Experimental Determination of Hydrogen Isotope Transport Parameters in Vanadium
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
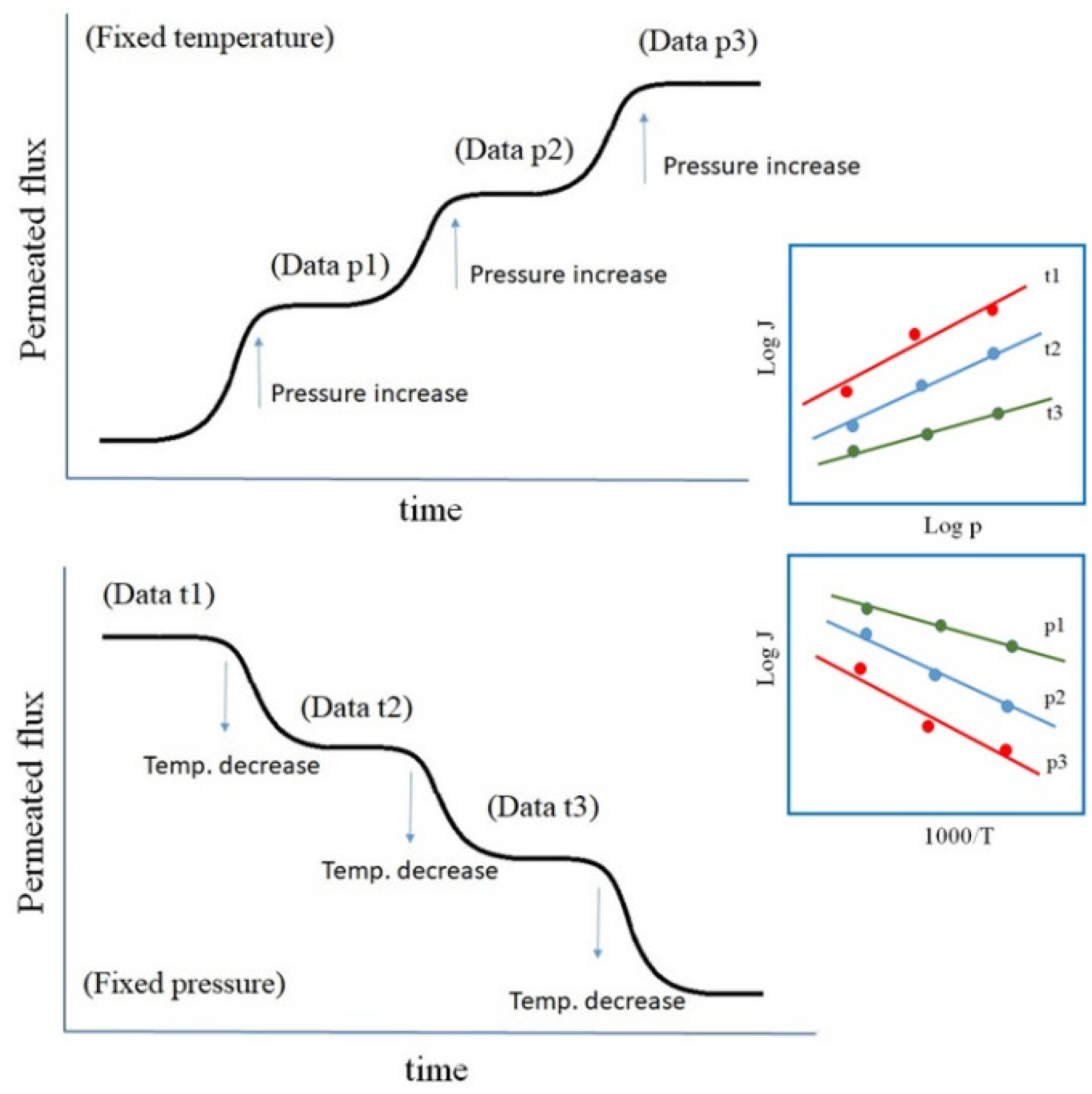
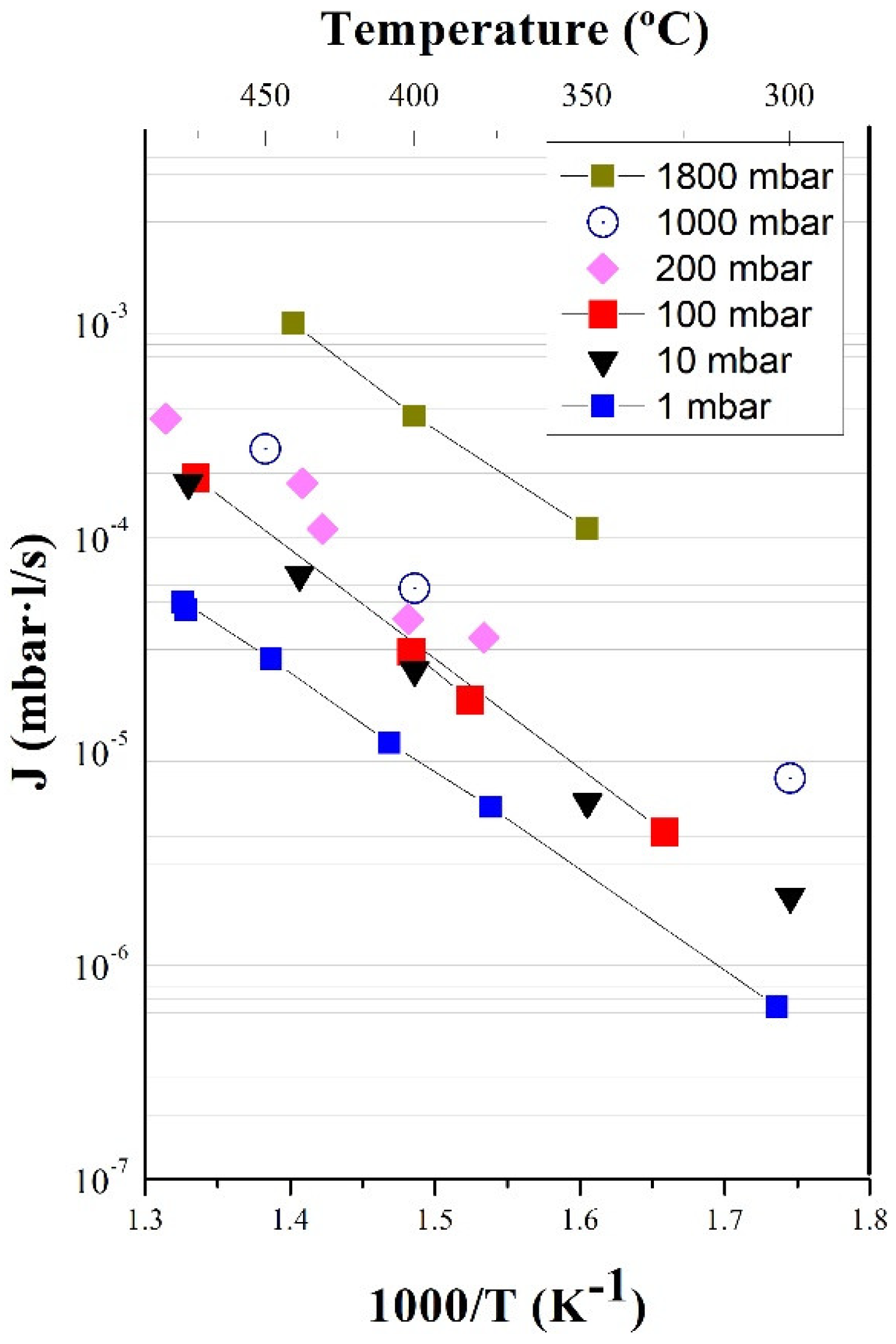
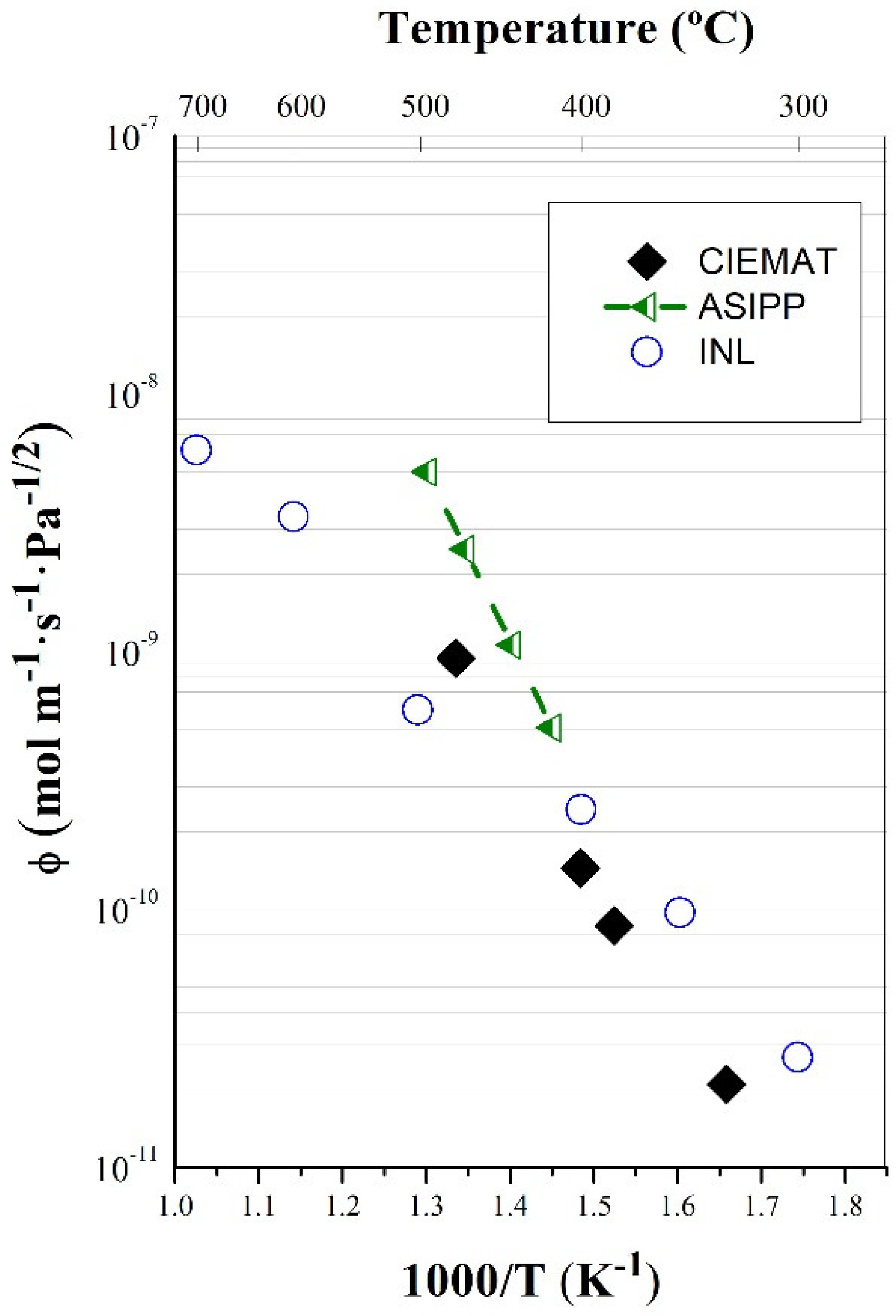
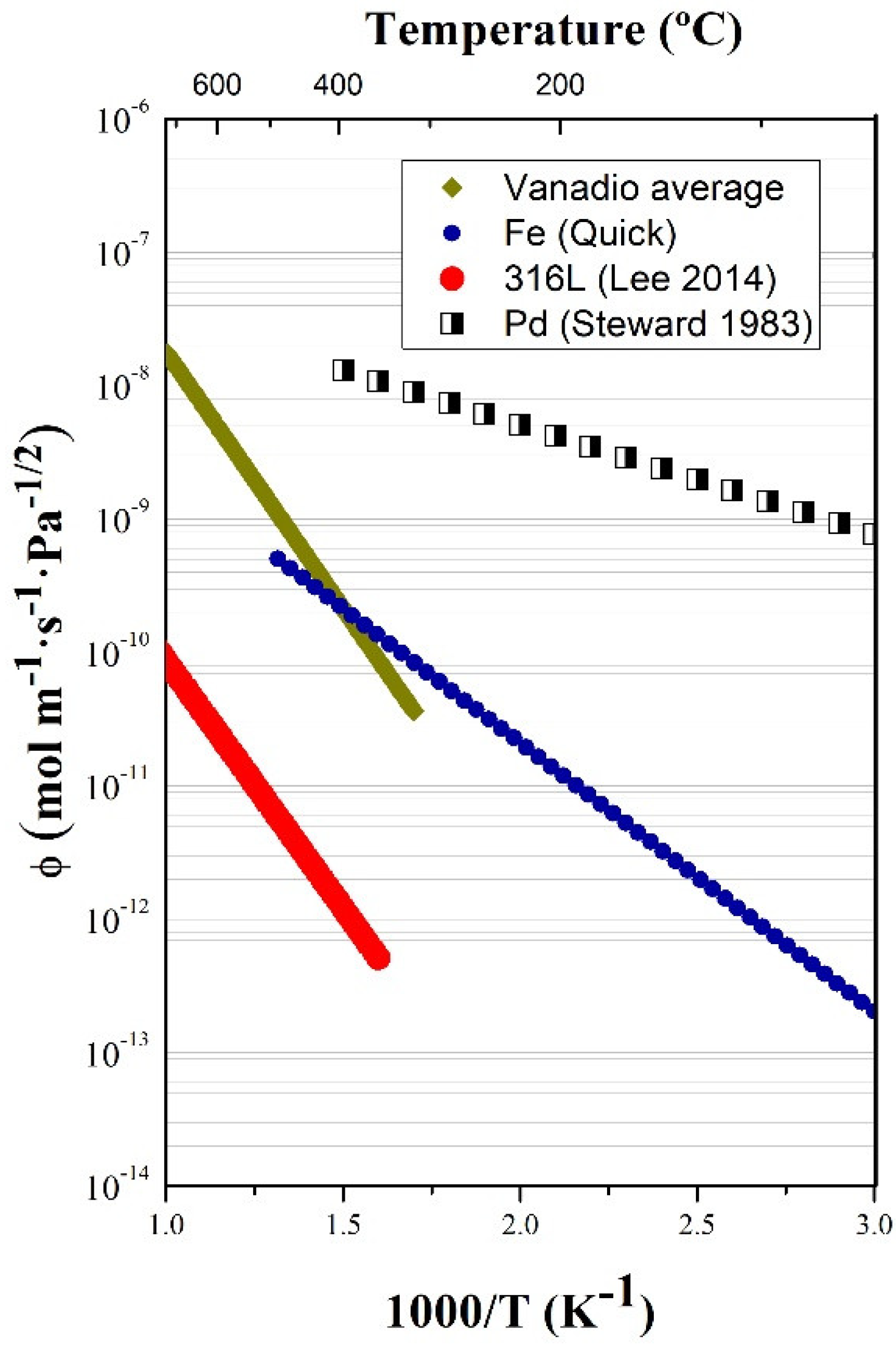
3.1. Permeability
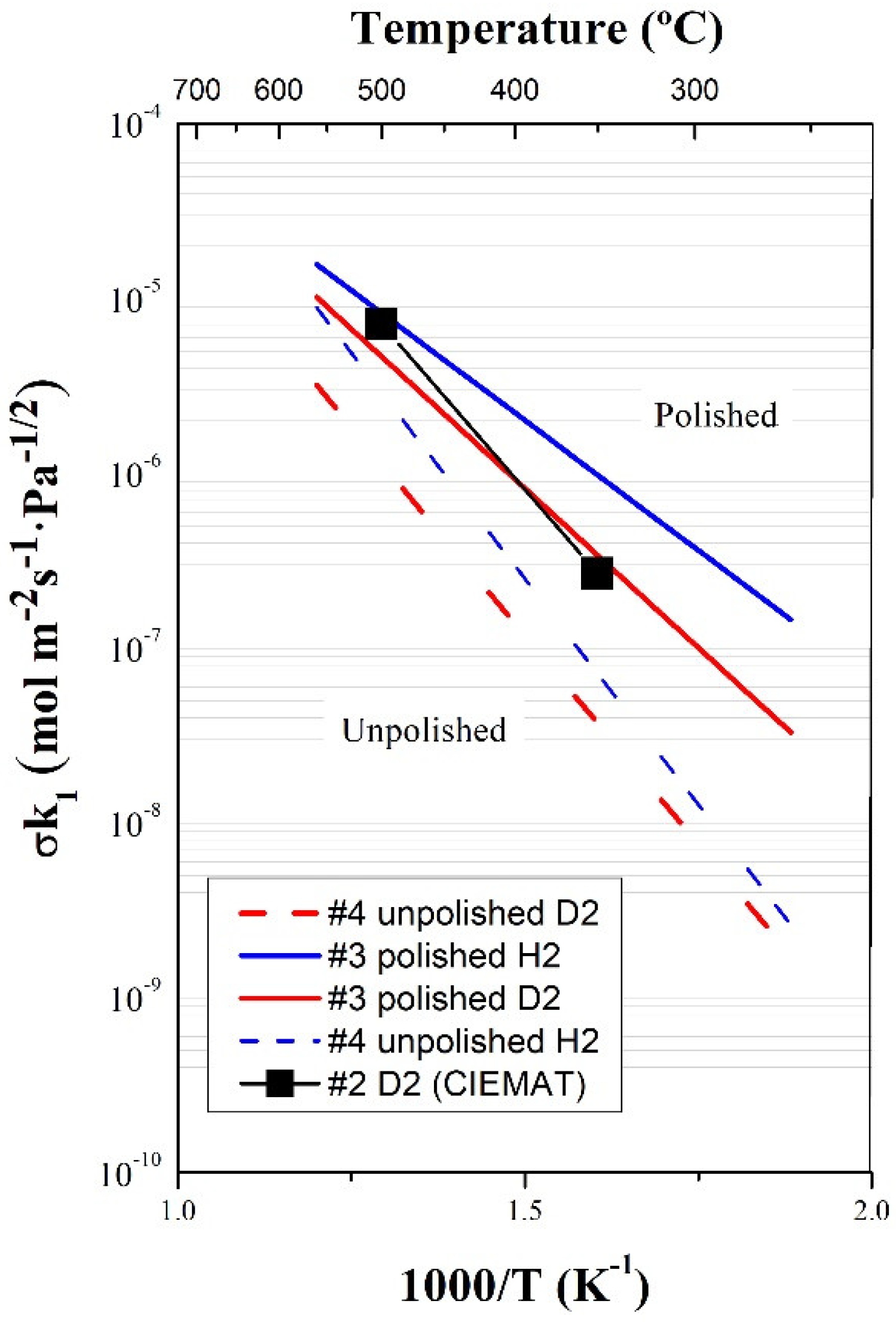
3.2. Surface Rate Constants
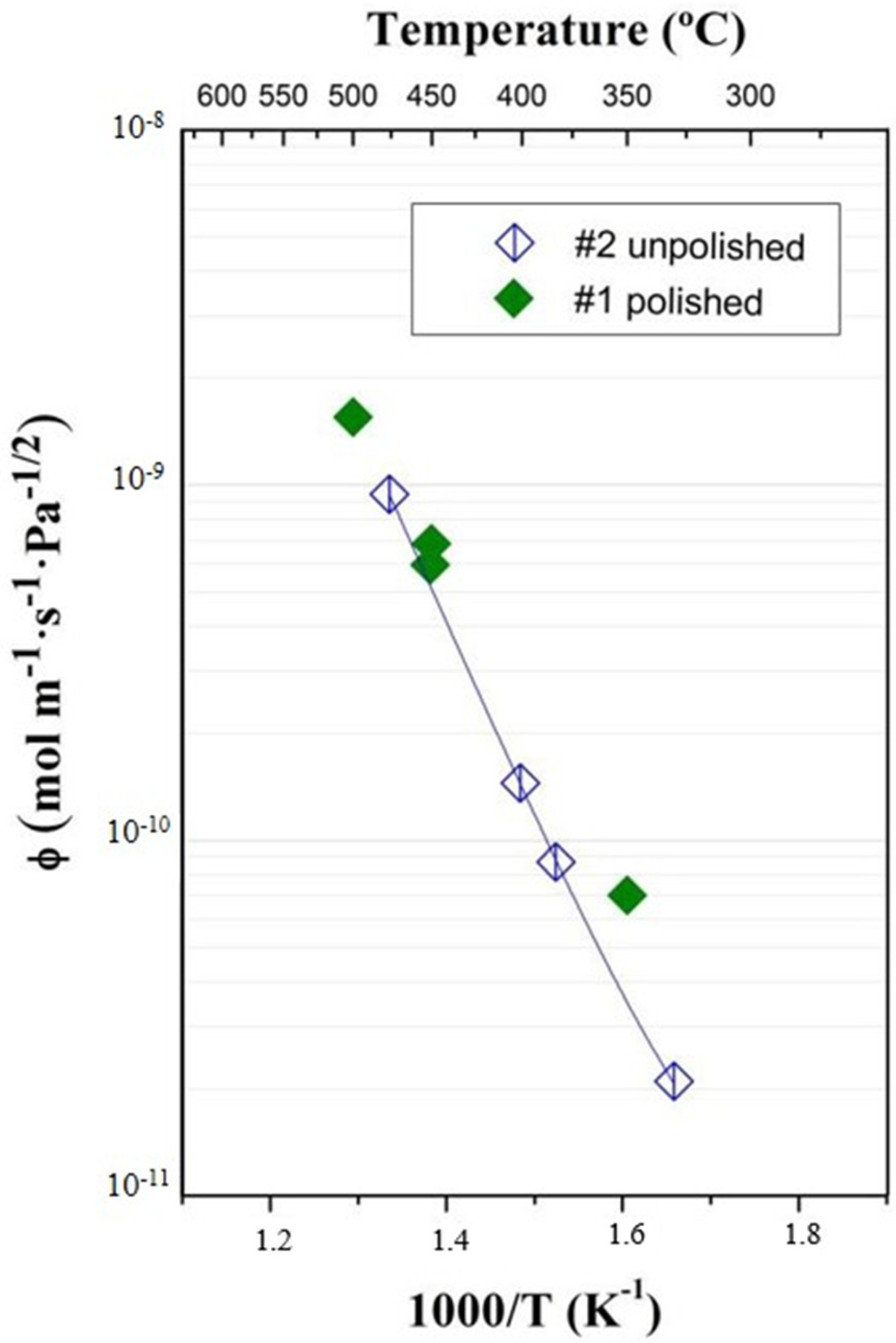
3.3. Surface Polishing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASIPP | Institute of Plasma Physics Chinese Academy of Sciences |
| CIEMAT | Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas |
| DLR | Diffusion Limited Regime |
| RMS | Root Mean Square |
| SLR | Surface Limited Regime |
| TDS | Thermally Desorption Spectroscopy |
| UPV/EHU | University of the Basque Country |
References
