arumugam, S.; Wen, J.; Pitchaimani, V.; Karuppagounder, V.; Afrin, M.R.; Sreedhar, R.; Miyashita, S.; Nomoto, M.; Ueno, K.; et al. Effect of carvedilol against myocardial injury due to ischemia-reperfusion of the brain in rats. Exp. Mol. Pathol. 2015, 98, 558–562. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Xiu, A.-Y.; Meng, D.-X.; Wang, S.-N.; Zhang, C.-Q. Carvedilol attenuates carbon tetrachloride-induced liver fibrosis and hepatic sinusoidal capillarization in mice. Drug Des. Devel. Ther. 2019, 13, 2667–2676. [Google Scholar] [CrossRef]
- McDowell, H.R.; Chuah, C.S.; Tripathi, D.; Stanley, A.J.; Forrest, E.H.; Hayes, P.C. Carvedilol is associated with improved survival in patients with cirrhosis: A long-term follow-up study. Aliment. Pharmacol. Ther. 2021, 53, 531–539. [Google Scholar] [CrossRef]
- Kumar, M.; Kainth, S.; Choudhury, A.; Maiwall, R.; Mitra, L.G.; Saluja, V.; Agarwal, P.M.; Shasthry, S.M.; Jindal, A.; Bhardwaj, A.; et al. Treatment with carvedilol improves survival of patients with acute-on-chronic liver failure: A randomized controlled trial. Hepatol. Int. 2019, 13, 800–813. [Google Scholar] [CrossRef]
- Li, Q.; Song, Y.; Wang, Q.; Chen, J.; Gao, J.; Tan, H.; Li, S.; Wu, Y.; Yang, H.; Huang, H.; et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics 2021, 11, 3916–3931. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Chao, J.; Bader, M.; Chao, L. Differential role of kinin B1 and B2 receptors in ischemia-induced apoptosis and ventricular remodeling. Peptides 2007, 28, 1383–1389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.; Lin, S.; Wu, J.; Lai, J.; Li, L. A study of the clinical application value of ultrasound and electrocardiogram in the differential diagnosis of cardiomyopathy. Am. J. Transl. Res. 2021, 13, 5200–5207. [Google Scholar] [PubMed]
- Li, S.; Liu, W.; Huang, R.; Wu, W. Preparation and release characteristics in vitro carvedilol pulsed-release tablets. Chin Hosp Pharm J. 2018, 38, 1458–1461. [Google Scholar] [CrossRef]
- Prichard, B.N. Carvedilol in ischaemic heart disease. Cardiology 1993, 82 (Suppl. 3), 34–39. [Google Scholar] [CrossRef]
- Moe, G. Carvedilol in the treatment of chronic heart failure. Expert Opin. Pharmacother. 2001, 2, 831–843. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Yang, X.; Wang, T.; Xu, M.; Huang, Z.; Yu, R.; Jiang, Y.; Zhou, Y.; Shi, J. Platelet-Membrane-Encapsulated Carvedilol with Improved Targeting Ability for Relieving Myocardial Ischemia–Reperfusion Injury. Membranes 2022, 12, 605. https://doi.org/10.3390/membranes12060605
Zhou T, Yang X, Wang T, Xu M, Huang Z, Yu R, Jiang Y, Zhou Y, Shi J. Platelet-Membrane-Encapsulated Carvedilol with Improved Targeting Ability for Relieving Myocardial Ischemia–Reperfusion Injury. Membranes. 2022; 12(6):605. https://doi.org/10.3390/membranes12060605
Chicago/Turabian StyleZhou, Tingting, Xuechao Yang, Tianyi Wang, Mingming Xu, Zhanghao Huang, Runze Yu, Yi Jiang, Youlang Zhou, and Jiahai Shi. 2022. "Platelet-Membrane-Encapsulated Carvedilol with Improved Targeting Ability for Relieving Myocardial Ischemia–Reperfusion Injury" Membranes 12, no. 6: 605. https://doi.org/10.3390/membranes12060605
APA StyleZhou, T., Yang, X., Wang, T., Xu, M., Huang, Z., Yu, R., Jiang, Y., Zhou, Y., & Shi, J. (2022). Platelet-Membrane-Encapsulated Carvedilol with Improved Targeting Ability for Relieving Myocardial Ischemia–Reperfusion Injury. Membranes, 12(6), 605. https://doi.org/10.3390/membranes12060605





