The Recent Development in Chemoresistive-Based Heterostructure Gas Sensor Technology, Their Future Opportunities and Challenges: A Review
Abstract
:1. Introduction
2. Performance of Gas Sensors
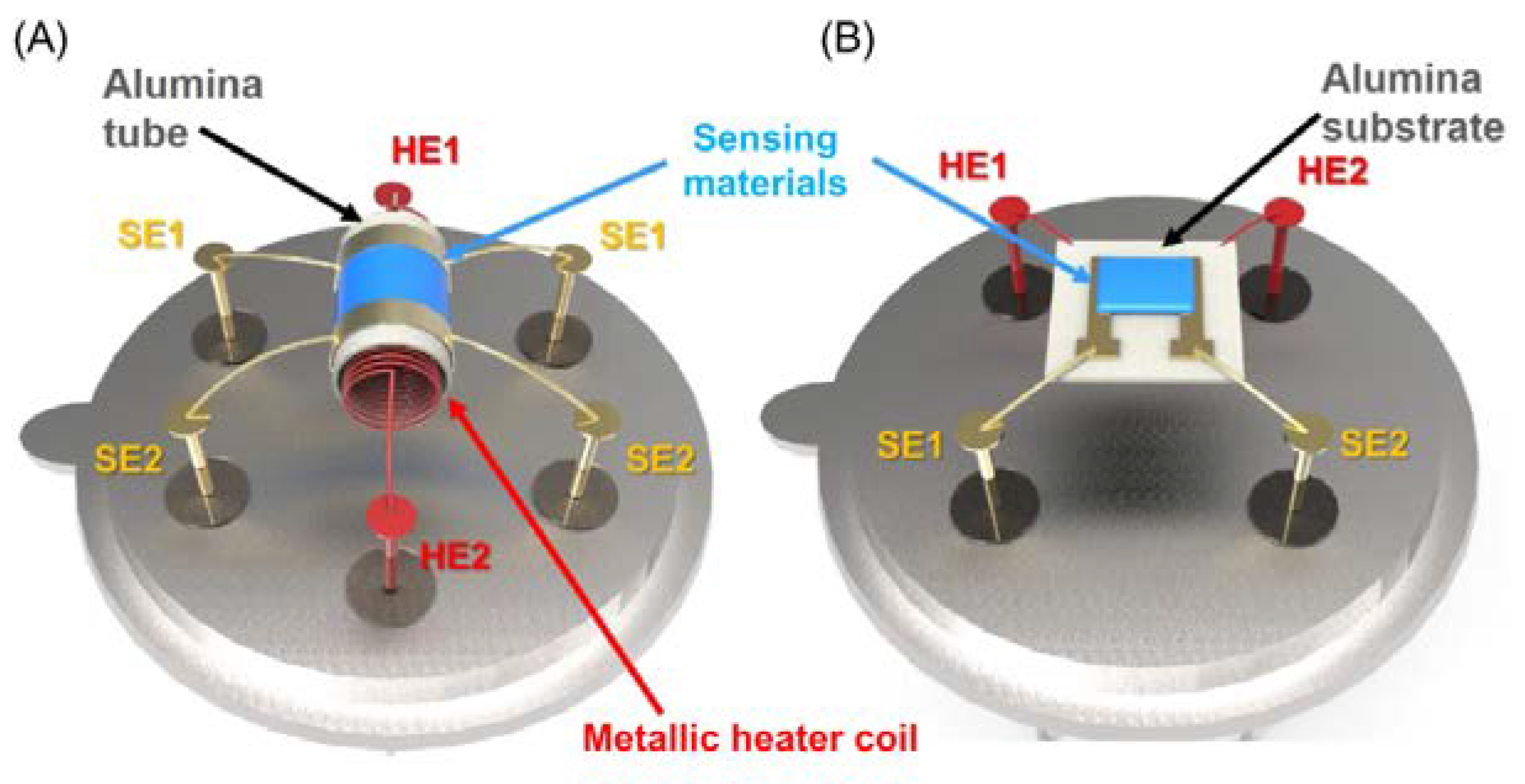
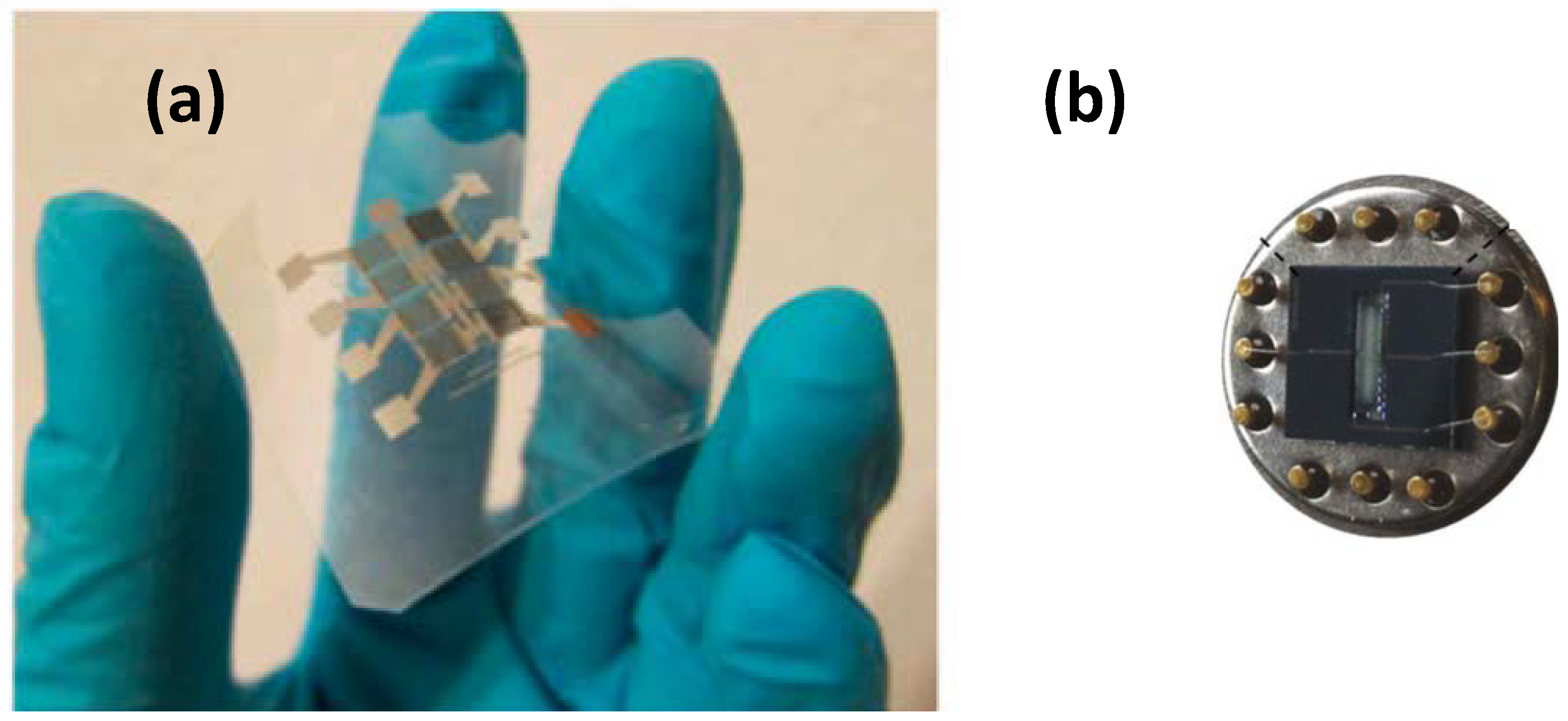
3. Gas-Sensing Devices
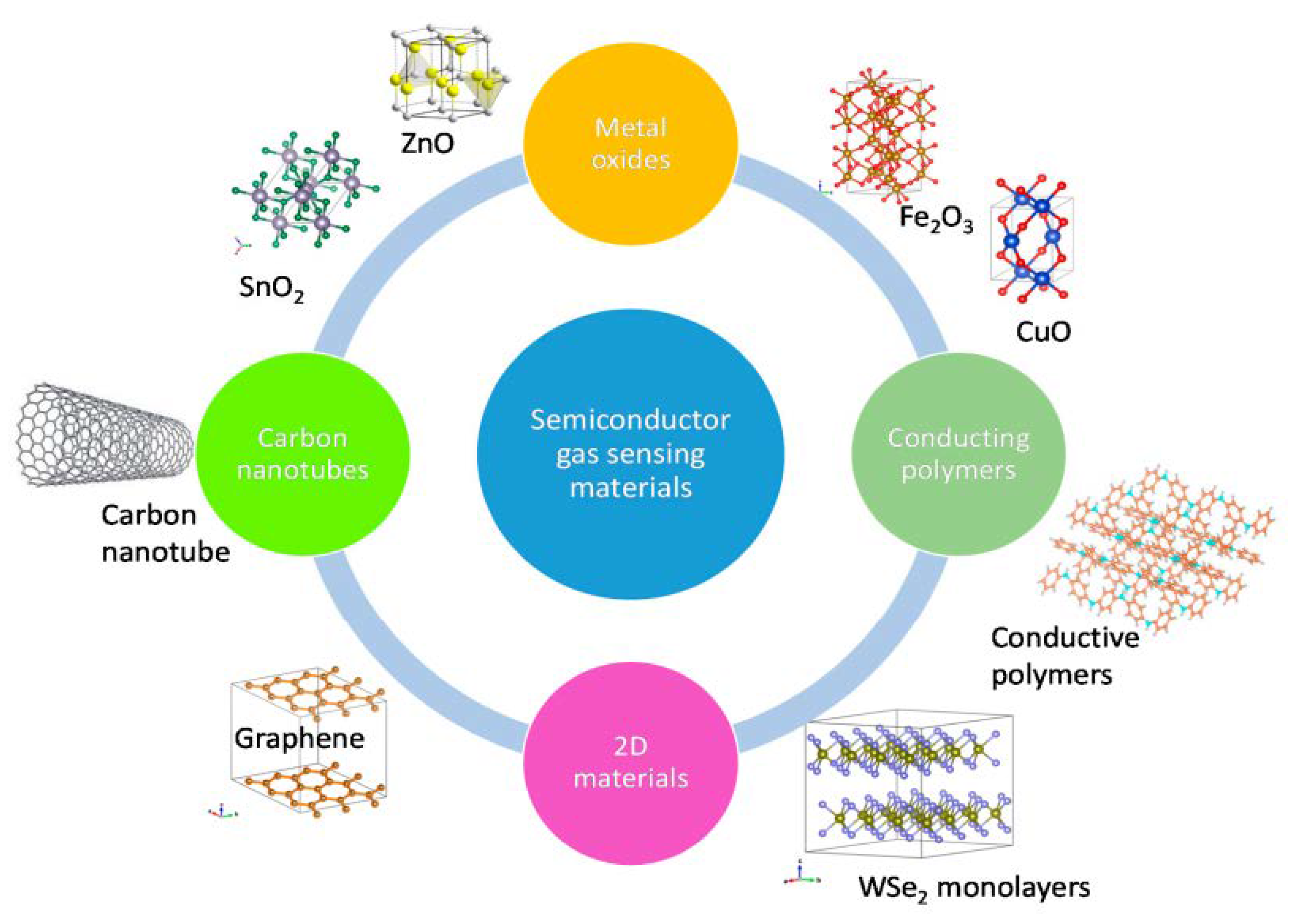
4. Gas-Sensing Materials
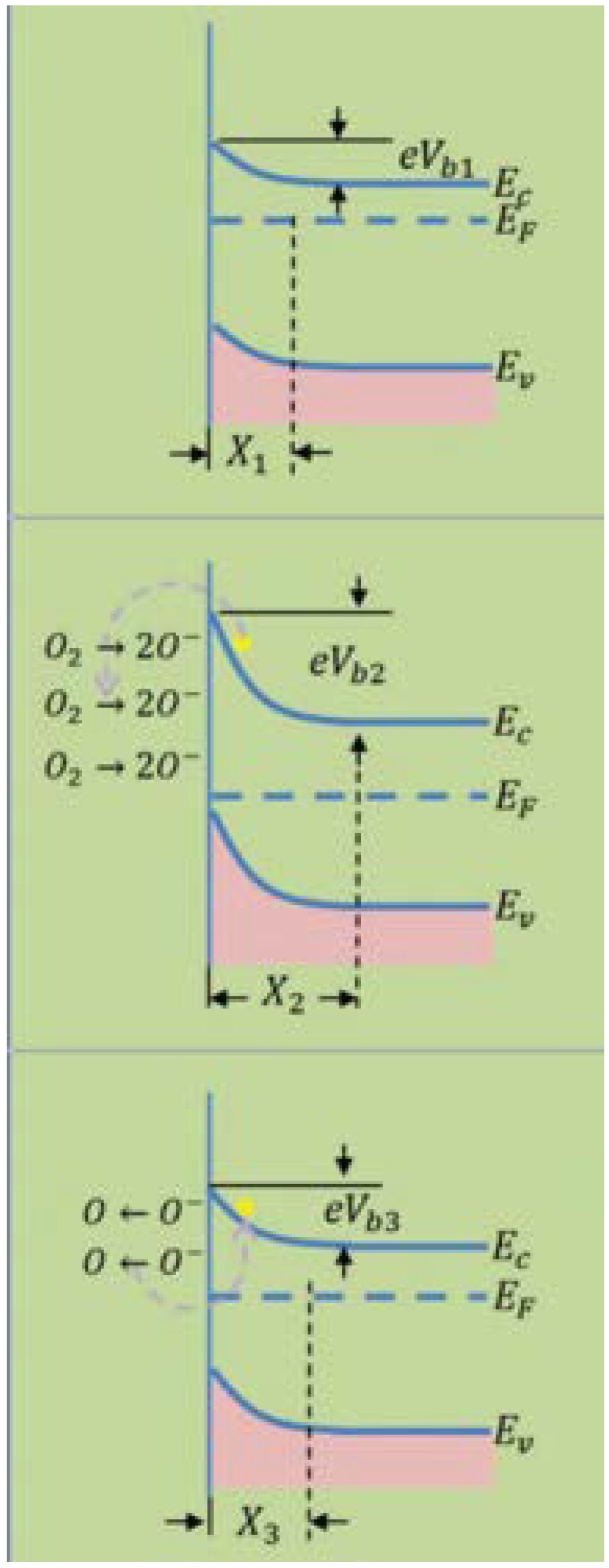
4.1. Metal Oxide Semiconductors
4.2. Conducting Polymers
4.3. Graphene and Carbon Nanotubes
5. Strategies for Improving Gas-Sensing Properties
5.1. Bilayer or Multilayered Heterostructure
5.2. Mixed Compounds
5.3. Decorated Structure
5.4. Core–Shell Structures
5.5. Branched 1D Heterostructures
6. Conclusions, Future Trends, and Challenges
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawasaki, H.; Ueda, T.; Suda, Y.; Ohshima, T. Properties of metal doped tungsten oxide thin films for NOx gas sensors grown by PLD method combined with sputtering process. Sens. Actuators B Chem. 2004, 100, 266–269. [Google Scholar] [CrossRef]
- West, D.L.; Montgomery, F.C.; Armstrong, T.R. Use of La0. 85Sr0. 15CrO3 in high-temperature NOx sensing elements. Sens. Actuators B Chem. 2005, 106, 758–765. [Google Scholar]
- Gatari, M.J. First WHO global conference on air pollution and health: A brief report. Clean Air J. 2019, 29, 7. [Google Scholar] [CrossRef]
- Anukunprasert, T.; Saiwan, C.; Traversa, E. The development of gas sensor for carbon monoxide monitoring using nanostructure of Nb-TiO2. Sci. Technol. Adv. Mater. 2005, 6, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yuan, Z.; Song, J.; Zheng, C. Improvement and mechanism for the fast response of a Pt/TiO2 gas sensor. Sens. Actuators B Chem. 2010, 148, 87–92. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor gas sensors: Dry synthesis and application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef] [PubMed]
- Blazy, V.; de Guardia, A.; Benoist, J.C.; Daumoin, M.; Guiziou, F.; Lemasle, M.; Wolbert, D.; Barrington, S. Correlation of chemical composition and odor concentration for emissions from pig slaughterhouse sludge composting and storage. Chem. Eng. J. 2015, 276, 398–409. [Google Scholar] [CrossRef]
- Hunter, G.W.; Akbar, S.; Bhansali, S.; Daniele, M.; Erb, P.D.; Johnson, K.; Liu, C.-C.; Miller, D.; Oralkan, O.; Hesketh, P.J.; et al. Editors’ choice—Critical review—A critical review of solid state gas sensors. J. Electrochem. Soc. 2020, 167, 37570. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Taguchi, N. Gas Detecting Devices. U.S. Patent 3,631,436, 28 December 1971. [Google Scholar]
- Neri, G.; Donato, N. Resistive gas sensors. Wiley Encycl. Electr. Electron. Eng. 1999, 1–12. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Schüler, M.; Sauerwald, T.; Schütze, A. A novel approach for detecting HMDSO poisoning of metal oxide gas sensors and improving their stability by temperature cycled operation. J. Sens. Sens. Syst. 2015, 4, 305–311. [Google Scholar] [CrossRef]
- Carmona, E.N.; Sberveglieri, V.; Ponzoni, A.; Galstyan, V.; Zappa, D.; Pulvirenti, A.; Comini, E. Detection of food and skin pathogen microbiota by means of an electronic nose based on metal oxide chemiresistors. Sens. Actuators B Chem. 2017, 238, 1224–1230. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Liu, M.; Zhang, C.; Chen, W. Three-dimensional mesoporous graphene aerogel-supported SnO2 nanocrystals for high-performance NO2 gas sensing at low temperature. Anal. Chem. 2015, 87, 1638–1645. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Zhang, R.; Gao, X.; He, S.; Liu, M.; Li, X.; Chen, W. 2D ultrathin Co3O4 nanosheet array deposited on 3D carbon foam for enhanced ethanol gas sensing application. Sens. Actuators B Chem. 2017, 244, 664–672. [Google Scholar] [CrossRef]
- Jin, Z.; Xiao, M.; Bao, Z.; Wang, P.; Wang, J. A general approach to mesoporous metal oxide microspheres loaded with noble metal nanoparticles. Angew. Chem. 2012, 124, 6512–6516. [Google Scholar] [CrossRef]
