The Biological Performance of a Novel Electrokinetic-Assisted Membrane Photobioreactor (EK-MPBR) for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microalgae and Culture Conditions
2.3. Operating Conditions
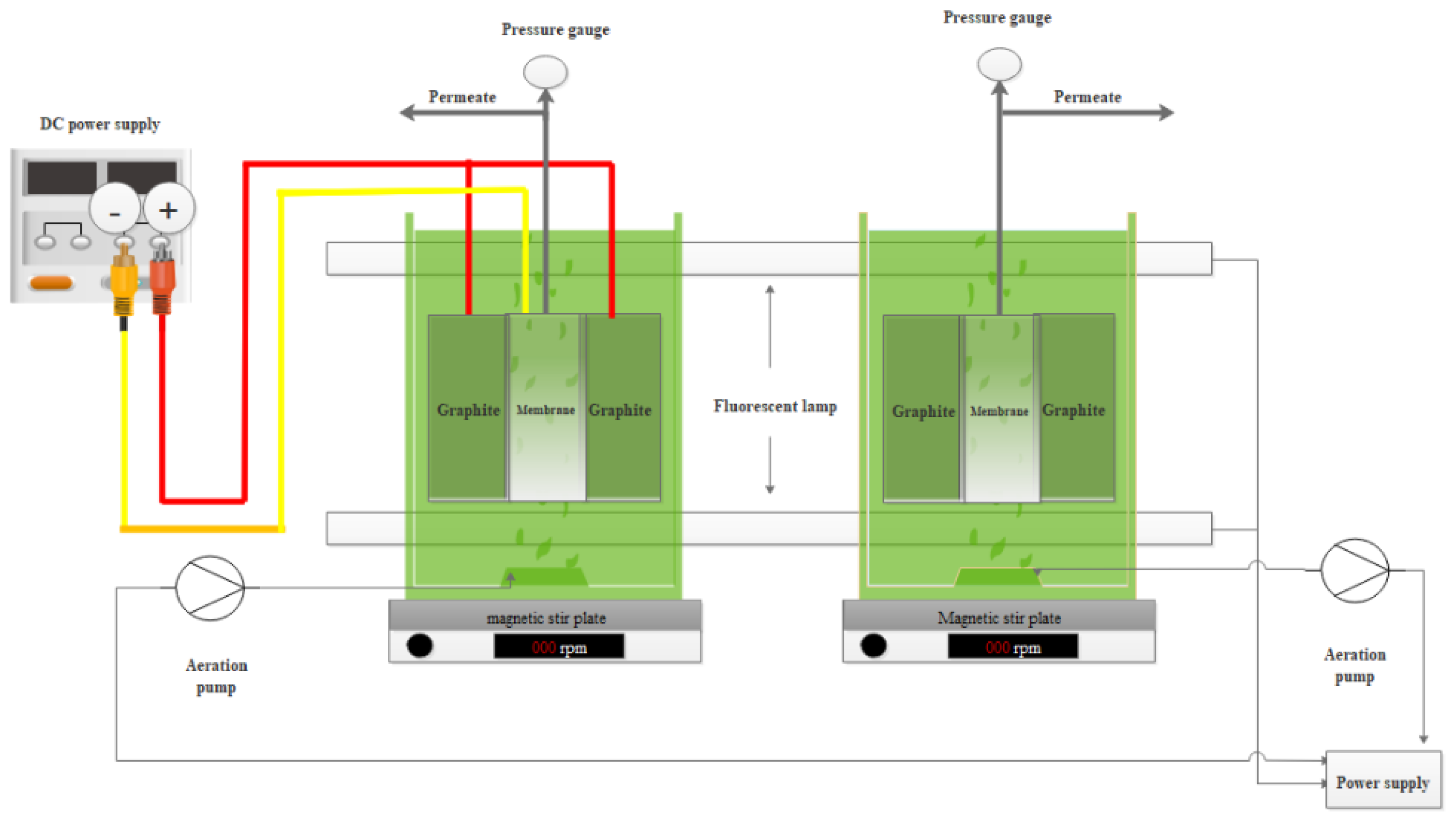
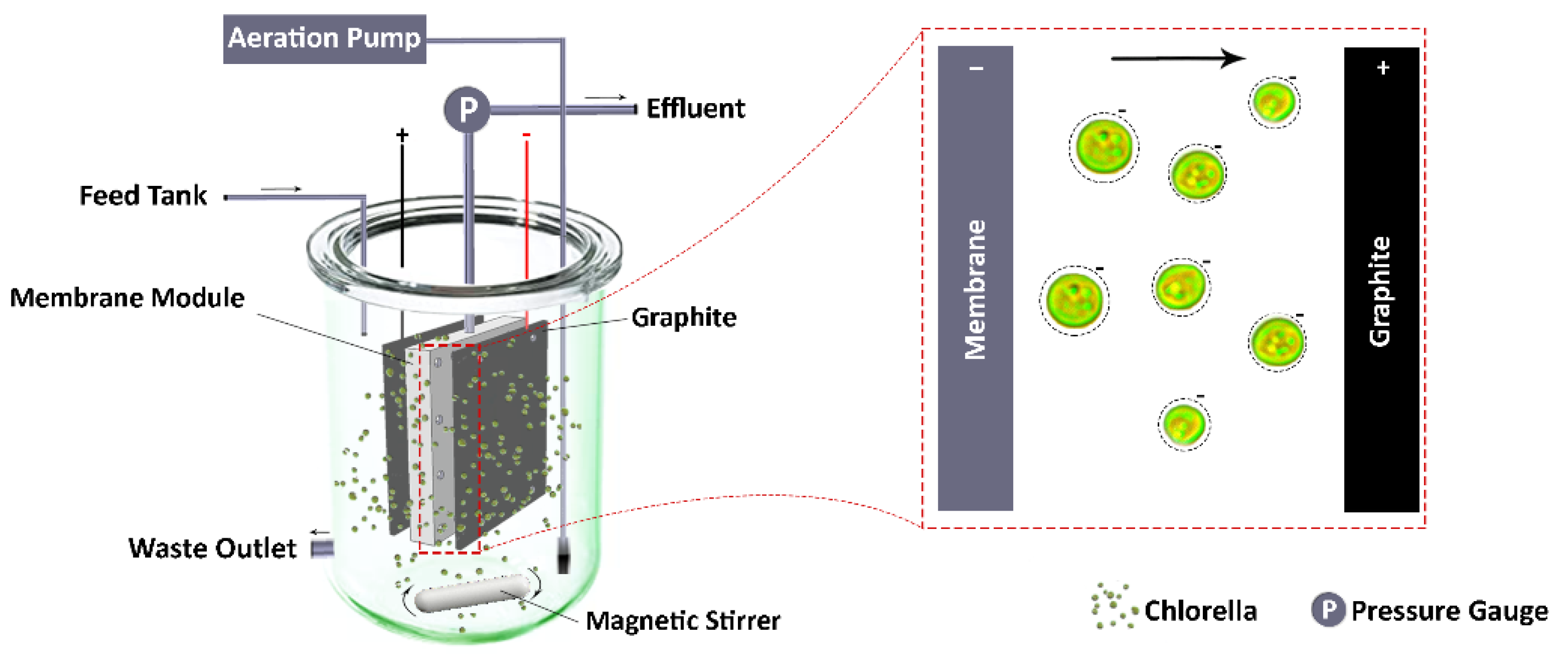
2.4. Experimental Set-Up
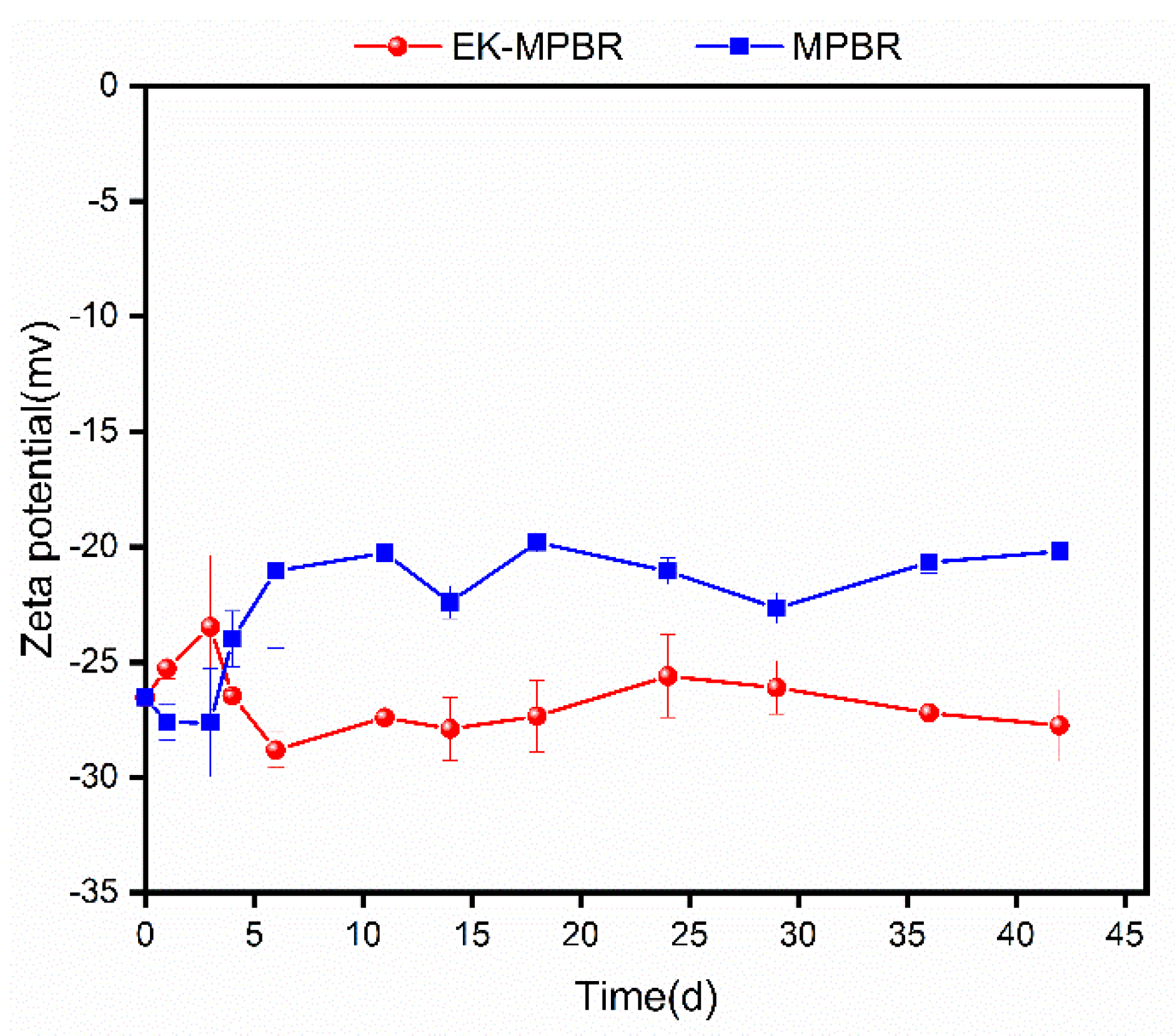
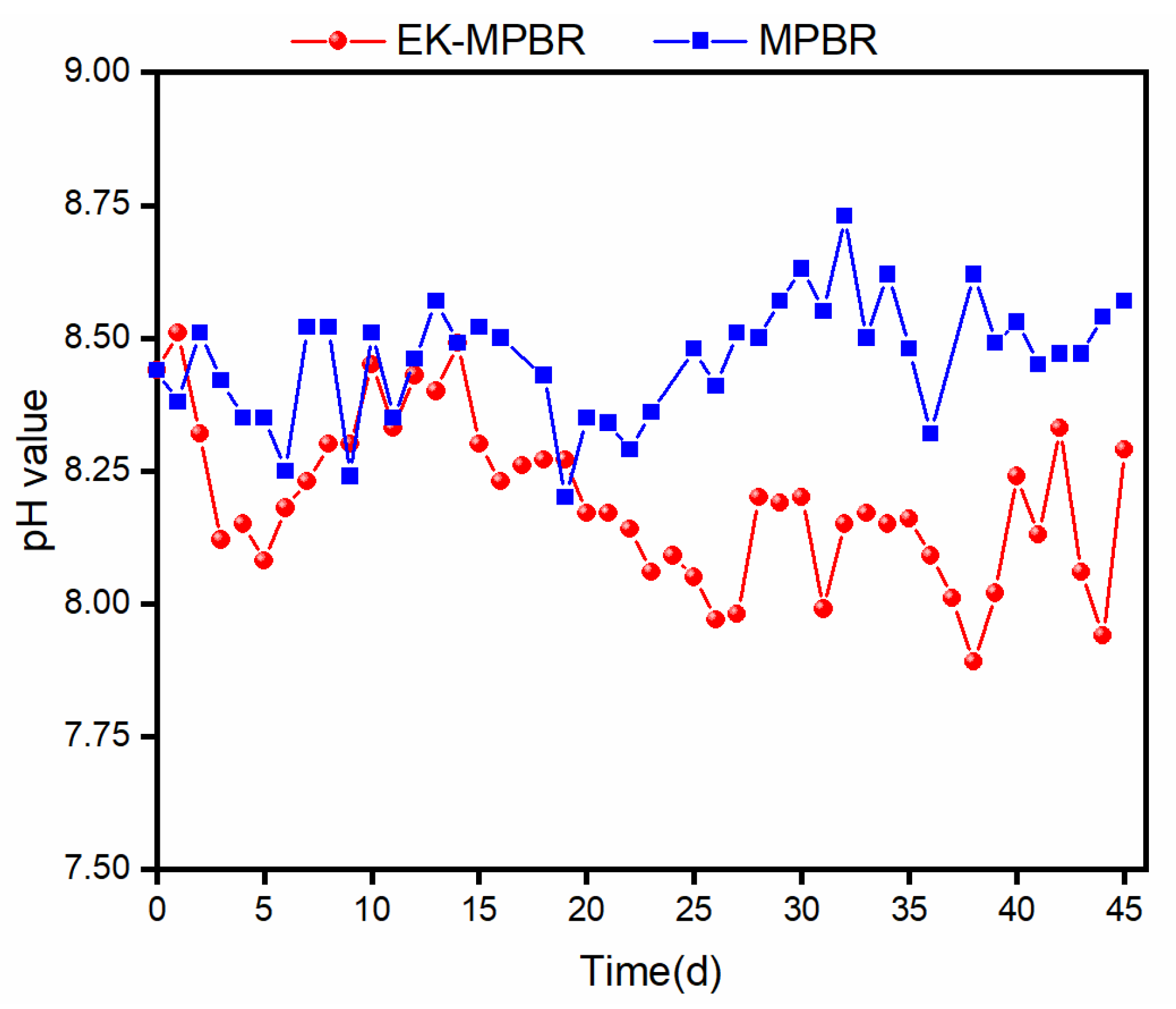
2.5. Zeta Potential and Routine Analysis
2.6. Determination of Biomass Characteristics
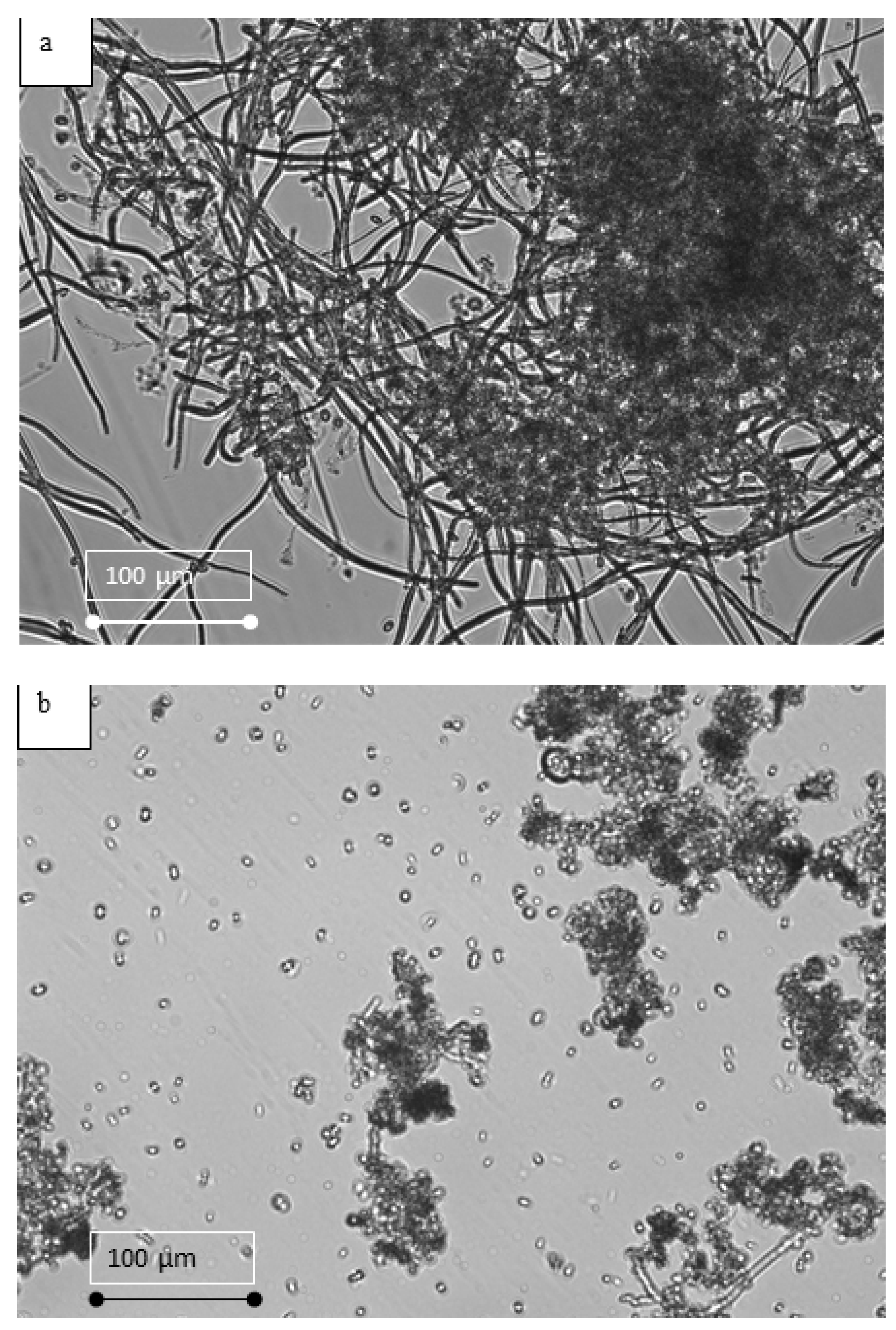
2.7. Particle Size Distribution and Microalgae Structure
2.8. Statistical Analysis
3. Results and Discussion
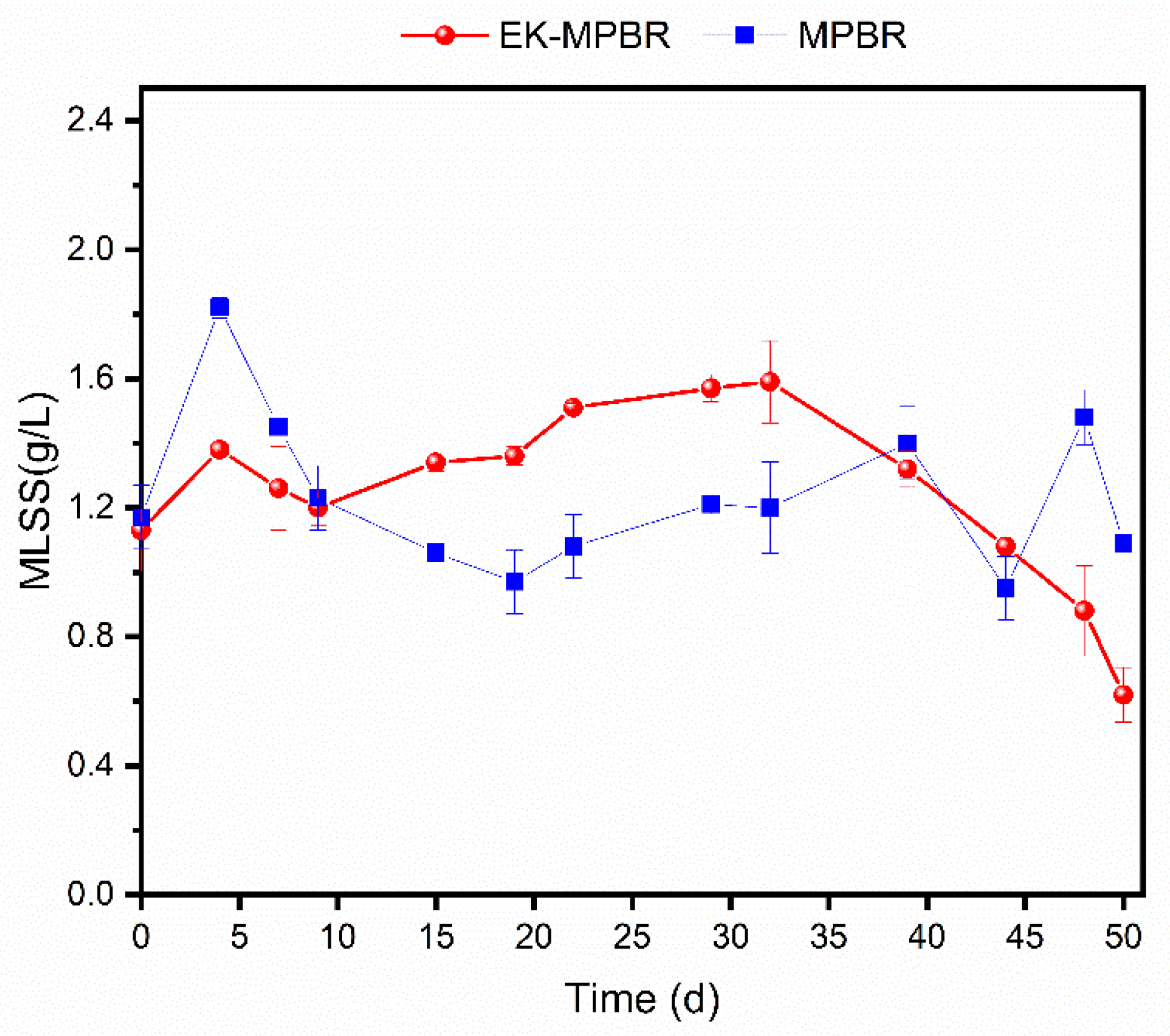
3.1. Effect of EF Treatment on Biomass Production
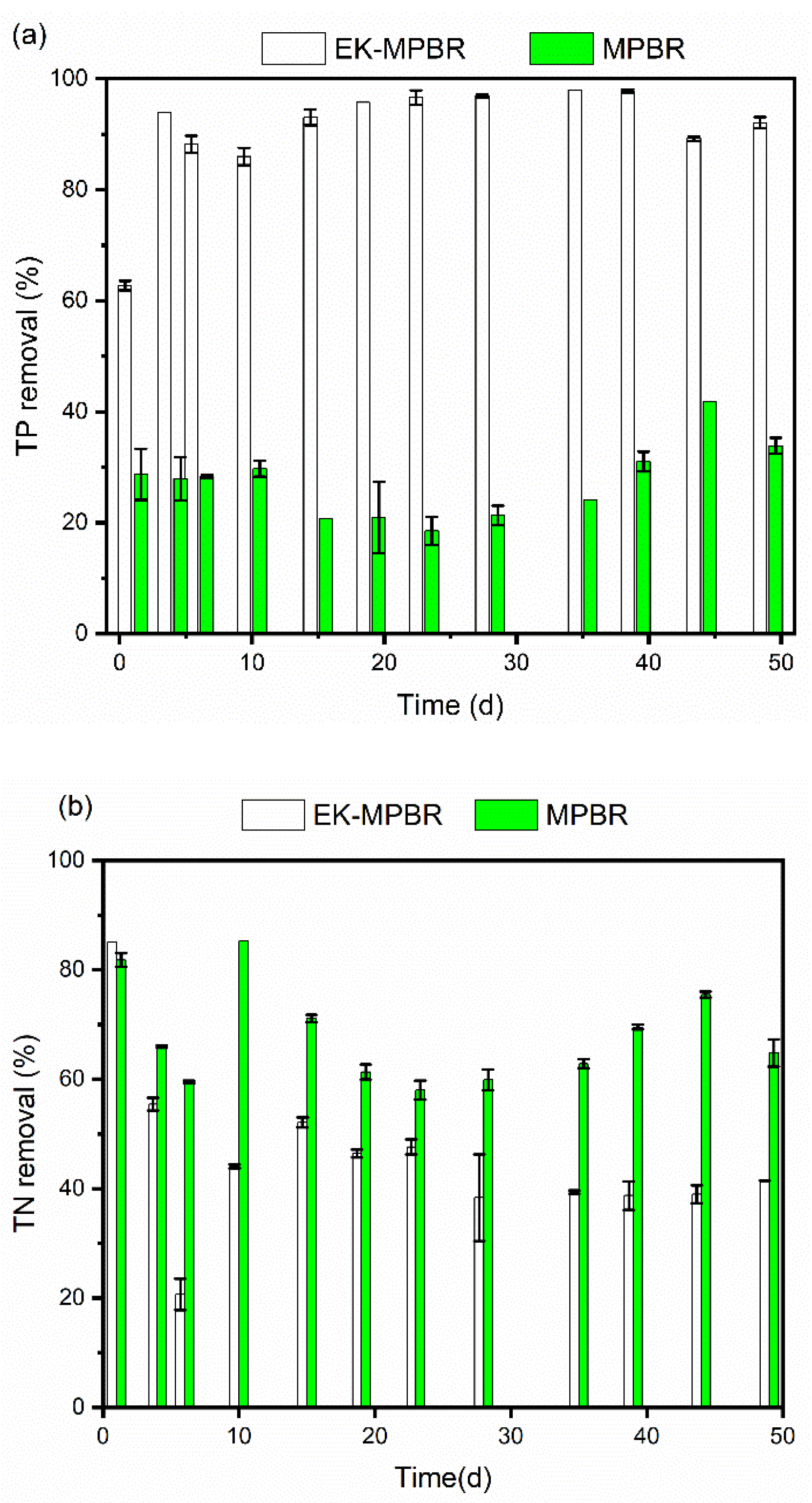
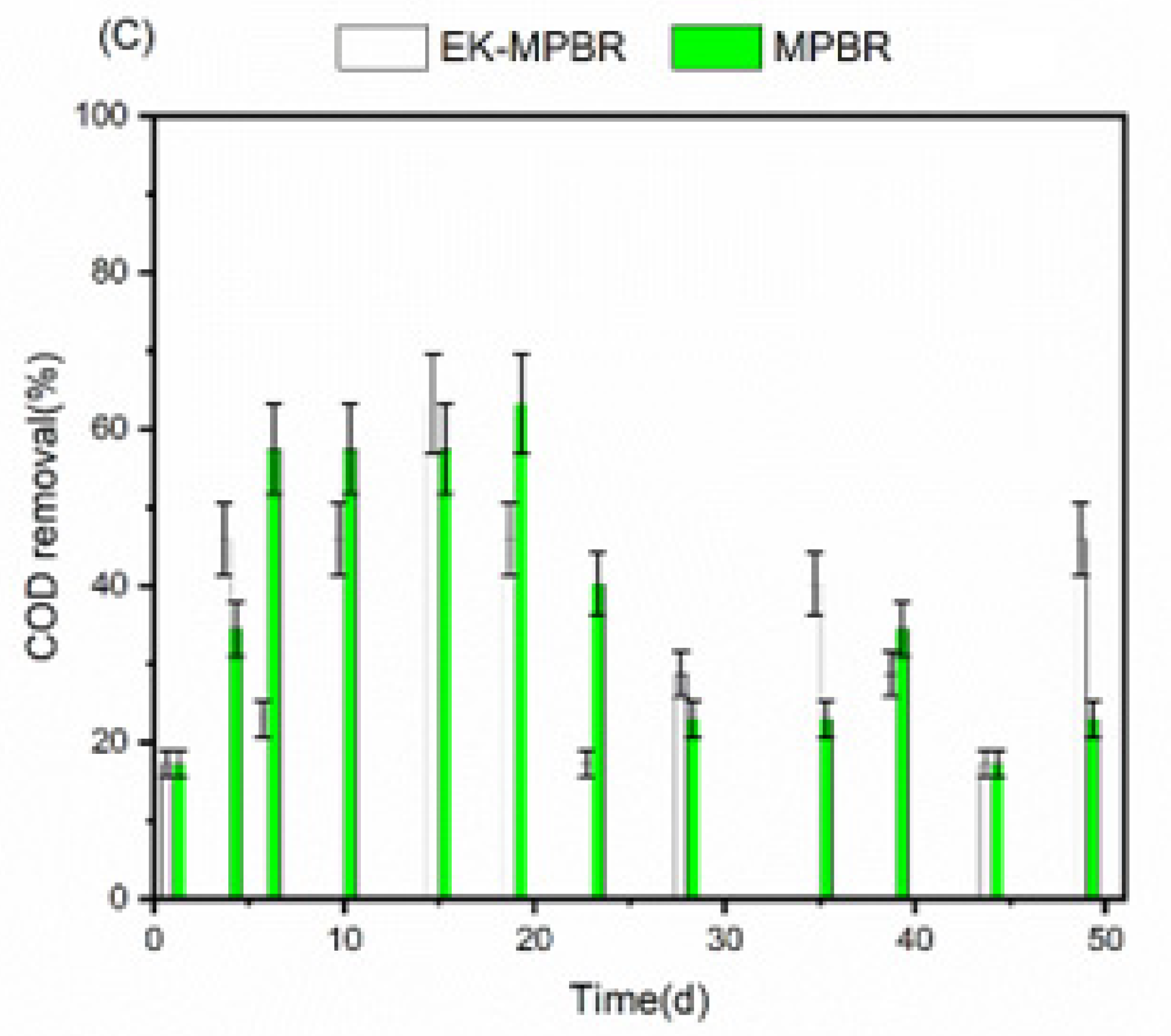
3.2. Nutrient Removal and Wastewater Treatment Potential
3.3. Effect of EF on the Physiology of Microalgae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Touliabah, H.E.-S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A Review of Microalgae-and Cyanobacteria-Based Biodegradation of Organic Pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef] [PubMed]
- Corpuz, M.V.A.; Borea, L.; Senatore, V.; Castrogiovanni, F.; Buonerba, A.; Oliva, G.; Ballesteros, F., Jr.; Zarra, T.; Belgiorno, V.; Choo, K.-H. Wastewater treatment and fouling control in an electro algae-activated sludge membrane bioreactor. Sci. Total Environ. 2021, 786, 147475. [Google Scholar] [CrossRef] [PubMed]
- Lavrinovičs, A.; Murby, F.; Zīverte, E.; Mežule, L.; Juhna, T. Increasing Phosphorus Uptake Efficiency by Phosphorus-Starved Microalgae for Municipal Wastewater Post-Treatment. Microorganisms 2021, 9, 1598. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.A.; Sousa, H.; Vale, F.; Simões, M. Microalgae-based bioremediation of wastewaters-Influencing parameters and mathematical growth modelling. Chem. Eng. J. 2021, 425, 131412. [Google Scholar] [CrossRef]
- Priyadharshini, S.D.; Babu, P.S.; Manikandan, S.; Subbaiya, R.; Govarthanan, M.; Karmegam, N. Phycoremediation of wastewater for pollutant removal: A green approach to environmental protection and long-term remediation. Environ. Pollut. 2021, 290, 117989. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, X.; Wang, X.; Wang, C.; Faustin, F.; Liu, W. Characterization of the start-up of single and two-stage Anammox processes with real low-strength wastewater treatment. Chemosphere 2020, 245, 125572. [Google Scholar] [CrossRef]
