Zero Discharge of Dyes and Regeneration of a Washing Solution in Membrane-Based Dye Removal by Cold Plasma Treatment
Abstract
:1. Introduction
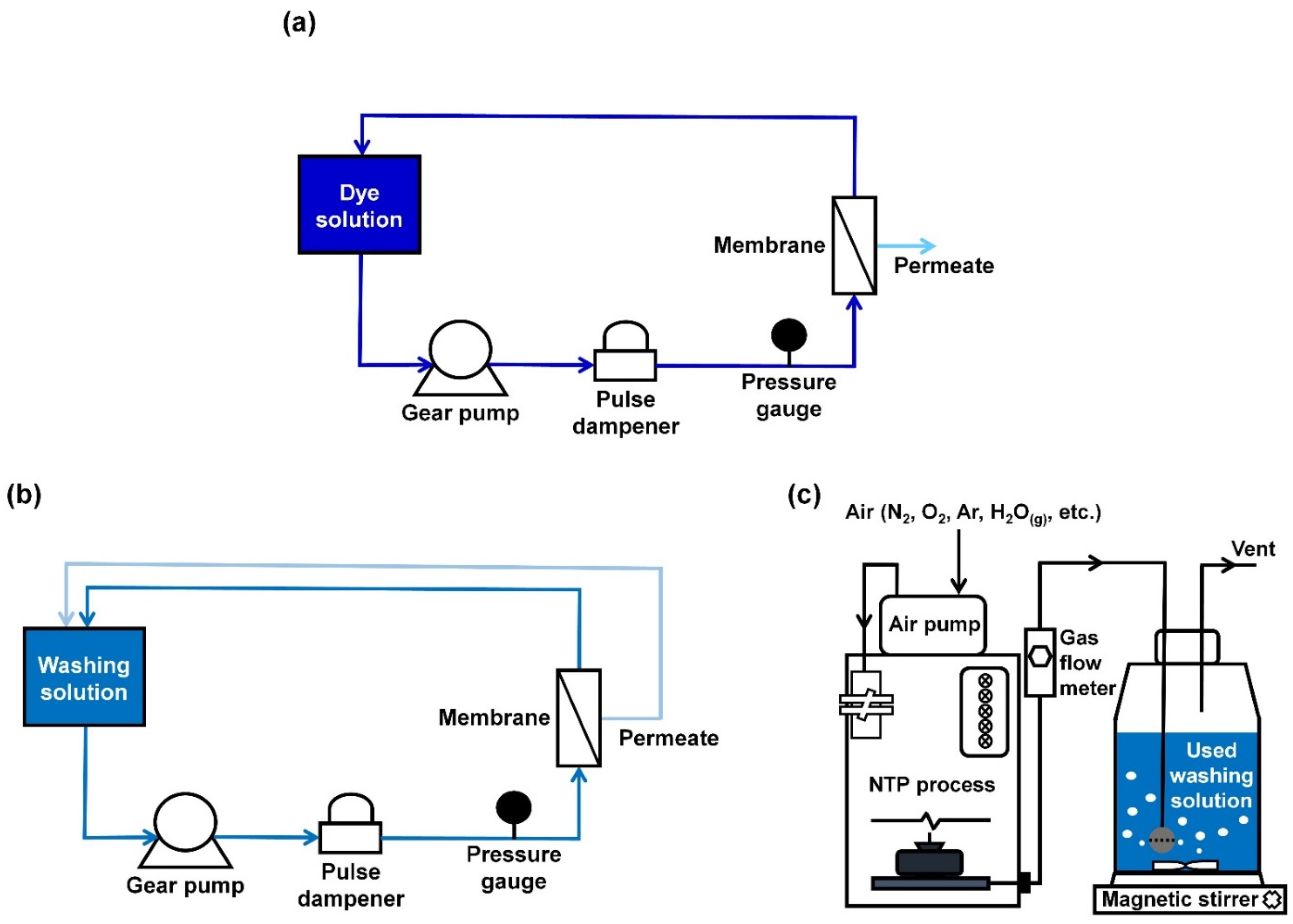
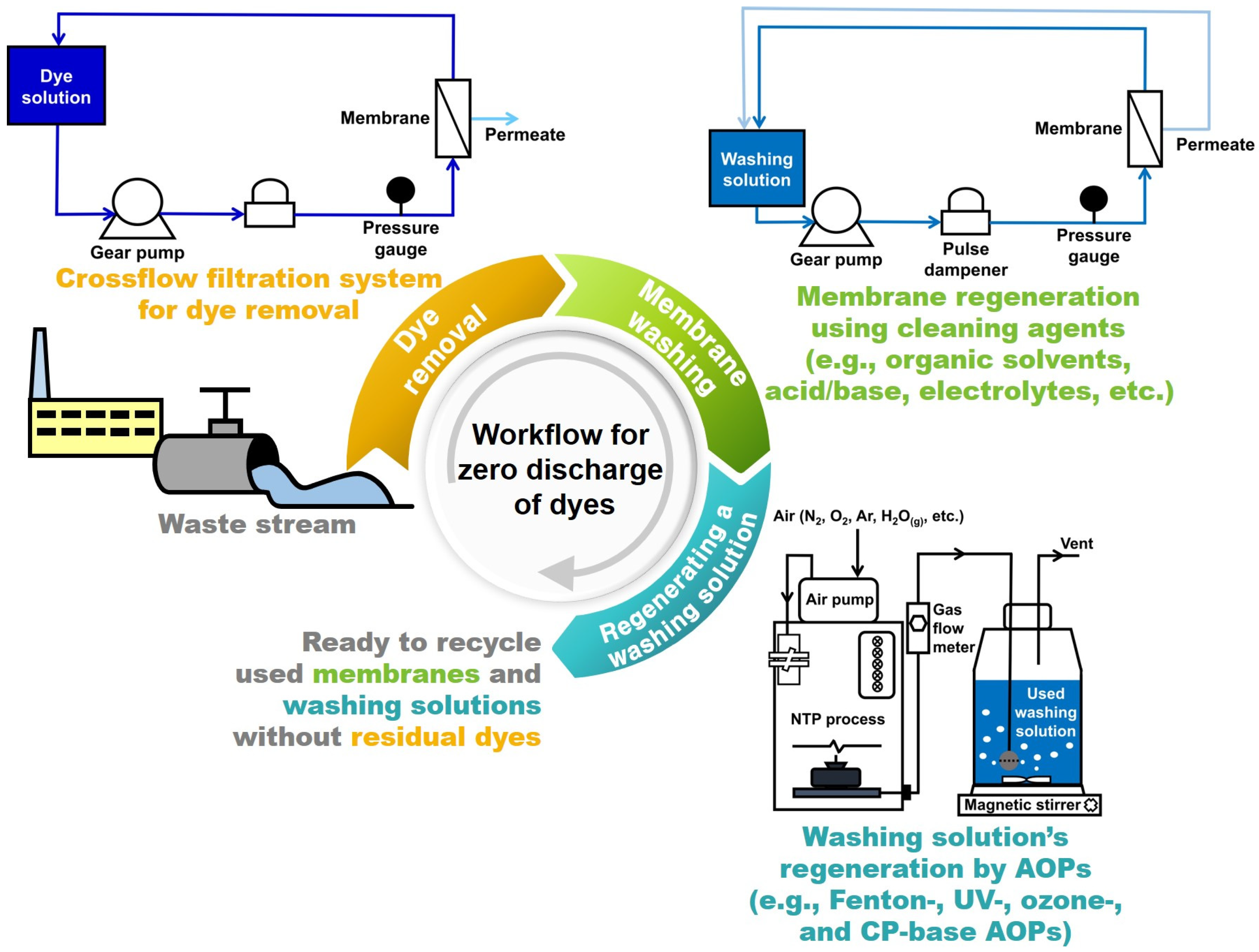
2. Materials and Methods
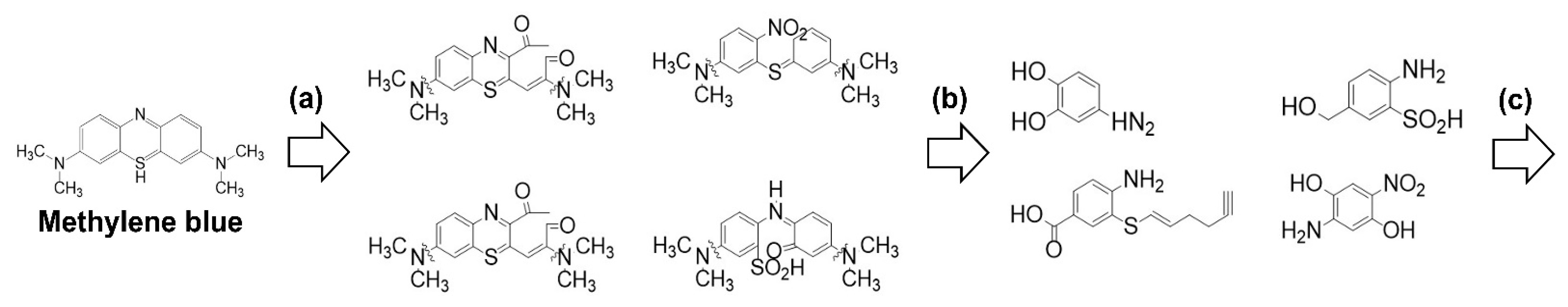
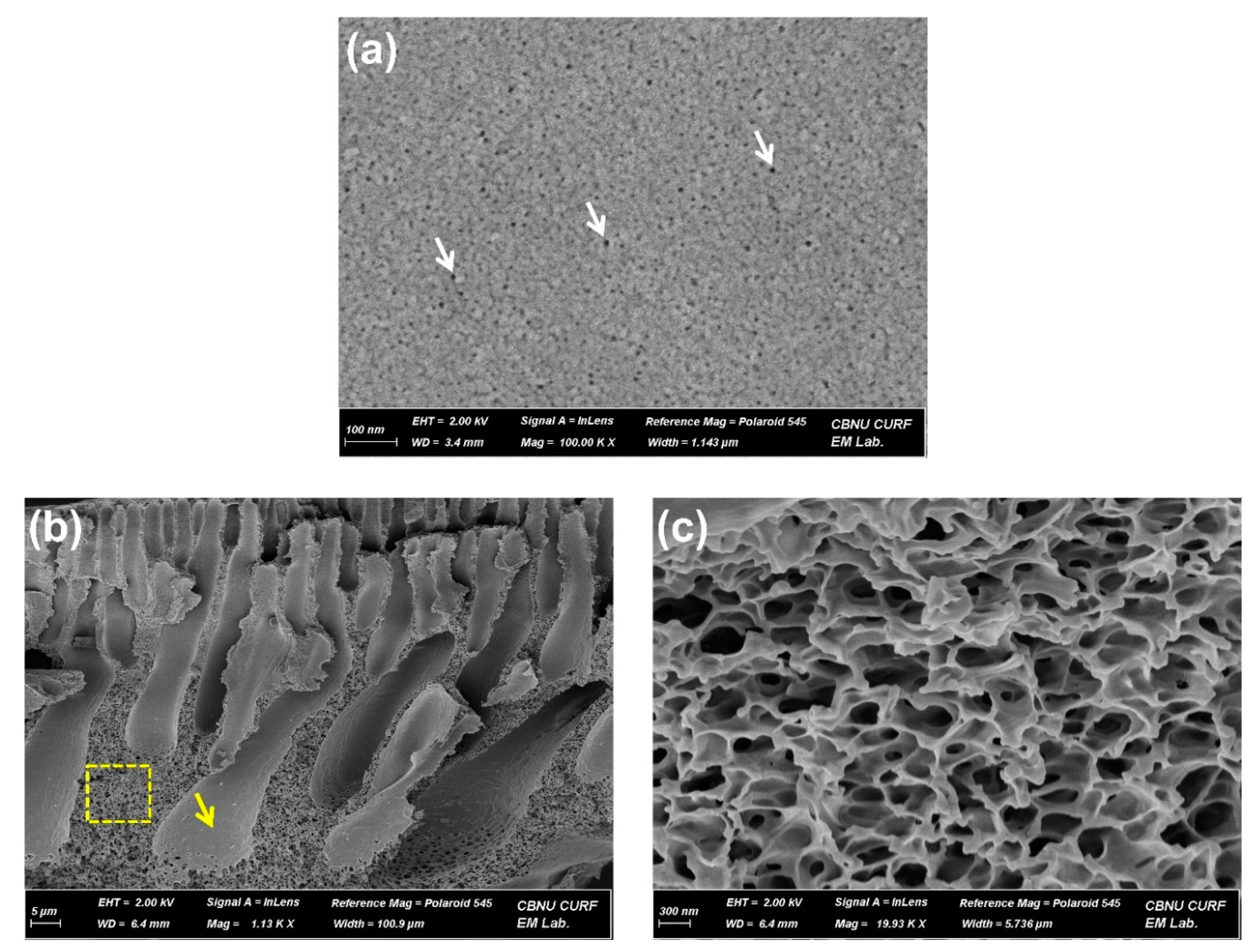
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Service, R.F. Desalination freshens up. Science 2006, 313, 1088–1090. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lee, S.M.; Wang, R.; Lee, J. Emerging materials to prepare mixed matrix membranes for pollutant removal in water. Membranes 2021, 11, 508. [Google Scholar] [CrossRef]
- Kim, T.-K.; Kim, T.; Lee, I.; Choi, K.; Zoh, K.-D. Removal of tetramethylammonium hydroxide (TMAH) in semiconductor wastewater using the nano-ozone H2O2 process. J. Hazard. Mater. 2021, 409, 123759. [Google Scholar] [CrossRef]
- Li, C.; Ma, H.; Venkateswaran, S.; Hsiao, B.S. Highly efficient and sustainable carboxylated cellulose filters for removal of cationic dyes/heavy metals ions. Chem. Eng. J. 2020, 389, 123458. [Google Scholar] [CrossRef]
- Zereshki, S.; Daraei, P.; Shokri, A. Application of edible paraffin oil for cationic dye removal from water using emulsion liquid membrane. J. Hazard. Mater. 2018, 356, 1–8. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Jaikumar, V. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Nandi, D.; Parameswaranpillai, J.; Lee, J.; Shivanna, J.M.; Nithya, R.; Siengchin, S. Adsorption of Cationic Dye onto ZSM-5 Zeolite-Based Bio Membrane: Characterizations, Kinetics and Adsorption Isotherm. J. Polym. Environ. 2022, 1–14. [Google Scholar] [CrossRef]
