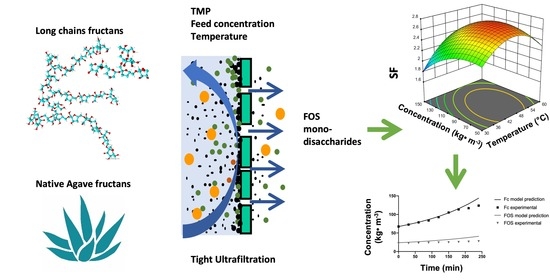
Effect of Operating Conditions and Fructans Size Distribution on Tight Ultrafiltration Process for Agave Fructans Fractionation: Optimization and Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Solution
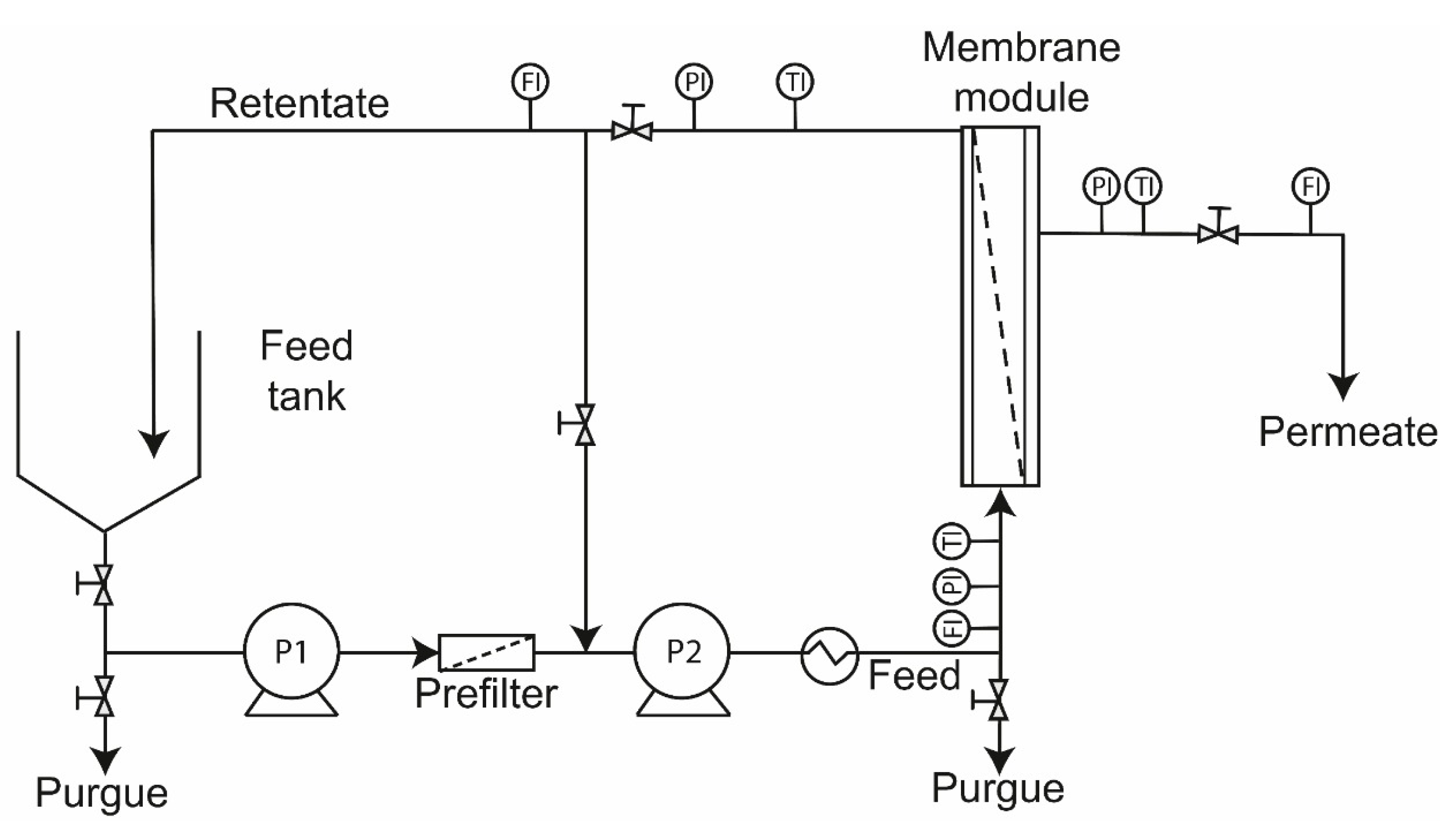
2.2. Membrane System and Procedures
2.3. Experimental Box-Behnken Design
2.4. Mathematical Modeling
- −
- Unsteady state.