- Karthik, V.; Saravanan, K.; Bharathi, P.; Dharanya, V.; Meiaraj, C. An overview of treatments for the removal of textile dyes. J. Chem. Pharm. Sci. 2014, 7, 301–307. [Google Scholar]
- Verma, R.; Suthar, S.; Chand, N.; Mutiyar, P.K. Phycoremediation of milk processing wastewater and lipid-rich biomass production using Chlorella vulgaris under continuous batch system. Sci. Total Environ. 2022, 833, 155110. [Google Scholar] [CrossRef]
- Bilad, M.; Azizo, A.; Wirzal, M.; Jia, L.J.; Putra, Z.; Nordin, N.; Mavukkandy, M.; Jasni, M.; Yusoff, A. Tackling membrane fouling in microalgae filtration using nylon 6, 6 nanofiber membrane. J. Environ. Manag. 2018, 223, 23–28. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.-H.; Zeng, G.-M.; Feng, L.-J.; Liu, J.-Z.; Liu, M.; Cai, H.-W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Lee, J.-C.; Baek, K.; Kim, H.-W. Semi-continuous operation and fouling characteristics of submerged membrane photobioreactor (SMPBR) for tertiary treatment of livestock wastewater. J. Clean. Prod. 2018, 180, 244–251. [Google Scholar] [CrossRef]
- Walter, U.; Beyer, M.; Klein, J.; Rehm, H.-J. Degradation of pyrene byRhodococcus sp. UW1. Appl. Microbiol. Biotechnol. 1991, 34, 671–676. [Google Scholar] [CrossRef]
- Nezammahalleh, H.; Ghanati, F.; Adams, T.A., II; Nosrati, M.; Shojaosadati, S.A. Effect of moderate static electric field on the growth and metabolism of Chlorella vulgaris. Bioresour. Technol. 2016, 218, 700–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra-Maia, R.; Bernard, O.; Gonçalves, A.; Bensalem, S.; Lopes, F. Influence of temperature on Chlorella vulgaris growth and mortality rates in a photobioreactor. Algal Res. 2016, 18, 352–359. [Google Scholar] [CrossRef]
- Zhang, M.; Lee, E.; Vonghia, E.; Hong, Y.; Liao, B. Introduction to aerobic membrane bioreactors: Current status and recent developments. In Current Developments in Biotechnology and Bioengineering; Ng, H.Y., Ng, T.C.A., Ngo, H.H., Mannina, G., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–23. [Google Scholar]
- Bokhary, A.; Maleki, E.; Hong, Y.; Hai, F.I.; Liao, B. Anaerobic membrane bioreactors: Basic process design and operation. In Current Developments in Biotechnology and Bioengineering; Ng, H.Y., Ng, T.C.A., Ngo, H.H., Mannina, G., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–54. [Google Scholar]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Simultaneous microalgae cultivation and wastewater treatment in submerged membrane photobioreactors: A review. Algal Res. 2017, 24, 425–437. [Google Scholar] [CrossRef]