- Arunkumar, S.; Hou, T.; Kim, Y.-B.; Choi, B.; Park, S.H.; Jung, S.; Lee, D.-W. Au Decorated ZnO hierarchical architectures: Facile synthesis, tunable morphology and enhanced CO detection at room temperature. Sens. Actuators B Chem. 2017, 243, 990–1001. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, O.S.; Park, S.J.; Park, E.Y.; You, S.A.; Yoon, H.; Jang, J. Fabrication of ultrafine metal-oxide-decorated carbon nanofibers for DMMP sensor application. ACS Nano 2011, 5, 7992–8001. [Google Scholar] [CrossRef]
- Gotoh, K.; Kinumoto, T.; Fujii, E.; Yamamoto, A.; Hashimoto, H.; Ohkubo, T.; Itadani, A.; Kuroda, Y.; Ishida, H. Exfoliated graphene sheets decorated with metal/metal oxide nanoparticles: Simple preparation from cation exchanged graphite oxide. Carbon 2011, 49, 1118–1125. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sens. Actuators B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Karnati, P.; Akbar, S.; Morris, P.A. Conduction mechanisms in one dimensional core-shell nanostructures for gas sensing: A review. Sens. Actuators B Chem. 2019, 295, 127–143. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Kaur, N.; Zappa, D.; Ferroni, M.; Poli, N.; Campanini, M.; Negrea, R.; Comini, E. Branch-like NiO/ZnO heterostructures for VOC sensing. Sens. Actuators B Chem. 2018, 262, 477–485. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, C.; Shi, X.; Cao, M.; Jin, H. The synthesis and selective gas sensing characteristics of SnO2/α-Fe2O3 hierarchical nanostructures. Nanotechnology 2008, 19, 205603. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Zhang, K.; Feng, X.; Wang, M. Porous TiO2 nanobelts coated with mixed transition-metal oxides Sn3O4 nanosheets core-shell composites as high-performance anode materials of lithium ion batteries. Electrochim. Acta 2018, 259, 131–142. [Google Scholar] [CrossRef]
- Wang, X.; Sang, Y.; Wang, D.; Ji, S.; Liu, H. Enhanced gas sensing property of SnO2 nanoparticles by constructing the SnO2–TiO2 nanobelt heterostructure. J. Alloys Compd. 2015, 639, 571–576. [Google Scholar] [CrossRef]
- Bhowmick, S.; Kunte, S.S.; Bhowmick, K.C. The smallest organocatalyst in highly enantioselective direct aldol reaction in wet solvent-free conditions. RSC Adv. 2014, 4, 24311–24315. [Google Scholar] [CrossRef]
- Rakshit, T.; Santra, S.; Manna, I.; Ray, S.K. Enhanced sensitivity and selectivity of brush-like SnO2 nanowire/ZnO nanorod heterostructure based sensors for volatile organic compounds. RSC Adv. 2014, 4, 36749–36756. [Google Scholar] [CrossRef]
- Zhai, T.; Xu, H.; Li, W.; Yu, H.; Chen, Z.; Wang, J.; Cao, B. Low-temperature in-situ growth of SnO2 nanosheets and its high triethylamine sensing response by constructing Au-loaded ZnO/SnO2 heterostructure. J. Alloys Compd. 2018, 737, 603–612. [Google Scholar] [CrossRef]
- Umar, A.; Hahn, Y.-B. Metal Oxide Nanostructures and Their Applications; American Scientific Publication: Los Angeles, CA, USA, 2010; Volume 5. [Google Scholar]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooker, S.A. Others Nanotechnology Advantages Applied to Gas Sensor Development. In Proceedings of the The nanoparticles 2002 Conference Proceedings, Washington, WA, USA, 26–28 August 2002; pp. 1–7. [Google Scholar]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Lee, J.-H. 4-Technological realization of semiconducting metal oxide–based gas sensors. In Gas Sensors Based on Conducting Metal Oxides; Barsan, N., Schierbaum, K., Eds.; Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–216. ISBN 978-0-12-811224-3. [Google Scholar] [CrossRef]
- Lorwongtragool, P.; Sowade, E.; Watthanawisuth, N.; Baumann, R.R.; Kerdcharoen, T. A Novel Wearable Electronic Nose for Healthcare Based on Flexible Printed Chemical Sensor Array. Sensors 2014, 14, 19700–19712. [Google Scholar] [CrossRef] [Green Version]
- Stoycheva, T.; Annanouch, F.E.; Gràcia, I.; Llobet, E.; Blackman, C.; Correig, X.; Vallejos, S. Micromachined gas sensors based on tungsten oxide nanoneedles directly integrated via aerosol assisted CVD. Sens. Actuators B Chem. 2014, 198, 210–218. [Google Scholar] [CrossRef]
- Li, T.; Zeng, W.; Long, H.; Wang, Z. Nanosheet-assembled hierarchical SnO2 nanostructures for efficient gas-sensing applications. Sens. Actuators B Chem. 2016, 231, 120–128. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Jeong, J.K. Recent progress in high performance and reliable n-type transition metal oxide-based thin film transistors. Semicond. Sci. Technol. 2015, 30, 24002. [Google Scholar] [CrossRef]
