Effect of Melissa officinalis L. Essential Oil Nanoemulsions on Structure and Properties of Carboxymethyl Chitosan/Locust Bean Gum Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
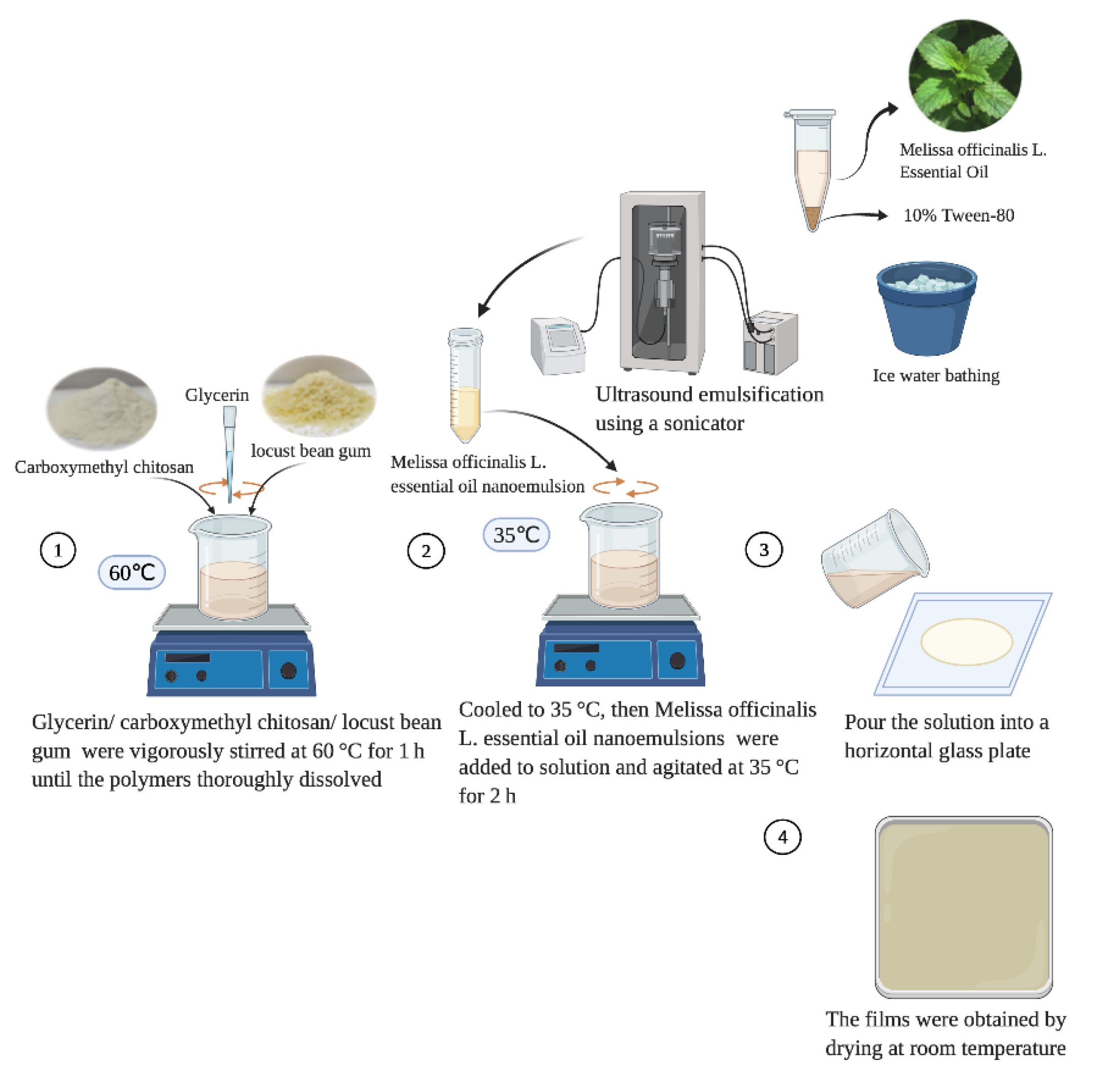
2.2. Fabrication of MOEO Emulsions
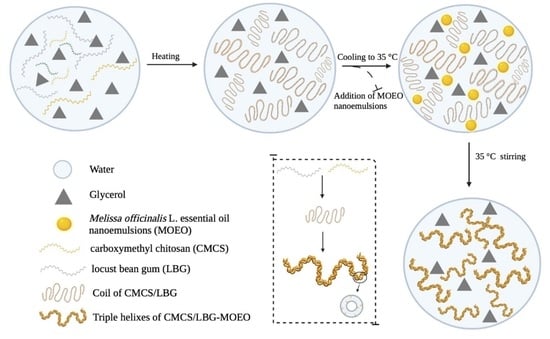
2.3. Preparation of Active Films
2.4. Characterization of Emulsion
2.4.1. Dynamic Light Scattering (DLS)
2.4.2. Transmission Electron Microscopy (TEM)
2.5. Physical and Mechanical Properties of Active Films
2.5.1. Thickness
2.5.2. Moisture Content and Water Solubility
2.5.3. Mechanical Properties
2.5.4. Water Vapor Permeability (WVP)
2.5.5. Oxygen Barrier Properties
2.6. Optical Properties
2.6.1. Color
2.6.2. Opacity
2.7. Contact Angle Measurements
2.8. Scanning Electron Microscopy (SEM)
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. Characterization of Antioxidant and Antibacterial Properties
2.10.1. DPPH Radical Scavenging Assay
2.10.2. Antibacterial Properties
2.11. Statistical Analysis
3. Results
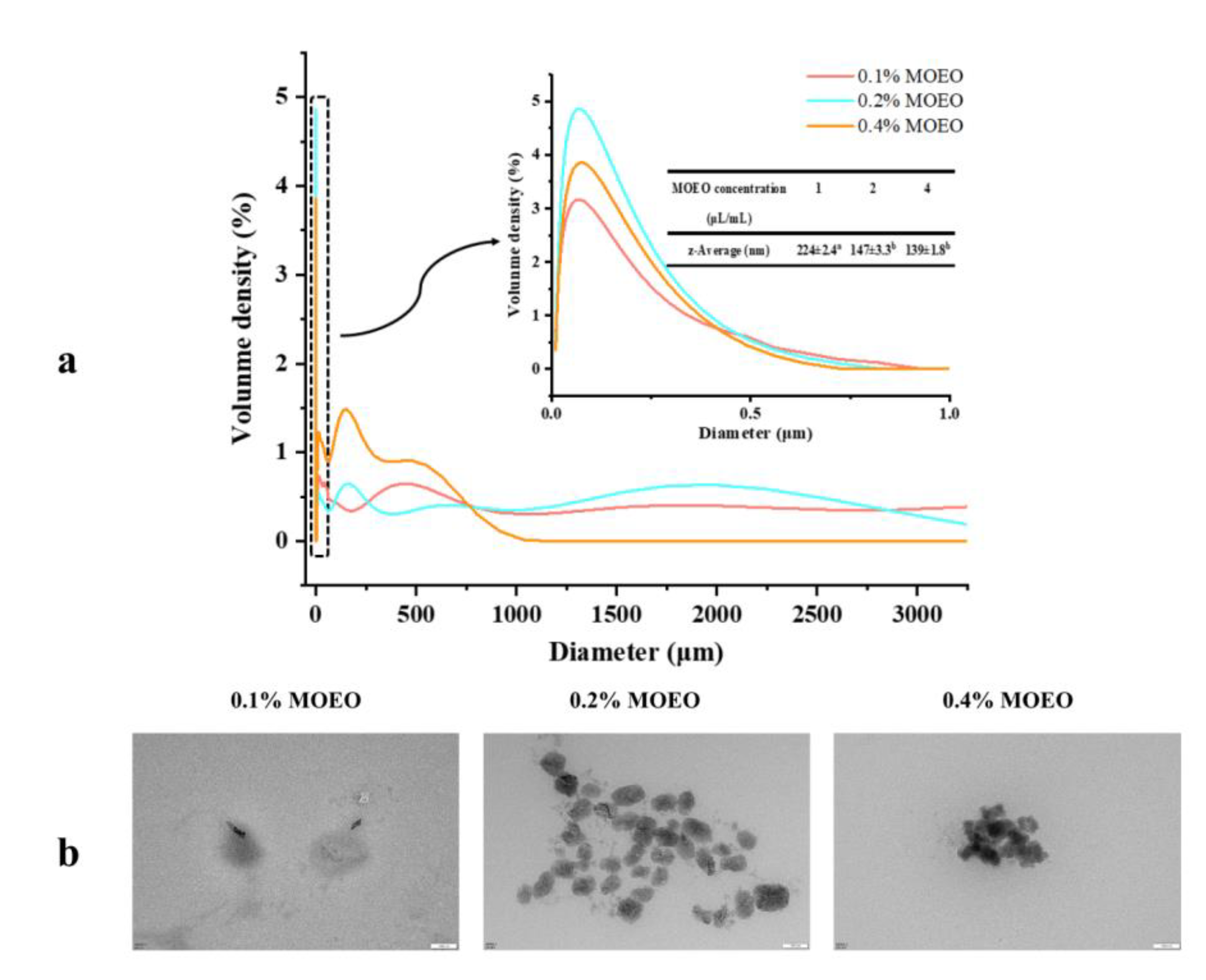
3.1. Characterization of MOEO Nanoemulsion
3.2. Physical Properties of Films
3.2.1. The Thickness of Films
3.2.2. Mechanical Properties
3.2.3. WVP and Water Contact Angle
3.2.4. Moisture Content and Solubility
3.2.5. Oxygen Barrier Properties
3.3. Color Properties
3.4. FTIR Results
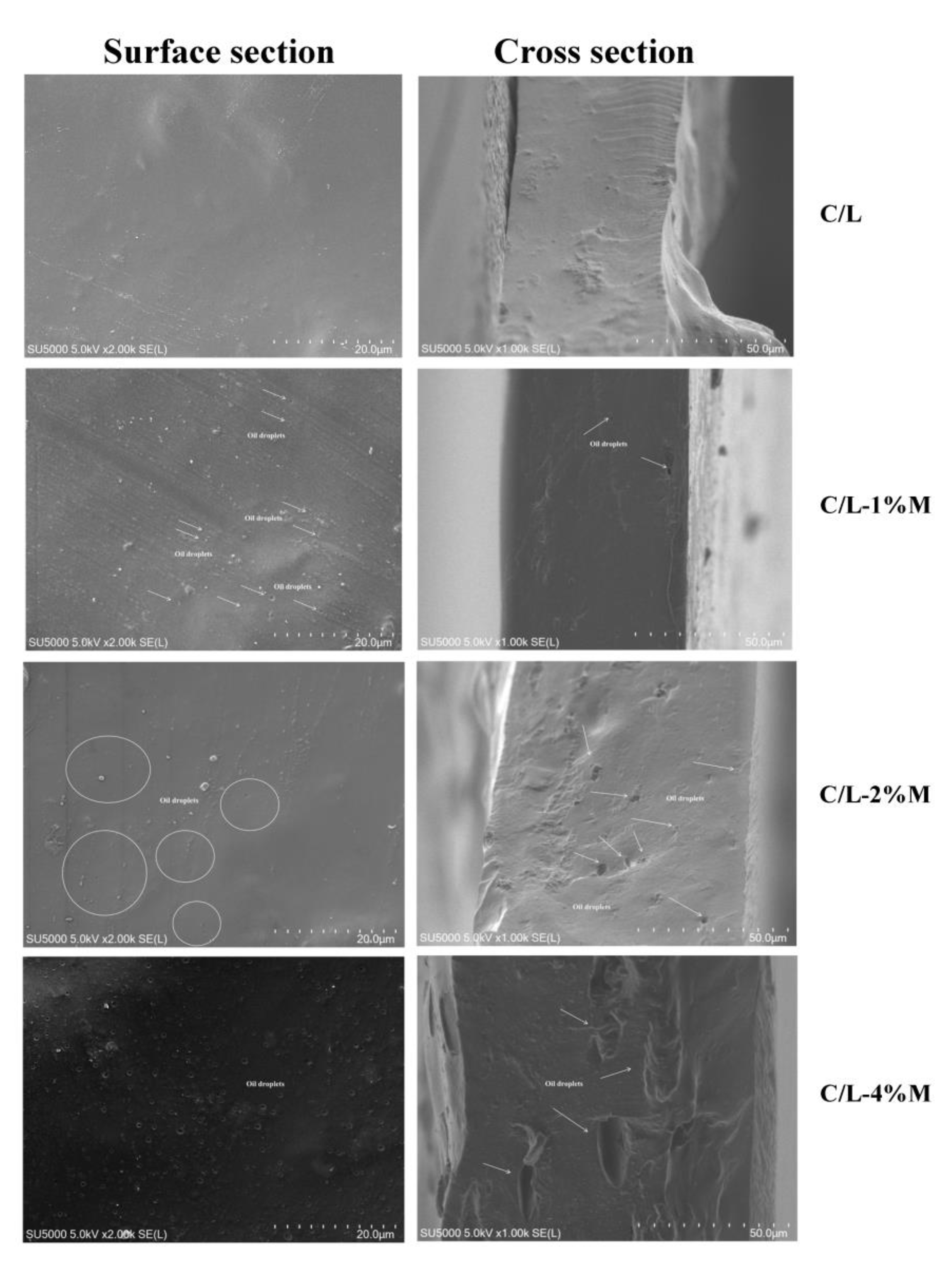
3.5. SEM
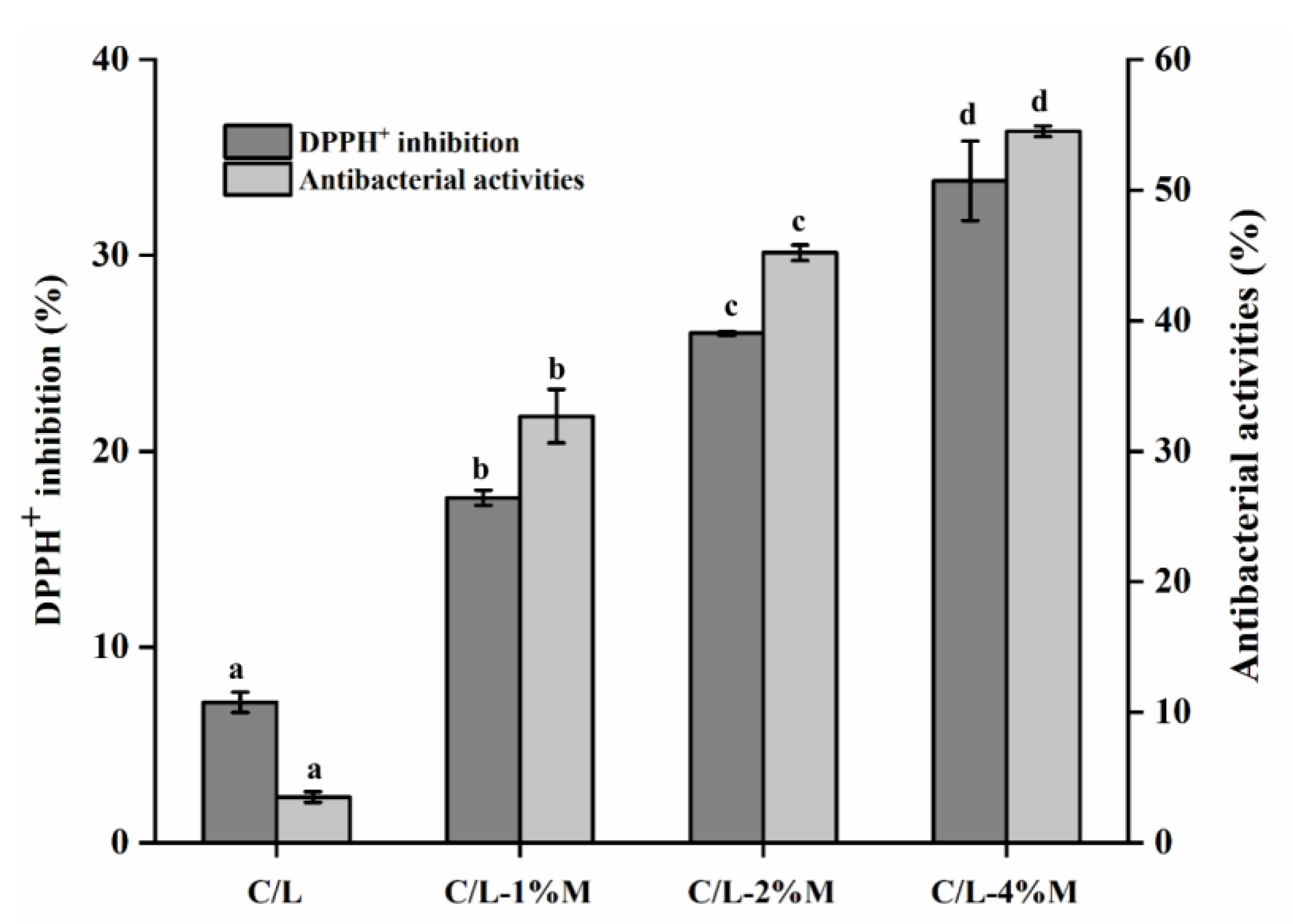
3.6. Antioxidant and Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Yang, W.; Zhao, X.; Chen, S.; Qian, G.; Jiang, G.; Ren, S.; Li, S. Composite films with excellent mechanical, antioxidant and UV-shielding properties prepared from oligomeric proanthocyanidin nanospheres and poly(vinyl alcohol). Ind. Crops Prod. 2021, 172, 114054. [Google Scholar] [CrossRef]