- Bilad, M.; Arafat, H.A.; Vankelecom, I.F. Membrane technology in microalgae cultivation and harvesting: A review. Biotechnol. Adv. 2014, 32, 1283–1300. [Google Scholar] [CrossRef]
- Zhang, M.; Leung, K.-T.; Lin, H.; Liao, B. Membrane fouling in a microalgal-bacterial membrane photobioreactor: Effects of P-availability controlled by N:P ratio. Chemosphere 2021, 282, 131015. [Google Scholar] [CrossRef]
- Katırcıoğlu Sınmaz, G.; Erden, B.; Şengil, İ. Cultivation of Chlorella vulgaris in alkaline condition for biodiesel feedstock after biological treatment of poultry slaughterhouse wastewater. Int. J. Environ. Sci. Technol. 2022, 86. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.; Ahmad, S.; Singh, H.M.; Pathak, V.V.; Tyagi, V.; Kumar, K.; Sari, A. Utilization of Chlorella pyrenoidosa for Remediation of Common Effluent Treatment Plant Wastewater in Coupling with Co-relational Study: An Experimental Approach. Bull. Environ. Contam. Toxicol. 2022, 108, 507–517. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yang, M.; Wang, C. Nutrient deprivation enhances lipid content in marine microalgae. Bioresour. Technol. 2013, 147, 484–491. [Google Scholar] [CrossRef] [PubMed]
- San Pedro, A.; González-López, C.; Acién, F.; Molina-Grima, E. Marine microalgae selection and culture conditions optimization for biodiesel production. Bioresour. Technol. 2013, 134, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.J.; Gagnon, G.A.; Jamieson, R.C. Microalgae growth and phosphorus uptake in wastewater under simulated cold region conditions. Ecol. Eng. 2016, 95, 588–593. [Google Scholar] [CrossRef]
- Arrojo, M.Á.; Regaldo, L.; Calvo Orquín, J.; Figueroa, F.L.; Abdala Díaz, R.T. Potential of the microalgae Chlorella fusca (Trebouxiophyceae, Chlorophyta) for biomass production and urban wastewater phycoremediation. AMB Express 2022, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Salgado, E.M.; Gonçalves, A.L.; Sánchez-Soberón, F.; Ratola, N.; Pires, J.C. Microalgal cultures for the bioremediation of urban wastewaters in the presence of siloxanes. Int. J. Environ. Res. Public Health 2022, 19, 2634. [Google Scholar] [CrossRef]
- Huang, H.; Zhong, S.; Wen, S.; Luo, C.; Long, T. Improving the efficiency of wastewater treatment and microalgae production for biofuels. Resour. Conserv. Recycl. 2022, 178, 106094. [Google Scholar] [CrossRef]
