Sustainable Treatment and Resource Recovery of Anion Exchange Spent Brine by Pilot-Scale Electrodialysis and Ultrafiltration
Abstract
:1. Introduction
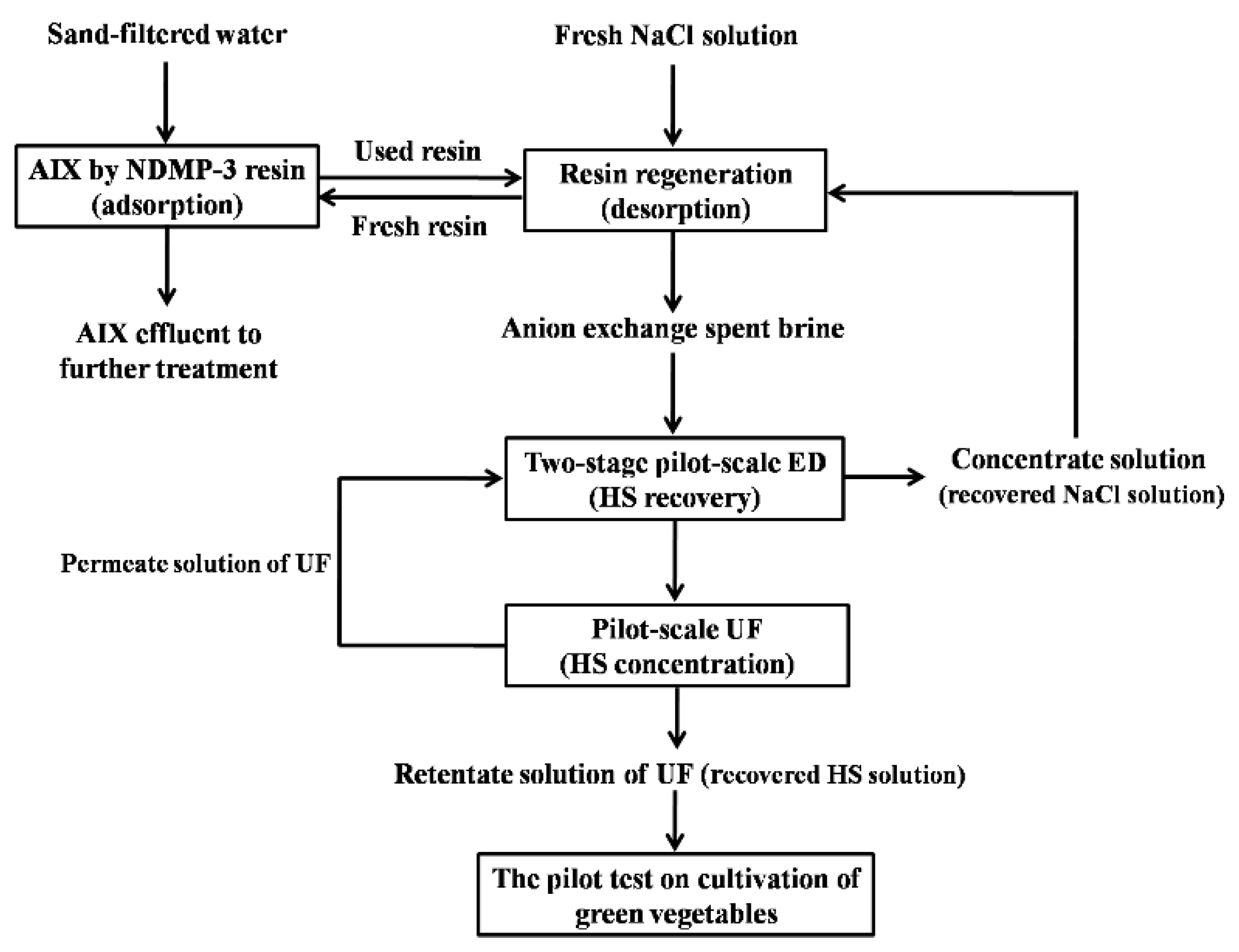
2. Materials and Methods
2.1. The Anion Exchange Spent Brine
2.2. Separation Test by Two-Stage Pilot-Scale ED
2.3. Resin Regeneration Test of Recovered NaCl Solution
2.4. Pilot-Scale UF Test for HS Concentration
2.5. Pilot Test on the Cultivation of Green Vegetables with HS Liquid Fertilizer