- −
- The transport of solutes is carried out in a single direction (flat plate).
- −
- Osmotic pressures of macromolecular solutions are negligible
- −
- The apparent rejection is constant throughout the process.
- −
- The process is in limited flow conditions.
2.5. Determination of Size Distribution of Agave Fructans
3. Results
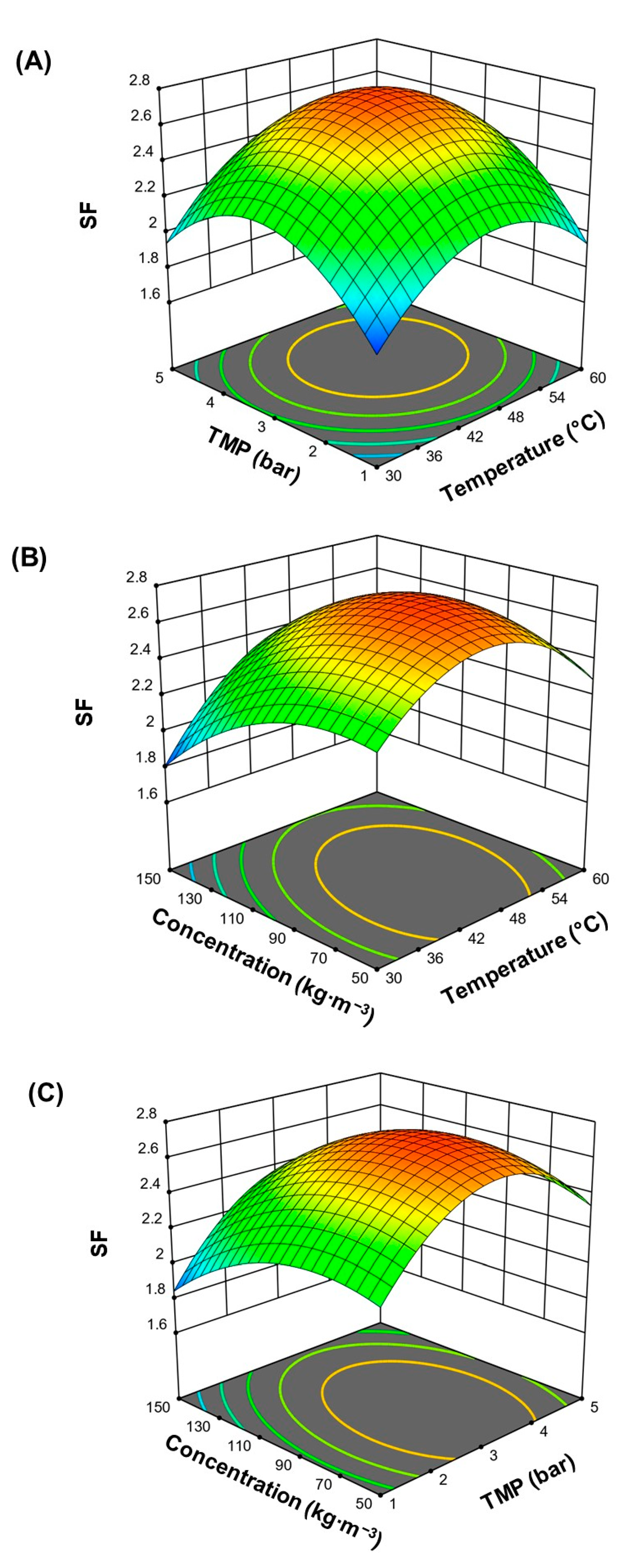
3.1. Experimental Box-Behnken Design: Effect of Operating Conditions on the Separation Factor
3.2. Effect of Operating Conditions on the Permeate Flux
3.3. Optimization of Responses
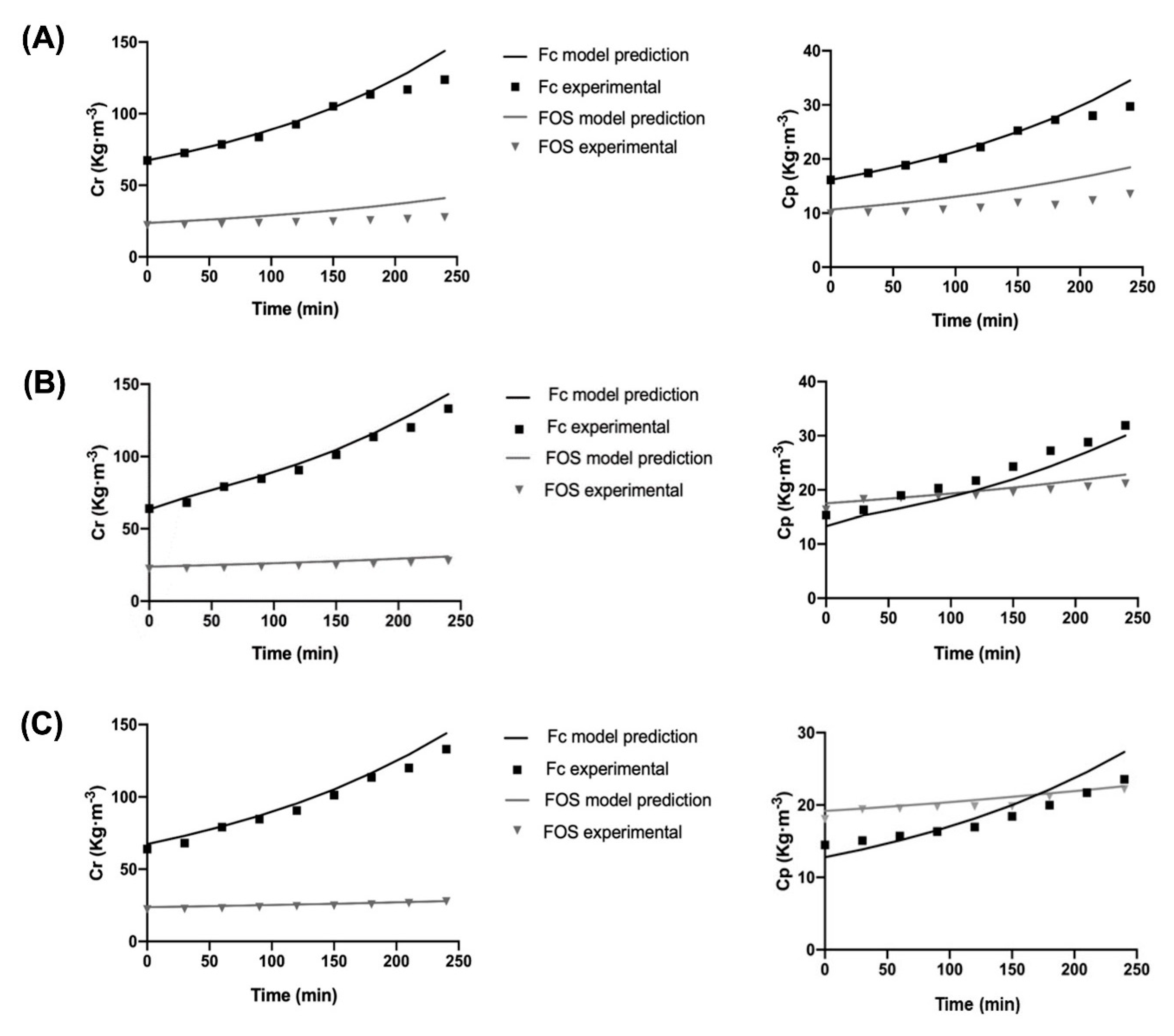
3.4. Mathematical Modeling for Different Native Fructans Size Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Praznik, W.; Loeppert, R.; Viernstein, H.; Haslberger, A.G.; Unger, F.M. Dietary Fiber and Prebiotics. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K., Mérillon, J.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 891–925. [Google Scholar] [CrossRef]
- Sankarapandian, V.; Nitharsan, K.; Parangusadoss, K.; Gangadaran, P. Prebiotic Potential and Value-Added Products Derived from Spirulina Laxissima SV001—A Step towards Healthy Living. BioTech 2022, 11, 13. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Fernández, Á.; Galicia-Lagunas, N.; Rodríguez-Alegría, M.E.; Olvera, C.; López-Munguía, A. Production of Functional Oligosaccharides through Limited Acid Hydrolysis of Agave Fructans. Food Chem. 2011, 129, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vilet, L.; Bostyn, S.; Flores-Montaño, J.L.; Camacho-Ruiz, R.M. Size-Exclusion Chromatography (HPLC-SEC) Technique Optimization by Simplex Method to Estimate Molecular Weight Distribution of Agave Fructans. Food Chem. 2017, 237, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Hernández, C.I.; Aguirre-Rivera, J.R.; Juárez-Flores, B.I.; Ortiz-Pérez, M.D.; Becerra-Jiménez, J. Extraction and Characterization of Agave Salmiana Otto Ex Salm-Dyck Fructans. Rev. Chapingo Ser. Ciencias For. Ambient. 2015, 22, 59–72. [Google Scholar] [CrossRef]
- Arrizon, J.; Morel, S.; Gschaedler, A.; Monsan, P. Comparison of the Water-Soluble Carbohydrate Composition and Fructan Structures of Agave Tequilana Plants of Different Ages. Food Chem. 2010, 122, 123–130. [Google Scholar] [CrossRef]
- Ramnani, P.; Costabile, A.; Bustillo, A.G.R.; Gibson, G.R. A Randomised, Double- Blind, Cross-over Study Investigating the Prebiotic Effect of Agave Fructans in Healthy Human Subjects. J. Nutr. Sci. 2015, 4, e10. [Google Scholar] [CrossRef] [Green Version]