- Novoa, A.F.; Fortunato, L.; Rehman, Z.U.; Leiknes, T. Evaluating the effect of hydraulic retention time on fouling development and biomass characteristics in an algal membrane photobioreactor treating a secondary wastewater effluent. Bioresour. Technol. 2020, 309, 123348. [Google Scholar] [CrossRef]
- Qiu, S.; Yu, Z.; Hu, Y.; Chen, Z.; Guo, J.; Xia, W.; Ge, S. An evolved native microalgal consortium-snow system for the bioremediation of biogas and centrate wastewater: Start-up, optimization and stabilization. Water Res. 2021, 196, 117038. [Google Scholar] [CrossRef]
- Gordalina, M.; Pinheiro, H.M.; Mateus, M.; da Fonseca, M.M.R.; Cesário, M.T. Macroalgae as Protein Sources—A Review on Protein Bioactivity, Extraction, Purification and Characterization. Appl. Sci. 2021, 11, 7969. [Google Scholar] [CrossRef]
- Buchmann, L.; Böcker, L.; Frey, W.; Haberkorn, I.; Nyffeler, M.; Mathys, A. Energy input assessment for nanosecond pulsed electric field processing and its application in a case study with Chlorella vulgaris. Innov. Food Sci. Emerg. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Zhang, J.; Satti, A.; Chen, X.; Xiao, K.; Sun, J.; Yan, X.; Liang, P.; Zhang, X.; Huang, X. Low-voltage electric field applied into MBR for fouling suppression: Performance and mechanisms. Chem. Eng. J. 2015, 273, 223–230. [Google Scholar] [CrossRef]
- Canelli, G.; Tevere, S.; Jaquenod, L.; Dionisi, F.; Rohfritsch, Z.; Bolten, C.J.; Neutsch, L.; Mathys, A. A novel strategy to simultaneously enhance bioaccessible lipids and antioxidants in hetero/mixotrophic Chlorella vulgaris as functional ingredient. Bioresour. Technol. 2022, 347, 126744. [Google Scholar] [CrossRef] [PubMed]
- Salamati Mashhad, N. Investigation of Activated Sludge Properties under Different Electrical Field and in the Presence of Calcium. Master’s Thesis, Concordia University, Montreal, QC, Canada, November 2010. [Google Scholar]
- Su, F.; Liang, Y.; Liu, G.; Mota Filho, C.R.; Hu, C.; Qu, J. Enhancement of anti-fouling and contaminant removal in an electro-membrane bioreactor: Significance of electrocoagulation and electric field. Sep. Purif. Technol. 2020, 248, 117077. [Google Scholar] [CrossRef]
- Giwa, A.; Dindi, A.; Kujawa, J. Membrane bioreactors and electrochemical processes for treatment of wastewaters containing heavy metal ions, organics, micropollutants and dyes: Recent developments. J. Hazard. Mater. 2019, 370, 172–195. [Google Scholar] [CrossRef]
- Asif, M.B.; Maqbool, T.; Zhang, Z. Electrochemical membrane bioreactors: State-of-the-art and future prospects. Sci. Total Environ. 2020, 741, 140233. [Google Scholar] [CrossRef]