- Sipatov, I.S.; Sidorov, N.; Pastukhov, E.A.; Gabis, I.; Piven, V.A.; Esin, A.A.; Pryanichnikov, S.; Vostryakov, A.A. Hydrogen permeability and structure of vanadium alloy membranes. Pet. Chem. 2017, 57, 483–488. [Google Scholar] [CrossRef]
- Gallucci, F.F.; Fernandez, E.E.; Corengia, P.; Annaland, M.M.V.S. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Phair, J.W.; Donelson, R. Developments and design of novel (non-palladium-based) metal membranes for hydrogen separation. Ind. Eng. Chem. Res. 2006, 45, 5657–5674. [Google Scholar] [CrossRef]
- Dolan, M. Non-Pd BCC alloy membranes for industrial hydrogen separation. J. Membr. Sci. 2010, 362, 12–28. [Google Scholar] [CrossRef]
- Suzuki, A.; Yukawa, H. Quantitative Evaluations of Hydrogen Diffusivity in V-X. Membranes 2021, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Iyoha, O.; Enick, R.; Killmeyer, R.; Howard, B.; Ciocco, M.; Morreale, B. H2 production from simulated coal syngas containing H2S in multi-tubular Pd and 80wt% Pd–20wt% Cu membrane reactors at 1173 K. J. Membr. Sci. 2007, 306, 103–115. [Google Scholar] [CrossRef]