- Urías-Silvas, J.E.; Cani, P.D.; Delmée, E.; Neyrinck, A.; López, M.G.; Delzenne, N.M. Physiological Effects of Dietary Fructans Extracted from Agave tequilana Gto. and Dasylirion Spp. Br. J. Nutr. 2008, 99, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Sáyago-Ayerdi, S.G.; Mateos, R.; Ortiz-Basurto, R.I.; Largo, C.; Serrano, J.; Granado-Serrano, A.B.; Sarriá, B.; Bravo, L.; Tabernero, M. Effects of Consuming Diets Containing Agave Tequilana Dietary Fibre and Jamaica Calyces on Body Weight Gain and Redox Status in Hypercholesterolemic Rats. Food Chem. 2014, 148, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Padilla-Camberos, E.; Barragán-Álvarez, C.P.; Diaz-Martinez, N.E.; Rathod, V.; Flores-Fernández, J.M. Effects of Agave Fructans (Agave Tequilana Weber Var. Azul) on Body Fat and Serum Lipids in Obesity. Plant Foods Hum. Nutr. 2018, 73, 34–39. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Urías-Silvas, J.E.; Morales-Hernández, N. The Role of Agave Fructans in Health and Food Applications: A Review. Trends Food Sci. Technol. 2021, 114, 585–598. [Google Scholar] [CrossRef]
- Crispín-Isidro, G.; Lobato-Calleros, C.; Espinosa-Andrews, H.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of Inulin and Agave Fructans Addition on the Rheological, Microstructural and Sensory Properties of Reduced-Fat Stirred Yogurt. LWT-Food Sci. Technol. 2015, 62, 438–444. [Google Scholar] [CrossRef]
- Palatnik, D.R.; Aldrete Herrera, P.; Rinaldoni, A.N.; Ortiz Basurto, R.I.; Campderrós, M.E. Development of Reduced-Fat Cheeses with the Addition of Agave Fructans. Int. J. Dairy Technol. 2017, 70, 212–219. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; López, M.G. Evaluation of Agave Angustifolia Fructans as Fat Replacer in the Cookies Manufacture. LWT-Food Sci. Technol. 2017, 77, 100–109. [Google Scholar] [CrossRef]
- Ortiz-Basurto, R.I.; Rubio-Ibarra, M.E.; Ragazzo-Sanchez, J.A.; Beristain, C.I.; Jiménez-Fernández, M. Microencapsulation of Eugenia uniflora L. Juice by Spray Drying Using Fructans with Different Degrees of Polymerisation. Carbohydr. Polym. 2017, 175, 603–609. [Google Scholar] [CrossRef]
- Jimenez-Sánchez, D.E.; Calderón-Santoyo, M.; Ortiz-Basurto, R.I.; Bautista-Rosales, P.U.; Ragazzo-Sánchez, J.A. Effect of Maltodextrin Reduction and Native Agave Fructans Addition on the Physicochemical Properties of Spray-Dried Mango and Pineapple Juices. Food Sci. Technol. Int. 2018, 24, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Reiner, J.; Fleischhacker, L.; Viernstein, H.; Loeppert, R.; Praznik, W. Growth of Selected Probiotic Strains with Fructans from Different Sources Relating to Degree of Polymerization and Structure. J. Funct. Foods 2016, 24, 264–275. [Google Scholar] [CrossRef]