- Zhang, M.; Leung, K.-T.; Lin, H.; Liao, B. The biological performance of a novel microalgal-bacterial membrane photobioreactor: Effects of HRT and N/P ratio. Chemosphere 2020, 261, 128199. [Google Scholar] [CrossRef]
- Muñoz, R.; Jacinto, M.; Guieysse, B.; Mattiasson, B. Combined carbon and nitrogen removal from acetonitrile using algal–bacterial bioreactors. Appl. Microbiol. Biotechnol. 2005, 67, 699–707. [Google Scholar] [CrossRef]
- Shaw, D.J. Introduction to Colloid and Surface Chemistry; Butterworths: Oxford, UK, 1980. [Google Scholar]
- Water Environment Federation; American Water Works Association; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005; Volume 21. [Google Scholar]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Assessment of membrane photobioreactor (MPBR) performance parameters and operating conditions. Water Res. 2018, 138, 169–180. [Google Scholar] [CrossRef]
- Jia, G.; Jing, H.; Hu, B.; Ju, Z.; He, S.; Shui, F.; Ce, J.; Xi, F.; Fa, F.; Hui, W. Monitoring and Analysis Methods for Water and Wastewater; China Environmental Science Press: Beijing, China, 2002; pp. 232–235. [Google Scholar]
- Haberkorn, I.; Buchmann, L.; Hiestand, M.; Mathys, A. Continuous nanosecond pulsed electric field treatments foster the upstream performance of Chlorella vulgaris-based biorefinery concepts. Bioresour. Technol. 2019, 293, 122029. [Google Scholar] [CrossRef]
- Buchmann, L.; Mathys, A. Perspective on pulsed electric field treatment in the bio-based industry. Front. Bioeng. Biotechnol. 2019, 7, 265. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, T.; Singh, H.; Kaur, G. Intermittent fasting–dietary restriction as a biological hormetin for health benefits. In The Science of Hormesis in Health and Longevity; Rattan, S.I.S., Kyriazi, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 99–104. [Google Scholar]
- Zong, E.; Wei, D.; Wan, H.; Zheng, S.; Xu, Z.; Zhu, D. Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide. Chem. Eng. J. 2013, 221, 193–203. [Google Scholar] [CrossRef]
- Wei, C. Nutrient Removal and Fouling Reduction in Electrokinetic Membrane Bioreactor at Various Temperatures. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2009. [Google Scholar]