- Subramanian, K.; Vadivu, K.S.; Subramaniyam, L.; Kumar, M.D. Synthesis, characterization, and analysis of bioplasticizer derived from Hibiscus rosa-sinensis leaves and cinnamon bark for poly (vinyl chloride) films. Ind. Crops Prod. 2022, 182, 114933. [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, C.; Cui, B.; Wang, J.; Yu, B.; Guo, L.; Dong, D. Mechanical and antimicrobial properties of high amylose corn starch/konjac glucomannan composite film enhanced by cinnamaldehyde/β-cyclodextrin complex. Ind. Crops Prod. 2021, 170, 113781. [Google Scholar] [CrossRef]
- Akkaya, N.E.; Ergun, C.; Saygun, A.; Yesilcubuk, N.; Akel-Sadoglu, N.; Kavakli, I.H.; Turkmen, H.S.; Catalgil-Giz, H. New biocompatible antibacterial wound dressing candidates; agar-locust bean gum and agar-salep films. Int. J. Biol. Macromol. 2020, 155, 430–438. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Singla, R.K.; Kang, N.; Kaur, G.; Kaur, H.; Kennedy, J.F. Carbamoylethyl locust bean gum: Synthesis, characterization and evaluation of its film forming potential. Int. J. Biol. Macromol. 2020, 149, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Grala, D.; Biernacki, K.; Freire, C.; Kuźniarska-Biernacka, I.; Souza, H.K.S.; Gonçalves, M.P. Effect of natural deep eutectic solvent and chitosan nanoparticles on physicochemical properties of locust bean gum films. Food Hydrocoll. 2022, 126, 107460. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ariffin, F.; Wijekoon, M.M.J.O.; Al-Hassan, A.A.; Dheyab, M.A.; Ghasemlou, M. Recent advances in extraction, modification, and application of chitosan in packaging industry. Carbohydr. Polym. 2022, 277, 118876. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, D.; Tu, J.; Zhong, Y.; Zhang, D.; Wang, Z.; Tao, X. Mechanisms of change in gel water-holding capacity of myofibrillar proteins affected by lipid oxidation: The role of protein unfolding and cross-linking. Food Chem. 2020, 344, 128587. [Google Scholar] [CrossRef]