- Márquez-Aguirre, A.L.; Camacho-Ruiz, R.M.; Arriaga-Alba, M.; Padilla-Camberos, E.; Kirchmayr, M.R.; Blasco, J.L.; González-Avila, M. Effects of Agave Tequilana Fructans with Different Degree of Polymerization Profiles on the Body Weight, Blood Lipids and Count of Fecal Lactobacilli/Bifidobacteria in Obese Mice. Food Funct. 2013, 4, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Aguirre, A.L.; Camacho-Ruíz, R.M.; Gutiérrez-Mercado, Y.K.; Padilla-Camberos, E.; González-Ávila, M.; Gálvez-Gastélum, F.J.; Díaz-Martínez, N.E.; Ortuño-Sahagún, D. Fructans from Agave Tequilana with a Lower Degree of Polymerization Prevent Weight Gain, Hyperglycemia and Liver Steatosis in High-Fat Diet-Induced Obese Mice. Plant Foods Hum. Nutr. 2016, 71, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Pérez, N.; Rodríguez-Mendiola, M.A.; De Alba, P.L.L.; Martínez, L.L.; Gutiérrez-Miceli, F.A.; Arias-Castro, C. Optimización de Los Procesos de Extracción y Fraccionamiento Por Grado de Polimerización de Fructanos, Obtenidos a Partir de Agave Tequilana Weber Var. Azul, Para La Obtención de Prebióticos. Gayana Bot. 2012, 69 (Suppl. 1), 31–39. [Google Scholar]
- Singh, R. Introduction to Membrane Technology. In Membrane Technology and Engineering for Water Purification, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 1–80. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of Ceramic and Polymeric Membrane Permeability and Fouling Using Surface Water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Diná Afonso, M.; Ferrer, J.; Bórquez, R. An Economic Assessment of Proteins Recovery from Fish Meal Effluents by Ultrafiltration. Trends Food Sci. Technol. 2004, 15, 506–512. [Google Scholar] [CrossRef]
- Walha, K.; Ben Amar, R.; Massé, A.; Bourseau, P.; Cardinal, M.; Cornet, J.; Prost, C.; Jaouen, P. Aromas Potentiality of Tuna Cooking Juice Concentrated by Nanofiltration. LWT-Food Sci. Technol. 2011, 44, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Grangeon, A.; Lescoche, P. Flat Ceramic Membranes for the Treatment of Dairy Products: Comparison with Tubular Ceramic Membranes. Lait 2000, 80, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhao, Y.; Zhou, S.; Xing, W.; Wong, F.S. Resistance Analysis for Ceramic Membrane Microfiltration of Raw Soy Sauce. J. Memb. Sci. 2007, 299, 122–129. [Google Scholar] [CrossRef]