- Zappa, D.; Bertuna, A.; Comini, E.; Kaur, N.; Poli, N.; Sberveglieri, V.; Sberveglieri, G. Metal oxide nanostructures: Preparation, characterization and functional applications as chemical sensors. Beilstein J. Nanotechnol. 2017, 8, 1205–1217. [Google Scholar] [CrossRef] [Green Version]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceramics 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Lou, Z.; Li, F.; Deng, J.; Wang, L.; Zhang, T. Branch-like hierarchical heterostructure (α-Fe2O3/TiO2): A novel sensing material for trimethylamine gas sensor. ACS Appl. Mater. Interfaces 2013, 5, 12310–12316. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V. Porous TiO2-based gas sensors for cyber chemical systems to provide security and medical diagnosis. Sensors 2017, 17, 2947. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Lu, H.; Hao, D.; Wang, L.; Wu, Z.; Wang, L.; Li, P.; Ye, J. Three-dimensional lupinus-like TiO2 nanorod@ Sn3O4 nanosheet hierarchical heterostructured arrays as photoanode for enhanced photoelectrochemical performance. ACS Appl. Mater. Interfaces 2017, 9, 38537–38544. [Google Scholar] [CrossRef]
- Verma, M.K.; Gupta, V. A highly sensitive SnO2–CuO multilayered sensor structure for detection of H2S gas. Sens. Actuators B Chem. 2012, 166, 378–385. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Hai, Z.; Wei, Z.; Detavernier, C.; Solano, E.; Verpoort, F.; Zhuiykov, S. ALD-Developed Plasmonic Two-Dimensional Au–WO3–TiO2 Heterojunction Architectonics for Design of Photovoltaic Devices. ACS Appl. Mater. Interfaces 2018, 10, 10304–10314. [Google Scholar] [CrossRef]
- Fang, J.; Zhu, Y.; Wu, D.; Zhang, C.; Xu, S.; Xiong, D.; Yang, P.; Wang, L.; Chu, P.K. Gas sensing properties of NiO/SnO2 heterojunction thin film. Sens. Actuators B Chem. 2017, 252, 1163–1168. [Google Scholar] [CrossRef]
- Yousefi, R.; Jamali-Sheini, F.; Zak, A.K.; Azarang, M. Growth and optical properties of ZnO-In2O3 heterostructure nanowires. Ceram. Int. 2013, 39, 5191–5196. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Tuantranont, A.; Thanachayanont, C.; Patthanasettakul, V.; Singjai, P. Electron beam evaporated carbon nanotube dispersed SnO2 thin film gas sensor. J. Electroceramics 2006, 17, 45–49. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, R.; Liu, S.; Song, J.; Chen, J.; Dong, B.; Song, H. NiO@ZnO heterostructured nanotubes: Coelectrospinning fabrication, characterization, and highly enhanced gas sensing properties. Inorg. Chem. 2012, 51, 7733–7740. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, S.; Yu, G.; Liu, S. Efficient hierarchical mixed Pd/SnO porous architecture deposited microheater for low power ethanol gas sensor. Sens. Actuators B Chem. 2018, 255, 2002–2010. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, H.; Chen, Y.; Jin, Z.; Xu, J.; Zhang, D. A Facile Synthesis of PdO-Decorated SnO2 Nanocomposites with Open Porous Hierarchical Architectures for Gas Sensors. J. Am. Ceram. Soc. 2016, 99, 3770–3774. [Google Scholar] [CrossRef]
- Sin, N.D.M.; Shafura, A.K.; Malek, M.F.; Mamat, M.H.; Rusop, M. Structural properties of ZnO/SnO2-composite-nanorod deposited using thermal chemical vapour deposition. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 28–29 March 2015; Volume 83, p. 12002. [Google Scholar]
- Ogawa, H.; Nishikawa, M.; Abe, A. Hall measurement studies and an electrical conduction model of tin oxide ultrafine particle films. J. Appl. Phys. 1982, 53, 4448–4455. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Pratsinis, S.E. Minimal cross-sensitivity to humidity during ethanol detection by SnO2–TiO2 solid solutions. Nanotechnology 2009, 20, 315502. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen sensors: Materials, methods, designs and applications. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Kim, H.-J.; Jeong, H.-M.; Lee, J.-H. Gas sensing characteristics of p-type Cr2O3 and Co3O4 nanofibers depending on inter-particle connectivity. Sens. Actuators B Chem. 2014, 202, 263–271. [Google Scholar] [CrossRef]