- Peters, B.J.; Hanke, S.; Day, C. Metal Foil Pump performance aspects in view of the implementation of Direct Internal Recycling for future fusion fuel cycles. Fusion Eng. Des. 2018, 136, 1467–1471. [Google Scholar] [CrossRef]
- Garcinuno, B.; Rapisarda, D.; Fernández-Berceruelo, I.; Jiménez-Rey, D.; Sanz, J.; Moreno, C.; Palermo, I.; Ibarra, Á. Design and fabrication of a Permeator Against Vacuum prototype for small scale testing at Lead-Lithium facility. Fusion Eng. Des. 2017, 124, 871–875. [Google Scholar] [CrossRef]
- Namba, T.; Miyaguchi, H.; Yamawaki, M.; Kanno, M. Hydrogen permeation through vanadium and the effect of surface impurity layer on it. J. Nucl. Mater. 1982, 105, 318–325. [Google Scholar] [CrossRef]
- Brimhall, J.L.; Simonen, E.P.; Jones, R.H. Data Base on Permeation, Diffusion and Concentration of Hydrogen Isotopes in Fusion Reactor Materials; DOE/ER—0313/16; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1994. [Google Scholar]
- Heinrich, R.R.; Johnson, C.E.; Crouthamel, C.E. Hydrogen Permeation Studies. II. Vanadium as a Hydrogen Electrode in a Lithium Hydride Cell. J. Electrochem. Soc. 1965, 112, 11. [Google Scholar]
- Steward, S.A. Review of Hydrogen Isotope Permeability through Materials; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1983. [Google Scholar]
- Reiter, F. Joint Research Centre Report EUR 1527 EN in Commision of The European Communities; ISPRA: Ispra, Italy, 1993. [Google Scholar]
- Malo, M.; Garcinuño, B.; Rapisarda, D. Experimental refutation of the deuterium permeability in vanadium, niobium and tantalum. Fusion Eng. Des. 2019, 146, 224–227. [Google Scholar] [CrossRef]
- D’Auria, V.; Dulla, S.; Ravetto, P.; Savoldi, L.; Utili, M.; Zanino, R. Design of a permeator-against-vacuum mock-up for the tritium extraction from PbLi at low speed. Fusion Eng. Des. 2017, 121, 198–203. [Google Scholar] [CrossRef]