- Nie, L.; Goh, K.; Wang, Y.; Lee, J.; Huang, Y.; Karahan, H.E.; Zhou, K.; Guiver, M.D.; Bae, T.-H. Realizing small-flake graphene oxide membranes for ultrafast size-dependent organic solvent nanofiltration. Sci. Adv. 2020, 6, eaaz9184. [Google Scholar] [CrossRef] [Green Version]
- Chandarana, H.; Kumar, P.S.; Seenuvasan, M.; Kumar, M.A. Kinetics, equilibrium and thermodynamic investigations of methylene blue dye removal using Casuarina equisetifolia pines. Chemosphere 2021, 285, 131480. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.; Purohit, L. Hetro-nanostructured Se-ZnO sustained with RGO nanosheets for enhanced photocatalytic degradation of p-Chlorophenol, p-Nitrophenol and Methylene blue. Sep. Purif. Technol. 2021, 275, 119219. [Google Scholar] [CrossRef]
- Bangari, R.S.; Yadav, A.; Bharadwaj, J.; Sinha, N. Boron nitride nanosheets incorporated polyvinylidene fluoride mixed matrix membranes for removal of methylene blue from aqueous stream. J. Environ. Chem. Eng. 2022, 10, 107052. [Google Scholar] [CrossRef]
- Dou, T.; Zang, L.; Zhang, Y.; Sun, Z.; Sun, L.; Wang, C. Hybrid g-C3N4 nanosheet/carbon paper membranes for the photocatalytic degradation of methylene blue. Mater. Lett. 2019, 244, 151–154. [Google Scholar] [CrossRef]
- Hashem, A.H.; Saied, E.; Hasanin, M.S. Green and ecofriendly bio-removal of methylene blue dye from aqueous solution using biologically activated banana peel waste. Sustain. Chem. Pharm. 2020, 18, 100333. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Adsorptive removal of organic pollutant methylene blue using polysaccharide-based composite hydrogels. Chemosphere 2022, 286, 131890. [Google Scholar] [CrossRef]
- Moradihamedani, P. Recent advances in dye removal from wastewater by membrane technology: A review. Polym. Bull. 2021, 4, 2603–2631. [Google Scholar] [CrossRef]