- de Assis, R.M.A.; Carneiro, J.J.; Medeiros, A.P.R.; de Carvalho, A.A.; da Cunha Honorato, A.; Carneiro, M.A.C.; Bertolucci, S.K.V.; Pinto, J.E.B.P. Arbuscular mycorrhizal fungi and organic manure enhance growth and accumulation of citral, total phenols, and flavonoids in Melissa officinalis L. Ind. Crops Prod. 2020, 158, 112981. [Google Scholar] [CrossRef]
- Son, Y.-J.; Park, J.-E.; Kim, J.; Yoo, G.; Nho, C.W. The changes in growth parameters, qualities, and chemical constituents of lemon balm (Melissa officinalis L.) cultivated in three different hydroponic systems. Ind. Crops Prod. 2021, 163, 113313. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.-W.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf B Biointerfaces 2014, 114, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Lomate, G.B.; Dandi, B.; Mishra, S. Development of antimicrobial LDPE/Cu nanocomposite food packaging film for extended shelf life of peda. Food Packag. Shelf Life 2018, 16, 211–219. [Google Scholar] [CrossRef]
- Keawpeng, I.; Paulraj, B.; Venkatachalam, K. Antioxidant and antimicrobial properties of mung bean phyto-film combined with longkong pericarp extract and sonication. Membranes 2022, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Ding, Z.; Mei, J.; Xie, J. Effect of cellobiose on the myofibrillar protein denaturation induced by pH changes during freeze-thaw cycles. Food Chem. 2022, 373, 131511. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Guo, Q.; Wu, Y.; Li, Y.; Yu, H. Characterization and antimicrobial properties of water chestnut starch-chitosan edible films. Int. J. Biol. Macromol. 2013, 61, 169–174. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of edible starch–chitosan film and its application in the storage of Mongolian cheese. Int. J. Biol. Macromol. 2013, 57, 17–21. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Y.; Chen, C.; Zhao, H.; Zhao, Y.; Jia, Y.; Li, J.; Li, Z. Preparation and characterization of a thin-film composite membrane modified by mXene nano-sheets. Membranes 2022, 12, 368. [Google Scholar] [CrossRef]