- Malo, M.; Valle, F.; Jiménez, F.; Moroño, A.; Hodgson, E.; Moreno, C. Hisotope thermo-diffusion in structural materials. Fusion Eng. Des. 2017, 124, 924–927. [Google Scholar] [CrossRef]
- D’Auria, V.; Dulla, S.; Ravetto, P.; Savoldi, L.; Utili, M.; Zanino, R. Interaction of Copper Alloys with Hydrogen. In Copper Alloys—Early Applications and Current Performance—Enhancing Processes; Collini, L., Ed.; Intechopen: London, UK, 2012; ISBN 978-953-51-0160-4. [Google Scholar]
- Peñalva, I.; Alberro, G.; Aranburu, J.; Legarda, F.; Sancho, J.; Vila, R.; Ortiz, C. Influence of the Cr content on the permeation of hydrogen in Fe alloys. J. Nucl. Mater. 2012, 442, S719–S722. [Google Scholar] [CrossRef]
- Esteban, G.A.; PerujoL, A.; Sedano, L.A.; Mancinelli, B. The Surface Rate Constants of Deuterium in the Reduced Activating Martensitic Steel OPTIFER-IV-b. J. Nucl. Mater. 2000, 282, 89–96. [Google Scholar] [CrossRef]
- Fuerst, T.F.; Humrickhouse, P.W.; Taylor, C.N.; Shimada, M. Surface effects on deuterium permeation through vanadium membranes. J. Membr. Sci. 2020, 620, 118949. [Google Scholar] [CrossRef]
- Quick, N.R.; Johnson, H.H. Hydrogen and deuterium in iron, 49–506 C. Acta Metall. 1976, 26, 903–907. [Google Scholar] [CrossRef]
- Lee, S.K.; Yun, S.H.; Joo, H.G.; Noh, S.J. Deuterium transport and isotope effects in type 316L stainless steel at high temperatures for nuclear fusion and nuclear hydrogen technology applications. Curr. Appl. Phys. 2014, 14, 1385–1388. [Google Scholar] [CrossRef]
- Pisarev, A.; Tsvetkov, I.; Yarko, S.; Tanabe, T. Hydrogen permeation through membranes with rough surface. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2006; Volume 837, p. 238. [Google Scholar]
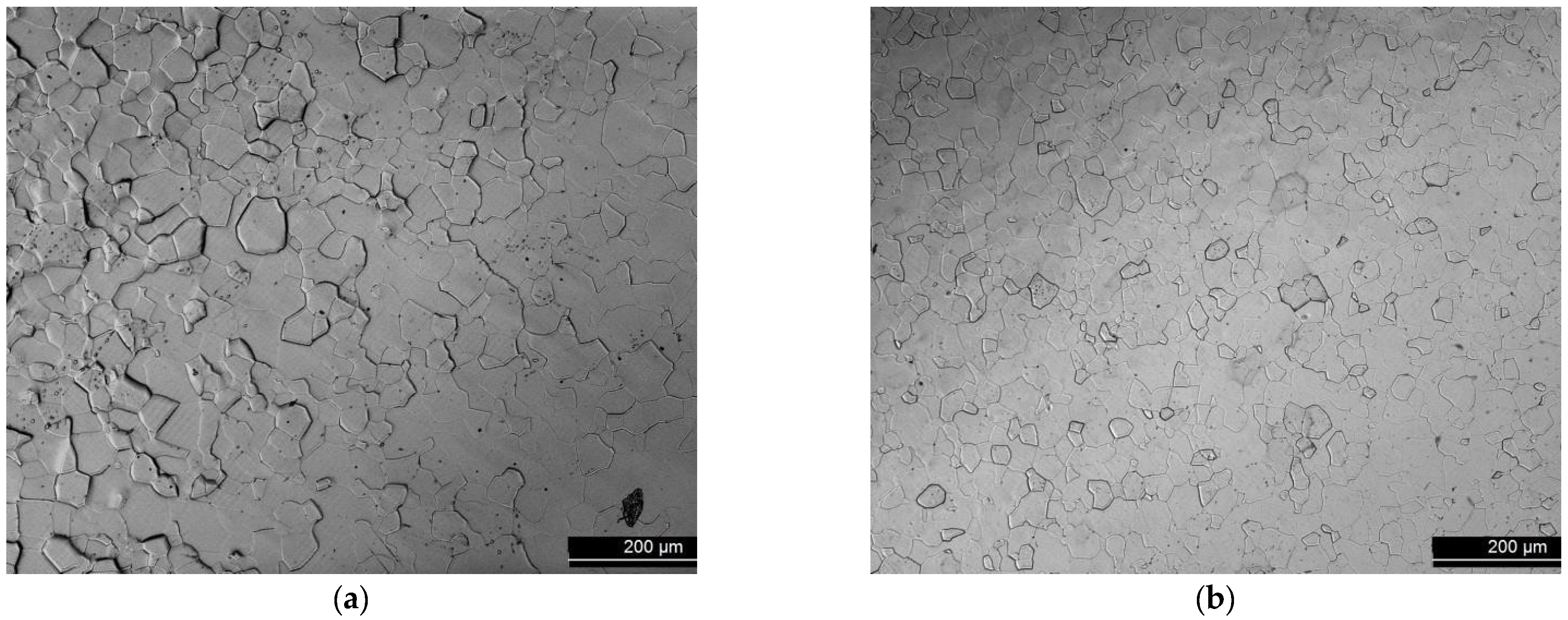