3. Results and Discussion
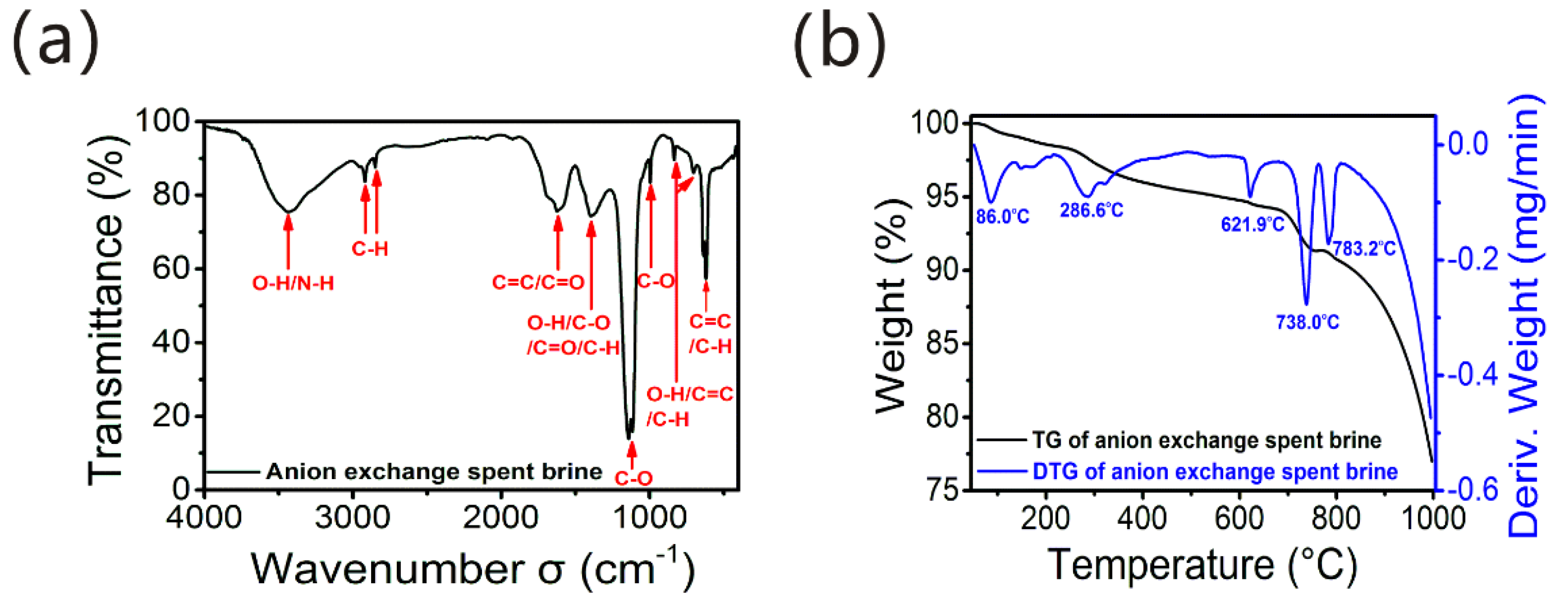
3.1. AIX Spent Brine Characterization with FT-IR and TGA
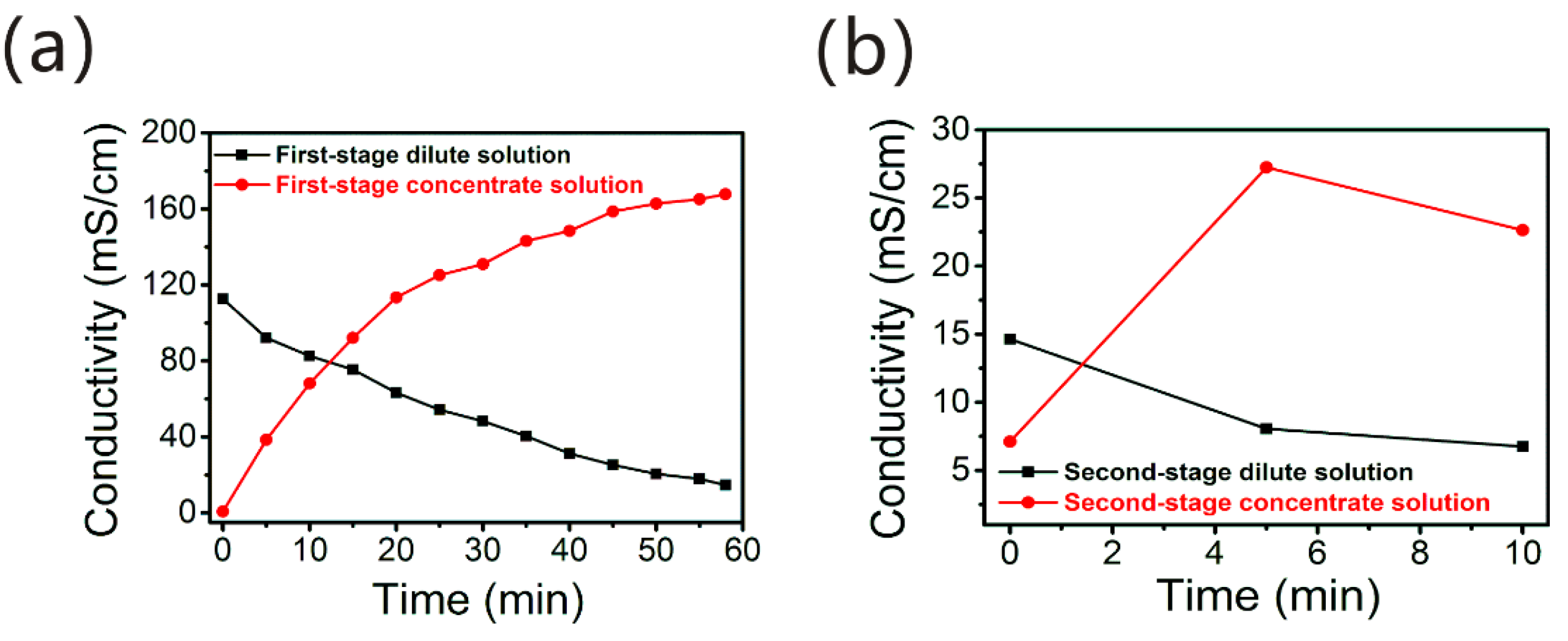
3.2. Separation Effect of NaCl and HSsin theTwo-Stage Pilot-Scale ED
3.3. Resin Regeneration Performance with Recovered NaCl Solution
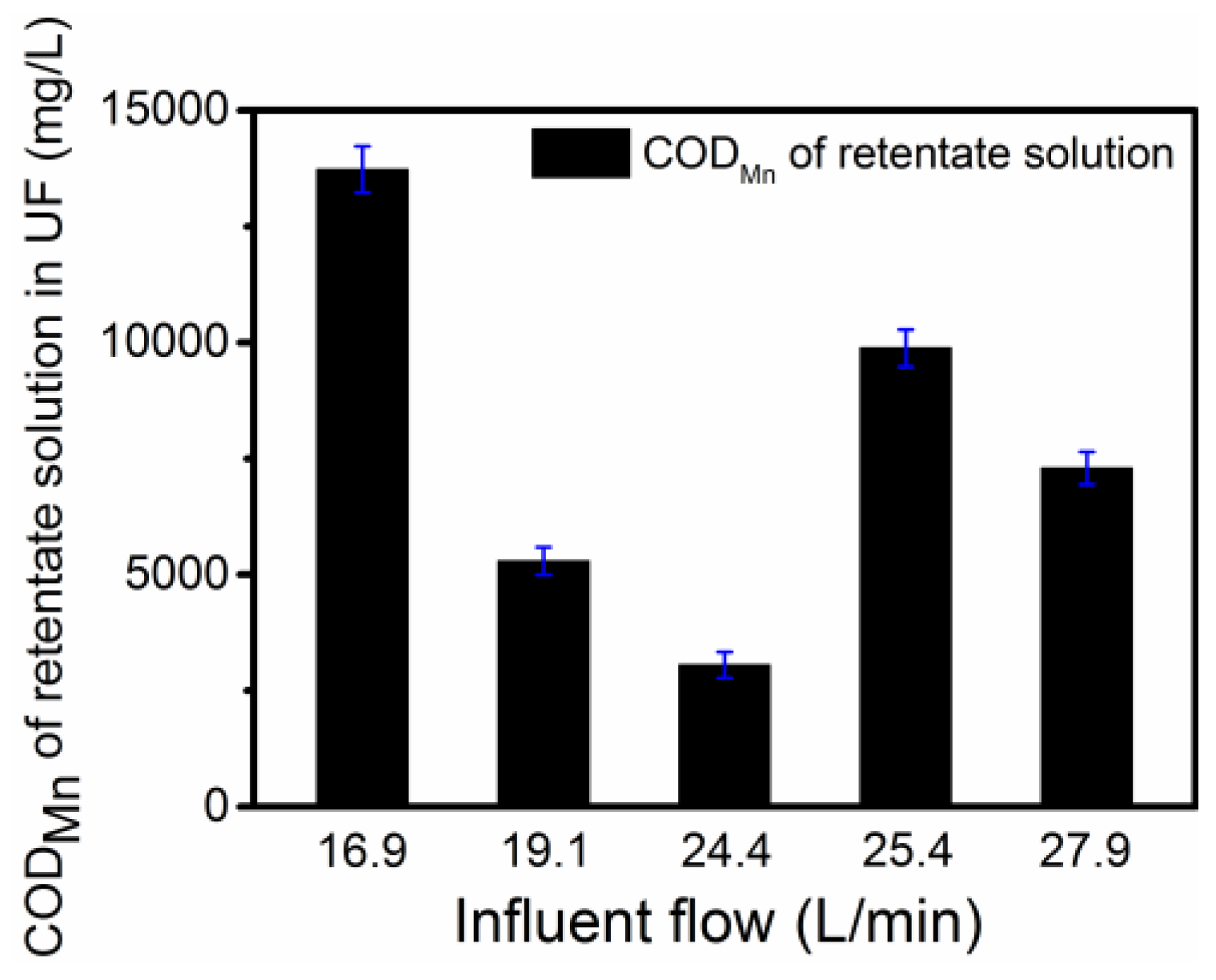
3.4. HS Concentration by Pilot-Scale UF
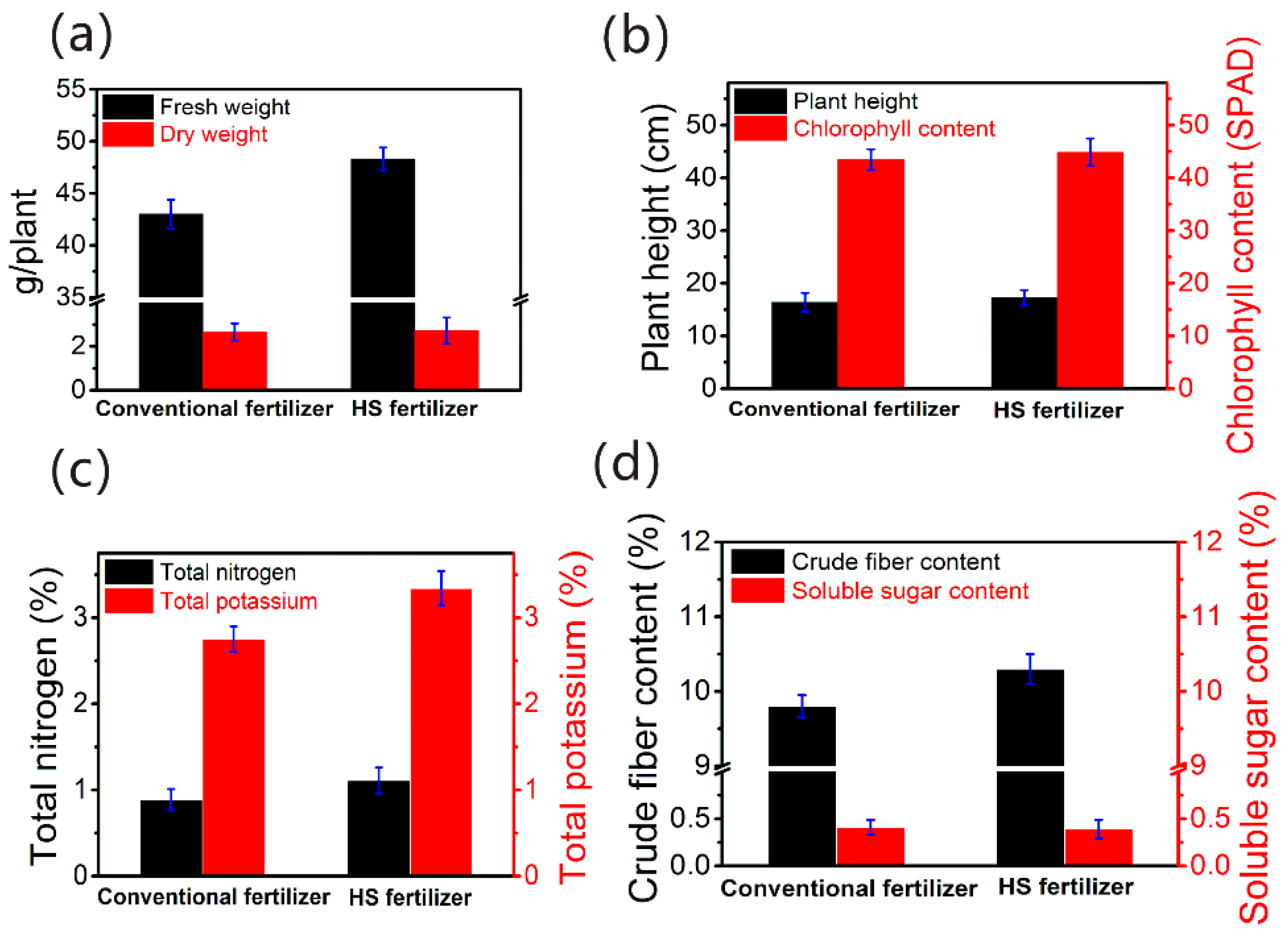