- Agarwal, C.; Kóczán, Z.; Börcsök, Z.; Halász, K.; Pásztory, Z. Valorization of Larix decidua Mill. bark by functionalizing bioextract onto chitosan films for sustainable active food packaging. Carbohydr. Polym. 2021, 271, 118409. [Google Scholar] [CrossRef]
- Rong, L.; Shen, M.; Wen, H.; Ren, Y.; Xiao, W.; Xie, J. Preparation and characterization of hyacinth bean starch film incorporated with TiO2 nanoparticles and Mesona chinensis Benth polysaccharide. Int. J. Biol. Macromol. 2021, 190, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zong, L.; Wang, J.; Xie, J. Microfibrillated cellulose reinforced starch/polyvinyl alcohol antimicrobial active films with controlled release behavior of cinnamaldehyde. Carbohydr. Polym. 2021, 272, 118448. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, S.; Chen, S.; Wang, C.; Liu, D.; Li, X. Antibacterial and antioxidant activities of sodium starch octenylsuccinate-based Pickering emulsion films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2020, 159, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Cheng, H.; Yu, H.; Mei, J.; Xie, J. Effect of multi-frequency ultrasound assisted thawing on the quality of large yellow croaker (Larimichthys crocea). Ultrason. Sonochem. 2022, 82, 105907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Recent advances in polysaccharides stabilized emulsions for encapsulation and delivery of bioactive food ingredients: A review. Carbohydr. Polym. 2020, 242, 116388. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Amini, A.M.; Zahedi, Y. Characterisation of a new biodegradable edible film based on sage seed gum: Influence of plasticiser type and concentration. Food Hydrocoll. 2015, 43, 290–298. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Zhang, C.; Show, P.L.; Du, A.; Fu, J.; Ashokkumar, V. Preparation and characterization of curdlan/polyvinyl alcohol/ thyme essential oil blending film and its application to chilled meat preservation. Carbohydr. Polym. 2020, 247, 116670. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de FF Soares, N.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Behjati, J.; Yazdanpanah, S. Nanoemulsion and emulsion vitamin D3 fortified edible film based on quince seed gum. Carbohydr. Polym. 2021, 262, 117948. [Google Scholar] [CrossRef]