- Le-Clech, P. Membrane bioreactors and their uses in wastewater treatments. Appl. Microbiol. Biotechnol. 2010, 88, 1253–1260. [Google Scholar] [CrossRef]
- Kraume, M.; Drews, A. Membrane bioreactors in waste water treatment–Status and trends. Chem. Eng. Technol. 2010, 33, 1251–1259. [Google Scholar] [CrossRef]
- Judd, S. The status of membrane bioreactor technology. Trends Biotechnol. 2008, 26, 109–116. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-M. Graphene Oxide Nanoplatelets Composite Membrane with Hydrophilic and Antifouling Properties for Wastewater Treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Lee, J.; Won, Y.-J.; Choi, D.-C.; Lee, S.; Park, P.-K.; Choo, K.-H.; Oh, H.-S.; Lee, C.-H. Micro-patterned membranes with enzymatic quorum quenching activity to control biofouling in an MBR for wastewater treatment. J. Membr. Sci. 2019, 592, 117365. [Google Scholar] [CrossRef]
- Sethunga, G.; Lee, J.; Wang, R.; Bae, T.-H. Influence of membrane characteristics and operating parameters on transport properties of dissolved methane in a hollow fiber membrane contactor for biogas recovery from anaerobic effluents. J. Membr. Sci. 2019, 589, 117263. [Google Scholar] [CrossRef]
- Mehmood, C.T.; Zhong, Z.; Zhou, H.; Xiao, Y. Constructing porous beads with modified polysulfone-alginate and TiO2 as a robust and recyclable photocatalyst for wastewater treatment. J. Water Process Eng. 2020, 38, 101601. [Google Scholar] [CrossRef]
- Rouquié, C.; Szymczyk, A.; Rabiller-Baudry, M.; Roberge, H.; Abellan, P.; Riaublanc, A.; Frappart, M.; Álvarez-Blanco, S.; Couallier, E. NaCl precleaning of microfiltration membranes fouled with oil-in-water emulsions: Impact on fouling dislodgment. Sep. Purif. Technol. 2022, 285, 120353. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Su, K.; Fan, T.; Cao, L. Mussel-inspired modification of PPS membrane to separate and remove the dyes from the wastewater. Chem. Eng. J. 2018, 341, 371–382. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Miao, Y.-E.; Tjiu, W.W.; Liu, T. Polydopamine-coated electrospun poly (vinyl alcohol)/poly (acrylic acid) membranes as efficient dye adsorbent with good recyclability. J. Hazard. Mater. 2015, 283, 730–739. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, L.; Zhao, Y.; Zhu, L. Interfacially crosslinked composite porous membranes for ultrafast removal of anionic dyes from water through permeating adsorption. J. Hazard. Mater. 2017, 337, 217–225. [Google Scholar] [CrossRef]
- Yang, F.; Sadam, H.; Zhang, Y.; Xia, J.; Yang, X.; Long, J.; Li, S.; Shao, L. A de novo sacrificial-MOF strategy to construct enhanced-flux nanofiltration membranes for efficient dye removal. Chem. Eng. Sci. 2020, 225, 115845. [Google Scholar] [CrossRef]
- Kim, H.-J.; Won, C.-H.; Kim, H.-W. Optimized Pretreatment of Non-Thermal Plasma for Advanced Sewage Oxidation. Int. J. Environ. Res. Public Health 2020, 17, 7694. [Google Scholar] [CrossRef]
- Lv, J.; Zhao, F.; Feng, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. The impact of particulate and soluble organic matter on physicochemical properties of extracellular polymeric substances in a microalga Neocystis mucosa SX. Algal Res. 2020, 51, 102064. [Google Scholar] [CrossRef]
- Kim, H.-J.; Won, C.-H.; Hong, Y.-P.; Lee, I.H.; Kim, H.-W. Energy-effective elimination of harmful microcystins by a non-thermal plasma process. Chemosphere 2021, 284, 131338. [Google Scholar] [CrossRef] [PubMed]
- Zeghioud, H.; Nguyen-Tri, P.; Khezami, L.; Amrane, A.; Assadi, A.A. Review on discharge Plasma for water treatment: Mechanism, reactor geometries, active species and combined processes. J. Water Process Eng. 2020, 38, 101664. [Google Scholar] [CrossRef]