- Almandoz, C.; Pagliero, C.; Ochoa, A.; Marchese, J. Corn Syrup Clarification by Microfiltration with Ceramic Membranes. J. Memb. Sci. 2010, 363, 87–95. [Google Scholar] [CrossRef]
- Hinkova, A.; Bubník, Z.; Kadlec, P.; Pridal, J. Potentials of Separation Membranes in the Sugar Industry. Sep. Purif. Technol. 2002, 26, 101–110. [Google Scholar] [CrossRef]
- Vegas, R.; Moure, A.; Domínguez, H.; Parajó, J.C.; Alvarez, J.R.; Luque, S. Evaluation of Ultra- and Nanofiltration for Refining Soluble Products from Rice Husk Xylan. Bioresour. Technol. 2008, 99, 5341–5351. [Google Scholar] [CrossRef]
- Aguirre Montesdeoca, V.; Janssen, A.E.M.; Boom, R.M.; Van der Padt, A. Fine Ultrafiltration of Concentrated Oligosaccharide Solutions—Hydration and Pore Size Distribution Effects. J. Memb. Sci. 2019, 580, 161–176. [Google Scholar] [CrossRef]
- Machado, M.T.C.; Trevisan, S.; Pimentel-Souza, J.D.R.; Pastore, G.M.; Hubinger, M.D. Clarification and Concentration of Oligosaccharides from Artichoke Extract by a Sequential Process with Microfiltration and Nanofiltration Membranes. J. Food Eng. 2016, 180, 120–128. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Santibañez, L.; Cassano, A.; Ruby-Figueroa, R.; Illanes, A. Purification of Galacto-Oligosaccharides (GOS) by Three-Stage Serial Nanofiltration Units under Critical Transmembrane Pressure Conditions. Chem. Eng. Res. Des. 2017, 117, 488–499. [Google Scholar] [CrossRef]
- Reynoso-Ponce, H.; Grajales-Lagunes, A.; Castillo-Andrade, A.; González-García, R.; Ruiz-Cabrera, M.A. Integration of Nanofiltration and Spray Drying Processes for Enhancing the Purity of Powdered Fructans from Agave Salmiana Juice. Powder Technol. 2017, 322, 96–105. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Moscosa-Santillán, M.; Grajales-Lagunes, A.; González-Chávez, M.; Bonnin-Paris, J.; Bostyn, S.; Ruiz-Cabrera, M. Sugars and Fructans Separation by Nanofiltration from Model Sugar Solution and Comparative Study with Natural Agave Juice. Sep. Sci. Technol. 2013, 48, 1768–1776. [Google Scholar] [CrossRef]
- Flores Montaño, J.L.; Camacho Ruiz, R.M.; Prado Ramírez, R.; Morena Vilet, L.; Luiz Santos, N.; Mendoza Rivera, M.; de los, Á.; Ballón Villagrá, A. Fructanos Fraccionados de Agave y Su Proceso de Obtención a Nivel Piloto e Industrial. MX Patent 375478 B, 15 October 2015. Available online: https://vidoc.impi.gob.mx/visor?usr=SIGA&texp=SI&tdoc=E&id=MX/a/2015/014523 (accessed on 28 April 2022).