- Shen, Y.; Ni, Z.-J.; Thakur, K.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Preparation and characterization of clove essential oil loaded nanoemulsion and pickering emulsion activated pullulan-gelatin based edible film. Int. J. Biol. Macromol. 2021, 181, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Zhou, C.; Li, C.; Siva, S.; Cui, H.; Lin, L. Chemical composition, antibacterial activity and study of the interaction mechanisms of the main compounds present in the Alpinia galanga rhizomes essential oil. Ind. Crops Prod. 2021, 165, 113441. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, Z.; Yang, J.; Zhang, M.; Cai, F.; Lu, P. ZnO nanoparticles stabilized oregano essential oil Pickering emulsion for functional cellulose nanofibrils packaging films with antimicrobial and antioxidant activity. Int. J. Biol. Macromol. 2021, 190, 433–440. [Google Scholar] [CrossRef]
- Chen, F.; Miao, X.; Lin, Z.; Xiu, Y.; Shi, L.; Zhang, Q.; Liang, D.; Lin, S.; He, B. Disruption of metabolic function and redox homeostasis as antibacterial mechanism of Lindera glauca fruit essential oil against Shigella flexneri. Food Contr. 2021, 130, 108282. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Y.; Li, Y. Development of tea extracts and chitosan composite films for active packaging materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Zahedi, Y.; Ghanbarzadeh, B.; Sedaghat, N. Physical properties of edible emulsified films based on pistachio globulin protein and fatty acids. J. Food Eng. 2010, 100, 102–108. [Google Scholar] [CrossRef]