| Sample | Area mm2 | Thickness | Surface | p (mbar) | Regime | Gas | Institute |
|---|---|---|---|---|---|---|---|
| #1 | 38 × 38 | 0.40 mm | Polished | 100–1800 | DLR/SLR | D2 | CIEMAT |
| #2 | 38 × 38 | 1.0 mm | Unpolished | 1–100 | DLR/SLR | D2 | CIEMAT |
| #3 | 30 × 30 | 0.60 mm | Polished | 0.3–1.8 | SLR | D2/H2 | UPV/EHU |
| #4 | 30 × 30 | 1.0 mm | Unpolished | 0.3–40.0 | SLR | D2/H2 | UPV/EHU |
| #5 | Φ 20 mm | 0.96 mm | Polished | 20–200 | DLR | D2 | ASIPP |
| Sample | Gas | σk1 (mol/(m2 s Pa)) * | σk2 (mol−1·m4 s−1) * |
|---|---|---|---|
| #3 | H | 4.72 × 10−2e(−6.72x) | 2.48e(−1.37×10x) |
| #3 | D | 1.92 × 10−1e(−8.23x) | 1.01 × 10e(−1.52×10x) |
| #4 | H | 1.41 × 10e(−1.19×10x) | 7.40 × 102e(−1.89×10x) |
| #4 | D | 1.73e(−1.10×10x) | 9.08 × 10e(+1.82×10x) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malo, M.; Peñalva, I.; Azkurreta, J.; Garcinuño, B.; Liu, H.-D.; Rapisarda, D.; Zhou, H.-S.; Luo, G.-N. Experimental Determination of Hydrogen Isotope Transport Parameters in Vanadium. Membranes 2022, 12, 579. https://doi.org/10.3390/membranes12060579
Malo M, Peñalva I, Azkurreta J, Garcinuño B, Liu H-D, Rapisarda D, Zhou H-S, Luo G-N. Experimental Determination of Hydrogen Isotope Transport Parameters in Vanadium. Membranes. 2022; 12(6):579. https://doi.org/10.3390/membranes12060579
Chicago/Turabian StyleMalo, Marta, Igor Peñalva, Jon Azkurreta, Belit Garcinuño, Hao-Dong Liu, David Rapisarda, Hai-Shan Zhou, and Guang-Nan Luo. 2022. "Experimental Determination of Hydrogen Isotope Transport Parameters in Vanadium" Membranes 12, no. 6: 579. https://doi.org/10.3390/membranes12060579
APA StyleMalo, M., Peñalva, I., Azkurreta, J., Garcinuño, B., Liu, H.-D., Rapisarda, D., Zhou, H.-S., & Luo, G.-N. (2022). Experimental Determination of Hydrogen Isotope Transport Parameters in Vanadium. Membranes, 12(6), 579. https://doi.org/10.3390/membranes12060579