- Kwak, D.-H.; Jee, S.-I.; Kim, H.-J.; Won, C.-H. Feasibility of glow discharge nonthermal plasma as an alternative pretreatment for low-carbon wastewater in a biological nutrient removal plant. Environ. Eng. Sci. 2018, 35, 1376–1386. [Google Scholar] [CrossRef]
- Park, R.; Kim, J.-G.; Kim, H.-W. Prediction of varying microcystins during non-thermal plasma oxidation of harvested microalgal biomass. J. Hazard. Mater. 2021, 403, 123596. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Dong, B.; Xu, M.; Chi, C.; Wang, C. Degradation of methylene blue using double-chamber dielectric barrier discharge reactor under different carrier gases. Chem. Eng. Sci. 2017, 168, 90–100. [Google Scholar] [CrossRef]
- Wu, L.; Xie, Q.; Lv, Y.; Wu, Z.; Liang, X.; Lu, M.; Nie, Y. Degradation of methylene blue via dielectric barrier discharge plasma treatment. Water 2019, 11, 1818. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jang, J.H.; Chae, H.-R.; Lee, S.H.; Lee, C.-H.; Park, P.-K.; Won, Y.-J.; Kim, I.-C. A facile route to enhance the water flux of a thin-film composite reverse osmosis membrane: Incorporating thickness-controlled graphene oxide into a highly porous support layer. J. Mater. Chem. A 2015, 3, 22053–22060. [Google Scholar] [CrossRef]
- Kim, H.-J.; Nam, G.-S.; Jang, J.-S.; Won, C.-H.; Kim, H.-W. Cold plasma treatment for efficient control over algal bloom products in surface water. Water 2019, 11, 1513. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-A.; Yang, B.; Park, C.; Choi, J.-W.; van Genuchten, C.M.; Lee, S.-H. Oxidation of microcystin-LR by the Fenton process: Kinetics, degradation intermediates, water quality and toxicity assessment. Chem. Eng. J. 2017, 309, 339–348. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.; Ismail, A.; Matsuura, T. Formation of thin film composite nanofiltration membrane: Effect of polysulfone substrate characteristics. Desalination 2013, 329, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Schwarze, M.; Schaefer, L.; Chiappisi, L.; Gradzielski, M. Micellar enhanced ultrafiltration (MEUF) of methylene blue with carboxylate surfactants. Sep. Purif. Technol. 2018, 199, 20–26. [Google Scholar] [CrossRef]
- Khumalo, N.P.; Vilakati, G.D.; Mhlanga, S.D.; Kuvarega, A.T.; Mamba, B.B.; Li, J.; Dlamini, D.S. Dual-functional ultrafiltration nano-enabled PSf/PVA membrane for the removal of Congo red dye. J. Water Process Eng. 2019, 31, 100878. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of dyes using graphene oxide (GO) mixed matrix membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, M.; Najjarizad-Peyvasti, S.; Khataee, A.; Karimi, A. Polyethersulfone ultrafiltration membranes incorporated with CeO2/GO nanocomposite for enhanced fouling resistance and dye separation. J. Environ. Chem. Eng. 2022, 10, 107533. [Google Scholar] [CrossRef]
- Salimi, A.; Roosta, A. Experimental solubility and thermodynamic aspects of methylene blue in different solvents. Thermochim. Acta 2019, 675, 134–139. [Google Scholar] [CrossRef]


| Pressure (Bar) | 1.5 |
| Crossflow rate (L min−1) | 1.8–2.1 L min−1 |
| Polymer (wt%) | Polysulfone (20 wt%) |
| Solvent | NMP |
| Effective membrane area (cm2) | 13.75 |
| Pure water flux (L m−2 h−1, n = 18) | 46.9 (±1.6) |
| CP Treatment Condition | CP Treatment Frequency | Methylene Blue | ||
|---|---|---|---|---|
| Equation | Kinetic Constant (k, min−1) | r2 | ||