- Guo, M.; Feng, L.; Liu, Y.; Zhang, L. Electrochemical simultaneous denitrification and removal of phosphorus from the effluent of a municipal wastewater treatment plant using cheap metal electrodes. Environ. Sci. Water Res. Technol. 2020, 6, 1095–1105. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Smith, E. Grey water treatment by a continuous process of an electrocoagulation unit and a submerged membrane bioreactor system. Chem. Eng. J. 2012, 198, 201–210. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Gao, B.; Yang, F. Minute electric field reduced membrane fouling and improved performance of membrane bioreactor. Sep. Purif. Technol. 2012, 86, 106–112. [Google Scholar] [CrossRef]
- Akamatsu, K.; Lu, W.; Sugawara, T.; Nakao, S.-i. Development of a novel fouling suppression system in membrane bioreactors using an intermittent electric field. Water Res. 2010, 44, 825–830. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess. Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Wu, J.; Chen, F.; Huang, X.; Geng, W.; Wen, X. Using inorganic coagulants to control membrane fouling in a submerged membrane bioreactor. Desalination 2006, 197, 124–136. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, H.; Husain, H. Use of chemical coagulants to control fouling potential for wastewater membrane bioreactor processes. Water Environ. Res. 2007, 79, 952–957. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Gao, B.; Yang, F.; Chellam, S. Fouling reductions in a membrane bioreactor using an intermittent electric field and cathodic membrane modified by vapor phase polymerized pyrrole. J. Membr. Sci. 2012, 394, 202–208. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Elektorowicz, M. Performance of the submerged membrane electro-bioreactor (SMEBR) with iron electrodes for wastewater treatment and fouling reduction. J. Membr. Sci. 2011, 379, 434–439. [Google Scholar] [CrossRef]
- Marbelia, L.; Bilad, M.R.; Passaris, I.; Discart, V.; Vandamme, D.; Beuckels, A.; Muylaert, K.; Vankelecom, I.F. Membrane photobioreactors for integrated microalgae cultivation and nutrient remediation of membrane bioreactors effluent. Bioresour. Technol. 2014, 163, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matho, C.; Schwarzenberger, K.; Eckert, K.; Keshavarzi, B.; Walther, T.; Steingroewer, J.; Krujatz, F. Bio-compatible flotation of Chlorella vulgaris: Study of zeta potential and flotation efficiency. Algal Res. 2019, 44, 101705. [Google Scholar] [CrossRef]