- Ghiasi, F.; Golmakani, M.-T.; Eskandari, M.H.; Hosseini, S.M.H. A new approach in the hydrophobic modification of polysaccharide-based edible films using structured oil nanoparticles. Ind. Crops Prod. 2020, 154, 112679. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Bhtaia, S.; Al-Azri, M.S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Mohan, S.; Sharma, A.; Behl, T. Development and characterization of chitosan and porphyran based composite edible films containing ginger essential oil. Polymers 2022, 14, 1782. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.S.; Scolaro, B.; Milne, G.L.; Castro, I.A. Oxidation products from omega-3 and omega-6 fatty acids during a simulated shelf life of edible oils. LWT 2019, 101, 113–122. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Wang, Y.; Tang, Q.; Xue, C.; Huang, Q. Oleogel-based Pickering emulsions stabilized by ovotransferrin–carboxymethyl chitosan nanoparticles for delivery of curcumin. LWT 2022, 157, 113121. [Google Scholar] [CrossRef]
- Hopkins, E.J.; Chang, C.; Lam, R.S.H.; Nickerson, M.T. Effects of flaxseed oil concentration on the performance of a soy protein isolate-based emulsion-type film. Food Res. Int. 2015, 67, 418–425. [Google Scholar] [CrossRef]
- Kadam, D.; Shah, N.; Palamthodi, S.; Lele, S.S. An investigation on the effect of polyphenolic extracts of Nigella sativa seedcake on physicochemical properties of chitosan-based films. Carbohydr. Polym. 2018, 192, 347–355. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Xiao, F.; Xia, L.; Li, L.; Jiang, S. Functional effectiveness of double essential oils@yam starch/microcrystalline cellulose as active antibacterial packaging. Int. J. Biol. Macromol. 2021, 186, 873–885. [Google Scholar] [CrossRef]
- Kang, J.; Cui, S.W.; Chen, J.; Phillips, G.O.; Wu, Y.; Wang, Q. New studies on gum ghatti (Anogeissus latifolia) part I. Fractionation, chemical and physical characterization of the gum. Food Hydrocoll. 2011, 25, 1984–1990. [Google Scholar] [CrossRef]