- Steinebach, H.; Kannan, S.; Rieth, L.; Solzbacher, F. H2 gas sensor performance of NiO at high temperatures in gas mixtures. Sens. Actuators B Chem. 2010, 151, 162–168. [Google Scholar] [CrossRef]
- Chou, P.-C.; Chen, H.-I.; Liu, I.-P.; Chen, C.-C.; Liou, J.-K.; Hsu, K.-S.; Liu, W.-C. On the ammonia gas sensing performance of a RF sputtered NiO thin-film sensor. IEEE Sens. J. 2015, 15, 3711–3715. [Google Scholar] [CrossRef]
- Liu, B.; Yang, H.; Zhao, H.; An, L.; Zhang, L.; Shi, R.; Wang, L.; Bao, L.; Chen, Y. Synthesis and enhanced gas-sensing properties of ultralong NiO nanowires assembled with NiO nanocrystals. Sens. Actuators B Chem. 2011, 156, 251–262. [Google Scholar] [CrossRef]
- Kaur, N.; Comini, E.; Zappa, D.; Poli, N.; Sberveglieri, G. Nickel oxide nanowires: Vapor liquid solid synthesis and integration into a gas sensing device. Nanotechnology 2016, 27, 205701. [Google Scholar] [CrossRef]
- Pokhrel, S.; Simion, C.E.; Quemener, V.; Barsan, N.; Weimar, U. Investigations of conduction mechanism in Cr2O3 gas sensing thick films by ac impedance spectroscopy and work function changes measurements. Sens. Actuators B Chem. 2008, 133, 78–83. [Google Scholar] [CrossRef]
- Ma, H.; Xu, Y.; Rong, Z.; Cheng, X.; Gao, S.; Zhang, X.; Zhao, H.; Huo, L. Highly toluene sensing performance based on monodispersed Cr2O3 porous microspheres. Sens. Actuators B Chem. 2012, 174, 325–331. [Google Scholar] [CrossRef]
- Nylander, C.; Armgarth, M.; Lundström, I. An ammonia detector based on a conducting polymer. Anal. Chem. Symp. Ser 1983, 17, 203–207. [Google Scholar]
- Chen, D.; Xu, Y.; Wang, J.; Zhang, L. Nerve gas sensor using film bulk acoustic resonator modified with a self-assembled Cu2+/11-mercaptoundecanoic acid bilayer. Sens. Actuators B Chem. 2010, 150, 483–486. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Fan, L.; Lan, Z.; Li, P.; Lin, J.; Hao, S. High-performance polypyrrole nanoparticles counter electrode for dye-sensitized solar cells. J. Power Sources 2008, 181, 172–176. [Google Scholar] [CrossRef]
- Li, B.; Lambeth, D.N. Chemical sensing using nanostructured polythiophene transistors. Nano Lett. 2008, 8, 3563–3567. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Shirakawa, H.; McDiarmid, A.; Heeger, A. Twenty-five years of conducting polymers. Chem. Commun. 2003, 2003, 1–4. [Google Scholar] [CrossRef]
- Swager, T.M. 50th anniversary perspective: Conducting/semiconducting conjugated polymers. A personal perspective on the past and the future. Macromolecules 2017, 50, 4867–4886. [Google Scholar] [CrossRef]
- Yang, Z.; Han, S.; Liu, Y.; Zhuang, X.; Akinwande, D.; Yu, J. Investigation of the atmosphere influence on device characteristics and NO2 sensing performance of organic field-effect transistors consisting of polymer bulk heterojunction. Org. Electron. 2018, 62, 114–120. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C.; Dzhardimalieva, G.I. Preparation and properties of nanostructured PANI thin film and its application as low temperature NO2 sensor. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1428–1433. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, A.; Baghaei Nejad, M.; Shahrokh Abadi, M.H.; Zou, Z.; Zheng, L.-R. Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Liu, C.; Noda, Z.; Sasaki, K.; Hayashi, K. Development of a polyaniline nanofiber-based carbon monoxide sensor for hydrogen fuel cell application. Int. J. Hydrogen Energy 2012, 37, 13529–13535. [Google Scholar] [CrossRef]
- Karmakar, N.; Fernandes, R.; Jain, S.; Patil, U.V.; Shimpi, N.G.; Bhat, N.V.; Kothari, D.C. Room temperature NO2 gas sensing properties of p-toluenesulfonic acid doped silver-polypyrrole nanocomposite. Sens. Actuators B Chem. 2017, 242, 118–126. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Bogue, R. Nanomaterials for gas sensing: A review of recent research. Sens. Rev. 2014, 34, 1–8. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple method of preparing graphene flakes by an arc-discharge method. J. Phys. Chem. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Russo, P.; Hu, A.; Compagnini, G. Synthesis, properties and potential applications of porous graphene: A review. Nano-Micro Lett. 2013, 5, 260–273. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, R.; Hu, N.; Chai, J.; Cheng, Y.; Zhang, L.; Wei, H.; Kong, E.S.-W.; Zhang, Y. The prospective two-dimensional graphene nanosheets: Preparation, functionalization and applications. Nano-Micro Lett. 2012, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, H.Y.; Lee, D.-S.; Choi, S.-Y.; Choi, C.-G. Flexible NO2 gas sensor using multilayer graphene films by chemical vapor deposition. Carbon Lett. 2013, 14, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Nemade, K.R.; Waghuley, S.A. Chemiresistive gas sensing by few-layered graphene. J. Electron. Mater. 2013, 42, 2857–2866. [Google Scholar] [CrossRef]
- Hu, N.; Wang, Y.; Chai, J.; Gao, R.; Yang, Z.; Kong, E.S.-W.; Zhang, Y. Gas sensor based on p-phenylenediamine reduced graphene oxide. Sens. Actuators B Chem. 2012, 163, 107–114. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Mittal, M.; Kumar, A. Carbon nanotube (CNT) gas sensors for emissions from fossil fuel burning. Sens. Actuators B Chem. 2014, 203, 349–362. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.; Chen, J. Nanocarbon-based gas sensors: Progress and challenges. J. Mater. Chem. A 2014, 2, 5573–5579. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yeow, J.T.W. A review of carbon nanotubes-based gas sensors. J. Sens. 2009, 2009, 493904. [Google Scholar] [CrossRef]
- Wongwiriyapan, W.; Honda, S.; Konishi, H.; Mizuta, T.; Ohmori, T.; Ito, T.; Maekawa, T.; Suzuki, K.; Ishikawa, H.; Murakami, T.; et al. Direct growth of single-walled carbon nanotube networks on alumina substrate: A novel route to ultrasensitive gas sensor fabrication. Jpn. J. Appl. Phys. 2005, 44, 8227. [Google Scholar] [CrossRef]
- Hoa, N.D.; Van Quy, N.; Cho, Y.; Kim, D. Porous single-wall carbon nanotube films formed by in situ arc-discharge deposition for gas sensors application. Sens. Actuators B Chem. 2009, 135, 656–663. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C.; et al. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Han, Z.J.; Mehdipour, H.; Li, X.; Shen, J.; Randeniya, L.; Yang, H.Y.; Ostrikov, K. SWCNT networks on nanoporous silica catalyst support: Morphological and connectivity control for nanoelectronic, gas-sensing, and biosensing devices. ACS Nano 2012, 6, 5809–5819. [Google Scholar] [CrossRef]
- Ahmadi, S.S.S.; Raissi, B.; Riahifar, R.; Yaghmaee, M.S.; Saadati, H.R.; Ghashghaie, S.; Javaheri, M. Fabrication of counter electrode of electrochemical CO gas sensor by electrophoretic deposition of MWCNT. J. Electrochem. Soc. 2015, 162, D3101. [Google Scholar] [CrossRef]
- Valentini, L.; Lozzi, L.; Cantalini, C.; Armentano, I.; Kenny, J.M.; Ottaviano, L.; Santucci, S. Effects of oxygen annealing on gas sensing properties of carbon nanotube thin films. Thin Solid Films 2003, 436, 95–100. [Google Scholar] [CrossRef]
- Chung, J.; Lee, K.-H.; Lee, J.; Troya, D.; Schatz, G.C. Multi-walled carbon nanotubes experiencing electrical breakdown as gas sensors. Nanotechnology 2004, 15, 1596. [Google Scholar] [CrossRef]
- Kawano, T.; Chiamori, H.C.; Suter, M.; Zhou, Q.; Sosnowchik, B.D.; Lin, L. An electrothermal carbon nanotube gas sensor. Nano Lett. 2007, 7, 3686–3690. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-C.; Chang, P.-C.; Chen, Y.-H.; Liu, C.-M.; Wu, C.-T.; Yen, H.-W.; Lin, W.-C. Reversible 90-Degree Rotation of Fe Magnetic Moment Using Hydrogen. Sci. Rep. 2018, 8, 3251. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, N.; Jiang, C.; Xia, B. Characterization of CuO-reduced graphene oxide sandwiched nanostructure and its hydrogen sensing characteristics. J. Mater. Sci. Mater. Electron. 2017, 28, 2763–2768. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, H.; Zhao, J.; Gao, S. Micro humidity sensors based on ZnO–In2O3 thin films with high performances. Sens. Actuators B Chem. 2012, 165, 76–81. [Google Scholar] [CrossRef]
- Larin, A.; Womble, P.C.; Dobrokhotov, V. Hybrid SnO2/TiO2 nanocomposites for selective detection of ultra-low hydrogen sulfide concentrations in complex backgrounds. Sensors 2016, 16, 1373. [Google Scholar] [CrossRef] [PubMed]