- Abt, V.; Gringel, F.; Han, A.; Neubauer, P.; Birkholz, M. Separation, characterization, and handling of microalgae by dielectrophoresis. Microorganisms 2020, 8, 540. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Huang, Y.; Liao, Q.; Xia, A.; Zhu, X.; Zhu, X. Analysis of the energy barrier between Chlorella vulgaris cells and their interfacial interactions with cationic starch under different pH and ionic strength. Bioresour. Technol. 2020, 304, 123012. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Price, N.T.; Pinilla, M.Á.G.; O’Shea, K.E. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Res. 2017, 123, 353–360. [Google Scholar] [CrossRef]
- Gizaw, A.; Zewge, F.; Kumar, A.; Mekonnen, A.; Tesfaye, M. A comprehensive review on nitrate and phosphate removal and recovery from aqueous solutions by adsorption. AQUA Water Infrastruct. Ecosyst. Soc. 2021, 70, 921–947. [Google Scholar] [CrossRef]
- Markou, G.; Depraetere, O.; Vandamme, D.; Muylaert, K. Cultivation of Chlorella vulgaris and Arthrospira platensis with recovered phosphorus from wastewater by means of zeolite sorption. Int. J. Mol. Sci. 2015, 16, 4250–4264. [Google Scholar] [CrossRef] [Green Version]
- González, L.E.; Cañizares, R.O.; Baena, S. Efficiency of ammonia and phosphorus removal from a Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour. Technol. 1997, 60, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Min, M.; Zhou, W.; Li, Y.; Mohr, M.; Cheng, Y.; Lei, H.; Liu, Y.; Lin, X.; Chen, P. Influence of exogenous CO2 on biomass and lipid accumulation of microalgae Auxenochlorella protothecoides cultivated in concentrated municipal wastewater. Appl. Biochem. Biotechnol. 2012, 166, 1661–1673. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Kuroda, M. Electric prompting and control of denitrification. Biotechnol. Bioeng. 1993, 42, 535–537. [Google Scholar] [CrossRef]
- Ibeid, S.; Elektorowicz, M.; Oleszkiewicz, J. Modification of activated sludge properties caused by application of continuous and intermittent current. Water Res. 2013, 47, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Griffith, R.; Li, W.; Peng, P.; Cheng, Y.; Chen, P.; Addy, M.M.; Liu, Y.; Ruan, R. A continuous flocculants-free electrolytic flotation system for microalgae harvesting. Bioresour. Technol. 2017, 238, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Marquez-Rocha, F.-J. Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 2004, 3, 21–34. [Google Scholar] [CrossRef] [Green Version]


| Water Quality Index | Average Value (mg/L) |
|---|---|
| Nitrogen | 25 ± 2 |
| Phosphorus (PO4 − P) | 3.5 ± 0.3 |
| Chemical Oxygen Demand | 20 ± 2.5 |
| Membrane Module | MPBR | EK-MPBR |
| Total membrane surface area | 0.03 m2 | 0.03 m2 |
| Membrane materials | Polyvinylidene fluoride (PVDF) | Polyvinylidene fluoride (PVDF) |