3.5. Pilot Test on the Cultivation of Green Vegetables with HS Liquid Fertilizer
4. Conclusions
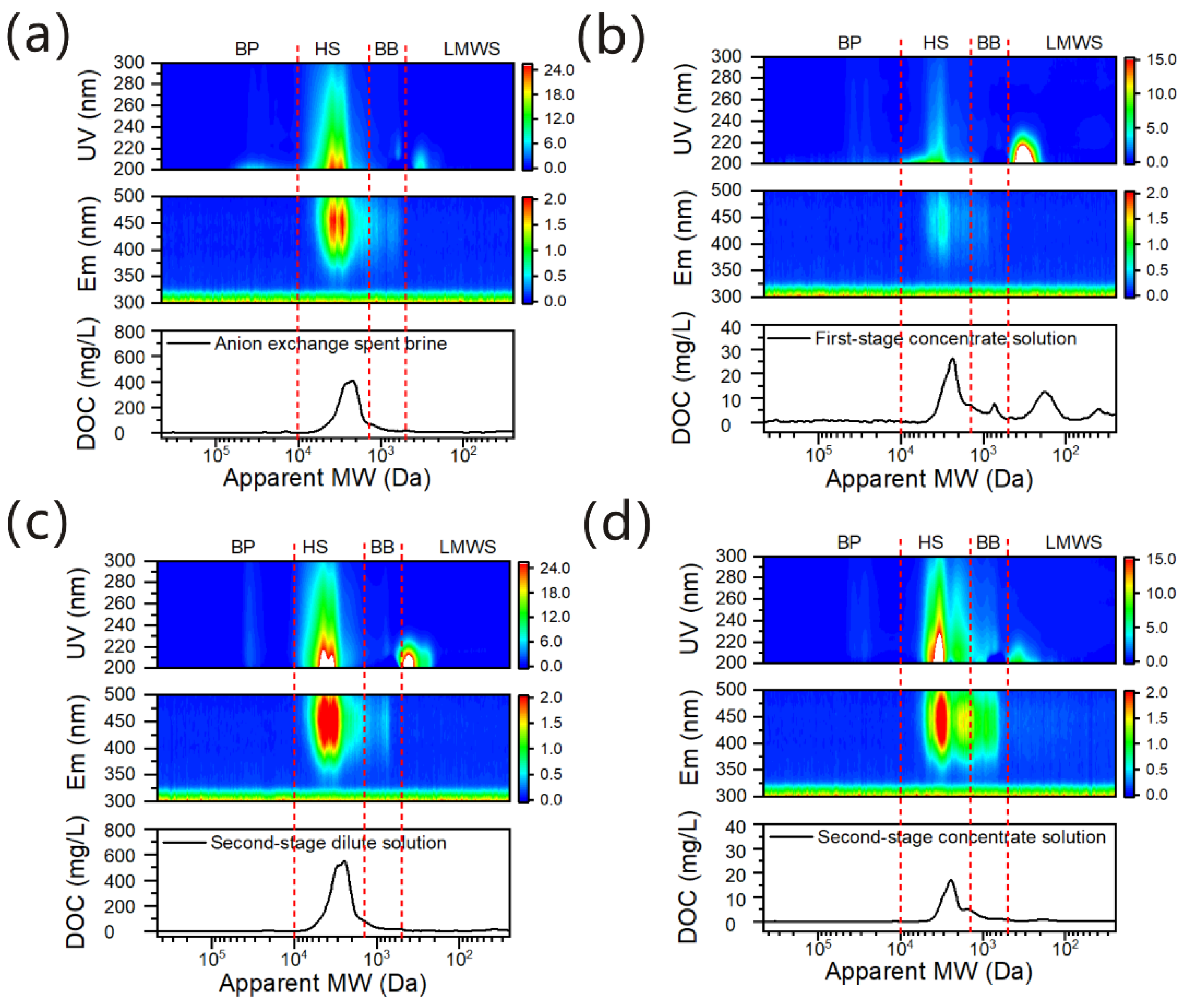
- AIX spent brine displayed absorption spectrum with typical HS bands, and had a higher proportion of aromatic rings, COO− groups and phenolic OH groups. The apparent molecular weight of HSs in AIX spent brine was located at ~7.2 kDa to ~1.3 kDa.
- ED can realize the effective separation of NaCl and HSs in AIX spent brine. There covered NaCl solution (≈15% w/w) could be applied as a regeneration agent of NDMP-3 resins. The NaCl content (0.49% w/w) in these cond-stage dilute solution can meet the industry standard requirement for the salinity of HS fertilizer.
- Some of the negatively charged and hydrophilic HSs and BB, low molecular weight and hydrophilic neutrals, and nitrate or nitrite could migrate from the dilute to the concentrate solution. The charge, molecular size, and hydrophilic property of organic matters as well as the ion exchange membrane properties could significantly affect the transport of organic substances during the ED process.
- The HS content in the retentate solution of UF was >30 g/L, which meets the HS content required of the Chinese professional standard NY1106-2010 for water-soluble fertilizers. The recovered HS solution was safe as a water-soluble fertilizer without phytotoxicity.