- Hoa, L.T.; Tien, H.N.; Luan, V.H.; Chung, J.S.; Hur, S.H. Fabrication of a novel 2D-graphene/2D-NiO nanosheet-based hybrid nanostructure and its use in highly sensitive NO2 sensors. Sens. Actuators B Chem. 2013, 185, 701–705. [Google Scholar] [CrossRef]
- Pasha, A.; Khasim, S.; Khan, F.A.; Dhananjaya, N. Fabrication of gas sensor device using poly (3, 4-ethylenedioxythiophene)-poly (styrenesulfonate)-doped reduced graphene oxide organic thin films for detection of ammonia gas at room temperature. Iran. Polym. J. 2019, 28, 183–192. [Google Scholar] [CrossRef]
- Evans, G.P.; Powell, M.J.; Johnson, I.D.; Howard, D.P.; Bauer, D.; Darr, J.A.; Parkin, I.P. Room temperature vanadium dioxide–carbon nanotube gas sensors made via continuous hydrothermal flow synthesis. Sens. Actuators B Chem. 2018, 255, 1119–1129. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Shen, F.; Tian, X.; Wang, D.; Zhang, T.; Chen, W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H 2 S gas sensing with ultrahigh sensitivity. Nanoscale 2013, 5, 1564–1569. [Google Scholar] [CrossRef]
- Wang, L.; Huang, H.; Xiao, S.; Cai, D.; Liu, Y.; Liu, B.; Wang, D.; Wang, C.; Li, H.; Wang, Y.; et al. Enhanced sensitivity and stability of room-temperature NH3 sensors using core-shell CeO2 nanoparticles@ cross-linked PANI with p–n heterojunctions. ACS Appl. Mater. Interfaces 2014, 6, 14131–14140. [Google Scholar] [CrossRef]
- Eising, M.; Cava, C.E.; Salvatierra, R.V.; Zarbin, A.J.G.; Roman, L.S. Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor. Sens. Actuators B Chem. 2017, 245, 25–33. [Google Scholar] [CrossRef]
- Ge, W.; Chang, Y.; Natarajan, V.; Feng, Z.; Zhan, J.; Ma, X. In2O3-SnO2 hybrid porous nanostructures delivering enhanced formaldehyde sensing performance. J. Alloys Compd. 2018, 746, 36–44. [Google Scholar] [CrossRef]
- Shaposhnik, D.; Pavelko, R.; Llobet, E.; Gispert-Guirado, F.; Vilanova, X. Hydrogen sensors on the basis of SnO2-TiO2 systems. Procedia Eng. 2011, 25, 1133–1136. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Li, D.; Han, D.; Luo, R.; Chen, A.; Chung, C.L. Preparation, characterization of WO3-SnO2 nanocomposites and their sensing properties for NO2. Sens. Actuators B Chem. 2010, 150, 749–755. [Google Scholar] [CrossRef]
- Gautam, M.; Jayatissa, A.H. Ammonia gas sensing behavior of graphene surface decorated with gold nanoparticles. Solid. State. Electron. 2012, 78, 159–165. [Google Scholar] [CrossRef]
- Liu, R.; Ding, H.; Lin, J.; Shen, F.; Cui, Z.; Zhang, T. Fabrication of platinum-decorated single-walled carbon nanotube based hydrogen sensors by aerosol jet printing. Nanotechnology 2012, 23, 505301. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, Z.; Zhang, J.; Pu, J.; Lin, Y.; Xu, S.; Shen, L.; Chen, Q.; Shi, W. Fully printed, rapid-response sensors based on chemically modified graphene for detecting NO2 at room temperature. ACS Appl. Mater. Interfaces 2014, 6, 7426–7433. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.Q.; Phan, P.Q.; Duong, H.N.; Nguyen, C.D.; Nguyen, L.H. Enhancement of NH3 gas sensitivity at room temperature by carbon nanotube-based sensor coated with Co nanoparticles. Sensors 2013, 13, 1754–1762. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.-Y.; Hsu, M.-C.; Su, P.-G.; Lin, H.-M.; Wu, R.-J.; Lai, H.-J. A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sens. Actuators B Chem. 2004, 101, 81–89. [Google Scholar] [CrossRef]
- Khalilian, M.; Abdi, Y.; Arzi, E. Formation of well-packed TiO2 nanoparticles on multiwall carbon nanotubes using CVD method to fabricate high sensitive gas sensors. J. Nanoparticle Res. 2011, 13, 5257–5264. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Jafri, R.I.; Arockiadoss, T.; Ramaprabhu, S. Nanostructured Pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor. Nanoscale 2009, 1, 382–386. [Google Scholar] [CrossRef]
- Tang, S.; Chen, W.; Zhang, H.; Song, Z.; Li, Y.; Wang, Y. The functionalized single-walled carbon nanotubes gas sensor with Pd nanoparticles for hydrogen detection in the high-voltage transformers. Front. Chem. 2020, 8, 174. [Google Scholar] [CrossRef]
- Shao, F.; Hoffmann, M.W.G.; Prades, J.D.; Zamani, R.; Arbiol, J.; Morante, J.R.; Varechkina, E.; Rumyantseva, M.; Gaskov, A.; Giebelhaus, I.; et al. Heterostructured p-CuO (nanoparticle)/n-SnO2 (nanowire) devices for selective H2S detection. Sens. Actuators B Chem. 2013, 181, 130–135. [Google Scholar] [CrossRef]