- Pérez Martínez, F.J.; Gonzalez Avila, M.; Camacho Ruiz, R.M.; Márquez Aguirre, A.L.; Alonso Segura, D.; Gschaedler Mathis, A.C.; Prado Ramírez, R.; Flores Montaño, J.L.; Mateos Díaz, J.C.; Arrizón Gaviño, J.P. Fructanos Fraccionados de Agave, Proceso de Obtención y Uso de Los Mismos. MX Patent 367976 B, 30 April 2013. Available online: https://vidoc.impi.gob.mx/visor?usr=SIGA&texp=SI&tdoc=E&id=MX/a/2013/004903 (accessed on 28 April 2022).
- Luiz-Santos, N.; Prado-Ramírez, R.; Arriola-Guevara, E.; Camacho-Ruiz, R.M.; Moreno-Vilet, L. Performance Evaluation of Tight Ultrafiltration Membrane Systems at Pilot Scale for Agave Fructans Fractionation and Purification. Membranes 2020, 10, 261. [Google Scholar] [CrossRef]
- Kuhn, R.C.; Maugeri Filho, F.; Silva, V.; Palacio, L.; Hernández, A.; Prádanos, P. Mass Transfer and Transport during Purification of Fructooligosaccharides by Nanofiltration. J. Memb. Sci. 2010, 365, 356–365. [Google Scholar] [CrossRef]
- González-Muñoz, M.J.; Parajó, J.C. Diafiltration of Eucalyptus Wood Autohydrolysis Liquors: Mathematical Modeling. J. Memb. Sci. 2010, 346, 98–104. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brand, G.C.; Silva, E.G.P.; Reis, P.S.; Souza, A.S.; Santos, W.N.L. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Ruby-figueroa, R.; Cassano, A.; Drioli, E. Ultrafiltration of Orange Press Liquor: Optimization of Operating Conditions for the Recovery of Antioxidant Compounds by Response Surface Methodology. Sep. Purif. Technol. 2012, 98, 255–261. [Google Scholar] [CrossRef]
- Montañez, J.; Venegas, J.; Vivar, M.; Ramos, E. Los Fructanos Contenidos en la Cabeza y en las Hojas del Agave Tequilana Weber AZUL. Bioagro 2011, 23, 199–206. [Google Scholar]
- Datta, D.; Bhattacharjee, S.; Nath, A.; Das, R.; Bhattacharjee, C.; Datta, S. Separation of Ovalbumin from Chicken Egg White Using Two-Stage Ultrafiltration Technique. Sep. Purif. Technol. 2009, 66, 353–361. [Google Scholar] [CrossRef]
- Foley, G. Minimisation of Process Time in Ultrafiltration and Continuous Diafiltration: The Effect of Incomplete Macrosolute Rejection. J. Memb. Sci. 1999, 163, 349–355. [Google Scholar] [CrossRef]
- Foley, G. Water Usage in Variable Volume Diafiltration: Comparison with Ultrafiltration and Constant Volume Diafiltration. Desalination 2006, 196, 160–163. [Google Scholar] [CrossRef]
- Vizzi, M. Membrane separations in biorefinery. Development of a General Predictive Model. Master’s Thesis, Ing. Scuola di Ingegneria Industriale e dell’ informazione. Politecnico Di Milano, Milano, Italy, 2016. Available online: https://www.politesi.polimi.it/handle/10589/131635 (accessed on 28 April 2022).