- Popescu, M.-C.; Dogaru, B.-I.; Goanta, M.; Timpu, D. Structural and morphological evaluation of CNC reinforced PVA/Starch biodegradable films. Int. J. Biol. Macromol. 2018, 116, 385–393. [Google Scholar] [CrossRef]
- Chu, Y.; Cheng, W.; Feng, X.; Gao, C.; Wu, D.; Meng, L.; Zhang, Y.; Tang, X. Fabrication, structure and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life. 2020, 25, 100547. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, K.; Gao, C.; Feng, X.; Cheng, W.; Wu, D.; Meng, L.; Yang, Y.; Shen, X.; Zhang, Y.; et al. Characterization of chitosan film with cinnamon essential oil emulsion co-stabilized by ethyl-Nα-lauroyl-l-arginate hydrochloride and hydroxypropyl-β-cyclodextrin. Int. J. Biol. Macromol. 2021, 188, 24–31. [Google Scholar] [CrossRef]
- Cazón, P.; Antoniewska, A.; Rutkowska, J.; Vázquez, M. Evaluation of easy-removing antioxidant films of chitosan with Melaleuca alternifolia essential oil. Int. J. Biol. Macromol. 2021, 186, 365–376. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Tmušić, N.; Stanojević, L.; Stanojević, J.; Cvetković, D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT 2022, 153, 112210. [Google Scholar] [CrossRef]
- Adouni, K.; Bendif, H.; Zouaoui, O.; Kraujalis, P.; Flamini, G.; Venskutonis, P.R.; Achour, L. Antioxidant activity of extracts obtained by high-pressure extraction procedures from Asparagus stipularis Forssk. South Afr. J. Bot. 2022, 146, 789–793. [Google Scholar] [CrossRef]

| Film Samples | Thickness (cm) | Tensile Strength (MPa) | Elongation at Break (%) | Moisture Content (%) | Water Solubility (%) | Water Vapor Permeability (×10−12 g * cm/(cm2 ⋅ s ⋅ Pa)) | Oxygen Transmission Rates (cm3/(m2 ⋅ 24 h ⋅ 0.1 MPa)) | Water Contact Angle (θ) |
|---|---|---|---|---|---|---|---|---|
| C/L | 0.0895 ± 0.0057 a | 13.44 ± 0.76 a | 18.49 ± 0.79 a | 19.59 ± 0.93 a | 56.32 ± 1.03 a | 7.47 ± 0.15 a | 12.43 ± 0.33 a | 75.13 ± 0.84 a |
| C/L-1%M | 0.0933 ± 0.0043 a | 5.68 ± 0.43 b | 25.30 ± 0.52 b | 19.17 ± 0.42 a | 34.26 ± 0.82 b | 8.14 ± 0.08 b | 8.47 ± 0.15 b | 78.25 ± 0.24 a |