- Mashock, M.; Yu, K.; Cui, S.; Mao, S.; Lu, G.; Chen, J. Modulating gas sensing properties of CuO nanowires through creation of discrete nanosized p–n junctions on their surfaces. ACS Appl. Mater. Interfaces 2012, 4, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Van Duy, N.; Chien, N.V.; Hoa, N.D.; Van Hieu, N.; Hjort, K.; Nguyen, H. On-chip growth of patterned ZnO nanorod sensors with PdO decoration for enhancement of hydrogen-sensing performance. Int. J. Hydrogen Energy 2017, 42, 16294–16304. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.; Ma, S.; Yan, S. Hierarchical heterostructure of porous NiO nanosheets on flower-like ZnO assembled by hexagonal nanorods for high-performance gas sensor. Ceram. Int. 2017, 43, 7508–7515. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, L.; Wang, L.; Sun, P.; Lu, H.; Liu, F.; Chuai, X.; Lu, G. Ultrasensitive and low detection limit of toluene gas sensor based on SnO2-decorated NiO nanostructure. Sens. Actuators B Chem. 2018, 255, 3505–3515. [Google Scholar] [CrossRef]
- Tan, W.; Tan, J.; Fan, L.; Yu, Z.; Qian, J.; Huang, X. Fe2O3-loaded NiO nanosheets for fast response/recovery and high response gas sensor. Sens. Actuators B Chem. 2018, 256, 282–293. [Google Scholar] [CrossRef]
- Cui, S.; Wen, Z.; Huang, X.; Chang, J.; Chen, J. Stabilizing MoS2 nanosheets through SnO2 nanocrystal decoration for high-performance gas sensing in air. Small 2015, 11, 2305–2313. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, Y.; You, H.; Hao, R.; Fang, J. Tip enrichment surface-enhanced Raman scattering based on the partial Leidenfrost phenomenon for the ultrasensitive nanosensors. Sens. Actuators B Chem. 2022, 355, 131250. [Google Scholar] [CrossRef]
- Zizzari, A.; Bianco, M.; del Mercato, L.L.; Sorarù, A.; Carraro, M.; Pellegrino, P.; Perrone, E.; Monteduro, A.G.; Bonchio, M.; Rinaldi, R.; et al. Highly sensitive membrane-based pressure sensors (MePS) for real-time monitoring of catalytic reactions. Anal. Chem. 2018, 90, 7659–7665. [Google Scholar] [CrossRef]
- Wang, J.; Lou, Y.; Wang, B.; Sun, Q.; Zhou, M.; Li, X. Highly sensitive, breathable, and flexible pressure sensor based on electrospun membrane with assistance of AgNW/TPU as composite dielectric layer. Sensors 2020, 20, 2459. [Google Scholar] [CrossRef]
- Woo, H.-S.; Na, C.W.; Kim, I.-D.; Lee, J.-H. Highly sensitive and selective trimethylamine sensor using one-dimensional ZnO–Cr2O3 hetero-nanostructures. Nanotechnology 2012, 23, 245501. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Sun, G.-J.; In Lee, W.; Kim, K.K.; Lee, C. Fabrication and NO2 gas sensing performance of TeO2-core/CuO-shell heterostructure nanorod sensors. Nanoscale Res. Lett. 2014, 9, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, A.; Mazady, A.; Anwar, M. Co-axial core–shell ZnMgO/ZnO NWs. Solid. State. Electron. 2015, 104, 126–130. [Google Scholar] [CrossRef]
- Liu, D.; Lin, L.; Chen, Q.; Zhou, H.; Wu, J. Low Power Consumption Gas Sensor Created from Silicon Nanowires/TiO2 Core–Shell Heterojunctions. ACS Sens. 2017, 2, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G. Study on TiO2-SnO2 core-shell heterostructure nanofibers with different work function and its application in gas sensor. Sens. Actuators B Chem. 2017, 248, 812–819. [Google Scholar] [CrossRef]
- Arafat, M.M.; Haseeb, A.; Akbar, S.A.; Quadir, M.Z. In-situ fabricated gas sensors based on one dimensional core-shell TiO2-Al2O3 nanostructures. Sens. Actuators B Chem. 2017, 238, 972–984. [Google Scholar] [CrossRef]
- Deng, J.; Yu, B.; Lou, Z.; Wang, L.; Wang, R.; Zhang, T. Facile synthesis and enhanced ethanol sensing properties of the brush-like ZnO-TiO2 heterojunctions nanofibers. Sens. Actuators B Chem. 2013, 184, 21–26. [Google Scholar] [CrossRef]
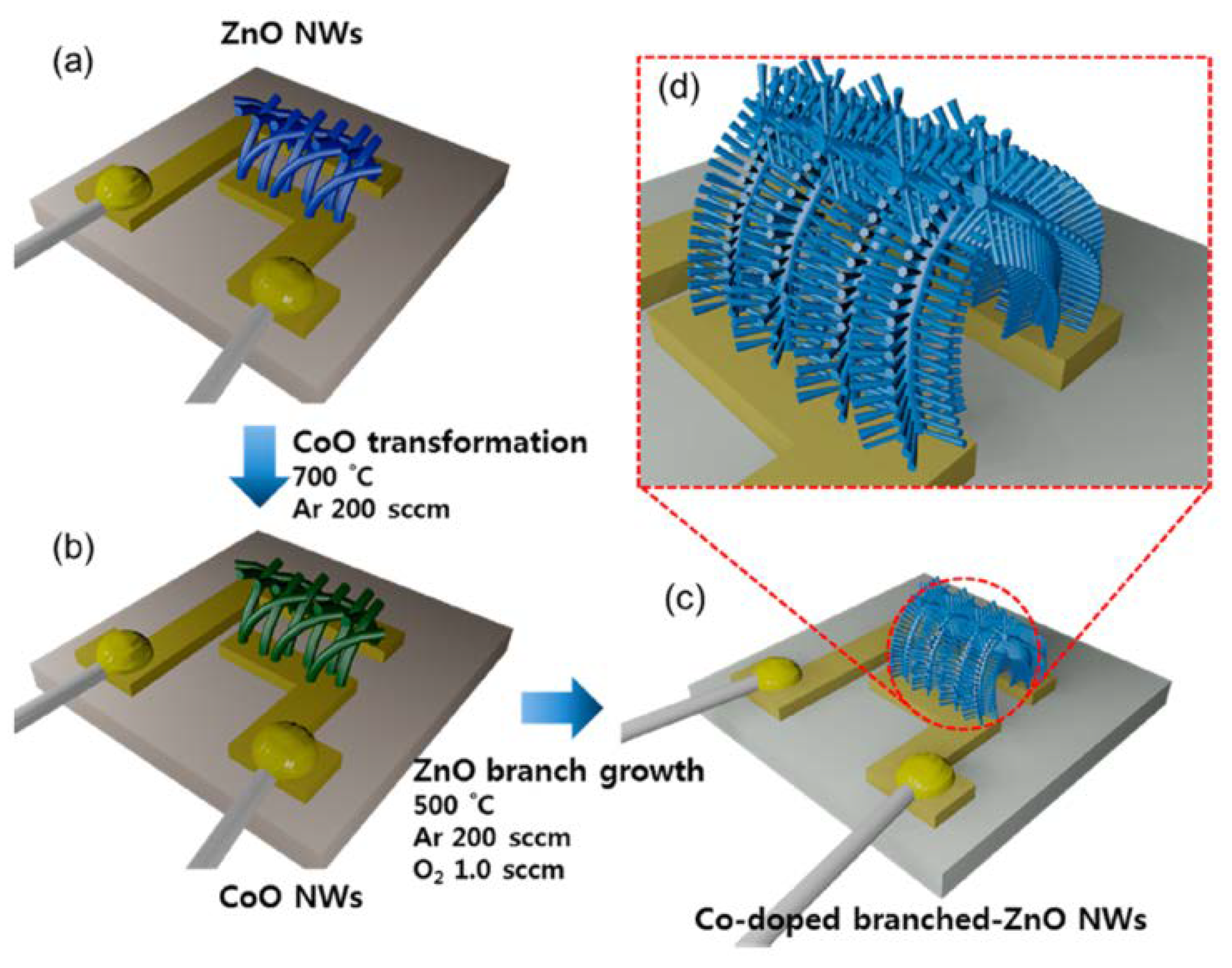
- Woo, H.-S.; Kwak, C.-H.; Chung, J.-H.; Lee, J.-H. Co-doped branched ZnO nanowires for ultraselective and sensitive detection of xylene. ACS Appl. Mater. Interfaces 2014, 6, 22553–22560. [Google Scholar] [CrossRef]

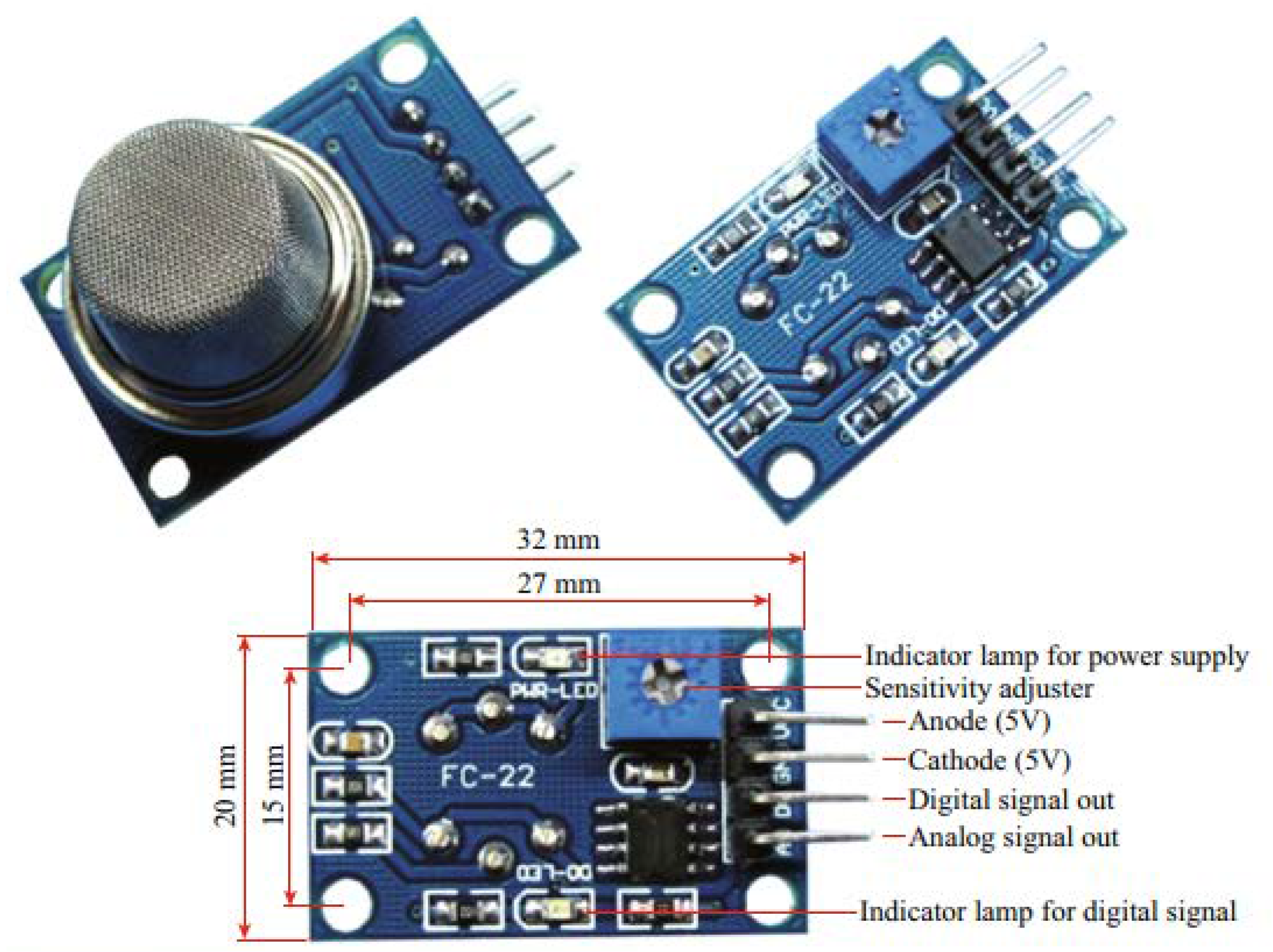