- Goulas, A.K.; Kapasakalidis, P.G.; Sinclair, H.R.; Rastall, R.A.; Grandison, A.S. Purification of Oligosaccharides by Nanofiltration. J. Memb. Sci. 2002, 209, 321–335. [Google Scholar] [CrossRef]
- Tsuru, T.; Izumi, S.; Yoshioka, T.; Asaeda, M. Temperature Effect on Transport Performance by Inorganic Nanofiltration Membranes. AIChE J. 2000, 46, 565–574. [Google Scholar] [CrossRef]
- Ponce, J.A.; Macías, E.R.; Soltero, J.A.; Fernández, V.V.; Zúñiga, V.; Escalona, H.B. Physical-Chemical and Non-Linear Rheological Properties of Aqueous Solutions of Agave Fructans. e-Gnosis 2008, 6, 1–23. [Google Scholar]
- Moreno-Vilet, L.; Bonnin-Paris, J.; Bostyn, S.; Ruiz-Cabrera, M.A.; Moscosa-Santillán, M. Assessment of Sugars Separation from a Model Carbohydrates Solution by Nanofiltration Using a Design of Experiments (DoE) Methodology. Sep. Purif. Technol. 2014, 131, 84–93. [Google Scholar] [CrossRef]
- Ben Amar, N.; Saidani, H.; Palmeri, J.; Deratani, A. Effect of Temperature on the Rejection of Neutral and Charged Solutes by Desal 5 DK Nanofiltration Membrane. Desalination 2009, 246, 294–303. [Google Scholar] [CrossRef]
- Dang, H.Q.; Price, W.E.; Nghiem, L.D. The Effects of Feed Solution Temperature on Pore Size and Trace Organic Contaminant Rejection by the Nanofiltration Membrane NF270. Sep. Purif. Technol. 2014, 125, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-González, F.; Parra-Montes de Oca, M.A.; Ávila-Reyes, S.V.; Camacho-Díaz, B.H.; Alamilla-Beltrán, L.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L. A Rheological Study of Chicory and Agave Tequilana Fructans for Use in Foods. LWT 2019, 115, 108137. [Google Scholar] [CrossRef]
- Bacchin, P.; Aimar, P.; Field, R.W. Critical and Sustainable Fluxes: Theory, Experiments and Applications. J. Memb. Sci. 2006, 281, 42–69. [Google Scholar] [CrossRef] [Green Version]
- Fernando, J.; Mario, C.; Heurística, E.; Usabilidad, D.E.L.A.; Para, D.E.S.; El, F.; Motriz, S.D.E.D.; Sánchez-Álvarez, J.F.; Zapata-Jaramillo, C.M.; Jiménez-Builes, J.A. Elección de la función de deseabilidad para diseños óptimos bajo restricciones. Rev. EIA 2017, 15, 171–187. [Google Scholar]
- Rizki, Z.; Janssen, A.E.M.; Boom, R.M.; van der Padt, A. Oligosaccharides Fractionation Cascades with 3 Outlet Streams. Sep. Purif. Technol. 2019, 221, 183–194. [Google Scholar] [CrossRef]
- Franck, A. Technological Functionality of Inulin and Oligofructose. Br. J. Nutr. 2002, 87, S287–S291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, R.L. Macromolecular Ultrafiltration with Microporous Membranes. Ind. Eng. Chem. Fundam. 1971, 10, 113–120. [Google Scholar] [CrossRef]

| Factors | Code | Variation Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature (°C) | X1 | 30 | 45 | 60 |
| TMP (bar) | X2 | 1 | 3 | 5 |
| Feed concentration (kg∙m−3) | X3 | 50 | 100 | 150 |
| Run | Temperature (°C) | TMP (bar) | Feed Concentration (kg∙m−3) | SF | Ji (kg∙h−1∙m−2) |
|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | |
| 1 | 45 (0) | 3 (0) | 100 (0) | 2.80 | 1.350 |
| 2 | 30 (−1) | 1 (−1) | 100 (0) | 1.75 | 0.270 |
| 3 | 45 (0) | 3 (0) | 100 (0) | 2.74 | 1.660 |
| 4 | 60 (1) | 3 (0) | 50 (−1) | 2.30 | 1.010 |
| 5 | 30 (−1) | 3 (0) | 150 (1) | 1.80 | 1.130 |
| 6 | 60 (1) | 1 (−1) | 100 (0) | 1.91 | 0.630 |
| 7 | 60 (1) | 3 (0) | 150 (1) | 2.25 | 2.220 |
| 8 | 60 (1) | 5 (1) | 100 (0) | 2.30 | 2.600 |
| 9 | 30 (−1) | 5 (1) | 100 (0) | 1.97 | 1.350 |
| 10 | 45 (0) | 5 (1) | 50 (−1) | 2.28 | 0.800 |
| 11 | 45 (0) | 5 (1) | 150 (1) | 2.14 | 1.510 |