| Membrane type | Flat sheet | Flat sheet |
| Mean membrane pore size | 0.4 µm | 0.4 µm |
| Operational Parameter | ||
| Working volume | 10 L | 10 L |
| Temperature | 25 ± 0.8 °C | 25 ± 0.8 °C |
| pH | 8.46 ± 0.5 | 8.46 ± 0.5 |
| Aeration rate | 2.16 ± 0.10 L/min | 2.16 ± 0.10 L/min |
| Illumination intensity | 8400 lux | 8400 lux |
| Voltage gradient | 0.62 ± 0.02 V/cm | |
| Current density | 0.261 A/m2 | |
| Electrodes surface area | 0.015 ± 0.008 m2 | |
| Electrodes distance | 0.03 m |
| Source of Water/Wastewater | Type of MPBR | Influent Concentration (mg/L) | Organic Loading Rate (mg/L·d) | HRT and SRT (d) | Membrane Pore Size | Removal Efficiency% | Ref. |
|---|---|---|---|---|---|---|---|
| Synthetic municipal wastewater effluent | MPBR | TN: 25 ± 2 TP: 3.5 ± 0.4 | TN: 10.58 ± 1.02 TP: 1.48 ± 0.2 | HRT: 2.5 SRT: 30 | 0.4 µm | 68 ± 3 of TN 41.81 ± 0.05 of TP | This Study |
| Synthetic municipal wastewater effluent | EK-MPBR | TN: 25 ± 2 TP: 3.5 ± 0.4 | TN: 10.58 ± 1.02 TP: 1.48 ± 0.2 | HRT: 2.5 SRT: 30 | 0.4 µm | 43 ± 2 of TN 97.98 ± 0.02 of TP | This Study |
| Synthetic municipal wastewater | MPBR | N/A | HRT: 2.5 d SRT:12.5 d | N/A | 50 of TN 50 of TP | [59] | |
| Synthetic municipal wastewater | MPBR | TN: 14.1 ± 0.5 TP: 2.5 ± 0.2 | HRT: 1 d SRT: 9 d | 0.04 μm | 31 of TN 30 of TP | [43] | |
| Synthetic municipal wastewater | MPBR | TN: 14.1 ± 0.5 TP: 2.5 ± 0.2 | HRT: 1 d SRT: 30 d | 0.04 μm | 32 of TN 25 of TP | [43] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amini, M.; Mohamedelhassan, E.; Liao, B. The Biological Performance of a Novel Electrokinetic-Assisted Membrane Photobioreactor (EK-MPBR) for Wastewater Treatment. Membranes 2022, 12, 587. https://doi.org/10.3390/membranes12060587
Amini M, Mohamedelhassan E, Liao B. The Biological Performance of a Novel Electrokinetic-Assisted Membrane Photobioreactor (EK-MPBR) for Wastewater Treatment. Membranes. 2022; 12(6):587. https://doi.org/10.3390/membranes12060587
Chicago/Turabian StyleAmini, Maryam, Eltayeb Mohamedelhassan, and Baoqiang Liao. 2022. "The Biological Performance of a Novel Electrokinetic-Assisted Membrane Photobioreactor (EK-MPBR) for Wastewater Treatment" Membranes 12, no. 6: 587. https://doi.org/10.3390/membranes12060587
APA StyleAmini, M., Mohamedelhassan, E., & Liao, B. (2022). The Biological Performance of a Novel Electrokinetic-Assisted Membrane Photobioreactor (EK-MPBR) for Wastewater Treatment. Membranes, 12(6), 587. https://doi.org/10.3390/membranes12060587