| No CP treatment between washing steps | CP treatment after triplicate washing | y = 116.4 (1 − e−0.0318x) | 0.0318 | 0.9869 |
| CP treatment between washing steps | 1st CP treatment | y = 101.2 (1 − e−0.3878x) | 0.3878 | 0.9981 |
| 2nd CP treatment | y = 80.1 (1 − e−0.1176x) | 0.1176 | 0.9933 | |
| 3rd CP treatment | y = 80.9 (1 − e−0.1231x) | 0.1231 | 0.9822 | |
| CP Treatment Condition | CP Treatment Frequency | Methylene Blue | ||
|---|---|---|---|---|
| Removal Rate (%) | Treatment Time (min) | EEO (kWh m−3 order−1) | ||
| No CP treatment between washing steps | CP treatment after triplicate washing | 44.4 | 15 | 20.3 |
| CP treatment between washing steps | 1st CP treatment | 53.1 | 2 | 3.4 |
| 2nd CP treatment | 47.8 | 8 | 10.2 | |
| 3rd CP treatment | 52.4 | 8 | 9.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Lee, U.; Kim, H.-W.; Cho, M.; Lee, J. Zero Discharge of Dyes and Regeneration of a Washing Solution in Membrane-Based Dye Removal by Cold Plasma Treatment. Membranes 2022, 12, 546. https://doi.org/10.3390/membranes12060546
Kim H-J, Lee U, Kim H-W, Cho M, Lee J. Zero Discharge of Dyes and Regeneration of a Washing Solution in Membrane-Based Dye Removal by Cold Plasma Treatment. Membranes. 2022; 12(6):546. https://doi.org/10.3390/membranes12060546
Chicago/Turabian StyleKim, Hee-Jun, Uje Lee, Hyun-Woo Kim, Min Cho, and Jaewoo Lee. 2022. "Zero Discharge of Dyes and Regeneration of a Washing Solution in Membrane-Based Dye Removal by Cold Plasma Treatment" Membranes 12, no. 6: 546. https://doi.org/10.3390/membranes12060546
APA StyleKim, H.-J., Lee, U., Kim, H.-W., Cho, M., & Lee, J. (2022). Zero Discharge of Dyes and Regeneration of a Washing Solution in Membrane-Based Dye Removal by Cold Plasma Treatment. Membranes, 12(6), 546. https://doi.org/10.3390/membranes12060546