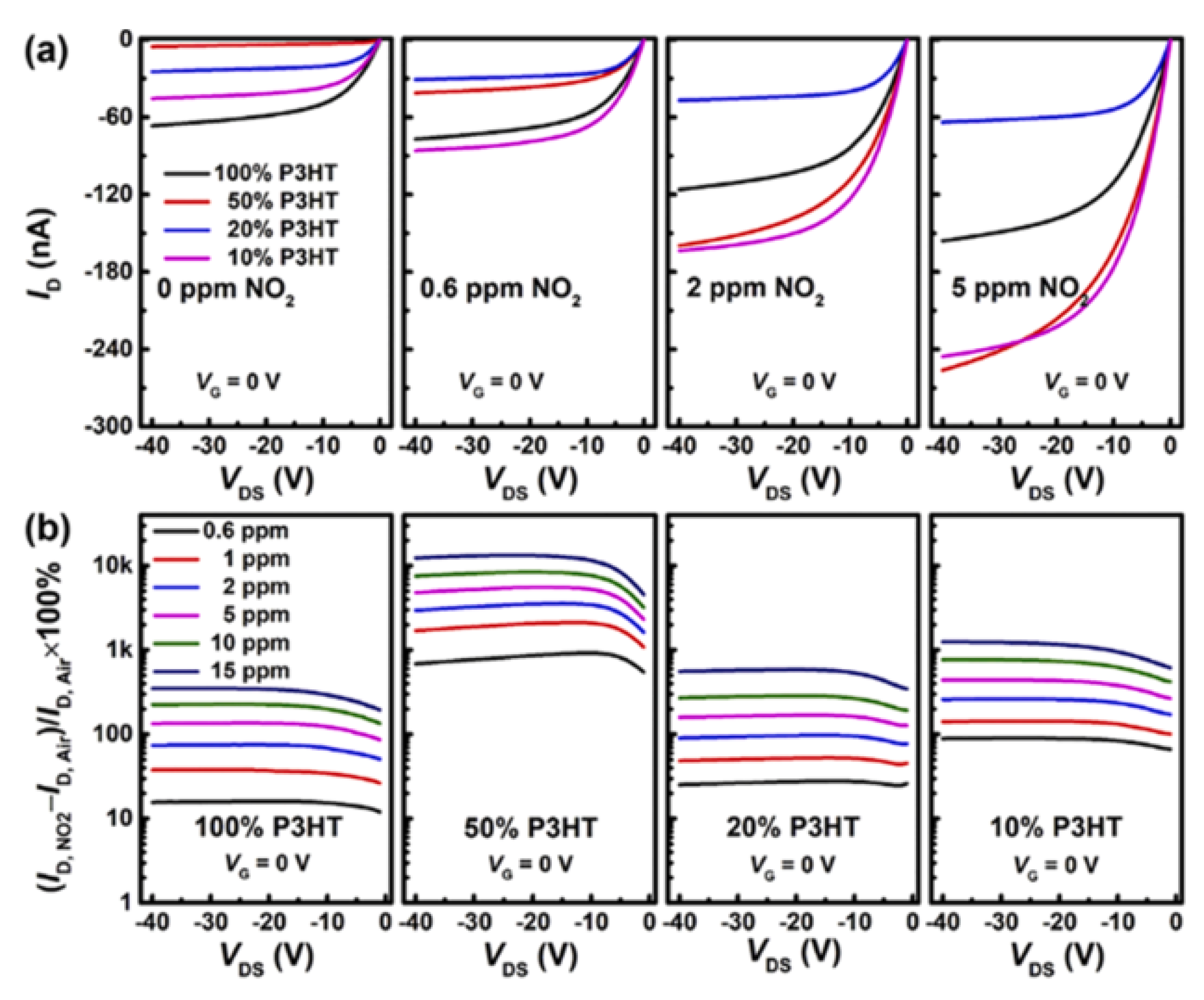
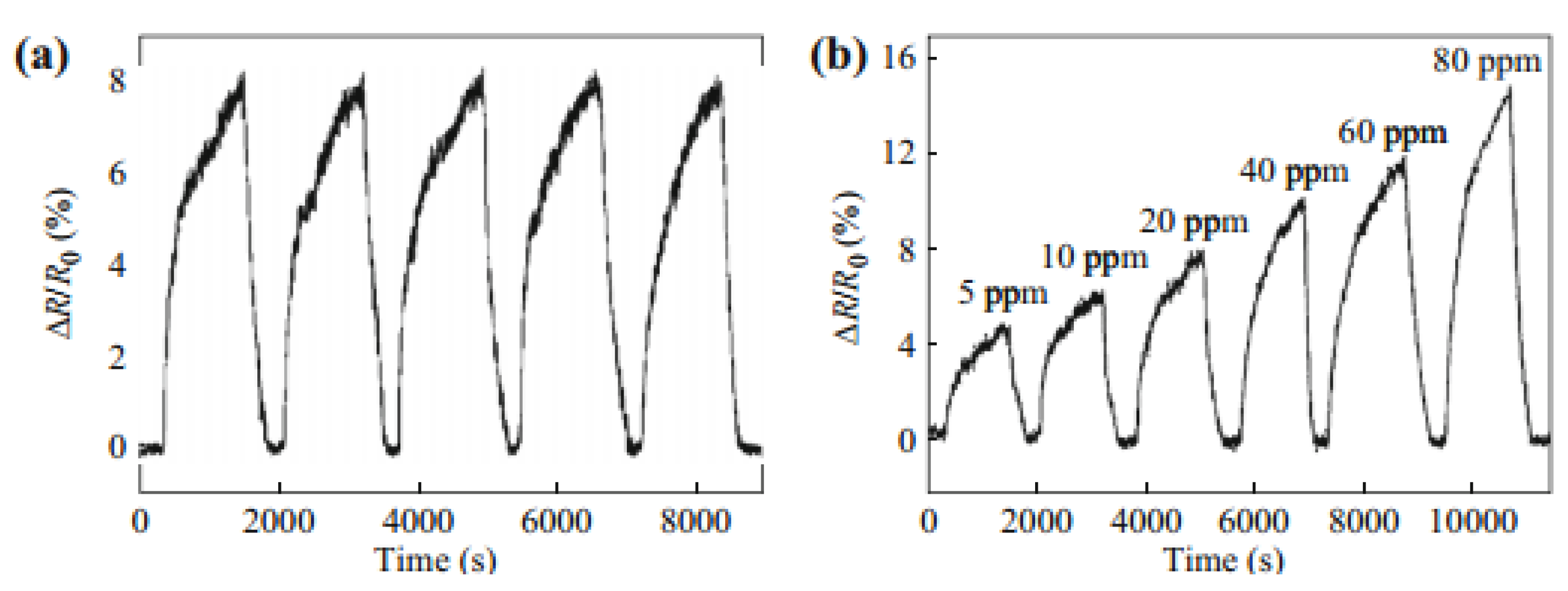
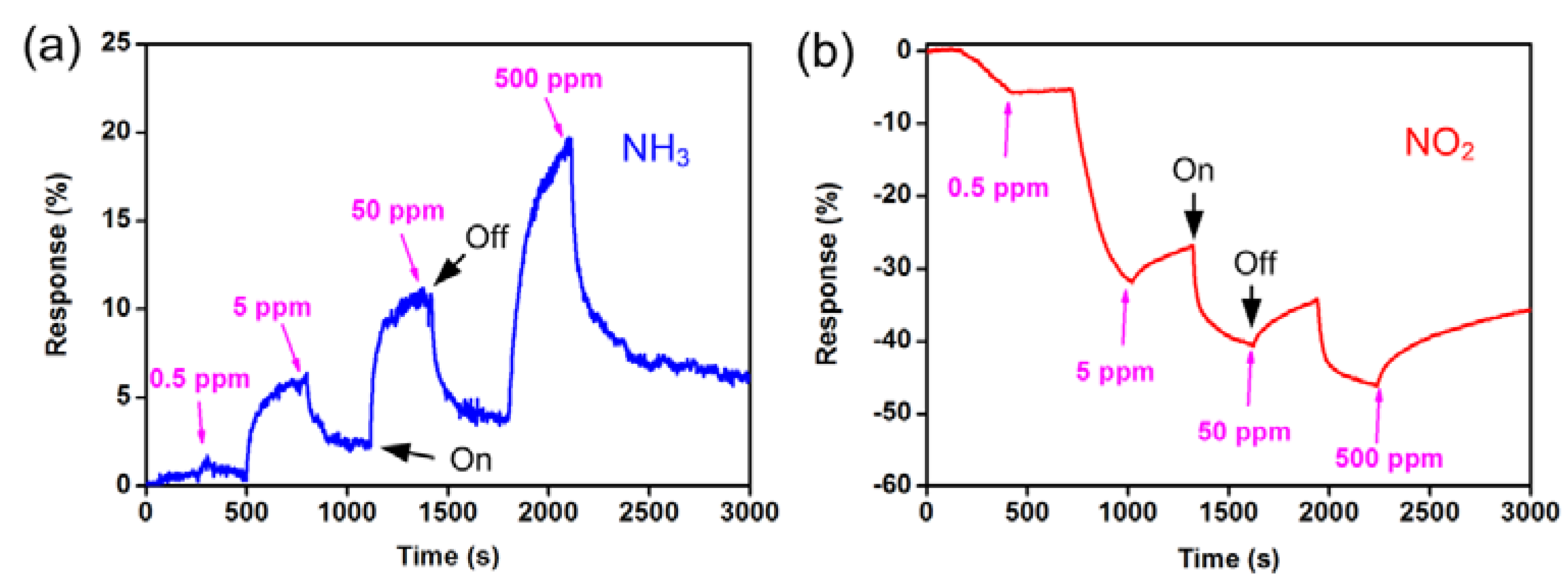
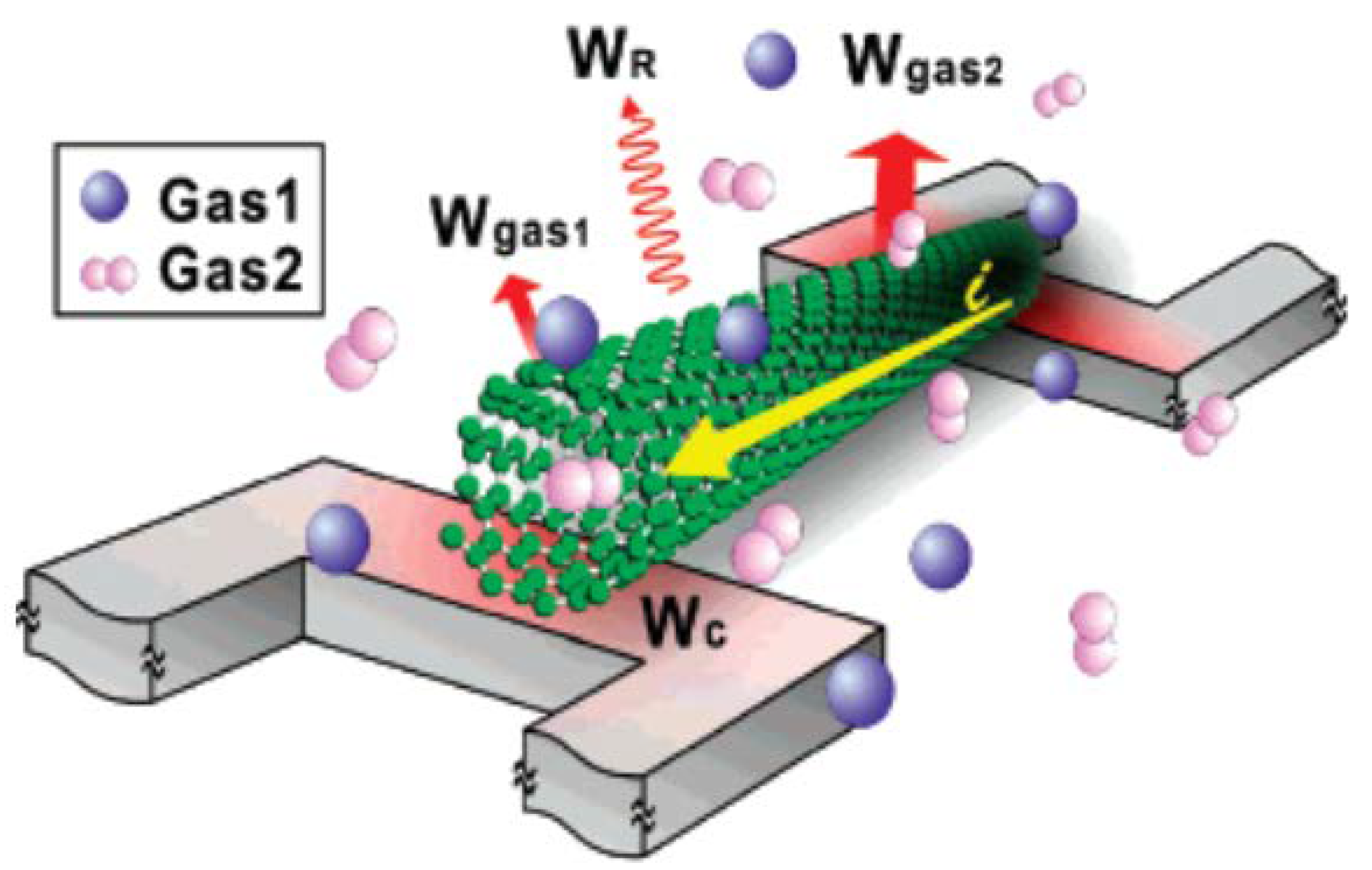
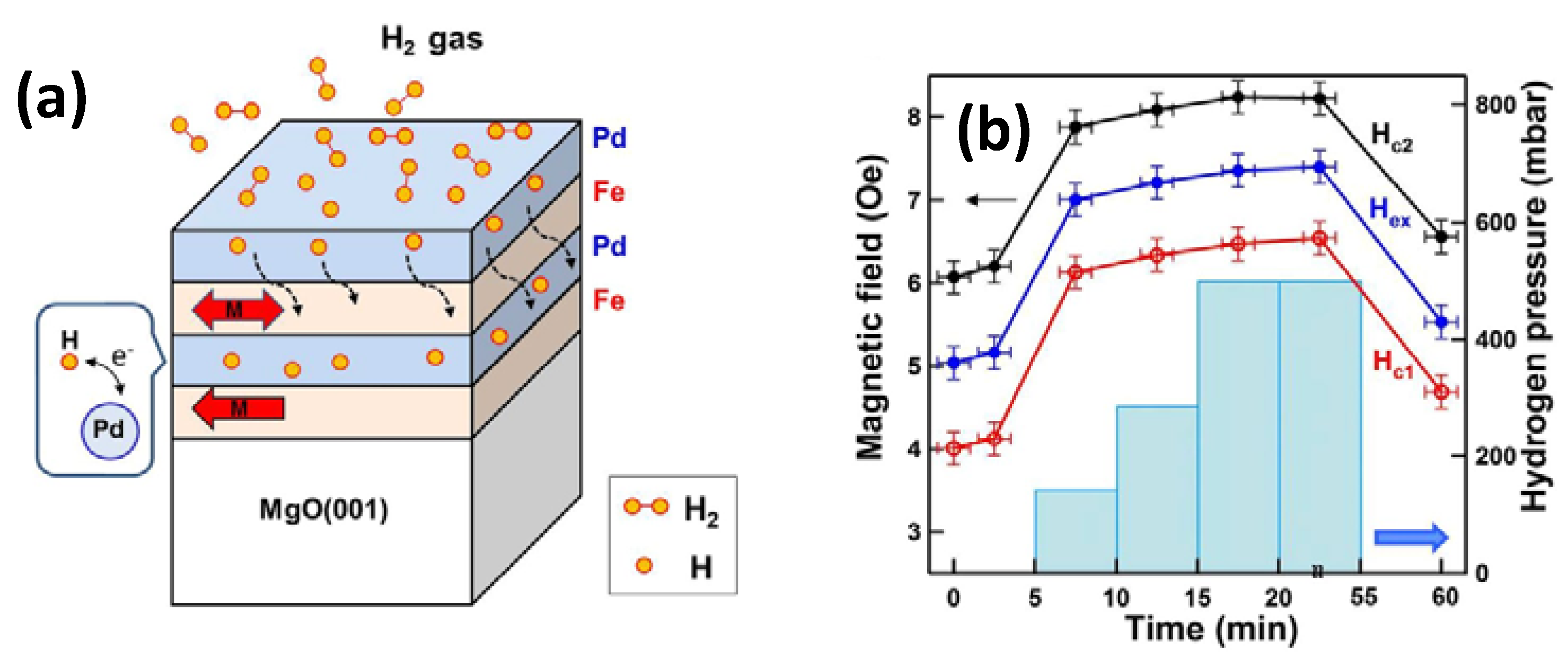

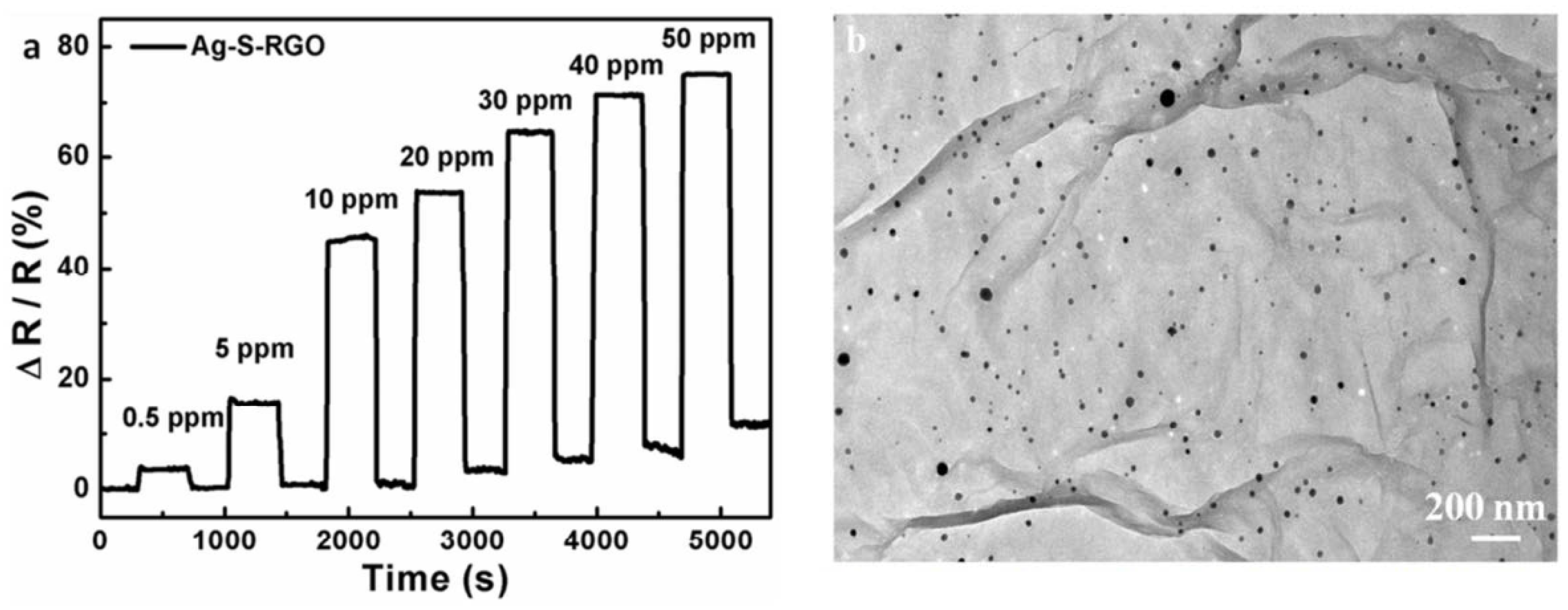
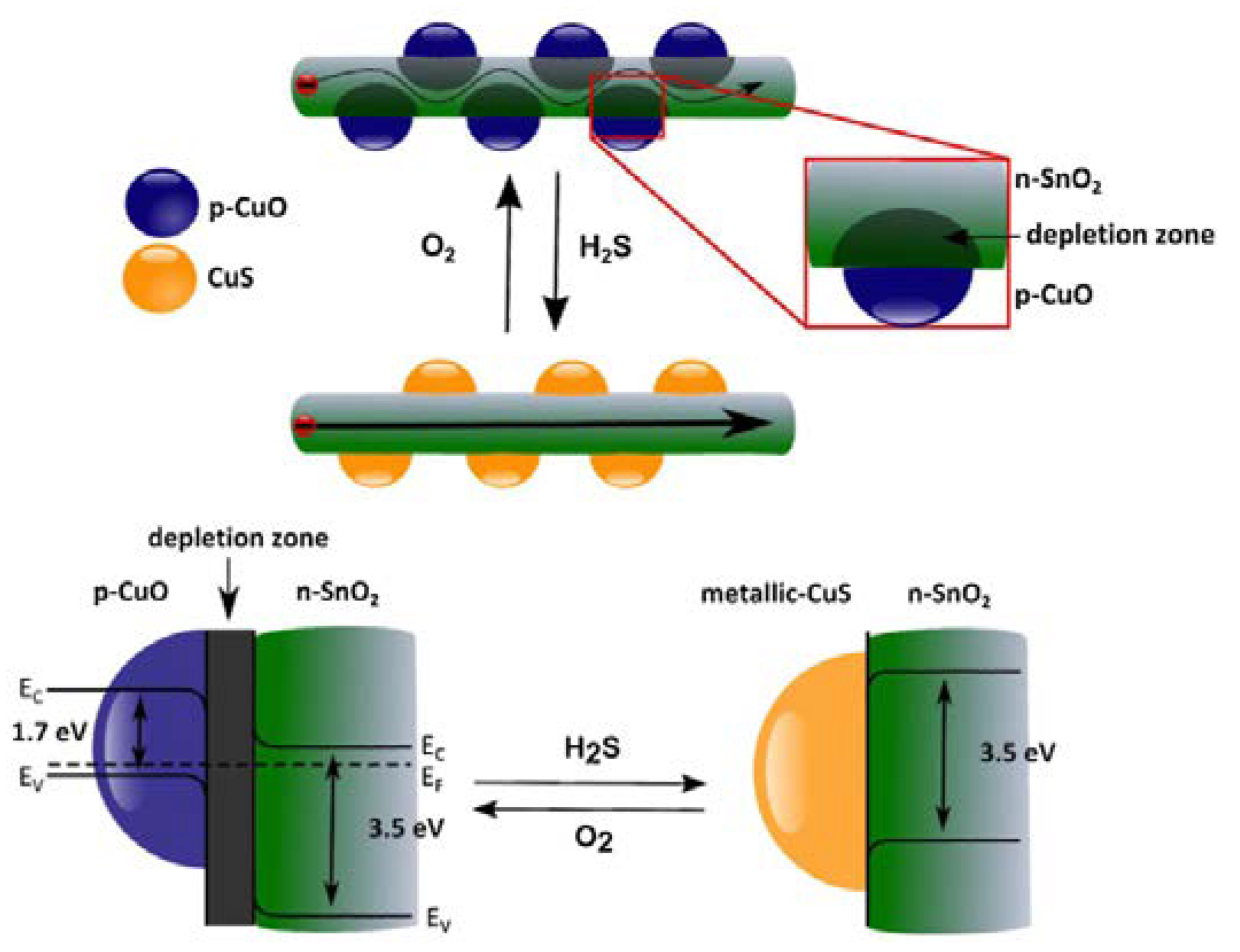
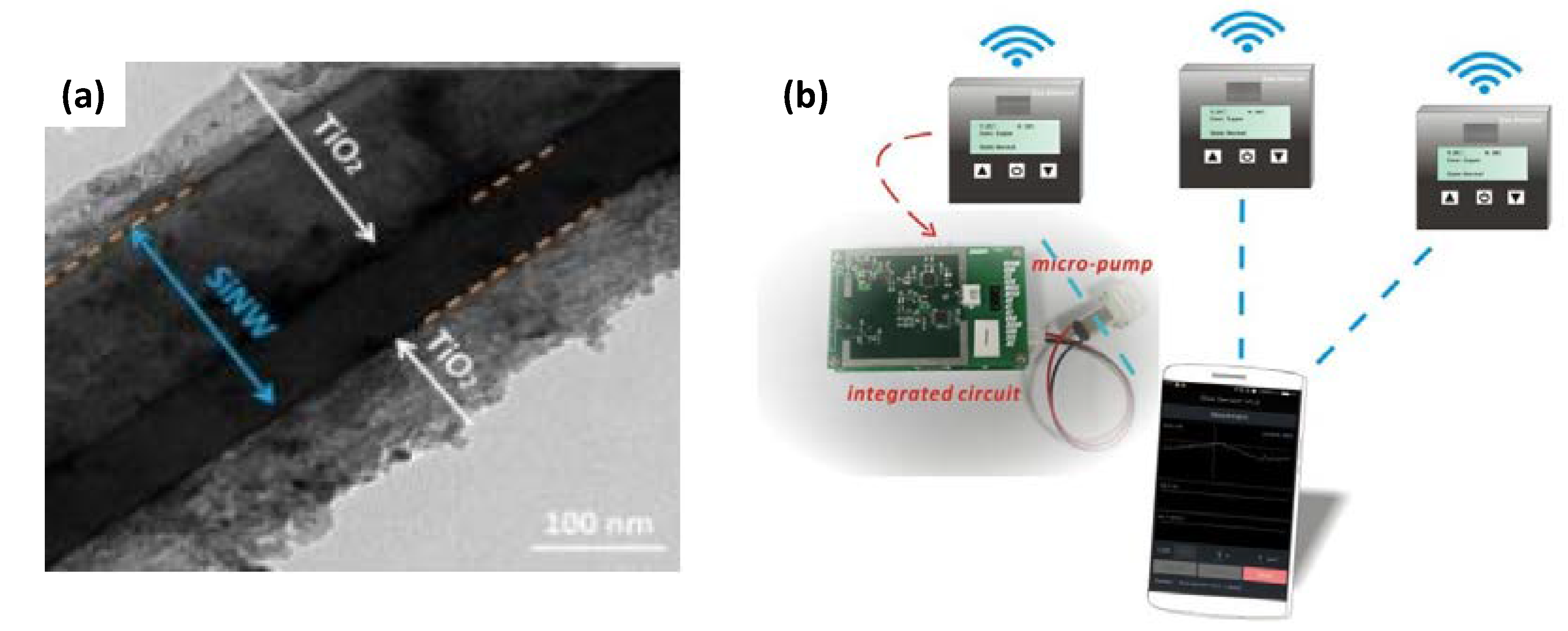
| Parameters | Types of Gas Sensors | ||||
|---|---|---|---|---|---|
| SMO Gas Sensors | Catalytic Combustion Gas Sensors | Electro Chemical Gas Sensors | Thermal Conductivity Gas Sensors | Infrared Absorption Gas Sensors | |
| Sensitivity | E | G | G | P | E |
| Accuracy | G | G | G | G | E |
| Selectivity | F | P | G | P | E |
| Response time | E | G | F | G | F |
| Stability | G | G | P | G | G |
| Durability | G | G | F | G | E |
| Maintenance | E | E | G | G | F |
| Cost | E | E | G | G | F |
| Suitability to portable instruments | E | G | F | G | P |
| Composition | Synthesis Route | Analyte Gas | Performance | Reference |
|---|---|---|---|---|
| PANI thin film | Chemical oxidative polymerization process | NO2 | 12.10% at 100 ppm Tres = 11 s Trec = 7 min | [77] |
| Ag-doped PPy thin film | In-situ oxidation | NO2 | 68% at 100 ppm NO2 Tres = 148 s Trec = 500 s | [80] |
| Single SWCNT | CVD | NO2 | 1000 fold @ 200 ppm Tres = 2–10 s Trec = 12 h | [92] |
| MWCNT | CVD | N2 | Tres = 10 s Trec = 10 s | [103] |
| Sulphonated rGO decorated with Ag nanoparticles | Wet chemical pathway | NO2 | 74.6% @ 50 ppm Tres = 12 s Trec = 20 s | [119] |
| Multilayered graphene | CVD | NO2 | 6% @ 1 ppm Tres = 1800 s | [89] |
| SnO2/CuO multilayered film | PLD | H2S | 2.7 × 104 @ 20 ppm Tres = 2 s | [50] |
| SnO2/TiO2 multilayered film | Sputtering | H2S | 1.6 × 104 @ 10 ppm Tres = 3.2 s Trec = 2.4 s | [107] |
| WO3/SnO2 mixed compund | Sol-precipitation | NO2 | 186 @ 200 ppm | [116] |
| VO2–SWCNT mixed compound | Hydrothermal flow synthesis | NH3 | 10% @ 45 ppm Tres = 7 s Trec = 30 min | [110] |
| PANI-CNT mixed compound | Interfacial polymerization | NH3 | 418% @ 4 ppm Tres = 18 s Trec = 46 s | [113] |
| SWCNT decorated with Pt nanoparticles | Aerosol jet printing | H2 | 4% @ 200 ppm | [118] |
| MWCNTs with TiO2 nanoparticles | CVD | O2 | 15% @ 1000 ppm Tres = 50 s Trec = 100 s | [122] |
| Fe2O3 decorated on NiO nanosheets | Thermal decomposition nanosheets | Ethanol | 170 @ 100 ppm Tres = 0.1 s Trec = 11.4 s | [130] |
| ZnO nanwires decorated with PdO | Hydrothermal and sputtering | H2 | 3.6 @ 500 ppm Tres = 207 s Trec = 92 s | [127] |
| Coreshell CuO@TeO2 nanorods | Thermal oxidation and sputtering | NO2 | 424.91 @ 10 ppm | [136] |
| SnO2@TiO2 core–shell nanofibers | Electro-spinning method | Acetone | 13.5 @ 100 ppm Tres = 2 s Trec = 60 s | [139] |
| NiO/ZnO-branched heterostructures | Vapor phase growth technique | Ethanol | 6.7 @ 50 ppm | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.W.; Pooja, P.; Aamir, M.; Souayeh, B.; Mushtaq, S.; Khan, M.S.; Amin, M.N.; Khan, K.; Shajahan, S. The Recent Development in Chemoresistive-Based Heterostructure Gas Sensor Technology, Their Future Opportunities and Challenges: A Review. Membranes 2022, 12, 555. https://doi.org/10.3390/membranes12060555
Alam MW, Pooja P, Aamir M, Souayeh B, Mushtaq S, Khan MS, Amin MN, Khan K, Shajahan S. The Recent Development in Chemoresistive-Based Heterostructure Gas Sensor Technology, Their Future Opportunities and Challenges: A Review. Membranes. 2022; 12(6):555. https://doi.org/10.3390/membranes12060555
Chicago/Turabian StyleAlam, Mir Waqas, Pheiroijam Pooja, Muhammad Aamir, Basma Souayeh, Shehla Mushtaq, Muhammad Shuaib Khan, Muhammad Nasir Amin, Kaffayatullah Khan, and Shanavas Shajahan. 2022. "The Recent Development in Chemoresistive-Based Heterostructure Gas Sensor Technology, Their Future Opportunities and Challenges: A Review" Membranes 12, no. 6: 555. https://doi.org/10.3390/membranes12060555
APA StyleAlam, M. W., Pooja, P., Aamir, M., Souayeh, B., Mushtaq, S., Khan, M. S., Amin, M. N., Khan, K., & Shajahan, S. (2022). The Recent Development in Chemoresistive-Based Heterostructure Gas Sensor Technology, Their Future Opportunities and Challenges: A Review. Membranes, 12(6), 555. https://doi.org/10.3390/membranes12060555