- The recovered AIX spent brine-derived HS liquid fertilizer could improve the growth of the green vegetables. These resulted from that the HSs in the AIX spent brine having high content oxygen-containing functional groups, low molecular size and hydrophilic properties, thus promoting the growth and metabolism of green vegetables.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Li, A.; Wang, J.; Shuang, C. Selection of magnetic anion exchange resins for the removal of dissolved organic and inorganic matters. J. Environ. Sci. 2012, 24, 1891–1899. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Li, A.; Wang, J.; Ma, R. Study on the removal of dissolved organic matters in the raw water by a new magnetic anion-exchange resin. Desalination Water Treat. 2014, 57, 572–581. [Google Scholar] [CrossRef]
- Sun, H.; Li, A.; Shi, P.; Cao, X.; Wang, C.; Cheng, S. Separation of NaCl and humic substances in anion exchange spent brine with electrodialysis. Desalination 2022, 523, 115442. [Google Scholar] [CrossRef]
- Caltran, I.; Rietveld, L.C.; Shorney-Darby, H.L.; Heijman, S.G.J. Separating NOM from salts in ion exchange brine with ceramic nanofiltration. Water Res. 2020, 179, 115894. [Google Scholar] [CrossRef]
- Ariono, D.; Purwasasmita, M.; Wenten, I.G. Brine Effluents: Characteristics, Environmental Impacts, and Their Handling. J. Eng. Technol. Sci. 2016, 48, 367–387. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H. Recovering humic substances from the dewatering effluent of thermally treated sludge and its performance as an organic fertilizer. Front. Environ. Sci. Eng. 2016, 10, 578–584. [Google Scholar] [CrossRef]
- Neale, P.A.; Schäfer, A.I. Magnetic ion exchange: Is there potential for international development? Desalination 2009, 248, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Panagopoulos, A.; Haralambous, K.J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Ahmed, M.; Shayya, W.H.; Hoey, D.; Mahendran, A.; Morris, R.; Al-Handaly, J. Use of evaporation ponds for brine disposal in desalination plants. Desalination 2000, 130, 155–168. [Google Scholar] [CrossRef]
- Mohamed, A.M.O.; Maraqa, M.; Al Handhaly, J. Impact of land disposal of reject brine from desalination plants on soil and groundwater. Desalination 2005, 182, 411–433. [Google Scholar] [CrossRef]
- Haddad, M.; Bazinet, L.; Barbeau, B. Eco-efficient treatment of ion exchange spent brine via electrodialysis to recover NaCl and minimize waste disposal. Sci. Total Environ. 2019, 690, 400–409. [Google Scholar] [CrossRef]
- Ye, W.; Liu, H.; Jiang, M.; Lin, J.; Ye, K.; Fang, S.; Xu, Y.; Zhao, S.; Van der Bruggen, B.; He, Z. Sustainable management of landfill leachate concentrate through recovering humic substance as liquid fertilizer by loose nanofiltration. Water Res. 2019, 157, 555–563. [Google Scholar] [CrossRef]
- Vaudevire, E.; Radmanesh, F.; Kolkman, A.; Vughs, D.; Cornelissen, E.; Post, J.; van der Meer, W. Fate and removal of trace pollutants from an anion exchange spent brine during the recovery process of natural organic matter and salts. Water Res. 2019, 154, 34–44. [Google Scholar] [CrossRef]
- Zhao, W.-Y.; Zhou, M.; Yan, B.; Sun, X.; Liu, Y.; Wang, Y.; Xu, T.; Zhang, Y. Waste Conversion and Resource Recovery from Wastewater by Ion Exchange Membranes: State-of-the-Art and Perspective. Ind. Eng. Chem. Res. 2018, 57, 6025–6039. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Turek, M.; Mitko, K.; Bandura-Zalska, B.; Ciecierska, K.; Dydo, P. Ultra-pure water production by integrated electrodialysis-ion exchange/electrodeionization. Membr. Water Treat. 2013, 4, 237–249. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Zhang, Z.; Xu, T. Electrodialysis of concentrated brine from RO plant to produce coarse salt and freshwater. J. Membr. Sci. 2014, 450, 323–330. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Wu, L.; Shehzad, M.A.; Jiang, C.; Fu, R.; Liu, Z.; Xu, T. Multistage-batch electrodialysis to concentrate high-salinity solutions: Process optimisation, water transport, and energy consumption. J. Membr. Sci. 2019, 570–571, 245–257. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Aladjem, C.; Valderrama, C.; Gibert, O.; Valero, F.; Centeno, C.M.; Larrotcha, E.; Cortina, J.L. Concentration of NaCl from seawater reverse osmosis brines for the chlor-alkali industry by electrodialysis. Desalination 2014, 342, 107–117. [Google Scholar] [CrossRef]