| C/L-2%M | 0.0995 ± 0.0090 ab | 4.59 ± 0.53 b | 25.91 ± 0.58 b | 18.79 ± 0.42 a | 26.67 ± 0.43 c | 8.31 ± 0.20 c | 9.34 ± 0.31 c | 81.41 ± 0.44 a |
| C/L-4%M | 0.1059 ± 0.0128 b | 7.32 ± 0.33 c | 27.97 ± 0.23 c | 18.34 ± 0.09 a | 25.43 ± 0.31 c | 8.67 ± 0.16 c | 9.42 ± 0.12 c | 83.86 ± 0.10 a |
| Film Samples | L* | a* | b* | ΔE | Chroma | Opacity |
|---|---|---|---|---|---|---|
| C/L | 90.06 ± 0.81 a | −3.81 ± 0.11 a | 13.44 ± 0.35 a | 9.14 ± 0.89 a | 13.97 ± 0.34 a | 0.34 ± 0.02 a |
| C/L-1%M | 87.81 ± 0.70 b | −4.02 ± 0.08 a | 16.26 ± 0.55 b | 12.74 ± 0.89 b | 16.75 ± 0.52 b | 0.48 ± 0.01 b |
| C/L-2%M | 88.42 ± 0.15 b | −3.87 ± 0.06 a | 16.40 ± 0.33 b | 12.49 ± 0.37 b | 16.85 ± 0.32 b | 0.31 ± 0.01 ac |
| C/L-4%M | 89.56 ± 0.63 ab | −3.96 ± 0.14 a | 16.47 ± 0.98 b | 11.92 ± 1.17 b | 16.94 ± 0.94 b | 0.30 ± 0.01 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Zhang, C.; Xie, Y.; Mei, J.; Xie, J. Effect of Melissa officinalis L. Essential Oil Nanoemulsions on Structure and Properties of Carboxymethyl Chitosan/Locust Bean Gum Composite Films. Membranes 2022, 12, 568. https://doi.org/10.3390/membranes12060568
Yu H, Zhang C, Xie Y, Mei J, Xie J. Effect of Melissa officinalis L. Essential Oil Nanoemulsions on Structure and Properties of Carboxymethyl Chitosan/Locust Bean Gum Composite Films. Membranes. 2022; 12(6):568. https://doi.org/10.3390/membranes12060568
Chicago/Turabian StyleYu, Huijie, Chi Zhang, Yao Xie, Jun Mei, and Jing Xie. 2022. "Effect of Melissa officinalis L. Essential Oil Nanoemulsions on Structure and Properties of Carboxymethyl Chitosan/Locust Bean Gum Composite Films" Membranes 12, no. 6: 568. https://doi.org/10.3390/membranes12060568
APA StyleYu, H., Zhang, C., Xie, Y., Mei, J., & Xie, J. (2022). Effect of Melissa officinalis L. Essential Oil Nanoemulsions on Structure and Properties of Carboxymethyl Chitosan/Locust Bean Gum Composite Films. Membranes, 12(6), 568. https://doi.org/10.3390/membranes12060568