| 12 | 45 (0) | 3 (0) | 100 (0) | 2.60 | 1.230 |
| 13 | 45 (0) | 1 (−1) | 150 (1) | 1.90 | 0.790 |
| 14 | 45 (0) | 1 (−1) | 50 (−1) | 2.22 | 0.380 |
| 15 | 30 (−1) | 3 (0) | 50 (−1) | 2.34 | 0.560 |
| 16 | 45 (0) | 3 (0) | 100 (0) | 2.70 | 1.110 |
| Source | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| X1 | 0.1013 | 1 | 0.1013 | 16.82 | 0.0064 * |
| X2 | 0.1035 | 1 | 0.1035 | 17.19 | 0.0060 * |
| X3 | 0.1378 | 1 | 0.1378 | 22.89 | 0.0030 * |
| X1X2 | 0.0072 | 1 | 0.0072 | 1.20 | 0.3153 |
| X1X3 | 0.0600 | 1 | 0.0600 | 9.97 | 0.0196 * |
| X2X3 | 0.0081 | 1 | 0.0081 | 1.35 | 0.2902 |
| X12 | 0.4761 | 1 | 0.4761 | 79.08 | 0.0001 * |
| X22 | 0.5852 | 1 | 0.5852 | 97.20 | 0.0001 * |
| X32 | 0.1482 | 1 | 0.1482 | 24.62 | 0.0025 * |
| Residual | 0.0361 | 6 | 0.0060 | ||
| Lack of Fit | 0.0149 | 3 | 0.0050 | 0.7040 | 0.6100 |
| Pure error | 0.0212 | 3 | 0.0071 | ||
| Total | 1.66 | 15 |
| Source | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| X1 | 1.24 | 1 | 1.24 | 9.96 | 0.0083 * |
| X2 | 2.19 | 1 | 2.19 | 17.62 | 0.0012 * |
| X3 | 1.05 | 1 | 1.05 | 8.44 | 0.0132 * |
| Residual | 1.49 | 12 | 0.1245 | ||
| Lack of Fit | 1.33 | 9 | 0.1474 | 2.64 | |
| Pure error | 0.1675 | 3 | 0.0558 | ||
| Total | 5.98 | 15 |
| Factors | Responses | ||
|---|---|---|---|
| SF and Ji | SF | ||
| Optimized coded level of variables | X1: temperatura (°C) X2: TMP (bar) X3: Concentration (kg∙m−3) | 53.55 4.12 120.16 | 46.81 3.27 85.70 |
| Predicted responses | Separation factor The flux of solute (kg fructans∙h−1∙m−2) | 2.57 1.82 | 2.74 |
| Overall desirability | 0.72 | 0.94 | |
| Experiment | Feed Sample | Operational Conditions | Predicted SF | Experimental SF | Difference | |
|---|---|---|---|---|---|---|
| DPav | FOS:Fc | |||||
| 1 | 15.4 | 0.35 | 45 °C, 3 bar, 100 kg∙m−3 | 2.71 | 3.50 | 0.79 |
| 2 | 14.6 | 0.39 | 30 °C, 5 bar, 50 kg∙m−3 | 2.05 | 5.78 | 3.73 |
| 3 | 16.8 | 0.36 | 60 °C, 5 bar, 50 kg∙m−3 | 1.53 | 1.63 | 0.1 |
| 4 | 13.5 | 0.44 | 54 °C, 4 bar, 120 kg∙m−3 | 2.56 | 3.25 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luiz-Santos, N.; Prado-Ramírez, R.; Camacho-Ruíz, R.M.; Guatemala-Morales, G.M.; Arriola-Guevara, E.; Moreno-Vilet, L. Effect of Operating Conditions and Fructans Size Distribution on Tight Ultrafiltration Process for Agave Fructans Fractionation: Optimization and Modeling. Membranes 2022, 12, 575. https://doi.org/10.3390/membranes12060575
Luiz-Santos N, Prado-Ramírez R, Camacho-Ruíz RM, Guatemala-Morales GM, Arriola-Guevara E, Moreno-Vilet L. Effect of Operating Conditions and Fructans Size Distribution on Tight Ultrafiltration Process for Agave Fructans Fractionation: Optimization and Modeling. Membranes. 2022; 12(6):575. https://doi.org/10.3390/membranes12060575
Chicago/Turabian StyleLuiz-Santos, Noe, Rogelio Prado-Ramírez, Rosa María Camacho-Ruíz, Guadalupe María Guatemala-Morales, Enrique Arriola-Guevara, and Lorena Moreno-Vilet. 2022. "Effect of Operating Conditions and Fructans Size Distribution on Tight Ultrafiltration Process for Agave Fructans Fractionation: Optimization and Modeling" Membranes 12, no. 6: 575. https://doi.org/10.3390/membranes12060575
APA StyleLuiz-Santos, N., Prado-Ramírez, R., Camacho-Ruíz, R. M., Guatemala-Morales, G. M., Arriola-Guevara, E., & Moreno-Vilet, L. (2022). Effect of Operating Conditions and Fructans Size Distribution on Tight Ultrafiltration Process for Agave Fructans Fractionation: Optimization and Modeling. Membranes, 12(6), 575. https://doi.org/10.3390/membranes12060575