- Kabsch-Korbutowicz, M.; Wisniewski, J.; Łakomska, S.; Urbanowska, A. Application of UF, NF and ED in natural organic matter removal from ion-exchange spent regenerant brine. Desalination 2011, 280, 428–431. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Li, X.; Lin, J.; Liao, Y.; Jin, Z. Recovery of humic substances from leachate nanofiltration concentrate by a two-stage process of tight ultrafiltration membrane. J. Clean. Prod. 2017, 161, 84–94. [Google Scholar] [CrossRef]
- Shuang, C.; Wang, M.; Zhou, Q.; Zhou, W.; Li, A. Enhanced adsorption and antifouling performance of anion-exchange resin by the effect of incorporated Fe3O4 for removing humic acid. Water Res. 2013, 47, 6406–6414. [Google Scholar] [CrossRef]
- Cai, M.-H.; Wu, Y.-P.; Ji, W.-X.; Han, Y.-Z.; Li, Y.; Wu, J.-C.; Shuang, C.-D.; Korshin, G.V.; Li, A.-M.; Li, W.-T. Characterizing property and treatability of dissolved effluent organic matter using size exclusion chromatography with an array of absorbance, fluorescence, organic nitrogen and organic carbon detectors. Chemosphere 2020, 243, 125321. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography—organic carbon detection—organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Núñez, L.A. Characterization of aquatic humic substances. Water Environ. J. 2011, 25, 163–170. [Google Scholar] [CrossRef]
- Samios, S.; Lekkas, T.; Nikolaou, A.; Golfinopoulos, S. Structural investigations of aquatic humic substances from different watersheds. Desalination 2007, 210, 125–137. [Google Scholar] [CrossRef]
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of biochar addition on the humic substances of composting manures. Waste Manag. 2016, 49, 545–552. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Davis, W.M.; Erickson, C.L.; Johnston, C.T.; Delfino, J.J.; Porter, J.E. Quantitative Fourier Transform Infrared spectroscopic investigation humic substance functional group composition. Chemosphere 1999, 38, 2913–2928. [Google Scholar] [CrossRef]
- Kim, H.-C.; Yu, M.-J.; Han, I. Multi-method study of the characteristic chemical nature of aquatic humic substances isolated from the Han River, Korea. Appl. Geochem. 2006, 21, 1226–1239. [Google Scholar] [CrossRef]
- Cristina, G.; Camelin, E.; Ottone, C.; Fraterrigo Garofalo, S.; Jorquera, L.; Castro, M.; Fino, D.; Schiappacasse, M.C.; Tommasi, T. Recovery of humic acids from anaerobic sewage sludge: Extraction, characterization and encapsulation in alginate beads. Int. J. Biol. Macromol. 2020, 164, 277–285. [Google Scholar] [CrossRef]
- Giovanela, M.; Crespo, J.S.; Antunes, M.; Adamatti, D.S.; Fernandes, A.N.; Barison, A.; da Silva, C.W.P.; Guégan, R.; Motelica-Heino, M.; Sierra, M.M.D. Chemical and spectroscopic characterization of humic acids extracted from the bottom sediments of a Brazilian subtropical microbasin. J. Mol. Struct. 2010, 981, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Esteves, V.I.; Duarte, A.C. Thermogravimetric properties of aquatic humic substances. Mar. Chem. 1999, 63, 225–233. [Google Scholar] [CrossRef]
- Swiech, W.M.; Hamerton, I.; Zeng, H.; Watson, D.J.; Mason, E.; Taylor, S.E. Water-based fractionation of a commercial humic acid. Solid-state and colloidal characterization of the solubility fractions. J. Colloid Interface Sci. 2017, 508, 28–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, J.; Zhang, F.; Yu, Y.; Zhang, J. Chemical characterization of humic substances isolated from mangrove swamp sediments: The Qinglan area of Hainan Island, China. Estuar. Coast. Shelf Sci. 2011, 93, 220–227. [Google Scholar] [CrossRef]
- de Souza, F.; Bragança, S.R. Extraction and characterization of humic acid from coal for the application as dispersant of ceramic powders. J. Mater. Res. Technol. 2018, 7, 254–260. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Li, W.T.; Chen, S.Y.; Xu, Z.X.; Li, Y.; Shuang, C.D.; Li, A.M. Characterization of dissolved organic matter in municipal wastewater using fluorescence PARAFAC analysis and chromatography multi-excitation/emission scan: A comparative study. Environ. Sci. Technol. 2014, 48, 2603–2609. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.W.K.; Drikas, M.; Korshin, G.; Amal, R. Multi-wavelength spectroscopic and chromatography study on the photocatalytic oxidation of natural organic matter. Water Res. 2010, 44, 2525–2532. [Google Scholar] [CrossRef]
- Leenheer, J.A.; Croué, J.-P. Peer reviewed: Characterizing aquatic dissolved organic matter. Environ. Sci. Technol. 2003, 37, 18A–26A. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Moon, S.-H.; Cho, J. Investigation of the adsorption and transport of natural organic matter (NOM) in ion-exchange membranes. Desalination 2003, 151, 11–20. [Google Scholar] [CrossRef]
- Bolto, B.; Dixon, D.; Eldridge, R.; King, S.; Linge, K. Removal of natural organic matter by ion exchange. Water Res. 2002, 36, 5057–5065. [Google Scholar] [CrossRef]
- Cromphout, J.; D’Haeseleer, V.; Closset, W.; Verdickt, L. Applicability of ion exchange for NOM removal from a sulfate-rich surface water incorporating full reuse of the brine. Water Supply 2012, 12, 878–887. [Google Scholar] [CrossRef]
- Beril Gönder, Z.; Arayici, S.; Barlas, H. Advanced treatment of pulp and paper mill wastewater by nanofiltration process: Effects of operating conditions on membrane fouling. Sep. Purif. Technol. 2011, 76, 292–302. [Google Scholar] [CrossRef]
- Akladious, S.A.; Mohamed, H.I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 2018, 236, 244–250. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279. [Google Scholar] [CrossRef]
- Delfine, S.; Tognetti, R.; Desiderio, E.; Alvino, A. Effect of foliar application of N and humic acids on growth and yield of durum wheat. Agron. Sustain. Dev. 2005, 25, 183–191. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Leite, J.M.; Pitumpe Arachchige, P.S.; Ciampitti, I.A.; Hettiarachchi, G.M.; Maurmann, L.; Trivelin, P.C.O.; Prasad, P.V.V.; Sunoj, S.V.J. Co-addition of humic substances and humic acids with urea enhances foliar nitrogen use efficiency in sugarcane (Saccharum officinarum L.). Heliyon 2020, 6, e05100. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; Garcia, C.A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garnica, M.; Urrutia, O.; Erro, J.; Zamarreño, A.M.; et al. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot- growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Aguirre, E.; Lemenager, D.; Bacaicoa, E.; Fuentes, M.; Baigorri, R.; Zamarreno, A.M.; Garcia-Mina, J.M. The root application of a purified leonardite humic acid modifies the transcriptional regulation of the main physiological root responses to Fe deficiency in Fe-sufficient cucumber plants. Plant. Physiol. Biochem. 2009, 47, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Dobbss, L.B.; Oliveira, A.L.; Chagas, J.G.; Aguiar, N.O.; Rumjanek, V.M.; Novotny, E.H.; Olivares, F.L.; Spaccini, R.; Piccolo, A. Chemical properties of humic matter as related to induction of plant lateral roots. Eur. J. Soil Sci. 2012, 63, 315–324. [Google Scholar] [CrossRef]
- Nardi, S.; Muscolo, A.; Vaccaro, S.; Baiano, S.; Spaccini, R.; Piccolo, A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol. Biochem. 2007, 39, 3138–3146. [Google Scholar] [CrossRef]

| Parameters | AIX Spent Brine | First-Stage Concentrate Solution | First-Stage Dilute Solution | Second-Stage Concentrate Solution | Second-Stage Dilute Solution |
|---|---|---|---|---|---|
| Conductivity (mS/cm) | 115.80 | 167.72 | 10.82 | 21.64 | 6.09 |
| pH | 8.27 | 8.21 | 8.11 | 8.25 | 8.18 |
| CODMn (mg/L) | 2572.3 | 241.1 | 4934.2 | 36.1 | 5158.7 |
| NaCl content (% w/w) | 9.53 | 14.92 | 0.68 | 1.48 | 0.49 |
| CODMn retention (%) | 95.5% (total) a | - | 95.8% | - | - |
| Desalination rate (%) | 94.7% (total) a | - | 90.7% | - | - |
| Parmeter | Recovery HS Solution |
|---|---|
| HS (g/L) | >30 |
| Cu (mg/L) | 0.842 |
| Zn (mg/L) | 0.920 |
| Cd (mg/L) | ND a |
| Pb (mg/L) | 0.27 |
| Cr (mg/L) | 0.71 |
| Hg (μg/L) | ND a |
| Fe (mg/L) | 15.5 |
| Mn (mg/L) | ND a |
| Mo (μg/L) | 532 |
| As (μg/L) | 37.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhu, D.; Shi, P.; Ji, W.; Cao, X.; Cheng, S.; Lou, Y.; Li, A. Sustainable Treatment and Resource Recovery of Anion Exchange Spent Brine by Pilot-Scale Electrodialysis and Ultrafiltration. Membranes 2022, 12, 273. https://doi.org/10.3390/membranes12030273
Sun H, Zhu D, Shi P, Ji W, Cao X, Cheng S, Lou Y, Li A. Sustainable Treatment and Resource Recovery of Anion Exchange Spent Brine by Pilot-Scale Electrodialysis and Ultrafiltration. Membranes. 2022; 12(3):273. https://doi.org/10.3390/membranes12030273
Chicago/Turabian StyleSun, Hongfang, Daoxu Zhu, Peng Shi, Wenxiang Ji, Xun Cao, Shi Cheng, Yufeng Lou, and Aimin Li. 2022. "Sustainable Treatment and Resource Recovery of Anion Exchange Spent Brine by Pilot-Scale Electrodialysis and Ultrafiltration" Membranes 12, no. 3: 273. https://doi.org/10.3390/membranes12030273
APA StyleSun, H., Zhu, D., Shi, P., Ji, W., Cao, X., Cheng, S., Lou, Y., & Li, A. (2022). Sustainable Treatment and Resource Recovery of Anion Exchange Spent Brine by Pilot-Scale Electrodialysis and Ultrafiltration. Membranes, 12(3), 273. https://doi.org/10.3390/membranes12030273





